Abstract
Drosophila peripheral nerves, similar structurally to the peripheral nerves of mammals, comprise a layer of axons and inner glia, surrounded by an outer perineurial glial layer. Although it is well established that intercellular communication occurs among cells within peripheral nerves, the signaling pathways used and the effects of this signaling on nerve structure and function remain incompletely understood. Here we demonstrate with genetic methods that the Drosophila peripheral nerve is a favorable system for the study of intercellular signaling. We show that growth of the perineurial glia is controlled by interactions among five genes: ine, which encodes a putative neurotransmitter transporter; eag, which encodes a potassium channel; push, which encodes a large, Zn2+-finger-containing protein; amn, which encodes a putative neuropeptide related to the pituitary adenylate cyclase activator peptide; and NF1, the Drosophila ortholog of the human gene responsible for type 1 neurofibromatosis. In other Drosophila systems, push and NF1 are required for signaling pathways mediated by Amn or the pituitary adenylate cyclase activator peptide. Our results support a model in which the Amn neuropeptide, acting through Push and NF1, inhibits perineurial glial growth, whereas the substrate neurotransmitter of Ine promotes perineurial glial growth. Defective intercellular signaling within peripheral nerves might underlie the formation of neurofibromas, the hallmark of neurofibromatosis.
Both mammalian and invertebrate peripheral nerves comprise a central core of motor and sensory axons surrounded by inner glia (termed Schwann cells in mammals and peripheral glia in Drosophila) and outer glia (termed perineurium in mammals, and perineurial glia in Drosophila; refs. 1 and 2). In Drosophila, the peripheral glia form the “blood–brain” permeability barrier (1). The role of the perineurial glia is less clear but might serve to provide structural support for the peripheral nerves. These cells communicate extensively by the release of and response to both small molecule and peptide neurotransmitters, as well as protein factors (3). For example, acetylcholine and ATP released by the frog motor nerve terminal in response to nerve activity increase intracellular [Ca2+] in neighboring perisynaptic Schwann cells (4). In addition, glutamate released from the squid giant neuron activates Schwann cell potassium currents by means of increased [cAMP] (5). Finally, Schwann cells release factors such as Desert Hedge Hog that organize the surrounding perineurium, and also release factors that regulate neuronal survival, neuronal excitability, synaptic transmission, and Schwann cell survival in the absence of neurons (6–8).
One disease in humans that might reflect impaired intercellular communication within peripheral nerves is type 1 neurofibromatosis, caused by mutations in the NF1 gene. This disease is characterized by the appearance of peripheral nerve sheath tumors called neurofibromas (reviewed in ref. 9). As is the case with other disorders involving tumor-suppressor genes, type 1 neurofibromatosis is inherited as a dominant disorder, and it is thought that tumors arise after spontaneous loss of the NF1+ allele within certain cells during somatic growth. However, neurofibromas exhibit properties that are not expected for those caused by loss of a tumor suppressor. For example, neurofibromas are heterogeneous and contain cells derived from each of the cell types normally found within peripheral nerves (neurons, Schwann cells, and perineurial cells). These cells are not clonally related and thus it seems highly unlikely that the NF1+ allele has been lost in each cell type. Rather, it seems more likely that phenotypically wild-type cells within these tumors overproliferate in response to factors released aberrantly from Nf1− cells. However, this possibility remains speculative and no detailed mechanism for the aberrant release of a diffusible factor has been proposed.
Here we report a genetic dissection of signaling pathways controlling the structure of the Drosophila peripheral nerve. We show that mutations in five distinct genes, in either single or particular double-mutant combination, increase the growth of the perineurial glial layer of the larval peripheral nerve. Two of these genes, amnesiac (amn) and inebriated (ine), are each likely to control neurotransmitter-mediated signaling pathways. amn encodes a putative neuropeptide related to the pituitary adenylate cyclase activator peptide (PACAP; ref. 10), whereas ine encodes a putative Na+/Cl−-dependent neurotransmitter transporter and is likely to be required for reuptake of a substrate neurotransmitter (11–13). These observations suggest that perineurial glial growth is controlled by two distinct neurotransmitters. Two additional genes, pushover (push) and NF1 (the Drosophila ortholog of the human NF1 gene), have each been implicated in other systems as downstream targets of Amn or PACAP (refs. 14–16; S. Hawley, personal communication). These observations suggest that push and NF1 might mediate the effects of Amn on perineurial glial growth. The fifth gene, eag, encodes a potassium channel that affects motor neuron excitability (17), suggesting a role for neuronal excitability in the control of perineurial glial growth. We also report the sequence of push, which encodes an extremely large membrane protein containing two Zn2+ fingers that is conserved throughout evolution. We propose a model for the control of perineurial glial growth by two interacting neurotransmitter-mediated signaling pathways. Furthermore, we suggest that neurofibromas, the hallmark of neurofibromatosis, might result from defective receipt of neurotransmitter signals within peripheral nerves.
Materials and Methods
Electron Microscopy.
Tissue sections were prepared as described (2). Because push mutations confer male sterility, it is not possible to construct a homozygous push mutant stock. Thus, to identify homozygous larvae, a stock was constructed in which the push, eag; push and ine push chromosomes were crossed into a yellow (y) background and placed in combination with the second chromosome balancer, CyO, that carried a P[y+] transgene. Homozygous larvae from these stocks were identified by yellow mouth hooks before dissection for electron microscopy. Wandering third instar larvae were grown at room temperature from uncrowded half-pint bottles and collected 1 or 2 days after the first wandering larvae appeared. For experiments involving heat shock, larvae were heat-shocked daily for 1 hour at 37°C. Larvae were dissected, fixed with glutaraldehyde and paraformaldehyde, stained with both 0.5% OsO4 and 2% uranyl acetate, and embedded in an eponate 12-araldite mixture. Ultrathin cross-sectional slices (pale gold, 75–125-nm-thick) were captured, poststained by using uranyl acetate and Reynolds lead citrate, and analyzed by using a JEOL or Hitachi (Tokyo) transmission electron microscope at either 60 or 80 kV. The thickness of the perineurial glial cell layer for a given nerve was determined by averaging the distance from the edge of the nerve to the boundary of the axon-containing lumen at 8 different positions: 12, 3, 6 and 9 o'clock and four additional measurements at points in between these positions. Measurements were not taken at positions where a perineurial glial cell nucleus was encountered. All statistical analyses were carried out by using STATVIEW v4.51 (Abacus Concepts, Berkeley, CA).
Cloning and Sequence Analysis of push.
DNA flanking push3420, a P element insertion that causes a hypomorphic push mutation (14, 18), was isolated by using a plasmid-rescue technique by transforming a dilute ligation of NheI-digested push3420 genomic DNA. The subcloned push3420 flanking DNA was used as a probe to screen both a lambda Drosophila genomic DNA library and a Drosophila testes cDNA library kindly donated by T. Hazelrigg (Columbia Univ., New York). Both of these libraries were screened according to protocols from Stratagene. A number of cDNA and genomic clones were isolated in the process of walking the entire gene. In addition, use was made of P1 clone DS002276 from the Berkeley Drosophila genome project, which contains the complete push gene. For sequencing, double-stranded DNA was prepared by using Qiagen (Hilden, Germany) plasmid purification kits, sequence reactions were performed by using the dye terminator chemistry from Perkin–Elmer Applied Biosystems (Foster City, CA), and were run on an automated DNA sequencer from the same company. Sequences were assembled by using sequencher software (Gene Codes, Ann Arbor, MI), and the completed cDNA was conceptually translated by using GCG software.
Results
Control of Perineurial Glial Growth by Interacting Pathways.
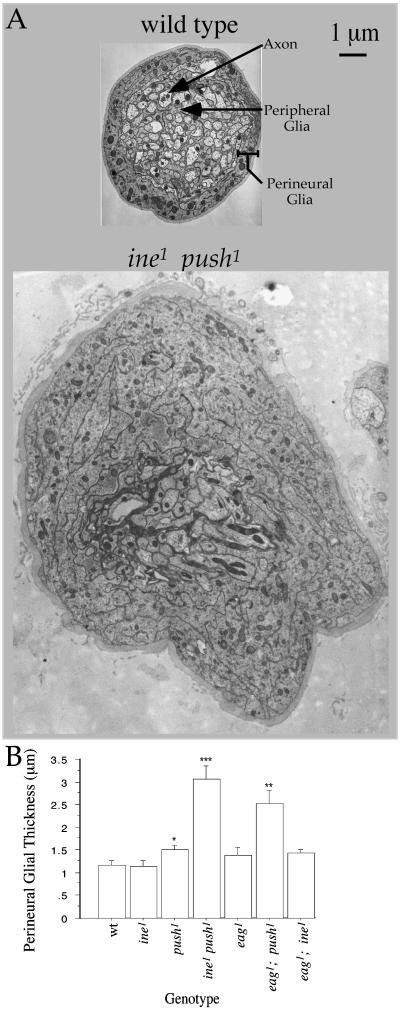
Mutations in two genes that affect neuronal excitability also affect the structure of the peripheral nerve: double mutants defective in inebriated (ine), which encodes a member of the Na+/Cl−-dependent neurotransmitter transporter family (11–13), and pushover (push) exhibit an extremely thickened nerve, which is a phenotype that is clearly visible with the dissecting microscope. To understand the cellular basis for this phenotype, we performed transmission electron microscopy on cross-sections of peripheral nerves. This analysis demonstrated that the push1 and ine1 push1 double mutants exhibited a normal axon and peripheral glial layer, but a thickened perineurial glial layer. This increased perineurial thickness was expressed only moderately in push1 but very strongly in the ine1 push1 double mutant (Fig. 1 and Fig. 5, which is published as supplemental data on the PNAS web site, www.pnas.org). This increase in thickness was accompanied by an increase in the number of mitochondria within perineurial glial thin sections (130 ± 19 in ine1 push1 vs. 30 ± 7 in wild type; P = 0.0001), suggesting that an increase in cell material accompanies this increased thickness. The ine1 push1 phenotype was significantly rescued in transgenic larvae expressing the 943-aa Ine isoform called Ine-P1 under the transcriptional control of the heat-shock promoter (13). In particular, perineurial glial thickness in ine1 push1; hs-ine-P1 larvae, even in the absence of heat shock, was reduced to 2.0 ± 0.2 μm (n = 11) from 3.1 ± 0.3 in ine1 push1 (n = 14, P value vs. ine1 push1; hs-ine-P1 = 0.008). The observed synergistic interaction between ine and push mutations suggests that each gene controls perineurial glial growth through partially redundant pathways.
Figure 1.
Effects of indicated mutants and double mutants on perineurial glial growth. Wild type indicates the isogenic parental control line of each mutant. (A) Cross sections of larval peripheral nerves of wild type and ine1 push1. Locations of the axon, peripheral glial, and perineurial glial layers are indicated. In ine1 push1, all of the cells that make up a peripheral nerve appear to be present and contain a normal number of axons (74.8 ± 1.8, n = 4) that are of normal cross-sectional area. (B) Means and standard errors of perineurial glial thickness for the indicated genotypes. The following pairwise combinations had statistically significant differences (two-tailed unpaired t test) in perineurial glial thickness: wild type vs. push1 (*, P = 0.04), ine1 push1 vs. ine1 or push1 (***, P < 0.0001), and eag1; push1 vs. eag1 or push1 (**, P = 0.0045 and 0.005, respectively).
In certain respects, mutations in ine confer phenotypes similar to mutations in the K+ channel structural gene eag. In particular, both eag and ine mutations interact synergistically with mutations in the K+ channel encoded by Shaker to cause a characteristic “indented thorax and down-turned wings” phenotype, which is not exhibited by any of the single mutants (19). Because of this phenotypic similarity, we tested the possibility that eag mutations might also affect perineurial glial thickness. We found that eag1 resembles ine1 in the control of perineurial glial growth: eag1; push1 double mutants, but not the eag1 single mutant, exhibited strongly potentiated perineurial glial growth. This increased growth was similar to, but less extreme than, what was observed in ine1 push1 (Figs. 1 and 5). eag1; push2 double mutants also exhibited a thickened perineurial glial layer (1.88 ± 0.15 μm, n = 14, P value vs. eag1 and push1 mutants = 0.04). In contrast, eag and ine mutations fail to display a comparable synergistic interaction: perineurial glial thickness in the eag1; ine1 double mutant was 1.43 ± 0.08 μm, which is not significantly different from the values of the eag1 or ine1 single mutants (P = 0.8 and 0.06, respectively).
Cloning and Sequence Analysis of push.
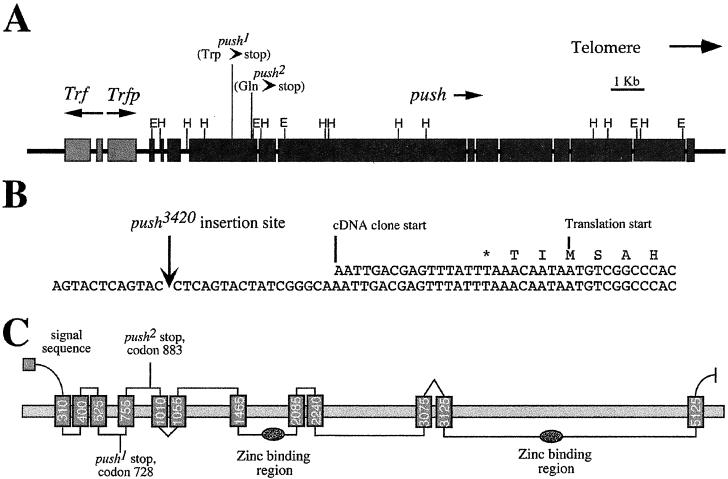
It was previously shown that a P element insertion termed push3420 caused a partial loss of function in push (14, 18). We used this P element as a starting point for the isolation of push DNA. We found that the P element lies between two ORFs, a centromere-proximal ORF called Trfp, and a novel centromere-distal ORF (20). To determine which ORF corresponded to push, we sequenced each ORF in the ethyl methanesulfonate (EMS)-induced push1 and push2 mutants and in the isogenic wild-type strain. The Trfp gene exhibited no sequence alterations in either push mutant. However, both push mutations were found in the novel ORF, and thus we assign this ORF as push; push1 and push2 mutations are both nonsense mutations (at codons 728 and 883 for push1 and push2, respectively). A putative TATA box is observed 260 bp upstream of the push3420 insertion site. Thus, it is possible that our most 5′ cDNA is incomplete at the 5′ end, and the push3420 P element is inserted in the 5′ untranslated region (UTR) of the push transcript. We found that push encodes an enormous 5,322-aa protein containing two potential Zn2+ binding domains and 12 potential transmembrane domains (Fig. 2).
Figure 2.
Structure of the push gene. (A) Scale diagram of the push locus including the positions of the Trf and Trfp genes. Boxes indicate exons, arrows indicate the direction of transcription of the genes, E indicates an EcoRI restriction enzyme site, and H indicates a HindIII restriction enzyme site. (B) Sequence around the push3420 P element insertion site showing the start of the most 5′ cDNA clone, the start of the push ORF, and the first methionine in that ORF (labeled “translation start”). (C) Hypothetical transmembrane structure of Push as predicted by tm pred (34). The horizontal box indicates the membrane bilayer, and the vertical boxes indicate putative transmembrane helices. The number on each helix represents the amino acid number at the approximate center of the helix. Positions of a putative signal sequence, the two nonsense mutations, and two putative zinc-binding regions are also indicated.
Push has many additional functions in Drosophila in addition to controlling neuronal excitability and perineurial glial growth. First, mutations in push cause male sterility (14). A P element insertion called purity of essence (poe), which also causes male sterility by means of the inhibition of sperm individuation, is found at the identical location to push3420, raising the possibility that push and poe are identical genes (21, 22). We found that push is transcribed in adult testes as well as the embryonic central nervous system (data not shown), which is consistent with this possibility. We confirmed this possibility by demonstrating that push and poe mutations fail to complement for the male sterility phenotype (data not shown). Second, push might have a role in eye development or function: an incomplete cDNA of push, encoding 4,100 amino acids and truncated at the N terminus, was identified previously under the name “Calossin.” This protein was identified on the basis of calmodulin binding and expression in the photoreceptor (23). Finally, genes similar to push are present in several multicellular organisms including Caenorhabditis elegans (GenBank accession no. AF003140), Arabidopsis, and humans (GenBank accession no. AB007931). However, the sequence of push provides few additional clues as to its function.
Mutations in push were identified independently on the basis of defective segregation of nonrecombinant chromosomes in the female meiosis (24). push was implicated in this process as an intermediate in a signaling pathway mediated by the PACAP-like neuropeptide encoded by amn (S. Hawley, personal communication). This observation raised the possibility that push likewise affects perineurial glial growth by acting as an intermediate from an Amn signal. Consistent with this hypothesis, we found that the amnX8 deletion mutation (25) increased perineurial glial thickness, and that this increase was significantly rescued in transgenic flies expressing amn+ (Fig. 3 and Fig. 6, which is published as supplemental data on the PNAS web site).
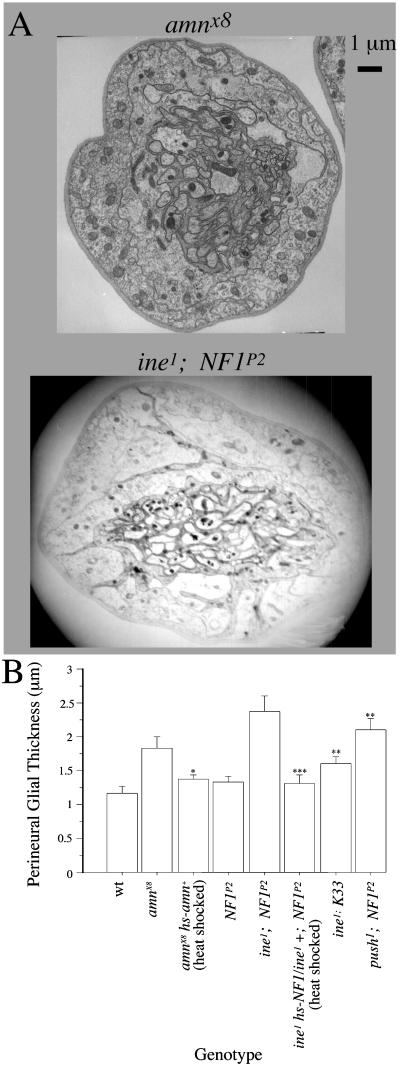
Figure 3.
Control of perineurial glial growth by amn and NF1. For both heat-shock control and heat-shock rescue experiments, lines were heat shocked daily for 1 hour at 37°C throughout development. We found that this heat-shocking protocol had no significant effect on perineurial glial thickness in either amnX8 or ine1: NF1P2 lacking the appropriate rescue construct. Perineurial glial thickness of amnX8 in the presence of heat shock was 1.82 ± 0.15 (n = 16) and in the absence of heat shock was 1.80 ± 0.07, n = 24. Perineurial glial thickness of ine1; NF1P2 in the presence of heat shock was 2.27 ± 0.17, n = 15, and in the absence of heat shock was 2.38 ± 0.22 (n = 12). (A) Electron micrographs of peripheral nerves of amnX8 and ine1; NF1P2 double mutants. (B) Means and standard errors of perineurial glial thickness for the indicated genotypes. The following pairwise combinations had statistically significant differences (two-tailed unpaired t test) in perineurial glial thickness: amnX8 or amnX8 heat shocked vs. amnX8 hs-amn+ heat shocked (*, P = 0.02), ine1; NF1P2 vs. ine1; K33 (**, P = 0.002), ine1; NF1P2, heat shocked vs. ine1 hs-NF1/ine1 +; NF1P2, heat shocked (***, P = 0.0003), and push1; NF1P2 vs. push1 or NF1P2 (**, P = 0.002 and 0.0005, respectively).
A second signaling pathway mediated by a PACAP-like neuropeptide was previously identified in Drosophila. In this pathway, the larval muscle responds to application of PACAP by activating a voltage-gated potassium channel (26). This activation requires NF1, the ortholog of the human gene responsible for type 1 neurofibromatosis (16). We tested the possibility that NF1 might affect perineurial glial growth. We found that the NF1P2-null mutant (15) exhibited strong potentiation of perineurial glial thickness in combination with ine1. This thickness was much greater than the thickness observed in ine1 mutants carrying K33, the NF1+ parent chromosome of NF1P2. The increased glial thickness of ine1; NF1P2 was fully rescued by heat-shock-induced expression of the NF1+ transgene (Figs. 3 and 6). However, unlike push, the phenotype of NF1P2 was potentiated only moderately by the eag1 mutation (not shown). In contrast, perineurial glial thickness in the push1; NF1P2 double mutant was 2.1 ± 0.15 μm, which is significantly thicker than either push1 or NF1P2 (P = 0.002 and 0.0005, respectively), but not significantly different from amnX8 (P = 0.33). These results are consistent with the possibility that push and NF1 mediate the amn signal through parallel partially redundant pathways.
Discussion
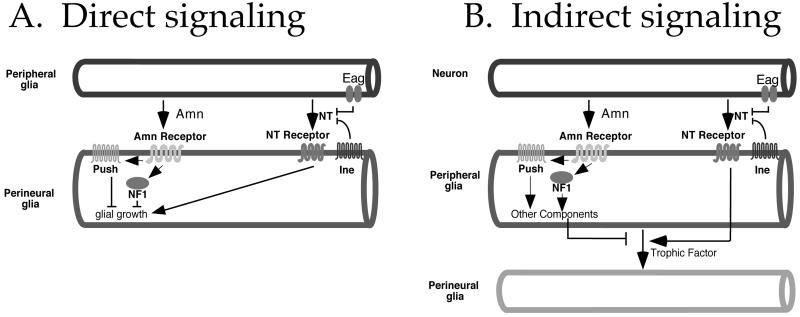
We have shown that mutations in five genes, in single- or double-mutant combination, increase growth of the perineurial glial layer of the Drosophila larval peripheral nerve. Our results are consistent with a model in which two neurotransmitter-mediated signaling pathways exert opposing effects on perineurial glial growth. One pathway, mediated by the Amn neuropeptide, inhibits perineurial glial growth. This pathway requires NF1 and Push activity. The second pathway, mediated by the substrate neurotransmitter of Ine (which we will call NT), promotes perineurial glial growth. In this pathway, mutations in ine or eag each increase signaling by NT: ine mutations increase NT signaling by eliminating the NT reuptake transporter thus increasing NT persistence, whereas eag mutations increase NT signaling by increasing NT release as a consequence of increased excitability. These pathways interact such that the most extreme effects on perineurial glial growth are observed when the NT pathway is overstimulated and the Amn pathway is disrupted simultaneously. The genetic interactions that form the basis for this interpretation require that the mutations under investigation be null. Although the eag1 mutation tested has not been characterized molecularly, the mutations in each of the other four genes that we analyzed are known to be or are strongly suspected to be null (11, 15, 25, this study). Direct neuron–perineurial glia signaling is unlikely because the peripheral glia, which form the blood–brain barrier, are expected to be an impervious barrier to intercellular traffic. Two alternative mechanisms could underlie this signaling. In the first mechanism (direct peripheral glia–perineurial glia signaling; Fig. 4A), the peripheral glia release each neurotransmitter, and the perineurial glia respond. In the second mechanism (indirect signaling; Fig. 4B), each neurotransmitter is released by neurons, and the peripheral glia respond by regulating the release of a trophic factor that acts on perineurial glia.
Figure 4.
Two models for the control of perineurial glial growth by neurotransmitters. (A) Direct signaling. The peripheral glia release both Amn and the substrate NT. The Amn neuropeptide inhibits perineurial glial growth via Push and NF1, whereas NT promotes perineurial glial growth. The Ine transporter attenuates the effects of this NT by reuptake. The Eag potassium channel, acting in peripheral glia, inhibits NT release by reducing excitability. (B) Indirect signaling. The peripheral glia release a trophic factor that causes increased cell growth among the perineurial glia. Release of this trophic factor is regulated by Amn and NT, each released from neurons, with opposing effects. Amn, acting through Push and Nf1, inhibits release of the trophic factor, whereas NT promotes release of the trophic factor. The placement of Push downstream of Amn is most consistent with previous studies (S. Hawley, personal communication), but we cannot rule out the possibility that Push acts in motor neurons, and its effect on perineurial glial growth results from increased release of NT or a distinct neurotransmitter. As above, Ine and Eag attenuate the effects of NT by performing reuptake and reducing release, respectively.
Although direct signaling seems to be the simplest possibility, indirect signaling is most consistent with previous studies. As described above, both invertebrate and mammalian motor neurons can release small molecule and peptide neurotransmitters that affect properties of Schwann cells. A similar motor nerve terminal-peripheral glia communication could occur in Drosophila, because first boutons at the larval neuromuscular junction are covered by peripheral glia (27). This observation raises the possibility that Drosophila peripheral glia might respond to Amn and NT released from motor nerve terminals, and propagate these signals along the length of the nerve via gap junctions. However, the alternative possibility of NT release from along the length of axons, as has been suggested in other systems (28, 29), cannot be ruled out. In addition, mammalian Schwann cells release trophic factors such as Desert Hedge Hog (Dhh) to induce growth of the surrounding perineurium (6), and astrocytes can respond to glutamate application by releasing a substance that affects blood vessels (30). Our model predicts that peripheral glia release a trophic factor that behaves similarly to Dhh. The prediction that Drosophila NF1 acts within peripheral glia is consistent with the likelihood that mammalian NF1 acts within Schwann cells as well (31, 32).
The possible effects of the thickened perineurial glia on motor neuron function are unclear. Mutations in four of the genes that affect perineurial glial thickness (eag, NF1, ine, and push) were each shown in previous studies to increase either neuronal or muscle membrane excitability (12–14, 16, 17, 19), which raises the possibility of a correlation between excitability and perineurial glial growth. However, we have been unable to detect any increases in neuronal excitability in the amn mutant or the ine; NF1 double mutant (greater than that conferred by the ine mutation alone), despite the presence of greatly thickened perineurial glia in these genotypes (data not shown). It is possible that the effects on neuronal excitability of these genotypes might be subtler than our assays can detect, or that the participation of these genes in both perineurial glial growth and excitability is coincidental.
Our results are consistent with the previous observations that push and NF1 act downstream of the Amn/PACAP receptor (ref. 16; S. Hawley, personal communication). However, the precise nature of the interactions among these proteins is unknown. Thus, it is possible that the interactions are direct, and that Push, the NF1-encoded protein Neurofibromin, and the Amn receptor bind to each other in a macromolecular complex. Alternatively, it is possible that Push and Neurofibromin mediate the effects of Amn only indirectly. In either case, the observation that the push1; NF1P2 double mutant exhibits a perineurial glial thickness much greater than push1 or NF1P2 alone is consistent with the possibility that Push and Neurofibromin mediate the Amn signal through parallel partially redundant pathways (Fig. 4).
The indirect signaling model described in Fig. 4B could explain the partial cell-nonautonomy of NF1 in neurofibroma formation. Neurofibromas most likely initiate in individuals heterozygous for NF1 mutations by loss of the NF1+ allele in Schwann cells. However, neurofibromas contain, in addition to Schwann cells, cells derived from fibroblasts, perineurial cells, and neurons, which are thought to remain phenotypically NF1+. We suggest that NF1 mutant Schwann cells cause the overproliferation of their wild-type neighbors by oversecreting trophic factors, and that this oversecretion might ultimately occur as a consequence of defective receipt of a neurotransmitter signal from neurons.
Supplementary Material
Acknowledgments
We are grateful to Tulle Hazelrigg for providing the testes cDNA library; Thomas Crowley for providing unpublished sequence data and the plasmid DNA flanking Trf1; Hank Adams at the Baylor College of Medicine Integrative Core Microscopy facility for assistance with electron microscopy; Joe Kramer, Heiner Matthies, and Scott Hawley for providing models and data before publication; Rhea Sumpter, Andrew Varga, and Patrick S. Thomas for assistance in various aspects of this project; Vanessa Auld and Phil Haydon for illuminating discussions; Andre Bernards (Harvard Medical School, Boston), Martin Burg, Bill Pak (Purdue University, Indianapolis), and Ulrike Heberlein (University of California, San Francisco) for fly stocks; Fei Lu of SeqWright (Houston) for advice on sequencing; Andre Bernards for comments on the manuscript; and Flybase, the Berkeley Drosophila genome project, and the Bloomington Drosophila stock center (Bloomington, IN) for fly stocks, clones, and information. This work was supported by National Institutes of Health Grants GM46566 and NS39984 and a grant from the National Neurofibromatosis Foundation (to M.S.), a National Institutes of Health Biotechnology Training Grant 5T32 GM08362 (to S.R.), and by a National Institutes of Health Research Service Award DC00139 (to D.S.H.).
Abbreviations
- PACAP
pituitary adenylate cyclase activator peptide
- NT
neurotransmitter of Ine
Note Added in Proof.
Phenotypes of mutants defective in BIG, the Arabidopsis ortholog of push, were recently reported (35).
Footnotes
References
- 1.Edwards J S, Swales L S, Bate M. J Comp Neurol. 1993;333:301–308. doi: 10.1002/cne.903330214. [DOI] [PubMed] [Google Scholar]
- 2.Hurd D D, Saxton W M. Genetics. 1996;144:1075–1085. doi: 10.1093/genetics/144.3.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jessen K R, Mirsky R. Trends Neurosci. 1999;22:402–410. doi: 10.1016/s0166-2236(98)01391-5. [DOI] [PubMed] [Google Scholar]
- 4.Jahromi B S, Robitaille F, Charlton M P. Neuron. 1992;8:1069–1077. doi: 10.1016/0896-6273(92)90128-z. [DOI] [PubMed] [Google Scholar]
- 5.Villegas J. In: Neuron–Glia Interrelations During Phylogeny II. Plasticity and Regeneration. Vernadakis A, Roots B I, editors. Totowa, NJ: Humana; 1995. pp. 95–127. [Google Scholar]
- 6.Parmantier E, Lynn B, Lawson D, Turmaine M, Namini S S, Chakrabarti L, McMahon A P, Jessen K R, Mirsky R. Neuron. 1999;23:713–724. doi: 10.1016/s0896-6273(01)80030-1. [DOI] [PubMed] [Google Scholar]
- 7.Pfrieger F W, Barres B A. Science. 1997;277:1684–1687. doi: 10.1126/science.277.5332.1684. [DOI] [PubMed] [Google Scholar]
- 8.Yuan L L, Ganetzky B. Science. 1999;283:1343–1345. doi: 10.1126/science.283.5406.1343. [DOI] [PubMed] [Google Scholar]
- 9.Viskochil D, White R, Cawthon R. Annu Rev Neurosci. 1993;16:183–205. doi: 10.1146/annurev.ne.16.030193.001151. [DOI] [PubMed] [Google Scholar]
- 10.Feany M B, Quinn WG. Science. 1995;268:869–873. doi: 10.1126/science.7754370. [DOI] [PubMed] [Google Scholar]
- 11.Soehnge H, Huang X, Becker M, Whitley P, Conover D, Stern M. Proc Natl Acad Sci USA. 1996;93:13262–13267. doi: 10.1073/pnas.93.23.13262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu C-F, Wong F. J Gen Physiol. 1977;69:705–724. doi: 10.1085/jgp.69.6.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burg M G, Geng C, Guan Y, Koliantz G, Pak W L. J Neurogenet. 1996;11:59–81. doi: 10.3109/01677069609107063. [DOI] [PubMed] [Google Scholar]
- 14.Richards S, Hillman T, Stern M. Genetics. 1996;142:1215–1222. doi: 10.1093/genetics/142.4.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The I, Hannigan G E, Cowley G S, Reginald S, Zhong Y, Gusella J F, Hariharan I K, Bernards A. Science. 1997;276:791–794. doi: 10.1126/science.276.5313.791. [DOI] [PubMed] [Google Scholar]
- 16.Guo H F, The I, Hannan F, Bernards A, Zhong Y. Science. 1997;276:795–798. doi: 10.1126/science.276.5313.795. [DOI] [PubMed] [Google Scholar]
- 17.Warmke J, Drysdale R, Ganetzky B. Science. 1991;252:1560–1562. doi: 10.1126/science.1840699. [DOI] [PubMed] [Google Scholar]
- 18.Riesgo-Escovar J, Woodward C, Gaines P, Carlson J. J Neurobiol. 1992;23:947–964. doi: 10.1002/neu.480230803. [DOI] [PubMed] [Google Scholar]
- 19.Stern M, Ganetzky B. J Neurogenet. 1992;8:157–172. doi: 10.3109/01677069209083445. [DOI] [PubMed] [Google Scholar]
- 20.Crowley T E, Hoey T, Liu J K, Jan Y N, Jan L Y, Tjian R. Nature(London) 1993;361:557–561. doi: 10.1038/361557a0. [DOI] [PubMed] [Google Scholar]
- 21.Castrillon D H, Gonczy P, Alexander S, Rawson R, Eberhart C G, Viswanathan S, DiNardo S, Wasserman S A. Genetics. 1993;135:489–505. doi: 10.1093/genetics/135.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fabrizio J J, Hime G, Lemmon S K, Bazinet C. Development (Cambridge, UK) 1998;125:1833–1843. doi: 10.1242/dev.125.10.1833. [DOI] [PubMed] [Google Scholar]
- 23.Xu X Z, Wes P D, Chen H, Li H S, Yu M, Morgan S, Liu Y, Montell C. J Biol Chem. 1998;273:31297–31307. doi: 10.1074/jbc.273.47.31297. [DOI] [PubMed] [Google Scholar]
- 24.Sekelsky J J, McKim K S, Messina L, French R L, Hurley W D, Arbel T, Chin G M, Deneen B, Force S J, Hari K L, et al. Genetics. 1999;152:529–542. doi: 10.1093/genetics/152.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore M S, DeZazzo J, Luk A Y, Tully T, Singh C M, Heberlein U. Cell. 1998;93:997–1007. doi: 10.1016/s0092-8674(00)81205-2. [DOI] [PubMed] [Google Scholar]
- 26.Zhong Y. Nature (London) 1995;375:588–592. doi: 10.1038/375588a0. [DOI] [PubMed] [Google Scholar]
- 27.Sepp K J, Schulte J, Auld V J. Glia. 2000;30:122–133. doi: 10.1002/(sici)1098-1136(200004)30:2<122::aid-glia2>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 28.Kriegler S, Chiu S Y. J Neurosci. 1993;13:4229–4245. doi: 10.1523/JNEUROSCI.13-10-04229.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy S, Minor R L, Wel G, Harrison D G. J Neurochem. 1990;55:349–351. doi: 10.1111/j.1471-4159.1990.tb08860.x. [DOI] [PubMed] [Google Scholar]
- 30.Lieberman E M, Sanzenbacher E. Neuroscience. 1992;47:931–939. doi: 10.1016/0306-4522(92)90041-y. [DOI] [PubMed] [Google Scholar]
- 31.Kluwe L, Friedrich R, Mautner V F. Genes Chromosomes Cancer. 1999;24:283–285. doi: 10.1002/(sici)1098-2264(199903)24:3<283::aid-gcc15>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 32.Sherman L S, Atit R, Rosenbaum T, Cox A D, Ratner N. J Biol Chem. 2000;275:30740–30745. doi: 10.1074/jbc.M001702200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cawthon R M, Weiss R, Xu G F, Viskochil D, Culver M, Stevens J, Robertson M, Dunn D, Gesteland R, O'Connell P, et al. Cell. 1990;62:193–201. doi: 10.1016/0092-8674(90)90253-b. [DOI] [PubMed] [Google Scholar]
- 34.Hofmann K, Stoffel W. Biol Chem Hoppe–Seyler. 1993;374:166. doi: 10.1515/bchm3.1993.374.7-12.507. [DOI] [PubMed] [Google Scholar]
- 35.Gil P, Dewey E, Friml J, Zhao Y, Snowden K, Putterill J, Palme K, Estelle M, Chory J. Gene Dev. 2001;15:1985–1997. doi: 10.1101/gad.905201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.