Abstract
Compared with males, females have lower BP before age 60, blunted hypertensive response to angiotensin II, and a leftward shift in pressure natriuresis. This study tested the concept that this female advantage associates with a distinct sexual dimorphic pattern of transporters along the nephron. We applied quantitative immunoblotting to generate profiles of transporters, channels, claudins, and selected regulators in both sexes and assessed the physiologic consequences of the differences. In rats, females excreted a saline load more rapidly than males did. Compared with the proximal tubule of males, the proximal tubule of females had greater phosphorylation of Na+/H+ exchanger isoform 3 (NHE3), distribution of NHE3 at the base of the microvilli, and less abundant expression of Na+/Pi cotransporter 2, claudin-2, and aquaporin 1. These changes associated with less bicarbonate reabsorption and higher lithium clearance in females. The distal nephrons of females had a higher abundance of total and phosphorylated Na+/Cl− cotransporter (NCC), claudin-7, and cleaved forms of epithelial Na+ channel (ENaC) α and γ subunits, which associated with a lower baseline plasma K+ concentration. A K+-rich meal increased the urinary K+ concentration and decreased the level of renal phosphorylated NCC in females. Notably, we observed similar abundance profiles in female versus male C57BL/6 mice. These results define sexual dimorphic phenotypes along the nephron and suggest that lower proximal reabsorption in female rats expedites excretion of a saline load and enhances NCC and ENaC abundance and activation, which may facilitate K+ secretion and set plasma K+ at a lower level.
Keywords: gender difference, Na transport, proximal tubule, potassium, distal tubule
There is consensus in the scientific community that human and experimental animal studies should be conducted in females as well as males because sex is a significant biologic variable.1 Understanding baseline sexual dimorphic phenotypes is critical to interpreting sex differences detected in studies of disease or genetic models. Because female physiology is optimized for successful reproduction which entails large fluctuations in vascular, hemodynamic, and renal function, there is no reason to expect that female kidneys will be indistinct from those of males.2,3 Differences between sexes extend beyond reproduction: women have lower BP than men,4 exhibit better renal ischemia tolerance,5,6 are protected from cardiovascular and renal disease relative to men before menopause,7,8 and excrete tubular enzymes cyclically reflecting changes in tubular architecture.9 Differences extend to experimental rodent models: females exhibit less hypertension and markers of renal injury after uninephrectomy,10 and present blunted hypertensive responses to angiotensin II (AngII) infusion associated with sex specific differences in renal immune infiltration.11–13 In males, testosterone elevates angiotensinogen mRNA14 and increases renal Na+ reabsorption.15 Studies in XX versus XY gonadal males and females indicate that chromosomes alone cannot account for sex differences in cardiovascular disease.16,17
The reduced response to AngII hypertension in females is also reflected in a leftward shift in the pressure natriuresis relationship, facilitated by higher angiotensin type 2 receptor expression in females.18 In males, testosterone contributes to a relative rightward shift in the pressure natriuresis curve.7 Pressure natriuresis, directly related to the BP set point, is ultimately a function of sodium transporter activity along the nephron.19–21 Studies comparing transporter abundance in both sexes concur that females have higher distal NCC abundance and activation by phosphorylation (NCCp) due to ovarian hormones and prolactin,22–25 but activated NCC does not explain lower BP. Previous studies of Na+ transporter regulation that include both sexes indicate animal- and strain-dependent differences.25–28
Apical transporters, claudins, and channels that reabsorb Na+ and water are expressed in a region-specific pattern along the nephron allowing for their analysis in homogenates, and activity in the apical membrane can often be inferred from covalent modifications such as phosphorylation and cleavage. Additionally, changes in transport activity in one region of the nephron can alter flow and driving force for transporter activation downstream (e.g., loop diuretics can increase collecting duct [CD] K+ secretion). In this study, we aimed to generate a useful, quantitative profile29 of renal Na+ transporters, channels, claudins, and associated regulators (including phosphorylation and cleavage states), in female and male rats and mice at baseline, and to determine the in vivo physiologic consequences of the differences identified. Results from the combined approaches indicate that, relative to males, females exhibit lower transporter abundance and activity in the proximal nephron and reciprocally higher abundance and activity markers in the distal nephron which predict a downstream shift in Na+ and water reabsorption. These results provide not only baseline sexual dimorphic information concerning nephron organization in rats and mice, but also provide insight into the female cardiovascular advantage.
Results
Female Rats Excrete a Saline Load More Rapidly, and Have Less Proximal Tubule Reabsorption than Males
The kidneys match Na+ and volume output to intake to minimize fluctuations in effective circulating volume.30 Acute responses of female versus male rats to an i.p. bolus of normal saline (6%–7% volume/body wt) were determined in metabolic cages. Diuretic and natriuretic responses were more rapid in female rats: females excreted approximately 30% and males <15% of the saline bolus at 3 hours, and by 5 hours, females excreted 48% and males 30%–33% (Figure 1, A and B). The rate at which a saline load is excreted is a function of GFR and renal Na+ transporter activity,31 which is notably suppressed in the PT of female rats.
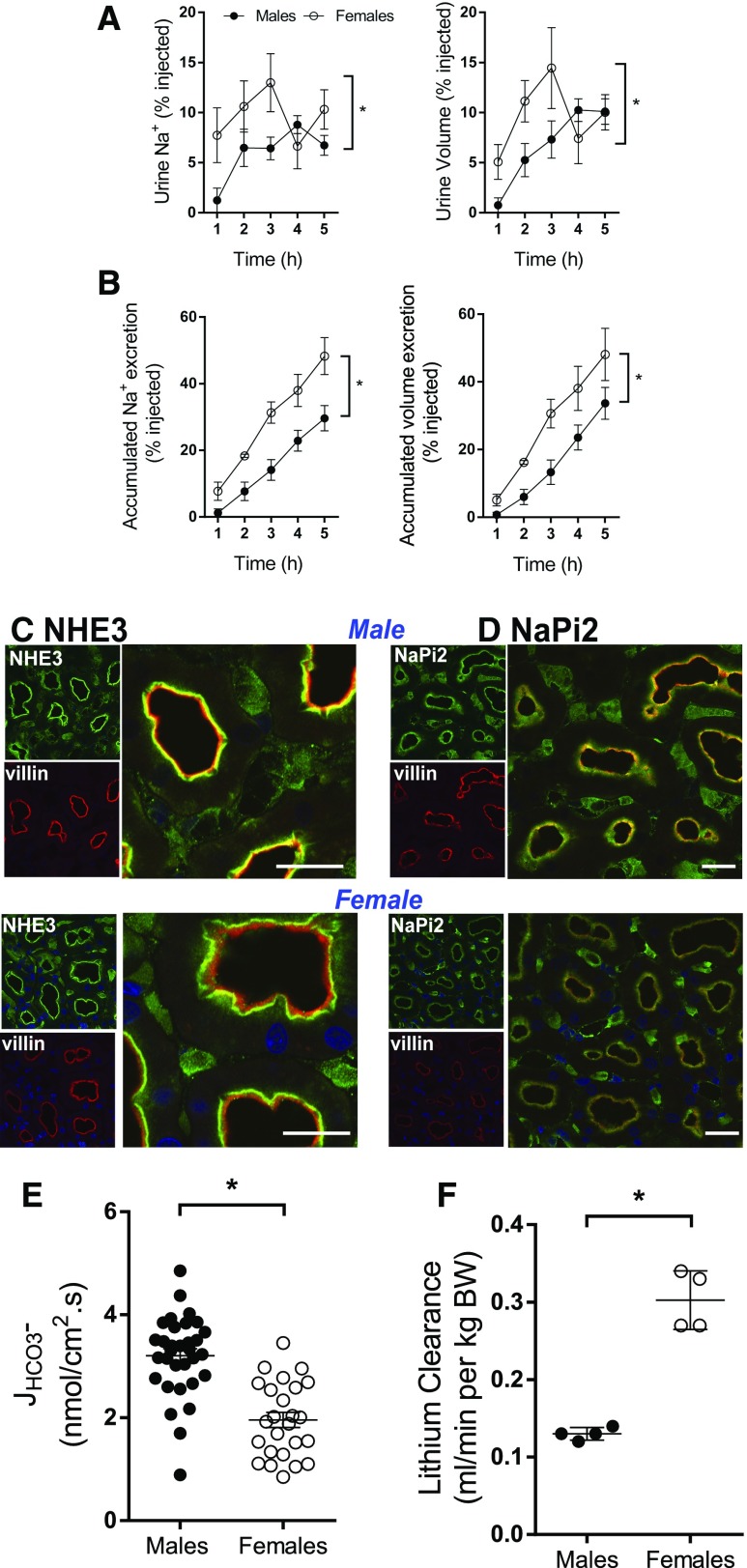
Figure 1.
Lower fractional sodium reabsorption in female versus male rats. Rats were challenged with an i.p. bolus of warmed saline equivalent of 6%–7% of their body weight and placed in metabolic cages for hourly urine collection. (A) Fraction of injected Na+ and volume excreted over 5-hour collection period. (B) Accumulated excretion of Na+ and volume. Data are expressed as mean±SEM, n=4 per group. *P<0.05. (C) NHE3 preferentially distributes to the base of the microvilli in females. Indirect immunofluorescence microscopy of NHE3 distribution and abundance in kidney samples from untreated male and female Sprague Dawley rats processed side by side on the same slide; NHE3 detected with polyclonal anti-NHE3 (green) and microvilli identified using monoclonal anti-villin (red). In males, the microvilli, primarily yellow, indicate colocalization of NHE3 with villin in the body of the villi, whereas in the females relatively more NHE3 is located at the base of the microvilli, revealing more red- (villin) stained microvilli. (D) NaPi2 exhibits similar distribution and lower abundance in female versus male rats. NaPi2, detected with polyclonal anti-NaPi2 (1:50, green), exhibited similar distribution in the body of microvilli overlaying villin in both sexes, but signal indicated lower abundance in females than males when viewed with the same settings. For (C and D), three different sets of rat kidneys were analyzed with similar results. Bar=20 µm. (E) Proximal tubule HCO3− reabsorption (JHCO3−), a measure of NHE3 activity, is lower in female than male rats. JHCO3− was evaluated by in vivo stationary microperfusion via continuous measurement of luminal pH (see Supplemental Material for details). Number of perfused tubules: males=32, and females=25. Values represent individual measurements and mean±SEM. *P<0.05 versus males. (F) CLi, a measure of volume flow leaving the proximal tubule, is greater in female than male rats. CLi was calculated according to the conventional expression: CLi = [U/P]Li × [Vu/body wt] (ml. min−1. kg BW−1). [U/P]Li denotes the urine-to-plasma concentration ratio of lithium and [Vu/body wt] is the rate of urine flow divided by the body weight. Values represent individual measurements and mean±SEM (n=4). *P<0.05 versus males.
PT transporter function was examined at levels of distribution, activity, and volume flow from PT. Cortex sections from male and female rats were stained and visualized on the same slide. Villin, the microvillar actin bundling protein, was used to delimit the location of the PT microvilli (red) (Figure 1C). In males, the Na+/H+ exchanger isoform 3 (NHE3, green) primarily colocalized in the PT brush border with villin (yellow signal), whereas in females, NHE3 was primarily distributed to the base of the microvilli (evident as a green band), exposing red-stained villin in the body of the villi (Figure 1C). NHE3 at the base of the villi is associated with less NHE3 activity.32–34 PT NaPi2 (green) colocalized in the microvilli with villin in both males and females (Figure 1D); less staining was evident in females than males.
To assess NHE3 activity, the rate of bicarbonate reabsorption (JHCO3−), which hinges on NHE3 activity, was determined by in vivo stationary microperfusion in native renal PT from both sexes (Supplemental Material). JHCO3− was significantly lower in females than males (2.0±0.1 versus 3.2±0.1 nmol/cm2.s; Figure 1E), supporting the conclusion that NHE3 activity is lower in females, consistent with its distribution at the base of the microvilli. Volume flow out of the PT was estimated from the clearance of endogenous lithium (CLi).35–37 CLi was twice as high in females versus males (Figure 1F). Taken together, the basal distribution of NHE3, lower JHCO3−, and higher CLi in females are consistent with their more rapid excretion of the saline challenge than males (Figure 1, A and B).
Profiles of Proximal Tubule and Loop of Henle Transporters and Related Proteins in Female versus Male Rats
Relative abundance of proteins was examined in homogenates of cortex and medulla by quantitative immunoblot after an overnight fast followed by a 3-hour K+-free meal, defined as baseline. Evidence for equivalent loading and linearity of the detection system is provided in Supplemental Figure 1, A and B and Supplemental Table 1. Transporter abundance as a function of stage of the estrus cycle (Supplemental Figures 2) did not reveal any apparent correlation; thus, results are presented without any adjustment for estrus stage.
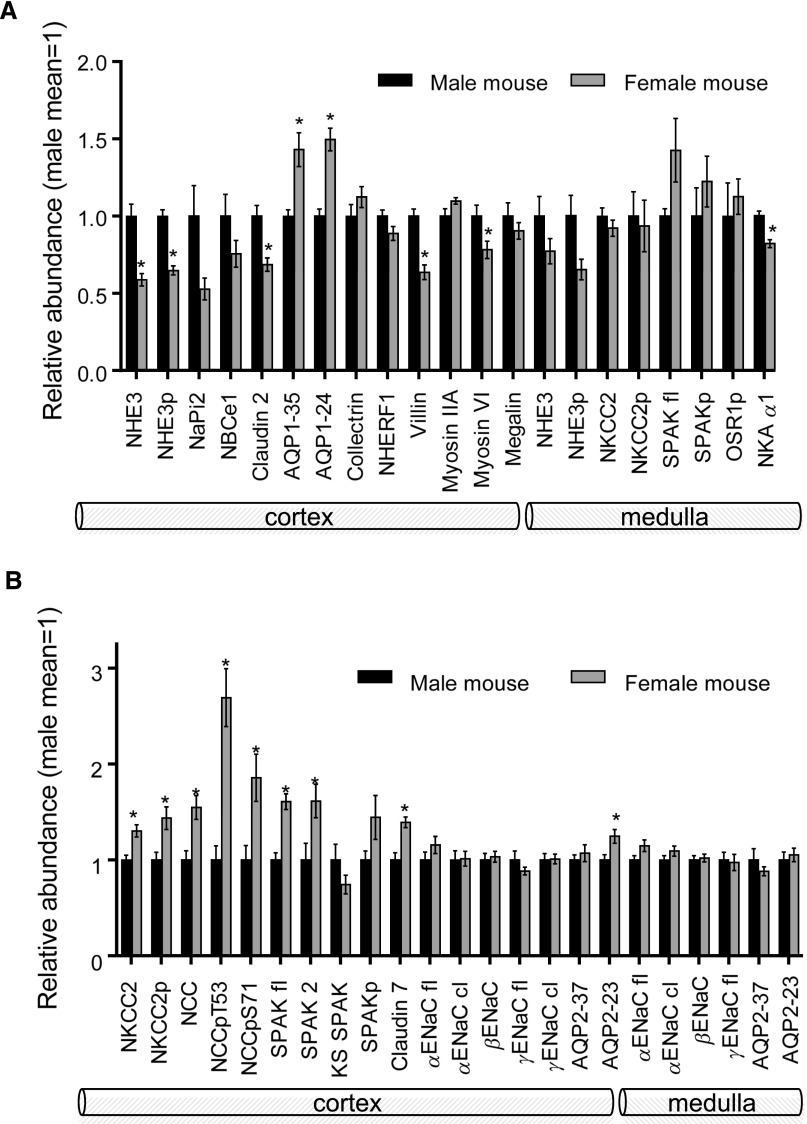
Figure 2A summarizes abundance of female versus male renal PT proteins. Although NHE3 was not significantly different, NHE3pS552, a marker for lower NHE3 activity at the microvillar base,34 was 30% higher in females, in line with immunohistochemistry (Figure 1C). NaPi2 abundance was 25% lower in females, supporting the immunohistochemistry (Figure 1D). Basolateral sodium bicarbonate cotransporter (NBCe1), key for PT HCO3− reabsorption38 and Na,K-ATPase, tended to be higher in females (NS). Cldn2, the tight junction protein effecting paracellular NaCl reabsorption in PT,39,40 was 60% lower in females. Likewise, AQP1, which effects constitutive water reabsorption across the PT,41 was lower by 60% (35 kD form) and 15% (24 kD form) in females versus males, verifying a recent report.42 Di-peptidyl peptidase IV (DPPIV), an activator of NHE3,43,44 was 50% more abundant in females; NHERF1, a scaffold protein that plays a role in activating NaPi245 and NHE3 activity,46 was 70% higher in females. Cytoskeletal-associated proteins also exhibited sexual dimorphism: in females, both myosin IIA and myosin VI (which transport cargo within microvilli)47,48 are 20% lower; the PT endocytic receptor megalin49 is 40% lower, and the microvillar actin bundling protein villin is 40% lower in females. Villin staining was assessed in three sets of male and female cortical samples treated on the same slide and viewed with the same settings (Figure 2B). In males, villin staining presented as wider bands and in females overall staining was less, confirming the immunoblot findings. Maunsbach’s classic electron microscopic analysis of proximal tubules did not note differences in microvilli height in females versus males.50
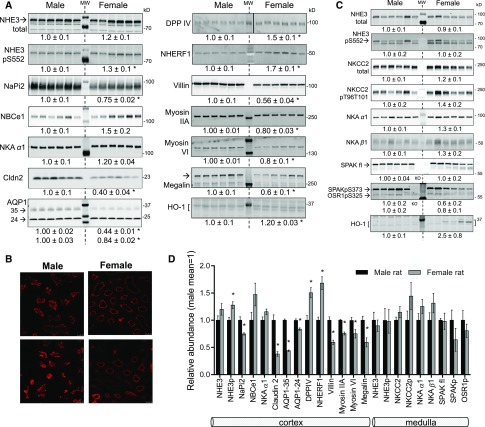
Figure 2.
Sexual dimorphic pattern of transporters, channels, regulators and cytoskeletal proteins along the proximal nephron. Immunoblots of renal homogenates. Both 1 and 1/2 amounts of protein were loaded (only one amount is displayed), see antibodies in Supplemental Table 1 for details. Density values, normalized to male group=1.0, displayed as mean±SEM (n=6). MW (molecular weight) indicates the lane loaded with prestained protein ladders (BioRad or Thermo Scientific). kD indicates apparent MW of the stained markers. *P<0.05 versus male. (A) Renal cortex samples, (B) evidence for shorter microvilli in females. Villin abundance and distribution in renal cortex samples from females and males collected, fixed, and processed at same time and viewed on same slide with identical settings. Villin was detected using anti-villin (1:100). Two different sets of rat kidneys were analyzed with similar results. Bar=25 µm. (C) Renal medulla samples. (D) Profile of transporters, structural and associated proteins, and regulators expressed along the proximal nephron summarized from (A and C). AQP1–35, -24, aquaporin 1 forms with apparent mobilities of -35 and -24 kD; Cldn2, claudin-2; DPPIV, dipeptidyl peptidase IV; fl, full length; HO-1, heme oxygenase-1; KO, SPAK−/−; NaPi2, Na+/Pi cotransporter isoform 2; NBCe1, Na+/HCO3− cotransporter 1; NHE3, Na+/H+ exchanger isoform 3; NHE3pS552, Na+/H+ exchanger isoform 3 phosphorylated at Ser 552; NHERF1, Na+/H+ exchanger regulatory factor 1; NKA, Na+,K+ - ATPase α1 and β1 subunits; NKCC2, Na+-K+- 2 Cl− cotransporter isoform 2; NKCC2pT96T101, NKCC2 phosphorylated at Thr 96 and 101; SPAK, STE20/SPS1-related proline alanine- rich kinase.
Does higher fractional delivery from PT provoke higher transporter abundance along the medullary thick ascending loop of Henle (mTAL) in females? In mTAL, Na+ reabsorption is mediated primarily by apical Na+-K+-2Cl− cotransporter isoform 2 (NKCC2), which can be phosphorylated and activated (NKCC2pT96T101, termed NKCC2p) by the kinase SPAK (Ste20/SPS-1 related proline-alanine rich kinase); mTAL reabsorption is also mediated by apical NHE3 and basolateral Na,K-ATPase (NKA α1 and NKA β1 subunits) drives transepithelial transport. Although there was a tendency for increased Na,K-ATPase subunits and NKCC2p (P>0.15), none of these transporters exhibited a pronounced sexual dimorphism in medulla (Figure 2C). Figure 2D provides a summary profile of proteins expressed from the PT through mTAL that indicates (for females): relatively inactivated NHE3 (higher NHE3pS552), and lower abundance of NaPi2, Cldn2, AQP1, villin, myosins, and megalin, all of which support the lower fractional reabsorption in PT and higher volume flow out of the PT (Figure 1). Immunoblot findings do not provide evidence for a compensatory higher level of transporters in mTAL in females; previous studies in perfused tubules (in males) indicate the potential for both flow-mediated increase51 and inhibition52 of mTAL transport rates.
Profiles of Distal Tubule to CD Transporters and Channels in Female versus Male Rats
Figure 3A summarizes results from cortical loop of Henle (cTAL) and distal convoluted tubule (DCT): As in mTAL, there was an insignificant tendency for higher NKCC2p in cTAL in females (P=0.08). As previously reported,24,27 this study confirms that DCT NCC total, NCCpT53, and NCCpS71 (both termed NCCp) are 60%, 200%, and 230% more abundant in females versus males (Figure 3A); full-length SPAK tended to be lower and the dominant negative KS-SPAK was 60% higher (SPAKp in rat cortex was not detectable). Increased delivery from proximal nephron and activation of NCC in females suggests the possibility of a potassium-deficient state.53 Claudin-7 (Cldn7), which likely facilitates Cl− transport through tight junctions from macula densa through CD,39 is 40% more abundant in females than males. In comparison, claudin-10 (Cldn10), expressed all along the nephron, was not different in females versus males (region-specific differences are possible).
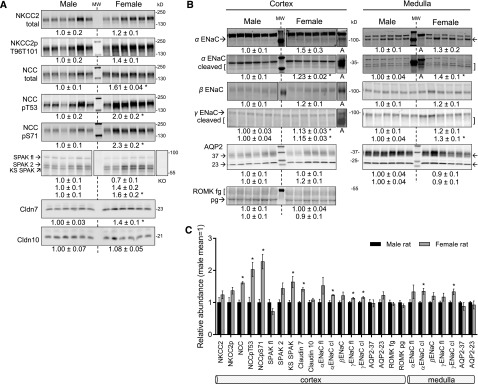
Figure 3.
Sexual dimorphic pattern of transporters, channels and regulator proteins along the distal tubule and collecting duct. Immunoblots of renal homogenates. Both 1 and 1/2 amounts of protein were loaded (only one amount is displayed), see antibodies in Supplemental Table 1 for details. Density values, normalized to male group=1.0, displayed as mean±SEM (n=6). *P<0.05 versus male. (A) Renal cortex samples probed for thick ascending limb and distal tubule cotransporters, kinases, and claudins. (B) Renal cortex and medulla samples probed for CD channels; “A” indicates samples from AngII-infused rats, which possess more abundant levels of activated ENaC subunits. (C) Profile of transporters, channels, claudins, and regulatory kinases expressed along the distal nephron and CD summarized from (A and B). AQP2–23, -37, aquaporin 2 forms with apparent mobilities of −23 and −37 kD; Cldn10, claudin 10; ENaC, epithelial Na+ channel; fl, full length; KO, SPAK−/−; KS-SPAK, kidney specific SPAK; NCC, Na+- Cl− cotransporter; NCCpT53 and NCCpS71, NCC phosphorylated at Thr 53 and Ser 71, respectively; ROMK, renal outer medullary K+ channel partial glycosylated (pg) and fully glycosylated (fg) forms; SPAK, STE20/SPS1-related proline alanine- rich kinase.
ENaC, expressed from the late DCT through the connecting segment and cortical CD, is composed of α, β, and γ subunits; proteolytic processing of α and γ subunits are markers for apical membrane channel activation.54,55 Full-length α ENaC subunit (weak signals in cortex) tended to be more abundant by 50% and 30% in female cortex and medulla, respectively (P=0.1–0.2). The cleaved (activated) form of α ENaC was significantly more abundant in females by 23% and 40% in cortex and medulla, respectively. There was a tendency for higher cortical and medullary β ENaC abundance in females (P=0.12). Full-length and cleaved (activated) γ ENaC subunit was 1.13- and 1.15-fold more abundant in female cortex, respectively; in medulla, γ ENaC cleavage was greater by 30% (Figure 3B). Taken together, these findings suggest activation of ENaC in females relative to males. Although greater ENaC cleavage could drive K+ secretion, K+ loss, and provoke NCC phosphorylation,56 no sexual dimorphism in renal outer medullary K+ channel (ROMK) abundance was evident (Figure 3B, apical distribution not determined). AQP2, controlling water reabsorption across the CD principal cells,57 presents as two bands of similar abundance in males and females (Figure 3B, apical distribution not determined). The profile of protein abundance from cTAL through medullary CD (Figure 3C) indicates significant activation of both NCC and ENaC as well as Cldn7 in female versus male rats, a pattern that could facilitate distal salt reabsorption in response to the higher volume flow from the proximal nephron and drive increased K+ secretion.
NCC Regulation by Potassium in Females
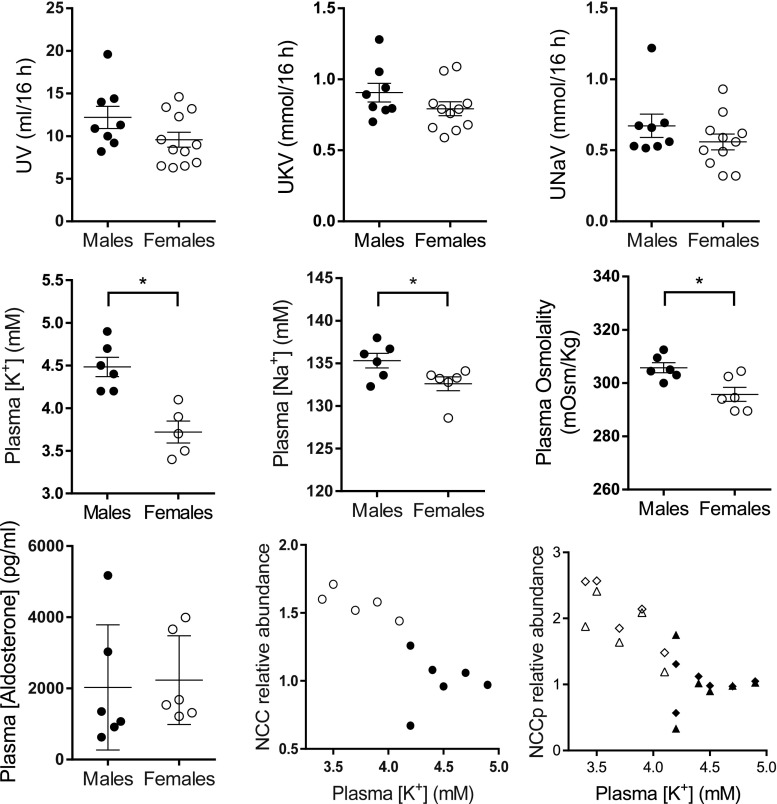
NCC activation in females versus males could reflect compensation for greater Na+ delivery from the proximal nephron, response to prevent K+ loss by reducing Na+ delivery to ENaC,56 and/or sex hormone regulation.24 We tested the hypothesis that K+ homeostasis was different in females than males by fasting rats overnight followed by a 0% K+ meal. Shown in Figure 4, no significant differences were detected in the overnight urinary output rate (UV), urine K+ (UKV), or urine Na+ (UNaV), reflecting similar intakes between sexes; measured food consumption during the 3-hour 0% K meal was similar (14±1 versus 12±1 g/3 h). Despite similar intakes and outputs, plasma [K+] was 18% lower in females than males (3.7±0.1 versus 4.5±0.1 mM), whereas plasma [Na+] (132.6±0.8 versus135.3±0.9 mM) and osmolality (296±3 versus 306±2 mOsm/Kg) were lower by only 2%–3%, confirming an earlier study.58 Plasma aldosterone levels were similar between sexes (2028±718 versus 2233±510 pg/ml) and there was no linear relationship with plasma [K+] (r=0.007, P=0.80). Across male and female rats at baseline, an inverse relationship is evident between plasma [K+] and NCC (r=0.62, P=0.004), NCCpT53 (r=0.50, P=0.01), and NCCpS71 (r=0.72, P<0.001) (Figure 4), supporting the notion that elevated NCCp in females is related to the lower baseline plasma [K+] as reported previously in male rats consuming altered K+ diets.59,60
Figure 4.
Physiologic correlates of sex-specific differences in renal transporters. Rats were fasted overnight with water, then fed a meal containing 0% K+ for 3 hours. Overnight, urine was collected in metabolic cages. At 3 hours, plasma was collected. Aldosterone was measured in plasma. Values represent individual measurements and mean±SEM (n=5–12). *P<0.05 versus males. The last two panels summarize abundance of renal cortical NCC and NCCp from Figure 3A as a function of plasma [K+]. Female rats (○, ◇, △) exhibit higher NCC and NCCp abundance along with lower plasma [K+] than male rats (●, ◆, ▲); NCC (●, ○), NCCpS71 (◇, ◆), NCCpS53 (▲, △). UKV, urinary K+ excretion; UNaV, urinary Na+ excretion; UV, urinary volume.
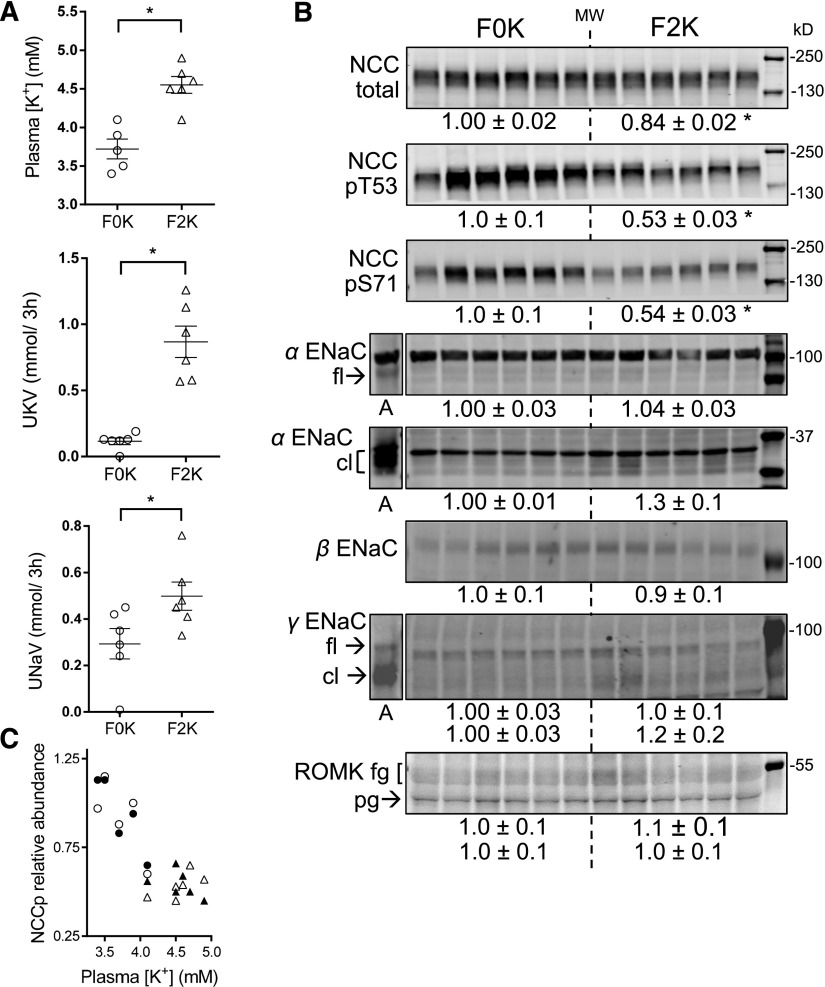
To assess whether the females defend this lower plasma [K+], we fed them a K+-rich meal. We previously reported that when male rats were fasted overnight and then fed a 3-hour meal containing either 0% or 2% K+, plasma [K+] reached 4.01±0.12 and 5.18±0.16 mM, respectively, and NCCp decreased 50% after the 2% K+ meal.59 In females subjected to identical protocols, plasma [K+] reached 3.7±0.1 and 4.6±0.1 mM, respectively (Figure 5A). Both UKV and UNaV rose after the 2% K+ meal in females (0.9±0.1 versus 0.11±0.03 mmol/3 h; 0.5±0.1 versus 0.3±0.1 mmol/3 h, respectively), demonstrating that females defend their lower plasma potassium set point with robust kaliuretic and natriuretic responses to a K+-rich meal. The K+-provoked natriuresis can be attributed to reduced proximal Na+ reabsorption61,62 as well as to NCC activity suppression. As in the males,59 the response was accompanied by decreases in abundance of NCC total (15%) and NCCp (50%) (Figure 5B). Delivery of more Na+ from the proximal nephron coupled to reduced DCT Na+ reabsorption shifts Na+ downstream to ENaC where activating cleavage of α and γ subunits is higher in females consistent with increased driving force for K+ secretion.53,63 An inverse relationship is evident between plasma [K+] and NCCpT53 (r=0.63, P=0.004) and NCCpS71 (r=0.83, P<0.001) (Figure 5C). No significant acute changes in ENaC or ROMK abundance were detected after the 2% K+ meal.
Figure 5.
Females respond to 3-hour 2% K meal with kaliuresis and reduced NCCp. Rats were fasted overnight and then fed either 2% K diet (F2K) or 0% KF0K diet in metabolic cages for 3 hours before they were anesthetized for blood and tissue collection and euthanized. (A) Plasma [K+], UKV (urinary K+ excretion), and UNaV (urinary Na+ excretion) increase in response to a K+-rich meal. (B) Immunoblots of renal cortex homogenates demonstrate that NCC and NCCp abundance decrease in response to a K+-rich meal. Both 1 and 1/2 amounts of protein were loaded (only one amount is displayed), see antibodies in Supplemental Table 1 for details. Density values, normalized to F0K=1.0, displayed as mean±SEM (n=6). *P<0.05 versus F0K group. (C) Abundance of renal cortical NCCp plotted as a function of plasma [K+] demonstrates that 2% K+-rich meal (△, ▲), versus 0% K+ meal (○, ●), increases plasma [K+] and depresses NCCpT53 (○, △) and NCCpS71 (●, ▲). cl, cleaved; fg, fully glycosylated; fl, full length; pg, partially glycosylated.
Sexual Dimorphism along the Mouse Nephron
To determine whether the sexual dimorphic differences identified in rats were also evident in mice, C57BL/6 mice were studied. A profile of transporters and regulators probed is summarized in Figure 6; methods and data are provided in Supplemental Figures 4–8. Along the PT, NHE3, NHE3p, Cldn2, villin, and myosin VI were significantly lower in abundance in females versus males by 20%–40%. Unlike rats, AQP1 was 40% more abundant and medullary NKA α1 was 20% lower in females (Figure 6A, blots in Supplemental Figure 4, A and B). Along the distal nephron, cortical NKCC2, NKCC2p, NCC, NCCp, SPAK, SPAK 2, and Cldn7 were more abundant in females versus males; unlike rats, there were no significant differences in CD ENaC subunits’ abundance or cleavage (Figure 6B, Supplemental Figure 5). Overall, similarities between female rats and mice include less proximal transporters (less NHE3 in mouse, more NHE3p in rat), Cldn2, villin, and myosin VI (Figures 2D and 6A), and more abundant transporters past the mTAL (more NKCC, NCC, and Cldn7 in mice, and more NCC, ENaC, and Cldn7 in rats) versus males (Figures 3C and 6B). In contrast, PT AQP1 (−35 kD) was 40% higher in female mice and 60% lower in female rats.
Figure 6.
Sexual dimorphic pattern of transporters, channels and regulators along the nephron in C57BL/6 mice, summarized from Supplemental Figures 4 and 5. (A) Renal cortex and medulla samples probed for proximal tubule and thick ascending limb proteins. (B) Renal cortex and medulla samples probed for DCT, connecting tubule, and CD proteins. Mean±SEM.
Unlike rats, plasma [K+] and [Na+] values overlapped in female and male mice after overnight fast and there was no relationship between plasma [K+] and NCCp across sexes (Supplemental Figure 6). Overnight UV, UKV, and UNaV were lower in females than males, likely reflecting less intake (Supplemental Figures 6 and 7). In response to a 2% (versus 0%) K+ meal (Supplemental Figure 7), plasma [K+] and UKV increased, and NCC and NCCp were reduced 50%–70% (Supplemental Figure 7B), as previously reported in males.64
Unlike rats, both sexes of mice excreted Na+ and volume at similar rates in response to a saline challenge (Supplemental Figure 8). Because the female mice had 30% less Cldn2 than males, we examined the response in Cldn2-deficient mice.40 By 5 hours after injection, the Cldn2 KO of both sexes excreted between 25% and 35% of the injected sodium and most of the volume load. Thus, no obvious sex difference in the excretion of a saline load was observed in mice of either sex, with or without Cldn2 with this strategy.
Discussion
Differences between female and male nephron organization and physiologic function are evident from our analyses: lower Na+ and water transporters and lower fractional reabsorption in the proximal nephron coupled with more abundant transporters in the distal nephron of female versus male rats, and similar profiles in mice. This pattern could facilitate the fluid retention adaptations required of pregnancy and lactation.3 Independently, the lower proximal fractional reabsorption and more rapid excretion of a saline challenge suggest that female rats will maintain salt balance at lower BP when faced with antinatriuretic stimuli or injury. The more robust natriuresis in response to a saline challenge in female rats is consistent with previous reports that Na+ and volume excretion are lower in male than female rats at similar renal perfusion pressures,65 that androgens stimulate proximal tubule reabsorption66 and distal ENaC expression,67 and that the pressure-natriuresis curve is shifted rightward in male versus female rodents.18
Implicating the PT in the more efficient natriuresis and diuresis, CLi a marker of volume flow from the proximal tubule,35 is twice as high in female rats (Figure 1F). As predicted from greater clearance at baseline, female rats have lower plasma [Li+] versus that measured in males (0.23±0.01 versus 0.36±0.01 mEq; P=0.002). Increased flow out of the PT provides an error signal, sensed at the macula densa, to increase afferent arteriole resistance to reduce GFR. Although not measured in this study, it has been previously reported that female rats present lower GFR and higher renal vascular resistance compared with males.68,69 The abundance of podocin, a glomerular protein, was 35% lower in cortical homogenate of females versus males (Supplemental Figure 3A).
HCO3− reabsorption from PT luminal fluid is a function of H+ secretion via apical NHE3. In male mice with a targeted deletion of renal proximal tubule NHE3, JHCO3− is reduced 35% and volume reabsorption reduced 27%.70 The similar differences in JHCO3− between females versus males and wild type versus NHE3 knockout males suggest significantly lower NHE3 activity in female versus male rats. Although total NHE3 was not different, NHE3pS552, a marker for NHE3 distribution to the base of the microvilli, was 30% greater in females (Figure 2A), and immunohistochemistry confirmed more basal NHE3 (Figure 1C). Brasen et al.33 recently modeled and measured NHE3 activity in male rats and concluded that NHE3 clustered at the base of the microvilli creates local unfavorable pH microdomains that significantly reduce NHE3 activity indicating the importance of covalent modification and transporter redistribution to NHE3 activity. Both the paracellular NaCl transporter Cldn2 and the apical and basolateral water channel AQP1 were 60% lower in females (Figure 2), and may facilitate the more rapid excretion of a saline load.
Maintaining a reserve pool of NHE3 at the base of the villi could provide a mechanism to rapidly increase Na+ reabsorption by redistribution of NHE3 into the body of the microvilli (e.g., during pregnancy, lactation, AngII stimulation), a hypothesis that remains to be tested.3,21 Likewise, the transporter responses of females to stimuli that, in males, redistribute NHE3 and NaPi2 to the microvillar base (acute hypertension, ACE inhibition, or PTH)21 or into the microvilli (AngII, sympathetic stimulation), remain to be determined. Although specific probes for AngII receptors are lacking, we assessed abundance of angiotensin-converting enzyme (ACE1) and angiotensin-converting enzyme 2 (ACE2) in cortical homogenates of both sexes of rats. Although it is reported that females have more Ang 1–7 and AT2R influence than males,71 abundance of ACE1 was 40% higher and that of ACE2 was 60% lower in females compared with males (Supplemental Figure 3A).
NHE3 activity also contributes titratable acids (TA) to the tubule lumen. In spite of lower NHE3 activity in the cortex (not evident in medulla), urinary titratable acidity (UTAV) was higher in females than males (Supplemental Figure 3B), suggesting other pathways for H+ secretion may be higher in females. It is conceivable that higher UTAV could be due to lower NaPi2-mediated inorganic phosphate (Pi) reabsorption, which is a main buffer of secreted H+. Indeed, NaPi2 abundance was 25% lower in females; however, there was no significant increase in urinary phosphate excretion (UPiV, Supplemental Figure 3B). These findings indicate that sexual dimorphic handling of acid and phosphate along the nephron warrant further in-depth analyses.
In contrast to rats, female mice did not excrete a saline challenge more rapidly than males (Supplemental Figure 8), despite 40% lower total NHE3 and villin and 30% lower Cldn2 (Supplemental Figure 4). NHE3 does appear to be distributed to the microvillar base in female mice (Supplemental Figure 4C), even though the NHE3pS552/NHE3 total ratio is unchanged. JHCO3− and CLi remain to be determined in female versus male mice. Lower Cldn2 does not seem to be directly involved in the time course of response to saline challenge (Supplemental Figure 8). An intriguing sex-specific difference is AQP1 abundance: the 35 kD glycosylated band is 60% lower in female rats (Figure 2A, confirming a recent report in Wistar rats),42 associated with more rapid excretion of a saline load, and is 40% higher in female mice (Supplemental Figure 4A), associated with no difference in excretion of a saline load.
We hypothesized that the TAL Na+ transporters would be more abundant in females to compensate for greater delivery from PT. No significantly higher abundance was evident in female versus male mTAL transporters (Figure 2C) in either rats or mice. Interestingly, a recent study in Cldn2 knockout mice detected compensatory upregulation of mTAL transport activity without an accompanying increase in NKCC2 or NKCC2p abundance.40
A downstream shift in Na+ reabsorption has metabolic consequences because it is more energetically favorable to reabsorb NaCl across the leaky PT, facilitated by the Cldn2 route,40 than along the tighter TAL, DCT, and CD.72 Lower fractional reabsorption of Na+ and HCO3− in female rats (Figure 1E) predicts a sexual dimorphism in oxygen utilization may exist in rats. Heme oxygenase–1 (HO-1) abundance, a marker of a beneficial adaptation to tissue stress,73 was 20% higher in female cortex and insignificantly higher (albeit 2.5-fold greater mean) in female medulla (Figure 2, A and C). This enzyme is induced in proximal tubules, glomeruli, and renal interstitium in response to oxidative stress and pathologic conditions,74–76 protecting renal medullary cells or tissues from ischemic injury or oxidant stress.
Further along the nephron in the cortical TAL, there was 40% greater NKCCp in female mice and a similar elevation in rats that did not reach significance (P=0.08), in agreement with a previous report of higher female NKCC2.77 In DCT, the previously reported higher female NCC and NCCp was evident in both mice and rats. Kaissling and Stanton78 determined that the distal cell volumes were increased when Na+ delivery was increased beyond the TAL. Recently, a study similarly concluded that higher NCC abundance in mice lacking angiotensin 1 receptors was due to increased NaCl delivery.79 Chen et al.23 showed that the urinary response to thiazides and binding of labeled metolazone to renal cortex was higher in female than in male rats. Higher NCC and NCCp abundance have been reported in intact female mice,24 and enhanced in ovariectomized female rats after estradiol administration.22 Female rats also displayed greater cleavage of α and γ ENaC subunits, not evident in mice. ENaC cleavage increases channel activity and amiloride-sensitive natriuresis.54,80,81 Cldn7, proposed to act as a Cl− pore providing the counter ion to Na+ transport via ENaC,39 is 40% more abundant in females (both rat and mouse). The distal nephron apical water channel AQP2 was similarly expressed in cortex and medulla of male and female rats (higher AQP2 23 kD band in female cortex). Taken together, these findings suggest that less fractional reabsorption of Na+ and H2O in PT drives higher fractional reabsorption along the distal nephron.
The lower plasma [K+] in female versus male rats could be driven by the greater ENaC subunit cleavage/activation, as we have recently described in AngII-infused male rats.56 The linear relationship between the lower plasma [K+] and higher NCCp in female and male rats suggests activation of NCCp in females may be a compensatory response to reduce Na+ delivery to ENaC where it drives K+ secretion. At baseline, females and males are reported to have similar aldosterone levels,82 confirmed in our assays (Figure 4). Direct measure of ENaC channel activity in split open tubules of both sexes would be necessary to make the connection between the ENaC activation, plasma [K+], and NCCp in the female rat. Previous studies have also reported lower plasma [K+] in female rats: A 1977 study in approximately 300 rats of each sex reported very similar values to ours,58 and Riazi et al.26 reported that ovariectomy raised plasma K+ which was reduced by estrogen replacement, implicating an estrogen dependency of the plasma [K+] set point. The female rats appear to defend the lower plasma [K+] as they exhibited robust kaliuretic and natriuretic responses accompanied by approximately 50% decreases in NCCp in response to a single K+-rich meal (Figure 5). This finding predicts there is a sexual dimorphism in the signaling cascade(s) that connects an increase in plasma [K+], detected by the Kir 4.1/5.1 heteromer in the DCT basolateral membrane, to NCC dephosphorylation.83,84 Females may actively adapt to maintain a lower plasma K+ set point to protect from hyperkalemia during pregnancy, when the dams must consume a great deal of food to support growing fetuses. In fact, despite high circulating aldosterone levels in late pregnancy, NCCp is reduced, likely due to the high dietary K+ intake.85
In summary, this report provides an overview of the sexual dimorphic characteristics of renal transporters and related physiologic differences. These baseline findings will be useful to investigators probing effects of gene manipulations or various pathologic stimuli in the two sexes. Some of the differences likely contribute to the “female advantage” observed before menopause.27,86,87 The effect of sex steroids, age, and renal hemodynamics on the profiles are missing pieces that will be important for understanding the genesis and consequences of the differences. Differences observed between sexes not discussed but warranting further investigation include: lower NaPi2 and higher levels of its regulator NHERF1, whether higher NBCe1 compensates for lower NHE3 to drive HCO3− reabsorption,38 the effect of lower megalin on protein transcytosis, and of higher DPPIV on incretin metabolism and NHE3 activation.44,88
Concise Methods
Animals and Metabolic Protocols
All animal procedures were approved by the Institutional Animal Care and Use Committee of the Keck School of Medicine of the University of Southern California and were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Experiments were performed on male and female Sprague Dawley rats (200–225 g) purchased from Harlan Laboratories (San Diego, CA). Rats were weighed and placed in metabolic cages (Tecniplast, Italy) for acclimation at 3:00 p.m. with free access to food and water. At 3:00 p.m. the following day, food was removed. Fasting overnight urine was collected from 3:00 p.m. to 7:00 a.m. Between 7:00 and 10:00 a.m. rats were provided free access to a meal containing 0.74% NaCl and no potassium (to avoid NCC downregulation59). In parallel, K+ adaptation responses were studied in rats provided a meal containing 4% KCl (approximately 2% K). Urine was collected during the 3 hours and pooled with bladder urine collected after euthanasia.59 After the meal, rats were anesthetized intramuscularly with 1:1 volume ratio of ketamine (40 mg/kg, Phoenix Pharmaceuticals, St. Joseph, MO) and xylazine (8 mg/kg, Lloyd laboratories, Shenandoah, IA). A blood sample was collected from the tail (avoiding hemolysis). Kidneys were rapidly removed, dissected into cortex and medulla, and homogenized as described,56 and detailed in the Supplemental Material. Blood was collected by cardiac puncture after kidney collection.
Saline Challenge
The diuretic and natriuretic responses to extracellular fluid volume expansion were examined in a second set of unfasted male and female rats. At 10:00 a.m. rats were acutely anesthetized with isoflurane, injected i.p. with a volume of 37°C 0.9% NaCl equivalent to 6%–7% of their body weight (v/w), and placed immediately in metabolic cages without food where they quickly woke up. Urine volume, collected hourly over 5 hours, was measured with a graduated pipette, [Na+] measured by flame photometry (Cole-Parmer, model 2655–10), and excretion results were expressed as the percentage of the Na+ and volume load injected.
Immunofluorescence
As previously described32 and detailed in the Supplemental Material, rats and mice were fasted for 3–4 hours, anesthetized, and infused with 4% BSA in 0.9% saline to maintain euvolemia. The left kidney was decapsulated, placed in a Plexiglas cup, bathed in situ with ice-cold PLP fixative, removed, bisected, postfixed in PLP fixative, and processed as described in detail in the Supplemental Material. Results shown are representative of three sets of male and female rats or mice examined.
Stationary in vivo Microperfusion
In vivo microperfusion experiments were carried out essentially as described previously89 and detailed in the Supplemental Material. In brief, tubules were perfused using a double-barreled micropipette: one side was filled with Sudan black-colored castor oil, the other with the luminal perfusion solution colored with 0.05% FD and C green. The intratubular pH was measured as the voltage difference between the two asymmetric sides of the H+ ion-sensitive microelectrode: the larger barrel contained an H+-sensitive ion-exchange resin (Fluka Chemika, Buchs, Switzerland), and the smaller barrel contained 1 mM KCl colored by FD and C green (reference barrel). Net bicarbonate reabsorption (JHCO3−) was measured by injecting a droplet of the luminal perfusion solution between the oil columns and following the intratubular pH changes toward the steady-state level ([pH]s).
Body Fluid Measurements
Urine volumes were measured with pipettes, urinary and plasma [Na+], [K+], and [Li+] were measured by flame photometry, and osmolality with a Precision Systems µ-Osmette. CLi,35 an inverse measure of proximal Na+ reabsorption, was calculated in the usual manner as urinary [Li+] × urine output/plasma [Li+]. Plasma aldosterone was measured by ELISA using a commercial kit (catalog # 501090, Cayman Chemical).
During terminal anesthesia, vaginal smears were obtained by lavage as described,90 and stage of estrous cycle evaluated by microscopy with 10× objective (Nikon TMS, Japan).
Quantitative Immunoblotting
As described,56 homogenates were denatured in SDS-PAGE sample buffer for 20 minutes at 60°C. Uniform protein concentration and loading were ascertained by resolving 12 µg of protein from each sample by SDS-PAGE, stained with Coomassie blue, and multiple random bands quantified and determined to be uniform (Supplemental Figure 1A).29,56 For blots, 1 and 1/2 amounts of protein were assayed to verify the linearity of detection system on each blot (Supplemental Figure 1B). Supplemental Table 1 catalogs the amounts assayed, antibodies, vendors, and dilutions. Blots were never stripped and reprobed. Signals were detected with the Odyssey Infrared Imaging System (LI-COR) and quantified by accompanying software. Arbitrary density units were normalized to mean intensity of control group, defined as 1.0. Because each sample was assayed twice (1 and 1/2 amounts), the normalized values were averaged and mean values compiled for statistical analysis.
Statistical Analyses
The differences between males and females were assessed by unpaired two-tailed t test, assuming unequal variance for immunoblot assessments, physiologic parameters, and microperfusion JHCO3− measurements. For saline test, two-way ANOVA followed by a post-test was used to analyze differences between groups. Statistical tests were calculated using GraphPad Prism 6.0 (San Diego, CA). Differences were regarded significant at P<0.05.
Disclosures
None.
Supplementary Material
Acknowledgments
This work is supported by National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) R01DK083785 and American Heart Association Grant in Aid Western States Affiliate 15GRNT23160003 to A.A.McD., NIDDK grant R01DK062283 to A.S.L.Y., and São Paulo Research Foundation grant # 2012/10146-0 to A.C.C.G.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017030295/-/DCSupplemental.
References
- 1.Miller VM, Reckelhoff JF: Sex as a biological variable: Now What?! Physiology (Bethesda) 31: 78–80, 2016 [DOI] [PubMed] [Google Scholar]
- 2.Maynard SE, Karumanchi SA, Thadhani R: Hypertension and Kidney Disease in Pregnancy. In: Brenner and Rector’s the Kidney, edited by Skorecki K, Chertow GM, Marsden PA, Taal MW, Yu AS, 10th Ed., Philadelphia, Elsevier, 2016 [Google Scholar]
- 3.West CA, Sasser JM, Baylis C: The enigma of continual plasma volume expansion in pregnancy: Critical role of the renin-angiotensin-aldosterone system. Am J Physiol Renal Physiol 311: F1125–F1134, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB; Writing Group Members; American Heart Association Statistics Committee; Stroke Statistics Subcommittee : Executive summary: Heart disease and stroke statistics--2016 update: A report from the American heart association. Circulation 133: 447–454, 2016 [DOI] [PubMed] [Google Scholar]
- 5.Aufhauser DD Jr, Wang Z, Murken DR, Bhatti TR, Wang Y, Ge G, Redfield RR 3rd, Abt PL, Wang L, Svoronos N, Thomasson A, Reese PP, Hancock WW, Levine MH: Improved renal ischemia tolerance in females influences kidney transplantation outcomes. J Clin Invest 126: 1968–1977, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanaka R, Yazawa M, Morikawa Y, Tsutsui H, Ohkita M, Yukimura T, Matsumura Y: Sex differences in ischaemia/reperfusion-induced acute kidney injury depends on the degradation of noradrenaline by monoamine oxidase. Clin Exp Pharmacol Physiol 44: 371–377, 2017 [DOI] [PubMed] [Google Scholar]
- 7.Reckelhoff JF: Gender differences in the regulation of blood pressure. Hypertension 37: 1199–1208, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Cobo G, Hecking M, Port FK, Exner I, Lindholm B, Stenvinkel P, Carrero JJ: Sex and gender differences in chronic kidney disease: Progression to end-stage renal disease and haemodialysis. Clin Sci (Lond) 130: 1147–1163, 2016 [DOI] [PubMed] [Google Scholar]
- 9.Seppi T, Prajczer S, Dörler MM, Eiter O, Hekl D, Nevinny-Stickel M, Skvortsova I, Gstraunthaler G, Lukas P, Lechner J: Sex differences in renal proximal tubular cell homeostasis. J Am Soc Nephrol 27: 3051–3062, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodríguez-Gómez I, Wangensteen R, Pérez-Abud R, Quesada A, Del Moral RG, Osuna A, O’Valle F, de Dios Luna J, Vargas F: Long-term consequences of uninephrectomy in male and female rats. Hypertension 60: 1458–1463, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Xue B, Pamidimukkala J, Hay M: Sex differences in the development of angiotensin II-induced hypertension in conscious mice. Am J Physiol Heart Circ Physiol 288: H2177–H2184, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Zimmerman MA, Sullivan JC: Hypertension: What’s sex got to do with it? Physiology (Bethesda) 28: 234–244, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gillis EE, Sullivan JC: Sex differences in hypertension: Recent advances. Hypertension 68: 1322–1327, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellison KE, Ingelfinger JR, Pivor M, Dzau VJ: Androgen regulation of rat renal angiotensinogen messenger RNA expression. J Clin Invest 83: 1941–1945, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quigley R: Androgens stimulate proximal tubule transport. Gend Med 5[Suppl A]: S114–S120, 2008. 18395677 [Google Scholar]
- 16.Arnold AP, Chen X: What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front Neuroendocrinol 30: 1–9, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maranon R, Reckelhoff JF: Sex and gender differences in control of blood pressure. Clin Sci (Lond) 125: 311–318, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mirabito KM, Hilliard LM, Kett MM, Brown RD, Booth SC, Widdop RE, Moritz KM, Evans RG, Denton KM: Sex- and age-related differences in the chronic pressure-natriuresis relationship: Role of the angiotensin type 2 receptor. Am J Physiol Renal Physiol 307: F901–F907, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Ivy JR, Bailey MA: Pressure natriuresis and the renal control of arterial blood pressure. J Physiol 592: 3955–3967, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McDonough AA, Nguyen MT: Maintaining balance under pressure: Integrated regulation of renal transporters during Hypertension. Hypertension 66: 450–455, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDonough AA: ISN forefronts symposium 2015: Maintaining balance under pressure-hypertension and the proximal tubule. Kidney Int Rep 1: 166–176, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verlander JW, Tran TM, Zhang L, Kaplan MR, Hebert SC: Estradiol enhances thiazide-sensitive NaCl cotransporter density in the apical plasma membrane of the distal convoluted tubule in ovariectomized rats. J Clin Invest 101: 1661–1669, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Z, Vaughn DA, Fanestil DD: Influence of gender on renal thiazide diuretic receptor density and response. J Am Soc Nephrol 5: 1112–1119, 1994 [DOI] [PubMed] [Google Scholar]
- 24.Rojas-Vega L, Reyes-Castro LA, Ramírez V, Bautista-Pérez R, Rafael C, Castañeda-Bueno M, Meade P, de Los Heros P, Arroyo-Garza I, Bernard V, Binart N, Bobadilla NA, Hadchouel J, Zambrano E, Gamba G: Ovarian hormones and prolactin increase renal NaCl cotransporter phosphorylation. Am J Physiol Renal Physiol 308: F799–F808, 2015 [DOI] [PubMed] [Google Scholar]
- 25.Riazi S, Madala-Halagappa VK, Hu X, Ecelbarger CA: Sex and body-type interactions in the regulation of renal sodium transporter levels, urinary excretion, and activity in lean and obese Zucker rats. Gend Med 3: 309–327, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Riazi S, Maric C, Ecelbarger CA: 17-beta Estradiol attenuates streptozotocin-induced diabetes and regulates the expression of renal sodium transporters. Kidney Int 69: 471–480, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Tiwari S, Li L, Riazi S, Halagappa VK, Ecelbarger CM: Sex and age result in differential regulation of the renal thiazide-sensitive NaCl cotransporter and the epithelial sodium channel in angiotensin II-infused mice. Am J Nephrol 30: 554–562, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tiwari S, Li L, Riazi S, Halagappa VK, Ecelbarger CM: Sex differences in adaptive downregulation of pre-macula densa sodium transporters with ANG II infusion in mice. Am J Physiol Renal Physiol 298: F187–F195, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDonough AA, Veiras LC, Minas JN, Ralph DL: Considerations when quantitating protein abundance by immunoblot. Am J Physiol Cell Physiol 308: C426–C433, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heer M, Baisch F, Kropp J, Gerzer R, Drummer C: High dietary sodium chloride consumption may not induce body fluid retention in humans. Am J Physiol Renal Physiol 278: F585–F595, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Weinstein SW, Szyjewicz J: Single-nephron function and renal oxygen consumption during rapid volume expansion. Am J Physiol 231: 1166–1172, 1976 [DOI] [PubMed] [Google Scholar]
- 32.Carneiro de Morais CP, Polidoro JZ, Ralph DL, Pessoa TD, Oliveira-Souza M, Barauna VG, Rebouças NA, Malnic G, McDonough AA, Girardi AC: Proximal tubule NHE3 activity is inhibited by beta-arrestin-biased angiotensin II type 1 receptor signaling. Am J Physiol Cell Physiol 309: C541–C550, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brasen JC, Burford JL, McDonough AA, Holstein-Rathlou NH, Peti-Peterdi J: Local pH domains regulate NHE3-mediated Na+ reabsorption in the renal proximal tubule. Am J Physiol Renal Physiol 307: F1249–F1262, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kocinsky HS, Dynia DW, Wang T, Aronson PS: NHE3 phosphorylation at serines 552 and 605 does not directly affect NHE3 activity. Am J Physiol Renal Physiol 293: F212–F218, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Thomsen K, Shirley DG: The validity of lithium clearance as an index of sodium and water delivery from the proximal tubules. Nephron 77: 125–138, 1997 [DOI] [PubMed] [Google Scholar]
- 36.Leong PK, Zhang Y, Yang LE, Holstein-Rathlou NH, McDonough AA: Diuretic response to acute hypertension is blunted during angiotensin II clamp. Am J Physiol Regul Integr Comp Physiol 283: R837–R842, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Leyssac PP, Christensen P: A comparison between endogenous and exogenous lithium clearance in the anaesthetized rat. Acta Physiol Scand 151: 173–179, 1994 [DOI] [PubMed] [Google Scholar]
- 38.Kurtz I, Zhu Q: Structure, function, and regulation of the SLC4 NBCe1 transporter and its role in causing proximal renal tubular acidosis. Curr Opin Nephrol Hypertens 22: 572–583, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu AS: Claudins and the kidney. J Am Soc Nephrol 26: 11–19, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pei L, Solis G, Nguyen MT, Kamat N, Magenheimer L, Zhuo M, Li J, Curry J, McDonough AA, Fields TA, Welch WJ, Yu AS: Paracellular epithelial sodium transport maximizes energy efficiency in the kidney. J Clin Invest 126: 2509–2518, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Magni F, Chinello C, Raimondo F, Mocarelli P, Kienle MG, Pitto M: AQP1 expression analysis in human diseases: Implications for proteomic characterization. Expert Rev Proteomics 5: 29–43, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Herak-Kramberger CM, Breljak D, Ljubojević M, Matokanović M, Lovrić M, Rogić D, Brzica H, Vrhovac I, Karaica D, Micek V, Dupor JI, Brown D, Sabolić I: Sex-dependent expression of water channel AQP1 along the rat nephron. Am J Physiol Renal Physiol 308: F809–F821, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Girardi AC, Fukuda LE, Rossoni LV, Malnic G, Rebouças NA: Dipeptidyl peptidase IV inhibition downregulates Na+ - H+ exchanger NHE3 in rat renal proximal tubule. Am J Physiol Renal Physiol 294: F414–F422, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Girardi AC, Knauf F, Demuth HU, Aronson PS: Role of dipeptidyl peptidase IV in regulating activity of Na+/H+ exchanger isoform NHE3 in proximal tubule cells. Am J Physiol Cell Physiol 287: C1238–C1245, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Blaine J, Weinman EJ, Cunningham R: The regulation of renal phosphate transport. Adv Chronic Kidney Dis 18: 77–84, 2011 [DOI] [PubMed] [Google Scholar]
- 46.He P, Zhao L, No YR, Karvar S, Yun CC: The NHERF1 PDZ1 domain and IRBIT interact and mediate the activation of Na+/H+ exchanger 3 by ANG II. Am J Physiol Renal Physiol 311: F343–F351, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang LE, Maunsbach AB, Leong PK, McDonough AA: Redistribution of myosin VI from top to base of proximal tubule microvilli during acute hypertension. J Am Soc Nephrol 16: 2890–2896, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Blaine J, Okamura K, Giral H, Breusegem S, Caldas Y, Millard A, Barry N, Levi M: PTH-induced internalization of apical membrane NaPi2a: Role of actin and myosin VI. Am J Physiol Cell Physiol 297: C1339–C1346, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nielsen R, Christensen EI, Birn H: Megalin and cubilin in proximal tubule protein reabsorption: From experimental models to human disease. Kidney Int 89: 58–67, 2016 [DOI] [PubMed] [Google Scholar]
- 50.Maunsbach AB: Observations on the segmentation of the proximal tubule in the rat kidney. Comparison of results from phase contrast, fluorescence and electron microscopy. J Ultrastruct Res 16: 239–258, 1966 [DOI] [PubMed] [Google Scholar]
- 51.Morgan T, Berliner RW: A study by continuous microperfusion of water and electrolyte movements in the loop of Henle and distal tubule of the rat. Nephron 6: 388–405, 1969 [DOI] [PubMed] [Google Scholar]
- 52.Cabral PD, Garvin JL: Luminal flow regulates NO and O2(-) along the nephron. Am J Physiol Renal Physiol 300: F1047–F1053, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Terker AS, Zhang C, McCormick JA, Lazelle RA, Zhang C, Meermeier NP, Siler DA, Park HJ, Fu Y, Cohen DM, Weinstein AM, Wang WH, Yang CL, Ellison DH: Potassium modulates electrolyte balance and blood pressure through effects on distal cell voltage and chloride. Cell Metab 21: 39–50, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kleyman TR, Carattino MD, Hughey RP: ENaC at the cutting edge: Regulation of epithelial sodium channels by proteases. J Biol Chem 284: 20447–20451, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Palmer LG, Patel A, Frindt G: Regulation and dysregulation of epithelial Na+ channels. Clin Exp Nephrol 16: 35–43, 2012 [DOI] [PubMed] [Google Scholar]
- 56.Veiras LC, Han J, Ralph DL, McDonough AA: Potassium supplementation prevents sodium chloride cotransporter stimulation during angiotensin II hypertension. Hypertension 68: 904–912, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pearce D, Soundararajan R, Trimpert C, Kashlan OB, Deen PM, Kohan DE: Collecting duct principal cell transport processes and their regulation. Clin J Am Soc Nephrol 10: 135–146, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nachbaur J, Clarke MR, Provost JP, Dancla JL: Variations of sodium, potassium, and chloride plasma levels in the rat with age and sex. Lab Anim Sci 27: 972–975, 1977 [PubMed] [Google Scholar]
- 59.Rengarajan S, Lee DH, Oh YT, Delpire E, Youn JH, McDonough AA: Increasing plasma [K+] by intravenous potassium infusion reduces NCC phosphorylation and drives kaliuresis and natriuresis. Am J Physiol Renal Physiol 306: F1059–F1068, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Terker AS, Zhang C, Erspamer KJ, Gamba G, Yang CL, Ellison DH: Unique chloride-sensing properties of WNK4 permit the distal nephron to modulate potassium homeostasis. Kidney Int 89: 127–34, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brandis M, Keyes J, Windhager EE: Potassium-induced inhibition of proximal tubular fluid reabsorption in rats. Am J Physiol 222: 421–427, 1972 [DOI] [PubMed] [Google Scholar]
- 62.Weinstein AM: A mathematical model of the rat kidney: K(+)-induced natriuresis. Am J Physiol Renal Physiol 312: F925–F950, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Penton D, Czogalla J, Wengi A, Himmerkus N, Loffing-Cueni D, Carrel M, Rajaram RD, Staub O, Bleich M, Schweda F, Loffing J: Extracellular K(+) rapidly controls NaCl cotransporter phosphorylation in the native distal convoluted tubule by Cl(-) -dependent and independent mechanisms. J Physiol 594: 6319–6331, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sorensen MV, Grossmann S, Roesinger M, Gresko N, Todkar AP, Barmettler G, Ziegler U, Odermatt A, Loffing-Cueni D, Loffing J: Rapid dephosphorylation of the renal sodium chloride cotransporter in response to oral potassium intake in mice. Kidney Int 83: 811–824, 2013 [DOI] [PubMed] [Google Scholar]
- 65.Khraibi AA, Liang M, Berndt TJ: Role of gender on renal interstitial hydrostatic pressure and sodium excretion in rats. Am J Hypertens 14: 893–896, 2001 [DOI] [PubMed] [Google Scholar]
- 66.Quan A, Chakravarty S, Chen JK, Chen JC, Loleh S, Saini N, Harris RC, Capdevila J, Quigley R: Androgens augment proximal tubule transport. Am J Physiol Renal Physiol 287: F452–F459, 2004 [DOI] [PubMed] [Google Scholar]
- 67.Quinkler M, Bujalska IJ, Kaur K, Onyimba CU, Buhner S, Allolio B, Hughes SV, Hewison M, Stewart PM: Androgen receptor-mediated regulation of the alpha-subunit of the epithelial sodium channel in human kidney. Hypertension 46: 787–798, 2005 [DOI] [PubMed] [Google Scholar]
- 68.Munger K, Baylis C: Sex differences in renal hemodynamics in rats. Am J Physiol 254: F223–F231, 1988 [DOI] [PubMed] [Google Scholar]
- 69.Remuzzi A, Puntorieri S, Mazzoleni A, Remuzzi G: Sex related differences in glomerular ultrafiltration and proteinuria in Munich-Wistar rats. Kidney Int 34: 481–486, 1988 [DOI] [PubMed] [Google Scholar]
- 70.Li HC, Du Z, Barone S, Rubera I, McDonough AA, Tauc M, Zahedi K, Wang T, Soleimani M: Proximal tubule specific knockout of the Na+/H+ exchanger NHE3: Effects on bicarbonate absorption and ammonium excretion. J Mol Med (Berl) 91: 951–963, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hilliard LM, Nematbakhsh M, Kett MM, Teichman E, Sampson AK, Widdop RE, Evans RG, Denton KM: Gender differences in pressure-natriuresis and renal autoregulation: Role of the Angiotensin type 2 receptor. Hypertension 57: 275–282, 2011 [DOI] [PubMed] [Google Scholar]
- 72.Singh P, McDonough AA, Thomson SC: Metabolic basis of solute transport. In: Brenner and Rector’s The Kidney, 10th Ed., edited by Skorecki K, Chertow GM, Marsden PA, Taal MW, Yu AS, Philadelphia, Elsevier, 2016, pp 122–143 [Google Scholar]
- 73.Agarwal A, Bolisetty S: Adaptive responses to tissue injury: Role of heme oxygenase-1. Trans Am Clin Climatol Assoc 124: 111–122, 2013 [PMC free article] [PubMed] [Google Scholar]
- 74.Agarwal A, Nick HS: Renal response to tissue injury: Lessons from heme oxygenase-1 GeneAblation and expression. J Am Soc Nephrol 11: 965–973, 2000 [DOI] [PubMed] [Google Scholar]
- 75.Nath KA, Balla G, Vercellotti GM, Balla J, Jacob HS, Levitt MD, Rosenberg ME: Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. J Clin Invest 90: 267–270, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lever JM, Boddu R, George JF, Agarwal A: Heme oxygenase-1 in kidney health and disease. Antioxid Redox Signal 25: 165–183, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Musselman TM, Zhang Z, Masilamani SM: Differential regulation of the bumetanide-sensitive cotransporter (NKCC2) by ovarian hormones. Steroids 75: 760–765, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kaissling B, Stanton BA: Adaptation of distal tubule and collecting duct to increased sodium delivery. I. Ultrastructure. Am J Physiol 255: F1256–F1268, 1988 [DOI] [PubMed] [Google Scholar]
- 79.Li, J, Hatano, R, Xu, S, Wan, L, Yang, L, Weinstein, AM, Palmer, LG, Wang, T: Gender difference in electrolyte transport I: Role of AT1a receptor in thiazide sensitive Na-Cl cotransporter activity and expression in male and female mice [published online ahead of print May 31, 2017]. Am J Physiol Renal Physiol doi: 10.1152/ajprenal.00087.2017 [DOI] [PMC free article] [PubMed]
- 80.Svenningsen P, Friis UG, Bistrup C, Buhl KB, Jensen BL, Skøtt O: Physiological regulation of epithelial sodium channel by proteolysis. Curr Opin Nephrol Hypertens 20: 529–533, 2011 [DOI] [PubMed] [Google Scholar]
- 81.Picard N, Eladari D, El Moghrabi S, Planès C, Bourgeois S, Houillier P, Wang Q, Burnier M, Deschenes G, Knepper MA, Meneton P, Chambrey R: Defective ENaC processing and function in tissue kallikrein-deficient mice. J Biol Chem 283: 4602–4611, 2008 [DOI] [PubMed] [Google Scholar]
- 82.Bibeau K, Otis M, St-Louis J, Gallo-Payet N, Brochu M: Differential responses to salt supplementation in adult male and female rat adrenal glands following intrauterine growth restriction. J Endocrinol 209: 85–94, 2011 [DOI] [PubMed] [Google Scholar]
- 83.Penton D, Czogalla J, Wengi A, Himmerkus N, Loffing-Cueni D, Carrel M, Rajaram RD, Staub O, Bleich M, Schweda F, Loffing J: Extracellular K+ rapidly controls NaCl cotransporter phosphorylation in the native distal convoluted tubule by Cl- -dependent and -independent mechanisms. J Physiol 594: 6319–6331, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McDonough AA, Youn JH: Potassium homeostasis: The knowns, the unknowns, and the health benefits. Physiology (Bethesda) 32: 100–111, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.West CA, McDonough AA, Masilamani SM, Verlander JW, Baylis C: Renal NCC is unchanged in the midpregnant rat and decreased in the late pregnant rat despite avid renal Na+ retention. Am J Physiol Renal Physiol 309: F63–F70, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Oudar O, Elger M, Bankir L, Ganten D, Ganten U, Kriz W: Differences in rat kidney morphology between males, females and testosterone-treated females. Ren Physiol Biochem 14: 92–102, 1991 [DOI] [PubMed] [Google Scholar]
- 87.Baylis C: Sexual dimorphism in the aging kidney: Differences in the nitric oxide system. Nat Rev Nephrol 5: 384–396, 2009 [DOI] [PubMed] [Google Scholar]
- 88.Farah LX, Valentini V, Pessoa TD, Malnic G, McDonough AA, Girardi AC: The physiological role of glucagon-like peptide-1 in the regulation of renal function. Am J Physiol Renal Physiol 310: F123–F127, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Girardi AC, Titan SM, Malnic G, Rebouças NA: Chronic effect of parathyroid hormone on NHE3 expression in rat renal proximal tubules. Kidney Int 58: 1623–1631, 2000 [DOI] [PubMed] [Google Scholar]
- 90.Goldman JM, Murr AS, Cooper RL: The rodent estrous cycle: Characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol 80: 84–97, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.