Abstract
Events defining the progression to human type 1 diabetes (T1D) have remained elusive owing to the complex interaction between genetics, the immune system, and the environment. Type 1 interferons (T1-IFN) are known to be a constituent of the autoinflammatory milieu within the pancreas of patients with T1D. However, the capacity of IFNα/β to modulate human activated autoreactive CD8+ T-cell (cytotoxic T lymphocyte) responses within the islets of patients with T1D has not been investigated. Here, we engineer human β-cell–specific cytotoxic T lymphocytes and demonstrate that T1-IFN augments cytotoxicity by inducing rapid phosphorylation of STAT4, resulting in direct binding at the granzyme B promoter within 2 h of exposure. The current findings provide novel insights concerning the regulation of effector function by T1-IFN in human antigen-experienced CD8+ T cells and provide a mechanism by which the presence of T1-IFN potentiates diabetogenicity within the autoimmune islet.
Introduction
Essential for the development and implementation of effective therapies for prevention or reversal of type 1 diabetes (T1D) is a detailed understanding of the molecular pathways and cellular interactions that result in β-cell destruction. The hallmark pathological lesion of T1D is a heterogeneous inflammatory cell infiltrate termed insulitis (1,2). CD8+ T cells, a major component of insulitis, are widely believed to be the primary immune cell responsible for loss of insulin-producing β-cells (2,3). Studies in the NOD mouse model of T1D indicate that CD8+ T cells gain effector activity following islet entry, suggesting signals within the islet microenvironment potentiate lymphocytoxicity (4).
Type 1 interferons (T1-IFNs) provide a candidate signal responsible for facilitating β-cell destruction. Case studies describing the induction of autoantibodies and T1D in individuals receiving T1-IFN therapies for chronic hepatitis and cancer have been reported (5). IFNα subtypes have been detected in the islets and circulation of patients with T1D and possess the capacity to enhance expansion and differentiation of cytotoxic T lymphocytes (CTLs) (6–13). Beyond this, T1D-associated genes involved in the induction, signaling, and regulation of the IFNα/β signaling pathway include IFIH1, TYK2, STAT4, and PTPN2 (14). Although knockout of the IFNα receptor (IFNAR) in NOD mice has produced results to the contrary, a preponderance of evidence in preclinical models also supports a pathogenic role for T1-IFN in T1D (15–18). For example, CRISPR-Cas9 deletion of the IFNAR1 subunit in LEW.1WR1 rats delays spontaneous and polyinosinic-polycytidylic acid–induced diabetes (17). Additionally, studies revealed that overexpression of IFNα in pancreatic β-cells of nondiabetes-prone mice regulates the onset of diabetes in mice with severe insulitis, whereas expression of IFNβ in islets of NOD mice accelerated autoimmunity (19–21). However, little is known regarding the mechanisms by which these cytokines direct immune responses within this microenvironment.
T1-IFNs constitute an essential component of the innate immune response to viral infection and are known as potent immune modulators (22). This family of cytokines displays Janus-like activity with the ability to activate all seven STAT molecules downstream of IFNAR (23,24). T1-IFN is a critical signal for the development of full differentiation and cytotoxicity by mouse CTLs, which are dependent upon the balance between STAT1 and STAT4 signaling (6,25,26). At present, a robust delineation of the T1-IFN signaling mechanisms in human antigen-experienced CTLs has not been determined.
Over the past decade, the identification of T-cell receptors (TCR) specific for tumor antigens has enabled the successful cloning and use of TCR gene transfer for cancer adoptive cell therapies while also advancing the understanding of tumor-infiltrating lymphocyte biology (27). This methodology has been adapted for studies in T1D, where autoantigen-reactive TCRs from patients have been identified and cloned, allowing for the engineering of primary human CD8+ T cells that express a β-cell–specific αβ TCR (28,29). One prime example is the identification of a human CTL TCR specific for islet-specific glucose 6 phosphatase catalytic subunit (IGRP) that displays β-cell autoreactivity (30–32). For this study, we engineered IGRP-specific CTL avatars to investigate T1-IFN signaling mechanisms that regulate human CTL effector function immediately following T1-IFN exposure. The current findings define a novel mechanism where T1-IFNs potently induce cytotoxic function in human CTLs through rapid phosphorylation of STAT4, resulting in direct binding of phosphorylated STAT4 to the granzyme B (GZMB) promoter. These data also provide a mechanistic link between T1-IFNs found within the islet microenvironment and regulation of CTL function that favors autoimmune destruction of β-cells.
Research Design and Methods
Study Subjects
Peripheral blood mononuclear cell samples were obtained from normal healthy donors through the University of Florida Diabetes Institute study bank or from Leukopak samples obtained from LifeSouth Community Blood Centers (Table 1). Human islets from human HLA-A*0201–positive donors were obtained from the Integrated Islet Distribution Program. All studies were approved by the University of Florida Institutional Review Board.
Table 1.
Peripheral blood donor sex, age, and CD8+ T-cell transduction efficiency
| Sex | n | Mean age, years (range) | CD8+ T-cell transduction efficiency, % (range) |
|---|---|---|---|
| Female |
7 |
27.64 (14.17–38.00) |
75.47 (43.6–93.3) |
| Male | 12 | 29.07 (12.50–46.6) | 62.71 (37.5–84.2) |
Materials and Culture Media
T cells and BetaLox5 cells (βL5), an immortalized human β-cell line, were cultured in complete RPMI and complete DMEM (cDMEM) as previously published (33). Cytokines used for these studies included IFNα2 and IFNβ1 (PBL InterferonSource), recombinant human IFNγ (R&D Systems, Inc.), and IL-2 (Peprotech). Information regarding antibodies, TaqMan probes, and chromatin immunoprecipitation (ChIP) primer pairs used in this study can be found in Supplementary Tables 1–3, respectively. For all studies, cells were cultured at 37°C in an atmosphere containing 5% CO2.
Generation of Human Antigen-Specific CD8+ T-Cell Avatars
CD8+ T cells were negatively selected using RosetteSep Human CD8+ T Cell Enrichment Cocktail (Stemcell). Afterward, naive CD8+ T cells (CD8+CD45RA+CD45RO−) were isolated by FACS to 91.0 ± 2.5% purity (Aria III; BD Biosciences). Lentiviral transduction with vectors containing TCR expression constructs pCCL.IGRPopt.eGFP recognizing the autoantigen IGRP (29,34) or LV.Mart1.TCR.RK recognizing the non–β-cell antigen MART1 (27,35) and subsequent T-cell expansion were carried out as outlined in Supplementary Fig. 1. CTL avatars were cryopreserved after 9 days of expansion.
T1-IFN Treatment of CTLs
Following removal from liquid nitrogen and equilibration in cDMEM for 1 h, CTL avatars were treated with IFNα2, IFNβ1, or IFNγ or left untreated for 2 h. After treatment, CTL avatars were washed twice with PBS (Corning) and were either immediately lysed or resuspended in cDMEM for use in cellular assays.
Chromium Release Assays
Primary human islets or βL5 cells were used as target cells in the chromium release assays (CML) as previously described (36). Human islets from donors with HLA-A*02 were selected. Prior to plating, human islets were washed with PBS and dispersed in cell dissociation buffer (Life Technologies) for 10 min at 37°C with gentle pipetting every 3 min. Dispersed islet cells were plated at 40,000 cells/well. βL5 cells express IGRP in the context of HLA-A*0201 and were seeded at 10,000 cells/well in 96-well flat bottom plates. CML was performed as described previously using IGRP-CTL avatars as effectors (37). Specific lysis was calculated as follows: [(Experimental Release − Spontaneous Release)/(Maximum Release − Spontaneous Release)] × 100. For inhibition of granule exocytosis, CTLs were incubated with 100 nmol/L of concanamycin A (CMA) (Sigma-Aldrich) for 2 h. For inhibition of Fas ligand (FasL)-mediated killing or caspase activation, 0.5 µg/mL of anti-Fas blocking antibody or 50 µmol/L of the pan-caspase inhibitor, Z-VAD-FMK (BD Biosciences), respectively, was added to target cells 2 h prior to coculture with CTLs.
Flow Cytometry
Subsequent to cytokine treatment, cells were washed and cultured for an additional 4 h in cytokine-free cDMEM containing monensin. Intracellular flow cytometry staining was performed according to standard procedures for the Nuclear Factor Fixation and Permeabilization Buffer set (BioLegend). Live/Dead Near-Infrared dye (Thermo Fisher) was used to determine viability. Transduction efficiency was detected by EGFP expression and tetramer staining (Supplementary Fig. 2). CTLs were analyzed using a LSRFortessa (BD Biosciences) and FlowJo Software (TreeStar).
Western Blotting
Western blots were performed as previously described (38). Proteins were resolved on 4–20% SDS-PAGE gels. Blots were probed with primary antibodies (Supplementary Table 1); bands were detected using HRP-linked secondary antibodies and an enhanced chemiluminescence detection system (GE Healthcare). Images were captured with an Alpha Innotech FluorChem HD2.
CTL Inhibition
Treatment with rapamycin or lisofylline (Cayman Chemical) was performed for 16 h. Afterward cells were washed in PBS and treated with IFNα as described. Due to the ability of rapamycin to inhibit mTORc1, an important regulator of widespread protein synthesis, we normalized the GZMB protein expression to their respective controls to correct for decreased protein expression observed with rapamycin treatment alone (39).
Small Interfering RNA Knockdown
Accell SMARTpool small interfering RNAs (siRNAs) against STAT1, STAT4, GZMB, GAPDH, and nontargeting negative control siRNA, and siRNA delivery media were purchased from GE Healthcare Dharmacon. Knockdown in CTLs was performed in Accell siRNA delivery media supplemented with IL-2 (100 units/mL) according to the manufacturer’s protocol. After 72 h, cells were harvested for gene expression, flow cytometry, and functional assays. Specificity and delivery efficiency of the siRNAs were evaluated using nontargeting control siRNAs.
Gene Expression
Total RNA was isolated using the RNeasy Micro Kit (Qiagen) and reverse transcribed using the iScript cDNA Synthesis Kit (Bio-Rad) according to the manufacturer’s protocol. TaqMan gene expression primer and probe sets (Supplementary Table 2) were purchased from Applied Biosystems. All gene expression experiments were performed in quadruplicate on a Roche LightCycler 480 and normalized to 18S.
ChIP
Activated and expanded human CTLs (5 × 106) were treated with or without IFNα (1,000 units/mL) for 1 h. ChIP was carried out using the SimpleChIP Kit (Cell Signaling) per manufacturer’s instructions. Chromatin-protein complex immunoprecipitations were performed using 2 μg of antibody (Supplementary Table 1). Oligonucleotides specific to the promoter of GZMB were designed using Primer 3 (http://primer3.ut.ee). Immunoprecipitated DNA was quantified by qPCR using SYBR Green (Qiagen) on the Roche LightCycler 480. Data are expressed as a percentage of input.
Statistical Analysis
All statistical analyses were performed using Prism software (Graphpad). Statistical hypothesis tests were conducted by using nonparametric Wilcoxon signed rank test. Statistical significance was defined as P < 0.05.
Results
Acute Exposure of CTLs to T1-IFN Increases β-Cell Lysis
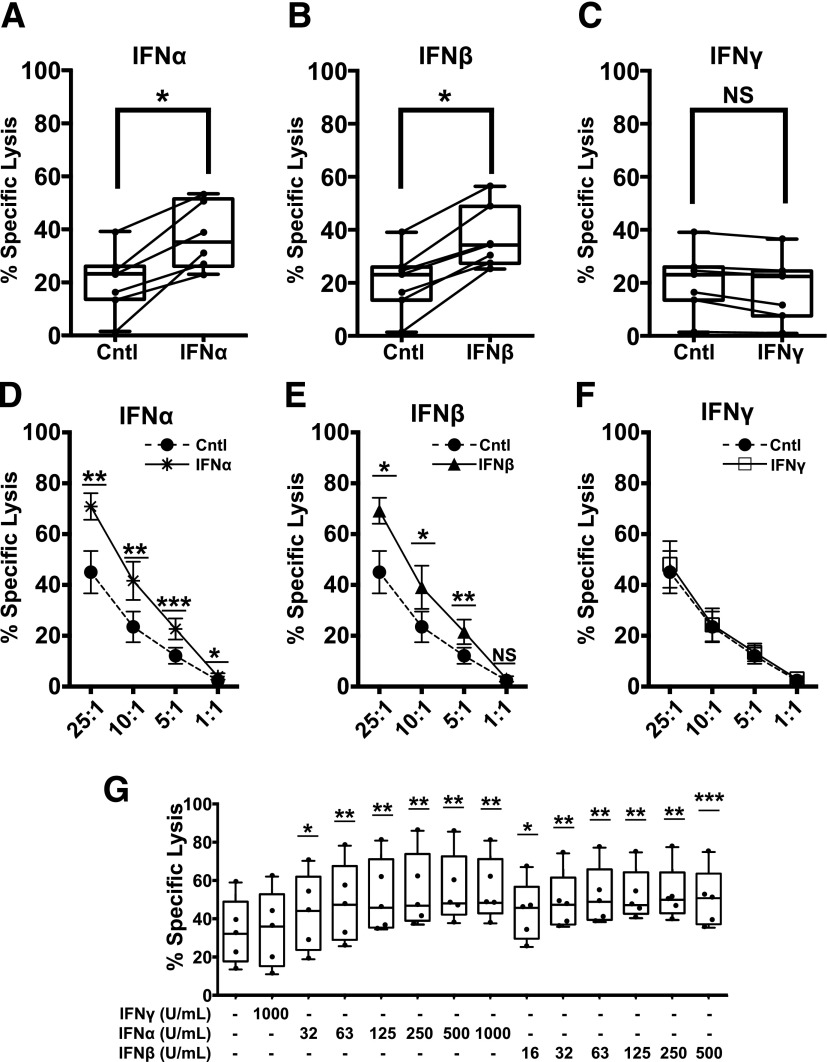
Although T1-IFNs are observed in the islets of deceased donors with T1D, no studies to date have elucidated the impact of these cytokines on infiltrating CTLs (8). To model CTL interactions with β-cells in vitro, we developed a protocol to generate antigen-specific CTLs by lentiviral TCR gene transfer using an IGRP-reactive human TCR (Supplementary Fig. 1) (30,32,34). These functional CTL avatars recognize IGRP(265–273) presented in the context of HLA-A*0201 as shown by dextramer staining and display markers characteristic of cytotoxic T effector cells (Supplementary Figs. 2–4). IGRP avatars were exposed to IFNα (1,000 units/mL), IFNβ (500 units/mL), or IFNγ (1,000 units/mL) for 2 h prior to use in cytotoxicity coculture assays using primary human islets as targets. IGRP avatars primed with IFNα (Fig. 1A) or IFNβ (Fig. 1B) displayed a significantly increased ability to lyse target primary human islets. In contrast, priming with the Type II IFN (IFNγ) did not enhance cytotoxicity (Fig. 1C). Moreover, this phenotype was maintained over several effector-to-target (E:T) ratios with IFNα (Fig. 1D) or IFNβ (Fig. 1E) enhancing the lysis of target βL5 cells at each ratio tested. Similar to the studies with primary islets as targets, priming the IGRP-CTL avatars with IFNγ for 2 h did not enhance cytotoxicity (Fig. 1F). Although significant donor-to-donor variation was observed in the ability of CTLs to lyse islet cells (Fig. 1A–C) or βL5 cells (Fig. 1D–F and Supplementary Table 2), overall the 2-h exposure of each donor’s CTLs to IFNα (Fig. 1A and D and Supplementary Table 2) and IFNβ (Fig. 1B and E, and Supplementary Table 2) resulted in an increased capacity to lyse target β-cells.
Figure 1.
Short-term exposure of autoreactive CTLs to T1-IFNs enhances cytotoxicity toward β-cells. IGRP-CTL avatars were exposed to IFNα, IFNβ, or IFNγ for 2 h. Cytokines were removed by washing, and then IGRP-CTL avatars were cocultured with dispersed primary human islets (A–C) or βL5 (D–F) for 16 h in a standard CML. A–C: Box-and-whisker plots represent the percentage of dispersed human islets lysed by IGRP-CTL avatars. Data for the interferon-mediated change in effector function for each individual T-cell donor is provided using a scatter plot with connecting lines. D–F: Lines represent the percentage of specific lysis induced by IGRP-CTL avatars primed with IFNα (1,000 units/mL) (A and D), IFNβ (500 units/mL) (B and E), or IFNγ (1,000 units/mL) (C and F) over several E:T ratios. G: IGRP-CTL avatars were primed with various concentrations of T1-IFN for 2 h and cocultured with βL5 at a 10:1 E:T for 16 h in CML. Data are plotted as mean ± SEM, where T cells from each donor (7 donors for A–C, 6 donors for D–F, and 5 donors for G) were weighted equally. There were at least three separate experiments for each donor. Statistical significance was assessed using a nonparametric paired t test with Wilcoxon post-test analysis. *P < 0.05; **P < 0.01; ***P < 0.001.
Patients with recent-onset T1D display increased serum concentrations of T1-IFN when compared with individuals without T1D. This may be indicative of T1-IFN within affected islets, where compartmentalized concentrations of these cytokines may be higher than detected in the sera (12,40). Thus, we performed a dose titration to investigate whether T1-IFNs could increase CTL activity at concentrations similar to those found in recent-onset patients. T1-IFN augmented CTL-targeted β-cell lysis in a dose-dependent manner (Fig. 1G), supporting the notion that exposure of autoreactive T cells to these cytokines enhances function.
Short-term Exposure of CTLs to T1-IFN Increases Cytotoxicity Through Enhanced GZMB Expression
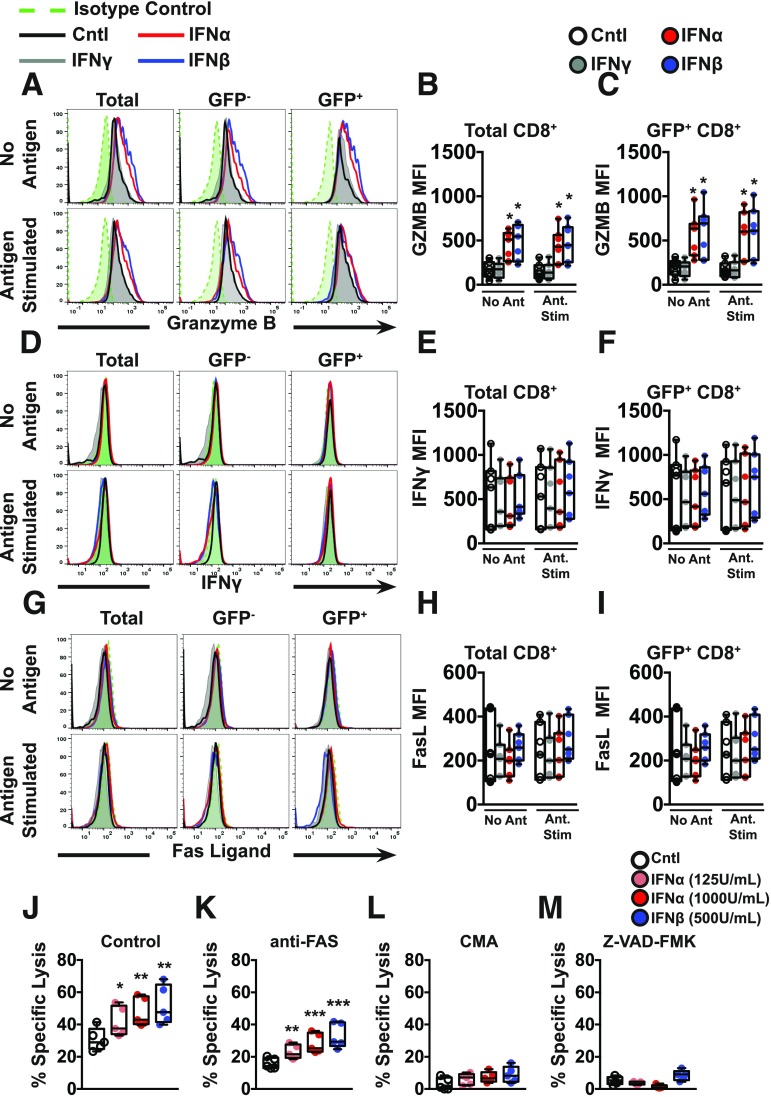
Licensed CTLs execute their cytotoxic activities through multiple mechanisms. Specifically, effector CTLs produce inflammatory cytokines and mediate targeted killing by expression of death ligands (i.e., FasL), as well as exocytosis of granule components including perforin and granzyme (41). To examine the underlying molecular pathways responsible for T1-IFN–induced cytolytic effector function, IGRP-reactive CTL avatars were treated with T1-IFN for 2 h and assessed for the expression of IFNγ, FasL, and GZMB by flow cytometry after 4 h of incubation in media (without antigen stimulation) or in the presence of βL5 cells (antigen stimulated) (Fig. 2A–I). No adverse effects on viability were observed following T1-IFN stimulation (Supplementary Fig. 5). GZMB was significantly increased upon T1-IFN priming of the IGRP-CTL avatars (Fig. 2A–C). No significant changes were observed in the expression of intracellular IFNγ (Fig. 2D–F) or surface FasL when compared with the nonprimed or IFNγ-primed controls (Fig. 2G–I). Given that we obtained a range of TCR transduction efficiencies with the IGRP-CTL avatars generated from the 19 donors (range 30–95%) (Table 1), we were able to gate on GFP reporter–positive or –negative CTLs, the latter serving as an internal control. This allowed us to assess the impact of TCR ligation on effector phenotype. For all three output measures (GZMB, IFNγ, and FasL), the TCR transduced and nontransduced cells were indistinguishable (Fig. 2). This observation suggests that T1-IFNs condition CTLs to enhance lytic potential through increasing GZMB protein levels in a manner that does not require concomitant ligation with cognate antigen and signaling through the TCR.
Figure 2.
GZMB expression is increased upon T1-IFN priming of autoreactive CTLs. A–I: IGRP-CTL avatars were primed with IFNα, IFNβ, or IFNγ for 2 h. Cytokines were removed by washing, and then IGRP-CTL avatars were incubated for an additional 4 h in media or stimulated by coculture with βL5 cells. Representative histograms and mean fluorescence intensities (MFI) for GZMB (A–C), IFNγ (D–F), and FasL (G–I) are displayed. J–M: To determine the contribution of pathways important for CTL-mediated killing, IGRP-CTL avatars were cocultured with βL5 cells (10:1 E:T) in the presence of inhibitors known to block CTL cytotoxic function. Bars represent the percentage of βL5 cell lysis by IGRP-CTL avatars in the absence of inhibitors (J), with anti-Fas antibody (K), with CMA (L), and with pan-caspase inhibitor, Z-VAD-FMK (M). For representative histograms (A, D, and G), all lines within a panel are from a single donor. Data plotted in B, C, E, F, and H–M are mean ± SEM, where T cells from each donor (7 donors for B, C, E, F, H, and I and 5 donors for J–M) were weighted equally. There were at least three separate experiments for each donor. Statistical significance was assessed by a nonparametric paired t test with Wilcoxon post-test analysis. *P < 0.05; **P < 0.01; ***P < 0.001.
Next, inhibitors were used to block T1-IFN–induced IGRP-CTL avatar cytotoxicity during CML (Fig. 2J–M). These included a neutralizing antibody to the Fas receptor on β-cells to block Fas–FasL interactions; CMA, a V-ATPase inhibitor to prevent the release of cytotoxic granules from CTLs, and Z-VAD-FMK, a pan-caspase inhibitor to counteract activation of caspases and granzymes within β-cells. Inhibition of Fas–FasL interactions did not abrogate the T1-IFN–induced gain in IGRP avatar cytotoxicity (Fig. 2K). However, prevention of granule exocytosis and release of GZMB by CTL avatars using CMA abolished the increased cytotoxicity seen in T1-IFN–primed IGRP-CTL avatars (Fig. 2L). Similarly, Z-VAD-FMK inhibition of protease activity (caspases and granzymes) in βL5 cells resulted in protection from IGRP-CTL avatar–mediated destruction (Fig. 2M).
Rapid T1-IFN Signaling Is STAT Dependent and Does Not Require T-bet, Eomes, or mTOR
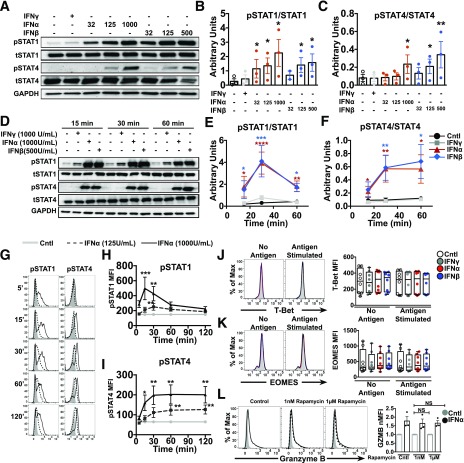
T1-IFNs use several noncanonical signaling pathways to induce their pleiotropic effects and have the ability to activate all members of the STAT family (24). STAT1 and STAT4 are heavily linked to effector T-cell function in murine models (42,43). Therefore, we hypothesized that STAT1 and STAT4 regulate T1-IFN responses in activated IGRP-CTL avatars. Thus, CTL avatars were treated with IFNγ (1,000 units/mL) or several concentrations of IFNα or IFNβ for 15 min. Phosphorylated and total STAT1 and STAT4 were assessed by Western blot. T1-IFNs induced a robust dose-dependent phosphorylation of both STAT1 and STAT4, whereas the total levels of STAT1 and STAT4 among the groups remained equal (Fig. 3A–C). Next, we performed time course analysis and observed that the phosphorylation of STAT1 peaks 30 min following T1-IFN exposure, while STAT4 phosphorylation remains highly elevated over the 1-h time course (Fig. 3D–F). We were able to confirm these results by phospho-flow cytometry, whereby activation of STAT1 was rapid, peaking between 15 and 30 min and returning to baseline by 2 h (Fig. 3G and H). STAT4 reached maximal activation by 30 min and remained elevated out to 2 h (Fig. 3G and I). IFNα was unable to induce phosphorylation of STAT5 and STAT6 but did display minimal activation of STAT3 (Supplementary Fig. 6), suggesting that STAT1 and STAT4 are the preferential T1-IFN signaling mediators in during acute IFNα exposure in activated IGRP-CTL avatars.
Figure 3.
T1-IFN induces phosphorylation of STAT1 and STAT4 in IGRP-CTL avatars. A–C: Western blot analysis of IGRP-CTL avatars treated with IFNα, IFNβ, or IFNγ for 15 min. Representative blots (A) and densitometry analysis of phosphorylated and total STAT1 (B) and STAT4 (C). D–F: Time course analysis of pSTAT1 and pSTAT4 activation is shown by Western blot (D). Densitometry was performed and was plotted against time for STAT1 (E) and STAT4 (F). Phospho-flow cytometry was performed on IGRP-CTL avatars treated with IFNα (125 units/mL and 1,000 units/mL) at several time points (5, 15, 30, 60, and 120 min). G: Representative histograms for pSTAT1 and pSTAT4 are displayed. H and I: Mean fluorescence intensities (MFI) of pSTAT1 and pSTAT4 are plotted against time. J–L: IGRP-CTL avatars were primed with T1-IFN for 2 h and assessed for T-bet and Eomes expression by flow cytometry. Representative histograms and MFI of T-bet (J) and Eomes (K) are displayed. L: IGRP-CTL avatars were pretreated with rapamycin for 16 h, primed with IFNα for 2 h, and subsequently assessed for GZMB expression by flow cytometry (representative histograms and MFI are plotted). For representative Western blots and histograms (A, D, G, and J–L), all lanes or lines within a panel are from a single donor. Data plotted in B, C, E, F, and H–L are mean ± SEM; T cells from each donor (at least 3 donors for B, C, E, F, and L; 5 donors for H and I; and 8 donors for J and K) were weighted equally. There were at least three separate experiments for each donor. Statistical significance was assessed by a nonparametric paired t test with Wilcoxon post-test analysis. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Previous studies performed in mouse models demonstrate that T1-IFNs regulate transcriptional programs induced by T-bet and Eomes, which are known to be essential for differentiation and development of human CTL effector function (25,44). Additionally, these transcription factors were upregulated in mouse CTLs exposed to IFNα and reported to specifically bind the promoters of IFNγ, perforin, and GZMB (45). As T1-IFNs rapidly act to increase effector function through the upregulation of GZMB in activated antigen-specific T cells, we reasoned that these cytokines could be indirectly inducing this phenotype through T-bet or Eomes. Therefore, we examined expression of T-bet and Eomes in IGRP-CTL avatars following a 2-h T1-IFN exposure followed by 4 h of incubation in media (without antigen stimulation) or in the presence of βL5 cells (antigen stimulated). Intriguingly, neither T-bet nor Eomes displayed increased expression after short-term incubation with IFNα or IFNβ (Fig. 3J and K) alone or in the presence of βL5 cells (antigen stimulated), suggesting that these transcription factors are not involved regulating GZMB following short-term T1-IFN treatment.
T1-IFNs have also been reported to regulate translation of interferon-stimulated genes through the activation of mTOR kinase activity (46). mTOR integrates environmental cues to direct cellular metabolism and plays a role in T-cell activation and differentiation (47). We assessed the impact of rapamycin-mediated mTOR inhibition on the rapid upregulation of GZMB detected after short-term T1-IFN treatment. Two concentrations of rapamycin were chosen to correlate with inhibition of mTOR complex 1 (at the lower concentration of 1 nmol/L) and inhibition of both mTORc1 and mTORc2 (at higher concentrations of 1 µmol/L) (48). IGRP-CTL avatars were pretreated for 16 h with rapamycin, primed with IFNα for 2 h, and assessed by flow cytometry. Rapamycin had no effect on CTL avatar viability (data not shown) or the T1-IFN–mediated increase in GZMB expression (Fig. 3L). These data suggest that regulation of GZMB by T1-IFN in CD8+ T cells is independent of TORC1 and TORC2 complexes.
Enhanced GZMB Expression Induced by T1-IFN Priming of Activated CTLs Is Dependent on STAT4 Activation
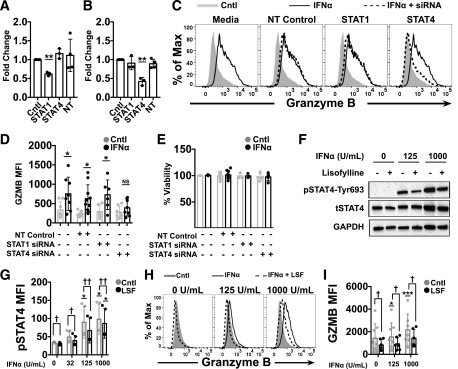
To investigate the whether the signaling mediators STAT1 and STAT4 are necessary for the T1-IFN induction of GZMB and heightened lysis of target cells, we used siRNAs to reduce the levels of STAT1 or STAT4. CTLs were transfected with STAT1- or STAT4-specific siRNAs for 72 h. qRT-PCR confirmed efficient and specific mRNA knockdown of STAT1 and STAT4 mRNA by their corresponding siRNAs (Fig. 4A and B). Next, siRNA-transfected IGRP-CTL avatars were primed with T1-IFN and assessed for GZMB production. Nontargeting control-transfected CTLs displayed an increase in GZMB after priming with IFNα (1,000 units/mL) (Fig. 4C and D). Transfection of IGRP avatars with siRNAs had no adverse effect on viability (Fig. 4E). CTL avatars transfected with STAT1-specific siRNA displayed similar levels of T1-IFN–induced GZMB when compared with controls, whereas CTL avatars where STAT4 was reduced by siRNA displayed a significant loss in T1-IFN–induced GZMB (Fig. 4C and E). These data confirm that STAT4 is critical for T1-IFN–induced amplification of CTL avatar effector function through GZMB production.
Figure 4.
Inhibition of STAT4 reverses T1-IFN induced cytotoxicity. IGRP-CTL avatars were transfected with Accell siRNAs specific for STAT1, STAT4, or nontargeting (NT) controls for 72 h. A and B: RT-PCR analysis to assess gene silencing was performed for STAT1 (A) and STAT4 (B). All values were normalized relative to 18S mRNA expression. C–E: IGRP-CTL siRNA transfectants were primed with T1-IFN and assessed for GZMB expression by flow cytometry. Representative histograms (C) and mean fluorescence intensities (MFI) for GZMB (D) are plotted. E: Viability analysis on siRNA-transfected CTLs is plotted. F–I: IGRP-CTL avatars were pretreated with lisofylline (LSF) and analyzed for pSTAT4 activation by Western blot (F) and phospho-flow cytometry (G). LSF-treated CTLs were primed with IFNα and analyzed for GZMB expression by flow cytometry. Representative histograms (H) and MFI (I) for GZMB are shown. For representative Western blots and histograms (C, F, and H), all lanes or lines within a panel are from a single donor. Data plotted in A, B, D, E, G, and I are mean ± SEM; T cells from each donor (3 donors for A and B; at least 5 donors for D, E, and G; and at least 7 donors for I) were weighted equally. There were at least three separate experiments for each donor. Statistical significance was determined with a nonparametric paired t test with Wilcoxon post-test analysis. *P < 0.05; **P < 0.01; ***P < 0.001 for comparison to control; †P < 0.05; ††P < 0.01 for comparison within IFNα dosage group. tSTAT, total STAT.
Lisofylline is an anti-inflammatory agent known to prevent IL-12–induced activation of STAT4 and has been used in combination with other therapeutics for the reversal of autoimmune diabetes in the NOD mouse (49,50). To confirm that STAT4 is critical for the early and rapid induction of GZMB by T1-IFN, we asked whether lisofylline could prevent T1-IFN–induced STAT4 activation in our model. IGRP-CTL avatars were pretreated for 16 h with lisofylline. Lisofylline exhibited no adverse effect on viability (data not shown). The ability of lisofylline to inhibit phosphorylation of STAT4 was confirmed by immunoblot and phospho-flow analysis (Fig. 4F and G). Lisofylline also prevented T1-IFN–mediated induction of GZMB (Fig. 4H and I). These data confirm the importance of STAT4 for GZMB production in response to T1-IFN and also suggest that lisofylline can act to reduce autoinflammation by modulating CTL responses to T1-IFN.
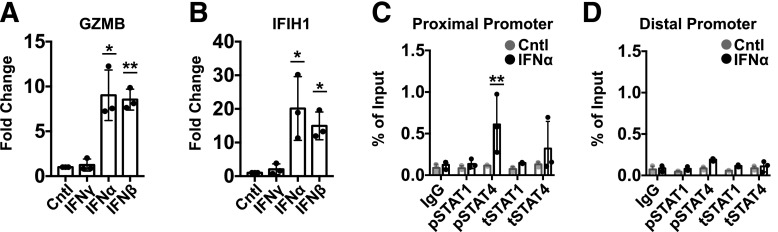
Activation of Phosphorylated STAT4-Tyr693 by T1-IFN Leads to Increased Transcription of GZMB Through Direct Promoter Binding
Previous studies conducted in primary human T cells showed that IFNα induced tyrosine phosphorylation of STAT4 (pTyr693) and that this activation led to DNA binding (51). We hypothesized that the rapid accumulation of CTL avatar effector function observed after short-term T1-IFN priming was due to binding of phosphorylated STAT4 (pSTAT4) to the GZMB promoter and transcriptional activation. To investigate this hypothesis, we first performed qRT-PCR to verify that GZMB transcript levels were increased with short-term priming by T1-IFN. Indeed, T1-IFN significantly increased GZMB expression (Fig. 5A). IGRP-CTL avatars also display a robust T1-IFN response with the induction of IFIH1, a well-known interferon-stimulated gene (Fig. 5B). Using the JASPAR database (52), we interrogated the GZMB promoter for putative binding sites for STAT1 and STAT4, which yielded one binding site 67–80 base pairs (bp) upstream of the GZMB transcription start site (TSS) and two additional distal binding sites within a region 625–650 bp upstream of the TSS. Oligonucleotides were designed to amplify regions of DNA that were in close proximity of these binding sites (schematics found in Supplementary Fig. 7). There was no observed binding of pSTAT1 or total STAT1 to the GZMB promoter following IFNα priming at either the proximal or distal site. This confirmed that STAT1, in this context, is not required for regulation of GZMB following acute T1-IFN exposure. Priming of activated CTL avatars with IFNα resulted in increased binding of pSTAT4-Tyr 693 but not total STAT4 to the GZMB promoter. pSTAT4 binding was only observed at the proximal binding site located 67–80 bp upstream of the GZMB TSS (Fig. 5C). Thus T1-IFN rapidly induces pSTAT4 that directly binds to the GZMB promoter (Fig. 5C) to increase transcription (Fig. 5A) and GZMB protein levels (Fig. 2), resulting in enhanced CTL cytolytic function (Fig. 1).
Figure 5.
T1-IFN induces pSTAT4 binding to the GZMB promoter to induce transcription. A and B: qRT-PCR analysis of GZMB (A) and IFIH1 (B) expression in IGRP-CTL avatars following 2-h treatment with interferons. C and D: ChIP-qPCR with SYBR Green was performed to assess the binding of pSTAT1 and pSTAT4 to the GZMB promoter in IGRP-CTL avatars after treatment with IFNα for 1 h. ChIP-qPCR representing the binding of STAT1 and STAT4 to the putative proximal promoter (C) and distal promoter (D) binding sites of GZMB is displayed. Data plotted are mean ± SEM; T cells from 3 donors were weighted equally. There were at least three separate experiments for each donor. Statistical significance was determined by a nonparametric paired t test with Wilcoxon post-test analysis. *P < 0.05; **P < 0.01.
Discussion
Here, we used the activated antigen-specific human CTLs to demonstrate that T1-IFN can induce a remarkably rapid acquisition of effector function through activation and direct binding of pSTAT4 to the GZMB promoter. Our data extend previous studies in murine and human T cells demonstrating that T1-IFN can act as a “third signal” cytokine (6,7,25). In contrast to previous reports, we used our model to define rapid T1-IFN signaling mechanisms after only 2 h of exposure, indicating that GZMB is a first responder, rapidly produced to arm antigen-experienced CTLs with an increased capacity to kill target cells. Unlike other reports, we did not detect any differences in production of IFNγ or FasL (6,7,25). It is likely that the kinetics for the generation of these effector molecules differs from GZMB and may occur by other T1-IFN–induced signaling pathways. Additionally, the diverse activation of downstream signaling pathways by T1-IFN may provoke a feed-forward amplification of this signal (53).
T1-IFNs regulate a complex network of signaling pathways, and in order to activate alternative T1-IFN–mediated signaling, there must be a disruption in the balance of classically activated STAT1 versus other STAT molecules (23,24). T1-IFN–mediated CTL responses in murine and human cells have been linked to STAT1 and STAT4 signaling downstream of the IFNAR (6,25,51). Phosphorylation of STAT4 can be induced through activation of TCR signaling and is reported to counteract the antiproliferative STAT1 response (42,43). This is the first study, to our knowledge, to characterize the kinetics of T1-IFN–mediated activation of pSTAT1 versus pSTAT4 in human antigen-experienced CTLs. By design, our in vitro experiments involve a controlled microenvironment, which limits our ability to definitively deduce signal transduction and cellular responses to the complex cytokine milieu within the pancreatic islet during T1D pathogenesis. In line with mouse models of viral infection, we show that activated human CTL avatars responding to T1-IFN favor STAT4-dependent signaling over the canonical STAT1-dependent signaling pathway (42). Therefore, these pathways likely function in a positive manner by enhancing clearance of viral infections, but conversely in autoimmune-prone individuals arm and enhance pathogenicity of β-cell–reactive CTLs.
Our results also mechanistically demonstrate that T1-IFN–induced signaling and activation of pSTAT4-Tyr693 lead to direct binding at the proximal promoter of GZMB. Previous studies investigating the transcriptional regulation of GZMB revealed that T-cell activation induces mRNA expression, which was mapped to a 243 bp promoter element upstream of the GZMB TSS (54,55). Mutational analysis and electrophoretic mobility shift assay studies confirmed the binding sites for several transcription factors involved in the activation and differentiation of T lymphocytes including AP-1, Runx1, Ikaros, and CREB1 (56,57). Apart from these studies, very little is known regarding the transcriptional regulation of GZMB. T1-IFN regulation of GZMB has been largely appreciated through studies carried out in murine and human T cells (6,7). However, the precise mechanisms and signal transduction pathways required for such induction are largely uncharacterized, typically noted as a consequence of widespread JAK/STAT signaling. In this investigation, our data provide further insight into this regulation. Survey of the GZMB promoter using the JASPAR database led to the identification several overlapping putative binding sites for STAT1 and STAT4. This was not surprising due to the fact that STAT molecules recognize palindromic core sequences with varied binding specificity attributed to nucleotide preference flanking this conserved region (58). It should be noted that the pSTAT4 promoter element −80/−67 bp upstream of the TSS, identified here, is within the T cell–inducible region identified by previous studies. Chromatin remodeling by T1-IFNs is crucial for the rapid induction of interferon-stimulated genes, which could explain the rapid changes occurring at the GZMB promoter (25,54).
Mounting evidence suggests that stimuli within the islet microenvironment contribute to CTL cytotoxicity and precipitate T1D. Studies in the NOD demonstrate that IGRP-reactive NY8.3 CD8+ T cells are initially activated in the pancreatic lymph node but only acquire full cytotoxic capacity when in the islet. This occurs independently of antigen presentation by β-cells (4). Over the years, investigators have hypothesized that proinflammatory cytokines contribute to disease with many efforts focused on elucidating contributions of TNFα, IL-1β, and IFNγ in the NOD mouse model. However, the only cytokine that displays a consistent increase in patients with T1D is IFNα (8,9). Our in vitro model has provided a potential mechanism as to how CD8+ T cells develop enhanced effector function when exposed to T1-IFN. Similar to NY8.3 CTLs found in the NOD islet, this increase in GZMB is global and independent of synergistic antigen-stimulation by the intended target cell. T1-IFN embodies an essential component of the innate immune response to viral infection, a well-known putative environmental factor that has long been associated with the islets of patients with T1D. Furthermore, T1D risk variants found in IFIH1, TYK2, and STAT4 are associated with constitutive activity, augmented T1-IFN signaling, and increased T1-IFN sensitivity, respectively (59–61). Taken together with our current findings, it is likely that a genetic predisposition skewed toward dysfunctional T1-IFN responses create an islet environment permissive to enhanced human β-cell–specific cytotoxicity. Although the pleiotropic actions of T1-IFN are designed to strengthen the immune response to viral pathogens, this response proves detrimental in the case of autoimmunity where the immune response is misdirected toward self and, in this way, can promote β-cell death in T1D.
Supplementary Material
Article Information
Acknowledgments. The authors would like to thank all of the study participants, as well as Amanda Posgai (University of Florida) and Robert L. Whitener (Stanford University) for critical review of the manuscript.
Funding. The current study was partially supported by grants from the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases (UC4DK104194, R01DK074656) and National Institute of Allergy and Infectious Diseases (P01A1042288). B.N.N. was also supported by an National Research Service Award individual fellowship from the National Institute of Diabetes and Digestive and Kidney Diseases (F30DK105788).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. B.N.N. conducted experiments. B.N.N., T.M.B., M.C.-S., and C.E.M. designed experiments. Data analysis, interpretation, and discussion were completed by B.N.N., T.M.B., B.Z., M.A.A., M.C.-S., and C.E.M. The manuscript was written and revised by B.N.N., T.M.B., M.A.A., M.C.-S., and C.E.M. The study was conceived by B.N.N. and C.E.M. C.E.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 76th Scientific Sessions of the American Diabetes Association, New Orleans, LA, 10–14 June 2016.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db17-0106/-/DC1.
References
- 1.Willcox A, Richardson SJ, Bone AJ, Foulis AK, Morgan NG. Analysis of islet inflammation in human type 1 diabetes. Clin Exp Immunol 2009;155:173–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Betts MR, Brenchley JM, Price DA, et al. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods 2003;281:65–78 [DOI] [PubMed] [Google Scholar]
- 3.Serreze DV, Leiter EH, Christianson GJ, Greiner D, Roopenian DC. Major histocompatibility complex class I-deficient NOD-B2mnull mice are diabetes and insulitis resistant. Diabetes 1994;43:505–509 [DOI] [PubMed] [Google Scholar]
- 4.Graham KL, Krishnamurthy B, Fynch S, et al. Autoreactive cytotoxic T lymphocytes acquire higher expression of cytotoxic effector markers in the islets of NOD mice after priming in pancreatic lymph nodes. Am J Pathol 2011;178:2716–2725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakamura K, Kawasaki E, Imagawa A, et al.; Research Committee on Type 1 Diabetes of the Japan Diabetes Society . Type 1 diabetes and interferon therapy: a nationwide survey in Japan. Diabetes Care 2011;34:2084–2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curtsinger JM, Valenzuela JO, Agarwal P, Lins D, Mescher MF, Type I. Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J Immunol 2005;174:4465–4469 [DOI] [PubMed] [Google Scholar]
- 7.Hervas-Stubbs S, Riezu-Boj JI, Gonzalez I, et al. Effects of IFN-α as a signal-3 cytokine on human naïve and antigen-experienced CD8(+) T cells. Eur J Immunol 2010;40:3389–3402 [DOI] [PubMed] [Google Scholar]
- 8.Foulis AK, Farquharson MA, Meager A. Immunoreactive alpha-interferon in insulin-secreting beta cells in type 1 diabetes mellitus. Lancet 1987;2:1423–1427 [DOI] [PubMed] [Google Scholar]
- 9.Huang X, Yuang J, Goddard A, et al. Interferon expression in the pancreases of patients with type I diabetes. Diabetes 1995;44:658–664 [DOI] [PubMed] [Google Scholar]
- 10.Lundberg M, Krogvold L, Kuric E, Dahl-Jørgensen K, Skog O. Expression of interferon-stimulated genes in insulitic pancreatic islets of patients recently diagnosed with type 1 diabetes. Diabetes 2016;65:3104–3110 [DOI] [PubMed] [Google Scholar]
- 11.Lindenmann J, Burke DC, Isaacs A. Studies on the production, mode of action and properties of interferon. Br J Exp Pathol 1957;38:551–562 [PMC free article] [PubMed] [Google Scholar]
- 12.Chehadeh W, Weill J, Vantyghem MC, et al. Increased level of interferon-alpha in blood of patients with insulin-dependent diabetes mellitus: relationship with coxsackievirus B infection. J Infect Dis 2000;181:1929–1939 [DOI] [PubMed] [Google Scholar]
- 13.Allen JS, Pang K, Skowera A, et al. Plasmacytoid dendritic cells are proportionally expanded at diagnosis of type 1 diabetes and enhance islet autoantigen presentation to T-cells through immune complex capture. Diabetes 2009;58:138–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Concannon P, Rich SS, Nepom GT. Genetics of type 1A diabetes. N Engl J Med 2009;360:1646–1654 [DOI] [PubMed] [Google Scholar]
- 15.Li Q, Xu B, Michie SA, Rubins KH, Schreriber RD, McDevitt HO. Interferon-alpha initiates type 1 diabetes in nonobese diabetic mice. Proc Natl Acad Sci U S A 2008;105:12439–12444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quah HS, Miranda-Hernandez S, Khoo A, et al. Deficiency in type I interferon signaling prevents the early interferon-induced gene signature in pancreatic islets but not type 1 diabetes in NOD mice. Diabetes 2014;63:1032–1040 [DOI] [PubMed] [Google Scholar]
- 17.Qaisar N, Lin S, Ryan G, et al. A critical role for the type I interferon receptor in virus-induced autoimmune diabetes in rats. Diabetes 2017;66:145–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lincez PJ, Shanina I, Horwitz MS. Reduced expression of the MDA5 Gene IFIH1 prevents autoimmune diabetes. Diabetes 2015;64:2184–2193 [DOI] [PubMed] [Google Scholar]
- 19.Pelegrin M, Devedjian JC, Costa C, et al. Evidence from transgenic mice that interferon-beta may be involved in the onset of diabetes mellitus. J Biol Chem 1998;273:12332–12340 [DOI] [PubMed] [Google Scholar]
- 20.Stewart TA, Hultgren B, Huang X, Pitts-Meek S, Hully J, MacLachlan NJ. Induction of type I diabetes by interferon-alpha in transgenic mice. Science 1993;260:1942–1946 [DOI] [PubMed] [Google Scholar]
- 21.Carrero JA, Calderon B, Towfic F, Artyomov MN, Unanue ER. Defining the transcriptional and cellular landscape of type 1 diabetes in the NOD mouse. PLoS One 2013;8:e59701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.González-Navajas JM, Lee J, David M, Raz E. Immunomodulatory functions of type I interferons. Nat Rev Immunol 2012;12:125–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kallal LE, Biron CA. Changing partners at the dance: variations in STAT concentrations for shaping cytokine function and immune responses to viral infections. JAK-STAT 2013;2:e23504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol 2005;5:375–386 [DOI] [PubMed] [Google Scholar]
- 25.Agarwal P, Raghavan A, Nandiwada SL, et al. Gene regulation and chromatin remodeling by IL-12 and type I IFN in programming for CD8 T cell effector function and memory. J Immunol 2009;183:1695–1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiao Z, Casey KA, Jameson SC, Curtsinger JM, Mescher MF. Programming for CD8 T cell memory development requires IL-12 or type I IFN. J Immunol 2009;182:2786–2794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morgan RA, Dudley ME, Wunderlich JR, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science 2006;314:126–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Babon JA, DeNicola ME, Blodgett DM, et al. Analysis of self-antigen specificity of islet-infiltrating T cells from human donors with type 1 diabetes. Nat Med 2016;22:1482–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Driver JP, Racine JJ, Ye C, et al. Interferon-γ limits diabetogenic CD8(+) T-cell effector responses in type 1 diabetes. Diabetes 2017;66:710–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Unger WW, Pinkse GG, Mulder-van der Kracht S, et al. Human clonal CD8 autoreactivity to an IGRP islet epitope shared between mice and men. Ann N Y Acad Sci 2007;1103:192–195 [DOI] [PubMed] [Google Scholar]
- 31.Jarchum I, Nichol L, Trucco M, Santamaria P, DiLorenzo TP. Identification of novel IGRP epitopes targeted in type 1 diabetes patients. Clin Immunol 2008;127:359–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Babad J, Mukherjee G, Follenzi A, et al. Generation of β cell-specific human cytotoxic T cells by lentiviral transduction and their survival in immunodeficient human leucocyte antigen-transgenic mice. Clin Exp Immunol 2015;179:398–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lightfoot YL, Chen J, Mathews CE. Role of the mitochondria in immune-mediated apoptotic death of the human pancreatic beta cell line betaLox5. PLoS One 2011;6:e20617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brusko TM, Koya RC, Zhu S, et al. Human antigen-specific regulatory T cells generated by T cell receptor gene transfer. PLoS One 2010;5:e11726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson LA, Morgan RA, Dudley ME, et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood; 2009;114:535–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takaki T, Marron MP, Mathews CE, et al. HLA-A*0201-restricted T cells from humanized NOD mice recognize autoantigens of potential clinical relevance to type 1 diabetes. J Immunol 2006;176:3257–3265 [DOI] [PubMed] [Google Scholar]
- 37.Chen J, Grieshaber S, Mathews CE. Methods to assess beta cell death mediated by cytotoxic T lymphocytes. J Vis Exp 2011;52:2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gusdon AM, Fernandez-Bueno GA, Wohlgemuth S, Fernandez J, Chen J, Mathews CE. Respiration and substrate transport rates as well as reactive oxygen species production distinguish mitochondria from brain and liver. BMC Biochem 2015;16:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell 2012;149:274–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xia CQ, Peng R, Chernatynskaya AV, et al. Increased IFN-α-producing plasmacytoid dendritic cells (pDCs) in human Th1-mediated type 1 diabetes: pDCs augment Th1 responses through IFN-α production. J Immunol 2014;193:1024–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chávez-Galán L, Arenas-Del Angel MC, Zenteno E, Chávez R, Lascurain R. Cell death mechanisms induced by cytotoxic lymphocytes. Cell Mol Immunol 2009;6:15–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gil MP, Ploquin MJ, Watford WT, et al. Regulating type 1 IFN effects in CD8 T cells during viral infections: changing STAT4 and STAT1 expression for function. Blood 2012;120:3718–3728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nguyen KB, Watford WT, Salomon R, et al. Critical role for STAT4 activation by type 1 interferons in the interferon-gamma response to viral infection. Science 2002;297:2063–2066 [DOI] [PubMed] [Google Scholar]
- 44.Kaech SM, Cui W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat Rev Immunol 2012;12:749–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Glimcher LH, Townsend MJ, Sullivan BM, Lord GM. Recent developments in the transcriptional regulation of cytolytic effector cells. Nat Rev Immunol 2004;4:900–911 [DOI] [PubMed] [Google Scholar]
- 46.Kaur S, Lal L, Sassano A, et al. Regulatory effects of mammalian target of rapamycin-activated pathways in type I and II interferon signaling. J Biol Chem 2007;282:1757–1768 [DOI] [PubMed] [Google Scholar]
- 47.Pollizzi KN, Powell JD. Regulation of T cells by mTOR: the known knowns and the known unknowns. Trends Immunol 2015;36:13–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sarbassov DD, Ali SM, Sengupta S, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell 2006;22:159–168 [DOI] [PubMed] [Google Scholar]
- 49.Yang Z, Chen M, Fialkow LB, Ellett JD, Wu R, Nadler JL. Inhibition of STAT4 activation by lisofylline is associated with the protection of autoimmune diabetes. Ann N Y Acad Sci 2003;1005:409–411 [DOI] [PubMed] [Google Scholar]
- 50.Yang Z, Chen M, Carter JD, et al. Combined treatment with lisofylline and exendin-4 reverses autoimmune diabetes. Biochem Biophys Res Commun 2006;344:1017–1022 [DOI] [PubMed] [Google Scholar]
- 51.Cho SS, Bacon CM, Sudarshan C, et al. Activation of STAT4 by IL-12 and IFN-alpha: evidence for the involvement of ligand-induced tyrosine and serine phosphorylation. J Immunol 1996;157:4781–4789 [PubMed] [Google Scholar]
- 52.Mathelier A, Fornes O, Arenillas DJ, et al. JASPAR 2016: a major expansion and update of the open-access database of transcription factor binding profiles. Nucleic Acids Res 2016;44(D1):D110–D115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ivashkiv LB, Donlin LT. Regulation of type I interferon responses. Nat Rev Immunol 2014;14:36–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hanson RD, Ley TJ. Transcriptional activation of the human cytotoxic serine protease gene CSP-B in T lymphocytes. Mol Cell Biol 1990;10:5655–5662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haddad P, Wargnier A, Bourge JF, Sasportes M, Paul P. A promoter element of the human serine esterase granzyme B gene controls specific transcription in activated T cells. Eur J Immunol 1993;23:625–629 [DOI] [PubMed] [Google Scholar]
- 56.Hanson RD, Grisolano JL, Ley TJ. Consensus AP-1 and CRE motifs upstream from the human cytotoxic serine protease B (CSP-B/CGL-1) gene synergize to activate transcription. Blood 1993;82:2749–2757 [PubMed] [Google Scholar]
- 57.Wargnier A, Legros-Maida S, Bosselut R, et al. Identification of human granzyme B promoter regulatory elements interacting with activated T-cell-specific proteins: implication of Ikaros and CBF binding sites in promoter activation. Proc Natl Acad Sci U S A 1995;92:6930–6934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamamoto K, Miura O, Hirosawa S, Miyasaka N. Binding sequence of STAT4: STAT4 complex recognizes the IFN-gamma activation site (GAS)-like sequence (T/A)TTCC(C/G)GGAA(T/A). Biochem Biophys Res Commun 1997;233:126–132 [DOI] [PubMed] [Google Scholar]
- 59.Funabiki M, Kato H, Miyachi Y, et al. Autoimmune disorders associated with gain of function of the intracellular sensor MDA5. Immunity 2014;40:199–212 [DOI] [PubMed] [Google Scholar]
- 60.Marroqui L, Dos Santos RS, Fløyel T, et al. TYK2, a candidate gene for type 1 diabetes, modulates apoptosis and the innate immune response in human pancreatic β-cells. Diabetes 2015;64:3808–3817 [DOI] [PubMed] [Google Scholar]
- 61.Kariuki SN, Kirou KA, MacDermott EJ, Barillas-Arias L, Crow MK, Niewold TB. Cutting edge: autoimmune disease risk variant of STAT4 confers increased sensitivity to IFN-alpha in lupus patients in vivo. J Immunol 2009;182:34–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.