Abstract
Carotenoids are plant pigment molecules that are potent antioxidants. Carotenoids cannot be synthesized de novo; therefore, their dietary intake and transport to various tissues are essential to harness their health benefits. Two of the three scavenger receptor class B (SRB) proteins, SR-B1 and CD36, have been implicated as carotenoid transporters in lower species and in various tissues of higher animals. The function of the third SRB protein, SR-B2, in carotenoid transport is unknown. Using surface plasmon resonance (SPR) analyses, we have determined that all three human SRB proteins are capable of binding the macular xanthophyll carotenoids; lutein, zeaxanthin, and meso-zeaxanthin. By over-expressing human SRB proteins in cells that do not endogenously express SRBs, we have determined that lutein uptake is enhanced in the presence of LDL and is mediated by SR-B1 and CD36. SR-B1, SR-B2, and CD36 were able to take up significant amounts of zeaxanthin as well as meso-zeaxanthin, and uptake was increased in the presence of HDL. Our analyses revealed no apparent differences in protein expression profiles of SRBs in central and peripheral regions of human donor tissues, indicating that carotenoid-binding proteins rather than transporters are likely to mediate selective accumulation of carotenoids into the macula.
Keywords: Lipoproteins, HDL, LDL, scavenger receptors, lutein, zeaxanthin, meso-zeaxanthin, eye, retina, macula, RPE
Graphical abstract
1. INTRODUCTION
Carotenoids are plant-derived pigment molecules that vertebrates cannot synthesize de-novo, which must be obtained exclusively from the diet. These compounds are potent anti-oxidants and are the precursors to compounds such as retinoids that are crucial for normal physiological functions [1,2]. In 2002, Harrison and colleagues showed that an epithelial transporter, SR-B1, in the intestine is responsible for carotenoid absorption [3]. In that same year, NinaD, a class B scavenger receptor (SRB) in Drosophila that shares homology to vertebrate CD36 and SR-B1, was determined to be responsible for carotenoid transport into the eye [4]. These two studies brought attention to the role of scavenger receptor class B proteins as carotenoid transporters in the intestine. Since then, studies have examined the effects of SR-B1 and CD36 on the uptake of carotenoids. Over-expression of SR-B1 or CD36 was sufficient for the uptake of pro-vitamin A carotenoids [5]. SR-B1 was shown to preferentially take up macular xanthophylls over carotenes [6]. Enhanced uptake of lutein in LDL complexes and zeaxanthin in HDL complexes was facilitated by endogenously expressed SR-B1 [7].
The area in the primate body with the highest carotenoid concentration is the macula lutea of the retina [2,8]. This region is yellow in color due to the abundance of carotenoids. There are only three carotenoids present in the macula – lutein, zeaxanthin, and meso-zeaxanthin, along with lower levels of their oxidative metabolites (Figure 1) [1]. These xanthophyll carotenoids are capable of filtering blue light from reaching the central retina, and they have strong antioxidant properties [9,10]. In addition, lutein and zeaxanthin supplements have been shown to increase macular pigment and alleviate the progression of age-related macular degeneration (AMD) [11], and supplements containing the non-dietary macular carotenoid meso-zeaxanthin have recently entered the market [12].
Figure 1.
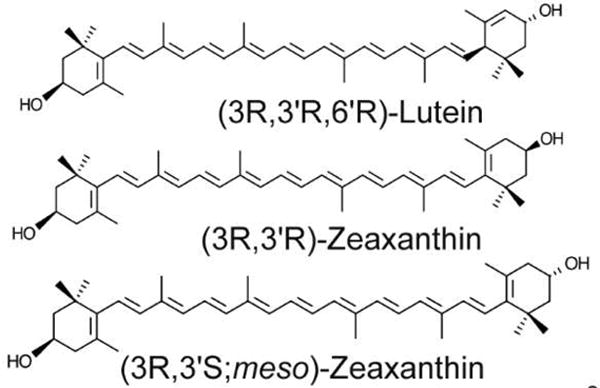
Structure of macular xanthophylls. Lutein, zeaxanthin, and meso-zeaxanthin are the three carotenoids present in the primate macula. They are structural isomers with the same molecular formula C40H56O2
Several studies have revealed that SR-B1 and CD36 can function as carotenoid transporters into the retina. The role of the third SRB family member, SR-B2, in carotenoid transport is unknown. Given the ability of all SRBs to bind similar ligands and the presence of all three in the primate retina [13], we hypothesized that SR-B2 may also be involved in carotenoid transport in mammals. In the present study, we have characterized the binding affinities of SRBs to the three macular xanthophyll carotenoids and determined their roles as transport proteins.
2. MATERIALS AND METHODS
Human tissue isolation
Human eyes from donors (ages ranging from 59 to 81) from the Utah Lions Eye Bank were isolated within 24-hours post-mortem. 6–8 mm punches of the macular retina, sub-macular RPE, peripheral retina, and peripheral RPE were obtained. The samples were stored in -80°C in RNALater (Thermo Fisher Scientific, Waltham, MA). Tissue procurement and handling were in compliance with the Declaration of Helsinki.
Protein isolation and Western blot
For total protein isolation, cells and tissues were homogenized at 4°C in RIPA buffer (25 mM NaCl, 0.5 mM EDTA, 25 mM Tris HCl (pH 7.2), 0.1% Tween 20) containing protease inhibitors. Cell debris was removed by centrifugation. BCA assay (Thermo Fisher Scientific) was carried out, and 20 μg of protein was resolved by 9% SDS-PAGE. Transfer was carried out to a 0.45 μm nitrocellulose membrane using a trans-blot SD semi-dry transfer cell (BioRad, Hercules, CA) at 25 V for 1 hour. Membranes were subsequently washed in TBS with 0.01% Tween 20 and blocked using Odyssey blocking buffer (LICOR Biotechnology, Lincoln, NE) containing 0.01% Tween 20 for 1 hour. Primary antibodies were diluted in the above blocking buffer, and the membranes were incubated overnight at 4°C. The antibodies used and their dilutions were as follows – 1:1000 dilution of rabbit monoclonal anti-SR-B1 (ab52629-Abcam, Cambridge, MA), 1:1000 dilution of goat polyclonal anti-SR-B2 (af1966-R&D Systems, Minneapolis, MN), 1:500 dilution of rabbit monoclonal anti-CD36 (14347, Cell Signaling Technology), 1:5000 dilution of mouse monoclonal anti-β-actin (8H10D10, Cell Signaling Technology), 1:2000 dilution of rabbit polyclonal anti-Na-K ATPase (3010, Cell Signaling Technology). Proteins were visualized using an Odyssey Image Analyzer (LICOR Biotechnology, Lincoln, NE) following incubation with IR dye conjugated secondary antibodies (LICOR Biotechnology) at 1:10000 dilutions for 1 hour at room temperature. For CD36, we ran two gels with the same lysate (at the same concentration) simultaneously. One membrane was blotted for CD36, whereas the other one was blotted for Na-K ATPase.
Immunohistochemistry
Eyes from 4–6 year old Macaca mulatta monkeys were obtained after perfusion fixation with 10% paraformaldehyde for 15 minutes. These animals were provided by other University of Utah reserchers whose IACUC protocols did not utilize ocular tissues after sacrifice. The eyes were dissected, and 10 μm thick cryosections of the tissue were obtained. The sections were rinsed in 0.1 M PBS with 0.1% Triton X-100 (PBT) and blocked in 10% donkey serum in PBT for 1 hour. Following this, primary antibody incubation was carried out overnight at 4°C. The antibodies and their dilutions were as follows - 1:100 dilution of rabbit monoclonal SRB1 (ab52629-Abcam), 1:500 dilution of goat polyclonal anti-SRB2 (af1966-R&D Systems), and 1:100 dilution of rabbit monoclonal anti-CD36 (14347, Cell Signaling Technology). The sections were rinsed in PBT, and incubations using FITC- or Cy3-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA) were conducted at 1:1000 dilution at room temperature for 2 hours. Control sections were incubated with only the corresponding secondary antibodies and not with primary antibodies. In order to quench autofluorescence, sections were treated with 0.1% Sudan black solution as previously described [14]
Cell culture and transient transfection
The ARPE-19 human RPE cell line and the HEK-293T human embryonic kidney cell line were purchased from ATCC (Rockville, MD). ARPE-19 cells were maintained in 1:1 Ham’s F12 medium:DMEM with 10% FBS and 1% penicillin streptomycin antibiotic mixture (Thermo Fisher Scientific). HEK-293Tcells were cultured in DMEM containing 10% FBS and 1% antibiotic mixture (Thermo Fisher Scientific). Both cell types were cultured in T-75 flasks until confluent. Cells were passaged using TrypLE Express (Thermo Fisher Scientific) and plated in 60 × 15 mm dishes for experiments. In order to ensure adequate differentiation, ARPE-19 cells were in culture for at least 6 weeks before experiments were conducted.
Over-expression was carried out by transient transfection of plasmids – pCMV-GFP SR-B1, pCMV-GFP SR-B2, and pCMV-GFP CD36 (Sino Biological Inc., Beijing, China). Cells were transfected with Jetprime reagent (Polyplus Transfection, Illkrich, France) following the manufacturer’s instructions. Transfection efficiencies were determined to be between 80–90% for each plasmid. Experiments were conducted after 48 hours of transfection.
Carotenoid delivery
Zeaxanthin was supplied by Zeavision (Chesterfield, MO), meso-zeaxanthin was from DSM Nutritional Products (Kaiseraugst, Switzerland), and lutein was provided by Kemin Health (Des Moines, IA). Carotenoid stocks were prepared in hexanes. 0.3% Tween 40 (Sigma-Aldrich) was added to appropriate volume of stocks, and the mixture was dried under nitrogen gas. For carotenoid delivery using HDL or LDL, either HDL (J64903) or LDL (J65039) (Alfa Aesar, Tewksbury, MA) were added to the dried stock solutions so that the final concentration of lipoproteins in the media were 10 μg/mL [7], and the mixture was vortexed overnight at 4°C in the dark. Serum-free medium was added, and the mixture was sonicated on ice for 1 hour and then vortexed at room temperature for 30 minutes. The media were filtered using 0.2 μm filters, and extracted to validate the concentration of carotenoids.
Cells were washed with serum-free media, and the mixtures of media with carotenoids and lipoproteins were added to the cells to initiate carotenoid uptake. The dishes were placed into a 37°C incubator for 30 minutes. The cells were subsequently washed with ice-cold 10 mM sodium taurocholate to remove any adsorbed carotenoids and then with 1X PBS. Cells were scraped into 1X PBS and centrifuged at 13000 rpm for 5 minutes. Excess liquid was removed, and carotenoid extraction was conducted.
Carotenoid extraction and HPLC analysis
Carotenoids were extracted following the addition of tetrahydrofuran (THF) containing 0.1% butylated hydroxytoluene (BHT). 1 mL of THF was added to the cell pellet from above, and this mixture was sonicated on ice (10 minutes) followed by vortexing for 5 minutes. The cell homogenates were centrifuged at maximum speed for 10 minutes, and the supernatant containing carotenoids was removed and dried under nitrogen gas. The above process was carried out three times to remove all carotenoid content in the cells. HPLC analyses were performed as previously described [15].
Surface plasmon resonance (SPR)
Recombinant proteins for CD36 (10752-H08H, Sino Biological Inc., Beijing, China), SR-B1 (11069-H08H, Sino Biological Inc., Beijing, China), and SR-B2 (BP001637-C532, Syd Labs, Natick, MA) were purchased. Proteins were immobilized onto hydroxyl gel modified sensor chips (Xantec, Dusseldorf, Germany) using standard amine coupling to obtain a density ranging from 6–12 kRU. Each of the three carotenoids (lutein, zeaxanthin, and meso-zeaxanthin) was dissolved in DMSO to obtain high concentration and then diluted to a final 5% DMSO concentration in running buffer. PBS with 0.05% Triton X-100 (for CD36 assay) or 1 mg/mL polyvinylpyrrolidone (PVP) (for SR-B1 and SR-B2 assays) was used as the running buffer. Carotenoid analytes’ concentrations ranged from 10–30 μM. Each of the analytes was run in triplicate with a flow rate of 100–200 μL/min. All analyses were carried out at 25°C using a SensiQ Pioneer optical biosensor (SensiQ Technologies Inc., Oklahoma City, OK). Data were collected at 10 Hz. SPR response data (sensorgrams) were zeroed at the beginning of each injection and double referenced. The responses were plotted against the analyte concentration and fit to a 1:1 (A+B=AB) binding model using Qdat analysis software (SensiQ Technologies) [16,17].
Statistical analysis
Statistical analyses were conducted using Graphpad Prism software (La Jolla, CA). Values are listed as mean ± SEM. Results were analyzed using Student t-tests.
3. RESULTS AND DISCUSSION
There are over 500 carotenoids present in nature. Humans and other primates consume about fifty of these, among which only three, lutein, zeaxanthin, and meso-zeaxanthin, are present in the macula. Such specificity in the accumulation of these three xanthophyll carotenoids into the eye suggests that this may be due to the function of specialized transport proteins and binding proteins [2,18,19]. Studies conducted using cell culture models as well as animal models have revealed that SR-B1 may be responsible for the transport of carotenoids from the intestine [3,4]. In addition, Sakudoh et al. showed that Cameo2, a CD36 orthologue, is responsible for selective accumulation of lutein in the cocoon [20,21]. Previous work has shown that SRB proteins, SR-B1 and CD36, can transport carotenoids into cells [3,4,20,21], but the role of SR-B2 in carotenoid transport has not been studied. In addition, studies on ARPE19 cells, an RPE cell line, have hypothesized that carotenoid uptake into these cells is mediated by SR-B1 [7]. In the current study, we chose to explore the roles of all three scavenger receptor proteins in carotenoid uptake.
In order to quantitatively assess the interactions between all three SRB proteins and carotenoids, we first determined the binding affinities of SRBs to macular xanthophylls on an SPR platform using human recombinant proteins of SR-B1, SR-B2, and CD36. Our results show that all three scavenger receptors bind macular xanthophylls with a binding affinity characteristic of transport proteins. As shown in Table 1 and Figure 2, SR-B1 and SR-B2 showed comparable affinities with all three carotenoids (Figure 2A–F). CD36 showed relatively stronger affinity toward macular xanthophylls, with KD values in the sub-micromolar range. As seen in the sensorgrams (Figure 2G, H, and I), this protein had a faster on-rate and slower off-rate with carotenoids. This resulted in a different kinetic profile of interaction than those of SR-B1 and SR-B2.
Table 1.
Equilibrium dissociation constants (KD) for carotenoid interactions with SRBs
| Lutein | Zeaxanthin | meso-Zeaxanthin | |
|---|---|---|---|
| SR-B1 | 2.04 ± 0.040 μM | 1.60 ± 0.100 μM | 3.10 ± 0.20 μM |
| SR-B2 | 2.51 ± 0.040 μM | 1.53 ± 0.040 μM | 3.90 ± 0.04 μM |
| CD36 | 0.73 ± 0.006 μM | 0.92 ± 0.007 μM | 1.66 ± 0.01 μM |
Figure 2.
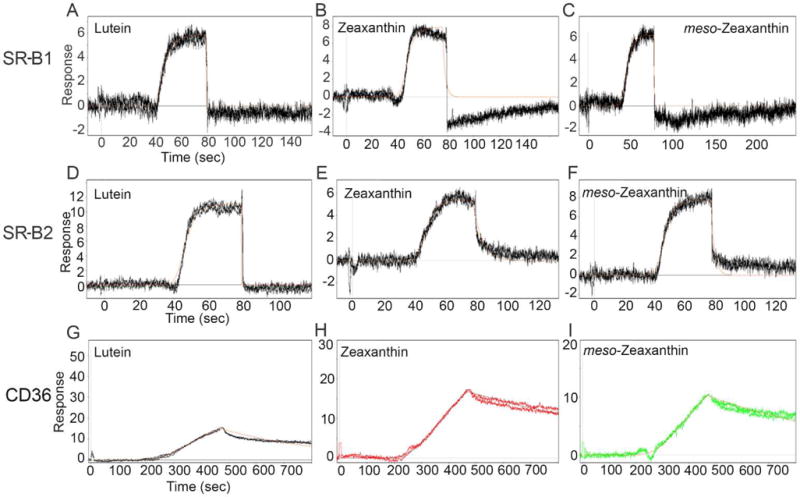
Surface plasmon resonance sensorgrams obtained for the binding interactions of carotenoids with SRB proteins. CD36 binding affinities toward all three carotenoids were stronger than those of SR-B1 and SR-B2 as evidenced by the slower off-rate in the sensorgrams (G, H, and I). Solid red lines through the curve show model fit for the calculation of the affinities of interaction.
Previous studies from our laboratory on the interactions between binding proteins and carotenoids have determined KD values in the sub-micromolar range. A low KD is indicative of stronger interaction between the ligand and the protein. For instance, KD values of StARD3 to lutein and GSTP1 to zeaxanthin were less than 0.6 μM [22,23]. However, binding affinities with carotenoids of a protein such as IRBP, a retinoid and carotenoid transporter that is present in the interphotoreceptor retinal space, was previously determined to be in the 1–2 μM range. Nonspecific binding interactions result in KD values that are higher than 10 μM. The KD values observed for SRB proteins here are in the same range as we observed for IRBP, indicating that SRBs bind carotenoids with affinities characteristic of transport proteins.
In the next set of experiments, we determined whether the binding affinity of SRBs for macular xanthophylls resulted in their enhanced uptake in a cell culture system. We chose to study carotenoid uptake in HEK-293T cells since they are free of endogenous SRBs (Figure 3), as opposed to the commonly used cell line, ARPE-19, which expresses both SR-B1 and SR-B2 endogenously (Figure 4). By over-expressing each SRB protein, and by using empty vector transfected cells as controls for all experiments, we were able to compare and contrast the SRB-mediated as well as non-SRB-mediated uptake of carotenoids into HEK-293T cells. Physiological levels of carotenoids in plasma are between 1 to 10 μM [24]; therefore, we tested the uptake using carotenoid concentrations ranging from 0.5 μM to 10 μM. Since carotenoids in circulation are present in mixed micelles of lipoproteins [25,26], we presented these molecules in LDL or HDL complexes to the cells.
Figure 3.
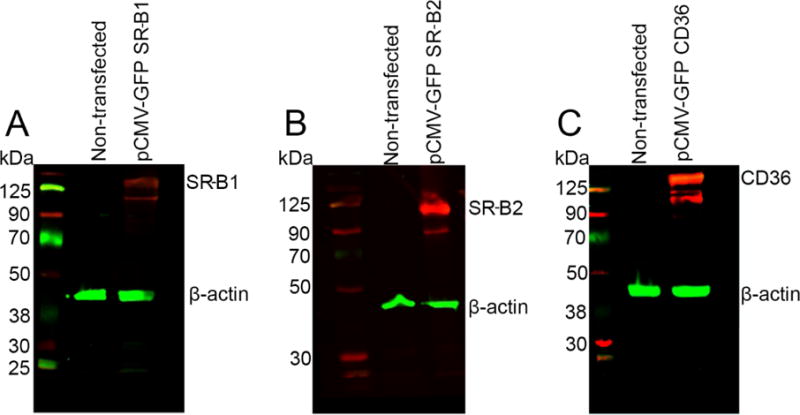
Western blot showing the endogenous and over-expressed SRB proteins in HEK-293Tcells. Endogenous expression of SR-B1, SR-B2 and CD36 was absent in these cells. Upon over-expression, SRBs were detected. β-Actin was used as loading control.
Figure 4.
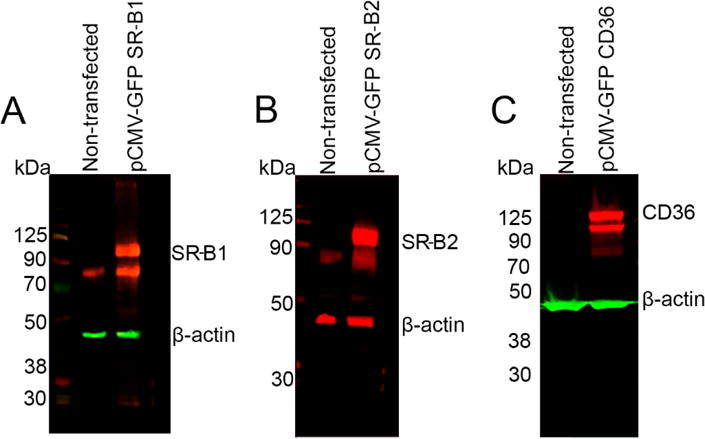
Western blot showing the endogenous and over-expressed SRB proteins in ARPE-19 cells. Endogenous expression of SR-B1, SR-B2 was detected, but CD36 was absent in these cells. Upon over-expression, SRBs were detected. β-Actin was used as loading control.
For lutein, uptake was significantly increased in cells over-expressing CD36 and SR-B1. SR-B2 over-expressing cells were able to take up this carotenoid at levels higher than the control cells, but the difference lacked statistical significance. We also noticed that the transport of LDL complexes with lutein were higher than transport of HDL complexes with lutein (Figure 5A and B).
Figure 5. Quantification of carotenoid uptake into HEK-293T cells over-expressing SRB proteins. Lutein uptake was increased in the presence of LDL, and zeaxanthin and meso-zeaxanthin uptake was higher in the presence of HDL. CD36 and SR-B1 were most efficient in the uptake of lutein and zeaxanthin, whereas SR-B2 showed significant uptake of zeaxanthin and meso-zeaxanthin.
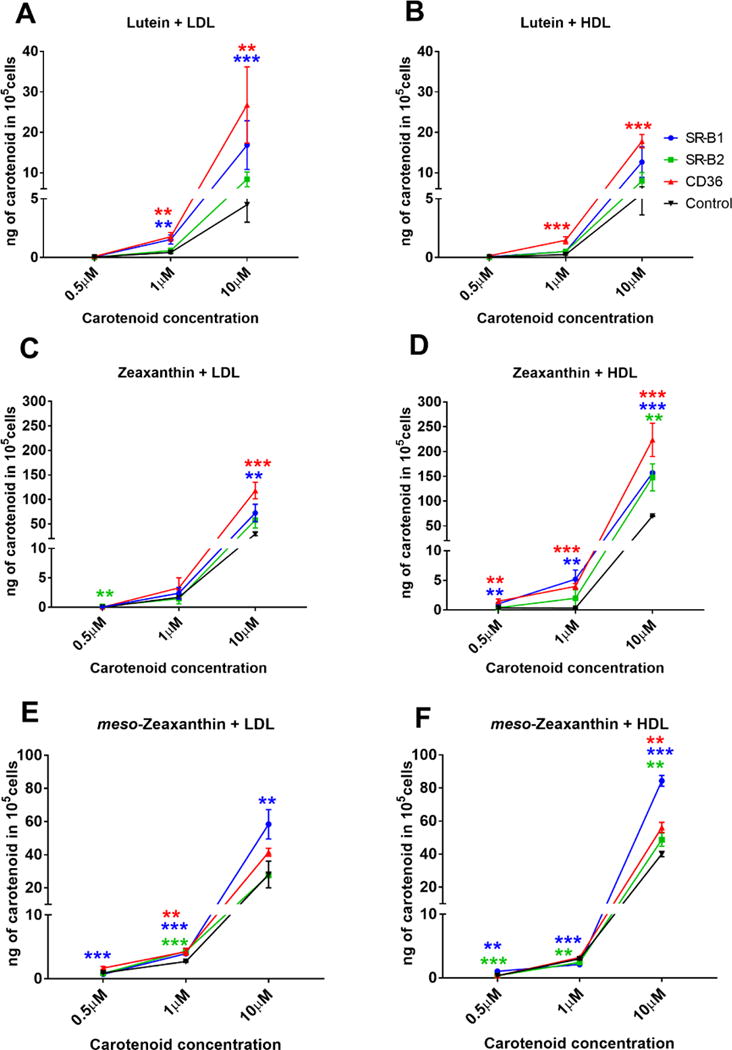
Carotenoid uptake in non-transfected cells may be due to passive diffusion. Xanthophyll uptake into the control cells may be mediated by passive diffusion. It is also likely that lipoprotein laden carotenoids are endocytosed into the control cells. Non-specific uptake into the control cells may also be mediated by taurocholate ** - p-value< 0.05, ***-p-value<0.0005
Treatment of SRB over-expressing cells with zeaxanthin in HDL resulted in higher uptake than when this carotenoid was presented to the cells in LDL (Figure 5C and D). Consistent with our observations for lutein, CD36 and SR-B1 over-expressing cells were able to take up significantly higher amounts of carotenoid than control cells. Interestingly, we noticed that the amount of zeaxanthin uptake into SR-B2 over-expressing cells was significant at higher concentrations of the carotenoid. In addition, the amount of zeaxanthin taken up by the cells was almost ten-fold higher than that of lutein.
With LDL as well as HDL, complexes of meso-zeaxanthin, SR-B1 over-expressing cells displayed the highest carotenoid uptake at all concentrations (Figure 5E and F). meso-Zeaxanthin, similar to zeaxanthin, was taken up better by cells in the presence of HDL. Interestingly, the uptake of HDL complexes of meso-zeaxanthin was higher in all three SRB over-expressing cells. SR-B2 over-expressing cells showed increased uptake of meso-zexanthin, and this trend was evident at all three concentrations of the carotenoid. Control cells treated with zeaxanthin or meso-zeaxanthin took up higher amounts of carotenoid than with lutein treatment. This suggests an inherent preference of zeaxanthins over lutein by these cells that may be SR-B independent.
Even though meso-zeaxanthin is not a common dietary component and is synthesized in the RPE enzymatically from lutein [28], supplements of this carotenoid are on the market [12]. It is hypothesized that consumption of such supplements may have positive effects on visual function [27], and protein mediated transport and deposition from the RPE to the retina would still be important. Identification that all three SRBs can function as meso-zeaxanthin transporters reveals a possible mechanism by which this carotenoid supplied by supplements may reach the retina.
With the understanding that SRB proteins can function as macular xanthophyll transporters, our next goal was to understand any differences in distribution of SRB proteins in the central and peripheral regions of human donor eye. We hypothesized that if SRB proteins are responsible for the selective accumulation of carotenoids in the macula, there will be differences in their protein expression profile in the macular and peripheral regions of the eye. Western blotting was conducted on human donor samples, and our data show the presence of SR-B1 and SR-B2 in peripheral retina, peripheral RPE, macular retina, and sub-macular RPE (Figure 6A and B). These proteins were determined using total lysates of the above-mentioned tissues. CD36, however, could not be detected in the total lysate. Thus, we carried out membrane protein isolation followed by Western blot. In the membrane fraction, we observed the presence of CD36 in the sub-macular RPE and peripheral RPE but not in macular retina or peripheral retina. Detection of CD36 after membrane protein isolation but not in the total tissue lysate indicates its low abundance (Figure 6C and D). We did not observe any differences in the expression patterns of SR-B1 and SR-B2 in the peripheral and macular regions of the eye. However, we noticed SR-B2 protein resolved at a slightly higher molecular weight in the RPE lysates. In addition, our data also revealed higher amounts of SR-B2 in the retina than in the RPE. Future studies may analyze the reasons behind the increased expression of this protein in the retina.
Figure 6.
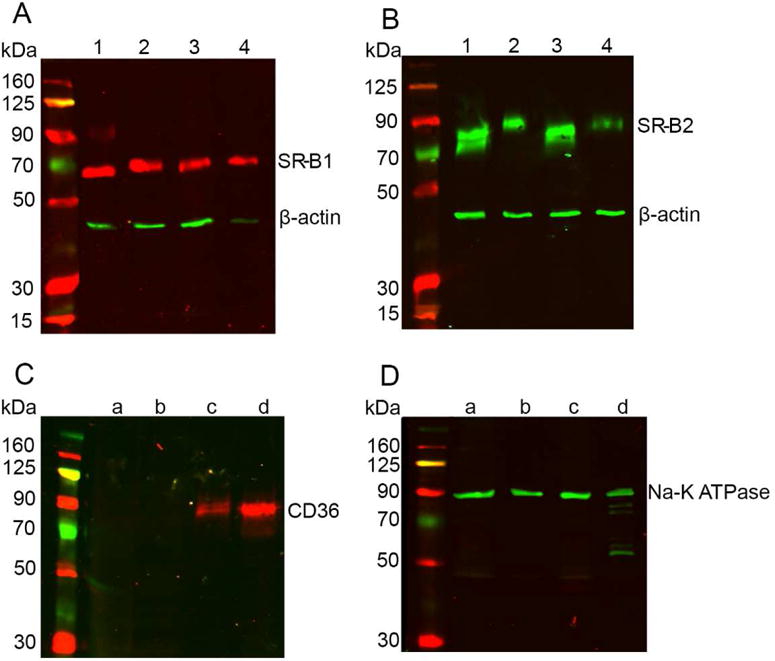
Western blots conducted on human tissues. SR-B1 and SR-B2 proteins (A and B) were detected in total tissue lysates of human macular retina (lane 1), sub-macular RPE (lane 2), peripheral retina (lane 3), and peripheral RPE (lane 4). β-actin was used as loading control. Membrane protein isolation resulted in the detection of CD36 (C) only in the peripheral RPE (lane c) and sub-macular RPE (lane d). Retinal tissue (lanes a and b) did not contain detectable CD36. Na-K ATPase was used as the loading control for membrane proteins. Panel D shows the presence of Na-K ATPase in all four tissues. Experiments were conducted in tissues from three different donors of ages ranging from 50–70 years with similar results. Results from one representative experiment are presented.
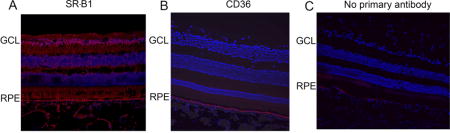
In order to determine the specific cell types in which these proteins are expressed, immunohistochemistry was conducted on sagittal sections of macaque eyes. SR-B1 was expressed throughout the retina and RPE (Figure 7A). SR-B2, like SR-B1, was expressed throughout the retinal layers. Strong SR-B2 expression was observed in the RPE layer and ganglion cell layer of the retina (Figure 8). Consistent with the Western blot data, CD36 expression was detected only in the RPE layer (Figure 7B).
Figure 7.
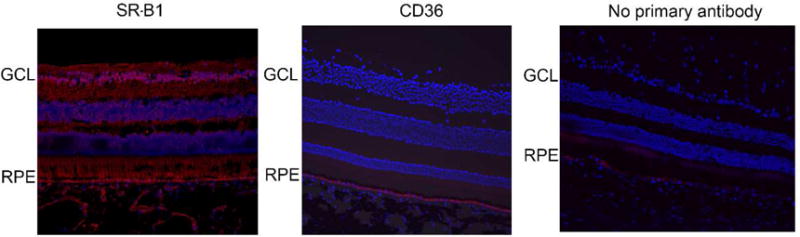
Immunohistochemistry showing expression of SR-B1 and CD36 in macaque eye sections. SR-B1 expression (red) was detected throughout the RPE and retinal layers. CD36 expression (red) was limited to the RPE layer. Both CD36 and SR-B1 antibodies were rabbit polyclonal. “No primary antibody” control sections were incubated with the secondary antibody alone. Nuclei are stained using DAPI in blue.
Figure 8.
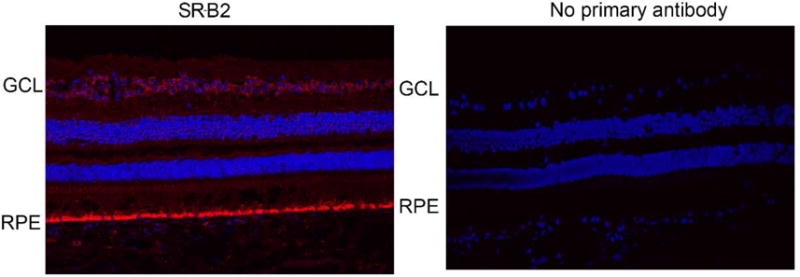
SR-B2 staining in macaque eye sections. Protein expression was strong in the RPE layer and in the ganglion cell layer. Weak expression was detected throughout the retina. SR-B2 expression (red) was determined using a goat polyclonal antibody. “No primary antibody” control sections were incubated with only the secondary antibody. Nuclei are stained using DAPI in blue.
Tserentsoodol et al. have previously shown the presence of all three SRB proteins in primate eye tissues, but we noticed inconsistencies in their Western blots and IHC. For instance, their IHC shows the presence of SR-B1 and SR-B2 in the retinal layers and in the RPE of primates. In their Western blot, SR-B1 was visible in the monkey neural retina, but no bands at the right molecular weight were present in the RPE fraction. Similarly, no bands that correspond to SR-B2 were present in the monkey retina and RPE. Their IHC detected the presence of CD36 in various retinal layers and not in the RPE, but in their Western blots, CD36 was not detected in the primate retina or RPE [13]. In the present study, we were able to observe the presence of SR-B1 and SR-B2 throughout the retina and RPE, while CD36 was only in the RPE. Our Western blots are in agreement with the IHC findings.
In the present study, we have shown that all three SRBs are expressed in the primate macula as well as peripheral regions. Interestingly, we did not observe any changes in expression profiles of these proteins in the macula and peripheral retinal tissues. Our analyses suggest that SRB proteins, even though they are capable of transporting macular xanthophylls, may not be responsible for their selective accumulation in the macula. Consistent with previous work [7], we also found that LDL complexes of lutein and HDL complexes of zeaxanthin and meso-zeaxathin are taken up better by cells in culture. With SPR analyses, we have quantitatively determined the binding affinities of SRBs to macular carotenoids.
In conclusion, we have shown that all three human SRBs are capable of binding and transporting all three macular carotenoids. Previous studies from our laboratory have identified and characterized carotenoid binding proteins that have restricted expression in the macula and not in the peripheral regions. GSTP1 was determined to be a zeaxanthin binding protein. Its strong expression was detected in the inner and outer plexiform layers of primate macula [19]. Similarly, work in our lab characterized StARD3 as a lutein binding protein [22]. Expression of this protein was enhanced in the primate macula, specifically in the photoreceptor inner segments. The present study reveals the mechanism by which carotenoids in circulation reach the peripheral or macular retina (Figure 9). The presence of higher levels of specific binding proteins in the macula is likely be responsible for the accumulation of carotenoids in this region.
Figure 9.
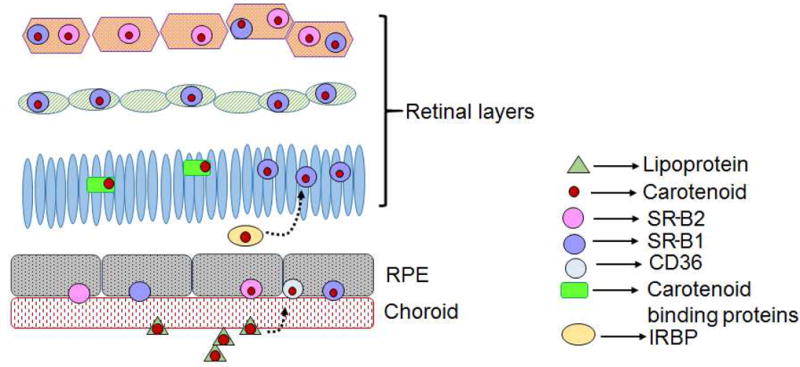
Proposed model of carotenoid uptake into the macula. Carotenoids in lipoproteins reach the RPE through the choroidal circulation. Here, SRB proteins bind and transport the carotenoids. From the RPE, transport proteins such as IRBP may function to shuttle the carotenoids into the retina. Once in the retina, binding proteins retain the carotenoids. Low level of CD36 protein was detected only in the RPE and not in the retina.
Highlights.
All three SRB proteins are capable of binding macular carotenoids
Over-expressed SRB proteins can transport macular carotenoids
LDL enhances lutein uptake, HDL increases zeaxanthin and meso-zeaxanthin uptake
All three SRB proteins are expressed in the primate eye
Acknowledgments
GRANT SUPPORT
The authors wish to acknowledge Wolfgang Baehr, Ph.D. and Jeanne Frederick, Ph.D. for expert advice during the preparation of this manuscript. This work was supported by NIH grants EY11600 and EY14800 (P.S.B.) and a Ruth L. Kirschstein NIH training grant T32EY024234 (R.S.), and an unrestricted departmental grant from Research to Prevent Blindness. Funding sources had no involvement in the conduct of research or preparation of this article.
Abbreviations
- AMD
Age-related macular degeneration
- CD36
Cluster determinant 36
- GSTP
Glutathione S-transferase Pi isoform
- IHC
immunohistochemistry
- PVP
Polyvinylpyrrolidone
- RPE
Retinal pigment epithelium
- SR-B1
Scavenger receptor class B protein 1
- SR-B2
Scavenger receptor class B protein 2
- SRB
Scavenger receptor class B proteins
- StARD3
steroidogenic acute regulatory domain 3
- SPR
Surface plasmon resonance
- THF
Tetrahydrofuran
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors declare no conflicts of interest
References
- 1.Bone RA, Landrum JT, Friedes LM, Gomez CM, Kilburn MD, Menendez E, Vidal I, Wang W. Distribution of lutein and zeaxanthin stereoisomers in the human retina. Exp Eye Res. 1997;64:211–8. doi: 10.1006/exer.1996.0210. [DOI] [PubMed] [Google Scholar]
- 2.Bernstein PS, Li B, Vachali PP, Gorusupudi A, Shyam R, Henriksen BS, Nolan JM. Lutein, zeaxanthin, and meso-zeaxanthin: The basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog Retin Eye Res. 2016;50:34–66. doi: 10.1016/j.preteyeres.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.During A, Hussain MM, Morel DW, Harrison EH. Carotenoid uptake and secretion by CaCo-2 cells: beta-carotene isomer selectivity and carotenoid interactions. J Lipid Res. 2002;43:1086–95. doi: 10.1194/jlr.m200068-jlr200. [DOI] [PubMed] [Google Scholar]
- 4.Kiefer C, Sumser E, Wernet MF, von Lintig J. A class B scavenger receptor mediates the cellular uptake of carotenoids in Drosophila. Proc Natl Acad Sci. 2002;99:10581–10586. doi: 10.1073/pnas.162182899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borel P, Lietz G, Goncalves A, Szabo de Edelenyi F, Lecompte S, Curtis P, Goumidi L, Caslake MJ, Miles EA, Packard C, Calder PC, Mathers JC, Minihane AM, Tourniaire F, Kesse-Guyot E, Galan P, Hercberg S, Breidenassel C, González Gross M, Moussa M, Meirhaeghe A, Reboul E. CD36 and SR-BI are involved in cellular uptake of provitamin A carotenoids by Caco-2 and HEK-293T cells, and some of their genetic variants are associated with plasma concentrations of these micronutrients in humans. J Nutr. 2013;143:448–56. doi: 10.3945/jn.112.172734. [DOI] [PubMed] [Google Scholar]
- 6.During A, Doraiswamy S, Harrison EH. Xanthophylls are preferentially taken up compared with β-carotene by retinal cells via a SRBI-dependent mechanism. J Lipid Res. 2008;49:1715–24. doi: 10.1194/jlr.M700580-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas SE, Harrison EH. Mechanisms of selective delivery of xanthophylls to retinal pigment epithelial cells by human lipoproteins. J Lipid Res. 2016;57:1865–1878. doi: 10.1194/jlr.M070193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Snodderly DM, Auran JD, Delori FC. The macular pigment. II. Spatial distribution in primate retinas. Invest Ophthalmol Vis Sci. 1984;25:674–85. [PubMed] [Google Scholar]
- 9.Junghans A, Sies H, Stahl W. Macular Pigments Lutein and Zeaxanthin as Blue Light Filters Studied in Liposomes. Arch Biochem Biophys. 2001;391:160–164. doi: 10.1006/abbi.2001.2411. [DOI] [PubMed] [Google Scholar]
- 10.Li B, Ahmed F, Bernstein PS. Studies on the singlet oxygen scavenging mechanism of human macular pigment. Arch Biochem Biophys. 2010;504:56–60. doi: 10.1016/j.abb.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chew EY, Clemons TE, SanGiovanni JP, Danis RP, Ferris FL, Elman MJ, Antoszyk AN, Ruby AJ, Orth D, Bressler SB, Fish GE, Hubbard GB, Klein ML, Chandra SR, Blodi BA, Domalpally A, Friberg T, Wong WT, Rosenfeld PJ, Agrón E, Toth CA, Bernstein PS, Sperduto RD, Sperduto RD. Secondary analyses of the effects of lutein/zeaxanthin on Age-related macular degeneration progression. JAMA Ophthalmol. 2014;132:142. doi: 10.1001/jamaophthalmol.2013.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Connolly EE, Beatty S, Loughman J, Howard AN, Louw MS, Nolan JM. Supplementation with All Three Macular Carotenoids: Response, Stability, and Safety, Invest. Opthalmol Vis Sci. 2011;52:9207. doi: 10.1167/iovs.11-8025. [DOI] [PubMed] [Google Scholar]
- 13.Tserentsoodol N, Gordiyenko NV, Pascual I, Lee JW, Fliesler SJ, Rodriguez IR. Intraretinal lipid transport is dependent on high density lipoprotein-like particles and class B scavenger receptors. Mol Vis. 2006;12:1319–33. [PubMed] [Google Scholar]
- 14.Sun Y, Yu H, Zheng D, Cao Q, Wang Y, Harris D, Wang Y. Sudan black B reduces autofluorescence in murine renal tissue. Arch Pathol Lab Med. 2011;135:1335–42. doi: 10.5858/arpa.2010-0549-OA. [DOI] [PubMed] [Google Scholar]
- 15.Gorusupudi A, Shyam R, Li B, Vachali P, Subhani YK, Nelson K, Bernstein PS. Developmentally regulated production of meso- Zeaxanthin in chicken retinal pigment epithelium/choroid and retina. Invest Opthalmol Vis Sci. 2016;57:1853. doi: 10.1167/iovs.16-19111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vachali P, Li B, Besch B, Bernstein P. Protein-flavonoid interaction studies by a taylor dispersion Surface Plasmon Resonance (SPR) technique: A novel method to assess biomolecular interactions. Biosensors. 2016;6:6. doi: 10.3390/bios6010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vachali PP, Besch BM, Gonzalez-Fernandez F, Bernstein PS. Carotenoids as possible interphotoreceptor retinoid-binding protein (IRBP) ligands: a surface plasmon resonance (SPR) based study. Arch Biochem Biophys. 2013;539:181–6. doi: 10.1016/j.abb.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li B, Vachali P, Bernstein PS. Human ocular carotenoid-binding proteins. Photochem Photobiol Sci. 2010;9:1418. doi: 10.1039/c0pp00126k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhosale P, Larson AJ, Frederick JM, Southwick K, Thulin CD, Bernstein PS. Identification and characterization of a Pi isoform of glutathione S-transferase (GSTP1) as a zeaxanthin-binding protein in the macula of the human eye. J Biol Chem. 2004;279:49447–54. doi: 10.1074/jbc.M405334200. [DOI] [PubMed] [Google Scholar]
- 20.Sakudoh T, Kuwazaki S, Iizuka T, Narukawa J, Yamamoto K, Uchino K, Sezutsu H, Banno Y, Tsuchida K. CD36 homolog divergence is responsible for the selectivity of carotenoid species migration to the silk gland of the silkworm Bombyx mori. J Lipid Res. 2013;54:482–495. doi: 10.1194/jlr.M032771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakudoh T, Iizuka T, Narukawa J, Sezutsu H, Kobayashi I, Kuwazaki S, Banno Y, Kitamura A, Sugiyama H, Takada N, Fujimoto H, Kadono-Okuda K, Mita K, Tamura T, Yamamoto K, Tsuchida K. A CD36-related transmembrane protein is coordinated with an intracellular lipid-binding protein in selective carotenoid transport for cocoon coloration. J Biol Chem. 2010;285:7739–7751. doi: 10.1074/jbc.M109.074435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li B, Vachali P, Frederick JM, Bernstein PS. Identification of StARD3 as a lutein-binding protein in the macula of the primate retina. Biochemistry. 2011;50:2541–9. doi: 10.1021/bi101906y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vachali P, Li B, Nelson K, Bernstein PS. Surface plasmon resonance (SPR) studies on the interactions of carotenoids and their binding proteins. Arch Biochem Biophys. 2012;519:32–37. doi: 10.1016/j.abb.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El-Sohemy A, Baylin A, Kabagambe E, Ascherio A, Spiegelman D, Campos H. Individual carotenoid concentrations in adipose tissue and plasma as biomarkers of dietary intake. Am J Clin Nutr. 2002;76:172–9. doi: 10.1093/ajcn/76.1.172. [DOI] [PubMed] [Google Scholar]
- 25.Borel P, Grolier P, Armand M, Partier A, Lafont H, Lairon D, Azais-Braesco V. Carotenoids in biological emulsions: solubility, surface-to-core distribution, and release from lipid droplets. J Lipid Res. 1996;37:250–61. [PubMed] [Google Scholar]
- 26.Tyssandier V, Lyan B, Borel P. Main factors governing the transfer of carotenoids from emulsion lipid droplets to micelles. Biochim Biophys Acta. 2001;1533:285–92. doi: 10.1016/s1388-1981(01)00163-9. [DOI] [PubMed] [Google Scholar]
- 27.Nolan JM, Meagher K, Kashani S, Beatty S. What is meso-zeaxanthin, and where does it come from? Eye (Lond) 2013;27:899–905. doi: 10.1038/eye.2013.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shyam R, Gorusupudi A, Nelson K, Horvath MP, Bernstein PS. RPE65 has an additional function as the lutein to meso-zeaxanthin isomerase in the vertebrate eye. PNAS. doi: 10.1073/pnas.1706332114. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]


