Abstract
The Unfolded Protein Response (UPR) is a cascade of intracellular stress signaling from the endoplasmic reticulum (ER) that protect the cells from the stress caused by accumulation of unfolded or misfolded proteins in the ER. Activating transcription factor 6 (ATF6) is one of primary UPR transducers that remodels the stressed cells through transcriptional regulation. Although the activation mechanism and biological roles of ATF6 have been well studied, the understanding of the negative or feedback regulation of ATF6 remains elusive. In this report, we showed that ATF6 protein can be modified by small ubiquitin-like modification (SUMOylation) and that the transcriptional activity of ATF6 is negatively regulated by SUMOylation. We identified that SUMOylation of ATF6 is significantly increased in the cells expressing misfolded cystic fibrosis transmembrane conductance regulator (CFTR) encoded by the mutant human CFTR gene (dF508CFTR). Further analyses revealed two highly conserved SUMOylation motifs within the trans-activation domain of ATF6 protein of human, mouse, or rat specie. The human ATF6 protein can be SUMOylated mediated through the small ubiquitin-like modifier protein 1 (SUMO-1) and E3 SUMO-protein ligase 1 (PIAS1) at the conserved sumoylation residue Lys149 that is located at the N-terminal of the activated form of ATF6 protein. Bimolecular fluorescence complementation (BiFC) analysis confirmed that the activated ATF6 protein can be SUMOylated and that the ATF6 sumoylation occurs in the nuclei. Moreover, trans-activation reporter analysis demonstrated that SUMOylation of the ATF6 protein at the conserved residue Lys14 represses the transcriptional activity of ATF6. In summary, our study revealed a negative regulation of the UPR transducer ATF6 through post-translational SUMOylation. The information from this study will not only increase our understanding of the fine-tuning regulation of the UPR signaling but will also be informative to the modulation of the UPR for therapeutic benefits.
Introduction
The unfolded Protein Response (UPR) is a set of intracellular signaling pathways from the endoplasmic reticulum (ER) that protect the cells from the stress caused by the accumulation of unfolded or misfolded proteins in the ER [1,2]. In mammalian cells, three ubiquitously-expressed ER transmembrane proteins, including IRE1α (inositol-requiring enzyme 1α), PERK (PKR-like ER kinase), and ATF6 (activating transcription factor 6), have been identified as the primary transducers of the UPR signaling. These transducers mediate distinct signaling pathways from the ER to cytosol and nucleus, leading to transcriptional and translational reprogramming of stressed cells. The UPR is critical for cells to make survival or death decision under ER stress conditions, and is closely associated with the initiation and progression of a variety of diseases, including metabolic disease, cardiovascular disease, neurodegenerative disease, and cancer [2,3]. Among others, the UPR pathway mediated through ATF6 has been implicated in regulating cell survival, ER capacity, lipid metabolism, as well as skeletal muscle function [4,5,6,7]. In response to ER stress, ATF6 is released from the ER membrane, and trafficks into the Golgi compartment where it is cleaved by site-1 protease (S1P) and site-2 protease (S2P), the same enzymes that processes sterol-response-element-binding protein 1 (SREBP1) for cholesterol biosynthesis [8,9]. Cleaved N-terminal ATF6 fragment transits into the nucleus and functions as a potent transcription factor to activate UPR target genes. Although the activation mechanism and pathophysiological roles of ATF6 have been extensively studied, the negative regulation of ATF6 activity has not been well understood.
SUMOylation (small ubiquitin-like modification) is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, and protein stability [10]. Similar to the process of ubiquitination, SUMO is first activated by the E1 enzyme SAE1 (SUMO1-activating enzyme subunit 1)-UBA2 (ubiquitin-like modifier activating enzyme 2), then is transferred on to the E2 enzyme UBC9 and subsequently conjugated to the lysine residues of target proteins via the activity of an E3 ligase, such as PIAS1 (protein inhibitor of activated STAT-1), RanBP2 (Ran-binding protein 2) or the polycomb group protein Pc2 [11]. A variety of proteins have been demonstrated to be SUMO targets based on the presence of a consensus SUMOylation core motif, Ψ-K-x-D/E (Ψ is a hydrophobic residue, X is any residue and E/D is aspartate/glutamate) [12]. Typically, only a small fraction of a protein is SUMOylated and this modification is rapidly reversed by the action of deSUMOylating enzymes. In many cases, SUMO modification of transcriptional regulators correlates with inhibition of transcription [13].
The cystic fibrosis transmembrane conductance regulator (CFTR) functions as a membrane chloride channel. CFTR mutations induce cystic fibrosis (CF), which is one of the most common human genetic disorders. Phenylalanine deletion at position of 508 on CFTR protein (delta F 508, dF508CFTR) is the major mutation of CFTR that resulted in protein misfolding. Misfolded dF508CFTR starts UPR then quickly degraded by ER-associated degradation pathway (ERAD), and cannot reach membrane [14]. Normally, dF508CFTR is a typical molecular model for UPR [15]. In this study, we demonstrated that SUMOylation of ATF6 is significantly increased in the cells expressing misfolded CFTR encoded by the mutant human CFTR gene (dF508CFTR). The activated form of ATF6 protein can be modified by SUMOylation mediated through SUMO1 and PIAS1, and the transcriptional activity of ATF6 is negatively regulated by the SUMOylation.
Materials and Methods
Materials
Chemicals were purchased from Sigma unless indicated otherwise. Synthetic oligonucleotides were purchased from Integrated DNA Technologies, Inc. (IA, USA). Antibodies against HA and Myc was purchased from Santa Cruz Biotechnologies (CA, USA). CFTR antibodies 217 and SUMO-1(#4930) was purchased from North Carolina University and Cell signaling separately. ATF antibody was from Dr. Zhang’s lab. HRP-labeled goat anti-mouse IgG (ab6789) and HRP-labeled goat anti-rabbit IgG (ab6741) were from Abcam. COS1 cells were cultured as recommended by the American Type Culture Collection. pCDNA3.1-WT CFTR and pCDNA3.1-dF508CFTR constructs were described previously [16]. Plasmid pCGN-ATF6(a) that expresses the activated form of human ATF6 protein was kindly provided by Dr. Ron Prywes at the Columbia University [17]. The site-directed mutagenesis kit was purchased from Stratagene (USA).
Site Mutagenesis
Site-directed mutagenesis to generate ATF6 mutants was performed using pCGN-ATF6(a) as the template. The PCR primers used for generating site mutation are K1R: 5'-CTGTACAGTTAAAGATATTAGGGCAGAACCTCAGCCA-3', and 5'-TGGCTGAGGTTCTGCCCTAATATCTTTAACTGTACAG-3'. The PCR primers used for generating site mutation are: K2R: 5'-GCGGAGCCACTGAGGGAAGATAAGCCTGT-3', and 5'-ACAGGCTTATCTTCCCTCAGTGGCTCCGC-3’. The PCR included the mutagenic primers, as well as the Pfu polymerase (Stratagene) and the program consisted of 13 cycles of 95 °C for 30 s, 55 °C for 30 s and 72 °C for 5 min. After DpnI digestion for 2–3 h, the PCR product was transformed into XL-Blue competent cells for cloning. The correct introduction of the mutants was confirmed by DNA sequencing.
IP-Western blot analysis
For immunoprecipitation, human cell or COS1 cell lysates (~1×107 cells) were mixed with the anti-ATF6 or an anti-HA antibody (1 µg) for 2 h, followed by the addition of 50 µl of protein A/G Plus-Sepharose beads for an additional hour at 4 °C. Immunoprecipitates were washed four times with NP-40 lysis buffer before denatured for SDS-PAGE (8% gel) and immune-blotting assay using the anti-SUMO1 or an anti-Myc antibody to detect the interaction between ATF6 and SUMO1.
BiFC analysis
For visualization of ATF6-SUMO1 interaction, the sequences encoding N-terminal amino acids residues 1–172 of enhanced yellow fluorescent protein (designated YN) were fused to the 5′ ends of the coding regions for SUMO1 or SUMO1(d) with the deletion of c-terminal glycines [18] to produce plasmids encoding YN-SUMO1 or YN-SUMO1(d). The activated human ATF6 protein encoded by the plasmid pCGN-ATF6(a) was fused to the complementary C-terminal YFP fragment to produce chimeric protein YC-ATF6. COS1 cells transfected with plasmids encoding the indicated combinations of fusion proteins were incubated at 37°C for 24 h and then transferred to 30°C for 4–16 h to promote fluorophore maturation. The fluorescence emissions of the cells were imaged as described [18,19].
Luciferase reporter analysis
The DNA plasmids for reporter analysis were co-transfected into MEF cells. At 36 hours post transfection, cell lysates were collected for measuring the luciferase and β-galactosidase (LacZ) activities using a Luciferase/β-Galactosidase Dual Light Reporter Assay kit according to the manufacturer's instructions (Applied Biosystems, MA, USA). The luciferase activities shown in Figure 1E were after normalization to the activities from the co-transfected pCMV-LacZ expression plasmid.
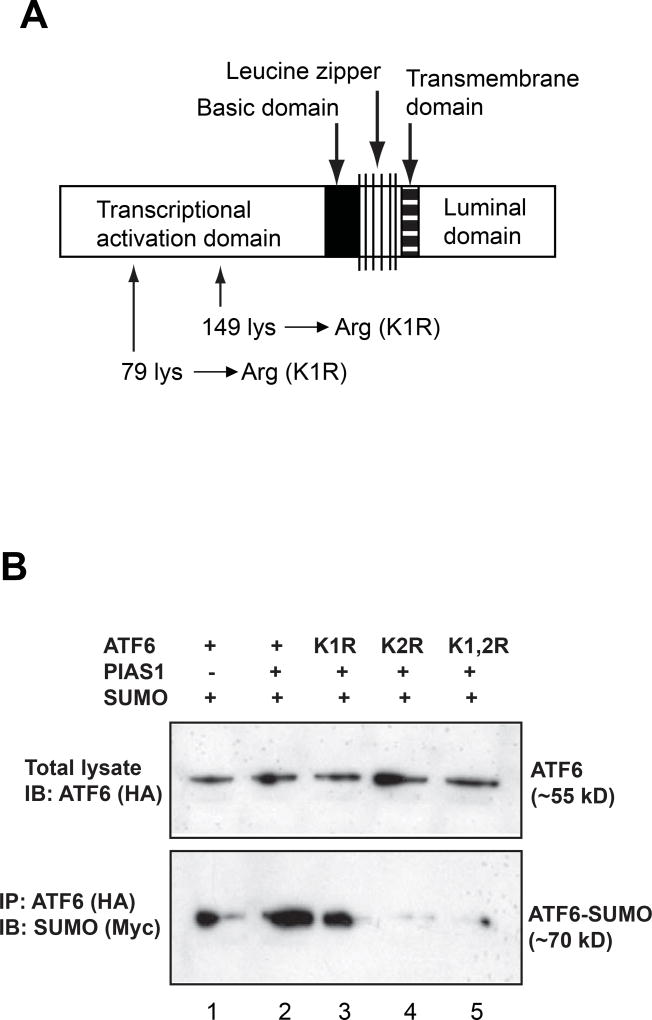
Figure 1.
(A) The domain structure of human ATF6 protein. The human ATF6 protein consists of 4 basic domains: an ER lumen domain, a transmembrane domain, a basic leucine zipper (bZIP) domain, and a trans-acivation domain. Two conserved sumoylation sites are located at the trans-activation domain. Site-mutagenesis was made by replacing the Lysine residue with Arginin at the two sumoylation sites, respectively (see Supplemental figure 1 for more details). (B) Western blot and immunoprecipetion (IP)-Western blot analyses of ATF6 expression and the interaction between ATF6 and SUMO-1. Plasmid construct pCGN-ATF6 expressing HA-tagged activated human ATF6 or its mutant isoform K1R, K2R, or K1,2R, construct pCMV-SUMO1 expressing Myc-tagged human SUMO-1, and construct expressing human PIAS1 were co-expressed in COS1 cells. Western blot analysis was performed to detect ATF6 expression in the transfected cells using an anti-HA antibody. To detect the interaction between SUMO-1 and ATF6 isforms, IP-Western blot analysis was performed using anti-HA antibody to pull down and anti-Myc antibody to detect the ATF6-SUMO complex.
Result
To explore the possibility of ATF6 SUMOylation, we first examined ATF6 protein sequence for potential SUMOylation sites. Indeed, two highly conserved SUMOylation motifs were identified within the trans-activation domain of ATF6 protein of human, mouse, or rat specie (Figure 1A and Supplemental figures 1 and 2). In human ATF6 protein, two SUMOylation residues are Lysine87 (IKAE) and Lysine149 (LKED) that are located in the trans-activation domain [8,17]. To illuminate the potential of ATF6 SUMOylation, site-directed mutagenesis was performed to replace Lysine87 (K1R) and/or Lysine149 (K2R) with arginine (Figure 1A and Supplemental figure 1). We then expressed an HA-tagged activated human ATF6 protein or its isoforms with mutations in Lysine87 (K1R), Lysine149 (K2R), or in both Lysine87 and Lysine149 (K1,2R), a Myc-tagged human SUMO-1 protein, and the E3 ligase PIAS1 in COS1 cells. Western blot analysis showed that the majority of forcedly-expressed ATF6 protein species are in a non-SUMOylated form with a molecular weight of approximately 55 kD (Figure 1B, upper panel). To detect whether there exists a SUMOylated form of ATF6, we performed immunoprecipetion (IP)-Western blot analysis to pull down ATF6-associated proteins using an anti-HA (ATF6) antibody. The immune-blot assay indicated that an anti-Myc (SUMO) antibody recognized an ATF6-SUMO complex with a molecular weight of approximately70 kD in the immuniprecipetes of the anti-HA (ATF6) from lysates of the cells expressing the activated ATF6 and SUMO-1 proteins (Figure 1B, lower panel lane 1). Apparently, SUMOylation increased the molecular mass of ATF6 protein by approximately 15 kDa. As anti-ATF6 failed to detect the shifted bands in the whole cell lysates (Figure 1B, upper panel), our results suggest that only a small portion of ATF6 is SUMOylated in transiently transfected cells. Moreover, when PIAS1 was over-expressed in the cells, an increase in ATF6 SUMOylation was evidenced (Figure 1B, lower panel lane 2), implying that PIAS1 serves as a potential E3 ligase for SUMOylation of ATF6. Interestingly, mutation in the conserved SUMOylation residue Lys87 (K1R) only slightly reduced the formation of ATF6-SUMO complex, while the mutations in the conserved SUMOylation residue Lys149 (K2R) or both Lys87 and Lys149 (K1,2R) almost completely abolished the SUMOylation event on the activated ATF6 (Figure 1B, lower panel lanes 3, 4 and 5). These results suggest that ATF6 can be SUMOylated through SUMO-1 and PIAS1 at the conserved sumoylation Lys149, but not Lys87, which is located at the N-terminal trans-activation domain of ATF6.
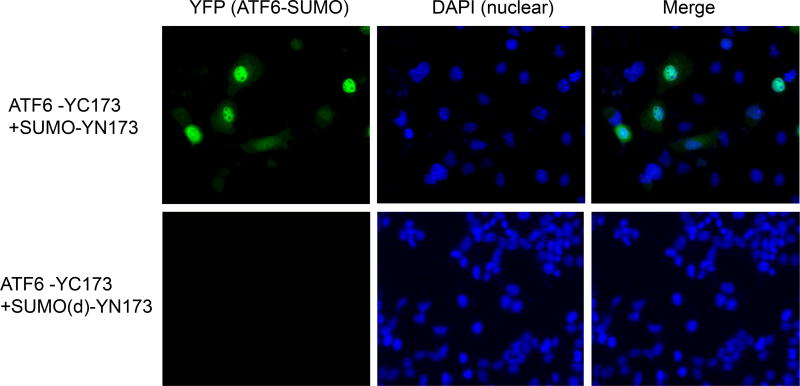
To confirm SUMOylation of ATF6 in living cells, we performed bimolecular fluorescence complementation (BiFC) analysis to visualize the ATF6 sumoylation event. BiFC is based on the association of fluorescent protein fragments that are attached to components of the same macromolecular complex (Supplemental figure 3). Interaction of these proteins will bring the fluorescent fragments within proximity, allowing the reporter protein to emit its fluorescent signal in living cells [18,19]. Therefore, this approach enables simultaneous visualization of intensity and subcellular localization of protein interactions/modifications. We fused the N-terminal fragment of the yellow fluorescent protein (YFP) to SUMO-1 and the complementary YFP fragment to the activated form of ATF6, and then expressed them in COS-1 cells. Fluorescence was observed predominantly in the nuclei of the cells expressing both ATF6 and SUMO-1 fusion proteins (Figure 2, upper panel). As a control, we used a SUMO-1 fluorescent fusion protein with the deletion of SUMO1 C-terminal double glycine residues, which is required for SUMO1 conjugation on the the lysine residue of a target protein [18]. Deletion of the C-terminus double glysine residues abolished the fluorescence complementation in COS-1 cells co-transfected with the ATF6 fusion protein (Figure 2, lower panel). Taken together, the BiFC analysis confirmed that the activated ATF6 protein can be SUMOylated and that the ATF6 sumoylation occurs in the nuclei.
Figure 2.
Visualization of SUMO-1 conjugated to ATF6 in living cells through bimolecular fluorescence complementation (BiFC) analysis. The activated form of human ATF6 protein fused with fluorescent fragment YC173 (ATF6-YC173) and human SUMO-1 protein or its C-terminal glycine residues-deleted isoform fused with fluorescent fragment YN173 (SUMO-YN173 or SUMO(d)-YN173) were co-expressed in COS-1 cells (Refs, see Supplemental figure 2 for more details). Green fluorescence images were acquired 36 h after plasmid transfection. The cell nuclei were stained with DAPI.
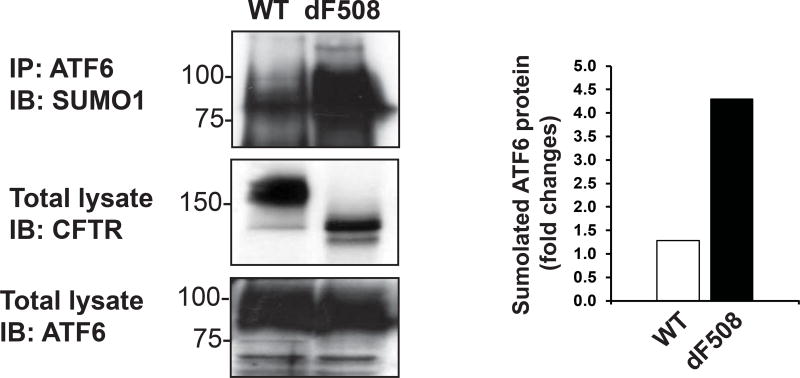
To identify whether SUMOylation of ATF6 is a cause of pathophysiological UPR related to human disease, we examined ATF6 sumoylation in the human tracheal cell line, CFBE41o-dF508CFTR, which stably expresses human mutant CFTR protein (dF508CFTR), and the control cell line, CFBE41o-WT CFTR, which stably expresses the wild-type human CFTR protein [14]. It’s known that dF508CFTR is a typical misfolded protein that could induce UPR and cleaned by ERAD pathway [15]. IP-Western blot analysis showed that SUMOylation of AFT6 protein was increased approximately 4 times in CFBE41o-dF508CFTR cells, compared to that in CFBE41o-WT CFTR cells (Figure 3). This not only confirmed the existence of ATF6 SUMOylation in pathophysiological UPR, but also suggested that the protective role of ATF6-mediated UPR may be diminished in CFTR syndrome where ER stress caused by misfolded CFTR protein gets prolonged.
Figure 3.
Sumoylation of ATF6 in the human tracheal cell line stably expressing CFBE41o-WT CFTR or CFBE41o-dF508CFTR. Total cellular lysates from CFBE41o-WT CFTR- or CFBE41o-dF508CFTR-expressing cells were immunoprecipitated using the anti-AFT6 antibody, and then probed with the anti-SUMO1 antibody to detect the sumoylated ATF6 protein (Upper panel). Expression of CFBE41o-WT CFTR and CFBE41o-dF508CFTR was detected by Western blot analysis using the anti-CFTR antibody (middle panel). The wild-type (WT) CFTR protein displayed C band (full glycosylation form, material band) and B band (core glycosylation form, immaterial band), and the dF508CFTR protein displayed B band and A band (none glycosylation form). As the control, expression of total AFT6 protein was also detected by Western blot analysis (lower panel). The graph beside the image showed the quantification of fold changes of sumoylated ATF6 potein levels, as determined by Western blot densitometry, in the CFBE41o-WT CFTR- or CFBE41o-dF508CFTR-expressing human tracheal cells.
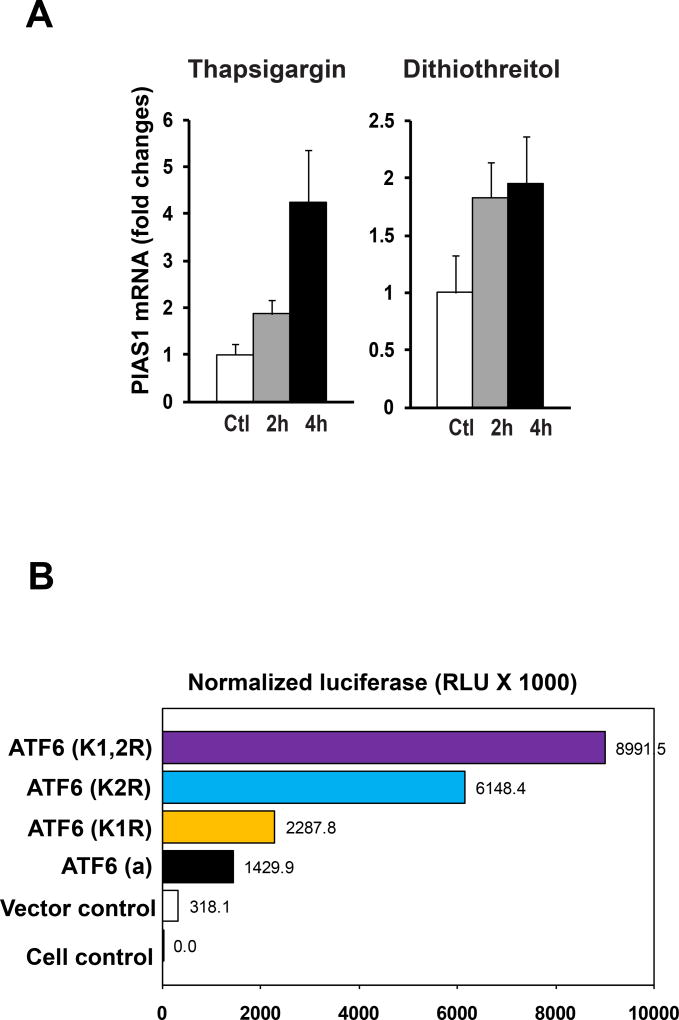
Further, we asked about the biological significance of ATF6 SUMOylation. As a UPR transducer, ATF6 functions as a transcription factor to recognize the unfolded protein response element (UPRE) in the promoter region of UPR target genes [17,20]. We hypothesized that SUMOylation may modulate the trans-activation activity of ATF6 on the UPRE-containing UPR gene promoters during UPR. To test this hypothesis, we first examined ER stress-triggered induction of PIAS1, the key regulatory E3 ligase that mediates ATF6 SUMOylation (Figure 1B) [11]. We challenged mouse embryonic fibroblast (MEF) with ER stress-inducing reagent thapsigargin or dithiothreitol [21]. In response to thapsigargin or dithiothreitol, expression of the gene encoding PIAS1 was significantly increased in MEF cells (Figure 4A), suggesting that induction of PIAS1 is ER stress-inducible. To determine whether SUMOylation regulates the transcriptional activity of ATF6, we analyzed the trans-activation activities of different isoforms of human ATF6 on the UPRE reporter in MEF cells. As expected, expression of the activated form of ATF6 increased the UPRE reporter activity (Figure 3B). Mutation of the conserved SUMOylation residue Lys87 (K1R) slightly increased the transcription activity of ATF6. However, mutations in the conserved SUMOylation residue Lys149 (K2R) increased the trans-activation activities of ATF6 by approximately 2 times relative to non-mutation ATF6 isoform, while mutations at both SUMOylation residues Lys87 and Lys149 (K1,2R) further increased ATF6 transcriptional activity (Figure 4B). These results suggest that the conserved SUMOylation residue Lys149, but not Lys87, is primarily responsible for the ATF6 SUMOylation that represses transcriptional activity of ATF6. This result is consistent with the IP-Western blot analysis, which indicated that the mutation at Lys149 abolished the majority of the interaction between ATF6 and SUMO-1 (Figure 1B). Since only a small portion of ATF6 proteins are SUMOylated as indicated by IP-Western analysis (Figure 1B), our findings suggest that SUMOlation may preferably occur in the functional ATF6.
Figure 4.
(A) Expression of the gene encoding PIAS1 is ER stress-inducible. Quantitative real-time PCR analysis of expression of the PIAS1 mRNA in MEF cells in response to thapsigargin (0,2µM) or dithiothreitol (5µg/ml) for 2 and 4 hours, respectively. Expression values were normalized to β-actin mRNA levels. Fold changes of mRNA are shown by comparing the mRNA expression values of treated cells to that of the control cells. Each bar denotes the mean ± STDEV (n=3 experiments). (B) Sumoylation represses the transcriptional activity of ATF6 on the UPRE-containing reporter. The plasmid vector control, the vector expressing the activated human ATF6 or its mutant isoforms K1R, K2R, or K1,2R, the luciferase reporter vector under the control of UPRE, and the internal control vector expressing β-galactosidase (LacZ) under the control of CMV (cytomegalovirus) promoter (pCMV-lacZ) were co-transfected into COS1 cells. The luciferase activities were measured at 36 hours post transfection, and the values were shown after the normalization to the internal control LacZ levels.
Discussion
In this study, we demonstrated that ER stress can cause sumoylation of the UPR transducer ATF6, a post-translational modification that usually represses activities of target proteins. This ER stress-induced post-translational modification represents a negative feedback regulation of the UPR signaling. In particularly, ATF6 is a major transcriptional activator of ERAD pathway. Sumoylation of ATF6 likely has important impact on the ER protein homeostasis that is associated with conformational diseases. In this perspective,
Mutation in the human gene encoding CFTR, a membrane chloride channel, can cause accumulation of misfolded CFTR protein and induce cystic fibrosis, one of the most common human genetic disorders [22]. Phenylalanine deletion at position of 508 of CFTR protein (dF508 CFTR) is the major CFTR mutation that results in misfolded CFTR protein in human patients. Misfolded dF508 CFTR protein is accumulated in the ER and subsequently causes UPR signaling and ERAD pathway [14,15]. ERAD is the key protective mechanism to maintain ER protein homeostasis in case of accumulation of misfolded proteins in the ER. Indeed, more than 99% of dF508CFTR protein can be degraded by ERAD, while only 25% of wild-type CFTR is degraded by the same pathway [23]. However, as shown in this study, human tracheal cells stably expressing the misfolded CFTR protein, dF508CFTR, exhibited significantly increased sumoylation of ATF6, a major transcriptional activator of ERAD program. This suggests that chronic ER stress, caused by constitutive expression of misfolded CFTR protein, eventually led to sumoylation of ATF6 and subsequent suppression of ERAD, thereby exacerbating the detrimental effect of prolonged ER stress.
In aggregate, our study suggested that the UPR transducer ATF6 can be SUMOylated and that the SUMO modification represses the transcriptional activity of ATF6. Together with our findings that ER stress induces PIAS1 expression, it is likely that PIAS1-mediated SUMOylation of ATF6 may serve as a negative feed-back regulation under ER stress. Similar to our observation, it was reported that SUMOylation also suppresses the transcriptional activity of another UPR trans-activator protein X-box binding protein 1 (XBP1) [24]. Given the potent transcriptional activities of XBP1 and ATF6, this negative feed-back through SUMOylation may be an important mechanism through which the stressed cells avoid harmful effects of the UPR signaling. Our work has raised lots of intriguing questions. In the future, it is important to elucidate the SUMO-mediated regulation of the UPR signaling in phathophysiological systems. The information gained from our study not only contributes to our understanding of the negative regulation of the UPR but also be informative to the modulation of the UPR for therapeutic benefits.
Supplementary Material
The Unfolded Protein Response transducer ATF6 protein is modified through SUMOylation under endoplasmic reticulum (ER) stress as a feedback regulation.
SUMOylation of ATF6 protein is mediated through the small ubiquitin-like modifier protein 1 (SUMO-1) and E3 SUMO-protein ligase 1 (PIAS1).
SUMOylation represses the transcriptional activity of ATF6.
Expression of misfolded cystic fibrosis transmembrane conductance regulator (CFTR) encoded by the mutant human CFTR gene (dF508CFTR) leads to ATF6 SUMOylation.
Acknowledgments
Portions of this work were supported by National Institutes of Health (NIH) grants DK090313 and ES017829 to KZ, NIH grants R01AI079056 to DF, NIH grant R01 AR066634 to DF and KZ, NIH grant HL133162 to FS, Department of Defense (DOD) grant BC095179P1 to KZ, and Cystic Fibrosis Foundation grant SUN15XX0 to FS.
Abbreviations
- ER
endoplasmic reticulum
- UPR
unfolded protein response
- ATF6
activating transcription factor 6
- CFTR
Cystic Fibrosis Transmembrane Conductance Regulator
- XBP1
X-box binding protein 1
- SUMOylation
small ubiquitin-like modification
- BiFc
bimolecular fluorescence complementation
- UPRE
unfolded protein response element
- PIAS1
protein inhibitor of activated STAT 1
- YFP
yellow fluorescent protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest
The authors X.H., Z.Y., K.Z., D.F. and F.S. have no conflicts of interest to claim
References
- 1.Zhang K, Kaufman RJ. Signaling the unfolded protein response from the endoplasmic reticulum. J Biol Chem. 2004;279:25935–25938. doi: 10.1074/jbc.R400008200. [DOI] [PubMed] [Google Scholar]
- 2.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 3.Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454:455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oyadomari S, Harding HP, Zhang Y, Oyadomari M, Ron D. Dephosphorylation of translation initiation factor 2alpha enhances glucose tolerance and attenuates hepatosteatosis in mice. Cell Metab. 2008;7:520–532. doi: 10.1016/j.cmet.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rutkowski DT, Wu J, Back SH, Callaghan MU, Ferris SP, Iqbal J, Clark R, Miao H, Hassler JR, Fornek J, Katze MG, Hussain MM, Song B, Swathirajan J, Wang J, Yau GD, Kaufman RJ. UPR pathways combine to prevent hepatic steatosis caused by ER stress-mediated suppression of transcriptional master regulators. Dev Cell. 2008;15:829–840. doi: 10.1016/j.devcel.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bommiasamy H, Back SH, Fagone P, Lee K, Meshinchi S, Vink E, Sriburi R, Frank M, Jackowski S, Kaufman RJ, Brewer JW. ATF6alpha induces XBP1-independent expansion of the endoplasmic reticulum. J Cell Sci. 2009;122:1626–1636. doi: 10.1242/jcs.045625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu J, Ruas JL, Estall JL, Rasbach KA, Choi JH, Ye L, Bostrom P, Tyra HM, Crawford RW, Campbell KP, Rutkowski DT, Kaufman RJ, Spiegelman BM. The unfolded protein response mediates adaptation to exercise in skeletal muscle through a PGC-1alpha/ATF6alpha complex. Cell Metab. 2011;13:160–169. doi: 10.1016/j.cmet.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haze K, Yoshida H, Yanagi H, Yura T, Mori K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol. Biol Cell. 1999;10:3787–3799. doi: 10.1091/mbc.10.11.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ye J, Rawson RB, Komuro R, Chen X, Dave UP, Prywes R, Brown MS, Goldstein JL. ER Stress Induces Cleavage of Membrane-Bound ATF6 by the Same Proteases that Process SREBPs. Mol Cell. 2000;6:1355–1364. doi: 10.1016/s1097-2765(00)00133-7. [DOI] [PubMed] [Google Scholar]
- 10.Hay RT. SUMO: a history of modification. Mol Cell. 2005;18:1–12. doi: 10.1016/j.molcel.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 11.Rytinki MM, Kaikkonen S, Pehkonen P, Jaaskelainen T, Palvimo JJ. PIAS proteins: pleiotropic interactors associated with SUMO. Cell Mol Life Sci. 2009;66:3029–3041. doi: 10.1007/s00018-009-0061-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodriguez MS, Dargemont C, Hay RT. SUMO-1 conjugation in vivo requires both a consensus modification motif and nuclear targeting. J Biol Chem. 2001;276:12654–12659. doi: 10.1074/jbc.M009476200. [DOI] [PubMed] [Google Scholar]
- 13.Geiss-Friedlander R, Melchior F. Concepts in sumoylation: a decade on. Nat Rev Mol Cell Biol. 2007;8:947–956. doi: 10.1038/nrm2293. [DOI] [PubMed] [Google Scholar]
- 14.Ramachandran S, Osterhaus SR, Parekh KR, Jacobi AM, Behlke MA, McCray PB., Jr SYVN1, NEDD8, and FBXO2 Proteins Regulate DeltaF508 Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Ubiquitin-mediated Proteasomal Degradation. J Biol Chem. 2016;291:25489–25504. doi: 10.1074/jbc.M116.754283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartoszewski R, Rab A, Fu L, Bartoszewska S, Collawn J, Bebok Z. CFTR expression regulation by the unfolded protein response. Methods Enzymol. 2011;491:3–24. doi: 10.1016/B978-0-12-385928-0.00001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun F, Zhang R, Gong X, Geng X, Drain PF, Frizzell RA. Derlin-1 promotes the efficient degradation of the cystic fibrosis transmembrane conductance regulator (CFTR) and CFTR folding mutants. J Biol Chem. 2006;281:36856–36863. doi: 10.1074/jbc.M607085200. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Shen J, Arenzana N, Tirasophon W, Kaufman RJ, Prywes R. Activation of ATF6 and an ATF6 DNA Binding Site by the Endoplasmic Reticulum Stress Response. J Biol Chem. 2000;275:27013–27020. doi: 10.1074/jbc.M003322200. [DOI] [PubMed] [Google Scholar]
- 18.Fang D, Kerppola TK. Ubiquitin-mediated fluorescence complementation reveals that Jun ubiquitinated by Itch/AIP4 is localized to lysosomes. Proc Natl Acad Sci U S A. 2004;101:14782–14787. doi: 10.1073/pnas.0404445101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu CD, Chinenov Y, Kerppola TK. Visualization of interactions among bZIP and Rel family proteins in living cells using bimolecular fluorescence complementation. Mol Cell. 2002;9:789–798. doi: 10.1016/s1097-2765(02)00496-3. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto K, Yoshida H, Kokame K, Kaufman RJ, Mori K. Differential contributions of ATF6 and XBP1 to the activation of endoplasmic reticulum stress-responsive cis-acting elements ERSE, UPRE and ERSE-II. J Biochem. 2004;136:343–350. doi: 10.1093/jb/mvh122. [DOI] [PubMed] [Google Scholar]
- 21.Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes. Dev. 1999;13:1211–1233. doi: 10.1101/gad.13.10.1211. [DOI] [PubMed] [Google Scholar]
- 22.Cheng SH, Gregory RJ, Marshall J, Paul S, Souza DW, White GA, O'Riordan CR, Smith AE. Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell. 1990;63:827–834. doi: 10.1016/0092-8674(90)90148-8. [DOI] [PubMed] [Google Scholar]
- 23.Henderson MJ, Vij N, Zeitlin PL. Ubiquitin C-terminal hydrolase-L1 protects cystic fibrosis transmembrane conductance regulator from early stages of proteasomal degradation. J Biol Chem. 2010;285:11314–11325. doi: 10.1074/jbc.M109.044057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen H, Qi L. SUMO modification regulates the transcriptional activity of XBP1. Biochem J. 2010;429:95–102. doi: 10.1042/BJ20100193. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.