Abstract
Dopamine signaling encodes reward learning and motivated behavior through modulation of synaptic signaling in the nucleus accumbens, and aberrations in these processes are thought to underlie obsessive behaviors associated with alcohol abuse. The nucleus accumbens is divided into core and shell sub-regions with overlapping but also divergent contributions to behavior. Here we optogenetically targeted dopamine projections to the accumbens allowing us to isolate stimulation of dopamine terminals ex vivo. We applied 5 pulse (phasic) light stimulations to probe intrinsic differences in dopamine release parameters across regions. Also, we exposed animals to 4 weeks of chronic intermittent ethanol vapor and measured phasic release. We found that initial release probability, uptake rate and autoreceptor inhibition were greater in the accumbens core compared to the shell, yet the shell showed greater phasic release ratios. Following chronic ethanol, uptake rates were increased in the core but not the shell, suggesting region-specific neuronal adaptations. Conversely, kappa opioid receptor function was upregulated in both regions to a similar extent, suggesting a local mechanism of kappa opioid receptor regulation that is generalized across the nucleus accumbens. These data suggest that dopamine axons in the nucleus accumbens core and shell display differences in intrinsic release parameters, and that ethanol-induced adaptations to dopamine neuron terminal fields may not be homogeneous. Also, chronic ethanol exposure induces an upregulation in kappa opioid receptor function, providing a mechanism for potential over-inhibition of accumbens dopamine signaling which may negatively impact downstream synaptic function and ultimately bias choice towards previously reinforced alcohol use behaviors.
Keywords: Dopamine, Nucleus Accumbens, Ethanol, Optogenetics, Voltammetry, Kappa Opioid Receptors
Introduction
Alcohol use disorders (AUD) and drug addiction share a common feature - persistent seeking and use despite delayed negative consequences on both physical and social health. These aberrant actions suggest abnormalities in the neural circuitry governing motivated behavior (Hyman et al. 2006; Stuber et al. 2010b), and have prompted research into elucidating ethanol induced neuroadaptations that may underlie the switch from casual to obsessive attention towards alcohol. An area of particular interest is the nucleus accumbens (NAc), which integrates diverse cognitive and limbic inputs onto motor plan-initiating output signals of the basal ganglia, implicating this region in motivation and behavioral selection. For instance, repeated drug exposure causes long term synaptic plasticity within the NAc that results in sensitized locomotor responses (Creed et al. 2015; Pascoli et al. 2011) and incubation of craving during withdrawal (Conrad et al. 2008), prompting increased attention on identifying the synaptic manifestations of chronic ethanol exposure in this region (Jeanes et al. 2014; Abrahao et al. 2013). Dopamine (DA) signaling in the NAc is thought to encode reward learning via modulation of synaptic signaling and gating aspects of synaptic plasticity (Shen et al. 2008; Surmeier et al. 2011; Tritsch and Sabatini 2012; Creed and Lüscher 2013), processes that are believed to ultimately bias decision making towards previously reinforced behaviors. Indeed, aberrations in DA signaling have been linked to obsessive/compulsive behaviors (Sesia et al. 2013) and behavioral rigidity (Beeler et al. 2014).
Kappa opioid (κ-opioid) receptors are expressed within the NAc where they inhibit DA signaling (Spanagel et al. 1992) upon activation by endogenous dynorphin, providing a negative feedback mechanism to regulate local DA levels (Steiner and Gerfen 1996). Further, the dynorphin/κ-opioid system appears to be upregulated following chronic ethanol exposure (Sirohi et al. 2012). Specifically, the κ-opioid receptor antagonist nor-binaltorphimine (nor-BNI) reduces the escalation of intake observed in alcohol dependent animals while having no effect on non-dependent animals (Walker and Koob 2008), suggesting that recruitment of κ-opioid receptor activity contributes to dependence and may provide a potential mechanism for ethanol-induced adaptations in DA transmission and the subsequent modulation of synaptic function (Shippenberg et al. 2007).
The NAc is divided into two sub-regions, the core and the shell, which receive unique assortments of afferent inputs and differentially contribute to reward aspects of behavior (Kelley 2004). The core is involved in reinforcement learning and adaptive instrumental behavior, while the shell is connected with viscero-endocrine effector systems involved in reward processing and motivational states (Kelley 1999). For example, by virtue of its afferent innervation from the ventral hippocampus (Britt et al. 2012) and efferent projections to the lateral hypothalamus (Kelley 2004) the shell is considered to be a component of the extended amygdala, a collection of structures heavily implicated in exaggerated stress and anxiety states during alcohol withdrawal (Koob 2013; Lovinger and Kash 2015). Both regions receive DA innervation from the ventral tegmental area (VTA); however, DA signaling does not appear to be homogenous across the NAc (Aragona et al. 2009). Acute drug and alcohol administration selectively increase DA release in the shell compared to the core (Di Chiara et al. 2004; Howard et al. 2008), and chronic drug exposure results in differential dopaminergic adaptations in the core and shell (Saddoris 2016; Saddoris et al. 2016). This is coupled with differences in phasic release parameters of DA terminal fields across regions (Jones et al. 1996a; Zhang et al. 2009), supporting region-specific heterogeneity in DA signaling.
One level of DA signal regulation occurs at the terminals, where expression of a variety of release-regulating heteroreceptors in the terminal membrane (Zhang and Sulzer 2012; Sulzer et al. 2016) allows local environmental influence of terminal physiology and results in diverse micro-domains within terminal fields (Wightman et al. 2007; Pickel 2000; Zhang et al. 2015; Tritsch et al. 2012). Fast-scan cyclic voltammetry (FSCV) is often used in ex vivo slice preparations to pharmacologically probe terminal receptor regulation of DA release and how terminal activity may be altered following chronic drug and alcohol administration (Ferris et al. 2013; Siciliano et al. 2015b; Calipari et al. 2015). However, relatively few of these investigations probe terminal fields in the medial NAc shell, partially due to the technical difficulties in obtaining robust, reliable DA release in this region. That is, a single pulse-stimulated DA signal is low in amplitude compared to neighboring regions such as the NAc core and dorsal striatum (Jones et al. 1996a). This can be overcome by applying multiple pulses in a stimulation train; however, electrical stimulation trains recruit modulation of DA terminals from concurrent excitation of the surrounding non-dopaminergic neuronal types within the tissue (Melchior et al. 2015a). This recruitment often distorts the stimulated DA signal and makes assessments of whether heteroreceptor modulation is occurring through direct (terminal receptors) or indirect (multi-synaptic) mechanisms difficult to determine. Here we use optogenetic tools to selectively light-stimulate DA terminals in the NAc, without concurrent electrical excitation of non-dopaminergic cells in the tissue, providing an isolated DA signal ex vivo.
The goal of these studies was to extend our investigations of chronic ethanol-induced alterations of DA signaling in the ventral striatum (Karkhanis et al. 2015; Rose et al. 2016; Siciliano et al. 2015a) across three novel parameters. 1) We measured DA signals in the NAc shell to provide a direct comparison to NAc core DA terminal fields in order to determine whether chronic ethanol exposure results in differential adaptations between regions. 2) We used targeted light stimulation, avoiding concurrent modulation of DA release by excitation of non-dopaminergic cells in the DA terminal field, to provide greater resolution with regard to direct vs indirect ethanol-induced DA terminal heteroreceptor adaptations. 3) We applied 5 pulse stimulation trains to approximate the phasic spikes in DA neurons that occur in response to discrete environmental cues in vivo.
Methods
Animals
Male Tyrosine Hydroxylase (TH)-internal ribosome entry site (IRES)-Cre Recombinase knock-in mice on a C57Bl/6J background (TH:Cre) were bred, genotyped for positive cre recombinase expression and maintained in group housing in the mouse colony. All animals were maintained according to the National Institutes of Health guidelines and all experimental protocols were approved by the Institutional Animal Care and Use Committee at Wake Forest University School of Medicine.
At 8–12 weeks of age mice were anesthetized with 100 mg/kg ketamine and 8 mg/kg xylazine and placed in a stereotaxic frame. A custom-made glass micropipette (80 μm outer diameter) was inserted directly above the VTA (coordinates from Bregma in mm: −3.3 AP, ±0.5 ML, −4.3 DV). Microinjections were administered using an air pressure injection system and consisted of applying small pulses of pressure (30 psi, 40–80 msec duration) to the infusion pipette. Individual injections were performed on each side of the midline resulting in bilateral VTA infusions, with each hemisphere of VTA receiving approximately 0.4 μl of AAV5-EF1α-hChR2(H134R)-eYFP (5.5 × 1012 virus molecules/ml; Virus Vector Core, University of North Carolina). Following surgery, mice were returned to the mouse colony, single housed, and maintained for a minimum of 14 days to recover from surgery and allow expression of channelrhodopsin-2 (ChR2).
Histology
Immunohistochemistry was used to verify ChR2 expression in dopamine axons in the nucleus accumbens as previously described (Melchior et al. 2015a). Mice were anesthetized with ketamine (100mg/kg) and xylazine (8 mg/kg) and transcardially perfused with phosphate-buffered saline (PBS) followed by 10% buffered formalin phosphate (Fischer Scientific, Waltham, MA, USA). Brains were then removed, submerged in 10% buffered formalin phosphate for an additional 24–48 hours, and subsequently transferred to 30% sucrose in PBS for 72 hours. Sections (40 μm) were obtained on a microtome (American Optical Company, Buffalo, NY, USA) and stored in PBS for immunohistochemistry.
Sections were permeablized in 0.3% triton (Sigma, St Louis, MO, USA) in PBS (PBS-Tx) for 2 hours, blocked in 5% normal goat serum (Vector Laboratories, Burlingame, CA, USA) in PBS-Tx, and incubated in primary antibody in the blocking solution for 24–48 hours. Primary antibodies include chicken anti-GFP (1 μg : 500 μl; Aves labs, Tigard, OR, USA) and rabbit anti-tyrosine hydroxylase (1 μg : 1000 μl; Cell Signaling, Danvers, MA, USA). Sections were rinsed and transferred to secondary antibody in blocking solution for 1.5 hours. Secondary antibodies include fluorescein-labeled goat anti-chicken IgY (Aves labs, 1 μg : 250 μl) and goat anti-rabbit alexa fluor 594 IgG (1 μg : 250 μl; Molecular Probes, Eugene, OR, USA). Sections were mounted on 1mm slides with Vectashield (Vector Labs) mounting medium and images were obtained with an Olympus BX-51 Microscope and Optronics Microfire digital camera (Goleta, CA, USA). Images were processed in Adobe Photoshop.
CIE
ChR2 expressing mice were administered the chronic intermittent ethanol (CIE) exposure protocol outlined on the website for the INIA-Stress research consortium (http://iniastress.org/dependence). Briefly, mice were injected with a ‘loading’ dose of ethanol (1.6 g/kg) and pyrazole (1 mmol/kg), an alcohol dehydrogenase inhibitor that stabilizes and maintains blood ethanol concentrations, and exposed to ethanol vapor in an air tight vapor chamber for 16 consecutive hours. This procedure is repeated, daily, for four consecutive days, followed by 3 days of exposure to room air with no injections - constituting one cycle of CIE. The ethanol treated group was exposed to 4 cycles of CIE spanning 4 consecutive weeks. An ‘air-treated’ control group was administered saline and pyrazole (1 mmol/kg) injections and exposed to room air across the 4 weeks with no exposure to ethanol. Following CIE or air-treatment, animals were sacrificed for voltammetry experiments. The CIE and air treatment groups were staggered such that each ethanol-treated animal had a complimentary air-treated control that was scheduled for voltammetry experiments the following day.
Blood ethanol concentrations (BEC) for animals exposed to ethanol vapor were measured at the end of each cycle for each animal to ensure that BECs were physiologically relevant. Blood samples were collected from the mice using the submandibular vein punch immediately after removal from the ethanol vapor chamber. Blood samples were assayed for ethanol concentration as described previously (Karkhanis et al. 2015).
Ex Vivo Voltammetry
Slice preparation and FSCV was performed as described previously (Ferris et al. 2012). On the morning following completion of the final cycle of CIE (72 hours after final ethanol exposure), animals were anesthetized with isoflurane, decapitated, and the brain rapidly removed and cooled in ice-cold, pre-oxygenated (95% O2/5% CO2) artificial cerebral spinal fluid (aCSF) consisting of (in mM): NaCl (126), KCl (2.5), NaH2PO4(1.2), CaCl2(2.4), MgCl2(1.2), NaHCO3(25), glucose (11), L-ascorbic acid (0.4) and pH was adjusted to 7.4. Multiple coronal slices (300 μm thick) containing the NAc (NAc) were prepared from each animal with a vibrating tissue slicer (Leica VT1000S; Leica Instruments, Nussloch, Germany). Slices were maintained in oxygenated aCSF at room temperature for 1 hour before transfer to a submersion recording chamber through which 32°C oxygenated aCSF was perfused at a rate of 1 ml/min. Two voltammetry rigs were used; slices were divided along the midline and each hemisphere was transferred to the recording chamber of the corresponding voltammetry rig. Thus, NAc core and shell recordings were occurring simultaneously, across two voltammetry rigs, from separate hemispheres of the same slice.
Carbon fiber microelectrodes (100–150 μM length, 7 μM diameter) were fabricated with a waterproof epoxied seal which results in reduced noise and increased stability, allowing a single electrode to be used across experiments. A total of 6 electrodes were used in this study, and the same electrode was used on consecutive days, for each corresponding animal in the experimental and control groups. The carbon fiber electrode was placed into the core or shell of the NAc approximately 100 μM below the surface. Light stimulation was delivered from an optic fiber coupled to a 100 mW, 473 nm blue laser. The optic fiber was positioned in the slice bath, above the tissue, and aimed to deliver light to the area of tissue immediately surrounding the recording electrode. DA was evoked by an optical pulse (~5 mW, 4 ms duration) applied as a single pulse or a 5 pulse train (20 Hz) every 5 min. Extracellular DA was monitored at the carbon fiber electrode every 100 msec using fast-scan cyclic voltammetry (Wightman et al., 1988) by applying a triangular waveform (−0.4 to +1.2 to −0.4 V vs Ag/AgCl, 400 V/s). Following experiments, recording electrodes were calibrated by measuring responses (in electrical current; nA) to a known concentration of DA (3 μM), using a flow-injection system. This was used to convert electrical current to DA concentration.
Experiments began with single pulse stimulations which were repeated every 5 minutes until the DA signal reached stability, defined by less than 10% variation in magnitude and not trending up or down across three successive recordings; stability occurs in approximately 90 minutes. Once stable single pulse evoked DA signals were obtained, 5 pulse (20 Hz) phasic stimulations were applied once every 5 minutes. In control slices, we found that signals remained stable for approximately 2 hours using this stimulation protocol, after which the peak amplitude would slowly decline (< 1%) with each successive stimulation. Once 5 pulse phasic stimulations were stable, the κ-opioid opioid receptor agonist U50,488 (30–1000 nM) or the D2 receptor agonist quinpirole (10–100 nM) was applied cumulatively to the brain slice. Recordings for each drug concentration were continued until the magnitude of DA release was again stable, before proceeding to the next concentration. Following U50,488, slices were washed in aCSF containing the κ-opioid receptor antagonist nor-binaltorphimine (nor-BNI; 30 μM) to reverse the U50,488 inhibition of DA release. To account for potential deterioration of baseline amplitude resulting from prolonged application of phasic light stimulations, the nor-BNI reversal for each group was normalized to the baseline magnitude and this correction was applied to each drug dose response accordingly.
All voltammetry data were collected and modeled using Demon Voltammetry and Analysis Software (Yorgason et al. 2011). Parameters of evoked levels of DA are determined based on Michaelis–Menten kinetics (Wightman et al. 1988), following standard voltammetric modeling procedures (Wu et al. 2001).
Statistics
Graph Pad Prism (version 5, La Jolla, CA, USA) was used to statistically analyze data sets and create graphs. Planned comparisons in DA release and uptake measures were analyzed using an unpaired two-tailed t-test. Drug concentration response curves were subjected to a two-way repeated measures ANOVA; concentration was the within subjects factor and experimental group was the between subjects factor. Differences between groups were tested using a Bonferroni post hoc test. All p values of < 0.05 were considered to be statistically significant.
Results
Histological analysis of ChR2 expression
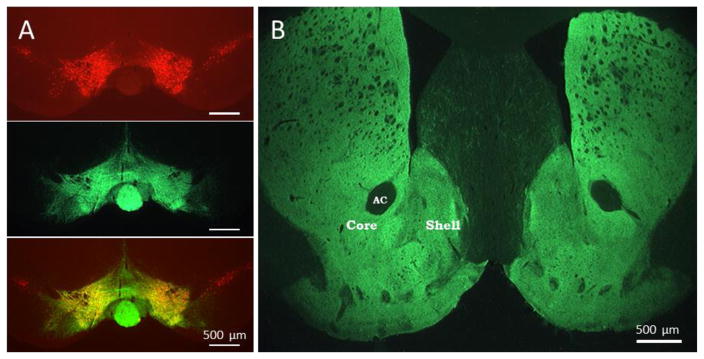
In order to target ChR2 expression in DA terminal fields in the nucleus accumbens, we used a viral construct with DNA encoding ChR2 expression in a manner dependent on cre-recombinase expression, and a transgenic mouse line that endogenously expresses cre-recombinase based on the transcription of TH mRNA (Tsai et al. 2009); thus, when used together, ChR2 will be targeted to dopamine precursor expressing neurons. Indeed, we found viral transfection of the VTA of TH:Cre mice resulted in ChR2 expression in midbrain DA neurons and their axonal projections to the NAc, as in previous studies (Melchior et al. 2015a). Figure 1 shows representative immunohistochemical images of ChR2 expression 6 weeks after viral injection. In the VTA, TH expression was immunolabeled with red fluorescence, allowing identification of midbrain DA neurons (Fig. 1A). ChR2 expression was immunolabeled with green fluorescence and robustly overlapped the TH expressing neurons in the VTA. Note that ChR2 expression did not extend laterally to the neighboring substantia nigra compacta, indicating accurate targeting of viral injections. In the ventral striatum, ChR2 expression was seen in the terminal projections of VTA DA neurons, which densely innervate the NAc core and shell regions (Fig. 1B).
Figure 1. Expression of ChR2 in ventral tegmental area and striatum.
A. Coronal midbrain section immunolabeled to show location of tyrosine hydroxylase expressing neurons in the VTA (red, top), ChR2-eYFP expression (green, middle) and a merged overlay showing the ChR2 expression throughout the site of injection in the VTA (bottom). B. Coronal section of striatum showing expression of ChR2 in the terminal fields of midbrain dopamine neurons (A). The ventral striatum shows robust ChR2 expression in both the nucleus accumbens core and shell regions. AC; anterior commissure. Scale bar = 500 μm.
An important consideration when using the TH:Cre mouse model is the ectopic expression of Cre-driven transgenes in midline VTA neurons that are not dopaminergic (Lammel et al. 2015; Stuber et al. 2015). This results from a population of cells within the medial VTA nuclei which express low levels of TH mRNA, sufficient to induce endogenous Cre recombinase expression, but do not translate detectable levels of TH protein and are not dopaminergic (Yamaguchi et al. 2015; Lammel et al. 2015). Thus, the population of ChR2-expressing projections to the accumbens may include glutamate- or GABA-releasing axons (Yamaguchi et al. 2015; Brown et al. 2012; Taylor et al. 2014). For the purposes of these studies, in which we are interested in presynaptic regulation of dopamine terminal release, our goal was to eliminate excitation of local accumbens elements which may profoundly modulate dopamine release such as acetylcholine and GABA interneurons, or retrograde release of neuropeptides or reactive oxygen species from repeated excitation of medium spiny neurons (Zhou et al. 2001; Steiner and Gerfen 1996; Patel and Rice 2012). While VTA glutamatergic projections may be included in the stimulation field, dopamine terminal fields do not receive direct glutamatergic input (Sulzer et al. 2016; Rice et al. 2011). Further, optogenetically targeted dopamine release is insensitive to blockade of nicotinic acetylcholine receptors and GABAB receptors (GABAA receptors are not expressed on dopamine terminals) in ex vivo preparations (Melchior et al. 2015), which suggests that non-dopaminergic projections from VTA are not cross-modulating dopamine terminals, either directly or indirectly through cholinergic interneurons (Kosillo et al. 2016) in this model.
Light-evoked DA release measured with voltammetry
Coronal slices containing the NAc were examined using voltammetry to measure light stimulated DA release (Melchior et al. 2015a). A single slice was divided into separate hemispheres and signals were measured at the same time using two separate voltammetry rigs. Within the slice, NAc core measurements were sampled at a placement slightly ventral and lateral to the anterior commissure. In the opposite hemisphere (randomly assigned as right or left), NAc shell measurements were sampled from the medial shell region, also slightly ventral to the level of the anterior commissure, on the same lateral plane as core samples. These locations are represented by the labels ‘core’ and ‘shell’ in Fig. 1B. Light stimulations applied to both regions resulted in increases in current (Fig. 2A) with peak changes in current detected at 0.6 V and −0.2 V on the voltage scan (Fig. 2A inset); these peaks are consistent with the oxidation and reduction profile of DA (Wightman et al. 1988). Color plot representations show the changes in current (z-axis) with respect to voltage (y-axis) and time (x-axis). Representative traces of 5 pulse (20 Hz) light stimulated DA release show characteristic signals obtained in the core and shell regions (Fig. 2A).
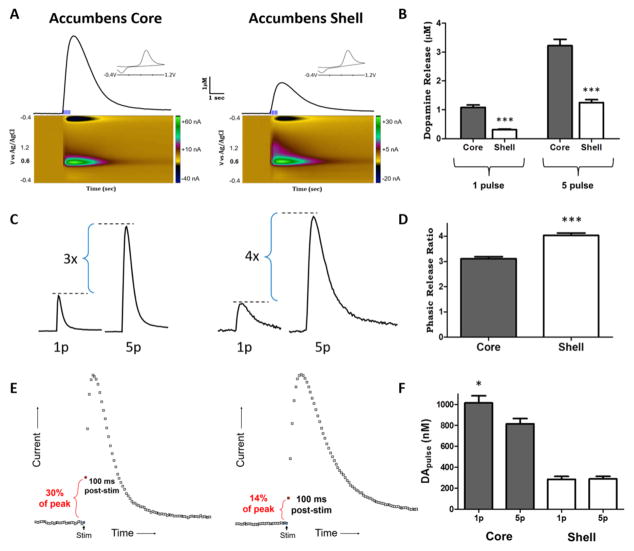
Figure 2. Light stimulated dopamine release parameters in the nucleus accumbens core and shell.
A. Representative traces of light-stimulated dopamine release in the ventral striatum. Five pulse, 20 Hz (phasic) light stimulations resulted in robust dopamine release in the nucleus accumbens core and shell. Color plots (below) display signals as current vs. voltage vs. time. Dopamine was identified by characteristic oxidation peaks at 0.6 V and reduction peaks at −0.2 V represented for both regions in the current vs. voltage plot (inset). Nucleus accumbens shell terminal fields show reduced stimulated dopamine release (peak height) and reduced dopamine uptake rates (descending portion of curve) compared to terminal fields in the nucleus accumbens core. Phasic light stimulations resulted in > 1 μM dopamine release magnitude in the accumbens shell. B. Grouped data showing dopamine release magnitude following a tonic (1 pulse) or phasic (5 pulse) light stimulation in the nucleus accumbens core and shell. Dopamine release was significantly less in the shell compared to the core for tonic and phasic stimulations. C. Representative traces show the relationship between 1p and 5p light stimulated dopamine release within nucleus accumbens core (left) and shell (right) terminal fields. D. Grouped data show phasic release as a factor of tonic release in the same field (5 pulse release / 1 pulse release). Phasic release ratios are significantly greater in the shell compared to the core. E. Representative traces show the difference in initial release velocity between the accumbens core (left) and shell (right). Dopamine measured 100 ms following the shell (14% of peak release). F. Grouped data showing that dopamine release per pulse was greater with a single pulse stimulation compared to 5 pulse stimulation in the core. Dopamine release per pulse was more uniform across stimulations in the shell. *p < 0.05, ***p < 0.001
DA release parameters: core versus shell
Stimulated DA signals in the NAc shell were smaller in amplitude compared to signals in the core (n = 60 total slices across 25 animals; 60/25). Single pulse light stimulations resulted in 1.08 ± 0.08 μM DA release in the core compared to 0.31 ± 0.03 μM DA release in the shell, which were significantly different between regions (t(57) = 9.0, p < 0.001; Fig. 2B). Similarly, 5 pulse light stimulations resulted in 3.22 ± 0.22 μM DA release in the core and 1.25 ± 0.10 μM DA release in the shell, which were also significantly different between regions (t(58) = 8.0, p < 0.001). Further, there was a significant interaction between region and pulse number (two-way anova; F(1,115) = 20.73, p < 0.001).
It has been reported that the shell shows a greater magnitude of phasic activation compared to the core (Zhang et al. 2009). That is, the ratio of multi-pulse stimulated release levels to single pulse stimulated release levels is enhanced in the shell. Here we calculated the ratio of 5 pulse (phasic) release magnitude to single pulse (tonic) release magnitude (5 p:1p ratio). The phasic release ratio was 3.10 ± 0.09 in the core and 4.09 ± 0.09 in the shell, thus the shell shows significantly greater phasic activation than the core (t(57) = 7.7, p < 0.001; Fig. 2C) in agreement with previous reports. In order to assess possible differences in release probability, we analyzed DA release per stimulus pulse (DAp) across groups (Fig. 2D). We found that DAp was significantly higher for a single pulse stimulation 1006 ± 70.8 nM compared to release per pulse across a 5 pulse stimulation train (814.4 ± 50.1 nM) in the core (t(59) = 2.2, p < 0.05). Conversely, in the shell, a single pulse stimulation results in a similar magnitude of DA release (293.2 ± 26.6 nM) compared to each pulse in a stimulation train (296.4 ± 24.0 nM; p > 0.05). There was a significant interaction between region and pulse number (two-way anova; F(1,115) = 4.20, p < 0.05).
Chronic ethanol effects on DA release
Next we wanted to determine whether chronic ethanol exposure resulted in changes in DA terminal parameters, specifically DA release, uptake rates and modulation by hetero-receptors. To this end, mice expressing ChR2 in DA neurons were exposed to chronic intermittent ethanol (CIE) exposure using ethanol vapor chambers (Becker and Lopez 2004). Mice were exposed to ethanol vapor for 16 hours a day, 4 days a week, for 4 consecutive weeks. BECs were measured at the end of each week and showed an average of 231.6 ± 14.7 mg/dL (mean ± SEM; n = 13). A separate group of ChR2 expressing mice were exposed to the identical protocol as the CIE treated animals except they were exposed to normal room air instead of ethanol vapor.
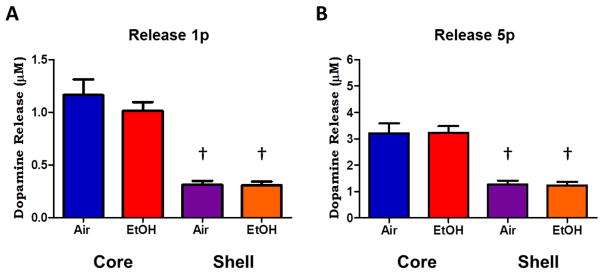
In order to determine if chronic ethanol exposure reduced DA terminal function, we assessed release using single or 5 pulse stimulations following CIE. We found no differences in light stimulated release in either the core or shell regions in ethanol treated animals. In the NAc core, single pulse light stimulations resulted in 1.01 ± 0.08 μM DA release in CIE treated animals (n = 16/13) which was not different from the 1.16 ± 0.15 μM DA release measured in air treated animals (n = 14/12; t(28) = 0.9, p > 0.05; Fig. 3A). Similarly, 5 pulse light stimulations resulted in 3.23 ± 0.25 μM DA release in CIE treated animals (n = 16/13) and 3.21 ± 0.37 μM DA release in air-treated animals (n = 15/12; t(29) = 0.03, p > 0.05; Fig. 3B). In the NAc shell, single pulse light stimulations resulted in 0.30 ± 0.03 μM DA release in CIE treated animals (n = 15/12) and 0.31 ± 0.03 μM DA release in air treated animals (n = 14/11; t(27) = 0.05, p > 0.05; Fig. 3A). Similarly, 5 pulse light stimulations resulted in 1.23 ± 0.14 μM DA release in CIE treated animals and 1.27 ± 0.15 μM DA release in air-treated animals (t(27) = 0.17, p > 0.05; Fig. 3B).
Figure 3. Light stimulated dopamine release following chronic ethanol exposure.
A. Grouped data showing dopamine release magnitude following a single pulse of light stimulation. Dopamine release was significantly greater in the core (red, blue) compared to the shell (purple, orange) regions; however there was no significant difference in stimulated release between ethanol-treated (red, orange) and air-treated controls (blue, purple) in either region. B. Stimulated dopamine release following five pulses of light stimulation show greater magnitude of dopamine release compared to single pulse stimulations (A). Dopamine release in the core is significantly greater than release in the shell, however, there were no differences in stimulated release between ethanol-treated and air-treated controls in either region. †p < 0.05 compared to core release.
Chronic ethanol effects on uptake rates
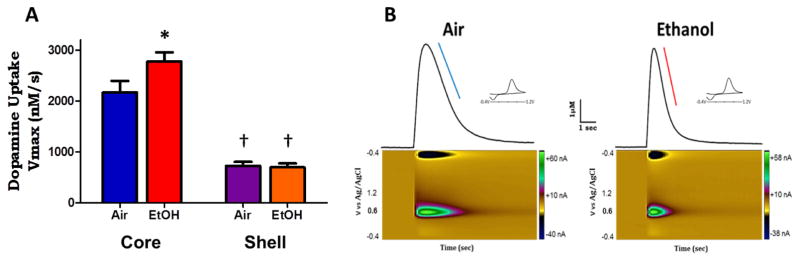
It has been reported that DA uptake rates are slower in the NAc shell compared to the NAc core in rodents (Jones et al. 1996b; Zhang et al. 2009). In order to examine this in our model we measured maximal uptake rates (Vmax) using 5 pulse light stimulations, which produced the largest amplitude DA release signals in each region. Consistent with previous reports, we found that uptake rates in the shell were slower than those in the core. In control animals (n = 15/12) Vmax in the core was 2172 ± 223 nM/s whereas in the shell (n = 14/11) Vmax was 726 ± 79 nM/s, which was significantly less compared to the core (t(27) = 5.9, p < 0.001; Fig. 4A).
Figure 4. Chronic ethanol results in increased uptake rates in accumbens core.
A. Grouped data showing maximal rate of uptake (Vmax) of dopamine (nM/s). Vmax is significantly faster in the core (blue, red) compared to the shell (purple, orange). Following ethanol-treatment the Vmax is significantly enhanced in the core compared to air-treated controls. However, ethanol treatment had no effect on Vmax in the shell compared to air-treated controls. B. Representative traces of five-pulse stimulated release in the nucleus accumbens core region of air-treated and ethanol-treated mice. Ethanol treatment had no effect on stimulated release magnitude, however, there was a significant increase in the uptake rates in the core of ethanol treated animals (right, red line) compared to air-treated control (left, blue line), evidenced by the increased steepness of slope in the descending curve of the measured dopamine signal. †p < 0.05 compared to core; *p < 0.05 compared to air.
Following chronic ethanol exposure we found that there was there was a significant interaction of treatment and region on uptake rates (F(1,56) = 4.18, p < 0.05). Specifically, we found that in ethanol treated animals (n = 16/13), Vmax in the core was 2780 ± 175 nM/s which was significantly increased compared to air treated animals in which the Vmax was 2172 ± 223 nM/s; (t(29) = 2.2, p < 0.05; Fig. 5A). Figure 4B shows representative traces of 5 pulse light stimulated DA signals in the NAc core of air and CIE treated animals, which produced similar magnitudes of release. There is a clear increase in steepness of slope on the descending limb of the DA signal, highlighted by the colored lines (blue, air-treated; red, CIE treated). In the NAc shell, however, we found no differences in Vmax between CIE treated animals (n= 15/12; 697 ± 78 nM/s DA/s) and air-treated controls (n = 14/11; 726 ± 79 nM/s; t(27) = 0.26, p > 0.05; Fig. 4A).
Figure 5. Chronic ethanol effects on dopamine autoreceptor function.
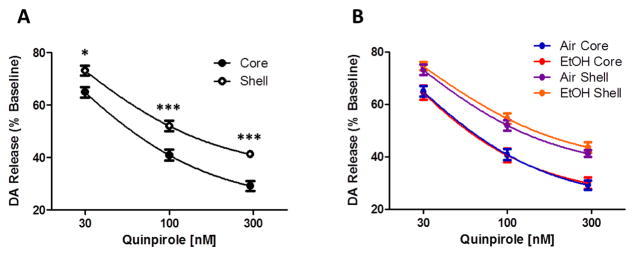
Dopamine terminal autoreceptor sensitivity is different between nucleus accumbens core and shell but not altered by chronic intermittent ethanol exposure. A. The D2/D3 agonist quinpirole dose dependently decreases 5 pulse light stimulated dopamine release to a greater extent in the nucleus accumbens core (open circle) compared to the shell (closed circle). B. Chronic intermittent ethanol exposure did not alter sensitivity to quinpirole in either the accumbens core or shell region. *p < 0.05, ***p < 0.001 compared to core. Air = air treated group, EtOH = CIE treated group.
Chronic ethanol effects on autoreceptors
Regulation of DA signaling is tightly controlled by D2/D3-type DA receptors located on DA terminals which act as autoreceptors, providing feedback-inhibition of DA release. Autoreceptor sensitivity has been shown to be altered following chronic exposure to stimulants (Lee et al. 1999; Mateo et al. 2005; Calipari et al. 2014). Here we wanted to assess autoreceptor function in both the NAc core and shell regions following chronic ethanol exposure.
To test autoreceptor sensitivity, we bath applied the D2/D3 receptor agonist quinpirole (30–300 nM); 5 pulse light stimulations were applied every five minutes and DA release was measured following incubation with each concentration of quinpirole. Quinpirole concentration effects were measured within-slice as a percentage of pre-drug baseline release in control animals (n = 7). Quinpirole induced a dose-responsive inhibition of 5 pulse light stimulated DA release across regions (concentration: F(2,24) = 1127, p < 0.001; Fig 5A). Surprisingly, we found a regional difference in the effect of quinpirole, with the core being significantly more sensitive than the shell (F(1,24) = 17.04, p < 0.01), including an interaction between concentration and region (F(2, 24) = 3.9, p < 0.05). Bonferroni post hoc analyses of individual concentrations revealed a significant regional difference in response to quinpirole at all doses tested: 30nM (p < 0.05), 100 nM (p < 0.001) and 300 nM (p < 0.001). These results showed that the NAc core was significantly more sensitive to autoreceptor regulation than the shell.
Next, we wanted to determine whether chronic ethanol exposure altered autoreceptor function in either region (Fig. 5B). The quinpirole induced inhibition of DA release in NAc core did not differ between CIE (n = 8) and air treated (n = 7) groups (F(1,26) = 0.01, p > 0.05). Similarly, in the NAc shell, quinpirole induced inhibition of DA release did not differ between CIE and air treated animals (F(1,26) = 0.74, p > 0.05). These results show that DA terminal autoreceptor regulation of optogenetically-evoked release is not altered 72 hours after chronic ethanol exposure using a CIE vapor paradigm.
Chronic ethanol effects on kappa opioid receptors
Chronic ethanol exposure has been suggested to upregulate the dynorphin/kappa opioid system receptor in vivo, a process that could include increased expression of dynorphin, the endogenous ligand for κ-opioid receptors (Sirohi et al. 2012; Shippenberg et al. 2007), or changes in sensitivity of κ-opioid receptors expressed within the NAc where activation decreases DA release (Spanagel et al. 1992; Sirohi et al. 2012). We used our model of targeted light stimulation of DA terminals to assess κ-opioid receptor modulation of DA release and the effects of chronic ethanol exposure on κ-opioid receptor sensitivity.
To test κ-opioid receptor inhibition of DA release we bath applied the κ-opioid receptor agonist U50,488 (0.03–1.0 μM) and subsequently reversed U50,488 effects with aCSF containing the κ-opioid receptor antagonist nor-BNI (30 μM). 5 pulse light stimulations were applied every 5 minutes and DA release was measured following incubation with each concentration of U50,488. U50,488 concentration effects were measured within-slice as a percentage of pre-drug baseline release and nor-BNI reversal.
In the NAc core, U50,488 inhibited DA release in a dose response manner (F(3, 42) = 56.3, p < 0.001). We found that CIE treated mice (n = 8) showed increased sensitivity to the κ-receptor agonist U50,488 (F(1, 42) = 7.55, p < 0.05) compared to air treated controls (n = 8; Fig. 6A). Post-hoc analysis revealed that there was a significant difference between the groups at the 0.3 μM (p < 0.05) and 1.0 μM (p < 0.01) concentrations of U50,488. Further, there was an interaction effect between group and drug dose (F(3, 42) = 3.82, p < 0.05) with the largest difference between groups being observed at the highest dose of U50,488 tested.
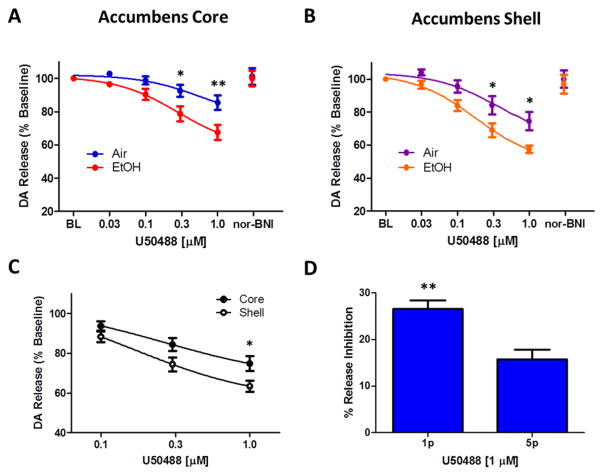
Figure 6. Chronic ethanol exposure increases kappa opioid receptor function in the nucleus accumbens core and shell.
The kappa opioid receptor agonist U50,488 decreases stimulated dopamine release in a dose dependent manner (0.03–1.0 μM), presented as the percent inhibition from baseline (BL) stimulated release; and this effect is reversed upon wash with the kappa opioid receptor antagonist nor-BNI (30 μM). A. In the nucleus accumbens core, chronic ethanol exposure (red) resulted in increased sensitivity to U50,488 compared to air-treated controls (blue). B. In the nucleus accumbens shell, chronic ethanol exposure (orange) resulted in increased sensitivity to U50,488 compared to air-treated controls (purple). C. The nucleus accumbens shell (open circle) is more sensitive to U50,488 than the accumbens core (closed circle). D. In the nucleus accumbens core of naïve animals, U50,488 (1 μM) inhibits single pulse light stimulated dopamine release significantly more than 5 pulse phasic stimulated release. *p < 0.05, **p<0.01, Air = air treated group, EtOH = CIE treated group.
In the NAc shell, U50,488 also inhibited 5 pulse light stimulated DA release (F(3, 42) = 104.5, p < 0.001). Similarly, we found that CIE treated mice showed increased sensitivity to the κ-opioid receptor agonist U50,488 (F(1, 42) = 6.94, p < 0.05) compared to air treated controls (Fig. 6B). Post-hoc analysis revealed that there was a significant difference between the groups at the 0.3 μM and 1.0 μM (p < 0.05) doses of U50,488. There was also an interaction effect between group and drug dose (F(3, 42) = 2.12, p < 0.05) with the largest difference between groups being observed at the highest dose of U50,488 tested.
In order to determine if there was a regional difference in sensitivity to κ-opioid receptor agonism we collapsed grouped data to compare U50,488 inhibition of dopamine release between the nucleus accumbens core and shell (Fig. 6C). We found that the NAc shell was more sensitive to U50,488 compared to the core (F1,56 = 4.7, p < 0.05), with post-hoc analysis of individual doses finding a significant difference at the highest dose tested (1 μM; p < 0.05). These data suggest that κ-opioid receptor modulation of DA release is greater in the NAc shell compared to the core; however, both regions show a similar magnitude of increased sensitivity to κ-opioid receptor modulation following chronic ethanol exposure.
Finally, we wanted to determine if κ-opioid receptor mediated inhibition differentially effects DA release resulting from single (tonic) stimulation or a 5 pulse train (phasic) stimulation. Receptor mediated modulation of DA terminal release is often overcome by increases in pulse number and/or frequency of stimulation (Zhang and Sulzer 2012). Here we wanted to see if κ-opioid receptor modulation of DA terminals follows those same principles. We ran a single dose of U50,488 on NAc core slices from naïve animals (n = 5) and compared the magnitude of inhibition between single pulse and 5 pulse light stimulation-induced DA release. Baseline DA release was established using 1 and 5 pulse stimulations in the same location in the slice, resulting in average of 1.1 ± 0.2 and 3.1 ± 0.8 μM DA signals respectively. U50,488 reduced single pulse light stimulated DA release by 26.6% ±1.8% and reduced 5 pulse stimulated release by 15.7% ± 2.1% of baseline in the same DA terminal fields (Fig. 6D). Thus, the inhibition produced by U50,488 was significantly greater on single pulse stimulated compared to multi-pulse burst stimulated release (paired t(4) = 3.9, p <0.05).
Discussion
Selectively targeting DA terminals for isolated stimulation ex vivo has advanced our understanding of DA signaling in the striatum, revealing unique characteristics previously masked by electrical stimulation (Stuber et al. 2010a; Tritsch et al. 2012). For example, DA release can be significantly enhanced by co-stimulation of local acetylcholine interneurons (Threlfell et al. 2012; Cachope et al. 2012; Melchior et al. 2015a) via nACh receptors on DA terminals. These receptors modulate tonic to phasic DA release ratios, which affect the amplitude of transient DA spikes in relation to basal DA levels (Zhang et al. 2009). Here we used optogenetic DA terminal stimulation, removing the influence of nACh activation that occurs with electrical stimulation (Melchior et al. 2015a), to compare signaling parameters between the NAc core and shell. We used single pulses and five-pulse trains to measure tonic and phasic DA release. We found that the core had greater amplitude DA release, but the shell has greater phasic-to-tonic DA release ratios. This is due, in part, to the core showing a paired pulse-like depression (McCool 2011), in which release probability is higher for a single pulse stimulation than for the pulses that follow it in a train. In the shell, the release per pulse is more equally distributed across pulses, suggesting a lower initial release probability compared to the core. This occurs in the absence of local microcircuit regulation, and instead suggests that the mechanisms are intrinsic to the terminals, likely due to differences in releasable pools and sensitivity to calcium (Sulzer et al. 2016; Brimblecombe et al. 2014). This highlights the different terminal properties of core- and shell-projecting DA axons (Jones et al. 1996b; Zhang et al. 2009). These differences also include increased uptake rates and increased sensitivity to autoreceptor regulation in the core. Uptake rates were measured using 5 pulse stimulated release, which results in large amplitude signals which allow accurate estimation of ‘maximal’ uptake rates at saturating DA concentrations (Wu et al. 2001). There were clearly reduced uptake rates in the shell, which is consistent with reduced DA transporter (DAT) expression (Coulter et al. 1996) in this region. We also measured D2-type autoreceptor function in the absence of concurrent stimulation of D2 expressing post synaptic neurons using optogenetic stimulation and found increased inhibition of presynaptic release in the core region. To our knowledge this is the first report of differences in autoreceptor sensitivity between accumbens sub-regions. Together, the increased tonic release, increased autoreceptor function and increased uptake rates suggest that DA signaling is more tightly regulated in the core. The shell has lower initial release probability but greater phasic-to-tonic release ratios, suggesting that DA release amplitude in this region tracks changes in DA neuron activity with more fidelity than the core.
We examined whether DA terminal characteristics were altered following chronic ethanol exposure. We have previously reported decreased electrically stimulated DA release in NAc core slices from mice following chronic intermittent ethanol exposure (Rose et al. 2016; Karkhanis et al. 2015); however, this finding did not extend to rats (Budygin et al. 2003) under a similar ethanol exposure paradigm, and there were regionally divergent effects in primates with a history of chronic daily ethanol self-administration (Siciliano et al. 2015a). These inconsistencies suggest that species, region and pattern of ethanol administration may be important in determining ethanol-induced changes in release parameters. Further, terminal neuroadaptations may develop from ethanol effects within divergent cellular microenvironments across regions (Robinson et al. 2009) in addition to direct ethanol actions on terminals, which would presumably apply uniformly across regions. Here we targeted only DA terminal fields with light stimulation and found no differences in stimulated release in either the NAc core or shell region following chronic ethanol treatment. An important caveat in the current study is that basal DA release magnitudes resulting from light stimulations are dependent on the amount of expression of ChR2 in the fields being stimulated. However, the variability of release across slices was comparable to studies using electrical stimulation (Rose et al. 2016), suggesting that there was consistency in the viral injection and expression of ChR2 between animals. Another consideration is that super-physiological Ca2+ entry during light stimulation (Zhang and Oertner 2007) occludes potential ethanol-induced decreases in terminal Ca2+ signaling and transmitter release. We previously demonstrated that in the presence of Na2+ channel blockade, Ca2+ entry during ChR2 activation is insufficient to induce measurable dopamine release (Melchior et al. 2015). Alternatively, due to the relatively slower kinetics of ChR2, the depolarization width is increased, which may result in increased Ca2+ entry and enhanced transmitter release (Zhang and Oertner 2007). However, using light stimulated release, we were able to detect differences in release parameters between the accumbens core and shell, supporting that this model is sufficiently sensitive to detect differences in Ca2+-mediated release events between populations. Therefore, we believe that potential ethanol-induced alterations in terminal Ca2+ signaling would be detected using light-stimulated release in ethanol-treated and control groups. Nonetheless, as the understanding of presynaptic ethanol targets advances, the caveats associated with light-stimulated transmitter release should be considered.
The lack of ethanol-related differences in stimulated DA release suggests no differences in basal terminal release capability or terminal field density. We hypothesize that the previously reported differences in stimulated DA release following chronic ethanol involve adaptations in the microcircuitry within the terminals fields. Electrical stimulation recruits local microcircuit modulation of dopamine release whereas targeted optical stimulation does not (Melchior et al. 2015a); thus, electrical stimulation may detect ethanol-induced changes in striatal modulation of dopamine release. For example, chronic ethanol may affect the release probability of GABAergic or cholinergic interneurons in the NAc, the simultaneous excitation of which inhibits and augments stimulated DA release, respectively (Melchior et al. 2015a). Prior ethanol exposure has been suggested to augment GABA interneuron inhibition of DA neurons in the ventral tegmental area (Melis et al. 2002). A similar mechanism may be occurring in the NAc where populations of GABAergic interneurons (Tepper et al. 2010) may modulate DA release via terminal GABAB receptors when using electrical stimulation (Pitman et al. 2014). Similarly, concurrent stimulation of local acetylcholine interneurons significantly augments stimulated DA release (Threlfell et al. 2012; Cachope et al. 2012), providing a means through with ethanol-induced inhibition of cholinergic transmission would be detected in the DA signal (Dohrman and Reiter 2003; Boutros et al. 2016). An increased number of GABAergic interneurons and a decreased density of cholinergic interneuron varicosities has been reported in the NAc of rats after chronic ethanol administration (Pereira et al. 2014). In light of this, the variability in release differences reported across species using local electrical stimulation may reflect ethanol-induced changes in indirect modulation of DA release probability through a multi-synaptic mechanism. This hypothesis is supported by a lack of direct effect of acute ethanol on DA terminals when applied using physiologically relevant doses and stimulation parameters (Budygin et al. 2001). Instead, long multi-pulse electrical stimulation trains are needed to detect ethanol inhibition of DA release (Yorgason et al. 2014), reinforcing the idea that recruitment of local circuitry is required. Together, these possibilities support the use of targeted stimulation methods, as utilized in this study, to probe specific mechanisms of ethanol induced release modulation in the NAc.
An additional mechanism through which chronic ethanol exposure may regulate DA signaling is modulation of DA uptake rates, which alters the magnitude and duration of DA signaling. Increased uptake rates have been reported in the NAc of mice (Rose et al. 2016), rats (Budygin et al. 2007) and monkeys (Siciliano et al. 2016) following chronic ethanol exposure, and these findings are supported by increased DAT protein tissue expression (Healey et al. 2008). Here we add to the consensus by showing increased uptake rates in the NAc core following a CIE vapor exposure paradigm. However, this effect did not extend to the medial shell of the same animals. A possible technical consideration is that DA release elicited in the shell was below the saturation level needed to accurately define changes in ‘maximal’ uptake rate (Wu et al. 2001); indeed, there was a 3 fold difference in release between the core and shell in the current study. However, ethanol-induced changes in uptake were detected in the core ex vivo using single pulse stimulations (Karkhanis et al. 2015) where DA release was of similar magnitude to that reported here in the shell (using phasic stimulations). Therefore, it appears more likely that there is a regional difference in ethanol-induced increases in uptake rates. For example, D2 autoreceptor activation can potentiate DAT trafficking to the membrane (Ford 2014; Wu et al. 2002), and may explain differential modulation of uptake rates across regions. The larger DA signals, and increased regulation by D2 autoreceptors in the core may translate into increased sensitivity of DAT trafficking in response to chronic ethanol insult.
Kappa opioid receptors are expressed in the NAc where they inhibit DA release from DA terminals (Spanagel et al. 1990), however, κ-opioid receptor modulation of afferent synaptic input can be cell-type specific. For example, in the bed nucleus of the stria terminalis, dynorphin expressing cells modulate afferent terminals arising from the basolateral amygdala but not those arising from the prefrontal cortex (Crowley et al. 2016). Conversely, in the ventral tegmental area, κ-opioid receptor agonists selectively decrease activity in DA neurons projecting to the prefrontal cortex but not those projecting to the NAc (but do regulate terminal release in NAc) (Margolis et al. 2006), further highlighting cell specific regulation by κ-opioid receptors and DA neuron heterogeneity based on projection region. Here we demonstrate that κ-opioid receptors have direct effects on dopamine terminals in the nucleus accumbens core and medial shell. Further, the κ-opioid receptor-mediated inhibition of dopamine release was sensitive to phasic stimulations, although showing reduced efficacy in attenuating 5 pulse-stimulated compared to single pulse-stimulated dopamine release. This is consistent with most heteroreceptor-mediated inhibitory regulation of dopamine terminals, which can be overcome with increases in pulse number or frequency (Zhang and Sulzer 2012; Melchior et al. 2015a). The inhibition of dopamine release produced by a κ-opioid receptor agonist on single pulse light-stimulated release was also less than what has been previously reported using electrical stimulation (Rose et al. 2016). Electrical stimulation likely recruits both direct and indirect effects of κ-opioid receptors on dopamine release. For example, it has been suggested that κ-opioid receptors are expressed on cholingergic interneurons (Schoffelmeer et al. 1997); therefore κ-opioid receptors may inhibit both dopamine terminal release and acetylcholine augmentation of dopamine release when both cell types are excited using electrical stimulation.
Within the NAc, κ-opioid receptor activation results in divergent reward-related behaviors dependent on the specific sub-region, i.e. hot-spot, being targeted (Al-Hasani et al. 2015; Castro and Berridge 2014) and suggests possible differential regulation of DA terminals. For example, κ-opioid receptor activation in the shell, but not the core, contributes to escalation of drug intake (Whitfield et al. 2015), a hallmark of dependence. Our results show that κ-opioid receptor-mediated inhibition of DA release is greater in the medial shell compared to the NAc core. This is in contrast to Ebner et al., who showed that salvinorin A caused greater decreases in DA release in the core versus the shell (Ebner et al. 2010); however, that study applied salvinorin A systemically in combination with electrical stimulation in vivo, thus incorporating multiple facets of potential κ-opioid receptor modulation of DA output in NAc. Here we provide a direct assessment of κ-opioid receptor inhibition of DA release in each region. Our detection of increased efficacy of κ-opioid receptor agonists in the shell is supported by κ-opioid receptor binding assays, which suggest a marginally higher density of κ-opioid receptor expression in the shell versus the core in rats (Mansour et al. 1995) and prairie voles (Resendez et al. 2016). However, κ-opioid receptors are distributed unevenly across cell types, and how that translates specifically to DA terminals across regions is unknown and may vary across species, sex and social housing arrangements. Indeed, our data suggest that κ-opioid receptor expression may be flexible and responsive to environmental influences.
Following chronic ethanol exposure, κ-opioid receptor sensitivity was increased in terminal fields of both the core and shell, and to a similar magnitude, suggesting that the mechanism regulating κ-opioid receptor functionality is local to the NAc and generalized across regions. Dynorphin is generated in the D1 receptor-expressing medium spiny neurons (D1-MSN) in response to D1 receptor activation and is released in order to provide feedback inhibition of over-excitation of D1-MSNs (Gerfen and Surmeier 2011; Steiner and Gerfen 1996). That is, dynorphin peptides are transported to recurrent collateral axons within the NAc in order to decrease DA or glutamate transmitter release via presynaptic κ-opioid receptors (Steiner and Gerfen 1993). κ-opioid receptors also desensitize in response to high levels of dynorphin, including those induced by a single injection of amphetamine, subsequently increasing DA signaling on MSNs in vivo (Xia et al. 2008). Chronically elevated DA levels across the daily 16 hour ethanol exposure that occurs with CIE (for 4 weeks) may induce repeated κ-opioid receptor desensitization, resulting in a subsequent upregulation of κ-opioid receptors in a manner similar to nicotine-induced nACh receptor upregulation (Fenster et al. 1999). An alternative compensatory mechanism to receptor upregulation may be changes in receptor coupling and efficacy, which were not tested here. Conversely, increased receptor function may also result from chronic antagonism of receptor activity (Jacobson et al. 1996; MacLennan et al. 1988). Acute ethanol actions on DA signaling in the NAc are biphasic, with increases in DA levels occurring with low-to-moderate doses of ethanol and decreases in DA levels occurring with high doses of ethanol that coincide with sedation (Imperato and Di Chiara 1986). Therefore, chronic exposure to high doses of ethanol may result in prolonged decreases in DA signaling and subsequent dynorphin signaling, which prime the system to be more sensitive once ethanol exposure has stopped. In order to fully understand the interplay between DA and dynorphin/κ-opioid systems in the NAc it will be important to have a clearer picture of temporal changes in DA activity across the duration of exposure. Other stress-inducing factors such as intracranial virus injection surgeries, subsequent social isolation housing and non-contingent ethanol exposure in the vapor chambers need also be considered with respect to alterations in dynorphin/κ-opioid systems.
Dopamine neurons encode learning and motivation via burst activity in response to unconditioned rewards and cues predicting conditioned rewards (Schultz 2002), and the amount of bursting scales with the magnitude of an expected reward (Morris et al. 2004; Eshel et al. 2016). Acutely, moderate doses of ethanol increase DA signaling in the NAc via increased activity in DA neurons in the ventral tegmental area. These acute increases in NAc DA gate plasticity at glutamatergic synapses encoding converging information about environmental contexts and cues paired with alcohol reward and strengthen context-associated alcohol use behaviors (Shen et al. 2008; Reynolds et al. 2001). Conversely, a general increase in κ-opioid inhibition of phasic dopamine responses in the NAc may result in reward-predicting stimuli being undervalued, negatively impacting behavioral flexibility. Thus, in the early period of abstinence following chronic ethanol exposure, a hypo-dopaminergic state occurs in the NAc (Diana et al. 1993; Volkow et al. 1996; Martinez et al. 2005; Volkow et al. 2007) and this is hypothesized to result in deficits in reward processing which may simultaneously be anhedonic (Schulteis et al. 1995; Danjo et al. 2014) and bias choice towards previously reinforced behaviors (Twining et al. 2015) over novel behaviors (Pierce et al. 1990; Rebec et al. 1997), resulting in continued and persistent alcohol use.
In summary, we have used an optogenetic model to target dopamine terminal stimulation in isolation within the heterogeneous environment in nucleus accumbens slices. We demonstrate that this model results in robust and reliable dopamine release that may be probed pharmacologically, providing a direct assessment of dopamine terminal function following chronic ethanol treatment. Using this model we show novel data that support intrinsic differences in release and regulation of dopamine terminals across accumbens sub-regions. Further we show that chronic ethanol exposure results in adaptations in distinct aspects of terminal regulation that may be uniform across the nucleus accumbens in some parameters and regionally selective in others. As the heterogeneity in dopamine neuron phenotype and accumbal field responses becomes more apparent (Juarez and Han 2016; Wightman et al. 2007), similar genetically targeted approaches to dissecting cell type-specific neuroadaptations will be useful to elucidate the molecular mechanisms that underlie neuronal plasticity and to develop accurately targeted pharmacotherapeutics.
Highlights.
Optogenetic stimulation induces phasic dopamine release in NAc slices.
Dopamine terminals in core and shell show intrinsic differences in release regulation.
Chronic ethanol increases dopamine uptake rates in the NAc core.
Chronic ethanol increases kappa opioid receptor inhibition of dopamine release.
Acknowledgments
This work was supported by National Institute of Health grants P50 DA006634 (SRJ), R01 DA030161 (SRJ), F31 AA023144 (JRM). The authors have no conflict of interest to report.
Abbreviations
- aCSF
artificial cerebral spinal fluid
- AUD
alcohol use disorder
- BEC
blood ethanol concentration
- ChR2
channelrhodopsin-2
- CIE
chronic intermittant ethanol
- DA
dopamine
- FSCV
fast scan cyclic voltammetry
- NAc
nucleus accumbens
- nor-BNI
nor-binaltorphimine
- PBS
phosphate buffered saline
- TH
tyrosine hydroxylase
- Vmax
maximal rate of uptake
- VTA
ventral tegmental area
- κ-opioid
kappa opioid receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrahao KP, Ariwodola OJ, Butler TR, Rau AR, Skelly MJ, Carter E, Alexander NP, McCool BA, Souza-Formigoni MLO, Weiner JL. Locomotor sensitization to ethanol impairs NMDA receptor-dependent synaptic plasticity in the nucleus accumbens and increases ethanol self-administration. J Neurosci Off J Soc Neurosci. 2013;33:4834–4842. doi: 10.1523/JNEUROSCI.5839-11.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hasani R, McCall JG, Shin G, Gomez AM, Schmitz GP, Bernardi JM, Pyo CO, et al. Distinct Subpopulations of Nucleus Accumbens Dynorphin Neurons Drive Aversion and Reward. Neuron. 2015;87:1063–1077. doi: 10.1016/j.neuron.2015.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Day JJ, Roitman MF, Cleaveland NA, Wightman RM, Carelli RM. Regional specificity in the real-time development of phasic dopamine transmission patterns during acquisition of a cue-cocaine association in rats. Eur J Neurosci. 2009;30:1889–1899. doi: 10.1111/j.1460-9568.2009.07027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC, Lopez MF. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin Exp Res. 2004;28:1829–1838. doi: 10.1097/01.alc.0000149977.95306.3a. [DOI] [PubMed] [Google Scholar]
- Beeler JA, Cools R, Luciana M, Ostlund SB, Petzinger G. A kinder, gentler dopamine… highlighting dopamine’s role in behavioral flexibility. Front Neurosci. 2014:8. doi: 10.3389/fnins.2014.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutros N, Semenova S, Markou A. Adolescent alcohol exposure decreased sensitivity to nicotine in adult Wistar rats. Addict Biol. 2016;21:826–834. doi: 10.1111/adb.12263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brimblecombe KR, Gracie CJ, Platt NJ, Cragg SJ. Gating of dopamine transmission by calcium and axonal N-, Q-, T- and L-type voltage-gated calcium channels differs between striatal domains. J Physiol. 2014 doi: 10.1113/jphysiol.2014.285890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt JP, Benaliouad F, McDevitt RA, Stuber GD, Wise RA, Bonci A. Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron. 2012;76:790–803. doi: 10.1016/j.neuron.2012.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MTC, Tan KR, O’Connor EC, Nikonenko I, Muller D, Luscher C. Ventral tegmental area GABA projections pause accumbal cholinergic interneurons to enhance associative learning. Nature. 2012;492:452–456. doi: 10.1038/nature11657. [DOI] [PubMed] [Google Scholar]
- Budygin EA, John CE, Mateo Y, Daunais JB, Friedman DP, Grant KA, Jones SR. Chronic ethanol exposure alters presynaptic dopamine function in the striatum of monkeys: a preliminary study. Synap N Y N. 2003;50:266–268. doi: 10.1002/syn.10269. [DOI] [PubMed] [Google Scholar]
- Budygin EA, Oleson EB, Mathews TA, Läck AK, Diaz MR, McCool BA, Jones SR. Effects of chronic alcohol exposure on dopamine uptake in rat nucleus accumbens and caudate putamen. Psychopharmacology (Berl) 2007;193:495–501. doi: 10.1007/s00213-007-0812-1. [DOI] [PubMed] [Google Scholar]
- Budygin EA, Phillips PE, Wightman RM, Jones SR. Terminal effects of ethanol on dopamine dynamics in rat nucleus accumbens: an in vitro voltammetric study. Synap N Y N. 2001;42:77–79. doi: 10.1002/syn.1101. [DOI] [PubMed] [Google Scholar]
- Cachope R, Mateo Y, Mathur BN, Irving J, Wang HL, Morales M, Lovinger DM, Cheer JF. Selective activation of cholinergic interneurons enhances accumbal phasic dopamine release: setting the tone for reward processing. Cell Rep. 2012;2:33–41. doi: 10.1016/j.celrep.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Ferris MJ, Siciliano CA, Jones SR. Differential influence of dopamine transport rate on the potencies of cocaine, amphetamine, and methylphenidate. ACS Chem Neurosci. 2015;6:155–162. doi: 10.1021/cn500262x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Sun H, Eldeeb K, Luessen DJ, Feng X, Howlett AC, Jones SR, Chen R. Amphetamine self-administration attenuates dopamine D2 autoreceptor function. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2014;39:1833–1842. doi: 10.1038/npp.2014.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro DC, Berridge KC. Opioid hedonic hotspot in nucleus accumbens shell: mu, delta, and kappa maps for enhancement of sweetness “liking” and “wanting. J Neurosci Off J Soc Neurosci. 2014;34:4239–4250. doi: 10.1523/JNEUROSCI.4458-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulter CL, Happe HK, Murrin LC. Postnatal development of the dopamine transporter: a quantitative autoradiographic study. Brain Res Dev Brain Res. 1996;92:172–181. doi: 10.1016/0165-3806(96)00004-1. [DOI] [PubMed] [Google Scholar]
- Creed MC, Lüscher C. Drug-evoked synaptic plasticity: beyond metaplasticity. Curr Opin Neurobiol. 2013;23:553–558. doi: 10.1016/j.conb.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Creed M, Pascoli VJ, Lüscher C. Addiction therapy. Refining deep brain stimulation to emulate optogenetic treatment of synaptic pathology. Science. 2015;347:659–664. doi: 10.1126/science.1260776. [DOI] [PubMed] [Google Scholar]
- Crowley NA, Bloodgood DW, Hardaway JA, Kendra AM, McCall JG, Al-Hasani R, McCall NM, et al. Dynorphin Controls the Gain of an Amygdalar Anxiety Circuit. Cell Rep. 2016;14:2774–2783. doi: 10.1016/j.celrep.2016.02.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danjo T, Yoshimi K, Funabiki K, Yawata S, Nakanishi S. Aversive behavior induced by optogenetic inactivation of ventral tegmental area dopamine neurons is mediated by dopamine D2 receptors in the nucleus accumbens. Proc Natl Acad Sci U S A. 2014;111:6455–6460. doi: 10.1073/pnas.1404323111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, Acquas E, Carboni E, Valentini V, Lecca D. Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology. 2004;47(Suppl 1):227–241. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Diana M, Pistis M, Carboni S, Gessa GL, Rossetti ZL. Profound decrement of mesolimbic dopaminergic neuronal activity during ethanol withdrawal syndrome in rats: electrophysiological and biochemical evidence. Proc Natl Acad Sci U S A. 1993;90:7966–7969. doi: 10.1073/pnas.90.17.7966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohrman And DP, Reiter CK. Chronic ethanol reduces nicotine-induced dopamine release in PC12 cells. Alcohol Clin Exp Res. 2003;27:1846–1851. doi: 10.1097/01.ALC.0000095923.41707.C8. [DOI] [PubMed] [Google Scholar]
- Ebner SR, Roitman MF, Potter DN, Rachlin AB, Chartoff EH. Depressive-like effects of the kappa opioid receptor agonist salvinorin A are associated with decreased phasic dopamine release in the nucleus accumbens. Psychopharmacology (Berl) 2010;210:241–252. doi: 10.1007/s00213-010-1836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshel N, Tian J, Bukwich M, Uchida N. Dopamine neurons share common response function for reward prediction error. Nat Neurosci. 2016;19:479–486. doi: 10.1038/nn.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenster CP, Whitworth TL, Sheffield EB, Quick MW, Lester RA. Upregulation of surface alpha4beta2 nicotinic receptors is initiated by receptor desensitization after chronic exposure to nicotine. J Neurosci Off J Soc Neurosci. 1999;19:4804–4814. doi: 10.1523/JNEUROSCI.19-12-04804.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris MJ, Calipari ES, Mateo Y, Melchior JR, Roberts DC, Jones SR. Cocaine self-administration produces pharmacodynamic tolerance: differential effects on the potency of dopamine transporter blockers, releasers, and methylphenidate. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2012;37:1708–1716. doi: 10.1038/npp.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris MJ, Calipari ES, Yorgason JT, Jones SR. Examining the complex regulation and drug-induced plasticity of dopamine release and uptake using voltammetry in brain slices. ACS Chem Neurosci. 2013;4:693–703. doi: 10.1021/cn400026v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford CP. The role of D2-autoreceptors in regulating dopamine neuron activity and transmission. Neuroscience. 2014;282:13–22. doi: 10.1016/j.neuroscience.2014.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healey JC, Winder DG, Kash TL. Chronic ethanol exposure leads to divergent control of dopaminergic synapses in distinct target regions. Alcohol Fayettev N. 2008;42:179–190. doi: 10.1016/j.alcohol.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard EC, Schier CJ, Wetzel JS, Duvauchelle CL, Gonzales RA. The shell of the nucleus accumbens has a higher dopamine response compared with the core after non-contingent intravenous ethanol administration. Neuroscience. 2008;154:1042–1053. doi: 10.1016/j.neuroscience.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Imperato A, Di Chiara G. Preferential stimulation of dopamine release in the nucleus accumbens of freely moving rats by ethanol. J Pharmacol Exp Ther. 1986;239:219–228. [PubMed] [Google Scholar]
- Jacobson KA, Lubitz DK, von Daly JW, Fredholm BB. Adenosine receptor ligands: differences with acute versus chronic treatment. Trends Pharmacol Sci. 1996;17:108–113. doi: 10.1016/0165-6147(96)10002-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanes ZM, Buske TR, Morrisett RA. CELL TYPE-SPECIFIC SYNAPTIC ENCODING OF ETHANOL EXPOSURE IN THE NUCLEUS ACCUMBENS SHELL. Neuroscience. 2014;277:184–195. doi: 10.1016/j.neuroscience.2014.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SR, O’Dell SJ, Marshall JF, Wightman RM. Functional and anatomical evidence for different dopamine dynamics in the core and shell of the nucleus accumbens in slices of rat brain. Synap N Y N. 1996a;23:224–231. doi: 10.1002/(SICI)1098-2396(199607)23:3<224::AID-SYN12>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Jones SR, O’Dell SJ, Marshall JF, Wightman RM. Functional and anatomical evidence for different dopamine dynamics in the core and shell of the nucleus accumbens in slices of rat brain. Synap N Y N. 1996b;23:224–231. doi: 10.1002/(SICI)1098-2396(199607)23:3<224::AID-SYN12>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Juarez B, Han MH. Diversity of Dopaminergic Neural Circuits in Response to Drug Exposure. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2016;41:2424–2446. doi: 10.1038/npp.2016.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkhanis AN, Rose JH, Huggins KN, Konstantopoulos JK, Jones SR. Chronic intermittent ethanol exposure reduces presynaptic dopamine neurotransmission in the mouse nucleus accumbens. Drug Alcohol Depend. 2015;150:24–30. doi: 10.1016/j.drugalcdep.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE. Functional specificity of ventral striatal compartments in appetitive behaviors. Ann N Y Acad Sci. 1999;877:71–90. doi: 10.1111/j.1749-6632.1999.tb09262.x. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev. 2004;27:765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Koob GF. Addiction is a Reward Deficit and Stress Surfeit Disorder. Front Psychiatry. 2013;4:72. doi: 10.3389/fpsyt.2013.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosillo P, Zhang Y-F, Threlfell S, Cragg SJ. Cortical Control of Striatal Dopamine Transmission via Striatal Cholinergic Interneurons. Cereb Cortex N Y N 1991. 2016 doi: 10.1093/cercor/bhw252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Steinberg EE, Földy C, Wall NR, Beier K, Luo L, Malenka RC. Diversity of transgenic mouse models for selective targeting of midbrain dopamine neurons. Neuron. 2015;85:429–438. doi: 10.1016/j.neuron.2014.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TH, Gao WY, Davidson C, Ellinwood EH. Altered activity of midbrain dopamine neurons following 7-day withdrawal from chronic cocaine abuse is normalized by D2 receptor stimulation during the early withdrawal phase. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 1999;21:127–136. doi: 10.1016/S0893-133X(99)00011-1. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, Kash TL. Mechanisms of Neuroplasticity and Ethanol’s Effects on Plasticity in the Striatum and Bed Nucleus of the Stria Terminalis. Alcohol Res Curr Rev. 2015;37:109–124. [PMC free article] [PubMed] [Google Scholar]
- MacLennan AJ, Atmadja S, Lee N, Fibiger HC. Chronic haloperidol administration increases the density of D2 dopamine receptors in the medial prefrontal cortex of the rat. Psychopharmacology (Berl) 1988;95:255–257. doi: 10.1007/BF00174519. [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Akil H, Watson SJ. Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implications. Trends Neurosci. 1995;18:22–29. doi: 10.1016/0166-2236(95)93946-u. [DOI] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Chefer VI, Shippenberg TS, Hjelmstad GO, Fields HL. Kappa opioids selectively control dopaminergic neurons projecting to the prefrontal cortex. Proc Natl Acad Sci U S A. 2006;103:2938–2942. doi: 10.1073/pnas.0511159103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Gil R, Slifstein M, Hwang DR, Huang Y, Perez A, Kegeles L, et al. Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biol Psychiatry. 2005;58:779–786. doi: 10.1016/j.biopsych.2005.04.044. [DOI] [PubMed] [Google Scholar]
- Mateo Y, Lack CM, Morgan D, Roberts DCS, Jones SR. Reduced dopamine terminal function and insensitivity to cocaine following cocaine binge self-administration and deprivation. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2005;30:1455–1463. doi: 10.1038/sj.npp.1300687. [DOI] [PubMed] [Google Scholar]
- McCool BA. Ethanol modulation of synaptic plasticity. Neuropharmacology. 2011;61:1097–1108. doi: 10.1016/j.neuropharm.2010.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchior JR, Ferris MJ, Stuber GD, Riddle DR, Jones SR. Optogenetic versus electrical stimulation of dopamine terminals in the nucleus accumbens reveals local modulation of presynaptic release. J Neurochem. 2015;134:833–844. doi: 10.1111/jnc.13177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis M, Camarini R, Ungless MA, Bonci A. Long-lasting potentiation of GABAergic synapses in dopamine neurons after a single in vivo ethanol exposure. J Neurosci Off J Soc Neurosci. 2002;22:2074–2082. doi: 10.1523/JNEUROSCI.22-06-02074.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris G, Arkadir D, Nevet A, Vaadia E, Bergman H. Coincident but distinct messages of midbrain dopamine and striatal tonically active neurons. Neuron. 2004;43:133–143. doi: 10.1016/j.neuron.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Pascoli V, Turiault M, Lüscher C. Reversal of cocaine-evoked synaptic potentiation resets drug-induced adaptive behaviour. Nature. 2011;481:71–75. doi: 10.1038/nature10709. [DOI] [PubMed] [Google Scholar]
- Patel JC, Rice ME. Classification of H2O2 as a neuromodulator that regulates striatal dopamine release on a subsecond time scale. ACS Chem Neurosci. 2012;3:991–1001. doi: 10.1021/cn300130b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira PA, Neves J, Vilela M, Sousa S, Cruz C, Madeira MD. Chronic alcohol consumption leads to neurochemical changes in the nucleus accumbens that are not fully reversed by withdrawal. Neurotoxicol Teratol. 2014;44:53–61. doi: 10.1016/j.ntt.2014.05.007. [DOI] [PubMed] [Google Scholar]
- Pickel VM. Research B.-PB, editor. Extrasynaptic distribution of monoamine transporters and receptors. Vol. 125. Elsevier; 2000. pp. 267–276. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Crawford CA, Nonneman AJ, Mattingly BA, Bardo MT. Effect of forebrain dopamine depletion on novelty-induced place preference behavior in rats. Pharmacol Biochem Behav. 1990;36:321–325. doi: 10.1016/0091-3057(90)90411-a. [DOI] [PubMed] [Google Scholar]
- Pitman KA, Puil E, Borgland SL. GABA(B) modulation of dopamine release in the nucleus accumbens core. Eur J Neurosci. 2014;40:3472–3480. doi: 10.1111/ejn.12733. [DOI] [PubMed] [Google Scholar]
- Rebec GV, Christensen JR, Guerra C, Bardo MT. Regional and temporal differences in real-time dopamine efflux in the nucleus accumbens during free-choice novelty. Brain Res. 1997;776:61–67. doi: 10.1016/s0006-8993(97)01004-4. [DOI] [PubMed] [Google Scholar]
- Resendez SL, Keyes PC, Day JJ, Hambro C, Austin CJ, Maina FK, Eidson L, et al. Dopamine and opioid systems interact within the nucleus accumbens to maintain monogamous pair bonds. eLife. 2016:5. doi: 10.7554/eLife.15325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JN, Hyland BI, Wickens JR. A cellular mechanism of reward-related learning. Nature. 2001;413:67–70. doi: 10.1038/35092560. [DOI] [PubMed] [Google Scholar]
- Rice ME, Patel JC, Cragg SJ. Dopamine release in the basal ganglia. Neuroscience. 2011;198:112–137. doi: 10.1016/j.neuroscience.2011.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DL, Howard EC, McConnell S, Gonzales RA, Wightman RM. Disparity between tonic and phasic ethanol-induced dopamine increases in the nucleus accumbens of rats. Alcohol Clin Exp Res. 2009;33:1187–1196. doi: 10.1111/j.1530-0277.2009.00942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JH, Karkhanis AN, Chen R, Gioia D, Lopez MF, Becker HC, McCool BA, Jones SR. Supersensitive Kappa Opioid Receptors Promotes Ethanol Withdrawal-Related Behaviors and Reduce Dopamine Signaling in the Nucleus Accumbens. Int J Neuropsychopharmacol. 2016:19. doi: 10.1093/ijnp/pyv127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saddoris MP. Terminal Dopamine Release Kinetics in the Accumbens Core and Shell Are Distinctly Altered after Withdrawal from Cocaine Self-Administration. eneuro. 2016;3 doi: 10.1523/ENEURO.0274-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saddoris MP, Wang X, Sugam JA, Carelli RM. Cocaine Self-Administration Experience Induces Pathological Phasic Accumbens Dopamine Signals and Abnormal Incentive Behaviors in Drug-Abstinent Rats. J Neurosci Off J Soc Neurosci. 2016;36:235–250. doi: 10.1523/JNEUROSCI.3468-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoffelmeer AN, Hogenboom F, Mulder AH. Kappa1- and kappa2-opioid receptors mediating presynaptic inhibition of dopamine and acetylcholine release in rat neostriatum. Br J Pharmacol. 1997;122:520–524. doi: 10.1038/sj.bjp.0701394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulteis G, Markou A, Cole M, Koob GF. Decreased brain reward produced by ethanol withdrawal. Proc Natl Acad Sci U S A. 1995;92:5880–5884. doi: 10.1073/pnas.92.13.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Sesia T, Bizup B, Grace AA. Evaluation of animal models of obsessive-compulsive disorder: correlation with phasic dopamine neuron activity. Int J Neuropsychopharmacol. 2013;16:1295–1307. doi: 10.1017/S146114571200154X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321:848–851. doi: 10.1126/science.1160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shippenberg TS, Zapata A, Chefer VI. Dynorphin and the pathophysiology of drug addiction. Pharmacol Ther. 2007;116:306–321. doi: 10.1016/j.pharmthera.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano CA, Calipari ES, Cuzon Carlson VC, Helms CM, Lovinger DM, Grant KA, Jones SR. Voluntary ethanol intake predicts κ-opioid receptor supersensitivity and regionally distinct dopaminergic adaptations in macaques. J Neurosci Off J Soc Neurosci. 2015a;35:5959–5968. doi: 10.1523/JNEUROSCI.4820-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano CA, Calipari ES, Ferris MJ, Jones SR. Adaptations of presynaptic dopamine terminals induced by psychostimulant self-administration. ACS Chem Neurosci. 2015b;6:27–36. doi: 10.1021/cn5002705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano CA, Calipari ES, Yorgason JT, Lovinger DM, Mateo Y, Jimenez VA, Helms CM, Grant KA, Jones SR. Increased presynaptic regulation of dopamine neurotransmission in the nucleus accumbens core following chronic ethanol self-administration in female macaques. Psychopharmacology (Berl) 2016;233:1435–1443. doi: 10.1007/s00213-016-4239-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirohi S, Bakalkin G, Walker BM. Alcohol-induced plasticity in the dynorphin/kappa-opioid receptor system. Front Mol Neurosci. 2012;5:95. doi: 10.3389/fnmol.2012.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Herz A, Shippenberg TS. The effects of opioid peptides on dopamine release in the nucleus accumbens: an in vivo microdialysis study. J Neurochem. 1990;55:1734–1740. doi: 10.1111/j.1471-4159.1990.tb04963.x. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Herz A, Shippenberg TS. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc Natl Acad Sci U S A. 1992;89:2046–2050. doi: 10.1073/pnas.89.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner H, Gerfen CR. Cocaine-induced c-fos messenger RNA is inversely related to dynorphin expression in striatum. J Neurosci Off J Soc Neurosci. 1993;13:5066–5081. doi: 10.1523/JNEUROSCI.13-12-05066.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner H, Gerfen CR. Dynorphin regulates D1 dopamine receptor-mediated responses in the striatum: relative contributions of pre- and postsynaptic mechanisms in dorsal and ventral striatum demonstrated by altered immediate-early gene induction. J Comp Neurol. 1996;376:530–541. doi: 10.1002/(SICI)1096-9861(19961223)376:4<530::AID-CNE3>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Hnasko TS, Britt JP, Edwards RH, Bonci A. Dopaminergic terminals in the nucleus accumbens but not the dorsal striatum corelease glutamate. J Neurosci Off J Soc Neurosci. 2010a;30:8229–8233. doi: 10.1523/JNEUROSCI.1754-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Hopf FW, Tye KM, Chen BT, Bonci A. Neuroplastic alterations in the limbic system following cocaine or alcohol exposure. Curr Top Behav Neurosci. 2010b;3:3–27. doi: 10.1007/7854_2009_23. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Stamatakis AM, Kantak PA. Considerations when using cre-driver rodent lines for studying ventral tegmental area circuitry. Neuron. 2015;85:439–445. doi: 10.1016/j.neuron.2014.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Cragg SJ, Rice ME. Striatal dopamine neurotransmission: regulation of release and uptake. Basal Ganglia. 2016;6:123–148. doi: 10.1016/j.baga.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Carrillo-Reid L, Bargas J. Dopaminergic modulation of striatal neurons, circuits, and assemblies. Neuroscience. 2011;198:3–18. doi: 10.1016/j.neuroscience.2011.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SR, Badurek S, Dileone RJ, Nashmi R, Minichiello L, Picciotto MR. GABAergic and glutamatergic efferents of the mouse ventral tegmental area. J Comp Neurol. 2014;522:3308–3334. doi: 10.1002/cne.23603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper JM, Tecuapetla F, Koós T, Ibáñez-Sandoval O. Heterogeneity and diversity of striatal GABAergic interneurons. Front Neuroanat. 2010;4:150. doi: 10.3389/fnana.2010.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfell S, Lalic T, Platt NJ, Jennings KA, Deisseroth K, Cragg SJ. Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron. 2012;75:58–64. doi: 10.1016/j.neuron.2012.04.038. [DOI] [PubMed] [Google Scholar]
- Tritsch NX, Ding JB, Sabatini BL. Dopaminergic neurons inhibit striatal output through non-canonical release of GABA. Nature. 2012;490:262–266. doi: 10.1038/nature11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch NX, Sabatini BL. Dopaminergic modulation of synaptic transmission in cortex and striatum. Neuron. 2012;76:33–50. doi: 10.1016/j.neuron.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai H-C, Zhang F, Adamantidis A, Stuber GD, Bonci A, Lecea L, de Deisseroth K. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324:1080–1084. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twining RC, Wheeler DS, Ebben AL, Jacobsen AJ, Robble MA, Mantsch JR, Wheeler RA. Aversive stimuli drive drug seeking in a state of low dopamine tone. Biol Psychiatry. 2015;77:895–902. doi: 10.1016/j.biopsych.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Hitzemann R, Ding YS, Pappas N, Shea C, Piscani K. Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcohol Clin Exp Res. 1996;20:1594–1598. doi: 10.1111/j.1530-0277.1996.tb05936.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Jayne M, Ma Y, Pradhan K, Wong C. Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J Neurosci Off J Soc Neurosci. 2007;27:12700–12706. doi: 10.1523/JNEUROSCI.3371-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Koob GF. Pharmacological evidence for a motivational role of kappa-opioid systems in ethanol dependence. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2008;33:643–652. doi: 10.1038/sj.npp.1301438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield TW, Schlosburg JE, Wee S, Gould A, George O, Grant Y, Zamora-Martinez ER, et al. κ Opioid receptors in the nucleus accumbens shell mediate escalation of methamphetamine intake. J Neurosci Off J Soc Neurosci. 2015;35:4296–4305. doi: 10.1523/JNEUROSCI.1978-13.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman RM, Amatore C, Engstrom RC, Hale PD, Kristensen EW, Kuhr WG, May LJ. Real-time characterization of dopamine overflow and uptake in the rat striatum. Neuroscience. 1988;25:513–523. doi: 10.1016/0306-4522(88)90255-2. [DOI] [PubMed] [Google Scholar]
- Wightman RM, Heien MLAV, Wassum KM, Sombers LA, Aragona BJ, Khan AS, Ariansen JL, Cheer JF, Phillips PEM, Carelli RM. Dopamine release is heterogeneous within microenvironments of the rat nucleus accumbens. Eur J Neurosci. 2007;26:2046–2054. doi: 10.1111/j.1460-9568.2007.05772.x. [DOI] [PubMed] [Google Scholar]
- Wu Q, Reith MEA, Walker QD, Kuhn CM, Carroll FI, Garris PA. Concurrent autoreceptor-mediated control of dopamine release and uptake during neurotransmission: an in vivo voltammetric study. J Neurosci Off J Soc Neurosci. 2002;22:6272–6281. doi: 10.1523/JNEUROSCI.22-14-06272.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Reith ME, Wightman RM, Kawagoe KT, Garris PA. Determination of release and uptake parameters from electrically evoked dopamine dynamics measured by real-time voltammetry. J Neurosci Methods. 2001;112:119–133. doi: 10.1016/s0165-0270(01)00459-9. [DOI] [PubMed] [Google Scholar]
- Xia Y, He L, Whistler JL, Hjelmstad GO. Acute amphetamine exposure selectively desensitizes kappa-opioid receptors in the nucleus accumbens. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2008;33:892–900. doi: 10.1038/sj.npp.1301463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Qi J, Wang HL, Zhang S, Morales M. Glutamatergic and dopaminergic neurons in the mouse ventral tegmental area. Eur J Neurosci. 2015;41:760–772. doi: 10.1111/ejn.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorgason JT, España RA, Jones SR. Demon voltammetry and analysis software: analysis of cocaine-induced alterations in dopamine signaling using multiple kinetic measures. J Neurosci Methods. 2011;202:158–164. doi: 10.1016/j.jneumeth.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorgason JT, Ferris MJ, Steffensen SC, Jones SR. Frequency-dependent effects of ethanol on dopamine release in the nucleus accumbens. Alcohol Clin Exp Res. 2014;38:438–447. doi: 10.1111/acer.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Sulzer D. Regulation of striatal dopamine release by presynaptic auto- and heteroreceptors. Basal Ganglia. 2012;2:5–13. doi: 10.1016/j.baga.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Doyon WM, Clark JJ, Phillips PEM, Dani JA. Controls of tonic and phasic dopamine transmission in the dorsal and ventral striatum. Mol Pharmacol. 2009;76:396–404. doi: 10.1124/mol.109.056317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Qi J, Li X, Wang HL, Britt JP, Hoffman AF, Bonci A, Lupica CR, Morales M. Dopaminergic and glutamatergic microdomains in a subset of rodent mesoaccumbens axons. Nat Neurosci. 2015;18:386–392. doi: 10.1038/nn.3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YP, Oertner TG. Optical induction of synaptic plasticity using a light-sensitive channel. Nat Methods. 2007;4:139–141. doi: 10.1038/nmeth988. [DOI] [PubMed] [Google Scholar]
- Zhou FM, Liang Y, Dani JA. Endogenous nicotinic cholinergic activity regulates dopamine release in the striatum. Nat Neurosci. 2001;4:1224–1229. doi: 10.1038/nn769. [DOI] [PubMed] [Google Scholar]