Abstract
Microvascular dysfunction (MVD) is considered a crucial pathway in the development and progression of cardiometabolic and renal disease and is associated with increased cardiovascular mortality. MVD often coexists with or even precedes macrovascular disease, possibly due to shared mechanisms of vascular damage, such as inflammatory processes and oxidative stress. One of the first events in MVD is endothelial dysfunction. With the use of different physiologic or pharmacologic stimuli, endothelium-dependent (micro)vascular reactivity can be studied. This reactivity depends on the balance between various mediators, including nitric oxide, endothelin, and prostanoids, among others. The measurement of microvascular (endothelial) function is important to understand the pathophysiologic mechanisms that contribute to MVD and the role of MVD in the development and progression of cardiometabolic/renal disease. Here, we review a selection of direct, noninvasive techniques for measuring human microcirculation, with a focus on methods, interpretation, and limitations from the perspective of chronic cardiometabolic and renal disease.
Keywords: Microvascular dysfunction, endothelium, Cardiometabolic disease, Renal disease, Pathophysiology
Noninvasive assessment of large arterial structure and function has been revolutionized by the development of vascular ultrasound. This has enabled broad application of measurement of carotid artery intima-media thickness and brachial artery flow-mediated, endothelium-dependent vasodilation in observational studies and clinical trials. In contrast, broadly applicable assessment of microvascular structure and function has lagged behind, because such measurements are technically demanding. Thus, assessment of microvascular function has relied, to an important extent, on the use of indirect biomarkers of microvascular endothelial function such as albuminuria and plasma or serum levels of molecules produced by the endothelium (e.g., vWf and soluble adhesion molecules). The interpretation, merits, and limitations of these biomarkers have been reviewed elsewhere.1,2
Technologic advances have now made noninvasive, direct assessment of microvascular function possible. This is important, because microvascular dysfunction (MVD) is considered a crucial pathway in the development and progression of both cardiometabolic3–5 and renal disease,6 and is associated with increased (cardiovascular) mortality.7,8 MVD often coexists with or even precedes macrovascular disease, possibly due to shared mechanisms of vascular damage.9 A key player in MVD is the endothelium.10,11 Classically, (micro)vascular endothelial function relates to endothelium-dependent vasodilation in response to physiologic or pharmacologic stimuli, which depends on the balance between various mediators such as nitric oxide (NO), endothelin, prostanoids, etc.12 Nevertheless, microvascular endothelium regulates not only vasomotor tone, but also permeability, coagulation, fibrinolysis, and proliferation.
Here, we review a selection of direct, noninvasive measurements of the microcirculation, with a focus on methods, interpretation, and limitations from the perspective of chronic cardiometabolic and renal disease.
The Microcirculation: Structure and Function
The microcirculation can be anatomically defined as blood vessels with a diameter<200–150 µm and comprises arterioles, capillaries, and venules. The function of the microcirculation is to distribute nutrients within, and collect waste products from, tissues. In addition, the microcirculation is involved in BP regulation because it is the major site of control of vascular resistance.13 Arterioles distribute blood within tissues according to local metabolic demand, using vasomotion as an essential mechanism. The actual exchange of fluid and solutes such as nutrients and hormones with the interstitium takes place in capillaries. Small venules not only collect capillary blood, but also play a role in determining capillary pressure. In addition, in many tissues, (postcapillary) venules are the preferential site for adhesion and diapedesis of leukocytes from blood into tissue.14,15
Here, we define microvascular function as any activity of microvessels either in the basal state or after stimulation. Microvascular function is the result of vessel wall components’ (smooth muscle cells, matrix, endothelium) structure and function, which are inextricably linked to neurogenic and local metabolic influences. Nevertheless, microvascular reactivity to various stimuli is often referred to as a “marker” of endothelial function, because it importantly involves endothelial vasomotor factors.
Exploring the Microcirculation in Humans
Noninvasive assessment of microvascular function is limited to a few organs: skin (using videomicroscopy, laser-Doppler flowmetry/imaging, or transcutaneous oxygen measurements), bulbar conjunctiva (using videomicroscopy), sublingual mucosa (using videomicroscopy), and retina (using fundus photography/videomicroscopy). Generalization of findings from one tissue to another should of course be done with caution. Although general functions of arterioles, capillaries, and venules are the same throughout the body, the organization of the microcirculation and the control of blood flow differ among tissues, depending on metabolic demand and specific organ functions. In addition, the position of a vessel segment in the vascular tree determines endothelial cell phenotype.15–17 Factors such as flow type, shear stress, local metabolic demands, and epigenetics shape the phenotype of the endothelium in the different parts of the (micro)circulation. For example, saphenous veins used in coronary artery bypass grafting and thus exposed to arterial flow conditions have been shown to increase endothelial NO synthase and reduce thrombomodulin production.17 Also, the lack of correlation between endothelial function measured in conduit arteries (using flow-mediated dilation) and in the microcirculation (e.g., retinal arteriolar dilation, postocclusive hyperemia in skin, and retinal arteriolar/venular diameters)18–20 may be related to differences in endothelial phenotype in the different parts of the vascular tree.
Capillary Microscopy
Skin is a unique site for simple and reproducible assessment of capillary structure and function, where intravital capillaroscopy can be used to directly visualize perfused nutritive capillaries. At the finger and toe nailfold, capillaries run in parallel to the skin surface, which enables evaluation of capillary morphology and measurement of blood flow and pressure. In all other parts of the skin, capillaries are orientated perpendicularly to skin surface, enabling quantification of capillary density. Only erythrocyte-filled capillaries can be visualized without dyes, using a bench-top or handheld digital videomicroscope with blue or green illumination (to enhance contrast of red blood cells) and a system magnification of approximately 100×. Classically, capillaries are visualized in the skin of the dorsal phalanges of the third or fourth finger, approximately 5 mm proximal to the nailfold. Besides baseline capillary density, functional capillary recruitment (increase in capillary density after arterial occlusion) and the maximum capillary density (during venous occlusion) can be assessed off-line manually or semiautomatically21,22 (Figure 1).
Figure 1.
Capillary density in skin on the dorsum of the finger. Images are stills from videomicroscopy clips of exactly the same visual field (1 mm2 of skin). Left: Baseline capillary density. Right: Capillary recruitment during postocclusive reactive hyperemia. The white arrows represent examples of nonperfused capillaries under baseline conditions that are recruited during postocclusive reactive hyperemia.
Functional capillary recruitment results from upstream arteriolar dilation involving a myogenic and endothelial response, and local metabolic factors. Maximal capillary recruitment during venous occlusion results from passive trapping of erythrocytes in the capillaries. Both recruitment capacities are physiologically relevant, because they correlate inversely with insulin resistance and BP.23–25 In addition, several studies have shown that microvascular responses observed in skin parallel those in muscle. For example, insulin augments capillary recruitment in both skin and muscle,26,27 whereas the presence of obesity or increased free fatty acid levels attenuates capillary recruitment.28,29 Capillary density changes may occur early and precede the occurrence of disease. For example, capillary densities and recruitment were lower in normotensive individuals with a family history of hypertension and in borderline hypertensive individuals versus controls.30,31 In more advanced disease, such as type 2 diabetes, hypertension, and advanced CKD, capillary rarefaction is also seen.21,32,33 In a healthy cohort (mean age approximately 62±5 years) it was shown that a diet with high intake of sweets was associated with lower capillary densities as compared with a diet with high intake of oil, poultry, and fish.34 In addition, in a population-based study (mean age approximately 60±9 years) we found that lower skin capillary density was independently associated with the presence of albuminuria, supporting a role of capillary rarefaction in the pathogenesis of albuminuria.35 In summary, these data suggest that skin capillary density and, in particular, recruitment capacity are associated with relevant physiologic outcomes. Changes can be measured in an early phase, before disease is clinically apparent. Reduced capillary recruitment often parallels other measures of MVD, e.g., in skin36 or in the kidney (albuminuria).
Laser-Doppler Flowmetry
Moving red blood cells in the superficial skin microvasculature give rise to a Doppler shift of monochromatic laser light, which is proportional to the concentration and speed of the blood cells. With use of this principle, relative changes in skin metabolic and thermoregulatory blood flow can be measured in a single spot (approximately 1 mm3 of skin) or in a larger skin area with laser-Doppler perfusion imaging, revealing spatial heterogeneity in microvascular perfusion.37 The laser-Doppler signal comes predominantly from small arterioles and venules, and to a lesser extent from capillaries.38
Baseline skin blood flow registrations can be used to evaluate flowmotion. Flowmotion is the result of vasomotion, an important component of microvascular function characterized by rhythmic changes in (precapillary) arteriolar diameter. Vasomotion leads to optimal flow distribution to various tissue regions for delivery of oxygen and nutrients,39,40 and reduces hydraulic resistance.41 The rhythmic changes in the perfusion signal can be analyzed with time-frequency methods (e.g., Fourier or Wavelet) to distinguish the contribution of different frequency domains to the signal. Typically, five domains can be distinguished, which relate to cardiac and breathing activity, and to (local) endothelial, myogenic, and neurogenic activity.42 Interest in flowmotion research in the clinical setting is relatively new. Small mechanistic studies have shown that flowmotion can be enhanced by insulin or after a meal,43,44 and that these reactions are diminished in obesity. In untreated hypertensive subjects, flowmotion is augmented and normalizes after treatment of hypertension.45 In several other diseases, e.g., peripheral arterial occlusive disease, diabetes, CKD with or without dialysis, or hypercholesterolemia, flowmotion has been found to be attenuated.46 In a population-based study we have shown that age and waist circumference are inversely, and BP is positively, associated with flowmotion, independent of various confounders.47 Vaso/flowmotion is undoubtedly an important function of the microcirculation. However, more study is needed to understand how cardiometabolic risk factors affect flowmotion signals. In addition, methodologic standardization is required for the calculation of the spectral value of the different frequency intervals.
Stimulated skin blood flow can also be measured, and gives reproducible measures of microvascular (maximal) response capacity.48 Both postocclusive and heat-induced reactive hyperemia are partly endothelium dependent49,50 (Figure 2). Next, endothelium-dependent and -independent reactivity can be measured as responses to acetylcholine (Ach) or sodium nitroprusside, respectively, applied with iontophoresis or microdialysis.51,52 Stimulated skin blood flow responses have been studied extensively. In healthy volunteers, the Ach- or heat-induced vasodilator response correlated with insulin sensitivity, but not with BP.23,25 In cross-sectional studies, the Ach-response has been found to be reduced in adults with obesity,53 but not in obese adolescents or overweight adults.54,55 Hypertensive, as compared with normotensive, individuals also show a reduced Ach-response.56,57 Several studies on both type 1 and 2 diabetes have shown reduced Ach- and heat-induced vasodilation, which is worse when complications are present.58–61 These vasodilator responses are inversely related to the level of glycemic control, and improve with intensified glucose control.58,62 In a population-based study, we have recently shown that the heat-induced vasodilator response is attenuated in prediabetes and even more in subjects with type 2 diabetes. This vasodilator response was inversely associated with fasting glucose levels, 2 hours postglucose load levels, and hemoglobin A1c levels, also after extensive adjustment for potential confounders.63 Finally, in patients with more advanced stages of disease, e.g., peripheral arterial occlusive disease, ESRD, or coronary artery disease, these skin vasodilator responses are reduced, but can be improved after treatment.64–66
Figure 2.
Typical registration of skin microvascular perfusion, measured with laser-Doppler flowmetry, before and during local heating of the skin in a healthy volunteer. After 2 minutes of baseline flow registration, skin heating to 44°C is started for 23 minutes. Time (minute) is depicted on the x axis and skin perfusion (arbitrary perfusion units, PU) on the y axis. The heat-induced skin hyperemic response is expressed as the percentage increase in average perfusion units during the 23-minute heating phase over the average baseline perfusion units.
In small studies, the skin vasodilator responses to arterial occlusion,67 heating, and Ach68 have been shown to be reduced in diabetic individuals and hypertensive patients with albuminuria, although contradicting results in relation to Ach exist.69 Similarly, reduced skin vasodilator responses have been observed in individuals with advanced CKD.70 However, for earlier CKD stages, results are unclear.71
In conclusion, skin (endothelium-dependent) vasodilator responses are easy-to-use, sensitive, and physiologically relevant measures of microvascular function. They can be used to detect early changes, even before disease is clinically apparent.
Retinal Imaging
Retinal imaging allows investigation of in vivo structure and function of arterioles, venules, and capillaries. Since the early 1920s, fundus photography has played a prominent role in diagnosis and follow-up of eye diseases. The widespread availability of this technique has facilitated its use in many mechanistic and epidemiologic studies. Widely used microvascular variables are the central retinal arteriolar/venular equivalents, presented separately or as a ratio (arteriolar venular ratio). Besides diameters, other measures of the retinal microvascular network have been studied, e.g., tortuosity, bifurcation angles and optimality, and fractal dimensions.72,73 Mechanisms of changes in retinal vessel diameters can be both functional and structural.74,75 For arterioles this involves changes in endothelial vasodilators (e.g., NO)76 and constrictors, and BP-related remodeling of the vessel wall.74,75 For venular widening, inflammatory signals and endothelial dysfunction have been suggested to be involved.74 A limitation of retinal microvascular analyses from a static image may be that vessel diameters change rhythmically due to vasomotion, which increases intra- and interindividual variability of single image diameter assessments. Recent developments in dynamic retinal imaging techniques have introduced the possibility to measure perfusion and microvessel constrictor responses to oxygen breathing or (endothelium-dependent) dilator responses to flicker light63,76–78 (Figure 3).
Figure 3.
Typical registration of diameter changes of a single retinal arteriole and venule before, during, and after a 30-second flicker light period (t=50 to t=80 seconds) in a healthy volunteer. Time (seconds) is depicted on the x axis and diameter (micrometers) on the y axis. The flicker light–induced vasodilator response is expressed as the average increase in diameter during flicker light as a percentage over baseline diameter.
There is a very large body of retinal microvascular studies in relation to cardiometabolic/renal risk factors and diseases. These include mechanistic, cross-sectional, population-based cohort, and longitudinal studies. For example, it has consistently been shown, across age groups, that both current and past higher BP are associated with reduced arteriolar diameters.79–81 Smaller arterioles may not only be an adaptive response to higher BP, but also predict (and possibly contribute to) the development of hypertension.82 The BP-related reduction in arteriolar diameter seems to be reversible. In hypertensive individuals, frequent fish consumption was associated with wider arteriolar and narrower venular diameters.83 In addition, 6–12 months of BP treatment resulted in wider arteriolar diameters.84,85 Finally, lifestyle interventions may also be a treatment option to normalize microvascular diameters.86
Data on retinal microvascular diameters and renal function or disease are less consistent. Cross-sectional data from an Asian population-based cohort showed an association between smaller arteriolar diameters and CKD (defined as eGFR of <60 ml/min per 1.73 m2 or the presence of micro/macroalbuminuria) independent of the presence of diabetes and hypertension.87 In addition, patients with CKD (stage 2–4) with small retinal arteriolar diameters were shown to develop more renal end points (function loss or start of dialysis) as compared with patients with larger arteriolar diameters.88 However, longitudinal population-based cohort studies did not find associations between baseline retinal arteriolar/venular diameters and incident CKD.6,89
Taken together, retinal microvascular diameters are relevant, sensitive, valid, reproducible, and consistent markers of microvascular function.
Flicker light can be used to enhance retinal metabolic activity, which, via neurovascular coupling, leads to endothelium-dependent vasodilation (involving NO) and increased blood flow.76,90 Recently, we reported, in a population-based setting, that the retinal arteriolar dilator response to flicker light was reduced in individuals with prediabetes and type 2 diabetes versus normoglycemic individuals.63 In small cross-sectional studies, similar findings of reduced retinal endothelial function have been found in individuals with hypertension, obesity, and coronary artery disease.91–93 Recently, it was shown, in patients with diabetes and/or cardiovascular disease, that retinal endothelial function correlates with creatinine clearance and eGFR.94 Because follow-up studies on retinal vasoreactivity are scarce, the prognostic value of these measurements remains to be explored. Nevertheless, microvascular (endothelial) reactivity data add valuable (patho)physiologic information to static retinal diameter/morphometry data.
Determinants of MVD
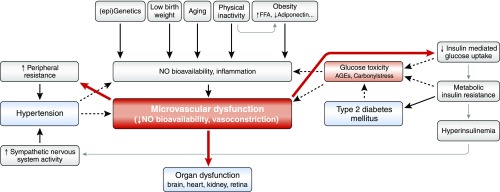
As stated above, dysfunction of the microcirculation may occur early and contribute to the development of disease. Several determinants of MVD have been identified (Figure 4).
Figure 4.
Hypothesis describing determinants contributing to MVD and subsequent organ dysfunction. We here define microvascular (dys)function being the result of vessel wall components’ (smooth muscle cells, matrix, endothelium) structure and function, and neurogenic and local metabolic influences. Early MVD leads to impaired insulin-mediated glucose uptake and raised peripheral resistance, which contributes to the development of insulin resistance/type 2 diabetes, and hypertension, respectively. The hyperglycemic milieu and hypertension in turn further aggravate MVD leading to a vicious cycle (dashed arrows). ↑, stimulated (levels of); ↓, reduced (levels of); AGEs, advanced glycation end products; FFA, free fatty acids.
Genetics
Normotensive offspring of hypertensive parents have structural and/or functional microvascular changes,95 with a lower number of skin capillaries (rarefaction) and reduced capillary recruitment capacity as compared with matched control individuals.30 In another study,96 both skin capillary density and heat-induced hyperemia were reduced in hypertensive individuals with hypertensive versus normotensive parents. Similarly, normotensive offspring of hypertensive parents had lower glomerular filtration reserve, which seemed to be related to lower NO formation.97 Importantly, lower glomerular filtration reserve is indicative of renal MVD with glomerular hyperfiltration. The latter may lead to glomerular capillary rarefaction and eventually the development of albuminuria as well as a decline in kidney function. Because hypertension is, at least in part, an inherited condition, the above findings thus suggest that genetic factors may contribute to MVD and that MVD in individuals with hypertension is of primary origin.
Low Birth Weight
Suboptimal intrauterine circumstances may result in low birth weight, which has been linked to cardiometabolic disease in adult life.98 Endothelial dysfunction, particularly reduced NO synthesis and NO scavenging by reactive oxygen species, may be a mechanism explaining these associations. Indeed, skin endothelium-dependent vasodilation to Ach has been found to be inversely associated with body weight and size in newborns.99,100 In contrast, functional and structural skin capillary densities seem to be higher in low birth weight as compared with normal birth weight newborns,101 although at prepubertal age this seems to be reversed.102 In addition, adults who were born preterm show reduced skin capillary densities,103 and retinal arteriolar diameters and vascularization.104,105
Similarly, low birth weight has been linked to the development of CKD later in life. This risk has been ascribed to a lower nephron number, which may result in an increased susceptibility to glomerular hypertension and a lower glomerular filtration reserve. Indeed, birth weight is positively associated with nephron number in neonates as well as adults.106
Physical Inactivity
Two meta-analyses have shown that endothelium-(in)dependent microvascular function is enhanced in both athletes and trained adults versus healthy controls.107,108 Vice versa, physical inactivity has been found to induce MVD acutely in healthy volunteers after bed rest.109,110 In addition, population-based cohort studies have shown that less physical activity/increased television viewing time is associated with wider retinal venular diameters.111,112 These data support the concept that regular exercise is associated with generalized improvement of microvascular function in the absence of disease. Although the exact mechanisms involved remain to be elucidated, increased shear stress/pressure and reduced oxidative stress levels due to physical activity have been proposed to contribute to augmented NO bioavailability and reduced activity of vasoconstrictor pathways.113,114 In line with these mechanisms of improved endothelial function, the Nurses’ Health Study found that higher levels of physical activity were associated with lower albumin-to-creatinine ratio.115 In addition, in patients with type 1 diabetes, higher levels of leisure-time physical activity were associated with less progression to renal failure (on the basis of urinary albumin excretion rate) and less incidence of microalbuminuria over 6 years of follow up.116
Obesity
Many studies have shown that MVD is present in obesity.117 Already at a young age, obesity is independently associated with smaller retinal arteriolar and wider venular diameters,118 which continues in adults.119 Wider retinal venular, but not smaller arteriolar, diameters may predict development of obesity.120 In addition, skin capillary recruitment capacity53 and impaired endothelium-dependent microvascular dilation in skin and muscle have been found in obese individuals.43,121,122 Several mechanisms may be involved in obesity-related MVD. Elevated free fatty acid levels augment skin MVD,28 and expanded/dysfunctional adipose tissue (1) releases inflammatory signals leading to reduced NO and increased endothelin-1 production; and (2) leads to changes in adipokine profile (less adiponectin and more leptin, resistin, and angiotensinogen).117,123 Visceral adipose tissue seems to be the most important source of this endocrine signaling to the microcirculation, but paracrine signaling from perivascular adipose tissue affects microvascular function as well.124
Relevant clinical consequences of obesity-related MVD are insulin resistance and raised BP.117 Subsequently, chronic hyperglycemia contributes to further deterioration of microvascular endothelial function.125 Raised BP contributes to endothelial dysfunction, arteriolar wall remodeling, and capillary/arteriolar rarefaction.95 Together, these conditions progressively aggravate each other in a vicious cycle. At the level of the kidney, MVD may lead to increased GFR and renal blood flow with glomerular hyperfiltration.126 The latter likely contributes to the development of secondary FSGS and loss of kidney function in individuals with (severe) obesity.127
Aging
The hallmark of aging is a gradual loss of functional reserve in all organs and tissues, including the (micro)vasculature. Investigating the independent effects of aging on the microcirculation is complex due to interrelationships of aging with increasing levels of cardiometabolic risk factors and incident cardiovascular disease. Longitudinal data from a population-based study showed reduced retinal arteriolar/venular diameters with increasing age, and a history of cardiovascular disease and CKD was associated with a change in venular diameter over time.128 In another cohort, age was independently associated with skin microvascular flowmotion.47 These findings in the systemic microcirculation parallel the significant loss of nephrons with aging observed in healthy kidney donors.129 Oxidative stress and inflammatory processes in the endothelium have been proposed to be the main drivers of MVD in aging.130
Future Directions
In this brief review, we focused on a few techniques only that are easy to apply, even in large-scale studies (Table 1). New developments may add valuable information to the status of microvascular function. First, the integrity of the endothelial surface layer (glycocalyx) is important in the glomerular barrier function.131,132 Endothelial activation leads to degradation of the glycocalyx with subsequent albuminuria, supporting the link between generalized endothelial activation, albuminuria, and renal/cardiometabolic disease.1,132 Using sidestream darkfield imaging, it is now possible to measure glycocalyx dimensions of the sublingual microcirculation in a clinical setting.133 For example, Dane et al.133 showed that patients with ESRD had a thinner glycocalyx versus healthy controls, and glycocalyx thickness correlated with eGFR. Interestingly, glycocalyx thickness in patients with a stable kidney transplantation was found to be in-between that of patients with ESRD and controls, suggesting reversal of endothelial dysfunction.133 Second, cerebral small vessel disease is a term used to describe pathologic, neuroimaging, and clinical features related to abnormalities of cerebral microvessels. Cerebral small vessel disease is associated with (incident) stroke, dementia, cognitive decline, and depression. With use of magnetic resonance imaging, various brain tissue abnormalities can be assessed (e.g., white matter hyperintensities, microbleeds, lacunar infarcts) which indirectly reflect microcirculatory function. For further reading, please see references.134,135 Third, near-infrared spectroscopy may be another interesting development. Near-infrared spectroscopy does not actually measure microvascular function, but measures O2 delivery and tissue capacity to use O2. Besides the skeletal muscle, this technique can also be applied to the brain, giving opportunities to study microcirculation-related end organ damage.136,137 Future longitudinal and population-based studies are needed to prove the validity of these techniques in measuring microvascular function.
Table 1.
Characteristics of a selection of noninvasive measurements of the human microcirculation, including endothelium-dependent (re)activity, which are clinically easy to perform and can be applied to large-scale studies
| Technique | Measured Variable | Unit | Duration | Advantages | Disadvantages |
|---|---|---|---|---|---|
| Skin videomicroscopy | Capillary density | number/mm2; %-change | Perf.: baseline, art and ven occlusion: approximately 11 min. Anal.: 15 min per ROIa/finger (semiautomatic)22; manually: 30 min. | Direct visualization of capillaries; measures functional reactivity | Difficult in dark skin; laborious analyses |
| Skin laser-doppler flowmetry | Perfusion | AU; %-change | Easy to perform; independent of skin pigmentation; measures functional reactivity | Indirect and relative measure of flow; mixed signal from arterioles and venules | |
| Flowmotion | Perf.: 15 min. | ||||
| Heat-induced hyperemia | Anal.: 5 min. | ||||
| Ach-induced hyperemia | Perf.: 25–30 min. | ||||
| Anal.: 5 min. | |||||
| Perf.: 10–15 min. | |||||
| Anal.: 5 min. | |||||
| Retinal photography | Microvessel: diameter; tortuosity; branching angle; fractal dimensions | AU | Perf.: 5 min. Anal.: diameters manually: 5–10 min; semiautomated: 1 min. Total set of variables automated: <1 min. | Direct visualization of arterioles, capillaries, venules; no mydriasis; easy to perform | Static single image: increased intra- and interindividual variability of vessel diameters (due to vasomotion) |
| Retinal videomicroscopy | AU; %-change | Direct visualization of arterioles, venules; measures functional reactivity | Mydriasis; requires good concentration/compliance of participant | ||
| Flicker light–induced vasodilation | Perf.: 5–10 min. | ||||
| Anal.: 2 min. |
Perf., performance; art and ven occlusion, arterial and venous occlusion using a finger cuff; anal., analyses; ROI, region of interest; AU, arbitrary units.
Usually 2–4 ROIs, in one or two fingers, are measured.
Conclusions
The studies reviewed here show that MVD is associated with many cardiometabolic/renal disease risk factors, and precedes and contributes to the development of disease. MVD measured in different tissues tends to show similar associations with cardiometabolic risk factors, suggesting that common pathophysiologic mechanisms (e.g., low grade inflammation, oxidative stress, etc.) are involved. It is, however, important to note that adaptation, to the same risk factor, may differ between vessel types. For example, cohort studies found BP to be inversely associated with retinal arteriolar but not, or even positively, with venular caliber.138,139 In addition, in a cross-sectional study comparing microvascular responses to a mixed meal in obese versus lean individuals, Ach-induced skin arteriolar/venular vasodilation (measured with laser Doppler flowmetry) was attenuated in the obese, whereas skin capillary recruitment capacity was unchanged.43
Most of the studies reviewed have a cross-sectional design. Hence, longitudinal observational and intervention studies are needed to unravel how MVD contributes to the development and progression of disease. The technology to do so is now available. For individual risk assessment, normative data for each technique are needed across the sexes and age ranges, as are standardized protocols for measurement and analysis of data, preferably with automated investigator-independent software.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Stehouwer CD, Smulders YM: Microalbuminuria and risk for cardiovascular disease: Analysis of potential mechanisms. J Am Soc Nephrol 17: 2106–2111, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Stehouwer CD: Is measurement of endothelial dysfunction clinically useful? Eur J Clin Invest 29: 459–461, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Chew SK, Xie J, Wang JJ: Retinal arteriolar diameter and the prevalence and incidence of hypertension: A systematic review and meta-analysis of their association. Curr Hypertens Rep 14: 144–151, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Wong TY, Klein R, Sharrett AR, Duncan BB, Couper DJ, Tielsch JM, Klein BE, Hubbard LD: Retinal arteriolar narrowing and risk of coronary heart disease in men and women. The sAtherosclerosis Risk in Communities study. JAMA 287: 1153–1159, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Wong TY, Klein R, Couper DJ, Cooper LS, Shahar E, Hubbard LD, Wofford MR, Sharrett AR: Retinal microvascular abnormalities and incident stroke: The Atherosclerosis Risk in Communities study. Lancet 358: 1134–1140, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Yau JW, Xie J, Kawasaki R, Kramer H, Shlipak M, Klein R, Klein B, Cotch MF, Wong TY: Retinal arteriolar narrowing and subsequent development of CKD Stage 3: The Multi-Ethnic Study of Atherosclerosis (MESA). Am J Kidney Dis 58: 39–46, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liew G, Mitchell P, Rochtchina E, Wong TY, Hsu W, Lee ML, Wainwright A, Wang JJ: Fractal analysis of retinal microvasculature and coronary heart disease mortality. Eur Heart J 32: 422–429, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Mutlu U, Ikram MK, Wolters FJ, Hofman A, Klaver CC, Ikram MA: Retinal microvasculature is associated with long-term survival in the general adult dutch population. Hypertension 67: 281–287, 2016 [DOI] [PubMed] [Google Scholar]
- 9.Krentz AJ, Clough G, Byrne CD: Vascular disease in the metabolic syndrome: Do we need to target the microcirculation to treat large vessel disease? J Vasc Res 46: 515–526, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Jacob M, Chappell D, Becker BF: Regulation of blood flow and volume exchange across the microcirculation. Crit Care 20: 319, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boulanger CM: Endothelium. Arterioscler Thromb Vasc Biol 36: e26–e31, 2016 [DOI] [PubMed] [Google Scholar]
- 12.Gutterman DD, Chabowski DS, Kadlec AO, Durand MJ, Freed JK, Ait-Aissa K, Beyer AM: The human microcirculation: Regulation of flow and beyond. Circ Res 118: 157–172, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pries AR, Secomb TW, Gaehtgens P: Structural autoregulation of terminal vascular beds: Vascular adaptation and development of hypertension. Hypertension 33: 153–161, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Ley K: Leukocyte recruitment as seen by intravital microscopy. In: Physiology of Inflammation, 1st Ed., edited by Ley K, New York, Oxford University Press, 2001, pp 303–337 [Google Scholar]
- 15.Aird WC: Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res 100: 158–173, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Chistiakov DA, Orekhov AN, Bobryshev YV: Effects of shear stress on endothelial cells: Go with the flow. Acta Physiol (Oxf) 219: 382–408, 2016 [DOI] [PubMed] [Google Scholar]
- 17.Aird WC: Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ Res 100: 174–190, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Domsic RT, Dezfulian C, Shoushtari A, Ivanco D, Kenny E, Kwoh CK, Medsger TA Jr, Champion HC: Endothelial dysfunction is present only in the microvasculature and microcirculation of early diffuse systemic sclerosis patients. Clin Exp Rheumatol 32[Suppl 86]: S-154–S-160, 2014 [PMC free article] [PubMed] [Google Scholar]
- 19.van Hecke MV, Dekker JM, Nijpels G, Stolk RP, Henry RM, Heine RJ, Bouter LM, Stehouwer CD, Polak BC: Are retinal microvascular abnormalities associated with large artery endothelial dysfunction and intima-media thickness? The Hoorn study. Clin Sci (Lond) 110: 597–604, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Meyer MF, Lieps D, Schatz H, Pfohl M: Impaired flow-mediated vasodilation in type 2 diabetes: Lack of relation to microvascular dysfunction. Microvasc Res 76: 61–65, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Serné EH, Gans RO, ter Maaten JC, Tangelder GJ, Donker AJ, Stehouwer CD: Impaired skin capillary recruitment in essential hypertension is caused by both functional and structural capillary rarefaction. Hypertension 38: 238–242, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Gronenschild EH, Muris DM, Schram MT, Karaca U, Stehouwer CD, Houben AJ: Semi-automatic assessment of skin capillary density: Proof of principle and validation. Microvasc Res 90: 192–198, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Serné EH, Stehouwer CD, ter Maaten JC, ter Wee PM, Rauwerda JA, Donker AJ, Gans RO: Microvascular function relates to insulin sensitivity and blood pressure in normal subjects. Circulation 99: 896–902, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Debbabi H, Uzan L, Mourad JJ, Safar M, Levy BI, Tibiriçà E: Increased skin capillary density in treated essential hypertensive patients. Am J Hypertens 19: 477–483, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Irving RJ, Walker BR, Noon JP, Watt GC, Webb DJ, Shore AC: Microvascular correlates of blood pressure, plasma glucose, and insulin resistance in health. Cardiovasc Res 53: 271–276, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Meijer RI, De Boer MP, Groen MR, Eringa EC, Rattigan S, Barrett EJ, Smulders YM, Serne EH: Insulin-induced microvascular recruitment in skin and muscle are related and both are associated with whole-body glucose uptake. Microcirculation 19: 494–500, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Jonk AM, Houben AJ, Schaper NC, de Leeuw PW, Serné EH, Smulders YM, Stehouwer CD: Angiotensin II enhances insulin-stimulated whole-body glucose disposal but impairs insulin-induced capillary recruitment in healthy volunteers. J Clin Endocrinol Metab 95: 3901–3908, 2010 [DOI] [PubMed] [Google Scholar]
- 28.de Jongh RT, Serné EH, Ijzerman RG, de Vries G, Stehouwer CD: Free fatty acid levels modulate microvascular function: Relevance for obesity-associated insulin resistance, hypertension, and microangiopathy. Diabetes 53: 2873–2882, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Clerk LH, Vincent MA, Jahn LA, Liu Z, Lindner JR, Barrett EJ: Obesity blunts insulin-mediated microvascular recruitment in human forearm muscle. Diabetes 55: 1436–1442, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Antonios TF, Rattray FM, Singer DR, Markandu ND, Mortimer PS, MacGregor GA: Rarefaction of skin capillaries in normotensive offspring of individuals with essential hypertension. Heart 89: 175–178, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Antonios TF, Singer DR, Markandu ND, Mortimer PS, MacGregor GA: Rarefaction of skin capillaries in borderline essential hypertension suggests an early structural abnormality. Hypertension 34: 655–658, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Tibiriçá E, Rodrigues E, Cobas RA, Gomes MB: Endothelial function in patients with type 1 diabetes evaluated by skin capillary recruitment. Microvasc Res 73: 107–112, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Thang OH, Serné EH, Grooteman MP, Smulders YM, ter Wee PM, Tangelder GJ, Nubé MJ: Capillary rarefaction in advanced chronic kidney disease is associated with high phosphorus and bicarbonate levels. Nephrol Dial Transplant 26: 3529–3536, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Karatzi K, Protogerou A, Kesse-Guyot E, Fezeu LK, Carette C, Blacher J, Levy BI, Galan P, Hercberg S, Czernichow S: Associations between dietary patterns and skin microcirculation in healthy subjects. Arterioscler Thromb Vasc Biol 34: 463–469, 2014 [DOI] [PubMed] [Google Scholar]
- 35.Martens RJ, Henry RM, Houben AJ, van der Kallen CJ, Kroon AA, Schalkwijk CG, Schram MT, Sep SJ, Schaper NC, Dagnelie PC, Muris DM, Gronenschild EH, van der Sande FM, Leunissen KM, Kooman JP, Stehouwer CD: Capillary rarefaction associates with albuminuria: The Maastricht study. J Am Soc Nephrol 27: 3748–3757, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Boer MP, Meijer RI, Newman J, Stehouwer CD, Eringa EC, Smulders YM, Serné EH: Insulin-induced changes in microvascular vasomotion and capillary recruitment are associated in humans. Microcirculation 21: 380–387, 2014 [DOI] [PubMed] [Google Scholar]
- 37.Allen J, Howell K: Microvascular imaging: Techniques and opportunities for clinical physiological measurements. Physiol Meas 35: R91–R141, 2014 [DOI] [PubMed] [Google Scholar]
- 38.Fagrell B: The relationship between macro- and microcirculation clinical aspects. Acta Pharmacol Toxicol (Copenh) 58[Suppl 2]: 67–72, 1986 [DOI] [PubMed] [Google Scholar]
- 39.Thorn CE, Kyte H, Slaff DW, Shore AC: An association between vasomotion and oxygen extraction. Am J Physiol Heart Circ Physiol 301: H442–H449, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aalkjær C, Boedtkjer D, Matchkov V: Vasomotion - what is currently thought? Acta Physiol (Oxf) 202: 253–269, 2011 [DOI] [PubMed] [Google Scholar]
- 41.Schmidt-Lucke C, Borgström P, Schmidt-Lucke JA: Low frequency flowmotion/(vasomotion) during patho-physiological conditions. Life Sci 71: 2713–2728, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Stefanovska A, Bracic M, Kvernmo HD: Wavelet analysis of oscillations in the peripheral blood circulation measured by laser Doppler technique. IEEE Trans Biomed Eng 46: 1230–1239, 1999 [DOI] [PubMed] [Google Scholar]
- 43.Jonk AM, Houben AJ, Schaper NC, de Leeuw PW, Serné EH, Smulders YM, Stehouwer CD: Obesity is associated with impaired endothelial function in the postprandial state. Microvasc Res 82: 423–429, 2011 [DOI] [PubMed] [Google Scholar]
- 44.de Jongh RT, Clark AD, IJzerman RG, Serné EH, de Vries G, Stehouwer CD: Physiological hyperinsulinaemia increases intramuscular microvascular reactive hyperaemia and vasomotion in healthy volunteers. Diabetologia 47: 978–986, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Rossi M, Bradbury A, Magagna A, Pesce M, Taddei S, Stefanovska A: Investigation of skin vasoreactivity and blood flow oscillations in hypertensive patients: Effect of short-term antihypertensive treatment. J Hypertens 29: 1569–1576, 2011 [DOI] [PubMed] [Google Scholar]
- 46.Rossi M, Carpi A, Galetta F, Franzoni F, Santoro G: Skin vasomotion investigation: A useful tool for clinical evaluation of microvascular endothelial function? Biomed Pharmacother 62: 541–545, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Muris DM, Houben AJ, Kroon AA, Henry RM, van der Kallen CJ, Sep SJ, Koster A, Dagnelie PC, Schram MT, Stehouwer CD: Age, waist circumference, and blood pressure are associated with skin microvascular flow motion: The Maastricht study. J Hypertens 32: 2439–2449, discussion 2449, 2014 [DOI] [PubMed] [Google Scholar]
- 48.Agarwal SC, Allen J, Murray A, Purcell IF: Comparative reproducibility of dermal microvascular blood flow changes in response to acetylcholine iontophoresis, hyperthermia and reactive hyperaemia. Physiol Meas 31: 1–11, 2010 [DOI] [PubMed] [Google Scholar]
- 49.Choi PJ, Brunt VE, Fujii N, Minson CT: New approach to measure cutaneous microvascular function: An improved test of NO-mediated vasodilation by thermal hyperemia. J Appl Physiol (1985) 117: 277–283, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cracowski JL, Gaillard-Bigot F, Cracowski C, Sors C, Roustit M, Millet C: Involvement of cytochrome epoxygenase metabolites in cutaneous postocclusive hyperemia in humans. J Appl Physiol (1985) 114: 245–251, 2013 [DOI] [PubMed] [Google Scholar]
- 51.Hodges GJ, Sparks PA: Contributions of endothelial nitric oxide synthase, noradrenaline, and neuropeptide Y to local warming-induced cutaneous vasodilatation in men. Microvasc Res 90: 128–134, 2013 [DOI] [PubMed] [Google Scholar]
- 52.Henricson J, Tesselaar E, Persson K, Nilsson G, Sjöberg F: Assessment of microvascular function by study of the dose-response effects of iontophoretically applied drugs (acetylcholine and sodium nitroprusside)--methods and comparison with in vitro studies. Microvasc Res 73: 143–149, 2007 [DOI] [PubMed] [Google Scholar]
- 53.de Jongh RT, Serné EH, IJzerman RG, de Vries G, Stehouwer CD: Impaired microvascular function in obesity: Implications for obesity-associated microangiopathy, hypertension, and insulin resistance. Circulation 109: 2529–2535, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Czernichow S, Greenfield JR, Galan P, Bastard JP, Charnaux N, Samaras K, Safar ME, Blacher J, Hercberg S, Levy BI: Microvascular dysfunction in healthy insulin-sensitive overweight individuals. J Hypertens 28: 325–332, 2010 [DOI] [PubMed] [Google Scholar]
- 55.Montero D, Walther G, Perez-Martin A, Mercier CS, Gayrard S, Vicente-Salar N, Sempere-Ortells JM, Martinez-Peinado P, Roche E, Vinet A: Effects of a lifestyle program on vascular reactivity in macro- and microcirculation in severely obese adolescents. J Clin Endocrinol Metab 99: 1019–1026, 2014 [DOI] [PubMed] [Google Scholar]
- 56.Serné EH, Gans RO, ter Maaten JC, ter Wee PM, Donker AJ, Stehouwer CD: Capillary recruitment is impaired in essential hypertension and relates to insulin’s metabolic and vascular actions. Cardiovasc Res 49: 161–168, 2001 [DOI] [PubMed] [Google Scholar]
- 57.Cupisti A, Rossi M, Placidi S, Fabbri A, Morelli E, Vagheggini G, Meola M, Barsotti G: Responses of the skin microcirculation to acetylcholine in patients with essential hypertension and in normotensive patients with chronic renal failure. Nephron 85: 114–119, 2000 [DOI] [PubMed] [Google Scholar]
- 58.Shah AS, Gao Z, Dolan LM, Dabelea D, D’Agostino RB Jr, Urbina EM: Assessing endothelial dysfunction in adolescents and young adults with type 1 diabetes mellitus using a non-invasive heat stimulus. Pediatr Diabetes 16: 434–440, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Heimhalt-El Hamriti M, Schreiver C, Noerenberg A, Scheffler J, Jacoby U, Haffner D, Fischer DC: Impaired skin microcirculation in paediatric patients with type 1 diabetes mellitus. Cardiovasc Diabetol 12: 115, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brooks BA, McLennan SV, Twigg SM, Yue DK: Detection and characterisation of microcirculatory abnormalities in the skin of diabetic patients with microvascular complications. Diab Vasc Dis Res 5: 30–35, 2008 [DOI] [PubMed] [Google Scholar]
- 61.Stansberry KB, Hill MA, Shapiro SA, McNitt PM, Bhatt BA, Vinik AI: Impairment of peripheral blood flow responses in diabetes resembles an enhanced aging effect. Diabetes Care 20: 1711–1716, 1997 [DOI] [PubMed] [Google Scholar]
- 62.Rathsman B, Jensen-Urstad K, Nyström T: Intensified insulin treatment is associated with improvement in skin microcirculation and ischaemic foot ulcer in patients with type 1 diabetes mellitus: A long-term follow-up study. Diabetologia 57: 1703–1710, 2014 [DOI] [PubMed] [Google Scholar]
- 63.Sörensen BM, Houben AJ, Berendschot TT, Schouten JS, Kroon AA, van der Kallen CJ, Henry RM, Koster A, Sep SJ, Dagnelie PC, Schaper NC, Schram MT, Stehouwer CD: Prediabetes and type 2 diabetes are associated with generalized microvascular dysfunction: The Maastricht study. Circulation 134: 1339–1352, 2016 [DOI] [PubMed] [Google Scholar]
- 64.Settergren M, Böhm F, Rydén L, Pernow J, Kalani M: Lipid lowering versus pleiotropic effects of statins on skin microvascular function in patients with dysglycaemia and coronary artery disease. J Intern Med 266: 492–498, 2009 [DOI] [PubMed] [Google Scholar]
- 65.Fronek A, DiTomasso DG, Allison M: Noninvasive assessment of endothelial activity in patients with peripheral arterial disease and cardiovascular risk factors. Endothelium 14: 199–205, 2007 [DOI] [PubMed] [Google Scholar]
- 66.Kruger A, Stewart J, Sahityani R, O’Riordan E, Thompson C, Adler S, Garrick R, Vallance P, Goligorsky MS: Laser Doppler flowmetry detection of endothelial dysfunction in end-stage renal disease patients: Correlation with cardiovascular risk. Kidney Int 70: 157–164, 2006 [DOI] [PubMed] [Google Scholar]
- 67.Houben AJ, Schaper NC, Slaaf DW, Tangelder GJ, Nieuwenhuijzen Kruseman AC: Skin blood cell flux in insulin-dependent diabetic subjects in relation to retinopathy or incipient nephropathy. Eur J Clin Invest 22: 67–72, 1992 [DOI] [PubMed] [Google Scholar]
- 68.Schmiedel O, Schroeter ML, Harvey JN: Microalbuminuria in Type 2 diabetes indicates impaired microvascular vasomotion and perfusion. Am J Physiol Heart Circ Physiol 293: H3424–H3431, 2007 [DOI] [PubMed] [Google Scholar]
- 69.Lim SC, Caballero AE, Smakowski P, LoGerfo FW, Horton ES, Veves A: Soluble intercellular adhesion molecule, vascular cell adhesion molecule, and impaired microvascular reactivity are early markers of vasculopathy in type 2 diabetic individuals without microalbuminuria. Diabetes Care 22: 1865–1870, 1999 [DOI] [PubMed] [Google Scholar]
- 70.Thang OH, Serné EH, Grooteman MP, Smulders YM, Ter Wee PM, Tangelder GJ, Nubé MJ: Premature aging of the microcirculation in patients with advanced chronic kidney disease. Nephron Extra 2: 283–292, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rossi M, Cupisti A, Di Maria C, Galetta F, Barsotti G, Santoro G: Blunted post-ischemic increase of the endothelial skin blood flowmotion component as early sign of endothelial dysfunction in chronic kidney disease patients. Microvasc Res 75: 315–322, 2008 [DOI] [PubMed] [Google Scholar]
- 72.Hughes AD, Wong TY, Witt N, Evans R, Thom SA, Klein BE, Chaturvedi N, Klein R: Determinants of retinal microvascular architecture in normal subjects. Microcirculation 16: 159–166, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Azemin MZ, Kumar DK, Wong TY, Wang JJ, Mitchell P, Kawasaki R, Wu H: Age-related rarefaction in the fractal dimension of retinal vessel. Neurobiol Aging 33: 194.e1–194.e4, 2012 [DOI] [PubMed] [Google Scholar]
- 74.Sun C, Wang JJ, Mackey DA, Wong TY: Retinal vascular caliber: Systemic, environmental, and genetic associations. Surv Ophthalmol 54: 74–95, 2009 [DOI] [PubMed] [Google Scholar]
- 75.Lehmann MV, Schmieder RE: Remodeling of retinal small arteries in hypertension. Am J Hypertens 24: 1267–1273, 2011 [DOI] [PubMed] [Google Scholar]
- 76.Dorner GT, Garhofer G, Kiss B, Polska E, Polak K, Riva CE, Schmetterer L: Nitric oxide regulates retinal vascular tone in humans. Am J Physiol Heart Circ Physiol 285: H631–H636, 2003 [DOI] [PubMed] [Google Scholar]
- 77.Delles C, Michelson G, Harazny J, Oehmer S, Hilgers KF, Schmieder RE: Impaired endothelial function of the retinal vasculature in hypertensive patients. Stroke 35: 1289–1293, 2004 [DOI] [PubMed] [Google Scholar]
- 78.Kiss B, Polska E, Dorner G, Polak K, Findl O, Mayrl GF, Eichler HG, Wolzt M, Schmetterer L: Retinal blood flow during hyperoxia in humans revisited: Concerted results using different measurement techniques. Microvasc Res 64: 75–85, 2002 [DOI] [PubMed] [Google Scholar]
- 79.Leung H, Wang JJ, Rochtchina E, Wong TY, Klein R, Mitchell P: Impact of current and past blood pressure on retinal arteriolar diameter in an older population. J Hypertens 22: 1543–1549, 2004 [DOI] [PubMed] [Google Scholar]
- 80.Sharrett AR, Hubbard LD, Cooper LS, Sorlie PD, Brothers RJ, Nieto FJ, Pinsky JL, Klein R: Retinal arteriolar diameters and elevated blood pressure: The Atherosclerosis Risk in Communities study. Am J Epidemiol 150: 263–270, 1999 [DOI] [PubMed] [Google Scholar]
- 81.Murgan I, Beyer S, Kotliar KE, Weber L, Bechtold-Dalla Pozza S, Dalla Pozza R, Wegner A, Sitnikova D, Stock K, Heemann U, Schmaderer C, Baumann M: Arterial and retinal vascular changes in hypertensive and prehypertensive adolescents. Am J Hypertens 26: 400–408, 2013 [DOI] [PubMed] [Google Scholar]
- 82.Ding J, Wai KL, McGeechan K, Ikram MK, Kawasaki R, Xie J, Klein R, Klein BB, Cotch MF, Wang JJ, Mitchell P, Shaw JE, Takamasa K, Sharrett AR, Wong TY; Meta-Eye Study Group : Retinal vascular caliber and the development of hypertension: A meta-analysis of individual participant data. J Hypertens 32: 207–215, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kaushik S, Wang JJ, Flood V, Liew G, Smith W, Mitchell P: Frequency of fish consumption, retinal microvascular signs and vascular mortality. Microcirculation 15: 27–36, 2008 [DOI] [PubMed] [Google Scholar]
- 84.Antonio PR, Marta PS, Luís DD, Antonio DP, Manuel ST, Rafael MS, Sonia GV, Manuel GP, Isabel MN, Carlos EN, Gabriel CT, Francisco GU: Factors associated with changes in retinal microcirculation after antihypertensive treatment. J Hum Hypertens 28: 310–315, 2014 [DOI] [PubMed] [Google Scholar]
- 85.Hughes AD, Stanton AV, Jabbar AS, Chapman N, Martinez-Perez ME, McG Thom SA: Effect of antihypertensive treatment on retinal microvascular changes in hypertension. J Hypertens 26: 1703–1707, 2008 [DOI] [PubMed] [Google Scholar]
- 86.Hanssen H, Nickel T, Drexel V, Hertel G, Emslander I, Sisic Z, Lorang D, Schuster T, Kotliar KE, Pressler A, Schmidt-Trucksäss A, Weis M, Halle M: Exercise-induced alterations of retinal vessel diameters and cardiovascular risk reduction in obesity. Atherosclerosis 216: 433–439, 2011 [DOI] [PubMed] [Google Scholar]
- 87.Sabanayagam C, Shankar A, Koh D, Chia KS, Saw SM, Lim SC, Tai ES, Wong TY: Retinal microvascular caliber and chronic kidney disease in an Asian population. Am J Epidemiol 169: 625–632, 2009 [DOI] [PubMed] [Google Scholar]
- 88.Baumann M, Burkhardt K, Heemann U: Microcirculatory marker for the prediction of renal end points: A prospective cohort study in patients with chronic kidney disease stage 2 to 4. Hypertension 64: 338–346, 2014 [DOI] [PubMed] [Google Scholar]
- 89.Sabanayagam C, Shankar A, Klein BE, Lee KE, Muntner P, Nieto FJ, Tsai MY, Cruickshanks KJ, Schubert CR, Brazy PC, Coresh J, Klein R: Bidirectional association of retinal vessel diameters and estimated GFR decline: The Beaver Dam CKD study. Am J Kidney Dis 57: 682–691, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Metea MR, Newman EA: Signalling within the neurovascular unit in the mammalian retina. Exp Physiol 92: 635–640, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nagel E, Vilser W, Lanzl I: Age, blood pressure, and vessel diameter as factors influencing the arterial retinal flicker response. Invest Ophthalmol Vis Sci 45: 1486–1492, 2004 [DOI] [PubMed] [Google Scholar]
- 92.Kotliar KE, Lanzl IM, Schmidt-Trucksäss A, Sitnikova D, Ali M, Blume K, Halle M, Hanssen H: Dynamic retinal vessel response to flicker in obesity: A methodological approach. Microvasc Res 81: 123–128, 2011 [DOI] [PubMed] [Google Scholar]
- 93.Al-Fiadh AH, Wong TY, Kawasaki R, Clark DJ, Patel SK, Freeman M, Wilson A, Burrell LM, Farouque O: Usefulness of retinal microvascular endothelial dysfunction as a predictor of coronary artery disease. Am J Cardiol 115: 609–613, 2015 [DOI] [PubMed] [Google Scholar]
- 94.Heitmar R, Varma C, De P, Lau YC, Blann AD: The relationship of systemic markers of renal function and vascular function with retinal blood vessel responses. Graefes Arch Clin Exp Ophthalmol 254: 2257–2265, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Levy BI, Schiffrin EL, Mourad JJ, Agostini D, Vicaut E, Safar ME, Struijker-Boudier HA: Impaired tissue perfusion: A pathology common to hypertension, obesity, and diabetes mellitus. Circulation 118: 968–976, 2008 [DOI] [PubMed] [Google Scholar]
- 96.Noon JP, Walker BR, Webb DJ, Shore AC, Holton DW, Edwards HV, Watt GC: Impaired microvascular dilatation and capillary rarefaction in young adults with a predisposition to high blood pressure. J Clin Invest 99: 1873–1879, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.O’Connor DT, Tyrell EA, Kailasam MT, Miller LM, Martinez JA, Henry RR, Parmer RJ, Gabbai FB: Early alteration in glomerular reserve in humans at genetic risk of essential hypertension: Mechanisms and consequences. Hypertension 37: 898–906, 2001 [DOI] [PubMed] [Google Scholar]
- 98.Gluckman PD, Hanson MA, Cooper C, Thornburg KL: Effect of in utero and early-life conditions on adult health and disease. N Engl J Med 359: 61–73, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Touwslager RN, Houben AJ, Gielen M, Zeegers MP, Stehouwer CD, Zimmermann LJ, Kessels AG, Gerver WJ, Blanco CE, Mulder AL: Endothelial vasodilatation in newborns is related to body size and maternal hypertension. J Hypertens 30: 124–131, 2012 [DOI] [PubMed] [Google Scholar]
- 100.Martin H, Gazelius B, Norman M: Impaired acetylcholine-induced vascular relaxation in low birth weight infants: Implications for adult hypertension? Pediatr Res 47: 457–462, 2000 [DOI] [PubMed] [Google Scholar]
- 101.D’Souza R, Raghuraman RP, Nathan P, Manyonda IT, Antonios TF: Low birth weight infants do not have capillary rarefaction at birth: Implications for early life influence on microcirculation. Hypertension 58: 847–851, 2011 [DOI] [PubMed] [Google Scholar]
- 102.Bonamy AK, Martin H, Jörneskog G, Norman M: Lower skin capillary density, normal endothelial function and higher blood pressure in children born preterm. J Intern Med 262: 635–642, 2007 [DOI] [PubMed] [Google Scholar]
- 103.Lewandowski AJ, Davis EF, Yu G, Digby JE, Boardman H, Whitworth P, Singhal A, Lucas A, McCormick K, Shore AC, Leeson P: Elevated blood pressure in preterm-born offspring associates with a distinct antiangiogenic state and microvascular abnormalities in adult life. Hypertension 65: 607–614, 2015 [DOI] [PubMed] [Google Scholar]
- 104.Hellström A, Dahlgren J, Marsál K, Ley D: Abnormal retinal vascular morphology in young adults following intrauterine growth restriction. Pediatrics 113: e77–e80, 2004 [DOI] [PubMed] [Google Scholar]
- 105.Liew G, Wang JJ, Duncan BB, Klein R, Sharrett AR, Brancati F, Yeh HC, Mitchell P, Wong TY; Atherosclerosis Risk in Communities Study : Low birthweight is associated with narrower arterioles in adults. Hypertension 51: 933–938, 2008 [DOI] [PubMed] [Google Scholar]
- 106.Luyckx VA, Brenner BM: The clinical importance of nephron mass. J Am Soc Nephrol 21: 898–910, 2010 [DOI] [PubMed] [Google Scholar]
- 107.Montero D, Walther G, Diaz-Cañestro C, Pyke KE, Padilla J: Microvascular dilator function in thletes: A Systematic Review and Meta-analysis. Med Sci Sports Exerc 47: 1485–1494, 2015 [DOI] [PubMed] [Google Scholar]
- 108.Lanting SM, Johnson NA, Baker MK, Caterson ID, Chuter VH: The effect of exercise training on cutaneous microvascular reactivity: A systematic review and meta-analysis J Sci Med Sport 20: 170–177, 2016 [DOI] [PubMed] [Google Scholar]
- 109.Hamburg NM, McMackin CJ, Huang AL, Shenouda SM, Widlansky ME, Schulz E, Gokce N, Ruderman NB, Keaney JF Jr, Vita JA: Physical inactivity rapidly induces insulin resistance and microvascular dysfunction in healthy volunteers. Arterioscler Thromb Vasc Biol 27: 2650–2656, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Demiot C, Dignat-George F, Fortrat JO, Sabatier F, Gharib C, Larina I, Gauquelin-Koch G, Hughson R, Custaud MA: WISE 2005: Chronic bed rest impairs microcirculatory endothelium in women. Am J Physiol Heart Circ Physiol 293: H3159–H3164, 2007 [DOI] [PubMed] [Google Scholar]
- 111.Tikellis G, Anuradha S, Klein R, Wong TY: Association between physical activity and retinal microvascular signs: The Atherosclerosis Risk in Communities (ARIC) study. Microcirculation 17: 381–393, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Anuradha S, Dunstan DW, Healy GN, Shaw JE, Zimmet PZ, Wong TY, Owen N: Physical activity, television viewing time, and retinal vascular caliber. Med Sci Sports Exerc 43: 280–286, 2011 [DOI] [PubMed] [Google Scholar]
- 113.Thijssen DH, Green DJ, Hopman MT: Blood vessel remodeling and physical inactivity in humans. J Appl Physiol (1985) 111: 1836–1845, 2011 [DOI] [PubMed] [Google Scholar]
- 114.Gielen S, Schuler G, Adams V: Cardiovascular effects of exercise training: Molecular mechanisms. Circulation 122: 1221–1238, 2010 [DOI] [PubMed] [Google Scholar]
- 115.Robinson ES, Fisher ND, Forman JP, Curhan GC: Physical activity and albuminuria. Am J Epidemiol 171: 515–521, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wadén J, Tikkanen HK, Forsblom C, Harjutsalo V, Thorn LM, Saraheimo M, Tolonen N, Rosengård-Bärlund M, Gordin D, Tikkanen HO, Groop PH; FinnDiane Study Group : Leisure-time physical activity and development and progression of diabetic nephropathy in type 1 diabetes: The FinnDiane study. Diabetologia 58: 929–936, 2015 [DOI] [PubMed] [Google Scholar]
- 117.Jonk AM, Houben AJ, de Jongh RT, Serné EH, Schaper NC, Stehouwer CD: Microvascular dysfunction in obesity: A potential mechanism in the pathogenesis of obesity-associated insulin resistance and hypertension. Physiology (Bethesda) 22: 252–260, 2007 [DOI] [PubMed] [Google Scholar]
- 118.Siegrist M, Hanssen H, Neidig M, Fuchs M, Lechner F, Stetten M, Blume K, Lammel C, Haller B, Vogeser M, Parhofer KG, Halle M: Association of leptin and insulin with childhood obesity and retinal vessel diameters. Int J Obes 38: 1241–1247, 2014 [DOI] [PubMed] [Google Scholar]
- 119.Boillot A, Zoungas S, Mitchell P, Klein R, Klein B, Ikram MK, Klaver C, Wang JJ, Gopinath B, Tai ES, Neubauer AS, Hercberg S, Brazionis L, Saw SM, Wong TY, Czernichow S; META-EYE Study Group : Obesity and the microvasculature: A systematic review and meta-analysis. PLoS One 8: e52708, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shankar A, Sabanayagam C, Klein BE, Klein R: Retinal microvascular changes and the risk of developing obesity: Population-based cohort study. Microcirculation 18: 655–662, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Keske MA, Clerk LH, Price WJ, Jahn LA, Barrett EJ: Obesity blunts microvascular recruitment in human forearm muscle after a mixed meal. Diabetes Care 32: 1672–1677, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD: Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest 97: 2601–2610, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Scalia R: The microcirculation in adipose tissue inflammation. Rev Endocr Metab Disord 14: 69–76, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Eringa EC, Bakker W, van Hinsbergh VW: Paracrine regulation of vascular tone, inflammation and insulin sensitivity by perivascular adipose tissue. Vascul Pharmacol 56: 204–209, 2012 [DOI] [PubMed] [Google Scholar]
- 125.Eringa EC, Serne EH, Meijer RI, Schalkwijk CG, Houben AJ, Stehouwer CD, Smulders YM, van Hinsbergh VW: Endothelial dysfunction in (pre)diabetes: Characteristics, causative mechanisms and pathogenic role in type 2 diabetes. Rev Endocr Metab Disord 14: 39–48, 2013 [DOI] [PubMed] [Google Scholar]
- 126.Zhang X, Lerman LO: Obesity and renovascular disease. Am J Physiol Renal Physiol 309: F273–F279, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kanasaki K, Kitada M, Kanasaki M, Koya D: The biological consequence of obesity on the kidney. Nephrol Dial Transplant 28[Suppl 4]: iv1–iv7, 2013 [DOI] [PubMed] [Google Scholar]
- 128.Myers CE, Klein R, Knudtson MD, Lee KE, Gangnon R, Wong TY, Klein BE: Determinants of retinal venular diameter: The Beaver Dam Eye study. Ophthalmology 119: 2563–2571, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Denic A, Lieske JC, Chakkera HA, Poggio ED, Alexander MP, Singh P, Kremers WK, Lerman LO, Rule AD: The substantial loss of nephrons in healthy human kidneys with aging. J Am Soc Nephrol 28: 313–320, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.El Assar M, Angulo J, Rodríguez-Mañas L: Oxidative stress and vascular inflammation in aging. Free Radic Biol Med 65: 380–401, 2013 [DOI] [PubMed] [Google Scholar]
- 131.Satchell SC, Tooke JE: What is the mechanism of microalbuminuria in diabetes: A role for the glomerular endothelium? Diabetologia 51: 714–725, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rabelink TJ, de Zeeuw D: The glycocalyx--linking albuminuria with renal and cardiovascular disease. Nat Rev Nephrol 11: 667–676, 2015 [DOI] [PubMed] [Google Scholar]
- 133.Dane MJ, Khairoun M, Lee DH, van den Berg BM, Eskens BJ, Boels MG, van Teeffelen JW, Rops AL, van der Vlag J, van Zonneveld AJ, Reinders ME, Vink H, Rabelink TJ: Association of kidney function with changes in the endothelial surface layer. Clin J Am Soc Nephrol 9: 698–704, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Østergaard L, Engedal TS, Moreton F, Hansen MB, Wardlaw JM, Dalkara T, Markus HS, Muir KW: Cerebral small vessel disease: Capillary pathways to stroke and cognitive decline. J Cereb Blood Flow Metab 36: 302–325, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wardlaw JM, Smith C, Dichgans M: Mechanisms of sporadic cerebral small vessel disease: Insights from neuroimaging. Lancet Neurol 12: 483–497, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kainerstorfer JM, Sassaroli A, Tgavalekos KT, Fantini S: Cerebral autoregulation in the microvasculature measured with near-infrared spectroscopy. J Cereb Blood Flow Metab 35: 959–966, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hamaoka T, McCully KK, Quaresima V, Yamamoto K, Chance B: Near-infrared spectroscopy/imaging for monitoring muscle oxygenation and oxidative metabolism in healthy and diseased humans. J Biomed Opt 12: 062105, 2007 [DOI] [PubMed] [Google Scholar]
- 138.Cheung CY, Tay WT, Mitchell P, Wang JJ, Hsu W, Lee ML, Lau QP, Zhu AL, Klein R, Saw SM, Wong TY: Quantitative and qualitative retinal microvascular characteristics and blood pressure. J Hypertens 29: 1380–1391, 2011 [DOI] [PubMed] [Google Scholar]
- 139.Kumagai K, Tabara Y, Yamashiro K, Miyake M, Akagi-Kurashige Y, Oishi M, Yoshikawa M, Kimura Y, Tsujikawa A, Takahashi Y, Setoh K, Kawaguchi T, Terao C, Yamada R, Kosugi S, Sekine A, Nakayama T, Matsuda F, Yoshimura N; Nagahama Study group : Central blood pressure relates more strongly to retinal arteriolar narrowing than brachial blood pressure: The Nagahama study. J Hypertens 33: 323–329, 2015 [DOI] [PubMed] [Google Scholar]