Abstract
Background
It is unclear whether, and to what extent, the striking cardiac morphological manifestations of endurance athletes are a result of exercise training or a genetically determined characteristic of talented athletes. We hypothesized that prolonged and intensive endurance training in previously sedentary healthy young individuals could induce cardiac remodeling similar to that observed cross-sectionally in elite endurance athletes.
Methods and Results
Twelve previously sedentary subjects (aged 29±6 years; 7 men and 5 women) trained progressively and intensively for 12 months such that they could compete in a marathon. Magnetic resonance images for assessment of right and left ventricular mass and volumes were obtained at baseline and after 3, 6, 9, and 12 months of training. Maximum oxygen uptake (V̇o2 max) and cardiac output at rest and during exercise (C2H2 rebreathing) were measured at the same time periods. Pulmonary artery catheterization was performed before and after 1 year of training, and pressure-volume and Starling curves were constructed during decreases (lower body negative pressure) and increases (saline infusion) in cardiac volume. Mean V̇o2 max rose from 40.3±1.6 to 48.7±2.5 mL/kg per minute after 1 year (P<0.00001), associated with an increase in both maximal cardiac output and stroke volume. Left and right ventricular mass increased progressively with training duration and intensity and reached levels similar to those observed in elite endurance athletes. In contrast, left ventricular volume did not change significantly until 6 months of training, although right ventricular volume increased progressively from the outset; Starling and pressure-volume curves approached but did not match those of elite athletes.
Conclusions
One year of prolonged and intensive endurance training leads to cardiac morphological adaptations in previously sedentary young subjects similar to those observed in elite endurance athletes; however, it is not sufficient to achieve similar levels of cardiac compliance and performance. Contrary to conventional thinking, the left ventricle responds to exercise with initial concentric but not eccentric remodeling during the first 6 to 9 months after commencement of endurance training depending on the duration and intensity of exercise. Thereafter, the left ventricle dilates and restores the baseline mass-to-volume ratio. In contrast, the right ventricle responds to endurance training with eccentric remodeling at all levels of training.
Keywords: exercise, exercise nutrition physiology, hypertrophy
The capacity to generate a high cardiac output is essential to support the very high rates of oxygen uptake required for excellence in endurance sports.1,2 Because the maximal heart rates of athletes compared with sedentary individuals are similar,3–5 virtually all of this increase in cardiac output is achieved by an exceptionally large maximal stroke volume (SV).5 This large SV in turn is mediated predominantly by a large left ventricular (LV) end-diastolic volume,5,6 which is the hallmark of the “athlete’s heart.”7
Cross-sectional cardiac magnetic resonance imaging (cMRI) studies have consistently reported increased LV and right ventricular (RV) mass and volumes in endurance-trained athletes compared with age- and sex-matched sedentary subjects.8–12 The magnitude of the change in ventricular remodeling is dependent on the degree of static and dynamic exercise required for the specific sport; such studies have typically identified “eccentric hypertrophy” in endurance-type athletes, with a balanced increase in volume and mass.13,14 This type of eccentric hypertrophy is associated with a marked increase in LV compliance in endurance athletes that facilitates the elaboration of large SVs during exercise via the Frank-Starling mechanism.15
It is controversial, however, whether this extreme cardiac remodeling is purely the result of prolonged endurance training or rather, in part, a genetically determined characteristic of a talented athlete.16 For example, although longitudinal training studies demonstrate increases in mass and volume with training,17,18 the magnitude of this increase and the associated increase in maximal aerobic power (maximum oxygen uptake [V̇o2max]) is well below that typically observed cross-sectionally in endurance athletes. Moreover, when endurance athletes detrain,19 even over prolonged periods of time, there is a reduction in both mass and volume but not to the degree typically seen in sedentary individuals. Prior reports about the angiotensin-converting enzyme allele polymorphism in athletes have fueled discussions about the genetic disposition to optimized cardiovascular adaptations in response to training.20–23 Alternatively, previous longitudinal studies may simply not have been prolonged or intensive enough to induce the same kind of cardiac adaptation in previously sedentary individuals as is typically observed in competitive athletes. The central hypothesis of the present study is that the cardiac adaptation to endurance training is a function of training load rather than genetic predisposition. To test this hypothesis, we prescribed a progressive training plan, based on a strategy derived from optimal training by competitive athletes, to healthy sedentary volunteers and assessed RV and LV adaptations using cMRI and right heart catheterization in response to a 12-month endurance training program designed to enable each subject to compete in a marathon.
Methods
Subjects
Twelve previously sedentary men (n=7) and women (n=5) with a mean age of 29±6 years completed all of the testing and training; 1 female subject completed all of the initial testing but became pregnant in the second quarter of her training and was excluded from further study. None of the participants had engaged previously in any regular endurance training; subjects were excluded if they exercised for >30 min/d >3 times per week regularly using either dynamic or static exercise. Subjects were screened with a careful history and physical examination, as well as with an ECG and echocardiogram. None of the subjects smoked, used recreational drugs, or had significant chronic medical problems. All subjects signed an informed consent form, which was approved by the institutional review boards of the University of Texas Southwestern Medical Center at Dallas and Presbyterian Hospital of Dallas.
Exercise Training
Endurance training was designed to enable all subjects to compete in a marathon at the end of a 12-month period (Figure 1). To determine specific training zones for optimal training prescription, heart rate at ventilatory and lactate thresholds and V̇o2max were determined by analysis of gas exchange, and lactate measurement was obtained during incremental treadmill tests performed at baseline and every 3 months to document exercise performance. “Maximal steady state” was estimated from the ventilatory and lactate thresholds according to standard criteria.24 On the basis of this maximal steady state heart rate and peak heart rate, 5 training zones were determined for individualized training prescription, as follows: zone 1=recovery, zone 2=base pace, zone 3=maximal steady state or “threshold,” zone 4=race pace/critical power, and zone 5=intervals, as described previously.25 During the early phases of the training program, the subjects trained 3 to 4 times per week for 30 to 45 minutes per session at base pace by brisk walking, slow jogging, swimming, or cycling. As the subjects became fitter, the duration of the base training sessions was prolonged, including the addition of 1 long run per week, which was performed at the lower end of the base pace heart rate range. In addition, during the second and third quarters of the training program, sessions of increased intensity (maximal steady state and interval sessions) were added first once, then twice, and occasionally 3 times per week. Interval sessions were followed the next day by recovery sessions to maximize performance gains. By the end of the year-long training program, subjects were exercising for 7 to 9 hours per week, including long runs of up to 3 hours plus regular interval sessions on the track and races. The purpose of this template was to maximize training efficiency and to provide a periodization of the training program. This strategy of varying the intensity and duration of training sessions within any given week (microcycle), applying periods of increasing stress followed by recovery from month to month (mesocycle) with an ultimate outcome goal of a specific competition (macrocycle), is routinely used by competitive athletes and is widely considered the optimal approach to training.26 Modifications of the training program occurred with some subjects; however, the overall pattern of training was consistent with this template in all. Duration of exercise and training heart rates were carefully monitored and documented with the use of heart rate monitors (Polar, Kempele, Finland).
Figure 1.
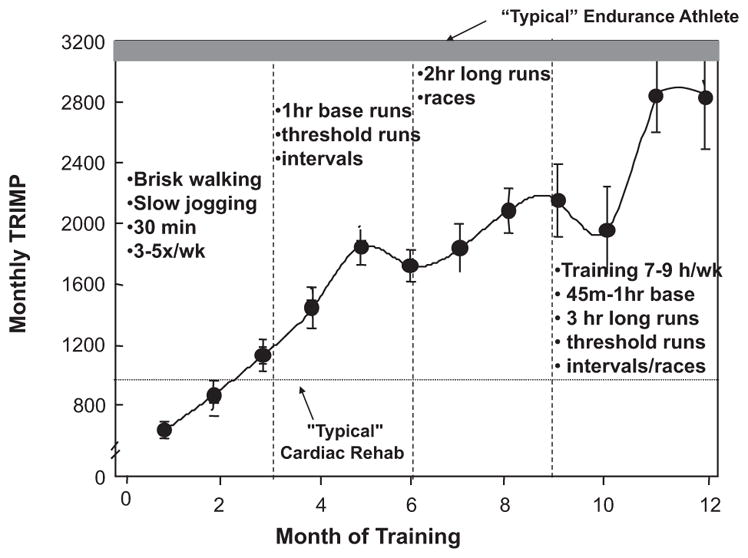
Average training impulse (TRIMP) scores per month for all subjects over the training year. Group values are mean±SD. Examples of training workouts are provided in each quarter (vertical dashed line); bottom dotted line represents the average monthly TRIMP that would be accomplished by a subject in a typical cardiac rehabilitation program exercising for 45 minutes at 75% of maximum predicted heart rate 3 times per week; upper bar represents the range of TRIMPs accumulated by typical middle-distance runners as quantified by studies performed in the senior author’s laboratory.41,42
To quantify the training stimulus, we used the method of Banister and Wenger27 for the calculation of the training impulse (TRIMP). This method multiplies the duration of a training session by the average heart rate achieved during that session, weighted for exercise intensity as a function of the heart rate reserve. Thus, exercise sessions of longer duration or higher intensity (higher heart rate and higher weighting factor), such as interval workouts, are assigned relatively higher TRIMP values than sessions of lower intensity. The details of this calculation as used in this study are available elsewhere,25 but, for example, a 30-minute workout at a heart rate of 150 in a young person with a resting heart rate of 50 and a maximum heart rate of 200 would equate to 46 TRIMPs; in contrast, a 60-minute workout at a heart rate of 140 would provide 73 TRIMPs, and a 28-minute interval workout at a heart rate of 190 would provide 100 TRIMPs.
cMRI Measurements
cMRI was performed on a 1.5-T Philips NT MRI scanner. Short-axis, gradient-echo, cine MRI sequences with a temporal resolution of 39 ms were obtained to calculate ventricular volumes, as described previously.28 Ventricular mass was computed as the difference between epicardial and endocardial areas multiplied by the density of heart muscle (1.05 g/mL).29 Previous studies in our laboratory and others have demonstrated that MRI with Simpson’s rule technique results in highly accurate and reproducible measurements of LV mass.29,30
For LV volume determination, the endocardial border of each slice was identified manually at end-diastole and end-systole, and volumes were calculated by summation.31 End-diastole was defined as the first frame in each sequence, and end-systole was defined as the frame with the smallest endocardial area. LV volumes were calculated with the use of Simpson’s rule technique, as described previously.31
Mean wall thickness for the entire LV included the papillary muscle and was calculated as described previously.32 For each short-axis slice, the epicardial area (LV chamber plus myocardial wall) and endocardial area (chamber area) were determined with the use of software on the imaging device. The “average” radius (r) for each area was calculated by approximating the cross section as a circle and using the equation for the area of a circle (Area=pr2 or r=ÖArea/p).
Exercise Testing
Measurements were made with the subjects standing quietly on a treadmill, at 2 submaximal steady state work rates (5 minutes each) at ≈33% and 66% of peak work rate, and during an incremental test to maximum rate. During initial familiarization sessions, 2 speeds were chosen for each subject: a brisk walking pace (usually 3.0–4.0 mph) and a comfortable jogging speed (generally 5–8 mph). These constant speeds were used for each subsequent test. After a brief rest, the incremental portion of the test was performed at the second sub-maximal speed, with the grade increased by 2% every 2 minutes until subjective exhaustion, despite vigorous encouragement. Measures of ventilatory gas exchange were made with the use of the Douglas bag technique. Gas fractions were analyzed by mass spectrometry (Marquette MGA1100), and ventilatory volume was measured by a calibrated dry-gas meter (Collins). V̇o2max was defined as the highest V̇o2 measured from at least a 40s Douglas bag. In nearly all cases, a plateau in V̇o2 was observed with increasing work rate, confirming the identification of V̇o2max.1 In addition, heart rate was monitored continuously via ECG (Polar), and fingertip capillary blood was obtained during the second minute of each stage for measurement of lactate concentration.
At baseline and at each quarterly testing session, all subjects underwent measurement of plasma volume with the use of the Evans Blue dye indicator dilution technique.33 Briefly, after at least 30 minutes of quiet, supine rest, a known quantity of Evans Blue dye was injected through a peripheral intravenous catheter, and venous blood was drawn at 10, 20, and 30 minutes after injection for the measurement of absorbance at 620 and 740 nm via spectrophotometry (DU 600, Beckman). Hematocrit was measured via microcapillary centrifuge, and blood volume was estimated by dividing plasma volume by 1-hematocrit, with appropriate corrections for trapped plasma and peripheral sampling.33
Cardiac Catheterization and Experimental Protocol
Catheterization was performed at baseline and after completion of the training period. All experiments were performed in the morning at least 2 hours after a light breakfast and at least 12 hours after the last caffeinated or alcoholic beverage in a quiet, environmentally controlled laboratory with an ambient temperature of 25°C. A 6F balloon-tipped fluid-filled catheter (Swan-Ganz, Baxter) was placed under fluoroscopic guidance through an antecubital vein into the pulmonary artery. All intracardiac pressures were referenced to atmospheric pressure with the pressure transducer (Transpac IV, Abbott) zero reading set at 5 cm below the sternal angle. The mean pulmonary capillary wedge pressure (PCWP) was determined visually at end expiration and was used as an estimation of LV end-diastolic pressure.
Cardiac output was measured with a modification of the acetylene rebreathing technique with acetylene used as the soluble gas and helium as the insoluble gas.34,35 SV was calculated from cardiac output and heart rate measured during rebreathing. LV end-diastolic volume (LVEDV) was measured with 2-dimensional echocardiography with the use of standard views and formulas as described by the American Society of Echocardiography.36 Images were obtained with an annular phased-array transducer with a frequency of 2.5 to 3.5 MHZ (Interspec Apogee CX) and stored on VCR tape for offline analysis by a skilled technician. Measurements of LV endocardial areas were made from the parasternal short-axis window at the level of the mitral valve and the papillary muscles and from the apical window in the 4-chamber view, with care taken to avoid foreshortening of the major axis. The major-axis distance was measured from the apex to the mitral annulus. For the calculation of LVEDV for each subject, either the modified Simpson’s rule method, the area length method, or the bullet model (cylinder hemiellipsoid) was chosen on the basis of which views provided the most optimal endocardial definition.37 The same formula was used for each individual subject throughout the study.
Testing Protocols
Cardiac filling was decreased by applying lower body negative pressure (LBNP), as described previously.15,38,39 Briefly, LBNP was achieved by placing the subject in a Plexiglas box sealed at the level of the iliac crest. Suction was provided with the use of a vacuum pump with a variable autotransformer. Measurements of PCWP, cardiac output (and therefore SV), LVEDV, heart rate, and blood pressure were made after 5 minutes each of −15 and −30 mm Hg LBNP. The LBNP was then released. After repeat baseline measurements to confirm a return to hemodynamic steady state (usually 20–30 minutes), cardiac filling was increased by rapid (200 mL/min) infusion of warm (37°C), isotonic saline. Measurements were repeated after 15 and 30 mL/kg had been infused.
Data were used to construct Starling (SV/PCWP) and pressure-volume (PCWP/LVEDV) curves. For the purposes of the present study, we characterized and here define explicitly 2 different but related mechanical properties of the heart during diastole: (1) Static stiffness or overall chamber stiffness (or its inverse compliance) refers to the stiffness constant S of the logarithmic equation describing the pressure-volume curve (see below); and (2) distensibility is used to mean the absolute LVEDV at a given distending pressure, independent of dP/dV, or S.
To characterize LV pressure-volume relations, we modeled the data in the present experiment according to the logarithmic equation described by Nikolic et al,40 as follows:
where P=PCWP, V=LVEDV, V0 is equilibrium volume or the volume at which P=0, Vm is the maximal volume obtained by this chamber, and S is a stiffness constant that describes the shape of the curve. In addition, pressure-volume curves were also calculated with the use of the difference between PCWP and right atrial pressure (PCW-right atrial pressure) as an index of transmural filling pressure41 to assess the contribution of pericardial constraint.
Statistical Analysis
Continuous variables measured over the 12-month study duration were analyzed longitudinally with a linear mixed effects model repeated measures analysis. The repeated measures model had 5 repeated measurements (time points at baseline and 3, 6, 9, and 12 months), and the study participant was modeled as a random effect. The covariance structure was selected on the basis of Akaike’s information criteria and model parsimony. Multiple comparisons were made with the use of the least squares mean contrasts that were derived from the mixed models and use the Hochberg-Bonferroni method to adjust for multiple testing. To express the dose–response relationship between the exercise stimulus, changes in peak oxygen uptake, and changes in cardiac dimensions, the relationship between the quarterly training impulse (monthly TRIMP) and cardiac adaptations at baseline and at 3, 6, 9, and 12 months was estimated with quadratic polynomial regression models. To account for the correlation between repeated observations within the same individuals, random effects linear regression models were used. Similar regression models were used to assess pressure-volume curves with the addition of a fixed effect to test for the difference and response interactions between the baseline and training curves. A 2-sided P value <0.05 was considered statistically significant. The analysis was performed with the use of SAS version 9.3 (SAS Institute, Cary, NC). Data are presented as mean±SD unless otherwise specified.
Results
All subjects successfully completed a marathon (n=10), Olympic distance triathlon (n=1), or 100-mile endurance cycling race (n=1) as the ultimate performance goal of the training program. The exercise training prescribed during this study led to significant changes in several resting parameters over the 12-month training period (Table 1). Lean body mass increased, whereas the overall weight of the subjects did not change, indicating a decrease in body fat. Plasma volume increased slightly over the 12-month period, although because the program had different start and stop dates over the course of the year in Texas, we speculate that the variable timing of heat acclimatization increased the variability of this outcome. Compared with baseline, heart rate significantly decreased (R-R interval significantly increased). Details of the hemodynamic and autonomic response to this training program have been reported previously.25
Table 1.
Resting Hemodynamics and Characteristics
| Baseline | 3 mo | 6 mo | 9 mo | 12 mo | P Value* | |
|---|---|---|---|---|---|---|
| Body weight, kg | 70±10 | 70±10 | 69±9 | 69±10 | 70±10 | 0.35 |
| Lean body mass, kg | 55.9±9.6 | 57.0±10.3 | 57.9±9.2† | 57.7±9.4† | 58.7±9.0† | 0.002 |
| Plasma volume, L | 2.87±0.40 | 2.97±0.57 | 3.01±0.47 | 3.08±0.49 | 3.10±0.61† | 0.003 |
| Heart rate, bpm | 67±6 | 66±6 | 68±8 | 66±9 | 60±5† | 0.004 |
| SBP, mm Hg | 113±6 | 113±8 | 107±4† | 109±8 | 116±7† | 0.001 |
| DBP, mm Hg | 67±6 | 63±7 | 61±3† | 66±9† | 68±6 | 0.0002 |
| Cardiac output, L/min | 6.5±1.1 | 7.1±1.1† | 7.6±1.2† | 7.5±1.5† | 6.7±1.1 | 0.0001 |
| TPR, dyne·s·cm5 | 1033±180 | 917±167† | 820±146† | 838±173† | 1026±168 | <0.0001 |
Results are presented as mean±SD. DBP indicates diastolic blood pressure; SBP, systolic blood pressure; and TPR, total peripheral resistance.
P value from repeated measures analysis time effect from baseline to 12 months.
Statistically significant difference compared with baseline from mixed model repeated measures analysis.
Training Impulse
An overview of the achieved TRIMP scores over the entire training period is given in Figure 1. Average TRIMP score increased from 676±215 in month 1 to 2528±925 in month 12. For comparison, competitive endurance runners achieve monthly TRIMP scores ≈2500–3500.42,43
Maximal Exercise
As expected, V̇o2max increased prominently with this training regimen, from 40.3 at baseline to 48.7 mL/kg per minute with training (Table 2; P<0.00001). Heart rate decreased at both submaximal and maximal work, whereas maximum SV increased with training, which resulted in an overall increase of maximum cardiac output with training.
Table 2.
Exercise Characteristics
| Baseline | 3 mo | 6 mo | 9 mo | 12 mo | P Value* | |
|---|---|---|---|---|---|---|
| V̇o2max, mL·kg−1·min−1 | 40.3±5.5 | 45.5±5.9† | 47.4±6.4† | 47.6±7.0† | 47.4±7.2† | <0.0001 |
| Peak heart rate, bpm | 197±12 | 187±8.0† | 188±9.2† | 185±9† | 186±9† | <0.0001 |
| Maximum stroke volume, mL | 98.1±18.2 | 108.2±21.6† | 113.7±18.9† | 115.1±25.3† | 113.6±23.2† | 0.002 |
| Maximum cardiac output, mL/min | 20.1±5.1 | 22.4±5.7† | 20.5±5.2 | 20.7±5.2 | 21.9±5.4† | 0.03 |
Results are presented as mean±SD. V̇o2max indicates maximum oxygen uptake.
P value from repeated measures analysis time effect from baseline to 12 months.
Statistically significant difference compared with baseline from mixed model repeated measures analysis.
LV Adaptations
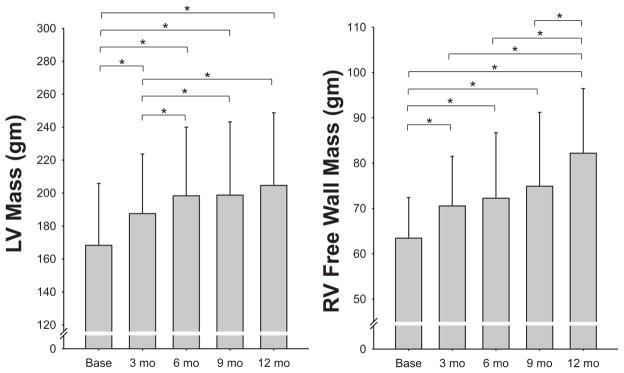
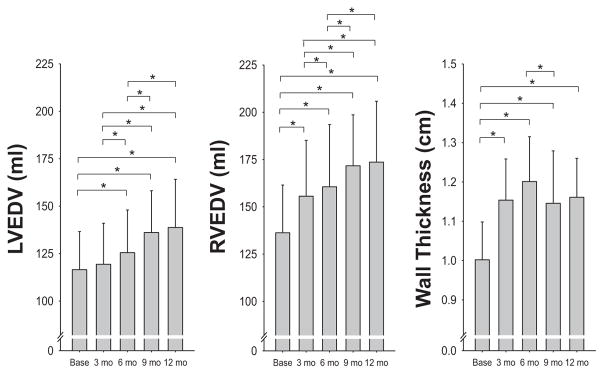
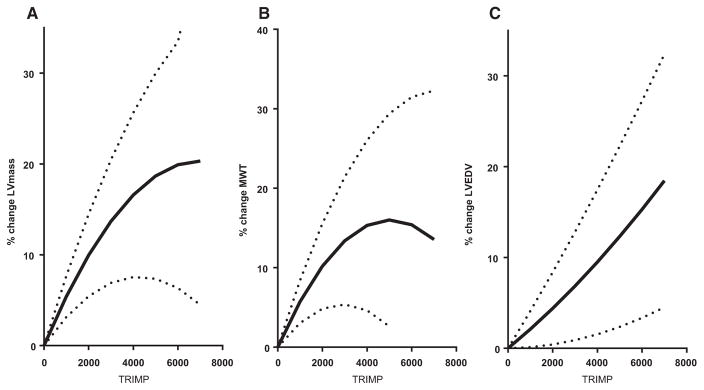
All cardiac morphology measurements reported in the tables and figures were derived from cMRI except for the pressure-volume curves, which, because of the invasive instrumentation and manipulations of cardiac loading (LBNP and saline infusion), were derived from echocardiography. Results for measurements of LV mass, LVEDV, and mean wall thickness are given in Figures 2 and 3. The largest increase in LV mass and mean wall thickness occurred in the first 6 months of endurance training. In contrast, LVEDV did not change significantly after 3 months, and it increased by only 8% after 6 months, reaching 39% of its maximum change within the testing period (Figure 3). The largest increase in LVEDV occurred during the last half of the training program, after the addition of interval sessions and long bouts lasting >60 minutes. Similarly, SV showed its largest quarterly increase between 6 and 9 months. Accordingly, the LV mass-to-volume ratio increased from 1.44 at baseline to 1.58 at 3 and 6 months (“concentric hypertrophy”) and then returned to values close to baseline after 9 and 12 months of training, leading ultimately to the classic eccentric hypertrophy of endurance athletes. The average relative LV mass achieved in these subjects approached a level close to that reported by the same laboratory in elite endurance athletes.44,45 When the mean quarterly values for LV mass were compared with the average TRIMP values obtained for each quarter, there was a close positive relationship between training impulse and development of LV mass (Figure 4). Ventricular ejection fraction at rest was 69% at baseline and 71%, 71%, 72%, and 71% after 3, 6, 9, and 12 months, respectively (statistically not significant).
Figure 2.
Changes in left ventricular (LV) mass (left) and right ventricular (RV) mass (right) measured by magnetic resonance imaging every 3 months during the 1-year training program. Mean±SD data (bars) are shown for each time point. Note differences in scale for each graph, with LV mass being ≈2.5 times the RV free wall mass. Overall P value from the linear mixed effects model repeated measures analysis, P<0.001 for each. *Post hoc comparisons for P<0.05. Individual data are reported separately in Figure I in the online-only Data Supplement.
Figure 3.
Mean±SD changes in left ventricular end-diastolic volume (LVEDV; left) and right ventricular end-diastolic volume (RVEDV; middle) by magnetic resonance imaging measured every 3 months during the training program. Note that in contrast to Figure 2 comparing LV to RV mass, the scale for LVEDV and RVEDV is the same. Mean wall thickness is shown on the right to facilitate a visual representation of the year-long changes in cardiac morphology. Overall P value from the linear mixed effects model repeated measures analysis, P<0.001 for each. *Post hoc comparisons for P<0.05. Individual data are reported separately in Figure II in the online-only Data Supplement.
Figure 4.
Quadratic regression analysis between average quarterly training impulse (TRIMP) values as a measure of training stimulus and left ventricular (LV) mass (left), LV mean wall thickness (MWT; middle), and LV end-diastolic volume (EDV; right). Solid black lines represent the random effect regression that uses all individual data points and models the study participant as a random effect to account for the lack of independence between observations on the same individual; dotted lines represent the 95% confidence limits for this regression. Individual data are reported separately in Figure III in the online-only Data Supplement.
RV Adaptations
Results for measurements of RV mass, RV end-diastolic volume, and SV are given in Table 3. In contrast to the changes in the LV, the mass and volume increased in an eccentric pattern at all levels of training such that the mass-to-volume ratio (0.46, 0.46, 0.45, 0.44, 0.47) did not change significantly throughout the training (Figures 2 and 3). The relative increase of mass (RV free wall only) and volume in the RV (30% and 27%) was more pronounced than in the LV (22% and 20% mean increase, respectively).
Table 3.
Adaptations of Left and Right Ventricular Mass, End-Diastolic Volume, Mean Wall Thickness, and Stroke Volume in Response to Endurance Training
| Baseline | 3 mo | 6 mo | 9 mo | 12 mo | P Value* | |
|---|---|---|---|---|---|---|
| LV mass, g | 168±38 | 188±36† | 198±42† | 199±45† | 203±46† | <0.0001 |
| LVEDV, mL | 117±20 | 119±22 | 125±23† | 136±22† | 138±27† | <0.0001 |
| LV MWT, cm | 1.00±0.10 | 1.15±0.10† | 1.20±0.11† | 1.15±0.13† | 1.16±0.10† | <0.0001 |
| LV SV, mL | 79±12 | 85±14† | 89±16† | 97±14† | 98±19† | <0.0001 |
| RV mass, g | 63±9 | 71±11† | 72±14† | 75±16† | 82±15† | 0.0005 |
| RVEDV, mL | 136±25 | 156±30† | 161±33† | 172±27† | 173±34† | <0.0001 |
| RV SV, mL | 78±12 | 87±14† | 90±17† | 97±14† | 97±16† | <0.0001 |
Results are presented as mean±SD. LV indicates left ventricular; LVEDV, left ventricular end-diastolic volume; MWT, mean wall thickness; RV, right ventricular; RVEDV, right ventricular end-diastolic volume; and SV, stroke volume.
P value from repeated measures analysis time effect from baseline to 12 months.
Statistically significant difference compared with baseline from mixed model repeated measures analysis.
Pressure-Volume Curves
These morphological adaptations caused a parallel shift of the pressure-volume curve to the right after the 12-month training period (Figure 5A). Thus, for any given filling pressure, LVEDV was greater after training compared with baseline (P=0.0001 for regression model), consistent with increased ventricular distensibility or reduction of pericardial constraint. Both maximum volume of the chamber and equilibrium volume increased with training, confirming the larger LV dimensions and consistent with the eccentric remodeling observed by MRI. There was no difference in the S value with endurance training, indicating that there was no change in overall chamber compliance; however, when the influence of the pericardium was taken into effect by estimating LV transmural pressure (PCWP–right atrial pressure),41 the shift in the pressure-volume curve to the right persisted (P=0.05; interaction P=0.08), particularly at higher filling volumes (Figure 5B). Together these curves suggest that a combination of reduced pericardial constraint and improved myocardial compliance occurred after training.
Figure 5.
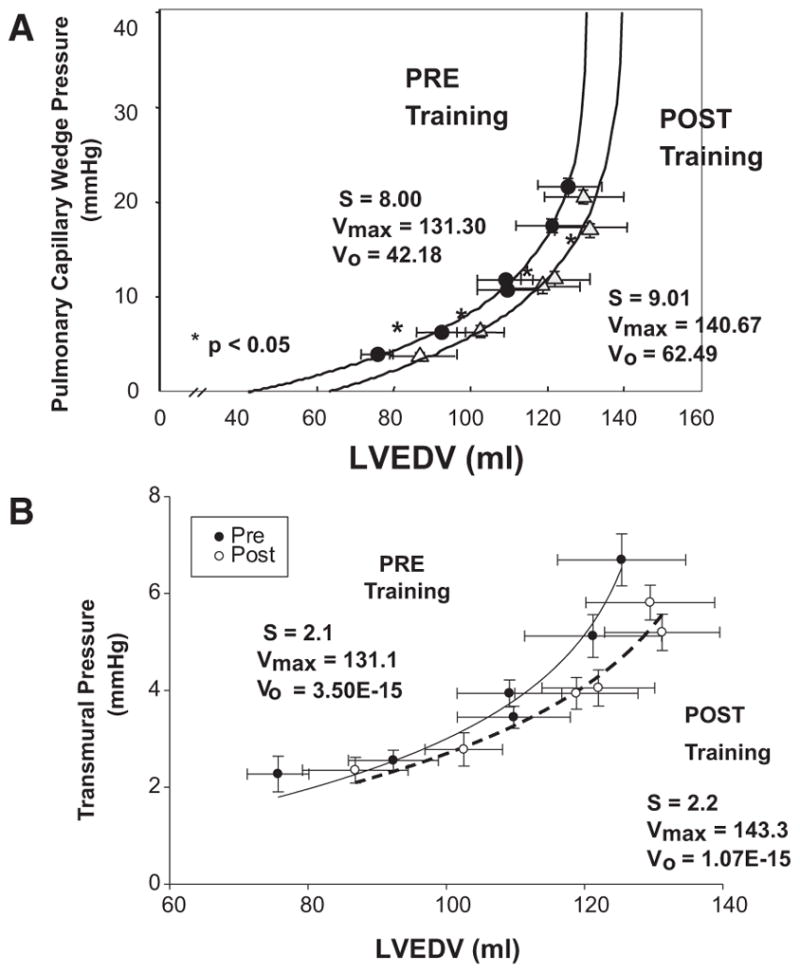
A, Pressure-volume curves representing pulmonary capillary wedge pressure as an index of left ventricular end-diastolic pressure vs left ventricular end-diastolic volume (LVEDV) obtained from 2-dimensional echocardiography over the range of left ventricular filling produced by lower body negative pressure (2 lowest values of pulmonary capillary wedge pressure), quiet baseline (2 middle values of pulmonary capillary wedge pressure), and rapid saline infusion (2 highest values of pulmonary capillary wedge pressure) with modeling of the pressure-volume curves, as described in the text. Each data point represents the mean±SE of all subjects before (filled symbol) and after (open symbol) 1 year of training. S indicates stiffness constant; Vmax, maximum volume obtained by this chamber; and Vo, equilibrium volume. *Statistically significant difference. B, Pressure-volume curves derived as in A but using the difference between pulmonary capillary wedge pressure and right atrial pressure as an index of transmural filling pressure.
Starling Curves
In accordance with the changes in the pressure-volume relationship, there was a significant increase in SV for any given filling pressure after 12 months of training (random effects regression P<0.0001; interaction P=0.6, consistent with a parallel upward shift; Figure 6). Maximum SV increased from 100 mL at baseline to 119 mL after training.
Figure 6.
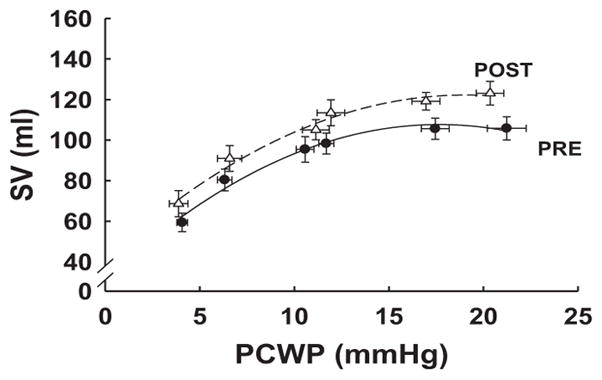
Starling curves representing pulmonary capillary wedge pressure (PCWP) as an index of left ventricular end-diastolic pressure vs stroke volume (SV) over range of left ventricular filling produced by lower body negative pressure (2 lowest values of PCWP), quiet baseline (2 middle values of PCWP), and rapid saline infusion (2 highest values of PCWP), as described in the text (before vs after, P<0.0001). Each data point represents the mean±SE of all subjects before (filled symbol) and after (open symbol) 1 year of training.
Discussion
The primary new findings from this study are as follows: (1) Prolonged and intensive endurance training in previously sedentary individuals resulted in a large increase in LV mass, approaching a level similar to that reported cross-sectionally in elite endurance athletes.8,44,45 (2) Contrary to conventional thinking, the LV responded to the initiation of endurance training with an increase in mass without a change in volume (concentric hypertrophy); an increase in LVEDV occurred only after 6 to 9 months of progressive training, restoring the baseline mass-to-volume ratio (eccentric hypertrophy). (3) In contrast to the LV, the RV responded to endurance training with a balanced increase in mass and volume, thereby maintaining a constant mass-to-volume ratio (eccentric hypertrophy) at all levels of training. (4) Despite these morphological adaptations, and although maximal cardiac output and V̇o2 max increased substantially during the training period, they did not reach levels typically observed in trained endurance athletes.5,8 (5) One year of intensive endurance training led to a modest increase in LV distensibility and compliance but remained substantially below that observed in elite athletes.15
Time Course of Change and Pattern of Cardiac Remodeling With Endurance Training
Cross-sectional cMRI studies examining the athlete’s heart have consistently demonstrated that endurance-trained athletes have increased LV and RV volumes and mass (eccentric hypertrophy) compared with age- and sex-matched healthy controls.8,11,46 Longitudinal cMRI studies confirmed that eccentric ventricular remodeling is inducible in previously sedentary younger individuals with moderate- to high-intensity endurance training over relatively brief periods of time.18,47,48
However, the extent of remodeling varied greatly among these reports, and no longitudinal study reported adaptations as seen in elite athletes. We speculated that the reason for the inconsistent and insufficient (compared with athletes) cardiac remodeling previously reported resided in the training methods and duration of training used in these studies. We hypothesized that cardiac remodeling might be induced in previously sedentary young subjects similar to that observed in elite endurance athletes when an “optimized” training schedule is applied and when sufficient time is given for morphological adaptations. In the present study, we employed a training program that is shared with many elite athletes worldwide, including careful periodization and with allowance of sufficient time for structural changes to occur.
A major new finding of our study is that the pattern of ventricular remodeling with prolonged endurance training was closely related to the intensity of exercise performed. During the first 6 months, when lower-intensity training was performed, the increased LV mass was secondary to the increase in mean wall thickness (concentric hypertrophy). Thereafter, when high-intensity interval training and longer-duration exercise were performed, the LV dilated and restored the mass-to-volume ratio close to baseline values (eccentric hypertrophy). In accordance with Laplace’s law,49 our finding that the LV responds to lower-intensity exercise with initial concentric hypertrophy suggests a primary “pressure overload” stimulus or perhaps an adaptive response to the increased cardiac work during training as a function of both increased heart rate and arterial pressure. Thus, contrary to a widely held belief in sports cardiology, endurance exercise may not be a primary LV “volume overload” stimulus; rather, the pattern of LV remodeling depends on the intensity and duration of the exercise performed (Figure 4).
In contrast to the changes in the LV, the RV mass and volume increased in an eccentric pattern at all levels of training such that the mass-to-volume ratio did not change significantly throughout the training period. This finding is likely attributable to the relatively greater wall stress imposed on the RV compared with the LV during incremental to peak endurance exercise. For example, LaGerche et al50 reported that the relative change in RV end-systolic wall stress from rest to peak exercise was significantly greater than the change in LV end-systolic wall stress (125% versus 14%, respectively) in endurance athletes and nonathletes alike. Although RV and LV diastolic wall stress were not measured in these studies, other investigators have shown that the percent change in pulmonary artery systolic pressure is markedly higher than the change in peak systemic pressure during exercise (182% versus 35%, respectively). In addition, RV end-diastolic volume and RV end-systolic volume are 2% and 15% greater than LVEDV and LV end-systolic volume during peak exercise, which together with the aforementioned pressures would result in greater change in diastolic wall stress.6 Accordingly, it appears that the RV is subject to both a pressure and volume load during acute submaximal and maximal endurance exercise, which, when repeated over years of training, may explain greater RV (compared with LV) remodeling reported in endurance-trained athletes.10
Relationship Between Training Intensity and LV Mass
The increase in cardiac mass was closely related to our training impulse score TRIMP. Although there seemed to be a linear relationship between TRIMP and cardiac mass for the first 9 months, the curve flattened toward the end of the training period. One explanation for the flattening of the curve may be the approximation of limits in cardiac growth, at least over the period of 1 year.51 Thus, at least in response to an intermittent stimulus of exercise training, more training might lead to only a small further increase in cardiac mass. On the other hand, one of the limitations of the TRIMP score is that it does not distinguish well between the contribution of intensity and duration. For example, a shorter and highly intense training session would yield the same score as a very prolonged but less intense session. It is conceivable, though, that these 2 types of training result in a different stimulus to myocardial growth and chamber remodeling. The most intense training sessions were maximized during months 7 to 9, whereas toward the end of the year the focus of training shifted to longer, less intense sessions to prepare for the requirements of the upcoming competition. Therefore, we cannot exclude the possibility that the change in training intensity near the end of the training year influenced the flattening of the LV mass/TRIMP relationship. Either of these hypotheses could also explain the apparent lack of continued increase or even a slight decrease in resting cardiac output (Table 1) during the last quarter of the training program, although multiple other factors could be involved in this observation, including changes in plasma volume, cardiovascular regulation, and perhaps even a slight degree of overtraining in some of these subjects.25
Improvement in Myocardial Compliance and V̇o2max With Endurance Training
Several investigators have shown that 6 months to 1 year of moderate-intensity endurance training increases V̇o2max in younger men and women (mean change=6 mL O2/kg per minute).18,52,53 We confirm these findings by showing that 1 year of endurance training increased V̇o2max by 8 mL/kg per minute, although the training loads used at the end of our year-long training program were substantially greater than in any previous study. Moreover, we extend prior work by demonstrating the mechanism for the improvement in aerobic power with training. Specifically, the increase in V̇o2max was primarily attributable to the increase in maximal cardiac output (Table 2) secondary to the increase in maximal SV because maximal heart rate was significantly lower after 1 year of training compared with baseline. Given that myocardial contractility improved minimally (preload recruitable stroke work at rest was unchanged; data not shown) with training, the increase in SV is likely attributable to increased LV filling. Figure 5 shows that 1 year of endurance training increased LVEDV during cardiac loading, such as might occur during exercise, although we were unable to measure LVEDV accurately during maximal exercise. A consequence of this adaptation is that it will allow for greater utilization of the Starling mechanism and concomitant increase in SV during maximal exercise. Our group has shown previously that endurance-trained athletes have greater myocardial compliance than controls.15 To the best of our knowledge, this is the first invasive hemodynamic study to report an improvement in myocardial compliance in previously sedentary individuals after 1 year of prolonged and intensive endurance exercise training.
Interestingly, although the relative cardiac mass of our subjects reached levels close to those of elite endurance athletes, their V̇o2 max at the end of the training period still was substantially below that of even mediocre endurance athletes. Furthermore, LV chamber compliance after 1 year of endurance training (Figure I in the online-only Data Supplement) was lower than that previously reported by our group for elite male athletes.15 Therefore, even after 1 year of training, LV diastolic filling may be limited by pericardial constraint. We speculate (but cannot prove) that it may take years for subjects to attain the same myocardial and or chamber compliance as elite athletes. It is also possible that training during growth and development, while the heart itself is growing along with body size, may be required to obtain the maximal cardiac training response and allow the full expression and elaboration of the athlete’s heart.
Limitations
Because we did not include a control group that did not train, we cannot exclude the possibility that some of the changes observed in this study were a function of time and day-to-day variability and not just endurance training. However, multiple studies from our group have shown that measures of cardiac compliance and exercise performance are highly reproducible and remarkably constant over periods of 1 year.38,39,54,55 In addition, our training program was progressive over time, with the specific goal to achieve a level of training typically practiced by competitive athletes. However, because of the intensive and invasive nature of our measurements, the total number of subjects studied was relatively small, and we did not include multiple groups of subjects that held their training constant at each quarterly “dose.” Therefore, we cannot distinguish the effects of progression of training duration and intensity from the effects of time alone.
Conclusions
One year of prolonged and intensive endurance training leads to cardiac morphological adaptations in previously sedentary young subjects similar to those observed in elite endurance athletes; however, it is not sufficient to achieve similar levels of cardiac compliance and performance. Contrary to conventional thinking, the LV responds to exercise with initial concentric but not eccentric remodeling during the first 6 to 9 months after commencement of endurance training depending on the duration and intensity of exercise. Thereafter, the LV dilates and restores the baseline mass-to-volume ratio. In contrast, the RV responds to endurance training with eccentric remodeling at all levels of training.
Supplementary Material
CLINICAL PERSPECTIVE.
Exercise training is an essential component of the treatment of nearly all cardiovascular diseases, leading to improved functional capacity and reduced cardiovascular morbidity and mortality via multiple mechanisms. Endurance athletes, representing the pinnacle of exercise training, have large, compliant hearts that pump large volumes of blood very quickly to support high rates of oxidative metabolism. Whether such an adaptation is attributable to prolonged and intense exercise training or is an inherited characteristic required to be a successful endurance athlete was hitherto unknown, in part because no one has attempted a long-term training study incorporating training durations and intensities typically practiced by competitive athletes. This study showed that there is quite remarkable plasticity in the heart and circulation: Prolonged and intense training led to increases in cardiac muscle mass equivalent to cross-sectional studies of endurance athletes. Intriguingly, increases in right ventricular mass and volume occur early in an endurance training program and may be permissive of an eventual increase in left ventricular volumes, allowing for the “classic” eccentric hypertrophy of the endurance athlete. However, even a year of training in a previously sedentary, healthy individual cannot generate the same aerobic power (maximum oxygen uptake) and cardiac compliance/enlargement of a successful endurance athlete. The clinician should be aware not only of the extent of cardiac plasticity but the dose–response nature of this adaptation: Lower doses of training lead primarily to increases in left ventricular mass without increases in volume, which only occur after the incorporation of training sessions involving long durations (hours) or high intensities.
Acknowledgments
Special thanks go to Matthew J. Morrow, MS, for outstanding work in successfully training the subjects; Fatima Franco, MD, for assistance with MRI analysis; Dr Qi Fu for help in constructing the figures; and Julie H. Zuckerman, RN, RDMS, for extraordinary nursing care during the invasive studies and performance of the ultrasound measurements.
Sources of Funding
This study was supported by the National Aeronautics and Space Administration Specialized Center for Research and Training grant NGW3582, the S. Finley Ewing Chair for Wellness at Presbyterian Hospital, and the Harry S. Moss Heart Chair for Cardiovascular Research.
Footnotes
The online-only Data Supplement is available with this article at http://circ.ahajournals.org/lookup/suppl/doi:10.1161/CIRCULATIONAHA.114.010775/-/DC1.
Disclosures
None.
Contributor Information
Armin Arbab-Zadeh, Institute for Exercise and Environmental Medicine, Texas Health Presbyterian Hospital, Dallas.
Merja Perhonen, Institute for Exercise and Environmental Medicine, Texas Health Presbyterian Hospital, Dallas.
Erin Howden, Institute for Exercise and Environmental Medicine, Texas Health Presbyterian Hospital, Dallas.
Ronald M. Peshock, University of Texas Southwestern Medical Center, Dallas.
Rong Zhang, Institute for Exercise and Environmental Medicine, Texas Health Presbyterian Hospital, Dallas. University of Texas Southwestern Medical Center, Dallas.
Beverly Adams-Huet, University of Texas Southwestern Medical Center, Dallas.
Mark J. Haykowsky, Faculty of Rehabilitation Medicine, University of Alberta, Edmonton, Alberta, Canada.
Benjamin D. Levine, University of Texas Southwestern Medical Center, Dallas.
References
- 1.Levine BD. VO2max: what do we know, and what do we still need to know? J Physiol. 2008;586:25–34. doi: 10.1113/jphysiol.2007.147629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitchell JH, Sproule BJ, Chapman CB. The physiological meaning of the maximal oxygen intake test. J Clin Invest. 1958;37:538–547. doi: 10.1172/JCI103636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levine BD, Buckey JC, Fritsch JM, Yancy CW, Jr, Watenpaugh DE, Snell PG, Lane LD, Eckberg DL, Blomqvist CG. Physical fitness and cardiovascular regulation: mechanisms of orthostatic intolerance. J Appl Physiol (1985) 1991;70:112–122. doi: 10.1152/jappl.1991.70.1.112. [DOI] [PubMed] [Google Scholar]
- 4.Di Bello V, Santoro G, Talarico L, Di Muro C, Caputo MT, Giorgi D, Bertini A, Bianchi M, Giusti C. Left ventricular function during exercise in athletes and in sedentary men. Med Sci Sports Exerc. 1996;28:190–196. doi: 10.1097/00005768-199602000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Stickland MK, Welsh RC, Petersen SR, Tyberg JV, Anderson WD, Jones RL, Taylor DA, Bouffard M, Haykowsky MJ. Does fitness level modulate the cardiovascular hemodynamic response to exercise? J Appl Physiol (1985) 2006;100:1895–1901. doi: 10.1152/japplphysiol.01485.2005. [DOI] [PubMed] [Google Scholar]
- 6.La Gerche A, Claessen G, Van de Bruaene A, Pattyn N, Van Cleemput J, Gewillig M, Bogaert J, Dymarkowski S, Claus P, Heidbuchel H. Cardiac MRI: a new gold standard for ventricular volume quantification during high-intensity exercise. Circ Cardiovasc Imaging. 2013;6:329–338. doi: 10.1161/CIRCIMAGING.112.980037. [DOI] [PubMed] [Google Scholar]
- 7.Thompson PD, Bruce D. Dill historical lecture: historical concepts of the athlete’s heart. Med Sci Sports Exerc. 2004;36:363–370. doi: 10.1249/01.mss.0000117117.67849.f6. [DOI] [PubMed] [Google Scholar]
- 8.Scharhag J, Schneider G, Urhausen A, Rochette V, Kramann B, Kindermann W. Athlete’s heart: right and left ventricular mass and function in male endurance athletes and untrained individuals determined by magnetic resonance imaging. J Am Coll Cardiol. 2002;40:1856–1863. doi: 10.1016/s0735-1097(02)02478-6. [DOI] [PubMed] [Google Scholar]
- 9.Scharf M, Brem MH, Wilhelm M, Schoepf UJ, Uder M, Lell MM. Atrial and ventricular functional and structural adaptations of the heart in elite triathletes assessed with cardiac MR imaging. Radiology. 2010;257:71–79. doi: 10.1148/radiol.10092377. [DOI] [PubMed] [Google Scholar]
- 10.La Gerche A, Heidbüchel H, Burns AT, Mooney DJ, Taylor AJ, Pfluger HB, Inder WJ, Macisaac AI, Prior DL. Disproportionate exercise load and remodeling of the athlete’s right ventricle. Med Sci Sports Exerc. 2011;43:974–981. doi: 10.1249/MSS.0b013e31820607a3. [DOI] [PubMed] [Google Scholar]
- 11.Luijkx T, Cramer MJ, Prakken NH, Buckens CF, Mosterd A, Rienks R, Backx FJ, Mali WP, Velthuis BK. Sport category is an important determinant of cardiac adaptation: an MRI study. Br J Sports Med. 2012;46:1119–1124. doi: 10.1136/bjsports-2011-090520. [DOI] [PubMed] [Google Scholar]
- 12.Utomi V, Oxborough D, Whyte GP, Somauroo J, Sharma S, Shave R, Atkinson G, George K. Systematic review and meta-analysis of training mode, imaging modality and body size influences on the morphology and function of the male athlete’s heart. Heart. 2013;99:1727–1733. doi: 10.1136/heartjnl-2012-303465. [DOI] [PubMed] [Google Scholar]
- 13.Prior DL, La Gerche A. The athlete’s heart. Heart. 2012;98:947–955. doi: 10.1136/heartjnl-2011-301329. [DOI] [PubMed] [Google Scholar]
- 14.Baggish AL, Wood MJ. Athlete’s heart and cardiovascular care of the athlete: scientific and clinical update. Circulation. 2011;123:2723–2735. doi: 10.1161/CIRCULATIONAHA.110.981571. [DOI] [PubMed] [Google Scholar]
- 15.Levine BD, Lane LD, Buckey JC, Friedman DB, Blomqvist CG. Left ventricular pressure-volume and Frank-Starling relations in endurance athletes: implications for orthostatic tolerance and exercise performance. Circulation. 1991;84:1016–1023. doi: 10.1161/01.cir.84.3.1016. [DOI] [PubMed] [Google Scholar]
- 16.Douglas PS. Citius, altius, fortius (the Olympic motto: swifter, higher, stronger) J Am Coll Cardiol. 2004;44:150–151. doi: 10.1016/j.jacc.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 17.Fagard RH. Athlete’s heart: a meta-analysis of the echocardiographic experience. Int J Sports Med. 1996;17(suppl 3):S140–S144. doi: 10.1055/s-2007-972915. [DOI] [PubMed] [Google Scholar]
- 18.Spence AL, Naylor LH, Carter HH, Buck CL, Dembo L, Murray CP, Watson P, Oxborough D, George KP, Green DJ. A prospective randomised longitudinal MRI study of left ventricular adaptation to endurance and resistance exercise training in humans. J Physiol. 2011;589(pt 22):5443–5452. doi: 10.1113/jphysiol.2011.217125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pelliccia A, Maron BJ, De Luca R, Di Paolo FM, Spataro A, Culasso F. Remodeling of left ventricular hypertrophy in elite athletes after long-term deconditioning. Circulation. 2002;105:944–949. doi: 10.1161/hc0802.104534. [DOI] [PubMed] [Google Scholar]
- 20.Myerson S, Hemingway H, Budget R, Martin J, Humphries S, Montgomery H. Human angiotensin I-converting enzyme gene and endurance performance. J Appl Physiol (1985) 1999;87:1313–1316. doi: 10.1152/jappl.1999.87.4.1313. [DOI] [PubMed] [Google Scholar]
- 21.Gayagay G, Yu B, Hambly B, Boston T, Hahn A, Celermajer DS, Trent RJ. Elite endurance athletes and the ACE I allele: the role of genes in athletic performance. Hum Genet. 1998;103:48–50. doi: 10.1007/s004390050781. [DOI] [PubMed] [Google Scholar]
- 22.Puthucheary Z, Skipworth JR, Rawal J, Loosemore M, Van Someren K, Montgomery HE. The ACE gene and human performance: 12 years on. Sports Med. 2011;41:433–448. doi: 10.2165/11588720-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 23.Di Mauro M, Izzicupo P, Santarelli F, Falone S, Pennelli A, Amicarelli F, Calafiore AM, Di Baldassarre A, Gallina S. ACE and AGTR1 polymorphisms and left ventricular hypertrophy in endurance athletes. Med Sci Sports Exerc. 2010;42:915–921. doi: 10.1249/MSS.0b013e3181c29e79. [DOI] [PubMed] [Google Scholar]
- 24.Anderson GS, Rhodes EC. A review of blood lactate and ventilatory methods of detecting transition thresholds. Sports Med. 1989;8:43–55. doi: 10.2165/00007256-198908010-00005. [DOI] [PubMed] [Google Scholar]
- 25.Iwasaki K, Zhang R, Zuckerman JH, Levine BD. Dose-response relationship of the cardiovascular adaptation to endurance training in healthy adults: how much training for what benefit? J Appl Physiol (1985) 2003;95:1575–1583. doi: 10.1152/japplphysiol.00482.2003. [DOI] [PubMed] [Google Scholar]
- 26.Vigil JI. Road to the Top. Albuquerque, NM: Creative Designs, Inc; 1995. [Google Scholar]
- 27.Banister EW, Wenger H. Monitoring training. In: MacDougall JD, Wenger HA, Green HG, editors. Physiological Testing of the Elite Athlete. Ottawa, Ontario, Canada: Canadian Association of Sport Sciences; 1982. [Google Scholar]
- 28.Hundley WG, Li HF, Willard JE, Landau C, Lange RA, Meshack BM, Hillis LD, Peshock RM. Magnetic resonance imaging assessment of the severity of mitral regurgitation: comparison with invasive techniques. Circulation. 1995;92:1151–1158. doi: 10.1161/01.cir.92.5.1151. [DOI] [PubMed] [Google Scholar]
- 29.Katz J, Milliken MC, Stray-Gundersen J, Buja LM, Parkey RW, Mitchell JH, Peshock RM. Estimation of human myocardial mass with MR imaging. Radiology. 1988;169:495–498. doi: 10.1148/radiology.169.2.2971985. [DOI] [PubMed] [Google Scholar]
- 30.Germain P, Roul G, Kastler B, Mossard JM, Bareiss P, Sacrez A. Inter-study variability in left ventricular mass measurement: comparison between M-mode echography and MRI. Eur Heart J. 1992;13:1011–1019. doi: 10.1093/oxfordjournals.eurheartj.a060307. [DOI] [PubMed] [Google Scholar]
- 31.Peshock RM, Willett DL, Sayad DE, Hundley WG, Chwialkowski MC, Clarke GD, Parkey RW. Quantitative MR imaging of the heart. Magn Reson Imaging Clin N Am. 1996;4:287–305. [PubMed] [Google Scholar]
- 32.Perhonen MA, Franco F, Lane LD, Buckey JC, Blomqvist CG, Zerwekh JE, Peshock RM, Weatherall PT, Levine BD. Cardiac atrophy after bed rest and spaceflight. J Appl Physiol (1985) 2001;91:645–653. doi: 10.1152/jappl.2001.91.2.645. [DOI] [PubMed] [Google Scholar]
- 33.Foldager N, Blomqvist CG. Repeated plasma volume determination with the Evans Blue dye dilution technique: the method and a computer program. Comput Biol Med. 1991;21:35–41. doi: 10.1016/0010-4825(91)90033-6. [DOI] [PubMed] [Google Scholar]
- 34.Triebwasser JH, Johnson RL, Burpo RP, Campbell JC, Reardon WC, Blomqvist CG. Noninvasive determination of cardiac output by a modified acetylene rebreathing procedure utilizing mass spectrometer measurements. Aviat Space Environ Med. 1977;48:203–209. [PubMed] [Google Scholar]
- 35.Jarvis SS, Levine BD, Prisk GK, Shykoff BE, Elliott AR, Rosow E, Blomqvist CG, Pawelczyk JA. Simultaneous determination of the accuracy and precision of closed-circuit cardiac output rebreathing techniques. J Appl Physiol (1985) 2007;103:867–874. doi: 10.1152/japplphysiol.01106.2006. [DOI] [PubMed] [Google Scholar]
- 36.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I, Silverman NH, Tajik AJ. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography: American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 37.Vuille C, Weyman A. General considerations, assessment of chamber size and function. In: Weyman A, editor. Principles and Practices of Echocardiography. Philadelphia, PA: Lea and Febiger; 1994. pp. 575–624. [Google Scholar]
- 38.Perhonen MA, Zuckerman JH, Levine BD. Deterioration of left ventricular chamber performance after bed rest: “cardiovascular deconditioning” or hypovolemia? Circulation. 2001;103:1851–1857. doi: 10.1161/01.cir.103.14.1851. [DOI] [PubMed] [Google Scholar]
- 39.Hastings JL, Krainski F, Snell PG, Pacini EL, Jain M, Bhella PS, Shibata S, Fu Q, Palmer MD, Levine BD. Effect of rowing ergometry and oral volume loading on cardiovascular structure and function during bed rest. J Appl Physiol (1985) 2012;112:1735–1743. doi: 10.1152/japplphysiol.00019.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nikolic S, Yellin EL, Tamura K, Vetter H, Tamura T, Meisner JS, Frater RW. Passive properties of canine left ventricle: diastolic stiffness and restoring forces [published correction appears in Circ Res. 1988;62:1059] Circ Res. 1988;62:1210–1222. doi: 10.1161/01.res.62.6.1210. [DOI] [PubMed] [Google Scholar]
- 41.Belenkie I, Kieser TM, Sas R, Smith ER, Tyberg JV. Evidence for left ventricular constraint during open heart surgery. Can J Cardiol. 2002;18:951–959. [PubMed] [Google Scholar]
- 42.Levine BD, Stray-Gundersen J. “Living high-training low”: effect of moderate-altitude acclimatization with low-altitude training on performance. J Appl Physiol (1985) 1997;83:102–112. doi: 10.1152/jappl.1997.83.1.102. [DOI] [PubMed] [Google Scholar]
- 43.Chapman RF, Stray-Gundersen J, Levine BD. Individual variation in response to altitude training. J Appl Physiol (1985) 1998;85:1448–1456. doi: 10.1152/jappl.1998.85.4.1448. [DOI] [PubMed] [Google Scholar]
- 44.Milliken MC, Stray-Gundersen J, Peshock RM, Katz J, Mitchell JH. Left ventricular mass as determined by magnetic resonance imaging in male endurance athletes. Am J Cardiol. 1988;62:301–305. doi: 10.1016/0002-9149(88)90228-7. [DOI] [PubMed] [Google Scholar]
- 45.Riley-Hagan M, Peshock RM, Stray-Gundersen J, Katz J, Ryschon TW, Mitchell JH. Left ventricular dimensions and mass using magnetic resonance imaging in female endurance athletes. Am J Cardiol. 1992;69:1067–1074. doi: 10.1016/0002-9149(92)90865-v. [DOI] [PubMed] [Google Scholar]
- 46.Perseghin G, De Cobelli F, Esposito A, Lattuada G, Terruzzi I, La Torre A, Belloni E, Canu T, Scifo P, Del Maschio A, Luzi L, Alberti G. Effect of the sporting discipline on the right and left ventricular morphology and function of elite male track runners: a magnetic resonance imaging and phosphorus 31 spectroscopy study. Am Heart J. 2007;154:937–942. doi: 10.1016/j.ahj.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 47.Matsuo T, Saotome K, Seino S, Shimojo N, Matsushita A, Iemitsu M, Ohshima H, Tanaka K, Mukai C. Effects of a low-volume aerobic-type interval exercise on VO2max and cardiac mass. Med Sci Sports Exerc. 2014;46:42–50. doi: 10.1249/MSS.0b013e3182a38da8. [DOI] [PubMed] [Google Scholar]
- 48.Vogelsang TW, Hanel B, Kristoffersen US, Petersen CL, Mehlsen J, Holmquist N, Larsson B, Kjaer A. Effect of eight weeks of endurance exercise training on right and left ventricular volume and mass in untrained obese subjects: a longitudinal MRI study. Scand J Med Sci Sports. 2008;18:354–359. doi: 10.1111/j.1600-0838.2007.00706.x. [DOI] [PubMed] [Google Scholar]
- 49.Grossman W, Jones D, McLaurin LP. Wall stress and patterns of hypertrophy in the human left ventricle. J Clin Invest. 1975;56:56–64. doi: 10.1172/JCI108079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.La Gerche A, Burns AT, Mooney DJ, Inder WJ, Taylor AJ, Bogaert J, Macisaac AI, Heidbüchel H, Prior DL. Exercise-induced right ventricular dysfunction and structural remodelling in endurance athletes. Eur Heart J. 2012;33:998–1006. doi: 10.1093/eurheartj/ehr397. [DOI] [PubMed] [Google Scholar]
- 51.Pelliccia A, Maron BJ, Spataro A, Proschan MA, Spirito P. The upper limit of physiologic cardiac hypertrophy in highly trained elite athletes. N Engl J Med. 1991;324:295–301. doi: 10.1056/NEJM199101313240504. [DOI] [PubMed] [Google Scholar]
- 52.Atomi Y, Miyashita M. Effect of training intensity in adult females. Eur J Appl Physiol Occup Physiol. 1980;44:109–116. doi: 10.1007/BF00421088. [DOI] [PubMed] [Google Scholar]
- 53.Scharhag-Rosenberger F, Meyer T, Walitzek S, Kindermann W. Time course of changes in endurance capacity: a 1-yr training study. Med Sci Sports Exerc. 2009;41:1130–1137. doi: 10.1249/MSS.0b013e3181935a11. [DOI] [PubMed] [Google Scholar]
- 54.Shibata S, Perhonen M, Levine BD. Supine cycling plus volume loading prevent cardiovascular deconditioning during bed rest. J Appl Physiol (1985) 2010;108:1177–1186. doi: 10.1152/japplphysiol.01408.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fujimoto N, Hastings JL, Carrick-Ranson G, Shafer KM, Shibata S, Bhella PS, Abdullah SM, Barkley KW, Adams-Huet B, Boyd KN, Livingston SA, Palmer D, Levine BD. Cardiovascular effects of 1 year of alagebrium and endurance exercise training in healthy older individuals. Circ Heart Fail. 2013;6:1155–1164. doi: 10.1161/CIRCHEARTFAILURE.113.000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.