Highlights
-
•
Carotid body tumors are rare neuroendocrine neoplasms derived from neural crest cells.
-
•
MRI imaging is considered to be the gold standard criterion for carotid body tumors diagnosis.
-
•
Surgical resection of carotid body tumors represents a special challenge to the surgeon.
Keywords: Carotid body tumor, Bilateral, Paraganglioma, Resection
Abstract
Introduction
Carotid body tumors also called carotid paragangliomas are rare neuroendocrine neoplasms derived from neural crest cells, approximately 3% of all paragangliomas occur in the head and neck area (Xiao and She, 2015); although they represent 65% of the head and neck paragangliomas (Georgiadis et al., 2008).
Presentation of case
We present the therapeutic management of a 65-year-old woman with bilateral carotid body tumors. The patient presented to medical clinic for unrelated signs and symptoms of weight loss, dyspepsia, and epigastric pain. Physical examination showed bilateral non-tender neck masses for which imaging studies were ordered resulting in the diagnosis of bilateral carotid tumor. Surgical resection was staged with one week of distance between each tumor resection.
Discussion
Carotid Body Tumors can arise from the paraganglia located within the adventitia of the medial aspect of the carotid bifurcation.
Resection is the only curative treatment. Carotid body tumors resection represents a special challenge due to potential neurovascular complications.
Conclusions
Surgical resection of carotid body tumors represents a special challenge to the surgeon because of the complex anatomical location of the tumor, including close relationship with the cranial nerves, involvement of the carotid vessels and large vascularization of the tumor. With the advance of diagnosis and improvement in surgical techniques as well as the understanding of biological behavior of tumors, surgical treatment has become a safer alternative for treating these tumors.
1. Background
Carotid body tumors are neuroendocrine neoplasms, derived embryonically from the neural crest cells of the autonomic nervous system, forming a group of neoplasms called paragangliomas. Parasympathetic paragangliomas are rare, with a prevalence of 1–2 per 100,000 population representing only 0.012% of all body tumors [1]. Approximately 3% of all paragangliomas occur in the head and neck area, the most anatomically prevalent sites found within the head and neck region are the carotid body, jugular body, the vagus nerve and along the glossopharyngeal nerve and its tympanic branch [1].
Carotid body tumors represent approximately 65% of head and neck paragangliomas [2]; about 5% of carotid body tumors are bilateral [3].
There are 3 different types of carotid body tumors described in the literature, Sporadic, Familial and Hyperplastic.
The most common type is the sporadic form, representing approximately 85% of carotid body tumors. The mean age of onset is reported to be 45 years.
The familial type can occur in 10–50% of the cases; in the familial group the age of onset is typically younger, being in the second to fourth decade, bilateral carotid body tumors are also related to the familial type, being found in 30–40% of the cases compared with 3–4% of the sporadic type [4].
The hyperplastic type is related in patients with chronic hypoxia, which includes, patients who have chronic obstructive pulmonary disease or cyanotic heart disease. Patients living at a high altitude (>5000 feet above sea level) are also related to the hyperplastic type [5].
Surgery is the only curative treatment for this type of tumors, being a special challenge to the surgeon due to the highly complex anatomic area of work, with vital structures being at risk of injury.
2. Case report
A 65-year-old woman presented to the clinic with history of weight loss, epigastric pain, and some degree of dyspepsia. The patient denied any history of hoarseness, dysphagia or voice changes. Past medical history and family history were unremarkable. On physical examination, the only pertinent finding was related to the neck, where on the right anterior triangle, a 4.0 × 3 cm non-tender, rubbery mass could be palpated over the common carotid artery. The mass was freely movable horizontally but not vertically (Fontaine sign). The left side of the neck was unremarkable. Neurological and cardiovascular examinations were intact.
Thyroid disease was suspected, for which general laboratory studies were sent, including thyroid function tests and neck ultrasound, the laboratory findings were unremarkable except for an elevation of the C- reactive protein; The ultrasound showed a single nodular cyst of the thyroid with benign characteristics, it also showed bilateral neck masses at the carotid bifurcation.
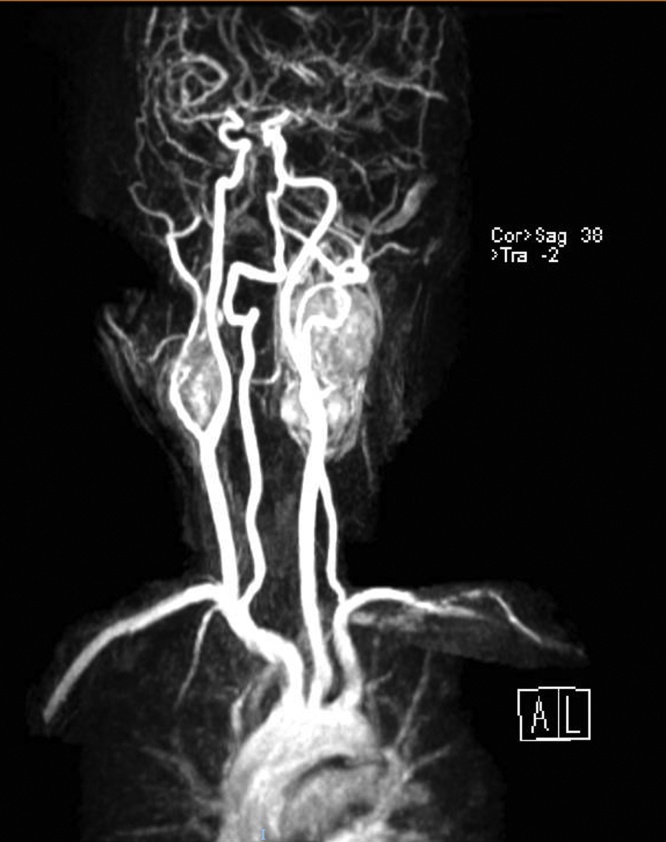
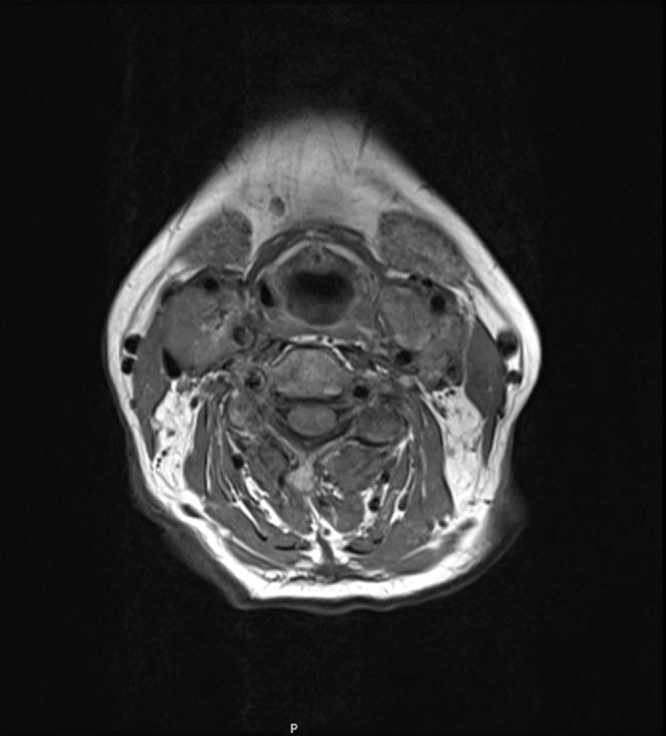
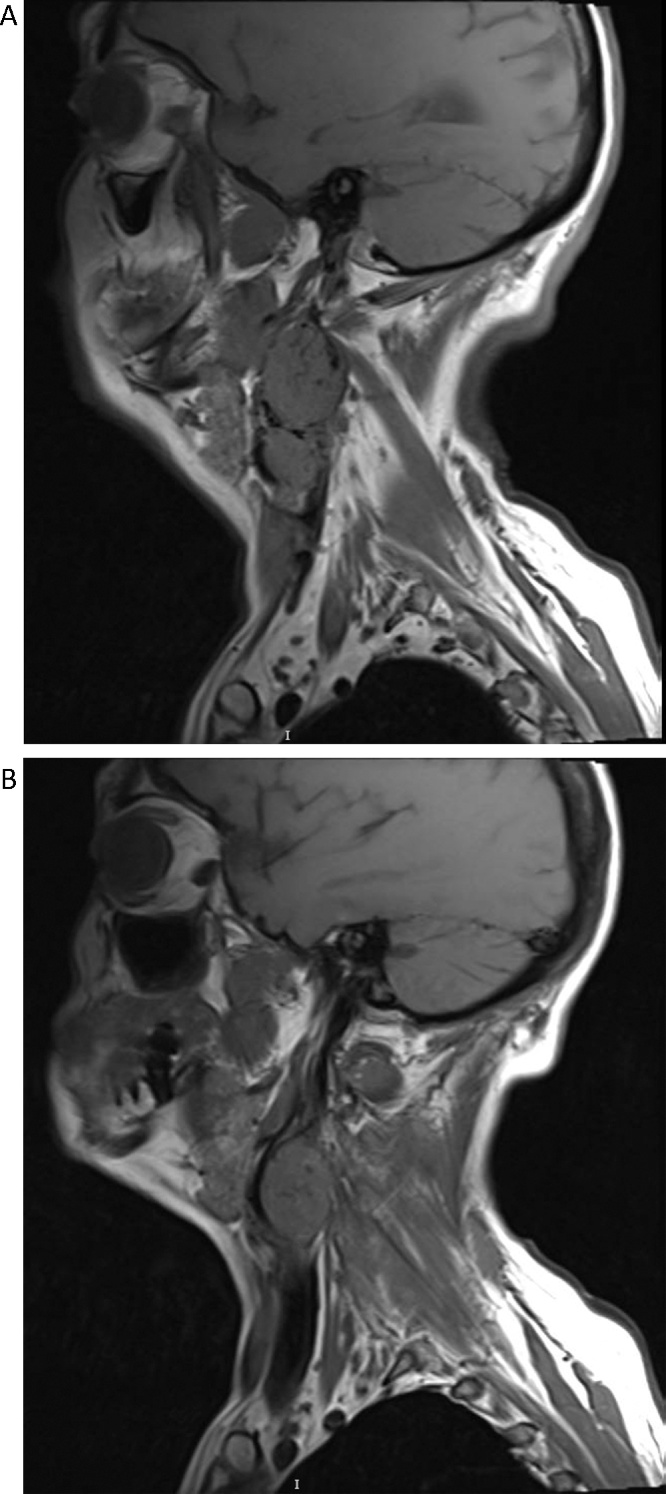
Further imaging evaluation was ordered, magnetic resonance imaging (MRI) and MR angiography showed the presence of two multilobulated, vascular masses located in both carotid spaces, anterior to the sternocleidomastoid muscle, at the level of the carotid bifurcation in T1 weighted images, with the characteristic heterogenic image of “salt and pepper” appearance (Fig. 1). Both lesions encased the external and internal carotid arteries giving a Shamblin type III classification (Fig. 2). The mass dimensions on the left carotid artery were 5.9 × 3.9 × 3.1 cm (Fig. 3A); on the right carotid artery the mass dimension were 2.3 × 3.0 × 2.6 cm (Fig. 3B). On the MRA sequence, we identify the feeder vessels in both lesions, which apparently depended on the ascending pharyngeal artery. These findings were consistent with bilateral carotid body tumors.
Fig. 1.
Magnetic resonance angiography showing bilateral carotid body tumors at the level of the carotid bifurcation.
Fig. 2.
Axial T-1 weighted MR image showing bilateral carotid body tumors; both with complete encasement of the ICA and ECA; Shamblin type III.
Fig. 3.
Sagittal T-1 weighted MRI of the left carotid artery (A) and right carotid artery (B) showing a mass at the level of the carotid bifurcation consistent of carotid body tumors.
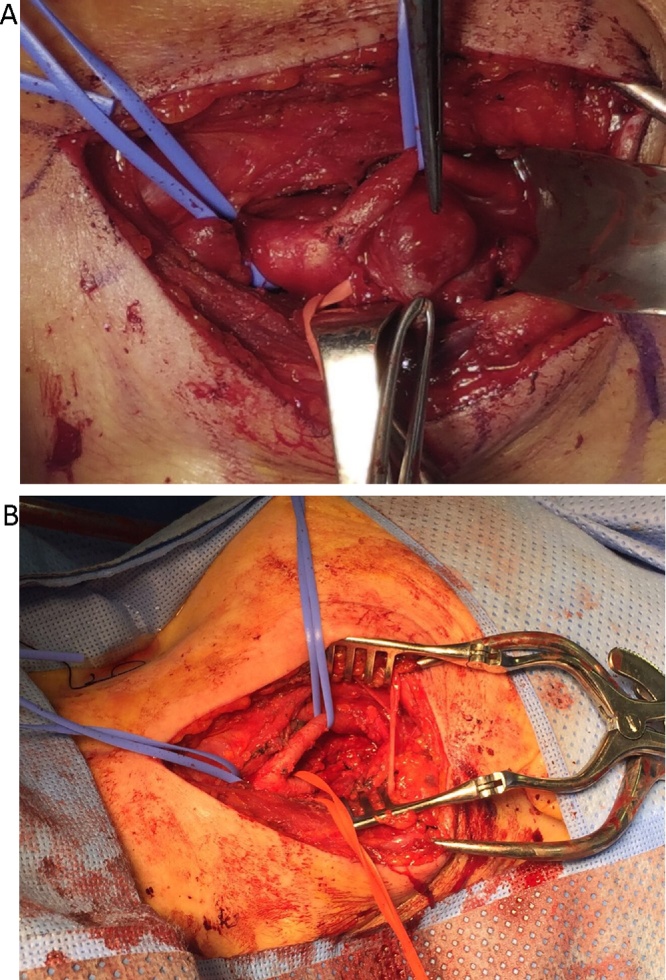
We recommend resecting the largest tumor first based on the reviewed literature. Therefore, the patient underwent surgical resection of the left-side tumor first. A left lateral cervical incision was made parallel to the anterior border of sternocleidomastoid muscle; careful dissection to expose the carotid vessels was critical to preserve all the surrounding structures (Fig. 4A). Proximal and distal control of the common carotid artery, internal carotid artery, and external carotid artery were performed before tumor resection. The inferior pole of the tumor was easily resected from the carotid arteries; we faced a special challenge with the resection of the superior pole of the tumor due to the extension of the tumor into the base of the skull, the Vagus nerve was identified at both the superior and inferior poles of the mass. All feeding vessels were ligated. The tumor was completely removed (Fig. 4B).
Fig. 4.
Intraoperative images of the tumor before resection (A) and after the tumor resection (B); note how all important structures must be identified before proceeding to the resection.
One week after the left tumor resection the patient had a second intervention to resect the right carotid body tumor, the same principles of the first intervention applied for this procedure. A right lateral cervical incision was made parallel to the anterior border of sternocleidomastoid muscle; followed careful dissection to expose the carotid vessels, proximal and distal control of the common carotid artery, internal carotid artery, and external carotid artery were performed before tumor resection. The right tumor was resected without any complications. All feeding vessels were ligated. The tumor was completely removed.
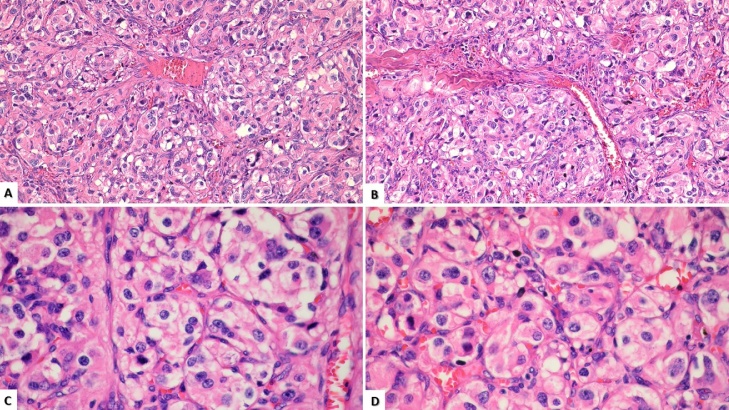
Both carotid body tumors were sent to pathology for analysis and final diagnosis (Fig. 5).
Fig. 5.
H&E 40 X showing classic architectural pattern of paragangliomas; consisting of well-defined solid nests of tumor cells, rounded by a fibro-vascular tissue “Zenballen configuration” (A and B). H&E 100X cytological characteristics of the tumor cells, showing a nucleus with moderate pleomorphism and abundant granular cytoplasm (C y D).
The patient presented post-operatively with mild dysphagia, voice changes and tongue deviation; nerve injury was suspected, therefore speech therapy was ordered. After a few sessions of therapy the patient dysphagia, voice changes, and tongue deviation improved.
3. Discussion
The carotid body originates from the embryological third branchial arch and from neuroectodermal derived neural crest lineage. The normal carotid body is ellipsoid, red-brown formation, located in the adventitia or periadventitial tissue at the bifurcation of the common carotid artery [6]. The healthy gland measures 3–5 mm in diameter and weighs less than 15 mg on average. The carotid body is mainly innervated by the Hering nerve, branch of the glossopharyngeal nerve, although many other important nerves pass in close proximity to the carotid body. The carotid body is highly vascular and receives its blood supply from feeder vessels running through the Mayer ligaments, predominantly from the ascending pharyngeal artery, which is branch of the external carotid artery. An alternative blood supply may be found in the vertebral artery.
Carotid body tumor is a rare neoplasm of the neuroendocrine system. Other glands of neural crest origin are seen in the adrenal medulla, neck, mediastinum, parapharyngeal spaces and retroperitoneum. Tumors involving these structures have been referred to as paraganglioma, glomus tumor, or chemodectoma. They have an incidence of approximately 1:30 000–1:100 000 in the general population, representing only 0.012% of all body tumors, in the majority of the cases they are considered benign, [1], [7], [8]. Malignant degeneration is rare and cannot be diagnosed histologically. Therefore, metastasis to a non-neuroendocrine tissue is regarded as the only true sign of malignancy [9]. Carotid body tumors are classified into sporadic, familial, and hyperplastic forms.
The most common type is the sporadic form, representing approximately 85% of carotid body tumors. The familial type can occur in 10–50% of the cases; in the familial group, the age of onset is typically younger being in the second to fourth decade. Bilateral carotid body tumors are also related to the familial type being found in 30–40% of the cases compared with 3–4% of the sporadic type.
The hyperplastic type is related in patients with chronic hypoxia, which includes, patients who have chronic obstructive pulmonary disease or cyanotic heart disease. Patients living at a high altitude (>5000 feet above sea level) are also related to the hyperplastic type [5].
Patients usually present between the fifth and seventh decades of life with an asymptomatic lateral neck mass in the anterior triangle of the neck. Carotid body tumors are slow-growing neoplasms that can remain asymptomatic for many years. The median growth rate is 1.0 mm/year and the median tumor doubling time is 4. 2 years, therefore a “wait and scan” policy might be justified in some cases [10].
On physical examination, the mass is typically palpated vertically fixed because of its attachment to the bifurcation of the common carotid (Fontaine sign). Sometimes a bruit can be felt over the anterior neck area; however, the absence of a bruit does not rule out a carotid body tumor [11].
Approximately 10% of the cases present with cranial nerve palsy with paralysis of the hypoglossal, glossopharyngeal, recurrent laryngeal, or spinal accessory nerve, or involvement of the sympathetic chain, therefore been associated with pain, hoarseness, dysphagia, Horner syndrome, or shoulder drop [12].
The diagnosis is made primarily by imaging techniques. Duplex ultrasound, CT scan, and MRI are all useful for the diagnosis. Carotid duplex ultrasound can localize a tumor adjacent to the carotid bifurcation, but CT or MRI is usually required to further delineate the relationship of the tumor to the adjacent structures. MRI imaging is considered to be the gold standard criterion for carotid body tumors diagnosis, where the tumor has a characteristic salt and pepper appearance on T1-weighted image.
Due to the highly vascular nature of the tumor, an open or closed biopsy should not be attempted and angiographic studies are recommended to better identify the feeder vessels and delineate the borders of the tumor due to the close proximity of the tumor to vital structures and allow for preoperative embolization of the feeder vessels, which has been reported to reduce intraoperative blood loss.
Shamblin in 1971 described a classification system for carotid body tumors based on the involvement of carotid vessels and tumor size [13]. Type I tumors are small tumors that are easily dissected from the adjacent vessels in a periadventitial plane and do not involve the surrounding vessels. Type II tumors are adherent or partially surround and compress the carotid vessels. Type III tumors have an intimate adherent relationship to the entire circumference of the carotid bifurcation, requiring partial or complete vessel resection and reconstruction.
In 1880 Reigner attempted the first excision of a carotid body tumor, but unfortunately, the patient died soon after the surgery [14]. In 1886, Maydl successfully resected the tumor but the patient became hemiplegic and aphasic due to stroke [15]. Albert performed the first successful carotid body tumor excision in 1889 without any complications reported in the case [16].
Surgical resection is the recommended treatment for carotid body tumors, with the main goal of excising the tumor and preventing local advancement.
Several important structures like the hypoglossal nerve, the pharyngeal and superior laryngeal rami of the vagus nerve, the accessory nerve, the glossopharyngeal nerve, the mandibular ramus of the facial nerve and the cervical sympathetic chain may be involved by carotid body tumors and need special attention and identification to be preserved during the surgical procedure.
A crucial step in carotid body tumor resection is superior and inferior control of the blood vessels. This includes identification of the common, internal and external carotid arteries and internal jugular vein.
The most common complication associated with surgery is related to postoperative cranial nerve dysfunction. The risk of cranial nerve palsy as a complication of carotid body tumor resection has been reported to range from 10% to 40% [17].
During resection of the carotid body tumor, special attention and identification of the surrounding nerves are crucial in avoiding inadvertent injuries, especially the superior laryngeal nerve, which has been reported to be the most injured nerve during dissection. The highest risk patients for cranial nerve palsy are the ones who have tumors larger than 5 cm and/or type III by Shamblin classification [18]. The patient might suffer from some aspiration problems and voice changes due to nerve injury.
Patients with bilateral carotid body tumors require special attention; in these patients the recommended procedure is staged excision, removing the largest tumor first [19], and re-operating the second tumor later. Doing it simultaneously carries the risk of experience labile blood pressure postoperatively, which is difficult to control medically due to bilateral Hering nerves excisions.
4. Conclusions
Carotid body tumors are rare neuroendocrine neoplasms, meticulous surgical techniques, as well as knowledge of the anatomy of the region, are of paramount importance for decreasing cranial nerves lesions during carotid body tumor resection surgery.
Bilateral carotid body tumors resections require staging procedures to reduce the risk of cardiovascular and neurological issues.
Conflict of interest
There was no conflict of interest.
Funding
There was no sponsorship for this study.
Ethical approval
Ethical approval wasn’t required by our institution for reporting this case; therefore we consent the patient about reporting the case and obtaining the approval.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.This work has been reported in line with the SCARE criteria (Agha et al., 2016 [20]).
Author contribution
Burgess, Alfred MD: Study design.
Calderon, Moises MD: Study design.
Jafif-Cojab, Marcos MD: Data collection, data analysis, writing the paper.
Balanza, Ricardo MD: Data collection, data analysis.
Jorge, Diego MD: Data collection, data analysis.
Guarantor
Burgess, Alfred MD.
Footnotes
This work has been reported in line with the SCARE criteria (Agha et al., 2016 [20]).
References
- 1.Xiao Zebin, She Dejun. Multiple paragangliomas of head and neck associated with hepatic paraganglioma: a case report. BMC Med. Imaging. 2015;15:38. doi: 10.1186/s12880-015-0082-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Georgiadis George S. J. Vasc. Surg. 2008;47:874–880. doi: 10.1016/j.jvs.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 3.Karatas E. Synchronous bilateral carotid body tumor and vagal paraganglioma a case report and review of literature. Auris Nasus Larynx. 2008;35:171–175. doi: 10.1016/j.anl.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 4.Demir Tolga, Uyar Ibrahim. Five year follow up of a patient with bilateral carotid body tumors after unilateral surgical resection. Am. J. Case Rep. 2014;15:426–430. doi: 10.12659/AJCR.891150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sajid M.S. A multicenter review of carotid body tumour management. Eur. J. Vasc. Endovasc. Surg. 2007;34:127–130. doi: 10.1016/j.ejvs.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 6.Fennessy B.G., Kozakewich H.P., Silvera M. The presentation and management of multiple paraganglioma in head and neck. Ir. J. Med. Sci. 2011;180:757–760. doi: 10.1007/s11845-009-0338-0. [DOI] [PubMed] [Google Scholar]
- 7.Boedeker C.C. Paragangliomas and paraganglioma syndromes. GMS Curr. Top Otorhinolaryngol. Head Neck Surg. 2011;10 doi: 10.3205/cto000076. Doc03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kollert M., Minovi A.A., Draf W., Bockmühl U. Cervical paragangliomas-tumor control and long-term functional results after surgery. Skull Base. 2006;16:185–191. doi: 10.1055/s-2006-950386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warshawski S.J., de Souza F.M. The carotid body tumor. J. Otolaryngol. 1989;18:306–310. [PubMed] [Google Scholar]
- 10.Jansen J.C., Van den Berg R., Kulper A., Van der Mey A.G., Zwindermn A.H., Cornelisse C.J. Estimation of growth rate in patients with head and neck paragangliomas influences the treatment proposal. Cancer. 2000;88:2811–2816. [PubMed] [Google Scholar]
- 11.Athanasiou A., Liappis C.D., Rapidis A.D., Fassolis A., Stavrianos S.D., Kokkalis G. Carotid body tumor: review of the literature and report of a case with a rare sensorineural symptomatology. J. Oral Maxillofac. Surg. 2007;65:1388–1393. doi: 10.1016/j.joms.2005.12.036. [DOI] [PubMed] [Google Scholar]
- 12.Isik A.C., Imamoglu M., Erem C., Sari A. Paragangliomas of the head and neck. Med. Principle Pract. 2007;16:209–214. doi: 10.1159/000100392. [DOI] [PubMed] [Google Scholar]
- 13.Shamblin W.R., ReMine W.H., Sheps S.G., Harrison E.G., Jr Carotid body tumor (chemodectoma). Clinicopathologic analysis of ninety cases. Am. J. Surg. 1971;122:732–739. doi: 10.1016/0002-9610(71)90436-3. [DOI] [PubMed] [Google Scholar]
- 14.Riegner (1880). Cited in: F.H. Lahey, K.W. Warren. A long-term appraisal of carotid body tumors with remarks on their removal. Surg. Gynec. Obstet. 1951, 92:481. [PubMed]
- 15.Maydl (1886). Cited in: J.J. Byrne. Carotid body and allied tumors. Am. J. Surg. 1958, 95:371. [DOI] [PubMed]
- 16.Albert (1889). Cited in: E.F. Staats, R.L. Brown, R.R. Smith. Laryngoscope. 1966, 76:907. [DOI] [PubMed]
- 17.Kollert M., Minovi A.A., Draf W., Bockmühl U. Cervical paragangliomas-tumor control and long-term functional results after surgery. Skull Base. 2006;16:185–191. doi: 10.1055/s-2006-950386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arya S., Rao V., Juvekar S., Dcruz A.K. Carotid body tumors: objective criteria to predict the Shamblin group on MR imaging. AJNR Am. J. Neuroradiol. 2008;29(August (7)):1349–1354. doi: 10.3174/ajnr.A1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Velegrakis G., Kalikakis G., Karampekios S. Bilateral paraganglioma of the vagus nerve. HNO. 2001;49:471–475. doi: 10.1007/s001060170099. [DOI] [PubMed] [Google Scholar]
- 20.Agha R.A., Fowler A.J., Saetta A., Barai I., Rajmohan S., Orgill D.P., for the SCARE Group The SCARE statement: consensus-based surgical case report guidelines. Int. J. Surg. 2016 doi: 10.1016/j.ijsu.2016.08.014. [DOI] [PubMed] [Google Scholar]