Abstract
Motor stereotypies are abnormally repetitive behaviors that can develop with excessive dopaminergic stimulation and are features of some neurologic disorders. To investigate the mechanisms required for the induction of stereotypy, we examined the responses of dopamine-deficient (DD) mice to increasing doses of the dopamine precursor l-DOPA. DD mice lack the ability to synthesize dopamine (DA) specifically in dopaminergic neurons yet exhibit robust hyperlocomotion relative to wild-type (WT) mice when treated with l-DOPA, which restores striatal DA tissue content to ≈10% of WT levels. To further elevate brain DA content in DD mice, we administered the peripheral l-amino acid decarboxylase inhibitor carbidopa along with l-DOPA (C/l-DOPA). When striatal DA levels reached >50% of WT levels, a transition from hyperlocomotion to intense, focused stereotypy was observed that was correlated with an induction of c-fos mRNA in the ventrolateral and central striatum as well as the somatosensory cortex. WT mice were unaffected by C/l-DOPA treatments. A D1, but not a D2, receptor antagonist attenuated both the C/l-DOPA-induced stereotypy and the c-fos induction. Consistent with these results, stereotypy could be induced in DD mice by a D1, but not by a D2, receptor agonist, with neither agonist inducing stereotypy in WT mice. Intrastriatal injection of a D1 receptor antagonist ameliorated the stereotypy and c-fos induction by C/l-DOPA. These results indicate that activation of D1 receptors on a specific population of striatal neurons is required for the induction of stereotypy in DD mice.
Motor stereotypies, which are excessive and repetitive behaviors, can be induced with repeated or high doses of psychomotor stimulants and are features of some psychiatric and neurologic disorders such as schizophrenia, obsessive-compulsive disorder, and Lesch–Nyhan disease (1, 2). The last is characterized by a substantial reduction in brain dopamine (DA) levels early in development, with subsequent behavioral spasticity and self-injurious behavior (2). Stereotypic behaviors due to l-DOPA therapy have also been observed in Parkinson's disease patients (3). Lesion experiments using the catecholamine neurotoxin 6-hydroxydopamine (6-OHDA), in adult or neonatal rats, reproduced some of the motor dysfunctions of Parkinson's and Lesch–Nyhan diseases, respectively (4–6). In each case, the chronic reduction of DA leads to a compensatory increase in the sensitivity to DA (7–9). The hypersensitivity to DA promotes susceptibility to stereotypy when dopaminergic circuits are activated, as may occur in schizophrenia (10) and with chronic exposure to psychomotor stimulants like cocaine and amphetamine (11).
The role of DA and the striatum in the expression of stereotypy is well established, primarily from studies of animal models of dopaminergic supersensitivity (12–14). Yet, there are substantial differences, depending on the model system studied, particularly in the relative roles of D1 and D2 receptors. In 6-OHDA lesion experiments, the severity of the stereotypy that can be elicited by DA receptor agonists and the contributions of D1 and D2 receptors depends on the developmental timing of the lesion and the extent of surviving dopaminergic neurons (6, 15). In animals sensitized to amphetamine or lesioned with 6-OHDA, there is an enhancement of both pre- and postsynaptic dopamine function (6, 11). Hence, the mechanisms underlying the induction of stereotypy remain unclear, and several unresolved questions persist: (i) What are the relative roles of D1 and D2 receptors in the induction of stereotypic behaviors when dopaminergic neurons remain intact? (ii) Is postsynaptic stimulation of DA receptors sufficient to induce stereotypy?
To address these questions, we used mice with a selective inactivation of tyrosine hydroxylase in dopaminergic neurons, thus eliminating DA but not norepinephrine or epinephrine synthesis (16). Dopamine-deficient (DD) mice are unique in that the loss of DA is specific and complete, yet dopaminergic neurons remain intact and presumably are capable of regulated neurotransmitter release. DD mice are born normally, but by 3–4 weeks of age they become hypoactive and aphagic and will die without intervention. Daily administration of l-DOPA restores striatal DA to ≈10% of normal levels for a few hours and results in an intense bout of locomotion, during which time the mice eat enough to survive (16, 17). This hypersensitivity is due to enhanced responsiveness of postsynaptic neurons to DA with no change in the abundance of DA receptors (18). DD mice were used to address the above questions because the presence of intact dopaminergic neurons made it possible to control DA levels by exogenous l-DOPA administration, and in the absence of DA, specific receptor agonists could be used to selectively activate either D1 or D2 receptors.
Materials and Methods
Behavioral Studies.
Mice were maintained under conditions approved by the National Institutes of Health and the University of Washington Animal Care Committee. The wild-type (WT) and DD mice used were 3- to 8-month-old male and female mice maintained on a mixed 129/SvEv × C57BL/6J background. The DD mice (Th−/−, DbhTh/+) were created as described (16). The WT mice used were littermate controls of the DD mice and were any combination of the following genotypes: Th+/+ or Th+/− and Dbh+/+ or DbhTh/+. They were housed singly or in pairs and maintained on a 12-h light/dark cycle with lights on at 07:00. Food and water were freely available.
Drug Administration.
Haloperidol (SoloPak Laboratories, Franklin Park, IL) and SCH23390 (Research Biochemicals) were dissolved in 10 mM sodium phosphate, 150 mM NaCl, pH 7.5 (PBS). (±)SKF81297 and quinpirole (Research Biochemicals) were dissolved in water. Agonists and antagonists were injected in a volume of 10 μl/g. l-DOPA (Sigma) was prepared as a 1.5 mg/ml stock in a 2.5 mg/ml ascorbic acid solution dissolved in PBS. S-(−)carbidopa (Sigma) was dissolved in the l-DOPA solution. Unless noted, all drugs were administered i.p. in a volume not exceeding 1 ml. All antagonists were delivered 15 min before either l-DOPA or carbidopa plus l-DOPA (C/l-DOPA). DD mice were used 24 h after their last l-DOPA (50 mg/kg) injection.
Locomotor Activity.
Activity chambers (40 × 20 × 20 cm) were placed in a rack containing four infrared photobeams spaced 8.8 cm apart, and photobeam interruptions were recorded electronically with pasf software (San Diego Instruments). One ambulation was defined as two different sequential beam breaks and was used as a relative measure of distance traveled (0.088 m per ambulation). Mice naive to the activity chambers were allowed to acclimate for 1 day before experiments and were then placed in the chambers 2 h before treatment each subsequent day.
Stereotypy.
Stereotypic behavior was recorded simultaneously with locomotor activity. Mice were videotaped for a 5-min interval at a specified time point after the administration of the indicated dopaminergic drug. An observer blind to the treatments scored the videotapes by glancing at the tape every 10 s during that 5-min interval and recording the behavior. A modification of the stereotypy rating scale used by Creese and Iverson (19) for amphetamine-treated rats was used: 0, no movement; 1, ambulations; 2, eating; 3, sniffing; 4, rearing; 5, vigorous grooming; 6, taffy pulling. “Taffy pulling” is defined as a continuous movement of clasped forepaws toward and then away from the mouth (5).
Dopamine Measurements.
WT and DD mice were killed by CO2 asphyxiation, and the brains were immediately removed and placed in ice-cold PBS for 2 min. Brain regions of interest were dissected on a glass plate kept on wet ice, frozen in Eppendorf tubes in dry ice, and stored at −80°C until analysis. Dopamine and 3,4-dihydroxyphenylacetic acid measurements were performed by HPLC with electrochemical detection as described (20).
In Situ Hybridization.
At a specified time after drug treatment, mice were killed and the brains were removed and frozen in cold isopentane kept on dry ice. Coronal sections (20 μm) were cut with a cryostat, thaw-mounted on Fisherbrand Superfrost microscope slides, and kept at −80°C until they were used in the hybridization assay. Tissue sections were processed as described (21). For the detection of c-fos mRNA, an antisense RNA probe labeled with [35S]UTP was transcribed with SP6 RNA polymerase from a linearized rat c-fos cDNA template. Tissue sections were hybridized at 58°C and washed at 62°C. The final concentration of the probe was 1.5 pmol probe/ml hybridization mix. After washing and dehydration through a graded alcohol series, slides were apposed to Hyperfilm-βmax (Amersham Pharmacia) for 6–10 days before development. Sense strand controls for the c-fos riboprobe produced no detectable signal above background.
Cannula Implantation.
Mice were anesthetized with ketamine/xylazine (6.5 mg/ml and 0.44 mg/ml, i.p.) at a dose of 20 μl/g (body weight). Animals were placed in a stereotaxic frame (Anilam Instruments, Brea, CA), and the head was leveled, with bregma, lambda, and the sagittal suture as landmarks. For intracerebroventricular cannula placement, a single 24-gauge cannula was placed in the lateral ventricle: −0.22 anterior–posterior, 1.00 lateral, and 2.30 dorsal—ventral, with bregma as the reference point (22). For bilateral intrastriatal cannula placement, two 24-gauge cannulas were lowered simultaneously to coordinates +0.86 anterior–posterior, ±2.00 medial–lateral, +3.25 dorsal–ventral. Animals were allowed to recover for 1 week before experimentation. Injections were done in a volume of 1 μl per side at a constant rate of 0.33 μl/min, with 30-gauge injector needles and an infusion pump (KD Scientific, New Hope, PA). Cannula placement was verified after the experiments by cresyl violet staining of 20-μm coronal sections through the appropriate regions.
Results
Behavioral and Neurochemical Responses to Increasing Brain DA Content.
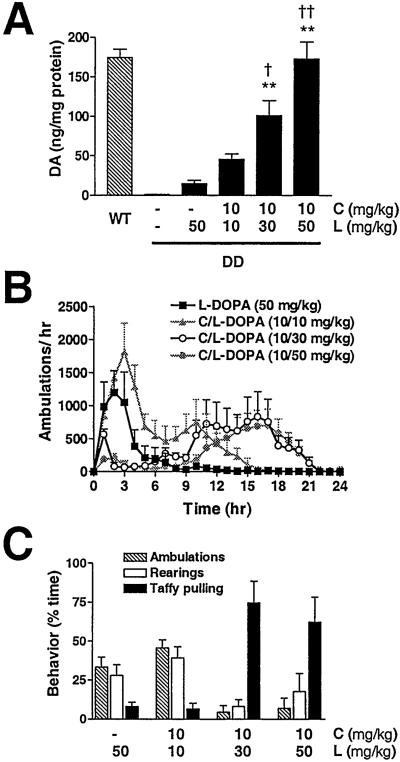
Adult DD mice were treated with l-DOPA (50 mg/kg) or with carbidopa (10 mg/kg) and increasing concentrations of l-DOPA (10, 30, or 50 mg/kg) (C/l-DOPA 10/10, 10/30, or 10/50, respectively). Carbidopa blocks peripheral l-aromatic amino acid decarboxylase activity, thus allowing more l-DOPA to reach the brain. Three hours after drug administration, tissue DA content was determined (Fig. 1A). l-DOPA (50 mg/kg) increased striatal DA content to 9% of WT levels. C/l-DOPA at ratios of 10/10, 10/30, and 10/50 increased striatal DA to 26%, 58%, and 100% of WT levels, respectively. DA levels were relatively low (≈2 ng/mg protein) in the midbrain of WT mice, and C/l-DOPA at ratios of 10/30 and 10/50 elevated midbrain DA levels in the DD mice to 150% and 390% of WT, respectively (data not shown). Tissue content of DA is not always a reflection of release, but the ratio of the DA metabolite 3,4-dihydroxyphenylacetic acid (DOPAC) to DA is an index of DA turnover rate and is correlated with DA release. The DOPAC/DA ratio for WT mice (0.038 ± 0.003 SEM) was similar to that for mice treated with l-DOPA or C/l-DOPA at 10/10 or 10/30. However, DD mice treated with 10/50 C/l-DOPA had increased DA turnover (DOPAC/DA = 0.129 ± 0.012 SEM).
Figure 1.
Behavioral and neurochemical response of DD mice to increasing doses of l-DOPA. (A) DA levels measured by HPLC from striatal tissue isolated 3 h after drug treatments (n = 4–9 per group). **, P < 0.001 compared with DD mice plus l-DOPA (50 mg/kg); † and ††, P < 0.05 and P < 0.001 compared with DD mice plus C/l-DOPA (10/10 mg/kg). One-way ANOVA with Tukey's post hoc test. (B) Locomotor dose response of DD mice (n = 8) over 24 h. Untreated WT mice average ≈50 ambulations per hour. (C) Behavior of DD mice (n = 6) is reported as the percentage of time spent ambulating, rearing, or taffy pulling during a 5-min period 3 h after C/l-DOPA administration. C, carbidopa; L, l-DOPA. Data are expressed as means + SEM.
In a separate experiment, DD mice were treated with l-DOPA or C/l-DOPA, locomotor activity was measured for 24 h, and stereotypic behaviors were recorded by videotape 3 h after drug administration (Fig. 1 B and C). C/l-DOPA at 10/10 induced twice as much locomotor activity over a 24-h period as l-DOPA alone (Fig. 1B), but the expression of rearing and taffy pulling was the same (Fig. 1C). C/l-DOPA (10/30 and 10/50), however, induced a dramatic shift in behavior: there was an abbreviated period of locomotor hyperactivity that became increasingly interspersed with stereotypic behaviors like rearing, vigorous grooming, and taffy pulling (an extremely robust behavior where the mice sit back on their hind legs and repeatedly draw their front legs up to and away from their mouths) to the exclusion of locomotion (Fig. 1 B and C). At 3 h after C/l-DOPA (10/30 or 10/50), DD mice were spending 75% or 64% of their time taffy pulling, respectively. At ≈8 h, mice treated with C/l-DOPA (10/30 or 10/50) emerged from taffy pulling and became hyperactive for the following 10–12 h (Fig. 1B).
Treatment of DD mice with carbidopa alone had no effect on locomotion or feeding compared with saline injection (data not shown). WT mice failed to show any behavioral responses to l-DOPA or C/l-DOPA (10/50) that differed from saline injection (data not shown).
Induction of Striatal c-fos mRNA.
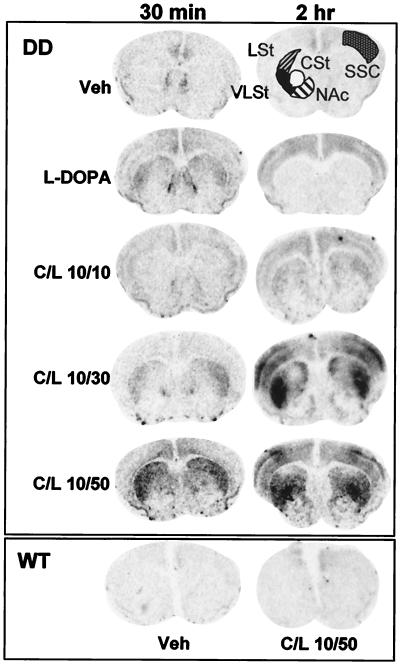
The induction of the immediate early gene c-fos is a reflection of increased cAMP and Ca2+-dependent intracellular signaling and is often used as a marker of neuronal activation (23). To assess the amount and neuroanatomical pattern of c-fos expression during the early locomotor and later stereotypic phases of behavior, DD mice were treated with saline, l-DOPA (50 mg/kg), or C/l-DOPA combinations and were killed 30 min or 2 h later. c-fos mRNA was assessed in coronal sections at the level of the midstriatum by in situ hybridization (Fig. 2). c-fos was induced in the lateral striatum at 30 min by l-DOPA (50 mg/kg) or C/l-DOPA (10/30 or 10/50). There was no detectable c-fos mRNA in the striatum at 30 min with the 10/10 C/l-DOPA dose. Low levels of c-fos mRNA were detected in the cingulate cortex, but not in the striatum, of DD mice treated with vehicle.
Figure 2.
Temporal and spatial expression of c-fos mRNA in response to increasing DA concentrations. Coronal sections are from mice killed 30 min or 2 h after drug injection. LSt, lateral striatum; VLSt, ventrolateral striatum; CSt, central striatum; NAc, nucleus accumbens; SSC, somatosensory cortex.
After 2 h, c-fos mRNA was gone from the striatum of l-DOPA-treated animals but present throughout the cortex. However, it was now detectable in the lateral striatum of 10/10 C/l-DOPA-treated animals. Interestingly, 2 h after C/l-DOPA at 10/30 and 10/50, the two groups that transition into stereotypy, there was a remarkable induction of c-fos in the ventrolateral striatum (VLSt), central striatum (CSt), and somatosensory cortex (SSC). There was little c-fos induction in the nucleus accumbens at either time point. WT mice treated with 10/50 C/l-DOPA had a low level of c-fos induction in the cingulate cortex (Fig. 2).
Dopamine D1, but Not D2, Receptor Antagonists Reduce Stereotypy.
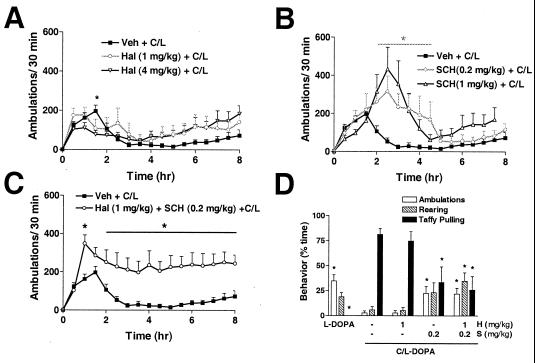
To determine the contribution of D1 and D2 receptors to C/l-DOPA-induced stereotypy, antagonists were administered separately or together 15 min before 10/50 C/l-DOPA. A low dose of haloperidol (1 mg/kg), a D2 receptor antagonist, had no significant effect on either locomotor activity (Fig. 3A) or the percentage of time the animals spent rearing or taffy pulling compared with C/l-DOPA alone (Fig. 3D), although there was a slight trend toward increased locomotor activity during the expression of focused stereotypies from 4 to 8 h (Fig. 3A). A higher dose of haloperidol (4 mg/kg) reduced locomotor activity slightly at 1.5 h after C/l-DOPA administration (Fig. 3A) but had no effect on the stereotypic response (data not shown). The D1 receptor antagonist, SCH23390 (0.2 mg/kg), prolonged the period of locomotor activity and delayed the C/l-DOPA-induced transition from only locomotion to only taffy pulling from 2 to 5 h (Fig. 3B). It reduced the percentage of time the animals spent taffy pulling at 3 h by 48% (Fig. 3D). The combination of haloperidol (1 mg/kg) and SCH23390 (0.2 mg/kg) completely prevented the decline in locomotion to only taffy pulling typically observed between 2 and 6 h (Fig. 3C) and reduced taffy pulling at 3 h by 55%, similar to the D1 receptor antagonist alone (Fig. 3D).
Figure 3.
Effect of DA receptor antagonists on C/l-DOPA-induced locomotion and stereotypy in DD mice. (A–C) C/L, C/l-DOPA (10/50 mg/kg) (■, n = 16). *, P < 0.05 compared with Veh + C/L; two-way ANOVA (drug × time) with matched samples and a Bonferroni post hoc test. (A) Hal, haloperidol (n = 8): 1 mg/kg (○) or 4 mg/kg (▿). (B) SCH, SCH23390 (n = 8): 0.2 mg/kg (○) or 1.0 mg/kg (▵). (C) Haloperidol (1 mg/kg) and SCH23390 (0.2 mg/kg) plus C/l-DOPA (10/50 mg/kg) (○, n = 8). (D) Behavior was videotaped at 3 h and scored for indicated behaviors. Mice were treated with SCH23390 (S) (0.2 mg/kg), haloperidol (H) (1 mg/kg), or both antagonists 15 min before C/l-DOPA (10/50). *, P < 0.05 compared with C/l-DOPA alone. One-way ANOVA with matched samples was done for each behavior with Dunnett's post hoc test.
Effect of Dopamine Receptor Antagonists on C/l-DOPA-Induced c-fos mRNA.
To test the hypothesis that induction of c-fos by C/l-DOPA is correlated with the behavioral response, the effects of DA receptor antagonists on C/l-DOPA-induced c-fos were assayed. Haloperidol (1 mg/kg), SCH23390 (0.2 mg/kg), or both antagonists were administered to DD mice 15 min before C/l-DOPA (10/50), and 2 h later the mice were killed for in situ hybridization. Haloperidol did not change the level or anatomical distribution of c-fos expression in the striatum or the SSC (Fig. 4D) compared with C/l-DOPA (10/50) alone (Fig. 4C). However, SCH23390 reduced c-fos expression in the VLSt and CSt—the regions of strongest signal in C/l-DOPA-treated animals—as well as the SSC (Fig. 4E). c-fos mRNA was still detectable in the lateral striatum and was greater than saline-treated controls (Fig. 4B). Haloperidol plus SCH23390 also reduced c-fos induction (Fig. 4F), and there were no obvious differences in this group versus SCH23390 alone plus C/l-DOPA.
Figure 4.
Effect of DA receptor antagonists on c-fos mRNA in DD mice 2 h after C/l-DOPA injection. Antagonists were administered 15 min before C/l-DOPA. (A) Representative schematic of coronal brain section. Ctx, cortex; CPu, caudate putamen; NAc, nucleus accumbens. (B) Saline-injected control. (C) C/l-DOPA (10/50 mg/kg). (D) Haloperidol (1 mg/kg) and C/l-DOPA. (E) SCH23390 (0.2 mg/kg) and C/l-DOPA. (F) Haloperidol (1 mg/kg), SCH23390 (0.2 mg/kg), and C/l-DOPA.
Intrastriatal Injection of a D1 Antagonist Blocks Stereotypy and c-fos Induction.
To determine whether the induction of c-fos was due to stimulation of striatal D1 receptors, SCH23390 (0.2 μg) was administered into the right lateral striatum 15 min before C/l-DOPA injection, and the mice were killed 2 h later for in situ hybridization. c-fos mRNA expression was reduced in the right striatum compared with the vehicle-treated left side (Fig. 5B). There was a lack of c-fos expression on both sides directly around the location of the cannula due to tissue damage, which can be seen in the cresyl violet-stained brain section (Fig. 5C). The extent of c-fos blockade by SCH23390 extended both dorsomedially and ventrolaterally to the cannula site (Fig. 5B) and rostrally and caudally (not shown). Slight c-fos expression was detected in the SSC, although the cannula track obscured much of it (Fig. 5B). The behavior of DD mice injected unilaterally with the D1 receptor antagonist plus C/l-DOPA (10/50) was a combination of turning ipsilateral to the SCH23390-injected side and bilateral stereotypy (data not shown).
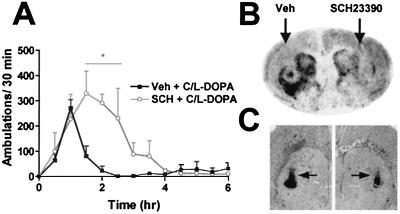
Figure 5.
Intrastriatal microinjection of SCH23390 reduces C/l-DOPA-induced stereotypy and c-fos expression. SCH23390 was administered via cannula 15 min before C/l-DOPA (10/50 mg/kg, i.p.). (A) Locomotor activity of DD mice (n = 3) treated with bilateral injections of SCH23390 (0.2 μg per side) and C/l-DOPA (10/50 mg/kg, i.p.) (○) or vehicle and C/l-DOPA (10/50 mg/kg, i.p.) (■) [*, P < 0.05, two-way ANOVA (drug × time) with Bonferroni's post hoc test]. (B) c-fos mRNA expression after unilateral injection of SCH23390 (0.2 μg on the right side) and C/l-DOPA (10/50 mg/kg, i.p.). (C) Cresyl violet stain of adjacent coronal section adjacent to B. Arrows indicate cannula tracks.
In a separate experiment, bilateral, intrastriatal administration of SCH23390 (0.2 μg per side) increased locomotor activity and delayed the onset of stereotypy induced by peripheral C/l-DOPA (Fig. 5A), similar to what was observed with systemic SCH23390.
A D1, but Not a D2, Receptor Agonist Induces Stereotypy in DD Mice.
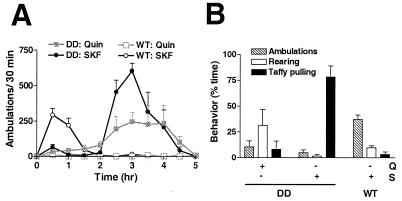
The D1 receptor agonist SKF81297 (10 mg/kg, i.p.) or the D2 receptor agonist quinpirole (1 mg/kg, i.p.) was administered to WT and DD mice, and then locomotor activity and stereotypy were assessed (Fig. 6 A and B). The doses of SKF81297 and quinpirole were chosen to be higher than those used to elicit locomotor behavior in DD mice (18). Taffy pulling was observed in DD mice almost immediately after SKF81297 injection and was scored at 15 min (Fig. 6B). At this time point, DD mice were primarily engaged in taffy pulling (78% of time), with some ambulations and rearing (Fig. 6B). At 2 h, they emerged from stereotypy and became hyperactive for the next 3 h (Fig. 6A). WT mice treated with SKF81297 were hyperlocomotive (Fig. 6A) compared with saline-treated WT mice (data not shown) for the first 2 h but did not exhibit taffy pulling (Fig. 6B). DD mice treated with quinpirole initially displayed behaviors such as splaying of the hind legs, sniffing, uncoordinated locomotion, and rearing (Fig. 6B). These behaviors evolved into more coordinated hyperlocomotion at ≈1.5 h, lasting until ≈3.5 h (Fig. 6A). WT mice treated with quinpirole appeared to be asleep or resting and did not engage in any stereotypic behaviors during the videotaped interval. Locomotor activity of quinpirole-treated WT mice was significantly reduced compared with saline-treated mice for the first hour after drug injection (data not shown).
Figure 6.
Effect of DA receptor agonists on locomotion and stereotypy. (A) Locomotor time course (n = 4) of quinpirole (1 mg/kg, i.p.) on DD mice (■) and WT mice (□) and SKF81297 (10 mg/kg, i.p.) on DD mice (●) and WT mice (○). (B) Stereotypy was recorded 15 min after agonist injection. Q, quinpirole (1 mg/kg, i.p.); S, SKF81297 (10 mg/kg, i.p.).
Discussion
Locomotor activity and stereotypic behaviors are thought to be competing motor activities that are mediated by distinct mechanisms (24). The present studies demonstrate that a transition from locomotor activity to intense stereotypic behavior occurs in DD mice when striatal DA is restored to >50% of WT levels, suggesting a threshold for the initiation of stereotypy. The time course of behavioral activation in DD mice by C/l-DOPA probably corresponds to the rise and fall of striatal DA levels. For example, as DA levels rise after DD mice are treated with C/l-DOPA at 10/30 or 10/50, the animals initially display hyperactive locomotion, but as the DA levels continue to rise toward 50% of WT levels they lapse into stereotypy, which lasts until DA levels fall below the threshold for stereotypy. At that point the mice become hyperlocomotive again until DA levels subside to <3% of WT, and then the mice become hypoactive. Because the DD mice are supersensitive to DA receptor agonists (16, 18), hyperactivity and stereotypy are achieved when DA levels are much less than WT levels. Consequently, none of the treatments that induce robust stereotypy in DD mice do so in WT mice.
Previous studies have shown that rats lesioned with 6-OHDA as neonates or adults or animals treated repeatedly with psychomotor stimulants become supersensitive to DA receptor agonists (5, 7, 11, 24). The emergence of focused stereotypies in these animals in response to DA receptor agonists is a common behavioral end point, suggesting similar underlying mechanisms. Rats lesioned as adults with 6-OHDA initially exhibit aphagia and akinesia, but a recovery of function usually arises from augmented synthesis and release of DA from the surviving dopaminergic terminals (6, 25), as well as enhanced signaling by postsynaptic striatal neurons. D2 receptor levels are increased, whereas D1 receptors are unchanged or decreased (26, 27). Adult-lesioned rats are primarily supersensitive to D2 agonists; quinpirole is able to induce stereotypic behaviors such as sniffing and rearing, but not taffy pulling (6). Rats lesioned neonatally with 6-OHDA have few behavioral deficits as adults, and the sparing of function seems to depend on remaining dopaminergic terminals (28). DA receptor levels are unchanged in neonatally 6-OHDA-lesioned rats (29), yet they are supersensitive primarily to D1 receptor activation (30), although this supersensitivity requires priming of the D1 receptor with repeated agonist exposure (31). Both D1 and D2 agonists can elicit stereotypic behaviors, including taffy pulling (15), and high doses of l-DOPA induce self-injurious behavior, which is thought to resemble that seen in Lesch–Nyhan disease (5). In our study, self-injurious behavior was occasionally observed in DD mice treated with C/l-DOPA (10/50). There are generally no changes in D1 or D2 receptor levels in rats sensitized to amphetamine (11). Amphetamine-induced hyperactivity and stereotypic behaviors can be blocked by either D1 or D2 receptor antagonists, and once sensitization has been induced, stereotypy can be elicited by agonists of either DA receptor (24, 32).
DD mice are also supersensitive to DA receptor agonists. We have shown that there is no recovery of function (16), unlike in neonatally 6-OHDA-lesioned rats. Despite the chronic absence of DA, there are no gross deficiencies in neurodevelopment or neuroanatomy; furthermore, D1 and D2 receptor levels and DA transporter levels are normal (16, 18). DD mice become hyperactive in response to either D1 or D2 receptor agonists, without priming (16, 18). Note that DD mice exhibit increased locomotor activity in response to a D2 receptor agonist, whereas locomotion is inhibited in WT mice, because D2 autoreceptor-induced inhibition of DA release is inconsequential in DD mice lacking DA. l-DOPA-induced locomotor activity can be attenuated by either D1 or D2 receptor antagonists (17, 18). These data highlight the importance of both D1 and D2 receptor activation for locomotor activity in DD mice.
Activation of the D1 receptor is primarily responsible for the induction of stereotypy in DD mice because taffy pulling is largely blocked by the D1 receptor antagonist SCH23390 but not by the D2 antagonist haloperidol. Furthermore, in the total absence of presynaptic DA, stimulation of striatal D1 receptors is sufficient to drive the animals into taffy pulling. The induction of taffy pulling by SKF81297 occurs almost immediately, suggesting that it does not depend on transcriptional or translational activation by DA. The combination of D1 and D2 receptor antagonists blocked the transition from locomotion to taffy pulling more effectively than the D1 receptor antagonist alone, which may reflect a contribution of D2 receptor signaling to maintenance, but not initiation, of stereotypy. The importance of D1 receptors for initiating stereotypy is substantiated by D1 receptor knockout mice, which are resistant to cocaine-induced stereotypy (33). Mice lacking D2 receptors manifest normal hyperlocomotion in response to amphetamine, but stereotypic responses have not been reported (34). The data for DD mice with intact dopaminergic neurons suggest, therefore, that either D1 or D2 receptor agonists can elicit locomotion, but only the D1 receptor can initiate the transition to stereotypy.
The dramatic induction of c-fos in the ventrolateral and central striatum, as well as the SSC, by C/l-DOPA (10/50) in DD mice suggests that these regions are important for mediating stereotypy, in agreement with reports that oral stereotypies can be induced by direct injection of amphetamine into the VLSt of rats (13). The induction of c-fos in the SSC is most likely due to the continuous and intense sensory stimulation of the vibrissa that occurs with taffy pulling. Psychostimulant-induced stereotypy has been correlated with an increased ratio of striosome (patches of the striatum enriched in D1 receptors) to matrix Fos expression (35). We predict that C/l-DOPA-induced c-fos mRNA expression may be primarily striosomal in DD mice as well.
Previous work has shown that a D1 agonist induces a uniform distribution of Fos protein expression in the striatum and nucleus accumbens of DD mice (18). This uniform distribution of Fos protein expression suggests that all D1 receptor-containing neurons are equally sensitive to D1 receptor stimulation, whereas the distinct regional patterns of c-fos mRNA observed in our studies after C/l-DOPA treatment reflect activity-dependent and regulated release of DA from dopaminergic neurons. We suggest that convergence of glutamate stimulation from cortical and thalamic inputs along with enhanced DA release to the VLSt and CSt initiates the transition to stereotypy. Preferential activation of these striatonigral neurons may become self-perpetuating while also inhibiting dopaminergic and cortical inputs to other striatal regions, which could account for the repetition of some activities to the exclusion of others and region-specific induction of c-fos. A circuit-based inhibition of inputs to the nucleus accumbens could explain the lack of c-fos expression in that region. It is worth noting that most movement-related striatonigral neurons decrease their firing rate as stereotypy emerges after a high dose of amphetamine (36), suggesting that D1 receptor activation does not necessarily translate into an increase in neuronal firing.
We considered the possibility that initiation of taffy pulling in DD mice may depend on activation of D1 receptors in the substantia nigra because midbrain DA levels were almost 4 times greater in C/l-DOPA-treated DD mice than in WT mice. In support of this hypothesis, l-DOPA-induced rotations can be blocked by intranigral infusion of SCH23390 (37). However; intrastriatal microinjection of SCH23390 delayed the C/l-DOPA transition from locomotor activity to stereotypy and reduced the induction of c-fos mRNA. Thus, we favor the interpretation that excessive D1 receptor activation in the VLSt and CSt is primarily responsible for inducing stereotypy in DD mice.
We have described behavioral and molecular responses of DD mice to increasing concentrations of DA and have shown that activation of postsynaptic striatal D1 receptors above a certain threshold is both necessary and sufficient for the induction and expression of the focused stereotypic behavior, taffy pulling. The cascade of events downstream of D1 receptor stimulation leads to increases in gene expression, for example, c-fos and neuropeptides such as neurotensin (E.H.C., unpublished observations), which could further modulate behavioral responses. Future studies identifying the molecular mechanisms underlying the supersensitivity in DD mice that facilitates the expression of DA-mediated stereotyped behaviors may help identify neurochemical alterations in disorders such as schizophrenia and amphetamine-induced psychosis.
Acknowledgments
We thank William Alaynick for cannula production and maintenance of animals and Douglas Kim and Mark Szczypka for helpful discussions. This work was supported in part by National Institutes of Health Grant NS-20311 (to D.M.D.), and a Department of Veterans Affairs Merit Review grant (to A.M.M.). E.H.C. was supported by a Molecular Pharmacology training grant.
Abbreviations
- DA
dopamine
- DD
dopamine-deficient
- C/l-DOPA
carbidopa plus l-DOPA
- 6-OHDA
6-hydroxydopamine
- WT
wild type
- VLSt
ventrolateral striatum
- CSt
central striatum
- SSC
somatosensory cortex
References
- 1.Ridley R M. Prog Neurobiol. 1994;44:221–231. doi: 10.1016/0301-0082(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 2.Visser J E, Bar P R, Jinnah H A. Brain Res Brain Res Rev. 2000;32:449–475. doi: 10.1016/s0165-0173(99)00094-6. [DOI] [PubMed] [Google Scholar]
- 3.Wolters E C. Neurology. 1999;52:S10–S13. [PubMed] [Google Scholar]
- 4.Ungerstedt U. Acta Physiol Scand Suppl. 1971;367:95–122. doi: 10.1111/j.1365-201x.1971.tb11001.x. [DOI] [PubMed] [Google Scholar]
- 5.Breese G R, Baumeister A A, McCown T J, Emerick S G, Frye G D, Crotty K, Mueller R A. J Pharmacol Exp Ther. 1984;231:343–354. [PMC free article] [PubMed] [Google Scholar]
- 6.Joyce J N, Frohna P A, Neal-Beliveau B S. Neurosci Biobehav Rev. 1996;20:453–486. doi: 10.1016/0149-7634(95)00025-9. [DOI] [PubMed] [Google Scholar]
- 7.Ungerstedt U. Acta Physiol Scand Suppl. 1971;367:69–93. doi: 10.1111/j.1365-201x.1971.tb11000.x. [DOI] [PubMed] [Google Scholar]
- 8.Zigmond M J, Stricker E M. Experientia. 1980;36:436–438. doi: 10.1007/BF01975133. [DOI] [PubMed] [Google Scholar]
- 9.Jinnah H A, Gage F H, Friedmann T. Behav Neurosci. 1991;105:1004–1012. doi: 10.1037//0735-7044.105.6.1004. [DOI] [PubMed] [Google Scholar]
- 10.Carlsson A, Waters N, Holm-Waters S, Tedroff J, Nilsson M, Carlsson M L. Annu Rev Pharmacol Toxicol. 2001;41:237–260. doi: 10.1146/annurev.pharmtox.41.1.237. [DOI] [PubMed] [Google Scholar]
- 11.Pierce R C, Kalivas P W. Brain Res Brain Res Rev. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- 12.Creese I, Iversen S D. Nat New Biol. 1972;238:247–248. doi: 10.1038/newbio238247a0. [DOI] [PubMed] [Google Scholar]
- 13.Kelley A E, Lang C G, Gauthier A M. Psychopharmacology (Berlin) 1988;95:556–559. doi: 10.1007/BF00172976. [DOI] [PubMed] [Google Scholar]
- 14.Conti L H, Segal D S, Kuczenski R. Psychopharmacology (Berlin) 1997;130:183–188. doi: 10.1007/s002130050227. [DOI] [PubMed] [Google Scholar]
- 15.Kostrzewa R M. Neurosci Biobehav Rev. 1995;19:1–17. doi: 10.1016/0149-7634(94)00019-w. [DOI] [PubMed] [Google Scholar]
- 16.Zhou Q-Y, Palmiter R D. Cell. 1995;83:1197–1209. doi: 10.1016/0092-8674(95)90145-0. [DOI] [PubMed] [Google Scholar]
- 17.Szczypka M S, Rainey M A, Kim D S, Alaynick W A, Marck B T, Matsumoto A M, Palmiter R D. Proc Natl Acad Sci USA. 1999;96:12138–12143. doi: 10.1073/pnas.96.21.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim D S, Szczypka M S, Palmiter R D. J Neurosci. 2000;20:4405–4413. doi: 10.1523/JNEUROSCI.20-12-04405.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Creese I, Iverson S D. Brain Res. 1973;55:369–382. doi: 10.1016/0006-8993(73)90302-8. [DOI] [PubMed] [Google Scholar]
- 20.Thomas S A, Marck B T, Palmiter R D, Matsumoto A M. J Neurochem. 1998;70:2468–2476. doi: 10.1046/j.1471-4159.1998.70062468.x. [DOI] [PubMed] [Google Scholar]
- 21.Merchant K M, Dorsa D M. Proc Natl Acad Sci USA. 1993;90:3447–3451. doi: 10.1073/pnas.90.8.3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franklin K B J, Paxinos G T. The Mouse Brain in Stereotaxic Coordinates. New York: Academic; 1997. [Google Scholar]
- 23.Dragunow M, Faull R. J Neurosci Methods. 1989;29:261–265. doi: 10.1016/0165-0270(89)90150-7. [DOI] [PubMed] [Google Scholar]
- 24.Segal D S, Mandell A J. Pharmacol Biochem Behav. 1974;2:249–255. doi: 10.1016/0091-3057(74)90060-4. [DOI] [PubMed] [Google Scholar]
- 25.Neve K A, Kozlowski M R, Marshall J F. Brain Res. 1982;244:33–44. doi: 10.1016/0006-8993(82)90901-5. [DOI] [PubMed] [Google Scholar]
- 26.Ariano M A. Brain Res. 1988;443:204–214. doi: 10.1016/0006-8993(88)91614-9. [DOI] [PubMed] [Google Scholar]
- 27.Joyce J N. Exp Neurol. 1991;113:261–276. doi: 10.1016/0014-4886(91)90016-6. [DOI] [PubMed] [Google Scholar]
- 28.Erinoff L, MacPhail R C, Heller A, Seiden L S. Brain Res. 1979;164:195–205. doi: 10.1016/0006-8993(79)90015-5. [DOI] [PubMed] [Google Scholar]
- 29.Breese G R, Duncan G E, Napier T C, Bondy S C, Iorio L C, Mueller R A. J Pharmacol Exp Ther. 1987;240:167–176. [PMC free article] [PubMed] [Google Scholar]
- 30.Breese G R, Napier T C, Mueller R A. J Pharmacol Exp Ther. 1985;234:447–455. [PubMed] [Google Scholar]
- 31.Criswell H, Mueller R A, Breese G R. J Neurosci. 1989;9:125–133. doi: 10.1523/JNEUROSCI.09-01-00125.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuczenski R, Segal D S. Brain Res. 1999;822:164–174. doi: 10.1016/s0006-8993(99)01149-x. [DOI] [PubMed] [Google Scholar]
- 33.Xu M, Hu X, Cooper D C, Moratalla R, Graybiel A M, White F J, Tonegawa S. Cell. 1994;79:945–955. doi: 10.1016/0092-8674(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 34.Chen J F, Moratalla R, Impagnatiello F, Grandy D K, Cuellar B, Rubinstein M, Beilstein M A, Hackett E, Fink J S, Low M J, et al. Proc Natl Acad Sci USA. 2001;98:1970–1975. doi: 10.1073/pnas.98.4.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Canales J J, Graybiel A M. Nat Neurosci. 2000;3:377–383. doi: 10.1038/73949. [DOI] [PubMed] [Google Scholar]
- 36.Ryan L J, Young S J, Segal D S, Groves P M. Behav Neurosci. 1989;103:3–14. doi: 10.1037//0735-7044.103.1.3. [DOI] [PubMed] [Google Scholar]
- 37.Robertson G S, Robertson H A. J Neurosci. 1989;9:3326–3331. doi: 10.1523/JNEUROSCI.09-09-03326.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]