Abstract
Despite evidence of insulin resistance and β-cell dysfunction in glucose metabolism in youth with prediabetes, the relationship between adipose tissue insulin sensitivity (ATIS) and β-cell function remains unknown. We investigated whole-body lipolysis, ATIS, and β-cell function relative to ATIS (adipose disposition index [DI]) in obese youth with impaired glucose tolerance (IGT) versus normal glucose tolerance (NGT). Whole-body lipolysis (glycerol appearance rate [GlyRa], [2H5]glycerol at baseline and during a hyperinsulinemic-euglycemic clamp), lipid oxidation (indirect calorimetry), insulin secretion (2-h hyperglycemic clamp), and body composition (dual-energy X-ray absorptiometry) were examined. Adipose DI was calculated as ATIS: (1/GlyRa × fasting insulin) × first-phase insulin secretion. Despite similar percent body fat, youth with IGT versus NGT had higher GlyRa, lower ATIS at baseline and during hyperinsulinemia, and higher lipid oxidation. Adipose DI was ∼43% lower in youth with IGT and correlated positively with glucose DI. The lower ATIS and diminished adipose DI in IGT versus NGT is in line with the compromised glucose metabolism reflected in impaired β-cell function relative to peripheral insulin resistance. We conclude that youth with IGT manifest a global decline in insulin sensitivity, including impaired insulin action in suppressing lipolysis and lipid oxidation, accompanied by β-cell dysfunction in fat and glucose metabolism, enhancing their risk of type 2 diabetes.
Introduction
A key pathophysiological factor for youth with prediabetes and type 2 diabetes is pancreatic β-cell dysfunction against the backdrop of insulin resistance (1,2). This impairment in glucose-stimulated β-cell function relative to insulin sensitivity (IS) can be detected even in youth with normal glucose tolerance (NGT) (3,4). Short-term elevation of free fatty acids (FFAs) rapidly induces insulin resistance and β-cell lipotoxicity in youth (5,6), signifying an important relationship between lipid metabolism, IS, and insulin secretion.
Given the important role of insulin in adipose tissue lipolysis (7), an accurate assessment of the relationship between adipose tissue IS (ATIS) and β-cell function would broaden our understanding of adipocyte and β-cell dysfunction in youth with prediabetes and type 2 diabetes. A recent study in obese adults suggests that relating adipose insulin resistance to glucose-stimulated insulin secretion provides a novel index of β-cell function (8). However, pathophysiological alterations in lipolysis and the balance between ATIS and β-cell function remain poorly elucidated in youth with dysglycemia.
Therefore, the purpose of this study was to examine the following: 1) whole-body lipolysis, measured with [2H5]glycerol tracer, fasting and during a hyperinsulinemic-euglycemic clamp; 2) lipid oxidation by indirect calorimetry; and 3) the relationship between ATIS and β-cell function (i.e., adipose disposition index [DI]) in obese youth with impaired glucose tolerance (IGT) versus NGT.
Research Design and Methods
Participants
A total of 138 adolescents (12 overweight and 126 obese) from our National Institutes of Health–funded K24 grant investigating insulin resistance in childhood were examined (2–4). The study was approved by the institutional review board of the University of Pittsburgh, and written informed parental consent and child assent were obtained from all participants.
Procedures
All participants underwent medical history, physical examination, and hematological and biochemical tests at the Pediatric Clinical and Translational Research Center of Children’s Hospital of Pittsburgh. Pubertal development was assessed using Tanner criteria (9). Body composition was evaluated with dual-energy X-ray absorptiometry.
Metabolic Studies
Details of metabolic studies and biochemical measurements (glucose, insulin, FFA, lipids, HbA1c, and enrichments of glycerol and glucose) are described in the Supplementary Data. In brief, all participants underwent a hyperinsulinemic-euglycemic clamp combined with stable isotopes and continuous indirect calorimetry and a hyperglycemic clamp after 10–12 h of fasting within a 1–4-week period in random order (2,3). Fasting hepatic glucose production (HGP) was measured by [6,6-2H2]glucose and whole-body lipolysis by [2H5]glycerol as before (10). Peripheral IS was evaluated during a 3-h hyperinsulinemic (80 mU/m2/min)-euglycemic clamp (2–4). Substrate oxidation was measured by continuous indirect calorimetry (Deltatrac Metabolic Monitor; SensorMedics, Anaheim, CA) as previously described (10). First- and second-phase insulin secretion were assessed during a 2-h hyperglycemic (225 mg/dL) clamp (2–4). Glucose tolerance status was determined with HbA1c and/or a 2-h oral glucose tolerance test (OGTT; 1.75 g/kg, maximum 75 g) (11). Fasting blood samples were obtained for lipid profile.
Calculations
Endogenous glycerol appearance rate (GlyRa), indicative of whole-body lipolysis, was calculated as published by us (10). ATIS was calculated as 1/(GlyRa × insulin) at fasting and during hyperinsulinemic-euglycemic clamp steady state. Adipose DI was calculated as fasting ATIS × first-phase insulin secretion. Fasting HGP was calculated during the last 30 min of the 2-h isotope infusion (12). Hepatic IS was calculated as 1/(fasting HGP × insulin) (13). Insulin-stimulated glucose disposal (Rd) was calculated to be equal to the rate of exogenous glucose infusion during the final 30 min of the hyperinsulinemic-euglycemic clamp. Peripheral IS was calculated as (Rd/steady-state clamp insulin) × 100 (12). Insulin-stimulated carbohydrate and lipid oxidation rates were calculated from indirect calorimetry as before (10). Nonoxidative glucose disposal was estimated as total Rd − glucose oxidation rate. During the hyperglycemic clamp, first- and second-phase insulin were calculated during the first 10 min and 15–120 min, respectively (12).
Statistical Analysis
Independent-samples t tests and χ2 analyses were used to compare IGT versus NGT. Spearman correlation analysis was used for bivariate relationships between ATIS/adipose DI and metabolic characteristics. Data that did not meet normality assumptions were log10 transformed; untransformed data are presented for ease of interpretation. Data are mean ± SEM with P ≤ 0.05.
Results
Physical and Metabolic Characteristics of Obese Youth With IGT Versus NGT
There were no differences in age, sex, race, Tanner stage, and percent body fat between the two groups. Youth with IGT versus NGT had higher BMI and fat mass. HbA1c, fasting glucose, and lipids were not different except for lower HDL and higher fasting insulin and a trend for higher FFA in youth with IGT (Table 1).
Table 1.
Physical and metabolic characteristics in obese youth with IGT vs. NGT
| NGT (n = 97) | IGT (n = 41) | P | |
|---|---|---|---|
| Physical characteristics | |||
| Age (years) | 14.3 ± 0.2 | 14.7 ± 0.4 | NS |
| Sex (male/female), n (%) | 35 (36)/62 (64) | 9 (22)/32 (78) | NS |
| Race (AA/AW), n (%) | 46 (47)/51 (53) | 13 (32)/28 (68) | NS |
| Tanner stage (III/IV/V), n (%) | 26 (27)/18 (18)/53 (55) | 4 (10)/7 (17)/30 (73) | NS |
| BMI (kg/m2) | 34.4 ± 0.6 | 36.9 ± 1.0 | 0.029 |
| BMI z score | 2.20 ± 0.05 | 2.35 ± 0.05 | 0.018 |
| Total fat mass (kg) | 38.5 ± 1.3 | 43.2 ± 1.7 | 0.046 |
| Fat-free mass (kg) | 48.0 ± 1.0 | 51.0 ± 1.6 | NS |
| Percent body fat (%) | 42.4 ± 0.8 | 44.5 ± 0.8 | NS |
| Total cholesterol (mg/dL) | 161.6 ± 3.2 | 172.9 ± 5.1 | NS |
| Triglyceride (mg/dL) | 116.2 ± 5.5 | 140.4 ± 13.7 | NS |
| HDL (mg/dL) | 41.7 ± 0.9 | 38.2 ± 1.4 | 0.034 |
| LDL (mg/dL) | 96.8 ± 2.9 | 106.9 ± 4.7 | NS |
| VLDL (mg/dL) | 23.2 ± 1.1 | 26.1 ± 2.4 | NS |
| Metabolic characteristics | |||
| HbA1c (%) | 5.26 ± 0.04 | 5.35 ± 0.07 | NS |
| Fasting glucose (mg/dL) | 95.8 ± 0.6 | 97.5 ± 1.2 | NS |
| Fasting insulin (µU/mL) | 35.6 ± 1.6 | 49.6 ± 4.6 | 0.002 |
| Fasting FFAs (mmol/L) | 0.30 ± 0.01 | 0.35 ± 0.02 | 0.070 |
| Steady-state glucose (mg/dL) | 100.6 ± 0.2 | 100.3 ± 0.3 | NS |
| Steady-state insulin (µU/mL) | 283.5 ± 9.5 | 308.0 ± 13.4 | NS |
| Hyperglycemic clamp parameters | |||
| First-phase insulin concentration (µU/mL) | 248.9 ± 19.6 | 219.3 ± 18.8 | NS |
| Second-phase insulin concentration (µU/mL) | 285.5 ± 15.9 | 323.5 ± 28.7 | NS |
Values are reported as mean ± SEM or n (%). AA, African American; AW, American white.
Whole-Body Lipolysis, Lipid Oxidation, and ATIS
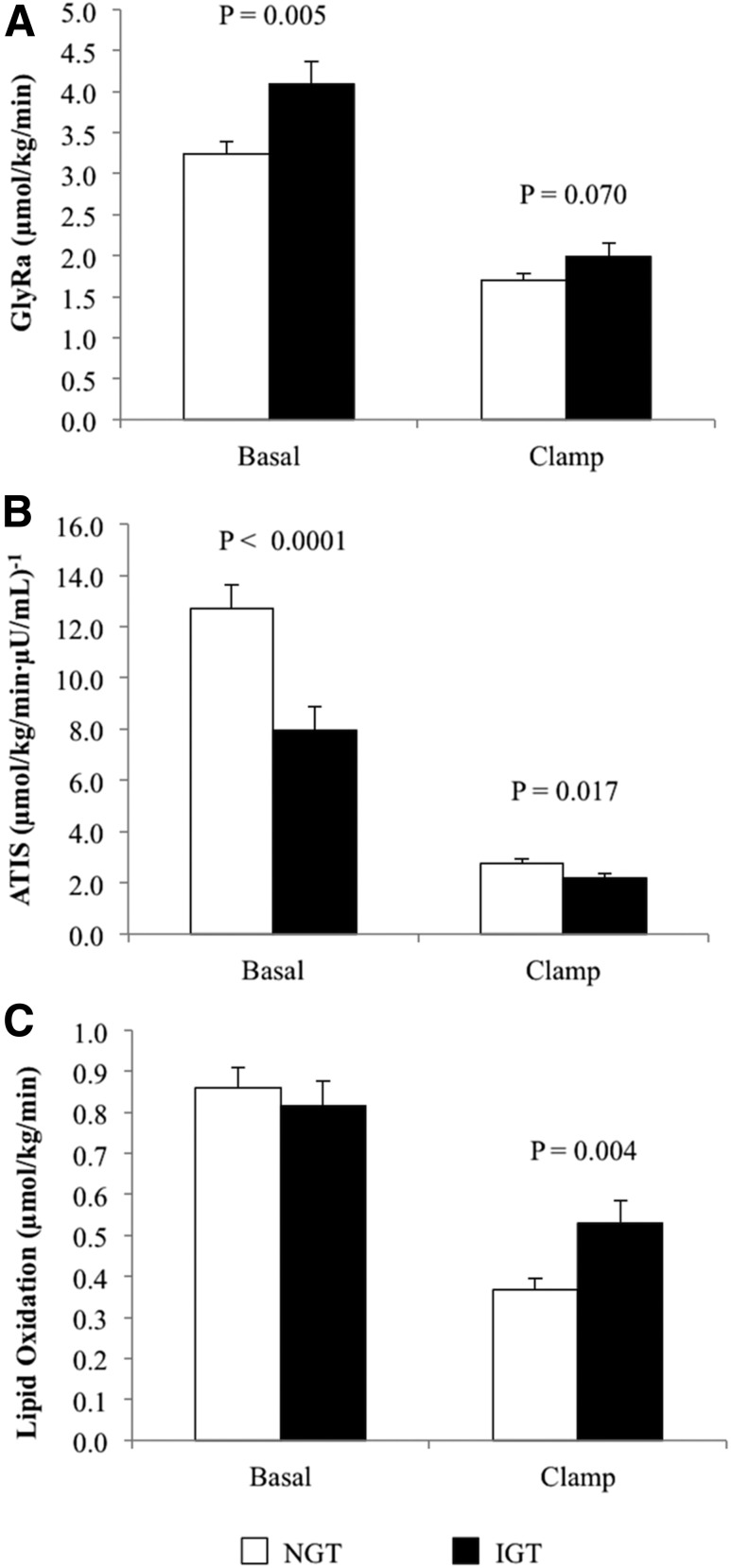
Despite an ∼1.4-fold higher fasting insulin in youth with IGT, basal GlyRa was higher compared with NGT (Fig. 1A). During the hyperinsulinemic-euglycemic clamp, there was a tendency for higher GlyRa in IGT versus NGT (Fig. 1A). Expressing GlyRa per total body, per kilogram fat-free mass, or per kilogram fat mass yielded consistent results (Supplementary Table 1). Fasting and clamp ATIS were lower in IGT versus NGT (Fig. 1B). Lipid oxidation was similar at baseline but higher in IGT during hyperinsulinemia (Fig. 1C). Percent suppression in lipid oxidation from baseline to clamp steady state was lower in youth with IGT versus NGT (NGT 59.6 ± 5.1 vs. IGT 35.1 ± 6.3%, P = 0.009).
Figure 1.
A: Whole-body lipolysis, fasting and during the last 30 min of the hyperinsulinemic-euglycemic clamp. B: ATIS, fasting and during the hyperinsulinemic-euglycemic clamp. C: Lipid oxidation, fasting and during the hyperinsulinemic-euglycemic clamp in youth with IGT vs. NGT.
β-Cell Function Relative to ATIS
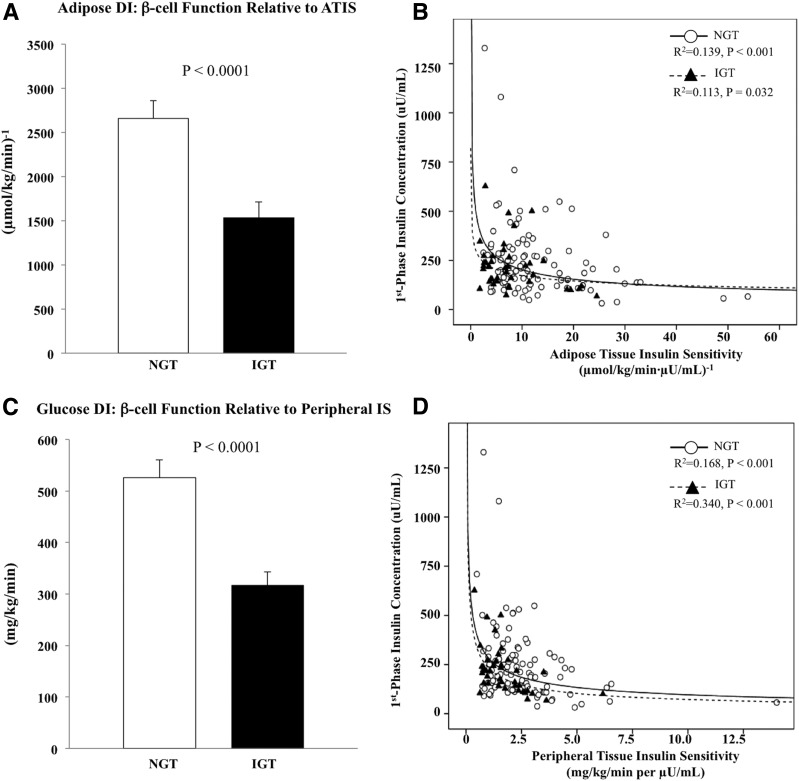
Adipose DI was lower in IGT versus NGT (Fig. 2A). The hyperbolic curves (ATIS × first-phase insulin) represent the nonlinear fitted regression lines derived from individual data points of NGT (R2 = 0.139) and IGT (R2 = 0.113, all P < 0.05) (Fig. 2B). Adipose DI using second-phase insulin yielded consistent results (NGT 2,963.7 ± 163.1 vs. IGT 2,192.2 ± 269.1 [µmol/kg/min]−1, P = 0.001). The lower adipose DI in IGT versus NGT mirrors the glucose DI (Fig. 2C and D).
Figure 2.
A: Adipose DI, i.e., β-cell function relative to ATIS. B: Hyperbolic relationship between ATIS and first-phase insulin concentration during the hyperglycemic clamp. C: Glucose DI, i.e., β-cell function relative to peripheral IS. D: Hyperbolic relationship between peripheral IS and first-phase insulin concentration during the hyperglycemic clamp in youth with IGT vs. NGT.
Hepatic and Peripheral IS, Insulin Secretion, and Glucose DI
Fasting HGP was not different between the two groups; however, hepatic IS was lower in IGT versus NGT (Table 2). Insulin-stimulated total, oxidative, and nonoxidative glucose disposal and peripheral IS were lower in IGT versus NGT (Table 2). During the hyperglycemic clamp, first- and second-phase insulin were not different between the two groups (Table 1). However, glucose DI (peripheral IS × first-phase insulin) was lower in youth with IGT (Fig. 2C).
Table 2.
Hepatic and peripheral tissue IS of glucose metabolism in obese youth with IGT vs. NGT
| NGT (n = 97) | IGT (n = 41) | P | |
|---|---|---|---|
| Fasting HGP (mg/kg/min) | 2.3 ± 0.1 | 2.4 ± 0.1 | NS |
| Hepatic IS (mg/kg/min ⋅ µU/mL)−1 | 16.0 ± 0.8 | 11.8 ± 1.0 | 0.001 |
| Insulin-stimulated glucose disposal (mg/kg/min) | 6.1 ± 0.2 | 4.7 ± 0.3 | <0.0001 |
| Insulin-stimulated glucose oxidation (mg/kg/min) | 2.7 ± 0.1 | 2.3 ± 0.1 | 0.006 |
| Insulin-stimulated nonoxidative glucose disposal (mg/kg/min) | 3.3 ± 0.2 | 2.6 ± 0.3 | 0.028 |
| Peripheral IS (mg/kg/min per µU/mL) | 2.5 ± 0.2 | 1.7 ± 0.2 | 0.001 |
Values are reported as the mean ± SEM.
Correlations of ATIS and Adipose DI With Glucose Metabolism
ATIS correlated with hepatic IS (r = 0.711, P < 0.0001) and peripheral IS (r = 0.615, P < 0.0001). The suppression in lipolysis during hyperinsulinemia correlated with total glucose disposal (r = 0.383, P < 0.0001) and oxidative (r = 0.230, P = 0.009) and nonoxidative disposal (r = 0.334, P < 0.0001). Adipose DI correlated with glucose DI in the total group (r = 0.702, P < 0.0001) and in each group separately (NGT r = 0.688, P < 0.0001; IGT r = 0.612, P < 0.0001).
Conclusions
We show that obese youth with IGT versus NGT have higher whole-body lipolysis and lower ATIS at baseline despite ∼40% higher fasting insulin concentrations. Further, youth with IGT have impaired insulin action in suppressing lipid oxidation. Together with adipose tissue insulin resistance, adipose DI is lower by ∼43% in IGT versus NGT and correlates directly with glucose DI (β-cell function relative to peripheral/skeletal muscle IS).
To date, there are no pediatric data with respect to the quantitative relationship of adipose tissue lipolysis, ATIS, and β-cell function in youth with prediabetes or type 2 diabetes. Studies in adults show adipocyte dysfunction in obesity, prediabetes, and type 2 diabetes (14–16). A study in pediatrics using FFA and insulin concentrations as surrogate markers of adipose tissue insulin resistance demonstrated worsening adipose insulin resistance, manifested in greater FFA area under the OGTT curve, with worsening glucose tolerance (17). Herein we hypothesized that youth with IGT will manifest not only dysregulation of lipolysis and impaired ATIS but also impaired β-cell function or compromised adipose DI.
Using [2H5]glycerol for robust assessment of whole-body lipolysis (18), our observation of increased fasting lipolysis (∼22%) and reduced ATIS (∼38%) in youth with IGT versus NGT is in agreement with adult studies (8,14–16) and the only pediatric publication (17). However, most of these studies used fasting plasma FFA to generate an index of adipose tissue insulin resistance (14–17). A study in adults using [1-14C]palmitate turnover (8) showed increased fasting lipolysis and higher adipose insulin resistance in subjects with type 2 diabetes versus their counterparts without diabetes, consistent with our fasting data in youth with IGT versus NGT. With concomitant fasting hyperinsulinemia, our findings of higher lipolysis but similar HGP in IGT versus NGT suggest that dysregulation of lipolysis could be an early/sensitive sign of metabolic disturbances. Given that obese youth with NGT in our study have 32–45% higher fasting GlyRa compared with what is reported in obese adults (19,20), future studies should evaluate lipid metabolism between phenotypically matched adults and youth along the spectrum of glucose tolerance. This may shed light on our recent observation that obese adolescents with IGT have 50% lower hepatic and peripheral IS than equally obese adults with IGT (21). Besides increased fasting lipolysis, youth with IGT have diminished suppression in lipolysis (∼22% lower ATIS) and in lipid oxidation (∼41% lower suppression from baseline) during hyperinsulinemia.
Only one study in adults examined the relationship between ATIS, derived from either palmitate turnover or fasting FFA, and insulin secretion, but during an OGTT, in adults without diabetes versus with diabetes (8). Our study supplements the adult literature and advances the pediatric literature by the novel observation that youth with IGT versus NGT have an impaired β-cell function relative to ATIS, with 43% lower adipose DI. Besides lipotoxicity potentially impairing β-cell function (5,6), one could speculate that adipocyte dysfunction through altered secretion of anti/proinflammatory adipokines could contribute to β-cell dysfunction (17,22). This lower adipose DI is commensurate with previously reported impaired β-cell function relative to peripheral IS with respect to glucose metabolism in youth with IGT (2). Thus, adipose DI can be yet another index of β-cell function that deteriorates from NGT to IGT, mirroring the declining glucose DI.
When we assessed the relationship between whole-body lipolysis and ATIS with pathophysiological alterations of glucose metabolism, hepatic and peripheral IS showed strong correlations with ATIS, pointing to a global insulin resistance in youth with IGT. Additionally, increased fat oxidation during the clamp, characteristic of youth with IGT, correlated with decreased peripheral IS (r = −0.337, P < 0.0001), suggestive of a tight relationship between lipolysis/fat oxidation and glucose metabolism. In high-fat diet–fed mice, partial inhibition of lipolysis improves glucose metabolism and IS, attesting to a causal relationship between the two (23).
The strengths of the present investigation include the following: 1) the use of [2H5]glycerol to examine whole-body lipolysis; 2) examining youth with IGT instead of type 2 diabetes to avoid confounding modulators of ATIS and β-cell function such as various treatments, weight loss preceding the diagnosis of type 2 diabetes, and degree of hyperglycemia and lipemia; 3) evaluating whole-body lipolysis not only during fasting but also during hyperinsulinemia; 4) complementing the evaluation of lipolysis with measurement of fat oxidation using calorimetry; 5) a first-time characterization of the relationship between ATIS and β-cell function; and 6) complementing ATIS evaluation with examination of peripheral IS and β-cell function with respect to glucose metabolism. Potential perceived limitations would be that in order to minimize participant burden, we did not use a multistep (low and high dose) hyperinsulinemic clamp, which would have prolonged the duration of the clamp, to measure submaximal suppression of lipolysis (24). A recent study, however, shows a strong correlation between ATIS measured by a one-step hyperinsulinemic-euglycemic clamp versus a multistep clamp (25). Another alleged weakness could be that we did not study youth with type 2 diabetes; however, our rational for not doing so is listed under strengths. We believe that IGT is a “virgin” state of type 2 diabetes that allows one to examine pathopysiological alterations “unadulterated” with treatment or gluco/lipotoxicity.
In summary, obese youth with IGT versus NGT are resistant to the antilipolytic effect of insulin in addition to having skeletal muscle insulin resistance. This insulin resistance is combined with impaired β-cell function relative to ATIS and coexists with β-cell dysfunction with respect to glucose metabolism. These findings signify global impairments in IS and in β-cell function in obese youth with IGT. Longitudinal studies are needed to determine the impact of adipocyte dysfunction on the natural history of prediabetes and type 2 diabetes in youth, and the effect of therapeutic interventions that target inhibition of lipolysis to prevent and/or treat type 2 diabetes.
Supplementary Material
Article Information
Acknowledgments. The authors thank the children who participated in this study and their parents, Nancy Guerra (Children’s Hospital of Pittsburgh) for her assistance, Resa Stauffer (Children’s Hospital of Pittsburgh) for her laboratory expertise, and the nursing staff of the Pediatric Clinical and Translational Research Center (Children’s Hospital of Pittsburgh) for their outstanding care of the participants and meticulous attention to the research.
Funding. This study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development grants K24-HD01357 and R01-HD27503 to S.A., National Center for Advancing Translational Sciences Clinical and Translational Science Award UL1TR000005, and National Center for Research Resources grant UL1RR024153 to the General Clinical Research Center.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. J.Y.K. and S.A. designed the study, analyzed the data, and wrote the manuscript. A.N. and S.F.M. collected and maintained the database and reviewed the manuscript. H.T. and F.B. contributed data and reviewed the manuscript. All authors approved the manuscript in its final version. S.A. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 77th Scientific Sessions of the American Diabetes Association, San Diego, CA, 9–13 June 2017.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db17-0551/-/DC1.
References
- 1.Hannon TS, Arslanian SA. The changing face of diabetes in youth: lessons learned from studies of type 2 diabetes. Ann N Y Acad Sci 2015;1353:113–137 [DOI] [PubMed] [Google Scholar]
- 2.Bacha F, Gungor N, Lee S, Arslanian SA. In vivo insulin sensitivity and secretion in obese youth: what are the differences between normal glucose tolerance, impaired glucose tolerance, and type 2 diabetes? Diabetes Care 2009;32:100–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tfayli H, Lee S, Arslanian S. Declining beta-cell function relative to insulin sensitivity with increasing fasting glucose levels in the nondiabetic range in children. Diabetes Care 2010;33:2024–2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burns SF, Bacha F, Lee SJ, Tfayli H, Gungor N, Arslanian SA. Declining β-cell function relative to insulin sensitivity with escalating OGTT 2-h glucose concentrations in the nondiabetic through the diabetic range in overweight youth. Diabetes Care 2011;34:2033–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michaliszyn SF, Bonadonna RC, Sjaarda LA, Lee S, Farchoukh L, Arslanian SA. β-Cell lipotoxicity in response to free fatty acid elevation in prepubertal youth: African American versus Caucasian contrast. Diabetes 2013;62:2917–2922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hughan KS, Bonadonna RC, Lee S, Michaliszyn SF, Arslanian SA. β-Cell lipotoxicity after an overnight intravenous lipid challenge and free fatty acid elevation in African American versus American white overweight/obese adolescents. J Clin Endocrinol Metab 2013;98:2062–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Groop LC, Bonadonna RC, DelPrato S, et al. Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J Clin Invest 1989;84:205–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malin SK, Kashyap SR, Hammel J, Miyazaki Y, DeFronzo RA, Kirwan JP. Adjusting glucose-stimulated insulin secretion for adipose insulin resistance: an index of β-cell function in obese adults. Diabetes Care 2014;37:2940–2946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanner JM. Growth and maturation during adolescence. Nutr Rev 1981;39:43–55 [DOI] [PubMed] [Google Scholar]
- 10.Arslanian SA, Kalhan SC. Correlations between fatty acid and glucose metabolism. Potential explanation of insulin resistance of puberty. Diabetes 1994;43:908–914 [DOI] [PubMed] [Google Scholar]
- 11.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2014;37(Suppl. 1):S81–S90 [DOI] [PubMed] [Google Scholar]
- 12.Arslanian SA, Lewy VD, Danadian K. Glucose intolerance in obese adolescents with polycystic ovary syndrome: roles of insulin resistance and beta-cell dysfunction and risk of cardiovascular disease. J Clin Endocrinol Metab 2001;86:66–71 [DOI] [PubMed] [Google Scholar]
- 13.Miyazaki Y, Glass L, Triplitt C, Wajcberg E, Mandarino LJ, DeFronzo RA. Abdominal fat distribution and peripheral and hepatic insulin resistance in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab 2002;283:E1135–E1143 [DOI] [PubMed] [Google Scholar]
- 14.Abdul-Ghani MA, Molina-Carrion M, Jani R, Jenkinson C, Defronzo RA. Adipocytes in subjects with impaired fasting glucose and impaired glucose tolerance are resistant to the anti-lipolytic effect of insulin. Acta Diabetol 2008;45:147–150 [DOI] [PubMed] [Google Scholar]
- 15.Barchetta I, Angelico F, Del Ben M, et al. Phenotypical heterogeneity linked to adipose tissue dysfunction in patients with Type 2 diabetes. Clin Sci (Lond) 2016;130:1753–1762 [DOI] [PubMed] [Google Scholar]
- 16.Gastaldelli A, Gaggini M, DeFronzo RA. Role of adipose tissue insulin resistance in the natural history of type 2 diabetes: results From the San Antonio Metabolism Study. Diabetes 2017;66:815–822 [DOI] [PubMed] [Google Scholar]
- 17.Hershkop K, Besor O, Santoro N, Pierpont B, Caprio S, Weiss R. Adipose insulin resistance in obese adolescents across the spectrum of glucose tolerance. J Clin Endocrinol Metab 2016;101:2423–2431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Søndergaard E, Jensen MD. Quantification of adipose tissue insulin sensitivity. J Investig Med 2016;64:989–991 [DOI] [PubMed] [Google Scholar]
- 19.Camastra S, Gastaldelli A, Mari A, et al. Early and longer term effects of gastric bypass surgery on tissue-specific insulin sensitivity and beta cell function in morbidly obese patients with and without type 2 diabetes. Diabetologia 2011;54:2093–2102 [DOI] [PubMed] [Google Scholar]
- 20.Ter Horst KW, van Galen KA, Gilijamse PW, et al. Methods for quantifying adipose tissue insulin resistance in overweight/obese humans. Int J Obes 2017;41:1288–1294 [DOI] [PubMed] [Google Scholar]
- 21.Arslanian S, Kim JY, Nasr A, et al. Insulin sensitivity across the lifespan from obese adolescents to obese adults with impaired glucose tolerance: who is worse off? Pediatr Diabetes. 20 July 2017. [Epub ahead of print]. DOI: 10.1111/pedi.12562 [DOI] [PubMed] [Google Scholar]
- 22.Cusi K. The role of adipose tissue and lipotoxicity in the pathogenesis of type 2 diabetes. Curr Diab Rep 2010;10:306–315 [DOI] [PubMed] [Google Scholar]
- 23.Girousse A, Tavernier G, Valle C, et al. Partial inhibition of adipose tissue lipolysis improves glucose metabolism and insulin sensitivity without alteration of fat mass. PLoS Biol 2013;11:e1001485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jensen MD, Nielsen S. Insulin dose response analysis of free fatty acid kinetics. Metabolism 2007;56:68–76 [DOI] [PubMed] [Google Scholar]
- 25.Søndergaard E, Espinosa De Ycaza AE, Morgan-Bathke M, Jensen MD. How to measure adipose tissue insulin sensitivity. J Clin Endocrinol Metab 2017;102:1193–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.