Abstract
Patients in the United States frequently initiate hemodialysis with a central venous catheter (CVC) and subsequently undergo placement of a new arteriovenous fistula (AVF) or arteriovenous graft (AVG). Little is known about the clinical and economic effects of initial vascular access choice. We identified 479 patients starting hemodialysis with a CVC at a large medical center (during 2004–2012) who subsequently had an AVF (n=295) or AVG (n=105) placed or no arteriovenous access (CVC group, n=71). Compared with patients receiving an AVG, those receiving an AVF had more frequent surgical access procedures per year (1.01 [95% confidence interval, 0.95 to 1.08] versus 0.62 [95% confidence interval, 0.55 to 0.70]; P<0.001) but a similar frequency of percutaneous access procedures per year. Patients receiving an AVF had a higher median annual cost (interquartile range) of surgical access procedures than those receiving an AVG ($4857 [$2523–$8835] versus $2819 [$1411–$4274]; P<0.001), whereas the annual cost of percutaneous access procedures was similar in both groups. The AVF group had a higher median overall annual access-related cost than the AVG group ($10,642 [$5406–$19,878] versus $6810 [$3718–$13,651]; P=0.001) after controlling for patient age, sex, race, and diabetes. The CVC group had the highest median annual overall access-related cost ($28,709 [$11,793–$66,917]; P<0.001), largely attributable to the high frequency of hospitalizations due to catheter-related bacteremia. In conclusion, among patients initiating hemodialysis with a CVC, the annual cost of access-related procedures and complications is higher in patients who initially receive an AVF versus an AVG.
Keywords: arteriovenous access, arteriovenous fistula, arteriovenous graft
Arteriovenous fistulas (AVFs) are considered superior to arteriovenous grafts (AVGs) because they have a longer cumulative patency for dialysis and require fewer interventions to maintain this patency.1 However, this conclusion may be misleading because it focuses only on those vascular accesses that have been successfully cannulated for dialysis. It does not consider the frequency of procedures required to achieve AVF maturation, the relatively high rate of AVFs that fail to mature despite percutaneous or surgical interventions to promote their maturation, or the effect of prolonged catheter dependence arising from AVF failure.2 Of the 110,000 patients initiating hemodialysis annually in the United States, only approximately 20% start dialysis with an AVF or AVG, whereas the remaining 80% start with a central venous catheter (CVC).3 About three quarters of the latter group do not have a secondary (maturing) vascular access. Such patients remain catheter dependent until a new AVF or AVG has been placed and is ready for dialysis use. Prolonged catheter dependence is associated with an increased risk of infection, hospitalization, morbidity, and mortality.4–8 Catheters frequently require replacement because of infection or dysfunction. Patients with AVFs typically require more interventions than those with AVGs before successful use for dialysis,9 and a substantially longer time to first cannulation.10 Conversely, patients with AVGs require more frequent interventions (angioplasty, thrombectomy, or surgical revision) than those with AVFs to maintain long-term access patency for dialysis after successful access cannulation.
Clearly, these numerous vascular access procedures and catheter infections represent a substantial expense to the health care system, but the literature quantifying the burden of vascular access procedures and their economic consequences is sparse and dated.11,12 The economic cost of vascular access may be influenced by several recent changes in the past few years, including the increasing burden of comorbidity in patients initiating hemodialysis, initiatives to increase AVF utilization in patients on dialysis (many of them being at high risk for AVF nonmaturation), and the shift of access surgery and interventional procedures from the inpatient to the outpatient setting. Recent Center of Medicare and Medicaid Services initiatives to shift reimbursement from fee-for-service to global capitation models require a better understanding of the economic effect of different vascular access management strategies. Accurate quantification of the consequences of each strategy may provide a rational basis for selecting a particular one in certain subsets of patients on hemodialysis. The goal of this observational study was to quantify the clinical and economic effect of placement of an AVF versus an AVG in patients initiating hemodialysis with a catheter.
Results
Patient Characteristics
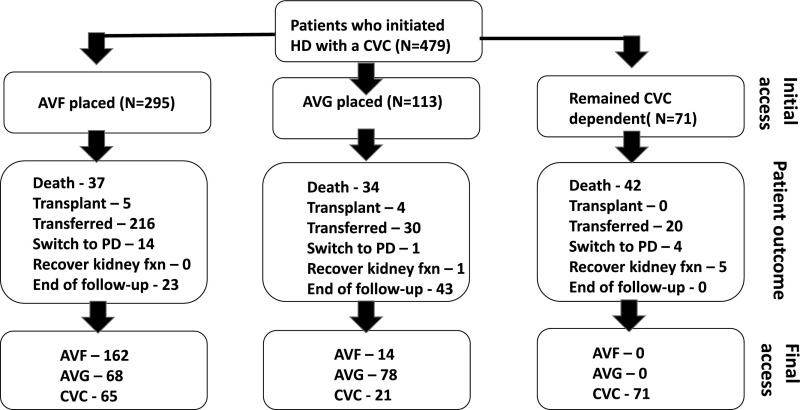
The study cohort included 479 patients with ESRD patients who did not have access surgery before starting dialysis, and initiated hemodialysis with a CVC. Of this cohort, 295 patients subsequently had an AVF placed (172 radiocephalic, 117 brachiocephalic, and six brachiobasilic), 113 had an AVG placed, and 71 never underwent placement of an arteriovenous access (CVC group). Patient follow-up was from the time of dialysis initiation to the time of patient death, kidney transplant, transfer to an outside dialysis unit, switch to peritoneal dialysis, recovery of kidney function, or end of follow-up period (December 31, 2015) (Figure 1). The proportion of patients dying during follow-up in the AVF, AVG, and CVC groups was 13%, 30%, and 59%, respectively. The median patient follow-up in years was similar in the AVF versus AVG group (2.44 [interquartile range (IQR), 1.03–5.01] versus 3.76 years [IQR, 1.70–5.56]; P=0.02). It was substantially shorter in the CVC group (0.51 years [IQR, 0.13–1.22]; P<0.001 versus AVF and AVG groups). Compared with patients undergoing AVF placement after initiation of hemodialysis, those undergoing AVG placement or no arteriovenous access placement (CVC group) were less likely to be men (Table 1). The three groups were similar in terms of age, race, and diabetes, hypertension, and cardiovascular comorbidities.
Figure 1.
Flowsheet of patients included in the analysis, including initial vascular access placed, patient events during follow-up, and vascular access in use at the end of patient follow-up. fxn, function; HD, hemodialysis; PD, peritoneal dialysis.
Table 1.
Comparison of baseline demographics of patients with an initial AVF surgery, AVG surgery, or no access surgery
| Parameter | AVF | AVG | CVC | P Value |
|---|---|---|---|---|
| No of patients | 295 | 113 | 71 | |
| Age in yr, mean±SD | 52±14 | 54±14 | 56±16 | 0.05 |
| Age ≥65 yr, n (%) | 53 (18) | 27 (24) | 20 (28) | 0.11 |
| Men, n (%) | 178 (60) | 43 (38) | 31 (44) | <0.001 |
| Black, n (%) | 260 (88) | 98 (87) | 61 (86) | 0.85 |
| Diabetes, n (%) | 145 (49) | 67 (59) | 36 (51) | 0.18 |
| Hypertension, n (%) | 281 (95) | 108 (96) | 70 (98) | 0.44 |
| Coronary artery disease, n (%) | 66 (22) | 26 (23) | 23 (32) | 0.20 |
| Heart failure, n (%) | 81 (27) | 32 (28) | 23 (32) | 0.26 |
| Cerebrovascular disease, n (%) | 40 (14) | 17 (15) | 7 (10) | 0.71 |
| Peripheral vascular disease, n (%) | 31 (10) | 16 (14) | 8 (11) | 0.58 |
Subsequent Access Placements and Final Access Outcomes
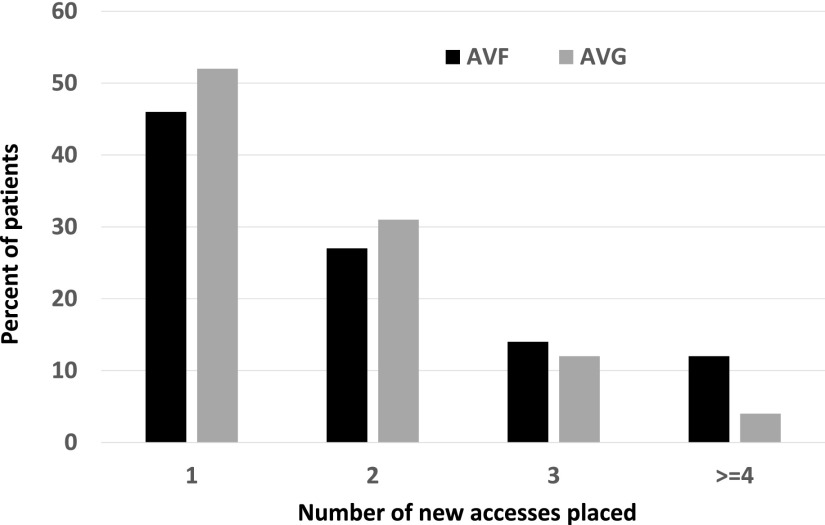
Many patients underwent placement of multiple vascular accesses during follow-up. The initial access was still in use at the end of patient follow-up in only 39.7% of patients whose initial access was an AVF and 45.1% of those whose initial access was an AVG. Among patients who initially received an AVF, 27% required two access placements, 14% required three access placements, and 12% required four or more access placements (Figure 2). Similarly, among patients who initially received an AVG, 31% required two access placements, 12% required three access placements, and 4% required four or more access placements. Among patients whose initial access was an AVF, the final access in use at the end of follow-up was an AVF in 55%, an AVG in 23%, and a CVC in 22% (Figure 1). Among patients whose initial access was an AVG, the final access at the end of follow-up was an AVF in 12%, an AVG in 69%, and a CVC in 18%.
Figure 2.
Patients initiating hemodialysis with a CVC frequently require multiple AVF and AVG placements during follow-up.
Frequency of Access Procedures and Complications
Among the 295 patients who initially received an AVF, 105 (36%) experienced an AVF that failed to mature. Moreover, of the AVFs that were successfully used for hemodialysis, 86 (46%) required an intervention to promote maturation. The median duration of CVC dependence was approximately 2 months shorter in the AVG group, compared with the AVF and CVC groups (126 [IQR, 46–357], 190 [IQR, 1122–364], and 185 [IQR, 49–446] days, respectively; P<0.001). We quantified the annual frequency of access procedures during patient follow-up. Compared with patients whose initial access was an AVG, those who initially received an AVF had a similar annual frequency of CVC exchange and angioplasty, but a lower rate of thrombectomy (Table 2). The overall rate of percutaneous access procedures was similar for the AVF and AVG groups. Compared with the AVG group, the AVF group had a higher frequency of new access creation, surgical access revision, and total surgical access procedures. The overall frequency of percutaneous and surgical access procedures was similar in patients whose initial access was an AVF or an AVG. Finally, the frequency of all catheter-related bacteremias (CRBs) and CRBs requiring hospitalization was similar for the AVF and AVG groups. Despite a similar median duration of catheter dependence (approximately 6 months), patients in the CVC group had a substantially higher frequency of CVC exchanges, CRBs, and CRBs requiring hospitalization, compared with patients who initially received an AVF.
Table 2.
Comparison of annual frequency of vascular access procedures and CRB between patents with an initial AVF surgery, AVG surgery, or no access surgery
| Parameter | AVF | AVG | CVC | P Value | P Value |
|---|---|---|---|---|---|
| AVF versus AVG | AVF versus CVC | ||||
| No. of patients | 295 | 113 | 71 | ||
| CVC exchange, n per patient-yr (95% CI) | 0.49 (0.44 to 0.53) | 0.50 (0.44 to 0.57) | 3.55 (3.09 to 4.07) | 0.19 | <0.001 |
| Angioplasty, n per patient-yr (95% CI) | 0.63 (0.58 to 0.68) | 0.58 (0.52 to 0.66) | – | 0.68 | – |
| Thrombectomy, n per patient-yr (95% CI) | 0.35 (0.31 to 0.38) | 0.52 (0.45 to 0.59) | – | 0.004 | – |
| All percutaneous procedures, n per patient-yr (95% CI) | 1.46 (1.39 to 1.54) | 1.61 (1.49 to 1.73) | 3.55 (3.09 to 4.07) | 0.06 | 0.002 |
| Surgical access revisions, n per patient-yr (95% CI) | 0.42 (0.38 to 0.47) | 0.19 (0.15 to 0.24) | – | <0.001 | – |
| Access creations, n per patient-yr (95% CI) | 0.59 (0.54 to 0.64) | 0.43 (0.37 to 0.49) | – | <0.001 | – |
| All surgical procedures, n per patient-yr (95% CI) | 1.01 (0.95 to 1.08) | 0.62 (0.55 to 0.70) | – | <0.001 | – |
| All access procedures, n per patient-yr (95% CI) | 2.48 (2.38 to 2.58) | 2.23 (2.09 to 2.37) | – | 0.69 | – |
| CRBs, n per patient-yr (95% CI) | 0.60 (0.55 to 0.65) | 0.60 (0.53 to 0.68) | 4.63 (4.09 to 5.21) | 0.23 | <0.001 |
| Hospitalizations for CRB, n per patient-yr (95% CI) | 0.24 (0.22 to 0.28) | 0.18 (0.14 to 0.22) | 2.06 (1.71 to 2.46) | 0.32 | <0.001 |
The frequency of access events or procedures between subgroups (AVF versus AVG, AVF versus CVC, and failed versus successful AVFs) was assessed using negative binomial models to account for the relatively high number of patients with zero counts. 95% CI, 95% confidence interval.
Cost of Access Procedures and Complications
We calculated the cost of all percutaneous and surgical access procedures, as well as the cost of hospitalization for CRB. Compared with patients whose initial access was an AVG, those receiving an AVF incurred a higher median annual cost of surgical access procedures, a similar cost of percutaneous access procedures, and a similar cost for CRB hospitalizations (Table 3). The median total annual access cost (percutaneous procedures, surgical procedures, and CRB hospitalizations) was $3832 greater in the AVF than in the AVG group (P=0.001). The median access cost for the first year of follow-up was $16,602 for the AVF cohort versus $14,817 for the AVG cohort (P=0.06). For the subsequent years of follow-up, the median annual cost was $2832 for the AVF cohort and $2524 for the AVG cohort (P=0.94). The cost of access care over the duration of patient follow-up was similar for the AVF group ($24,713 over a median follow-up of 2.44 years) and the AVG group ($28,064 over a median of 3.76 years; P=0.64). The annual cost of access care was not significantly associated with patient age, sex, race, or diabetes (data not shown). Using multinomial regression to predict annual access cost, with initial access type (AVF versus AVG), patient age, sex, race, and diabetes in the model, access type was the only variable significantly predicting access-related cost (P<0.001). Finally, there was no interaction between patient sex and access type (AVF or AVG) when using access cost as the outcome (P=0.65).
Table 3.
Median annual vascular access-related costs in patients with an initial AVF, an initial AVG, or no access surgery
| Parameter | AVF | AVG | CVC | P Value, AVG versus AVF | P Value, CVC versus AVF |
|---|---|---|---|---|---|
| No. of patients | 295 | 113 | 71 | ||
| Percutaneous procedures, median $/yr [IQR] | 1879 [371–4290] | 2438 [444–4593] | 3860 [1586–7759] | 0.28 | <0.001 |
| Surgical access procedures, median $/yr [IQR] | 4857 [2523–8835] | 2819 [1411–4274] | – | <0.001 | – |
| Hospitalization for CRB, median $/yr [IQR] | 0 [0–6345] | 0 [0–7171] | 26,709 [9746–56,794] | 0.13 | <0.001 |
| Total access-related cost, median $/yr [IQR] | 10,642 [5406–19,878] | 6810 [3718–13,651] | 28,709 [11,793–66,917] | 0.001 | <0.001 |
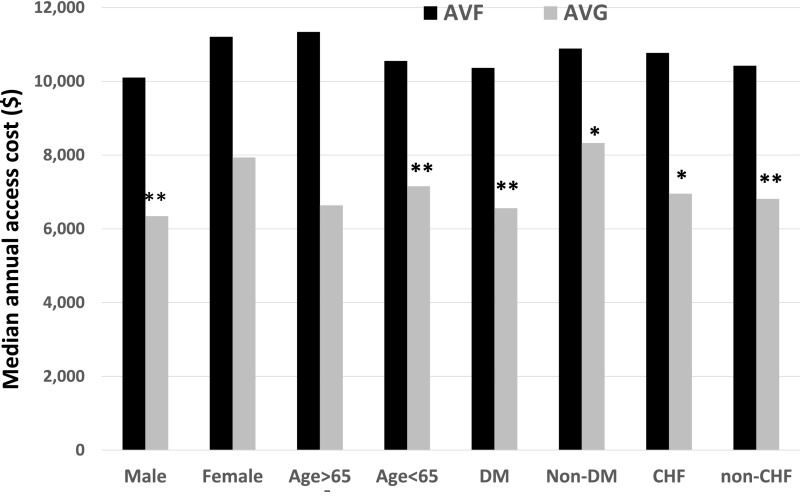
The excess annual cost of access care between AVF and AVG groups was evident for multiple patient subgroups, and was statistically significant for men, younger patients, patients with diabetes, and patients with congestive heart failure (Figure 3). In addition, the cost was not different whether the initial AVF was in the forearm or upper arm (median annual cost, $10,441 versus $10,856; P=0.57). The median annual cost of access-related procedures was nearly three-fold higher in the CVC group than in the AVF group. This difference was primarily attributable to the cost of hospitalization for CRB in the CVC group.
Figure 3.
The annual cost is higher in patients who initially receive an AVF rather than an AVG in multiple patient sub-groups. *P<0.05; **P<0.01 for AVG versus AVF. CHF, congestive heart failure; DM, diabetes mellitus.
The excess cost of access care in the AVF group was driven by the subgroup of patients whose initial AVF failed. Compared with patients with a successful AVF, those with a failed AVF had a significantly higher annual cost for percutaneous access procedures, surgical access procedures, and hospitalization for CRB, such that the total access cost was about twice as high (Table 4). Compared with patients receiving an AVG, those with a successful AVF had a lower annual cost for percutaneous procedures, a higher cost for surgical access procedures, and a similar overall cost for total access-related care. Patients who never received an arteriovenous access (CVC group) had an annual total access cost that was nearly triple that observed in the AVF group, and this difference was largely driven by the hospitalization costs for CRB.
Table 4.
Cost of access-related procedures in patients with an initial AVG, a successful AVF, or a failed AVF
| Parameter | AVG | AVF-S | AVF-F | P Value | P Value |
|---|---|---|---|---|---|
| AVF-S versus AVG | AVF-F versus AVF-S | ||||
| No. of patients | 113 | 190 | 105 | ||
| Percutaneous procedures, median $/yr [IQR] | 2438 [444–4593] | 1438 [0–3527] | 2892 [1277–5518] | <0.01 | <0.001 |
| Surgical access procedures, median $/yr [IQR] | 2819 [1411–4274] | 4675 [2277–8234] | 5501 [2948–10,281] | <0.001 | 0.03 |
| Hospitalization for CRB, median $/yr [IQR] | 0 [0–7171] | 0 [0–2020] | 5693 [1908–16,361] | 0.06 | <0.001 |
| Total access-related cost, median $/yr [IQR] | 6810 [3718–13,651] | 8146 [4014–14,397] | 16,652 [9938–33,053] | 0.46 | <0.001 |
AVF-S, successful arteriovenous fistula; AVF-F, failed arteriovenous fistula.
Discussion
Our study quantified the substantial burden of vascular access procedures and CRB, and their associated economic costs in patients initiating hemodialysis with a catheter who subsequently underwent placement of an AVF or AVG. Only 40% of the patients who initially received an AVF were still using the same AVF at the end of follow-up, and about one quarter required three or more new access creations during follow-up. The overall annual frequency of access-related procedures was similar in the AVF and AVG groups, but the relative frequency of different types of access procedures differed substantially between the two groups. In particular, patients with an AVF required more frequent surgical revisions to promote AVF maturation, and more frequent placement of a new access because of nonmaturation of the initial AVF. The high cost of these surgical procedures resulted in a median annual cost for vascular access care that was nearly $4000 greater in the patients whose initial access was an AVF than in those who initially received an AVG. The higher cost of access management in the AVF group was evident even in relatively low-risk groups, including men, younger patients, patients without diabetes, and those without congestive heart failure.
This analysis is relevant to the approximately 66,000 patients in the United States who initiate hemodialysis each year with a catheter and no secondary access. Although the more recent consensus vascular access guidelines now emphasize the importance of avoiding catheters, they continue to recommend preferential placement of an AVF rather than an AVG.13 Unfortunately, there has been a dearth of hard evidence to support or refute this recommendation. Our results suggest that creation of an AVF in patients initiating hemodialysis with a CVC is not associated with a lower frequency of access procedures, and actually incurs a higher annual cost for access management. The excess cost of placing an AVF first was driven primarily by the 36% of patients whose initial AVF failed to mature. The median annual cost of access-related management was twice as high in this cohort than in the patients whose initial AVF was used successfully for dialysis.
Few published studies have quantified the economic costs of vascular access procedures and complications. Feldman et al.11 reported that vascular access-related hospitalizations as a percent of all-cause hospitalization in patients on dialysis rose from 17% in 1986 to 20% in 1991, and projected that the cost would soon exceed $1 billion annually. Similarly, a single-center, prospective Canadian study by Manns et al.12 reported that 18.4% of all hospital admissions of patients on hemodialysis between 1999 and 2001 were related to vascular access complications. The median cost of access-related care during the first year of dialysis in patients initiating with a catheter was significantly higher for those receiving an AVG versus an AVF (CAN$8152 versus CAN$4641), and was actually lowest in those who remained catheter dependent (CAN$3812).12 This study did not report on access-related costs after the first year of dialysis. Drew et al.14 concluded that an AVF-first strategy was more cost-effective than an AVG-first strategy in most patient groups, particularly in young men without diabetes. However, the economic advantage of an AVF-first strategy was lessened among older patients and women with diabetes. Finally, Xue et al.7 used a Markov analysis to estimate access-related costs, and concluded that an initial AVF attempt was more cost-effective than an initial AVG attempt.
In contrast to these previous studies, our investigation concluded that an initial strategy of placing an AVG first was less costly. What might explain the discrepancy between our study and previous ones suggesting that AVFs are cheaper than AVGs? The Manns study12 reflects patients initiating hemodialysis in Canada over 15 years ago. It is likely that the vascular access practice patterns differ substantially from those present in the United States. For example, among Canadian patients initiating hemodialysis without pre-ESRD AVF creation, a subsequent arteriovenous access surgery is attempted in only 33%,15 compared with 85% (71 out of 479 patients) in our study. Likewise, AVGs account for only 11% of arteriovenous accesses placed in Canada,16 compared with 28% (113 out of 408 patients) in our study. The studies by Xue et al.7 and Drew et al.14 were on the basis of models that use several important assumptions. To the extent that these assumptions were not fulfilled, these models may result in inaccurate estimates of access-related costs. For example, they were limited to the operator costs for surgical or percutaneous access procedures, but did not include facility fees. The previous studies did not include the cost associated with interventions required to promote AVF maturation, which were required in nearly half of patients receiving an AVF in our study. They assumed that after a failed AVF or AVG, the patients would receive an access of the same type, whereas in reality patients often receive an AVG after a failed AVF or an AVF after a failed AVG. They also assumed that no more than two accesses would be attempted in a given patient. Our own data found that 26% of the patients who initially received an AVF had three or more access creations after initiating dialysis with a CVC.
There are additional considerations that may explain why we found a higher economic cost in patients whose initial access was an AVF. Patients initiating hemodialysis in recent years have considerably higher comorbidity than ones starting hemodialysis in previous years.17 This likely translates to poorer vascular quality and a higher likelihood of needing more frequent interventions to promote AVF maturation and maintain AVF patency for dialysis. In several recent studies, 35%–50% of AVFs required an intervention before successful use.9,18–20 AVFs that mature after an intervention have a shorter long-term patency and require more frequent interventions to maintain long-term patency for dialysis, thereby increasing the cost of access care.9,19 In fact, a recent study observed that AVFs requiring an intervention to achieve maturation actually had a shorter survival than AVGs that were used for hemodialysis without a prior intervention.9 The effect of interventions before successful use on AVF survival and frequency of subsequent AVF interventions was not factored into the cost models in previous publications.
How can one ameliorate the cost disadvantage of AVF over AVG in patients initiating hemodialysis with a CVC? Clearly, this would require implementing one or more of the following changes: (1) preferential placement of an AVG rather than an AVF in patients deemed to be at high risk of AVF nonmaturation, (2) enhanced surgical techniques or pharmacologic interventions to reduce AVF nonmaturation, and (3) enhanced processes of care to minimize the duration of catheter dependence in patients with AVF failure.
We observed substantially different death rates in the AVF, AVG, and CVC groups, consistent with several previous publications.4–8 This association should not be construed as evidence that the vascular access type directly affects patient survival. Rather, it is likely that patients with different types of vascular access have important differences in unmeasured comorbidities that are not captured even after efforts to adjust survival for baseline characteristics, or even matching for propensity scores. Two recent studies highlighted this issue. Brown et al.21 and Quinn et al.15 observed that the adjusted survival was higher in patients who initiated hemodialysis with a catheter but had an unsuccessful AVF placed before dialysis than in patients who initiated with a catheter without prior AVF surgery. In addition, Quinn et al.15 reported that among patients dialyzing with a CVC, only 2.3% of deaths were attributable to catheter complications.
The strengths of our study are the inclusion of all patients who initiated hemodialysis with a catheter and subsequently underwent placement of a vascular access; use of dedicated vascular access coordinators and a prospective vascular access database to ensure accurate and comprehensive accounting of all access procedures and complications, something that would be very difficult to obtain from an administrative database; and reliance on a small number of highly experienced access surgeons. This study used prospectively collected data to quantify the burden of access procedures and estimate the economic costs of procedures and access complications. This study also has some limitations. First, it represents the experience of a single large dialysis center, and the observations may not generalize to some dialysis centers. It is reassuring, however, that the proportion of patients with failed AVF (35.6%) in our study was remarkably similar to that reported recently for a nationally representative cohort of patients with CKD (35.9%).22 Of course, it will be important to validate our observations at other dialysis centers. The overall economic costs of AVFs would likely be lower in centers with a lower rate of AVF nonmaturation and higher in centers with a higher rate. Second, there may have been suboptimal processes of care that led to prolongation of catheter dependence in many of the patients. However, the long duration of catheter dependence in patients on hemodialysis who undergo AVF creation has been highlighted in studies of national databases.22 Third, the imbalance in the proportion of patients with AVFs and AVGs transferring to outside units may have affected the analysis. Fourth, we did not capture the cost of patients hospitalized with an infected AVG who did not require a surgical intervention, although this applies to only a small minority of patients with infected AVGs at our center.23 Finally, our findings do not apply to patients who undergo access placement before initiation of hemodialysis.
In summary, the results of this single-center study suggest that among patients initiating hemodialysis with a CVC, placing an AVF first is associated with a substantially greater cost (by almost $4000 annually) than that incurred by placing an AVG first. AVFs that failed to mature had a huge clinical and economic effect, and contributed substantially to the overall access-related cost in patients with an initial AVF placement. Our findings challenge the prevailing perception that AVFs are more cost-effective than AVGs, and suggest that in many patients, a strategy of placing AVFs may actually be more expensive than that of placing AVGs. Of course, this conclusion requires caution, as it is derived from an observational, single-center study. However, it provides equipoise to justify conducting a multicenter, randomized clinical trial of AVFs versus AVGs in patients initiating hemodialysis with a catheter.
Concise Methods
Vascular Access Management
The University of Alabama at Birmingham provides ESRD care for approximately 500 in-center patients on hemodialysis at ten units in the metropolitan Birmingham area. Patients underwent standardized preoperative vascular access mapping by the ultrasound department before undergoing vascular access surgery.24–26 Per our center’s protocol, creation of a vascular access required a minimal arterial diameter of 2.0 mm, a minimal venous diameter of 2.5 mm for AVFs and 4.0 mm for AVGs, and absence of stenosis or thrombosis in the draining vein of the target access.26 The surgeons used their clinical judgment to plan the optimal type and location of the vascular access, taking into account both the vascular anatomy and the patient characteristics. Patients receiving an AVF underwent a routine 6-week postoperative ultrasound to measure the AVF diameter and blood flow, and to identify potential anatomic abnormalities, such as a juxta-anastomotic stenosis or large accessory veins.17,27 Patients with clinically immature AVFs underwent percutaneous or surgical interventions in an attempt to promote AVF maturation.27 The initial AVF cannulation was performed 6–8 weeks after AVF creation, or at later time points if the AVF required subsequent interventions. AVGs were typically cannulated 2–3 weeks after their placement.
Catheter dysfunction was treated by instillation of tissue plasminogen activator (TPA) into the catheter lumen, and by catheter exchange when TPA was unsuccessful.28 CRB was treated with systemic antibiotics, in conjunction with an adjunctive antibiotic lock.29 If the CRB persisted, the catheter was replaced. Patients with CRB associated with sepsis or metastatic complications were hospitalized, whereas milder cases were treated in the outpatient setting. Catheters were removed once the AVF or AVG had been successfully used for dialysis for three consecutive sessions.
Data Collection
All vascular access procedures were scheduled by two full-time vascular access coordinators. They maintained a prospective, computerized vascular access database to track all vascular access procedures and their outcomes.24 Institutional Review Board approval was obtained before review of the patients’ medical records for research purposes. We retrospectively queried our access database to identify 705 patients who started hemodialysis with a CVC from January 1, 2004 to December 31, 2012 and subsequently had an AVF or AVG placed or continued to dialyze with a CVC. We excluded 63 patients who had undergone a vascular access placement before initiation of hemodialysis and 163 patients who transferred to an outside dialysis unit immediately after initiating dialysis. Of the remaining 479 patients, 295 received an AVF, 113 received an AVG, and 71 never underwent an access surgery (CVC group).
All patients were followed from the date of hemodialysis initiation with a CVC to the time of death, kidney transplant, transfer to an outside dialysis unit, switch to peritoneal dialysis, recovery of kidney function, or end of study follow-up (December 31, 2015). Each patient’s electronic medical record was reviewed to collect demographic and clinical information. The total duration of catheter dependence was determined for each patient. In some patients, this total included two or more discrete time segments of catheter dependence interposed between other periods of catheter independence. For example, one patient had an initial 4-month period of catheter dependence until his AVF matured and was successfully cannulated for dialysis. This was followed by a subsequent period of 3 months of catheter independence, during which the AVF was used for dialysis. The AVF then failed, and the patient resumed dialysis with a CVC for an additional 3 months, during which a second AVF was created and matured. Subsequently, the CVC was removed and the second AVF used for dialysis until the patient died. In this example, the total duration of catheter dependence was 7 months, consisting of two separate time intervals of 4 and 3 months, respectively.
We queried the prospective vascular access database to identify all percutaneous (interventional radiology) access procedures (CVC exchange, angioplasty, or surgical revision) and surgical access procedures (initial access placement, subsequent access placements if the first access failed, and surgical revisions to promote maturation or maintain long-term access patency). The frequency of each access procedure or event was calculated as the number of events divided by the number of years of follow-up.
Calculation of Overall Access Cost
All access costs were calculated from the date of hemodialysis initiation with a CVC to the end of patient follow-up. These costs included the following access procedures: initial access placement (AVF or AVG), interventions required to promote AVF maturation (angioplasty or surgical revision), interventions to maintain access patency for dialysis after successful use (angioplasty, thrombectomy, or surgical revision), surgery to place a new vascular access (AVF or AVG) if the initial or subsequent access failed, and interventions to promote maturation and maintain patency of all subsequent accesses. We also included the cost associated with CVC exchange due to catheter dysfunction or infection. We were not able to capture the cost of TPA instillation for catheter dysfunction. The cost of antibiotic administration was not calculated if the CRB was treated in the outpatient unit, as this cost is included in the dialysis bundle. If a patient with a CRB was hospitalized, we calculated the reimbursement for that admission. The overall cost of interventional radiology (percutaneous) access procedures included CVC exchanges, angioplasty, and thrombectomy. The overall cost of surgical access procedures included the cost of placement of the initial and subsequent accesses, as well as any surgical interventions to promote maturation or maintain access patency. The overall cost of access procedures was the sum of percutaneous and surgical access procedures. Finally, the overall access cost for a patient included the cost of all access procedures, as well as the cost of all hospitalizations for CRB. The annual cost of access per patient was calculated by dividing the total cost of access procedures and CRB hospitalizations by the total number of years of follow-up for that patient.
We utilized the outpatient prospective payment schedule to attach a dollar amount to each vascular access procedure (https://www.cms.gov/apps/physician-fee-schedule/search/search-criteria.aspx), and diagnosis-related groups codes to capture the cost of hospitalizations due to CRB. In patients whose CRB was treated in the outpatient arena, the cost was limited to administration of antibiotics with dialysis (cost included in the dialysis bundle), as well as the cost of the catheter replacement. The 2015 Center of Medicare and Medicaid Services reimbursements for access-related procedures and hospitalization for CRB are summarized in the Supplemental Table 1. For each patient, we calculated the overall access-related cost by multiplying the frequency of each procedure and catheter infection with its respective cost.
Statistical Analyses
Baseline patient characteristics were compared using chi-squared test for categorical variables and t tests for continuous variables. The exposure of interest for this analysis was the initial vascular access placed after initiation of hemodialysis with a CVC: an AVF, AVG, or no arteriovenous access (CVC). The primary outcome of our analysis was the overall annual access cost for the patient. Because access costs were not normally distributed, we compared the median values using nonparametric analysis (the Mann–Whitney U test), with adjustment for the covariates of interest, including patient age, sex, race, and diabetes. We used categorical analysis and multinomial regression (our outcome variable has multiple levels) models to determine whether the differences in annual access costs between vascular access groups (AVF, AVG, or CVC) were affected by the baseline covariates. The outcome variable has three levels: AVF, AVG, and CVC. We used multinomial regression (a logistic regression model with an outcome variable having three categories) to determine differences in annual access costs after adjusting for the other covariates in our model. The secondary outcomes were the annual frequency of various percutaneous and surgical access procedures, and the frequency of overall CRBs or CRBs requiring hospitalization. The frequency of access events or procedures between subgroups (AVF versus AVG, AVF versus CVC, and failed versus successful AVFs) was assessed using negative binomial models to account for the relatively high number of patients with zero counts.30 We evaluated three different model approaches, Poisson regression, negative binomial, and zero-inflated models, and on the basis of the criteria, the negative binomial resulted in the best fit. For comparing Poisson regression and the negative binomial models, we looked at the dispersion parameter using SAS software, and calculated confidence intervals and P values. The negative binomial dispersion parameter was estimated by maximum likelihood. If the dispersion parameter equals zero, the model reduces to the simpler Poisson model; if dispersion is greater than zero, the response variable is overdispersed; and if dispersion is less than zero, the response variable is underdispersed. On the basis of the 95% confidence limits for our dispersion parameter estimates, we can say that the dispersion estimate is significantly different from 0 and we are justified in using the negative binomial model, using a P value ≥0.05. The nonsignificant P value suggests that the negative binomial model is a good fit for the data. We also looked at models using robust SEM using a “repeated” statement in SAS. There were no differences in the results when using robust SEMs. For comparing the negative binomial model and the zero-inflated model parameters, we looked at the Akaike Information Criterion. P<0.05 was considered statistically significant.
Disclosures
T.L. is a consultant for Proteon Therapeutics. M.A. is a consultant for CorMedix.
Supplementary Material
Acknowledgments
A.A.-B. is supported by a postdoctoral institutional T-32 grant from the National Institutes of Health (NIH) National Institute of Diabetes, Digestive and Kidney Diseases (NIDDK) (T32 DK007545-23). T.L. is supported by an American Society of Nephrology Carl W. Gottschalk research scholar grant, University of Alabama Nephrology Research Center Anderson innovation award, and University of Alabama at Birmingham Center for Clinical and Translational Science multidisciplinary pilot award (1UL1TR001417-01). M.A. is supported by grant R01-DK-085027 from the NIDDK.
Portions of this article were presented at the National Kidney Foundation meeting in Boston, Massachusetts, April 27–May 1, 2016.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016060707/-/DCSupplemental.
References
- 1.KDOQI clinical practice guidelines and clinical practice recommendations for vascular access 2006. Am J Kidney Dis 48[Suppl 1]: S176–S322, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Allon M: Current management of vascular access. Clin J Am Soc Nephrol 2: 786–800, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Collins AJ, Foley RN, Gilbertson DT, Chen SC: The state of chronic kidney disease, ESRD, and morbidity and mortality in the first year of dialysis. Clin J Am Soc Nephrol 4[Suppl 1]: S5–S11, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Xue JL, Dahl D, Ebben JP, Collins AJ: The association of initial hemodialysis access type with mortality outcomes in elderly Medicare ESRD patients. Am J Kidney Dis 42: 1013–1019, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Allon M, Dinwiddie L, Lacson E Jr, Latos DL, Lok CE, Steinman T, Weiner DE: Medicare reimbursement policies and hemodialysis vascular access outcomes: A need for change. J Am Soc Nephrol 22: 426–430, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Lacson E Jr, Wang W, Lazarus JM, Hakim RM: Change in vascular access and mortality in maintenance hemodialysis patients. Am J Kidney Dis 54: 912–921, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Xue H, Lacson E Jr, Wang W, Curhan GC, Brunelli SM: Choice of vascular access among incident hemodialysis patients: A decision and cost-utility analysis. Clin J Am Soc Nephrol 5: 2289–2296, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradbury BD, Chen F, Furniss A, Pisoni RL, Keen M, Mapes D, Krishnan M: Conversion of vascular access type among incident hemodialysis patients: Description and association with mortality. Am J Kidney Dis 53: 804–814, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Harms JC, Rangarajan S, Young CJ, Barker-Finkel J, Allon M: Outcomes of arteriovenous fistulas and grafts with or without intervention before successful use. J Vasc Surg 64: 155–162, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saran R, Dykstra DM, Pisoni RL, Akiba T, Akizawa T, Canaud B, Chen K, Piera L, Saito A, Young EW: Timing of first cannulation and vascular access failure in haemodialysis: An analysis of practice patterns at dialysis facilities in the DOPPS. Nephrol Dial Transplant 19: 2334–2340, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Feldman HI, Kobrin S, Wasserstein A: Hemodialysis vascular access morbidity. J Am Soc Nephrol 7: 523–535, 1996 [DOI] [PubMed] [Google Scholar]
- 12.Manns B, Tonelli M, Yilmaz S, Lee H, Laupland K, Klarenbach S, Radkevich V, Murphy B: Establishment and maintenance of vascular access in incident hemodialysis patients: A prospective cost analysis. J Am Soc Nephrol 16: 201–209, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Wish JB: Catheter last, fistula not-so-first. J Am Soc Nephrol 26: 5–7, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drew DA, Lok CE, Cohen JT, Wagner M, Tangri N, Weiner DE: Vascular access choice in incident hemodialysis patients: A decision analysis. J Am Soc Nephrol 26: 183–191, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quinn RR, Oliver MJ, Devoe D, Poinen K, Kabani R, Kamar F, Mysore P, Lewin AM, Hiremath S, MacRae J, James MT, Miller L, Hemmelgarn BR, Moist LM, Garg AX, Chowdhury TT, Ravani P: The effect of predialysis fistula attempt on risk of all-cause and access-related death. J Am Soc Nephrol 28: 613–620, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lok CE, Sontrop JM, Tomlinson G, Rajan D, Cattral M, Oreopoulos G, Harris J, Moist L: Cumulative patency of contemporary fistulas versus grafts (2000-2010). Clin J Am Soc Nephrol 8: 810–818, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allon M, Robbin ML: Increasing arteriovenous fistulas in hemodialysis patients: Problems and solutions. Kidney Int 62: 1109–1124, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Falk A: Maintenance and salvage of arteriovenous fistulas. J Vasc Interv Radiol 17: 807–813, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Lee T, Ullah A, Allon M, Succop P, El-Khatib M, Munda R, Roy-Chaudhury P: Decreased cumulative access survival in arteriovenous fistulas requiring interventions to promote maturation. Clin J Am Soc Nephrol 6: 575–581, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asif A, Cherla G, Merrill D, Cipleu CD, Briones P, Pennell P: Conversion of tunneled hemodialysis catheter-consigned patients to arteriovenous fistula. Kidney Int 67: 2399–2406, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Brown RS, Patibandla BK, Goldfarb-Rumyantzev AS: The survival benefit of “Fistula First, Catheter Last” in hemodialysis is primarily due to patient factors. J Am Soc Nephrol 28: 645–652, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.United States Renal Data System : USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States, Bethesda, MD, National Institutes of Health, National Institutes of Diabetes and Digestive and Kidney Diseases, 2015 [Google Scholar]
- 23.Harish A, Allon M: Arteriovenous graft infection: A comparison of thigh and upper extremity grafts. Clin J Am Soc Nephrol 6: 1739–1743, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Allon M, Bailey R, Ballard R, Deierhoi MH, Hamrick K, Oser R, Rhynes VK, Robbin ML, Saddekni S, Zeigler ST: A multidisciplinary approach to hemodialysis access: Prospective evaluation. Kidney Int 53: 473–479, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Allon M, Lockhart ME, Lilly RZ, Gallichio MH, Young CJ, Barker J, Deierhoi MH, Robbin ML: Effect of preoperative sonographic mapping on vascular access outcomes in hemodialysis patients. Kidney Int 60: 2013–2020, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Robbin ML, Gallichio MH, Deierhoi MH, Young CJ, Weber TM, Allon M: US vascular mapping before hemodialysis access placement. Radiology 217: 83–88, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Singh P, Robbin ML, Lockhart ME, Allon M: Clinically immature arteriovenous hemodialysis fistulas: Effect of US on salvage. Radiology 246: 299–305, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Mokrzycki MH, Lok CE: Traditional and non-traditional strategies to optimize catheter function: Go with more flow. Kidney Int 78: 1218–1231, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Poole CV, Carlton D, Bimbo L, Allon M: Treatment of catheter-related bacteraemia with an antibiotic lock protocol: Effect of bacterial pathogen. Nephrol Dial Transplant 19: 1237–1244, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Weaver CG, Ravani P, Oliver MJ, Austin PC, Quinn RR: Analyzing hospitalization data: Potential limitations of poisson regression. Nephrol Dial Transplant 30: 1244–1249, 2015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.