Abstract
Type-1 Diabetes (T1D) is a devastating autoimmune disorder which results in the destruction of beta cells within the pancreas. A promising treatment strategy for T1D is the replacement of the lost beta cell mass through implantation of immune-isolated microencapsulated islets referred to as the bioartificial pancreas. The goal of this approach is to restore blood glucose regulation and prevent the long-term comorbidities of T1D without the need for immunosuppressants. A major requirement in the quest to achieve this goal is to address the oxygen needs of islet cells. Islets are highly metabolically active and require a significant amount of oxygen for normal function. During the process of isolation, microencapsulation, and processing prior to transplantation, the islets’ oxygen supply is disrupted, and a large amount of islet cells are therefore lost due to extended hypoxia, thus creating a major barrier to clinical success with this treatment. In this work, we have investigated the oxygen generating compounds, sodium percarbonate (SPO) and calcium peroxide (CPO) as potential supplemental oxygen sources for islets during isolation and encapsulation before and immediately after transplantation. First, SPO particles were used as an oxygen source for islets during isolation. Secondly, silicone films containing SPO were used to provide supplemental oxygen to islets for up to 4 days in culture. Finally, CPO was used as an oxygen source for encapsulated cells by co-encapsulating CPO particles with islets in permselective alginate microspheres. These studies provide an important proof of concept for the utilization of these oxygen generating materials to prevent beta cell death caused by hypoxia.
Keywords: Islets, Isolation Culture, Microencapsulation, Calcium Peroxide, Sodium Percarbonate, Oxygenation
Graphical Abstract
In this work, sodium percarbonate and calcium peroxide were utilized as an oxygen source to improve islet viability and functionality.
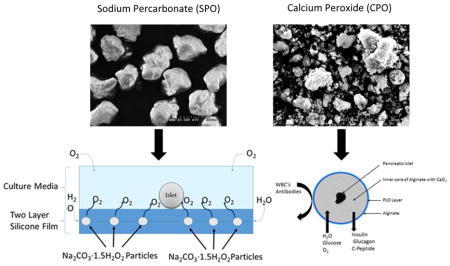
3) Introduction
Approximately three million Americans have T1D, which accounts for $14.9 billion in health care costs annually in the US 1. While insulin therapy can control blood sugar, it has been established that even long-term intensive insulin therapies cannot prevent the development of secondary complications of diabetes, including diabetic retinopathy, nephropathy, and neuropathy 2–4, in part as a result of poor glycemic control and the absence of C-peptide 5–7. Beta-cell replacement through islet transplantation has the potential to cure T1D as it can provide both insulin and C-peptide, and perhaps other factors. Additionally, the microencapsulation of islets in alginate microbeads with a perm-selective coating, such as poly-L-orthinine or poly-L-lysine, prior to transplantation may permit both allogeneic and xenogeneic transplantation without the use of immunosuppressants 8–10.
While immunoisolation by microencapsulation opens up more options for donor tissue sources and obviates the need for immunosuppressants, implanted grafts have had limited success due to severe hypoxic conditions, which starts from islet isolation through the immediate post-transplant period. The hypoxic conditions are especially harmful to the islets due to the fact that the islet βcells have high metabolic requirements; they require approximately 10 to 12 % of blood flow to the pancreas, while only accounting for 1 to 2 % of the gland’s weight 11, 12. Several strategies have been proposed to reduce hypoxic burden on islets including the use of angiogenic growth factors such as FGF-1 to improve angiogenesis and reduce time to engraftment 13 and implanting encapsulated islets within highly vascularized transplant sites, such as the omentum 14, 15. While these approaches have improved graft function, the hypoxic burden still remains a challenge. In order to prevent hypoxic damage to islets, a continuous supply of oxygen is needed from the beginning of the isolation process until revascularization occurs, typically 5 to 10 days post-transplantation 16. Other approaches for supplying oxygen to islets include the use of perfluorocarbons (PFCs) 17. PFCs have been used in a number of tissue-engineered constructs 18–20 as well as in pancreas and islet storage prior to transplantation 21, 22; however, because these materials cannot generate oxygen they require an oxygen reservoir which can be problematic in a closed environment 23. In addition, it has been proposed that a macrochamber can be engineered for islet transplantation that would allow for controlled and adequate oxygen supply while providing immunological protection of donor islets against the host immune system 24. The engineered macrochamber consists of islets immobilized in alginate hydrogel, a gas chamber, a gas permeable membrane, an external membrane and a mechanical support. The implantable device is refueled with oxygen via subdermally implantable access port, and has been shown to achieve normal blood glucose control in diabetic rats for periods up to 6 months 25. While the results are promising with this approach, a potential challenge with the macrodevice as with whole pancreas oxygenated externally by the 2-layer method, is that only a small proportion of the islets in the chamber may be oxygenated 26. Thus, a method to enhance oxygen supply to individual islets until revascularization after transplantation is highly desirable.
Chemically-generated oxygen is an alternative method of supplying oxygen to tissues through the controlled decomposition of peroxides to oxygen and water. The oxygen generating materials sodium percarbonate (SPO) and calcium peroxide (CPO) are of particular interest. SPO has been shown to be an oxygen-generator for tissue engineering applications 27, 28, and it rapidly degrades to produce O2 through the generation and decomposition of hydrogen peroxide as shown below:
| Equation 1 |
| Equation 2 |
CPO has also been shown to improve cell viability in hypoxic environments in vitro 29. In the presence of water, CPO will generate hydrogen peroxide which will degrade to oxygen and water in the two step reaction shown below in equations 3 and 4, at a rate much slower than SPO. A study by Pedraza et al., has shown CPO to be beneficial to islets for >3 weeks in culture when combined with silicone 30. Thus, this material could be utilized to provide oxygen to the encapsulated islet in low-oxygen environments for up to seven days.
| Equation 3 |
| Equation 4 |
The goal of the present study was to determine if we can utilize both SPO and CPO to reduce the hypoxic burden on islets, beginning with islet isolation and continuing 7 days after microencapsulation. To accomplish this objective, we conducted the following three studies: i) we examined whether the addition of SPO directly to reagents during islet isolation, could improve islet functionality and viability immediately following isolation; ii), we evaluated the use of culture wells lined with silicone films containing SPO particles to determine if oxygen release from these materials would also improve islet quality; iii), we assessed whether co-encapsulation of individual islets with CPO particles in alginate microbeads would improve islet quality following one week of culture in a low-oxygen environment.
4) Materials and Methods
a) Ethics Statement
This study was performed in strict accordance with the NIH guidelines for the care and use of laboratory animals (NIH Publication No. 85-23 Rev. 1985) and was approved by the Institutional Animal Care and Use Committee of Wake Forest University Health Sciences, Winston Salem, NC, USA.
b) Materials
Except for the materials listed below all materials and reagents were purchased from Sigma (St. Louis, MO, USA). All catalase used for the following experiments was derived from bovine liver, (Catalogue # C9322, Sigma, St. Louis, MO, USA). Collagenase P was purchased from Roche (Indianapolis, IN, USA). Density gradient solutions were prepared using Optiprep and University of Wisconsin solution (UWS; Perfadex, Xvivo Perfusion, Englewood, CO). High-mannuronic acid-low viscosity (LVM) alginate and high-guluronic acid-low viscosity (LVG) alginate were purchased from Nova-Matrix (Sandvika, Norway) and were reported by the manufacturer to have molecular weights 75–200 kDa and G/M ratios of ≤1 and ≥1.5, respectively. [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS) was purchased from Promega (Madison, WI, USA). 125I-insulin was purchased from Perkin Elmer (Waltham, MA).
c) Oxygen Release from Oxygen Generating Biomaterials
For study (i), oxygen release was measured from SPO particles at both 4°C and 37°C, with and without catalase, in the presence or absence of paraffin coatings. For study (ii), oxygen release was measured from the oxygen generating culture wells described below at 37°C in the presence of catalase. For study (iii), oxygen release was measured from CPO particles, which were encapsulated in 2% LVM (as described below) at 37°C with Catalase. In all cases, the same methodology was followed. Oxygen release measurements were taken using an Orion Four Star dissolved oxygen (DO) probe (Thermo Scientific, Waltham, MA, USA) in a sealed 2.6 L tank filled with phosphate buffered saline (PBS), pH 7.4. First, the PBS was degassed by boiling and cooling under a low vacuum to either 37 or 4 °C, and then the tank was submerged and sealed in the degassed PBS taking care that no air was present in the tank. In study (ii), culture wells were added prior to sealing the tank. After the tank was equilibrated to the appropriate temperature, 0.5 mL of an antibiotic-antimycotic (Clear Bath, Spectrum, Rancho Dominguez, CA, USA) was added through a one-way valve. The tank was then mixed using a stir bar set at 60 rpm, and once DO and temperature readings were stable, either CPO or SPO particles were added through the one-way valve; the probe then sampled every five minutes automatically and data was logged using the software Hyper Terminal (Hilgraeve, Monroe, MI). To determine release profiles, oxygen entering the system through diffusion, which was determined from control measurements, was subtracted, and oxygen generated from the materials was calculated as a percentage of the total possible amount of oxygen, which was determined using equations 1 and 2 above. These oxygen generation rates were then fit to Equation 5:
| Equation 5 |
This equation has been previously used to describe the release rates of solid peroxides, including calcium peroxide (CPO) and sodium percarbonate (SPO) 31, where C is the concentration of O2 (described here as the percentage of the total oxygen released), C* is saturation concentration of O2 (also described here as the percentage of the total oxygen generated), Cstart is the initial concentration of oxygen, ϰ is the generation rate (h−1), and t is the time (h). Since the starting solutions for these experiments were degassed, the initial concentration of oxygen is 0 mg/L, and Equation 6 shows the simplified expression:
| Equation 6 |
d) Rat Islet Isolation and Culture
Female Fisher 344 rats were purchased from Harlan (Dublin, VA) and housed 2 rats/cage in a temperature-controlled room with a 12-hour light dark cycle. The animals had unlimited access to food and water. Islet isolations were conducted as previously described 32, 33; however, a fresh 100 μM solution of SPO with 100 unit/mL Catalase was added to all reagents immediately prior to use. Figure 1 represents a sample of the SPO particles used in in our studies. Following euthanasia by CO2 asphyxiation, the rat abdomen was shaven and disinfected using 10% Betadine followed by 70% ethanol. A midline incision was made and the pancreatic duct was cannulated and infused with 10 mL of a 1 mg/mL Collagenase P solution with 100 U/mL catalase in Hanks Balanced Salts Solution (HBSS) with or without (control) 100 μM SPO. Following distention, the pancreas was harvested and placed in a dish with 2 mL of the digestion solution on ice. The tissue was then digested for 20 minutes at 37 °C in a shaking water bath. Following digestion, the tissue was filtered through a 500 μm nylon mesh into 20 mL of ice cold wash solution consisting of HBSS with 10% fetal bovine serum (FBS), 10 mM HEPES, 100 U/mL Catalase, with or without 100μM SPO. The tissue was then gently inverted for 45seconds and then centrifuged for 5 minutes at 250 g and 4 °C. The digestion solution was removed and the tissue was washed thrice with fresh wash solution.
Figure 1.
SEM images of coated and uncoated SPO particles. Figure 1A is a representative image of uncoated SPO particles with an average diameter of 330±8 μm, Figure 1B is a representative image of SPO particles coated with paraffin wax with an average diameter of 356±10 μm. Magnified images of the uncoated SPO particles reveal the crystalline structure of SPO as seen in Figure 1C; this structure was undetectable in coated SPO particles as seen in Figure 1D, indicating that the paraffin coating completely coated the SPO.
Following the washing steps, islets were purified using an Optiprep density gradient as previously described 15 with the addition of 100 U/mL Catalase and with or without 100 μM SPO. Pelleted tissue was re-suspended in 30 mL of a 1.1 g/mL density gradient solution prior to 8 mL of 1.09 g/mL and 10 mL of 1.046 g/mL solutions being gently layered on top. The columns were allowed 10 minutes to equilibrate at 4 °C prior to centrifugation at 380 g for 5 minutes with no brake. After centrifugation, islets were collected at the 1.045/1.09 interface, washed with wash solution, and then placed in culture in RPMI 1640 media with 10% FBS, 1 % penicillin/streptomycin, 10 mM HEPES, 100 U/mL catalase, and with or without 100 μM SPO under standard cultured conditions for 4 hours prior to viability analysis with MTS, dynamic perifusion, and qPCR. Following culture islet yield was determined by Dithizone staining, using a 1 mg/mL solution of Dithizone in a 1:5 (v/v) solution of dimethyl sulfoxide and PBS.
e) Porcine Islet Isolation and Culture
Porcine islets were isolated using the selective osmotic shock method 34. In this method, young adult pigs (20kg) were intravenously heparinized (400U/kg) then euthanized according to IACUC guidelines. Following euthanasia, the pancreas was removed and placed in sterile saline on ice until isolation. Pancreases were first washed with 1 L sterile PBS and incubated for 5 minutes in a 10% Betadine solution and washed again with fresh 1L sterile PBS. The tissue was then finely minced and incubated in RPMI-1640 containing 300 mM glucose and 2 mM Trolox for 20 minutes at room temperature. The tissue was then centrifuged at 200 g for 5 minutes and the high glucose solution was removed and replaced with RPMI-1640 without glucose. The tissue was washed thrice with the no glucose RPMI solution and then gently dissociated with a syringe for 15 minutes. The dissociated tissue was filtered first through a 500 μM filter and then through a 350 μM filter and washed three times prior to purification. After washing, the islets were purified using the same Optiprep method described above, without the addition of SPO or Catalase. For culture experiments using the oxygen generating culture wells, islets were immediately placed into culture on either experimental or control culture plates in an RPMI-1640 media with 20% porcine serum, 1 % penicillin/streptomycin, 10 mM HEPES, 1.5 mM Trolox, and 100 U/mL Catalase. Media were changed after overnight culture, then every 48 hours, and islets were collected after 1 and 4 days of culture for viability assessments by MTS, dynamic perifusion, and qPCR. For encapsulation experiments, islets were cultured overnight in the same media prior to encapsulation as described below.
f) Generation of PDMS-SPO Culture Wells
Oxygen generating culture wells (shown in figure 2) were prepared using the Sylgard 184 silicone elastomer kit (World Precision Instruments, Sarasota, FL). First, a 1 mm thick silicone film was made by pouring silicone (10:1 (wt/wt) silicone elastomer base: curing agent) into a 150 mm × 20 mm Petri dish, and then SPO particles with an average diameter of 1.25±0.25 mm were added on top of the silicone and allowed to cure at room temperature for 48 hours. After curing, a second 1 mm layer was added over the SPO particles and the construct was allowed to cure at room temperature for an additional 48 hours prior to sterilization via ethylene oxide.
Figure 2.
(A) Schematic representation of oxygen generating culture system. (B) SEM images of SPO particles; based on these images we found that our SPO particles had an average diameter of 1810 μm ± 90 μm (average ± SEM; n=20). (C) Culture dishes with silicone films containing no SPO (control) and 90 mg SPO.
g) Islet Co-encapsulation with CPO
Islets were encapsulated using a modified protocol previously described. CPO particles, as shown in figure 3A, were first incubated in PBS over night at 37°C and washed thrice with sterile PBS prior to encapsulation. Following the washing steps, CPO was added to 2% LVM at a concentration of 10 mg/mL with pig islets at a concentration of 3×103 islet/mL alginate in the presence of 100 U/mL Catalase. The cell suspension was then pumped thorough an 8 channel microfluidic device 35 at a flow rate of 0.5 mL/min with an air pressure of 4.5 psi. The resulting microbeads (shown without CPO particles in Figure 3B and with CPO particles in Figures 3C) were collected in a 100 mM calcium chloride bath in 10 mM HEPES and allowed to crosslink for 15 minutes prior to washing with HBSS. After washing, the microcapsules were then placed in an RPMI-1640 culture media with 20% porcine serum, 1% penicillin/streptomycin, 10 mM HEPES, and 1.5 mM Trolox and cultured in a hypoxic chamber at 37 °C with 5% CO2, 5% O2, and 90% N2 for one week prior to analysis with MTS, dynamic perifusion, and qPCR.
Figure 3.
(A) SEM images of CPO particles (B) Phase contrast image of microencapsulated islets in alginate microcapsules with poly-L-orthinine semipermeable coatings (control). (C) Phase contrast images of islets co-encapsulated with CPO.
h) MTS Assay
At the time points specified above for studies i and iii, MTS assays were used to determine islet viability. Five hundred islets from each group were handpicked and incubated in 300 μL of a 20% MTS working solution in culture media for 4 hours under standard conditions. After incubation, the islets were removed by centrifugation and the colorimetric change was measured by absorbance at 490 nm.
i) Dynamic perifusion of islets and measurement of insulin secretion
Perifusion experiments were conducted as previously described 36 with the following modifications. At the above mentioned time points, islets were handpicked into perifusion chambers (Biorep Technologies, Miami, FL, USA) with 200 islets per chamber. The islets were pre-perifused at the rate of 120 μL/min with a low glucose solution consisting of 125 mM NaCl, 5.9 mM KCl, 2.56 mM CaCl2, 1 mM MgCl2, 25 mM HEPES, 0.1% BSA and 3 mM glucose at pH=7.4 for 1 hour at 37°C. Following the 1 h pre-perifusion, basal effluent samples were collected every five minutes for 20 minutes before changing the glucose concentration in the perifusate to 16. 7 mM (300 mg/dL) glucose for 30 minutes stimulation of insulin secretion. During the high glucose perifusion, effluent samples were collected every 2 minutes. After 30-minute high glucose perifusion, the islets were returned to the basal 3.3 mM glucose perifusion for 20 minutes, and effluent perifusate samples were also collected every 5 minutes. All effluent samples were kept on ice during the perifusions and then stored at −20°C until they were analyzed by radioimmunoassay (RIA) for insulin content 37. For all perifusion experiments the stimulation index was calculated as a ratio of the average insulin secreted during the high glucose period normalized to the average insulin secreted during the basal glucose incubation period.
j) RNA Isolation, cDNA Synthesis, and Quantitative Polymerase chain reaction (qPCR)
Immediately after harvesting, islets were washed with ice-cold PBS; snap frozen in liquid nitrogen and stored a −80 °C until analysis. Total RNA was extracted from approximately 1000 islets per group using an RNAeasy kit (Qiagen, Sussex, UK) and quantified on a NanoDrop 2000 UV/Vis spectrophotometer (Thermo Scientific, Waltham, MA, USA). Reverse transcription to cDNA was performed using the Applied Biosystems High Capacity cDNA synthesis kit (Carlsbad, CA, USA). Each sample value was normalized to GAPDH and relative expressions of mRNAs calculated by the 2−ΔΔ Ct method. Levels of mRNA were measured using TaqMan probes on a 7300 Real Time PCR System (Applied Biosystems, Waltham, MA, USA). The probes used to detect Casp3, INS1, and GAPDH, were Rn00563902_m1, Rn02121433_g1 and Rn01775763_g1 for study i, and Ss03382792_u1, Ss03386682_u1, and Ss03374854_g1 for studies ii and iii, respectively.
k)
l) Statistical Analysis
Statistical assessments were performed on functional and histological data using GraphPad Prism software. Unless otherwise specified, all results were presented as the arithmetic mean ± standard error of the mean (SEM). For study (i), analysis of variance (ANOVA) was performed for all parameters. When an ANOVA revealed significance (p<0.05), a Bonferroni post-hoc test was performed. For studies (ii) and (iii) a Student’s paired t-test was used for comparisons with p<0.05 considered significant.
5) Results and Discussion
a) Oxygen Release from Oxygen Generating Materials
Oxygen generation profiles from SPO with or without incorporation into silicone film, as well as that released from CPO alone are shown in Figure 4, and a summary of the oxygen generation rates are shown in Table 1. We found that the release rate of SPO is temperature sensitive, with an approximate 3-fold increase in the rate of oxygen generated from SPO at 37°C compared to 4°C (Figures 4A and 4B). However, the biggest driver of the reaction appeared to be Catalase, which at 4°C, at a concentration of 100 U/mL increased the reaction rate approximately 40-fold. As expected, the activity of Catalase is also temperature sensitive, and consequently, the SPO with Catalase at 37°C had a release rate approximately 4-fold higher than at 4°C. The oxygen release profile at both temperatures for the purpose of islet isolation is interesting, considering that both temperatures are involved in the isolation process. The addition of the paraffin coating extended the release rate approximately 4-fold at 37°C and 3-fold at 4°C. Considering that fresh SPO is added during each stage of the isolation, the oxygen requirements for islets should at least be met, if not exceeded, during the entirety of this process. Oxygen release from silicone films containing SPO is shown in Figure 4C, with an oxygen generation rate of C=32.9(1-e−0.0114t). When compared to the oxygen measurements obtained from SPO particles alone as shown in Table 1, it is apparent that the incorporation of SPO particles into silicone delayed oxygen release from the SPO approximately ten-fold. Oxygen release measurements for encapsulated CPO particles in PBS with 100 U/mL of Catalase at 37 °C indicated that the most of the oxygen release from this oxygen generator occurred within the first four days of culture, as shown in Figure 4D. Using the best fit equation the release rate was determined to be, C=21.106*(1-e−0.0402·t).
Figure 4.
Oxygen measurements from oxygen generating biomaterials. (A) Oxygen release from SPO particles in PBS, pH=7.4, at 37°C without catalase (Black), with catalase (Red), and paraffin-coated with catalase (Green). (B) Oxygen release from SPO particles in PBS, pH=7.4, at 4°C without catalase (Black), with catalase (Red), and paraffin-coated with catalase (Green). Each line represents the (average ± SEM, n=5). (C) Oxygen generated from culture dishes with silicone bottoms containing SPO particles in PBS at 37 °C, measurements were taken in PBS at 37 °C with 100 U/mL catalase. (Average ± SEM, n=5) (D) Oxygen release profile for islets co-encapsulated with CPO in PBS, pH=7.4, at 37°C with 100 U/mL catalase. The O2 release is described as the percentage of total oxygen release, which was determined by the total theoretical amount of oxygen release per mg of SPO or CPO.
Table 1.
Release kinetics for oxygen generating particles. To describe the release rates of SPO and CPO, oxygen release measurements were fit to the following equation: C=C*(1-e−ϰt) + Cstart·e−ϰt, which has been previously used to describe the release rates of solid peroxides, including calcium peroxide and sodium percarbonate. In the equation, C is the concentration of O2 (described here as the percentage of the total oxygen released), C* is saturation concentration of O2 (also described here as the percentage of the total oxygen released), Cstart is the initial concentration of oxygen, ϰ is the release rates (h−1), t is the time (h). Since the starting solution for these experiments was degassed the initial concentration of oxygen is 0 mg/L, reducing the equation to: C=C*(1-e−ϰt).
| Group | ϰ (h−1) | Cmax (%) | r2 |
|---|---|---|---|
| SPO, 4°C | 0.136 | 36.2 | 0.82 |
| SPO, 37°C | 0.428 | 17.8 | 0.99 |
| SPO, 4°C + catalase | 5.73 | 91.8 | 0.92 |
| SPO, 37°C + catalase | 21.68 | 91.0 | 0.99 |
| Paraffin-coated SPO, 4°C + catalase | 1.75 | 69.3 | 0.98 |
| Paraffin-coated SPO, 37°C + catalase | 5.31 | 66.3 | 0.78 |
| Oxygen Generating Culture Wells | 0.01 | 32.9 | 0.96 |
| Encapsulated CPO | 0.04 | 21.1 | 0.982 |
b) Islet Isolations with Sodium Percarbonate
For our islet isolation experiments, there was no significant difference in cold ischemia times of the procured pancreases between the control group and the groups treated with SPO, which were 28.5±11.7 versus 24.5±2.06 minutes, respectively. Isolation and purification times between control and experimental groups were also comparable at 118.75±2.92 versus 114.5±2.06 minutes. We found no significant difference between the pre-purification yield per rat (Control: 1150±580 islets vs SPO: 1010±310 islets) and post-purification yields (Control: 510±60 islets vs SPO: 590±420 islets) for islets isolated with or without SPO (average ± standard deviation; n=5).
The addition of SPO during the islet isolation process resulted in higher insulin secretion rates by islets immediately following isolation compared to controls, as determined by dynamic perifusion tests shown in Figure 5A, where glucose stimulation indices (Figure 5B) were measured as 1.76±0.51 for control samples, 3.15±1.65 for islets isolated with SPO, and 2.89±0.58 for islets isolated with paraffin-coated SPO (p<0.05; average ± standard deviation, N=5). The metabolic activity for islets isolated in the presence of either coated or uncoated SPO was found to be higher relative to control as determined by the MTS assay results shown in Figure 5C (Control: 1.00±0.1; SPO: 1.33±0.14 islets; paraffin-coated SPO: 1.36±0.18 normalized abs. units, p<0.05; (mean ± standard deviation, N=4). Insulin production was also higher in beta cells isolated with SPO relative to control cells, as determined by INS1 expression, and shown in Figure 5D (Control: 49.5% ± 14.45%; SPO: 7.71±2.05 islets; paraffin-coated SPO: 5.81±1.63 fold increase, p<0.05; average ± standard deviation, N=3). We observed a significant difference in cell viability 24 hours following isolation under the different groups, as shown in Figure 5E, and quantitated in 5F (Control: 49% ± 14%; SPO: 87% ± 13%; paraffin-coated SPO: 84 ± 2 fold increase, p<0.05; mean± standard deviation, N=3). However, there was no significant difference immediately following the isolation. This later increase in viability may be the result of the SPO preventing an ischemic reperfusion injury which would significantly decrease the quality of islets following isolation 38 and significantly decrease the viability of islets following overnight culture 39.
Figure 5.
Effect of Addition of Sodium Percarbonate during islet isolation and purification on islet viability and functionality. (A) (B) (C) (D) MTS Results for islets isolated under normal conditions (control), SPO, and Paraffin-coated SPO. All groups were isolated with 100 U/mL of catalase. At 4 hours, islets isolated with SPO and paraffin-coated SPO were found to exhibit a significant increase in absorbance compared to control (p<0.05). Absorbance values are normalized to control values for each time point; values represent averages ± SEM, * indicates significance (p<0.05). (E) Representative live/dead images of dispersed islets 24 hours after isolation with either no additive (control), SPO, or paraffin-coated SPO. Green cells represent viable cells which stained positive for SYTO-10, and red cells represent dead cells which stained positive for Dead Red. (F) Quantification of live/dead images: data is depicted as percent viable cells, (Syto-10 positive cells/Syto-1 positive cells + Dead Red positive cells). At 4 hours following isolation there is no significant difference between groups (data not shown); however, by 24 hours there is a significant difference in viability for islets isolated with either SPO or paraffin-coated SPO compared to control. Data is displayed as average ± SEM, * indicates significance (P<0.05).
It is unclear whether the SPO was most effective during the cold ischemia period prior to isolation or during the actual isolation process itself; further studies will be needed to determine the period of greatest benefit. However, we hypothesize that SPO will be more beneficial during the cold ischemia time as a way to prevent reperfusion injury to organs than during the isolation, especially considering that, in the case of standard human islet isolations, which use a Ricordi chamber the process is presently done with an oxygenated perfusion loop. Interestingly, we did not observe an additional benefit to coating SPO with paraffin. This could be due to the fact that the burst release of oxygen from SPO is sufficient to improve islet quality during these isolations. Alternatively, this could be a result of the relatively short cold ischemia and isolation time; it is possible that with longer cold ischemia times the longer release of paraffin-coated SPO particles will provide more benefit to tissue than the uncoated SPO particles. We also found that there is a limit to the amount of SPO which can be utilized to prevent hypoxic damage to islets. The addition of too much SPO will result in damage to islets as a result of oxidative stress, which islets are especially susceptible to 40, 41. In initial studies, we found that exceeding 100 μM SPO typically resulted in the loss of cell viability as a result of oxidative stress. However, there are several antioxidants, in particular Trolox, which may allow for an increase in SPO concentration without causing significant damage 42.
c) Islet Culture with Oxygen Generating Culture Wells
The dynamic perifusion results are shown in Figures 6A–D. There was a modest increase in the GSI for islets cultured on dishes containing SPO after 1 day in culture. Thus, the GSI was 1.29 ± 0.29 in the control group compared to the experimental SPO group, which was 1.67 ± 0.35 (mean ± standard deviation, n=5, p>0.05 (see Figures 6A&B). However, there was a significant increase in the GSI after 4 days of culture as the GSI for the control group was 0.89 ± 0.24 compared to the experimental group, which was 1.58 ± 0.49 (mean ± standard deviation, n=5, p<0.05, Figures 6C&D). Live/dead staining, as shown in Figure 6E, indicates that islets cultured on standard control dishes began to develop necrotic cores starting by day 4, whereas islets cultured on SPO plates were still mostly viable over this time period in culture. The qPCR analysis of islets cultured on oxygen generating dishes following 24 hours of culture indicated that insulin was over expressed by 5.93±1.36-fold compared to the control. However, we found no difference in CASP3 expression between the control group and islets cultured on SPO.
Figure 6.
Effect of Sodium Percarbonate film during islet culture on islet viability and functionality. (A) Perifusion results for islets cultured on oxygen generating culture plates with or without SPO following 1 day in culture. Each line represents the mean insulin secretion rate/min normalized to the average basal secretion rate (Average ± SEM, n=5) (B) 1 day GSI results for islets on oxygen generating culture wells. (C) Perifusion results for islets cultured on oxygen generating culture plates with or without SPO following 4 days in culture. Each line represents the mean insulin secretion rate/min normalized to the average basal secretion rate (Average ± SEM, n=4) (D) 4 day GSI results for islets on oxygen generating culture wells. (E) Representative live/dead images of islets cultured on oxygen generating culture dishes without (control) or with SPO at 1 and 4 days of culture. Green cells represent viable cells which stained positive for CFDA-SE, and red cells represent dead cells which stained positive for PI. (F) qPCR results for islets cultured on oxygen generating culture plates following one day of culture. Bars represent average ± SEM, n=3.
We can conclude from this study that using culture systems with approximately 90 mg of SPO improved the viability and functionality of islets in culture. These findings are consistent with previously published results by Pedraza et al 30, where the authors used a similar approach to prevent hypoxic injury to islets in culture. In that study, CPO was utilized as the oxygen generating material and the authors were able to show an improvement in islet viability for up to 48 hours in culture. Additionally, we found that higher concentrations of SPO were detrimental to the viability and functionality of islets (data not shown). In this case, the most likely cause of the islet damage is excessive reactive oxygen species (ROS) produced from hydrogen peroxide, to which islets are particularly sensitive 43. Because of the risk of superoxide damage to islets, it is important that this system be utilized only in situations where hypoxia is a concern and may not be appropriate for use in well-oxygenated systems.
d) Islet Encapsulation with CPO
The results from study (iii) are shown in Figures 7 A–E. We found that following one week in culture, islets which were encapsulated with CPO were generally more viable compared to encapsulated islets without CPO. After 7 days of culture, the GSI results for islets co-encapsulated with CPO were 1.58±0.15 while control islets were 1.06±0.02, p<0.05 (Figures 7 A&B). MTS assay results, shown in Figure 7C, indicated that islets co-encapsulated with CPO were significantly more metabolically active when compared to the control: 1±0.50, versus 2.76±0.17 for the islets co-encapsulated with CPO (mean ± SEM, n=4, p<0.05). qPCR results indicated that there was an increase in insulin expression of 3.37±0.75 fold over control, but the expression of CASP3 was found to be not significantly different from the control (Figure 7D, mean ± SEM, n=4). Islet viability assessed by Live/Dead stains indicated that there was a significant increase over the control in cell viability and functionality in islets co-encapsulated with CPO following 7 days in culture with 5% O2, as shown in in the lower panel of Figure 7E.
Figure 7.
Effect of incorporating CPO into alginate microcapsules on islet viability and functionality. (A) Perifusion results for islets co-encapsulated with CPO or without (control) following one week in moderately hypoxic conditions. Each line represents the mean insulin secretion rate/min normalized to the average basal secretion rate (Average ± SEM, n=6). (B) GSI results for islets co-encapsulated with CPO after 7 days of culture. Each bar represents the average GSI per group, which is defined as the mean insulin secretion rate during the high glucose period divided by the basal insulin secretion rate. Error bars represent the SEM and * indicates significance (p<0.05). (C) MTS Results for islets co-encapsulated with and without CPO. (average ± SEM, n=4, asterisk indicates significance, p<0.05). (D) qPCR results for islets encapsulated with and without CPO following 7 days in hypoxic culture. Error bars represent average ± SEM, n=3, asterisk indicates significance, p<0.05. (E) Representative Live/Dead images of encapsulated islets following one week in moderately hypoxic conditions. Green cells represent viable cells which stained positive for CFDA-SE, and red cells represent dead cells which stained positive for PI.
While SPO was investigated as an oxygen generating material during islet isolation and culture the reaction rate of SPO was found to be too fast to be successfully utilized during the islet encapsulation process. Consequently, the slower-reacting CPO was utilized as an oxygen source during islet encapsulation. While incorporating CPO into alginate was initially challenging due to the presence of excess calcium that cross-linked the alginate prior to encapsulation, washing the CPO particles overnight in PBS was helpful in preventing early crosslinking, presumably because it reduced the burst release of CPO upon contact with alginate and also coated the CPO particles with calcium phosphate; however, further studies may be needed in order to clarify how the CPO particles interact with PBS in this process. The wash step prior to CPO incorporation in microbeads also prevented the burst release of oxygen from damaging islets, which has been previously shown to be a problem when using CPO as an oxygen source for tissue engineering applications. 44
For this set of experiments, we incorporated CPO particles at a concentration of 10 mg per mL of alginate. We also observed, as with SPO, that higher concentrations resulted in oxidative stress and cell death (data not shown). Moving forward with further studies using CPO in vivo it will be important to provide supplemental antioxidants along with the co-encapsulated CPO in order to prevent damage to the cells from free radicals. However, at the concentration we did not observe any deleterious effects from oxidative stress and our qPCR data indicated that there was no upregulation of CASP3 which has been shown to be upregulated in the presence of reactive oxidative species. 45, 46
6) Conclusion
The risk of hypoxia, while a major concern in islet transplantation, can be overcome through a wide variety of strategies. In this work, we have demonstrated that oxygen generating materials such as SPO or CPO provide a potentially viable mechanism to supplement oxygen to both naked and encapsulated islets used for transplantation in diabetic patients. While these initial results have shown that SPO and CPO can be used to improve islet viability and functionality and, additional work is required to control the oxygen generation rates from these materials in order to provide adequate supply of oxygen over an extended period of time without consequent damage to the tissue by oxidative stress.
Acknowledgments
The authors would like to acknowledge financial support from the National Institutes of Health (RO1 DK080897) and the Vila Rosenfeld Estate, Greenville NC for the work in Dr. Opara’s laboratory at the Wake Forest Institute for Regenerative Medicine. Also, research reported in this publication was supported by the National Institute of Biomedical Imaging and Bioengineering (NIBIB) of the National Institutes of Health under award # T32EB014836. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors are grateful to Dr. Benjamin Harrison for technical guidance in the use of the oxygen generating particles. Also, thanks to Dr James Jordan, Dwight Deal and Magan Lane at the Wake Forest Baptist Health Cardiothoracic Department for provision of porcine organs. Special thanks to Alyssa McQuilling for help with manuscript preparation and editing. Finally, we thank Casey Frey, Anne Farrow, and Jasmine Gonzalez for their assistance with the RIAs.
Footnotes
7) Conflicts of Interest
There are no conflicts to declare
9) References
- 1.PGf JDRF. Journal. 2011 [Google Scholar]
- 2.Kort Hd, Koning EJd, Rabelink TJ, Bruijn JA, Bajema Ingeborg M. BMJ. 2011;342:217. doi: 10.1136/bmj.d217. [DOI] [PubMed] [Google Scholar]
- 3.Opara EC, Mirmalek-Sani SH, Khanna O, Moya ML, Brey EM. J Investig Med. 2010;58:831–837. doi: 10.231/JIM.0b013e3181ed3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shamoon H, Duffy H, Fleischer N, Engel S, Saenger P, Strelzyn M, Litwak M, Wylierosett J, Farkash A, Geiger D, Engel H, Fleischman J, Pompi D, Ginsberg N, Glover M, Brisman M, Walker E, Thomashunis A, Gonzalez J, Genuth S, Brown E, Dahms W, Pugsley P, Mayer L, Kerr D, Landau B, Singerman L, Rice T, Novak M, Smithbrewer S, McConnell J, Drotar D, Woods D, Katirgi B, Litvene M, Brown C, Lusk M, Campbell R, Lackaye M, Richardson M, Levy B, Chang S, Heinheinemann M, Barron S, Astor L, Lebeck D, Brillon D, Diamond B, Vasilasdwoskin A, Laurenzi B, Foldi N, Rubin M, Flynn T, Reppucci V, Heise C, Sanchez A, Whitehouse F, Kruger D, Kahkonen D, Fachnie J, Fisk J, Carey J, Cox M, Ahmad B, Angus E, Campbell H, Fields D, Croswell M, Basha K, Chung P, Schoenherr A, Mobley M, Marchiori K, Francis J, Kelly J, Etzwiler D, Callahan P, Hollander P, Castle G, Bergenstal R, Spencer M, Nelson J, Bezecny L, Roethke C, Orban M, Ulrich C, Gill L, Morgan K, Laechelt J, Taylor F, Freking D, Towey A, Lieppman M, Rakes S, Mangum J, Cooper N, Upham P, Jacobson A, Crowell S, Wolfsdorf J, Beaser R, Ganda O, Rosenzweig J, Stewart C, Halford B, Friedlander E, Tarsy D, Arrigg P, Sharuk G, Shah S, Wu G, Cavallerano J, Poole R, Silver P, Cavicchi R, Fleming D, Marcus J, Griffiths C, Cappella N, Nathan D, Larkin M, Godine J, Lynch J, Norman D, McKitrick C, Haggen C, Delahanty L, Anderson E, Lou P, Taylor C, Cros D, Folino K, Brink S, Abbott K, Sicotte K, Service FJ, Schmidt A, Rizza R, Zimmerman B, Schwenk W, Mortenson J, Ziegler G, Lucas A, Hanson N, Sellnow S, Pach J, Stein D, Eickhoff B, Woodwick R, Tackmann R, Trautmann J, Rostvold J, Link T, Dyck P, Daube J, Colligan R, Windebank A, King J, Colwell J, Wood D, Mayfield R, Picket J, Chitwood M, Billings D, Dabney Y, Buse J, King L, Vale S, Thompson T, Bohm B, Lyons T, Hermayer K, Rice A, Molitch M, Schaefer B, Johnson C, Lyons J, Metzger B, Cohen B, Nishida T, Parque K, Yusim V, Moore M, Jampol L, Dineen K, Stahl J, Richine L, Weinberg D, Loose I, Kushner M, Morrison A, Jalbert A, Tildesley H, Leung S, Begg I, Johnson D, Lalani S, Kennedy T, Meadows G, Kolterman O, Lorenzi G, Jones K, Goldbaum M, Swenson M, Lyon R, Giotta M, Kadlec K, Reed R, Kirsch L, Goodman J, Cahill S, Clark T, Abram R, Sayner L, Ochabski R, Gloria R, Birchler G, Grant J, Grasse B, Christle L, Abreu B, Grant I, Heaton R, Zeitler R, Sivitz W, Bayless M, Schrott H, Olson N, Tindal B, Snetselaar L, Mueller D, Dudler A, Swartzendruber J, Hoffman R, Macindoe J, Kramer J, Weingeist T, Kimura A, Stone E, Grout T, Fountain C, Karakas S, Vogel C, Montague P, Keyser D, Mennen S, Doggett C, Rose G, Devet K, Muhle P, Kowarski A, Ostrowski D, Levin P, Chalew S, Hylton J, Younghyman D, Barlow M, Mayer R, Elman M, Lakhanpal V, Weiner B, Millar M, Blum S, Buie W, Mace B, Greene D, Martin C, Floyd J, Dunn F, Henry D, Bennett S, Lasichak A, Vine A, Albers J, Sandford T, Loftin J, Stevens M, Elner S, Martonyi C, McIver F, Stanley S, Willis J, Ryan K, Spiegelberg T, Nalepa S, Glasgow B, Chan E, Dotimas P, Bantle J, Mech M, Balles M, Kennedy W, Khan M, Knobloch W, Kwong C, McKenzie L, Olson J, Ramsay R, Robiner W, Warhol R, Genia A, McDonough G, McMichael B, Philiph D, Ponwith L, Sahinen R, Stinson E, Verness J, Fimreite L, Stein J, Goldstein D, Hall M, Burns T, Klachko D, Giangiacomo J, Rawlings S, Aston L, England J, Wiedmeyer H, Daugherty M, Lightfoot M, Wilson R, Griffing G, Gardner D, Conway R, Blinder K, Brownleeduffeck M, Palmer N, Gash L, Schade D, Johannes C, Reidy R, Bicknell J, Vogel A, Drumm D, Boyle P, Burge M, Jones N, Canady J, Nickell D, Baker L, Ilvescorressel P, Schwartz S, Braunstein S, McBride J, Brucker A, Rendle L, Brown M, Sladky J, Maschakcarey B, Lawley D, Nyberg W, Weeney L, Sandburg E, Byrd S, Aguado E, Mulholland N, Cahn D, Suscavage M, Egler J, Vaughnnorton M, Collins C, Mameniskis H, Drash A, Wesche J, Bratkowski M, Becker D, Arslanian S, Doft B, Lobes L, Rinkoff J, Warnicki J, Curtin D, Steinberg D, Vagstad G, Ryan C, Harris F, Steranchak L, Arch J, Kelly K, Ostroska P, Guiliani M, Good M, Williams T, Olsen K, Campbell A, Shipe C, Conwit R, Finegold D, Zaucha M, Malone J, Grove N, McMillan D, Babione L, Declue T, Pavan P, Korthals J, Solc H, Mangione A, Kitabchi A, Taylor L, Jones L, Pitts K, Bertorini T, Bittle J, Burghen G, Fisher J, Hughes T, Linn J, Meyer D, Murphy W, Justice M, Sherman A, Wright L, Murphy L, Raskin P, Strowig S, Basco M, Cercone S, Ramirez L, Anand R, Wilson C, Greenlee R, Anderson W, Mendelson E, Vanacek P, Howard J, Ousley C, Yates B, Conger D, Maguire B, Biggs M, Newton B, Sherill K, Zinman B, Barnie A, Ehrlich R, Daneman D, Perlman K, Leiter L, Gottesman I, Devenyi R, Mortimer C, Moffat K, Gordon A, Ferguson R, Camelon K, Simkins S, Littlefield C, Rodin G, Hartley K, Kwan J, Gnanapandithen D, Rogers S, Haye L, Rose J, Mezei S, Bunphy B, Maclean S, Mackeen L, Mandelcorn M, Nellis P, Ruttan L, Wilsonsmith D, Palmer J, Ginsberg J, Hirsch I, Kinyoun J, Doerr H, Mauseth R, Sweeney K, Vanottingham L, Thomson L, Greenbaum C, Sameshima L, Farkashirsch R, Rosenbaum G, Rubner N, Brown T, Kraft G, Broeckel J, Karlsen M, Khakpour D, Ramirez M, Smit B, Mix L, Dupre J, Colby P, Rodger W, Hramiak I, Jenner M, Canny C, Brown W, Smith T, Harth J, Bondy S, Beath S, McCabe S, Gouchie C, Blanchard K, McCallum J, Jung S, Suetang A, Lorenz R, Lipps J, McRae J, May J, May M, Campbell P, Feman S, Kilroy A, Pulliam C, Schlundt D, Jannasch K, Davis D, Cullen N, Adkins T, Snell M, Virts K, Quesenberry L, Santiago J, Levandoski L, White N, McGill J, Bubb J, Schmidt L, Strasberg Y, Casso M, Noetzel M, Olk R, Boniuk I, Grand M, Thomas M, Williams D, Nobel G, Kacizak R, Ort E, Dahl J, Breeding L, Hoffmeyer G, Bilyeu P, Blank J, Walters C, Bodnar J, Rodriguez P, Erickson M, Hedrick S, Tamborlane W, Ahern J, Sherwin R, Gatcomb P, Stoessel K, Held N, Ebersole J, Scanlon I, Louard R, Wildstein C, Bilodeau D, Fong K, Ottaviano D, Larson C, Crofford OB, Lachin J, Cleary P, Thompson D, Kenny D, Lan S, Lan G, Brenneman A, Owen W, Adams K, Arnold D, Campanell R, Loring N, Scheirer P, Lamas D, Dunegan C, Veeramachaneni H, Williams C, Abdulbaaqiy S, Kassoff A, Dorman J, Spielman R, Klein R, Siebert C, Silverman R, Pfeifer M, Schumer M, Moran M, Farquhar J, Rohlfing C, Davis M, Hubbard L, Magli Y, Thomas S, Onofrey J, Jensen K, Brothers R, Ansay S, Armstrong J, Badal D, Vanderhoofyoung M, Esser B, Geithman P, Hurlburt D, Reimers J, Kewley K, Miner K, Steffes M, Bucksa J, Catanzaro R, Lukes A, Bagovich G, Woodfill T, Crow R, Hughlett J, Swanson C, Buzzard I, Sielaff B, Pickering B, Schakel S, Herman W, Dasbach E, Songer T, Janes G, Deeb L, Ewart R, Orchard T, Clark C, Cutter G, Demets D, Ferris F, Furberg C, Horton E, Keen J, Lockwood D, Palmberg P, Rourke B, Tsiatis A, Levy R, Frank R, Grizzle J, Rubenstein A, Schneider J, Skyler J, Nathan DM, Crofford O, Rand L. New England Journal of Medicine. 1993;329:977–986. [Google Scholar]
- 5.Ekberg K, Brismar T, Johansson BL, Lindstrom P, Juntti-Berggren L, Norrby A, Berne C, Arnqvist HJ, Bolinder J, Wahren J. Diabetes Care. 2007;30:71–76. doi: 10.2337/dc06-1274. [DOI] [PubMed] [Google Scholar]
- 6.Hansen A, Johansson BL, Wahren J, von Bibra H. Diabetes. 2002;51:3077–3082. doi: 10.2337/diabetes.51.10.3077. [DOI] [PubMed] [Google Scholar]
- 7.Ido Y, Vindigni A, Chang K, Stramm L, Chance R, Heath WF, DiMarchi RD, Di Cera E, Williamson JR. Science. 1997;277:563–566. doi: 10.1126/science.277.5325.563. [DOI] [PubMed] [Google Scholar]
- 8.Lim F, Sun AM. Science. 1980;210:908–910. doi: 10.1126/science.6776628. [DOI] [PubMed] [Google Scholar]
- 9.Omer A, Duvivier-Kali VrF, Trivedi N, Wilmot K, Bonner-Weir S, Weir GC. Diabetes. 2003;52:69–75. doi: 10.2337/diabetes.52.1.69. [DOI] [PubMed] [Google Scholar]
- 10.Zimmermann H, Zimmermann D, Reuss R, Feilen PJ, Manz B, Katsen A, Weber M, Ihmig FR, Ehrhart F, Gessner P, Behringer M, Steinbach A, Wegner LH, Sukhorukov VL, Vásquez JA, Schneider S, Weber MM, Volke F, Wolf R, Zimmermann U. Journal of Materials Science: Materials in Medicine. 2005;16:491–501. doi: 10.1007/s10856-005-0523-2. [DOI] [PubMed] [Google Scholar]
- 11.Lifson N, KKG, MRR, LEJ Gastroenterology. 1980;79:466–473. [PubMed] [Google Scholar]
- 12.Jansson L, Hellerstrom C. Diabetologia. 1983;25:45–50. doi: 10.1007/BF00251896. [DOI] [PubMed] [Google Scholar]
- 13.McQuilling JP, Arenas-Herrera J, Childers C, Pareta RA, Khanna O, Jiang B, Brey EM, Farney AC, Opara EC. Transplant Proc. 2011;43:3262–3264. doi: 10.1016/j.transproceed.2011.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kin T, Korbutt GS, Rajotte RV. American Journal of Transplantation. 2003;3:281–285. doi: 10.1034/j.1600-6143.2003.00049.x. [DOI] [PubMed] [Google Scholar]
- 15.Pareta R, McQuilling JP, Sittadjody S, Jenkins R, Bowden S, Orlando G, Farney AC, Brey EM, Opara EC. Pancreas. 2014;43:605–613. doi: 10.1097/MPA.0000000000000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Menger MD, Jaeger S, Walter P, Feifel G, Hammersen F, Messmer K. Diabetes. 1989;38(Suppl 1):199–201. doi: 10.2337/diab.38.1.s199. [DOI] [PubMed] [Google Scholar]
- 17.Ness PM, Cushing MM. Arch Pathol Lab Med. 2007;131:734–741. doi: 10.5858/2007-131-734-OTPOAA. [DOI] [PubMed] [Google Scholar]
- 18.Tan Q, El-Badry AM, Contaldo C, Steiner R, Hillinger S, Welti M, Hilbe M, Spahn DR, Jaussi R, Higuera G, van Blitterswijk CA, Luo QQ, Weder W. Tissue Eng Part A. 2009;15:2471–2480. doi: 10.1089/ten.tea.2008.0461. [DOI] [PubMed] [Google Scholar]
- 19.Radisic M, Park H, Chen F, Salazar-Lazzaro JE, Wang YD, Dennis R, Langer R, Freed LE, Vunjak-Novakovic G. Tissue Eng. 2006;12:2077–2091. doi: 10.1089/ten.2006.12.2077. [DOI] [PubMed] [Google Scholar]
- 20.Chin K, Khattak SF, Bhatia SR, Roberts SC. Biotechnol Prog. 2008;24:358–366. doi: 10.1021/bp070160f. [DOI] [PubMed] [Google Scholar]
- 21.Ricordi C, Fraker C, Szust J, Al-Abdullah I, Poggioli R, Kirlew T, Khan A, Alejandro R. Transplantation. 2003;75:1524–1527. doi: 10.1097/01.TP.0000058813.95063.7A. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi T, Tanioka Y, Matsuda T, Toyama H, Kakinoki K, Li S, Hiraoka K, Fijino Y, Suzuki Y, Kuroda Y. Hepatogastroenterology. 2006;53:179–182. [PubMed] [Google Scholar]
- 23.Goh F, Gross JD, Simpson NE, Sambanis A. J Biotechnol. 2010;150:232–239. doi: 10.1016/j.jbiotec.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ludwig B, Rotem A, Schmid J, Weir GC, Colton CK, Brendel MD, Neufeld T, Block NL, Yavriyants K, Steffen A, Ludwig S, Chavakis T, Reichel A, Azarov D, Zimermann B, Maimon S, Balyura M, Rozenshtein T, Shabtay N, Vardi P, Bloch K, de Vos P, Schally AV, Bornstein SR, Barkai U. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:5022–5027. doi: 10.1073/pnas.1201868109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barkai U, Weir GC, Colton CK, Ludwig B, Bornstein SR, Brendel MD, Neufeld T, Bremer C, Leon A, Evron Y, Yavriyants K, Azarov D, Zimermann B, Maimon S, Shabtay N, Balyura M, Rozenshtein T, Vardi P, Bloch K, de Vos P, Rotem A. Cell transplantation. 2013;22:1463–1476. doi: 10.3727/096368912X657341. [DOI] [PubMed] [Google Scholar]
- 26.Papas KK, Hering BJ, Guenther L, Rappel MJ, Colton CK, Avgoustiniatos ES. Transplantation proceedings. 2005;37:3501–3504. doi: 10.1016/j.transproceed.2005.09.085. [DOI] [PubMed] [Google Scholar]
- 27.Ward CL, Corona BT, Yoo JJ, Harrison BS, Christ GJ. PLoS One. 2013;8:e72485. doi: 10.1371/journal.pone.0072485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harrison BS, Eberli D, Lee SJ, Atala A, Yoo JJ. Biomaterials. 2007;28:4628–4634. doi: 10.1016/j.biomaterials.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 29.Oh SH, Ward CL, Atala A, Yoo JJ, Harrison BS. Biomaterials. 2009;30:757–762. doi: 10.1016/j.biomaterials.2008.09.065. [DOI] [PubMed] [Google Scholar]
- 30.Pedraza E, Coronel MM, Fraker CA, Ricordi C, Stabler CL. Proc Natl Acad Sci U S A. 2012;109:4245–4250. doi: 10.1073/pnas.1113560109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waite A, Bonner J, Autenrieth R. Environmental Engineering Science. 1999;16:187–199. [Google Scholar]
- 32.Field J, Farney A, Sutherland DE. Transplantation. 1996;61:1554–1556. doi: 10.1097/00007890-199605270-00026. [DOI] [PubMed] [Google Scholar]
- 33.Lacy PE, Kostianovsky M. Diabetes. 1967;16:35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- 34.Atwater I, Guajardo M, Caviedes P, Jeffs S, Parrau D, Valencia M, Romero C, Arriagada C, Caamano E, Salas A, Olguin F, Atlagich M, Maas R, Mears D, Rojas E. Transplant Proc. 2010;42:381–386. doi: 10.1016/j.transproceed.2009.11.030. [DOI] [PubMed] [Google Scholar]
- 35.Tendulkar S, McQuilling JP, Childers C, Pareta R, Opara EC, Ramasubramanian MK. Transplant Proc. 2011;43:3184–3187. doi: 10.1016/j.transproceed.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mirmalek-Sani SH, Orlando G, McQuilling JP, Pareta R, Mack DL, Salvatori M, Farney AC, Stratta RJ, Atala A, Opara EC, Soker S. Biomaterials. 2013;34:5488–5495. doi: 10.1016/j.biomaterials.2013.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herbert V, Lau KS, Gottlieb CW, Bleicher SJ. J Clin Endocrinol Metab. 1965;25:1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- 38.Linn T, Schmitz J, Hauck-Schmalenberger I, Lai Y, Bretzel RG, Brandhorst H, Brandhorst D. Clin Exp Immunol. 2006;144:179–187. doi: 10.1111/j.1365-2249.2006.03066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hogan AR, Doni M, Molano RD, Ribeiro MM, Szeto A, Cobianchi L, Zahr-Akrawi E, Molina J, Fornoni A, Mendez AJ, Ricordi C, Pastori RL, Pileggi A. Cell Transplant. 2012;21:1349–1360. doi: 10.3727/096368911X623853. [DOI] [PubMed] [Google Scholar]
- 40.Opara EC. J R Soc Promot Health. 2002;122:28–34. doi: 10.1177/146642400212200112. [DOI] [PubMed] [Google Scholar]
- 41.Littman ED, Opara EC, Akwari OE. J Surg Res. 1995;59:694–698. doi: 10.1006/jsre.1995.1225. [DOI] [PubMed] [Google Scholar]
- 42.Stiegler P, Stadlbauer V, Hackl F, Schaffellner S, Iberer F, Greilberger J, Strunk D, Zelzer S, Lackner C, Tscheliessnigg K. Journal of Artificial Organs. 2010;13:38–47. doi: 10.1007/s10047-010-0488-x. [DOI] [PubMed] [Google Scholar]
- 43.Modak MA, Parab PB, Ghaskadbi SS. Pancreas. 2009;38:23–29. doi: 10.1097/MPA.0b013e318181da4e. [DOI] [PubMed] [Google Scholar]
- 44.Steg H, Buizer AT, Woudstra W, Veldhuizen AG, Bulstra SK, Grijpma DW, Kuijer R. J Mater Sci Mater Med. 2015;26:207. doi: 10.1007/s10856-015-5542-z. [DOI] [PubMed] [Google Scholar]
- 45.Wang M, Crager M, Pugazhenthi S. Exp Diabetes Res. 2012;2012:647914. doi: 10.1155/2012/647914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Movafagh S, Crook S, Vo K. J Cell Biochem. 2015;116:696–703. doi: 10.1002/jcb.25074. [DOI] [PubMed] [Google Scholar]









