Abstract
Background
Pathological cardiac hypertrophy induced by stresses such as aging and neurohumoral activation is an independent risk factor for heart failure and is considered a target for the treatment of heart failure. However, the mechanisms underlying pathological cardiac hypertrophy remain largely unknown. We aimed to investigate the roles of SIRT2 in aging-related and angiotensin II (Ang II)-induced pathological cardiac hypertrophy.
Methods
Male C57BL/6J wild-type (WT) and Sirt2 knockout (Sirt2-KO) mice were subjected to the investigation of aging-related cardiac hypertrophy. Cardiac hypertrophy was also induced by Ang II (1.3 mg/kg/day for four weeks) in male C57BL/6J Sirt2-KO mice, cardiac-specific SIRT2 transgenic (SIRT2-Tg) mice and their respective littermates (8~12-week-old). Metformin (200 mg/kg/day) was used to treat WT and Sirt2-KO mice that were infused with Ang II. Cardiac hypertrophy, fibrosis, and cardiac function were examined in these mice.
Results
SIRT2 protein expression levels were down-regulated in hypertrophic hearts from mice. Sirt2-KO markedly exaggerated cardiac hypertrophy and fibrosis as well as decreases in cardiac ejection fraction and fractional shortening in aged (24-month-old) mice and Ang II-infused mice. Conversely, cardiac-specific SIRT2 overexpression protected the hearts against Ang II-induced cardiac hypertrophy and fibrosis and rescued cardiac function. Mechanistically, SIRT2 maintained the activity of AMP-activated protein kinase (AMPK) in aged and Ang II-induced hypertrophic hearts in vivo as well as in cardiomyocytes in vitro. We identified the liver kinase B1 (LKB1), the major upstream kinase of AMPK, as the direct target of SIRT2. SIRT2 bound to LKB1 and deacetylated it at lysine 48, which promoted the phosphorylation of LKB1 and the subsequent activation of LKB1-AMPK signaling. Remarkably, the loss of SIRT2 blunted the response of AMPK to metformin treatment in mice infused with Ang II and repressed the metformin-mediated reduction of cardiac hypertrophy and protection of cardiac function.
Conclusions
SIRT2 promotes AMPK activation by deacetylating the kinase LKB1. Loss of SIRT2 reduces AMPK activation, promotes aging-related and Ang II-induced cardiac hypertrophy and blunts metformin-mediated cardioprotective effects. These findings indicate that SIRT2 will be a potential target for therapeutic interventions in aging and stress-induced cardiac hypertrophy.
Keywords: SIRT2, Aging, Angiotensin II, Cardiac hypertrophy, LKB1, AMPK, Deacetylation, Metformin
Introduction
Heart failure is a growing public health problem and a leading cause of morbidity and mortality in modern society. Pathological cardiac hypertrophy induced by aging and neurohumoral activation (e.g., angiotensin II [Ang II]) is an independent risk factor for heart failure.1, 2 Anti-aging strategies, such as caloric restriction, show cardiac benefits in rodents, monkeys, and humans.3–5 Clinically, neurohormone blocking drugs (including adrenergic receptor blockers, inhibitors of angiotensin converting enzyme, and blockers of the Ang II receptor AT1) are commonly used for treatment of the pathological hypertrophy and heart failure.6
Defects in myocardial metabolism appear to be a major contributor to aging-related and stress-induced cardiac hypertrophy and subsequent heart failure.7 Aging and aging-related cardiac hypertrophy are regulated by several core metabolic sensors, including AMP-activated protein kinase (AMPK), SIRT1, mammalian target of rapamycin (mTOR), and insulin-like growth factor 1 receptor (IGF1R).8 For instance, AMPK is a major regulatory kinase that directly controls numerous metabolic processes, including fatty acid oxidation and glycolysis. AMPK can also regulate other metabolic pathways, such as the SIRT1, mTOR, and peroxisome proliferator-activated receptor gamma (PPAR-γ) coactivator-1 alpha (PGC-1α) pathways.9, 10 AMPK contains one catalytic subunit (α) and two regulatory subunits (β and γ). Mutations in the AMPKγ subunit cause hypertrophic cardiomyopathy (HCM) in humans.9 AMPK deficiency contributes to cardiac hypertrophy induced by aging, neurohumoral activation, pressure overload and myocardial infarction.9, 11 Activation of AMPK by AICAR or metformin protects the heart from cardiac hypertrophy induced by aging and other stresses.12, 13
The Sirtuin family of proteins is a family of class III nicotinamide adenine dinucleotide (NAD+)-dependent deacetylase that deacetylates histone and non-histone proteins. Sirtuins regulate metabolism and aging-related diseases, including diabetes and cardiovascular diseases.14–16 Among the Sirtuins, the cytosol member SIRT2 is less characterized. SIRT2 is expressed in a wide range of tissues, particularly in metabolically relevant tissues, such as the heart, brain, and adipose tissue.17 SIRT2 plays essential roles in diverse aspects of metabolism.17 However, the pivotal target of SIRT2 in metabolic processes and the roles of SIRT2 in pathological cardiac hypertrophy remain unknown.
In the present study, we demonstrate that SIRT2 represses aging-related and stress-induced cardiac hypertrophy, at least in part, by maintaining signaling through the liver kinase B1 (LKB1)-AMPK pathway, the central pathway controlling various aspects of metabolism. We found that SIRT2 protein levels were reduced in the hearts of aged and Ang II-infused mice. Sirt2-KO promoted aging-related and Ang II-induced cardiac hypertrophy and fibrosis and caused cardiac dysfunction, whereas cardiac-specific SIRT2 overexpression inhibited Ang II-induced cardiac hypertrophy and rescued cardiac function. Moreover, we showed that SIRT2 bound to and deacetylated LKB1, which activated the LKB1-AMPK signaling pathway in the hearts of aged mice and mice infused with Ang II. Additionally, Sirt2-KO attenuated metformin-induced activation of AMPK signaling and, subsequently, the cardioprotective functions of metformin in Ang II-induced hypertrophic hearts.
Methods
Knockout and transgenic mice
All animal protocols were approved by the Animal Care and Use Committee at the Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences and Peking Union Medical College. Global germ-line Sirt2 knockout (Sirt2-KO) mice (C57BL/6J background; stock number: 012772) were purchased from Jackson Laboratories. Transgenic mice with cardiomyocyte-specific SIRT2 overexpression were generated as previously described.18 An expanded method for generation of transgenic mice is available in the online-only Data Supplement.
Cardiac hypertrophy model and metformin treatment
The young (4-month-old) and aged (24-month-old) WT and Sirt2-KO mice were subjected to pathological analysis of cardiac hypertrophy. Cardiac hypertrophy was also induced in 8~12 weeks old mice by chronic subcutaneous infusion of angiotensin II (Ang II, Sigma-Aldrich, #A9525) at a dose of 1.3 mg/kg/day using the ALZET® Osmotic Pumps (Model 2004) for four weeks. The control mice were infused with saline for four weeks. For metformin treatment, mice were randomized to be treated with either vehicle or metformin (Sigma-Aldrich, #PHR1084) at a dose of 200 mg/kg/day in the drinking water.
Histological analysis
For histological analysis, hearts were arrested with a 10% potassium chloride solution at end-diastole and then fixed in 4% paraformaldehyde. Fixed hearts were embedded in paraffin and cut transversely into 5 μm sections. Serial heart sections were stained with hematoxylin-eosin (H&E) or wheat germ agglutinin (WGA) (Invitrogen, #W11261) to measure myocyte cross-sectional areas. The degree of collagen deposition was detected by Picrosirius Red (PSR) Staining Kit (Abcam, #ab150681). Images were analyzed using a quantitative digital image analysis system (Image-Pro Plus 6.0).
Statistical analysis
All experiments were performed at least in triplicates unless otherwise stated. Homogeneity of the variance was assessed via F test (two groups) or Brown-Forsythe test (≥ three groups). Normality of the data was assessed via Shapiro-Wilk test. When reporting two groups with normal distribution, we used standard Student’s t test for equal variance or Welch t test for unequal variance. In the results with more than two groups, ANOVA or appropriate non-parametric tests were applied to analyze the difference. When one factor was involved, we used one-way ANOVA followed by Bonferroni post-hoc test when the assumptions (equal variance and normal distribution) are satisfied. Otherwise, we used the non-parametric test Kruskal-Wallis test followed by the Dunn’s post-hot test to correct for multiple comparisons. In results with two factors, two-way ANOVA followed by Bonferroni post-hoc test was applied for multiple comparisons when the assumptions (equal variances and normal distribution) are satisfied. Otherwise, we used the non-parametric test Scheirer-Ray-Hare test (an extension of the Kruskal-Wallis test) followed by the Dunn’s post-hot test to correct for multiple comparisons. The P values were adjusted for multiple comparisons where appropriate. P values of less than 0.05 were considered statistically significant. All statistical analyses were carried out using GraphPad Prism 7.0 or SPSS Version 21 software.
An expanded Methods section is available in the online-only Data Supplement.
Results
SIRT2 deficiency aggravates cardiac hypertrophy in aged mice
To investigate the role of SIRT2 in aging-related cardiac hypertrophy, the male Sirt2-KO mice (Supplementary Figure 1A–B) and their control wild-type (WT) littermates were maintained for two years. The body weights of WT and Sirt2-KO mice were comparable at 4 months and 24 months (Supplementary Figure 2A). Remarkably, aged Sirt2-KO mice showed more visible aging characteristics (such as hair browning) than their WT littermates (Figure 1A), indicating that Sirt2-KO might affect the health of aged mice.
Figure 1. Sirt2 deficiency aggravates cardiac hypertrophy in aged mice.
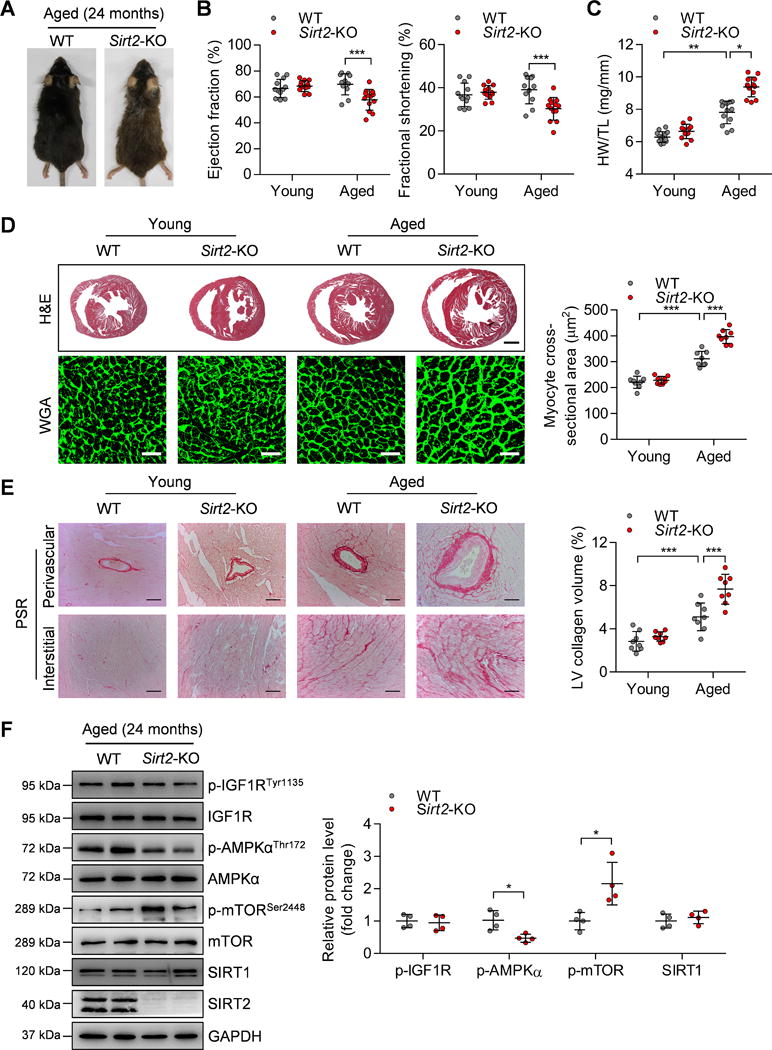
(A) Representative gross morphology of aged (24-month-old) WT and Sirt2-KO mice.
(B) Ejection fraction and fractional shortening of WT and Sirt2-KO mice at 4 months (young) or 24 months (aged). n=11~13. ***P<0.001.
(C) Heart weight-to-tibia length (HW/TL) ratios of WT and Sirt2-KO mice at 4 months (young) or 24 months (aged). n=12~13. *P<0.05, **P<0.01.
(D) Left: Hematoxylin-eosin (H&E, scale bar=1 mm) staining and wheat germ agglutinin (WGA, scale bar=30 μm) staining were performed to determine the hypertrophic growth of the hearts in young and aged WT and Sirt2-KO mice. Right: Quantification of cardiomyocyte size in young and aged WT and Sirt2-KO mice (n=8~10; ***P<0.001).
(E) Left: Picrosirius red (PSR, scale bar=50 μm) staining was performed to determine cardiac fibrosis in young and aged WT and Sirt2-KO mice. Right: Quantification of cardiac fibrosis in young and aged WT and Sirt2-KO mice (n=8~10; ***P<0.001).
(F) Left: Western blotting showing the expression levels of phosphorylated IGF1R, AMPK and mTOR and the total protein expression levels of IGF1R, AMPK, mTOR, and SIRT1. Right: Quantification of p-IGF1R, p-AMPK, p-mTOR, and SIRT1 levels (n=4; *P<0.05). IGF1R: Insulin-like growth factor 1 receptor; AMPK: AMP-activated protein kinase; mTOR: Mammalian target of rapamycin.
Young (4-month-old) and aged (24-month-old) mice were subjected to functional cardiac phenotyping. Significantly, Sirt2-KO caused echocardiography-detectable dysfunction in aged mice, as evidenced by decreases in cardiac ejection fraction and fractional shortening (Figure 1B). But Sirt2 deficiency did not affect heart rate, systolic blood pressure or diastolic blood pressure in aged mice (Supplementary Figure 2B–C). The aged WT mice had increased heart weight-to-tibia length (HW/TL) ratio compared with those of young mice, and HW/TL ratio was further enhanced in aged Sirt2-KO mice (Figure 1C). The heart weight-to-body weight (HW/BW) ratio was also increased in aged Sirt2-KO mice compared with that in aged WT mice (Supplementary Figure 2D). Next, hematoxylin-eosin (H&E), wheat germ agglutinin (WGA) and picrosirius red (PSR) staining were performed to analyze cardiac hypertrophy and fibrosis. The results showed that hypertrophic remodeling and fibrosis of the myocardial tissues were significant in aged WT mice compared with those in young WT mice. Notably, these abnormalities were enhanced in the hearts of aged Sirt2-KO mice compared with aged WT mice (Figure 1D–E). Hypertrophic remodeling and heart failure commonly lead to pulmonary remodeling. Indeed, Sirt2-KO increased body weight-normalized and tibia-length-normalized lung weights in aged mice (Supplementary Figure 2E), which is a sign of pulmonary congestion due to abnormal cardiac function.
Aging-dependent cardiac hypertrophy is regulated by several central metabolic regulators, including AMPK, SIRT1, mTOR, and IGF-1R.12, 19–21 Therefore, these core metabolic regulators were analyzed in the hearts of aged WT and Sirt2-KO mice. IGF1R phosphorylation at Tyr1135 and SIRT1 expression were comparable in the hearts of aged WT and Sirt2 KO mice. AMPK phosphorylation at Thr172 was significantly reduced, whereas mTOR phosphorylation at Ser2448, a negative downstream of AMPK,10 was increased in aged Sirt2-KO hearts compared with aged WT hearts (Figure 1F). These findings implicate that AMPK might be involved in the effect of SIRT2 on aging-related cardiac hypertrophy.
SIRT2 expression and activity are reduced during cardiac hypertrophy
To explore the potential functions of SIRT2 during cardiac hypertrophy induced by aging as described above and by other stresses, the expression and activity levels of SIRT2 were examined in aged and hypertrophic mouse hearts. SIRT2 protein levels were significantly down-regulated in aged (24-month-old) hearts compared with young (4-month-old) hearts (Figure 2A). The concentration of Ang II is upregulated in aged hearts, and activation of the renin-angiotensin system is one of the core mechanisms underlying cardiac aging in rodents.22 Chronic infusion of Ang II recurs the development of cardiac hypertrophy observed in aged mice.18 Therefore, cardiac hypertrophy in young (8~12-week-old) mice was induced by subcutaneously infusing Ang II (1.3 mg/kg/day) into the mice for four weeks. Ang II infusion significantly increased circulating Ang II serum levels (Supplementary Figure 3A). SIRT2 protein levels were decreased in Ang II-induced hypertrophic hearts (Figure 2A). Tubulin is a substrate of the deacetylase SIRT2.23 The level of acetylated Tubulin at lysine 40 was upregulated in aged and Ang II-induced hypertrophic hearts (Figure 2B), indicating that SIRT2 activity was decreased in hypertrophic hearts. Consistently, the enzymatic activity assay also demonstrated that SIRT2 activity was decreased in Ang II-induced hypertrophic hearts (Figure 2C).
Figure 2. SIRT2 expression and activity are down-regulated in hypertrophic hearts.
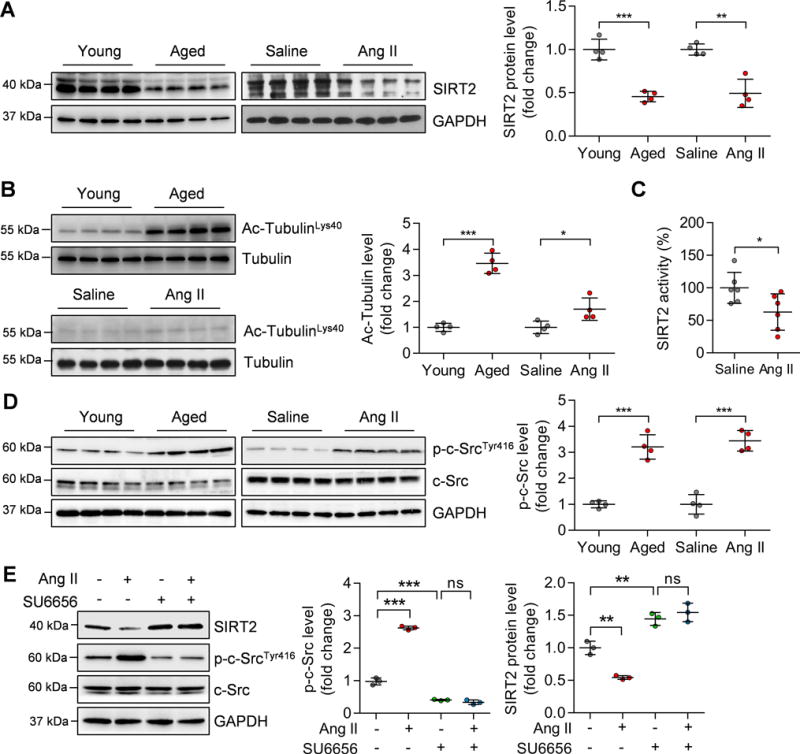
(A) Left: Representative western blotting showing changes in SIRT2 protein in aged (24-month-old) hearts and Ang II-induced hypertrophic hearts. Right: Quantification of SIRT2 protein levels (n=4; **P<0.01, ***P<0.001).
(B) Left: Representative western blotting showing changes in acetylated Tubulin levels in aged (24-month-old) hearts and Ang II-induced hypertrophic hearts. Right: Quantification of acetylated Tubulin levels (n=4; *P<0.05, ***P<0.001).
(C) SIRT2 activity in cardiac tissues of mice infused with saline or Ang II. The SIRT2 protein was purified by immunoprecipitation using an anti-SIRT2 antibody. Then an enzymatic assay was performed to determine SIRT2 protein activity (n=6; *P<0.05).
(D) Left: Representative western blotting results showing the phosphorylated level of c-Src in aged and Ang II-infused mouse hearts. Right: Quantification of phosphorylated level of c-Src (n=4; ***P<0.001).
(E) Left: Representative western blotting results showing the phosphorylated level of c-Src and level of SIRT2. Right: Quantification of phosphorylated c-Src and SIRT2 levels (**P<0.01, ***P<0.001, ns: not significant). Neonatal rat cardiomyocytes (NRCMs) were treated with Ang II (1 μM) for 24 hours with/without the presence of c-Src inhibitor SU6656 (5 μM).
However, Sirt2 mRNA levels were not changed in aged or Ang II-induced cardiac hypertrophy (Supplementary Figure 3B), implicating that the protein stability might be decreased in hypertrophic hearts. The kinase c-Src can promote phosphorylation of SIRT2 to reduce the protein stability and inactivate SIRT2.24 Importantly, c-Src can be activated by various hypertrophic stress (e.g. Ang II) and accelerates the development of cardiac hypertrophy.25 Indeed, c-Src was activated in aging- and Ang II-induced hypertrophic hearts as well as Ang II-treated neonatal rat cardiomyocytes (NRCMs) (Figure 2D–E). Strictly, inhibition of c-Src with its inhibitor SU6656 (5 μM) blocked Ang II-induced decrease in SIRT2 protein level in NRCMs (Figure 2E). Therefore, the decrease in SIRT2 protein level is at least in part mediated by c-Src activation during cardiac hypertrophy.
Sirt2 knockout aggravates Ang II-induced cardiac hypertrophy
To test whether SIRT2 deficiency contributes to maladaptive hypertrophy and heart remodeling in vivo, Sirt2-KO mice and their WT littermates (8~12-week-old) were exposed to sustained Ang II treatment for four weeks. When subjected to Ang II treatment, WT mice developed cardiac hypertrophy, as evidenced by decreased ejection fraction and fractional shortening and increased HW/BW and HW/TL ratios (Figure 3A–B). The H&E staining and WGA staining results also demonstrated that Ang II treatment increased cardiomyocyte size in WT mice (Figure 3C). Remarkably, these hypertrophic features were enhanced in the hearts of Sirt2-KO mice treated with Ang II (Figure 3A–C). Sirt2 deficiency also aggravated perivascular and interstitial fibrosis in the hypertrophic hearts (Figure 3D). Consistently, the quantitative real-time PCR (qRT-PCR) results showed that these hypertrophic pathological phenotypes were accompanied by the upregulation of hypertrophic genes, including atrial natriuretic polypeptide (Anp), brain natriuretic peptide (Bnp), myosin heavy chain beta (β-Mhc), α-sarcomeric actin (Acta1), interleukin 6 (IL-6), and regulator of calcineurin 1.4 (Rcan1.4), and fibrotic genes including connective tissue growth factor (Ctgf), collagen1a1 (Col1a1), and collagen3a1 (Col3a1) (Figure 3E). The effects of Sirt2-KO on heart rate and blood pressure were examined. Neither Ang II treatment nor Sirt2-KO affected the heart rate of the mice. Ang II treatment increased the systolic and diastolic blood pressures of the mice, while they were not affected by Sirt2-deficiency (Supplementary Figure 4A–B).
Figure 3. Sirt2-KO aggravates Ang II-induced cardiac hypertrophy.
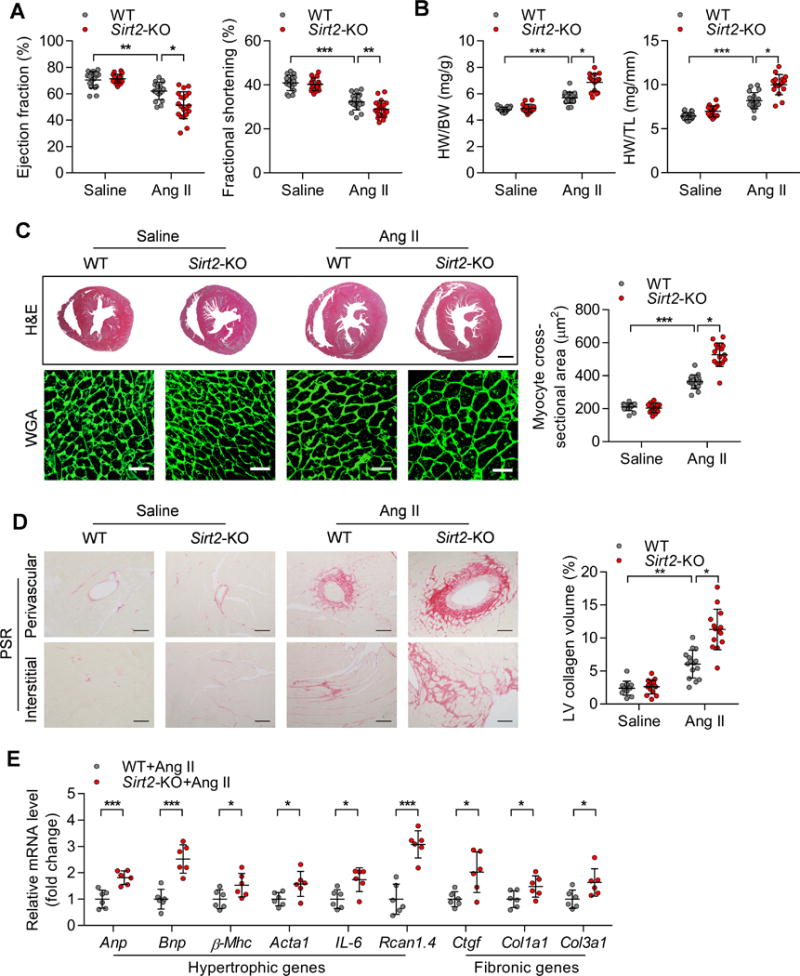
(A) Ejection fraction and fractional shortening of WT and Sirt2-KO mice treated with saline or Ang II (1.3 mg/kg/day) for four weeks (n=17~20; *P<0.05, **P<0.01, ***P<0.001).
(B) Heart weight-to-body weight (HW/BW) ratios and heart weight-to-tibia length (HW/TL) ratios of WT and Sirt2-KO mice treated with saline or Ang II (n=16~20; *P<0.05, ***P<0.001).
(C) Left: Hematoxylin-eosin (H&E, scale bar=1 mm) staining and wheat germ agglutinin (WGA, scale bar=30 μm) staining were performed to determine the hypertrophic growth of the hearts in WT and Sirt2-KO mice treated with saline or Ang II. Right: Quantification of cardiomyocyte size in WT and Sirt2-KO mice treated with saline or Ang II (n=13~17; *P<0.05, ***P<0.001).
(D) Left: Picrosirius red (PSR, scale bar=50 μm) staining was performed to determine cardiac fibrosis of the hearts from WT and Sirt2-KO mice treated with saline or Ang II. Right: Quantification of cardiac fibrosis in WT and Sirt2-KO mice treated with saline or Ang II (n=13~17; *P<0.05, **P<0.01).
(E) Quantitative real-time PCR (qRT-PCR) was performed to analyze the mRNA levels of hypertrophic (Anp, Bnp, β-Mhc, Acta1, IL-6 and Rcan1.4) and fibrosis (Ctgf, Col1a1 and Col3a1) genes (n=6; *P<0.05, ***P<0.001). Anp: Atrial natriuretic peptide; Bnp: brain natriuretic peptide; β-Mhc: Myosin heavy chain beta; Acta1: α-sarcomeric actin; IL-6: interleukin 6; Rcan1.4: regulator of calcineurin 1.4. Ctgf: Connective tissue growth factor; Col1a1: Alpha-1 type I collagen; Collagen 3a1.
SIRT2 overexpression represses Ang II-induced cardiac hypertrophy
We next investigated whether SIRT2 directly regulates cardiomyocyte hypertrophy using an in vitro model of cardiomyocyte hypertrophy induced by Ang II (1 μM Ang II treatment for 48 hours) in NRCMs. NRCMs were treated with AGK2 (10 μM), which specifically inhibited the activity of SIRT2 (Supplementary Figure 5A–B).26 SIRT2 inhibition with AGK2 promoted Ang II-induced increase in cardiomyocyte size and facilitated the expression of hypertrophic marker genes (Anp, Bnp, and β-Mhc) (Figure 4A–B). Next, SIRT2 was overexpressed in NRCMs with adenovirus to investigate whether SIRT2 overexpression could repress Ang II-induced hypertrophy in NRCMs (Supplementary Figure 5C). NRCMs with SIRT2 overexpression had a dampened hypertrophic response to Ang II treatment based on the analysis of cardiomyocyte size and the expression levels of Anp, Bnp, and β-Mhc (Figure 4C–D).
Figure 4. SIRT2 regulates Ang II-induced hypertrophy in neonatal rat cardiomyocytes (NRCMs).
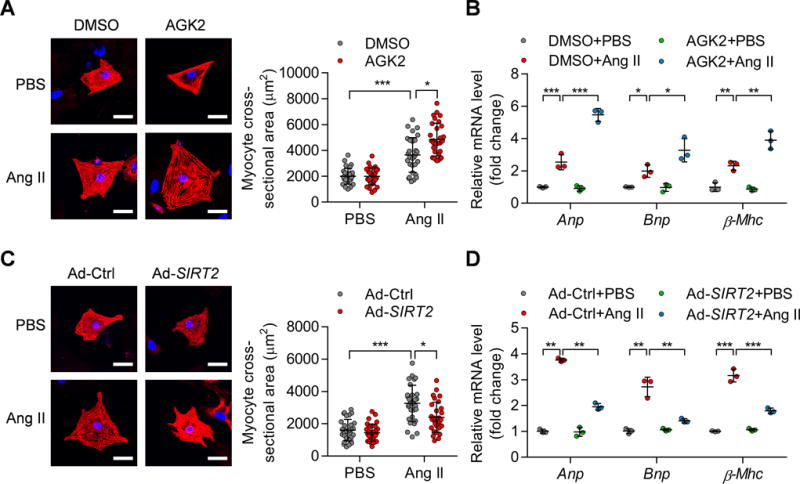
(A) NRCMs were treated with phosphate-buffered saline (PBS) or Ang II (1 μM) for 48 hours in the presence of AGK2 (10 μM) or DMSO. α-Actinin staining was performed to determine cell size. Representative images (Left) and quantification of cell size of total 30 NRCMs (Right) in each group are shown (scale bar=30 μm; *P<0.05, ***P<0.001).
(B) NRCMs were treated as shown in (A) and qRT-PCR was performed to analyze the mRNA levels of hypertrophic genes (Anp, Bnp, and β-Mhc). *P<0.05, **P<0.01, ***P<0.001.
(C) NRCMs were infected with the indicated adenovirus for 24 hours and then treated with PBS or Ang II (1 μM) for 48 hours. α-Actinin staining was performed to determine cell size. Representative images (Left) and quantification of cell size of total 30 NRCMs (Right) in each group are shown (scale bar=30 μm; *P<0.05, ***P<0.001). Ad-Ctrl: Control adenovirus; Ad-SIRT2: Adenovirus overexpressing human SIRT2.
(D) NRCMs were treated as shown in (C) and qRT-PCR was performed to analyze the expression of hypertrophic genes (Anp, Bnp, and β-Mhc). **P<0.01, ***P<0.001.
We further evaluated whether SIRT2 overexpression in cardiomyocytes could rescue cardiac function in vivo. To this end, we generated two lines of cardiac-specific SIRT2 transgenic (SIRT2-Tg) mice that express human SIRT2 under the control of the mouse α-myosin heavy chain promoter (Supplementary Figure 6A–D). These two lines of SIRT2-Tg mice had comparable SIRT2 protein levels (Supplementary Figure 6B) and developed normally without structural and functional defects. There were no significant differences between these two lines of transgenic mice. Therefore, we used one SIRT2-Tg mouse line (SIRT2-Tg1) for further studies. The male C57BL/6J SIRT2-Tg mice and their non-transgenic (N-Tg) littermates (8~12-week-old) were infused with Ang II for four weeks to induce cardiac hypertrophy. Cardiac-specific SIRT2 overexpression rescued the Ang II-induced decreases in ejection fraction and fractional shortening (Figure 5A) and inhibited the Ang II-induced increase in heart weights (Figure 5B). Furthermore, Ang II-mediated increases in cardiomyocyte size and cardiac fibrosis were significantly reduced in SIRT2-Tg mice (Figure 5C–D). In addition, the expression of hypertrophic and fibrotic genes was also reduced in the hypertrophic hearts of SIRT2-Tg mice (Figure 5E). Similar to the above results in aged mice and Ang II-infused mice with Sirt2 deficiency, SIRT2 overexpression did not change heart rate, systolic blood pressure or diastolic blood pressure (Supplementary Figure 7A–B).
Figure 5. Cardiac-specific SIRT2 overexpression represses Ang II-induced cardiac hypertrophy.
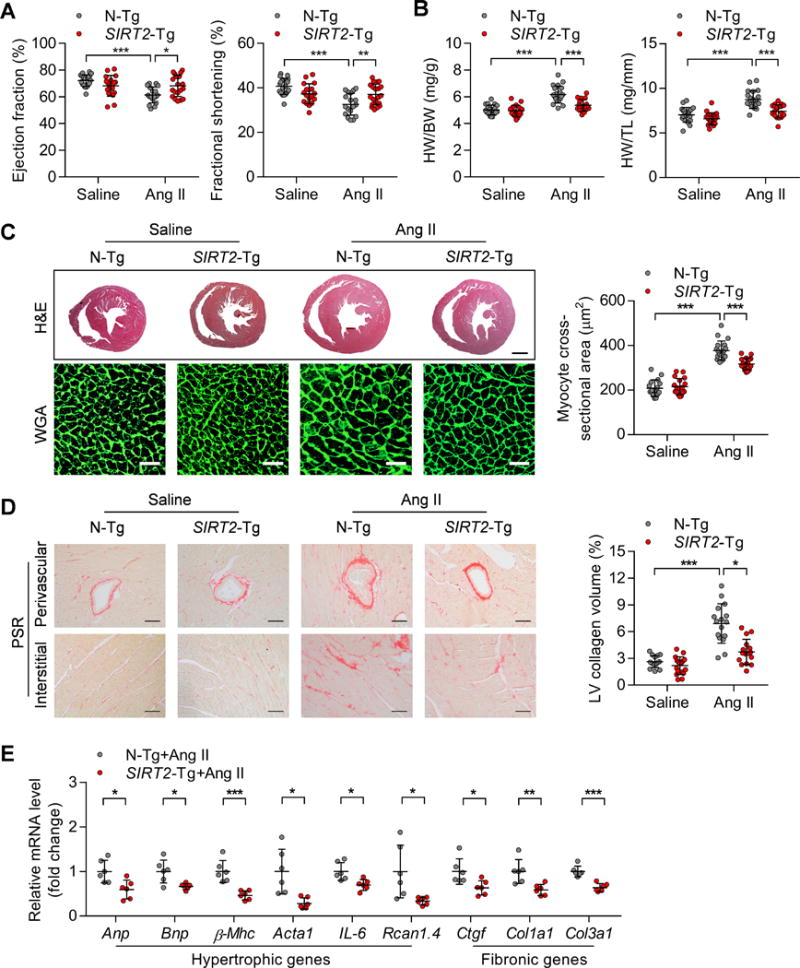
(A) Ejection fraction and fractional shortening in non-transgenic (N-Tg) and cardiac-specific SIRT2 transgenic (SIRT2-Tg) mice treated with saline or Ang II (1.3 mg/kg/day) for four weeks (n=18~20; *P<0.05, **P<0.01, *** P<0.001).
(B) Ratios of heart weight-to-body weight (HW/BW) or heart weight-to-tibia length (HW/TL) in N-Tg and SIRT2-Tg mice treated with saline or Ang II (n=18~20; ***P <0.001).
(C) Left: Hematoxylin-eosin (H&E, scale bar=1 mm) staining and wheat germ agglutinin (WGA, scale bar=30 μm) staining were performed to determine the hypertrophic growth of the hearts in N-Tg and SIRT2-Tg mice treated with saline or Ang II. Right: Quantification of cardiomyocyte size in N-Tg and SIRT2-Tg mice treated with saline or Ang II (n=15~18; ***P <0.001).
(D) Left: Picrosirius red (PSR, scale bar=50 μm) staining was performed to determine cardiac fibrosis of the hearts from N-Tg and SIRT2-Tg mice treated with saline or Ang II. Right: Quantification of cardiac fibrosis in N-Tg and SIRT2-Tg mice treated with saline or Ang II (n=15~18; *P<0.05, ***P <0.001).
(E) Expression of hypertrophic (Anp, Bnp, β-Mhc, Acta1, IL-6 and Rcan1.4) and fibrotic (Ctgf, Col1a1 and Col3a1) genes in the hearts of N-Tg and SIRT2-Tg mice treated with saline or Ang II (n=6; *P<0.05, ** P<0.01, ***P<0.001).
Collectively, SIRT2 in cardiomyocytes represses hypertrophy directly, and cardiac-specific SIRT2 overexpression inhibits Ang II-induced cardiac hypertrophy and rescues cardiac function.
SIRT2 maintains AMPK signaling in hypertrophic hearts
Among the various metabolic sensors analyzed, we observed that AMPK signaling was repressed in aged Sirt2-KO hearts compared with aged WT hearts (Figure 1F). The metabolic orchestrator AMPK controls diverse aspects of cardiac metabolism, including glycolysis, fatty acid metabolism, protein synthesis and autophagy. AMPK deficiency contributes to aging-dependent or stress-induced cardiac hypertrophy.9, 12 Therefore, we analyzed whether the defect in AMPK signaling might underlie the cardiac phenotype of SIRT2-modified mice upon Ang II infusion. The phosphorylation of AMPK and its downstream substrates acetyl-CoA carboxylase (ACC) and regulatory-associated protein of mTOR (Raptor) were analyzed. In Ang II-induced hypertrophic hearts, Sirt2-KO hearts had substantially decreased AMPK pathway activation, including down-regulation of AMPK phosphorylation at Thr172, ACC phosphorylation at Ser79, and Raptor phosphorylation at Ser792 (Figure 6A). In contrast, the AMPK signaling pathway was enhanced by SIRT2 overexpression in Ang II-induced hypertrophic hearts (Figure 6A). Similar results were observed in NRCMs because inhibition of SIRT2 activity with AGK2 reduced AMPK, ACC, and Raptor phosphorylation, whereas SIRT2 overexpression promoted the activation of AMPK signaling (Figure 6B).
Figure 6. SIRT2 maintains AMPK signaling in myocardial tissues.
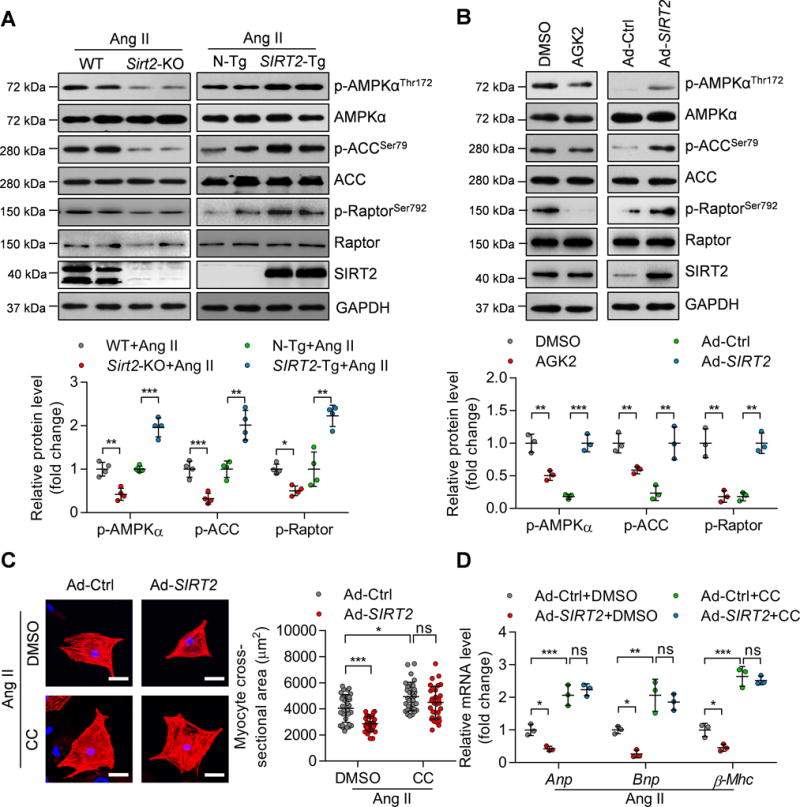
(A) Representative western blotting and quantitative results showing the phosphorylation levels of AMPK and the phosphorylation levels of its substrates ACC and Raptor in the hearts of WT, Sirt2-knockout, N-Tg and SIRT2-Tg mice infused with Ang II (n=4; *P<0.05, **P<0.01, ***P<0.001).
(B) Representative western blotting and quantification results showing the phosphorylation of AMPK, ACC, and Raptor in NRCMs. NRCMs were treated with the SIRT2 inhibitor AGK2 (10 μM) or with DMSO for 24 hours or infected with adenovirus overexpressing SIRT2 (Ad-SIRT2) or control adenovirus (Ad-Ctrl) for 24 hours (**P<0.01, ***P<0.001).
(C) NRCMs were infected with the indicated adenovirus for 24 hours and then treated with Ang II (1 μM) for 48 hours in the presence of the AMPK inhibitor compound C (CC, 10 μM) or DMSO. α-Actinin staining was performed to identify cells. Representative images (Left) and quantification of cell size of total 30 NRCMs in each group are shown (scale bar=30 μm; *P<0.05, ***P<0.001, ns: not significant).
(D) NRCMs were treated as shown in (C) and RNA was subjected to qRT-PCR to determine the mRNA level of hypertrophic genes (Anp, Bnp, and β-Mhc). *P<0.05, **P<0.01, ***P<0.001, ns: not significant.
Next, we analyzed whether SIRT2-mediated AMPK activation contributes to the effect of SIRT2 on cardiomyocyte hypertrophy. In Ang II-induced hypertrophic cardiomyocytes, treatment with AMPK inhibitor compound C (CC, 10 μM) increased cardiomyocyte size and the expression of hypertrophic genes (Anp, Bnp, and β-Mhc). Notably, AMPK inhibition blocked the protective function of SIRT2 in Ang II-induced cardiomyocyte hypertrophy, because cardiomyocyte size and gene expression pattern were not affected by SIRT2 overexpression in NRCMs in the presence of CC (Figure 6C–D). SIRT2 maintains the activation of AMPK signaling, which in turn contributes to the protective function of SIRT2 during cardiomyocyte hypertrophy.
Deacetylation of LKB1 is involved in SIRT2-mediated activation of AMPK
A previous report showed that the acetylation of AMPKα1 and α2 could repress AMPK phosphorylation.27 Therefore, we analyzed the effects of SIRT2 on the acetylation of these two subunits of AMPK in HEK293T cells by overexpressing AMPKα1 or α2 with/without SIRT2 co-overexpression. SIRT2 overexpression did not decrease the acetylation levels of AMPKα1 or α2 (Supplementary Figure 8), indicating that SIRT2 regulates AMPK activation indirectly.
Calcium/calmodulin-dependent protein kinase kinase β (CaMKKβ) and the liver kinase B1 (LKB1) activate AMPK by phosphorylating Thr172 within the kinase domain of the α subunit.10 We next investigated the potential participation of CaMKKβ in SIRT2-mediated activation of AMPK by inhibiting CaMKKβ using its inhibitor STO-609 (20 μM) in NRCMs. Indeed, STO-609 treatment reduced the phosphorylation of AMPK at Thr172 and the phosphorylation of ACC at Ser79. Interestingly, SIRT2 overexpression still increased AMPK and ACC phosphorylation in the presence of STO-609 (Supplementary Figure 9A), indicating that CaMKKβ did not contribute to SIRT2-mediated activation of AMPK. Additionally, STO-609 alone increased cardiomyocyte size and promoted the expression of hypertrophic genes (Anp, Bnp, β-Mhc) in the model of Ang II-induced hypertrophy in NRCMs. However, STO-609 treatment did not block the effects of SIRT2 on cardiomyocyte size and the expression of hypertrophic genes in the NRCMs (Supplementary Figure 9B–C). These results indicate that the effect of SIRT2 on AMPK activation is indirect and is independent of CaMKKβ.
Next, the effect of SIRT2 on AMPK phosphorylation was analyzed in HeLa cells, which lack LKB1 as a result of promoter methylation.28 SIRT2 overexpression did not promote AMPK phosphorylation in HeLa cells (Supplementary Figure 10A). We also confirmed that adenovirus-mediated knockdown of Lkb1 in NRCMs reduced AMPK phosphorylation and blocked SIRT2-mediated activation of AMPK (Supplementary Figure 10B). Therefore, we further studied the possibility that SIRT2 regulates LKB1 activation. Sirt2-KO reduced the phosphorylation of LKB1 at Ser428 in aged hearts and Ang II-induced hypertrophic hearts (Figure 7A). Conversely, cardiac-specific SIRT2 overexpression promoted LKB1 phosphorylation in Ang II-treated hearts (Figure 7A). SIRT2 also activated LKB1 in NRCMs, because SIRT2 inhibitor AGK2 reduced the LKB1 phosphorylation and SIRT2 overexpression promoted LKB1 phosphorylation (Supplementary Figure 10C). In addition, the immunofluorescence assay demonstrated that SIRT2 overexpression significantly increased the cytosol-to-nucleus ratio of endogenous LKB1 in cardiomyocytes (Figure 7B). Therefore, these findings revealed that SIRT2 promotes the phosphorylation of LKB1 and facilitates its translocation to the cytoplasm, where it interacts with its partners (STRAD and MO25) to phosphorylate AMPK.
Figure 7. SIRT2 deacetylates and activates LKB1.
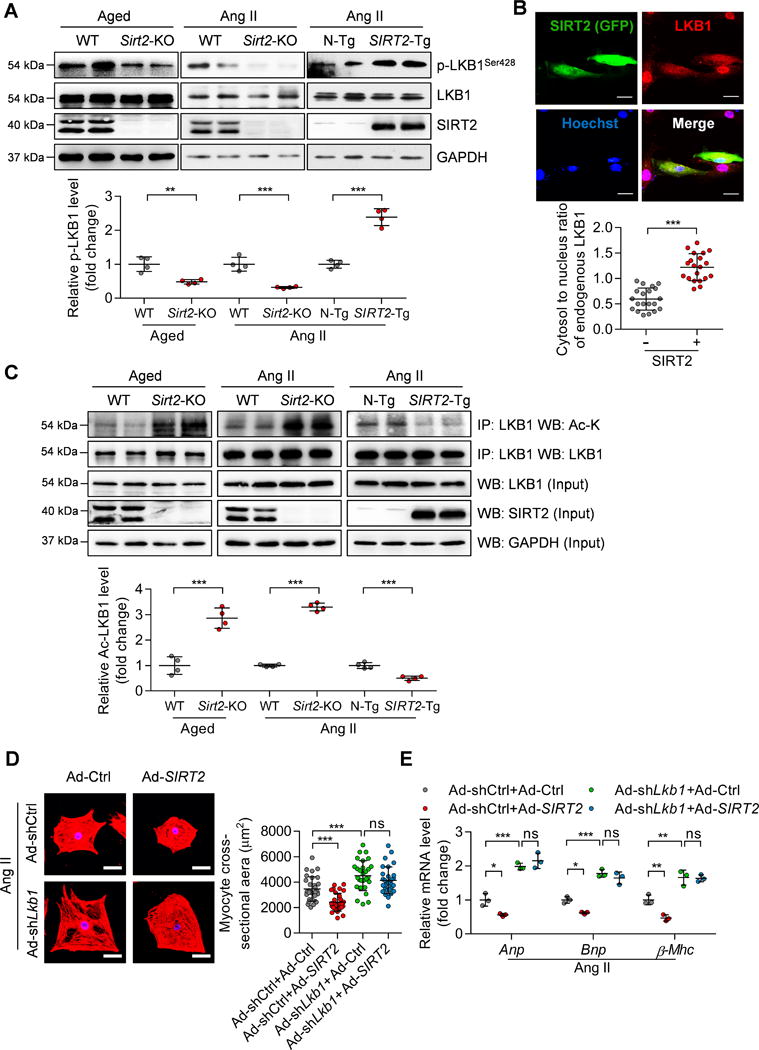
(A) Representative western blotting and quantitative results showing LKB1 phosphorylation at Ser428 in the hearts of aged WT and Sirt2-KO mice and the hearts of Ang II-induced WT, Sirt2-KO, N-Tg and SIRT2-Tg mice (n=4; **P<0.01, ***P<0.001).
(B) Subcellular location of LKB1 in NRCMs. NRCMs were infected with Ad-SIRT2 for 24 hours, followed by immunofluorescence to detect the SIRT2 and LKB1 protein. Top: Representative immunofluorescence showing the location of LKB1 (red) in NRCMs, SIRT2-overexpressing cells were indicated by green fluorescence protein (GFP, green), scale bar=30 μm. Bottom: The quantification of the cytosol-to-nuclear ratio of LKB1 protein in 20 NRCMs without (-) and with (+) SIRT2-overexpression (***P<0.001).
(C) Representative immunoprecipitation, western blotting and quantitative results showing LKB1 acetylation in hypertrophic hearts. Endogenous LKB1 was purified by immunoprecipitation with anti-LKB1 antibody from the hearts from aged WT and Sirt2-KO mice or WT, Sirt2-KO, N-Tg, and SIRT2-Tg mice infused with Ang II. Western blotting was performed with the indicated antibodies (n=4; ***P<0.001).
(D) NRCMs were infected with the indicated adenovirus for 24 hours and then treated with Ang II (1 μM) for 48 hours. α-Actinin staining was performed to determine cell size. Representative images (Left) and quantification of cell size of total 30 NRCMs (Right) in each group are shown (scale bar=30 μm; ***P<0.001, ns: not significant).
(E) NRCMs were treated as shown in (D) and qRT-PCR was performed to analyze the expression of hypertrophic genes (Anp, Bnp, and β-Mhc). *P<0.05, **P<0.01, ***P<0.001, ns: not significant.
We next investigated whether SIRT2 regulates LKB1 activation directly. SIRT2 could interact with LKB1 when overexpressed in HEK293T cells (Supplementary Figure 11A). We also found that the endogenous SIRT2 and LKB1 could interact with each other in cardiomyocytes (Supplementary Figure 11A). We next investigated whether the deacetylase SIRT2 could deacetylate LKB1. SIRT2 overexpression reduced the acetylation levels of exogenous LKB1 in HEK293T cells. In addition, adenovirus-mediated SIRT2 overexpression also reduced the acetylation levels of endogenous LKB1 in NRCMs (Supplementary Figure 11B). Furthermore, Sirt2-KO significantly increased the acetylation levels of LKB1 in the aging- or Ang II-induced hypertrophic hearts, whereas opposing results were observed in Ang II-infused SIRT2-Tg hearts (Figure 7C). These findings strongly support the notion that SIRT2 binds to and deacetylates LKB1. Lysine 48 of LKB1 was reported to be the key lysine that determined the phosphorylation of LKB1 at Ser428.29 We wanted to know whether lysine 48 is critical for the SIRT2-mediated deacetylation of LKB1 and the subsequent activation of LKB1 and AMPK. Therefore, lysine 48 (K48) of LKB1 was mutated to arginine 48 (R48) to generate a mutated LKB1 (LKB1K48R) protein. Overexpression of SIRT2 did not change the acetylated level of LKB1K48R in HEK293T cells (Supplementary Figure 11C), indicating that lysine 48 is the core target site for SIRT2. In addition, the K48R mutant increased LKB1 and AMPK phosphorylation and blunted the effects of SIRT2 on LKB1 and AMPK phosphorylation (Supplementary Figure 11D). Therefore, SIRT2-mediated deacetylation of lysine 48 of LKB1 is critical for activation of LKB1 and AMPK.
Finally, we investigated whether LKB1 is critically involved in SIRT2-mediated inhibition of hypertrophy in NRCMs. We knocked down Lkb1 in NRCMs with adenovirus and treated the cells with Ang II. Lkb1 knockdown promoted Ang II-induced increase in the size of NRCMs and expression of hypertrophic genes. Moreover, Lkb1 deficiency blocked SIRT2-mediated repression of cardiomyocyte hypertrophy and expression of hypertrophic genes in NRCMs (Figure 7D and E). Taken together, these findings suggest that LKB1 is crucial for SIRT2-mediated repression of cardiomyocyte hypertrophy, which may be the mechanism underlying SIRT2-mediated cardioprotection against aging- and Ang II-induced pathological cardiac hypertrophy.
SIRT2 contributes to the effects of metformin on AMPK activation and cardiac hypertrophy
Metformin is a first-line clinical drug for the treatment of diabetes, and it is associated with various cardiovascular benefits. AMPK is an important target for metformin and contributes to metformin-mediated cardioprotection.13 We investigated whether AMPK activation by metformin could reduce the effects of Sirt2-KO on cardiac hypertrophy and subsequently rescue heart function.
We induced hypertrophy in WT and Sirt2-KO mice by infusing Ang II. The mice of each genotype were randomly divided into two groups and treated with metformin (200 mg/kg/day) or vehicle in drinking water for four weeks. Supplementation of metformin significantly activated AMPK in the heart tissues of WT mice. Interestingly, AMPK activation was blunted in the hearts of Sirt2-knockout mice, as metformin did not significantly upregulate AMPK phosphorylation in these mice (Figure 8A), indicating that SIRT2 is essential for metformin-mediated AMPK activation.
Figure 8. Sirt2 deficiency blunts the cardioprotective function of metformin.
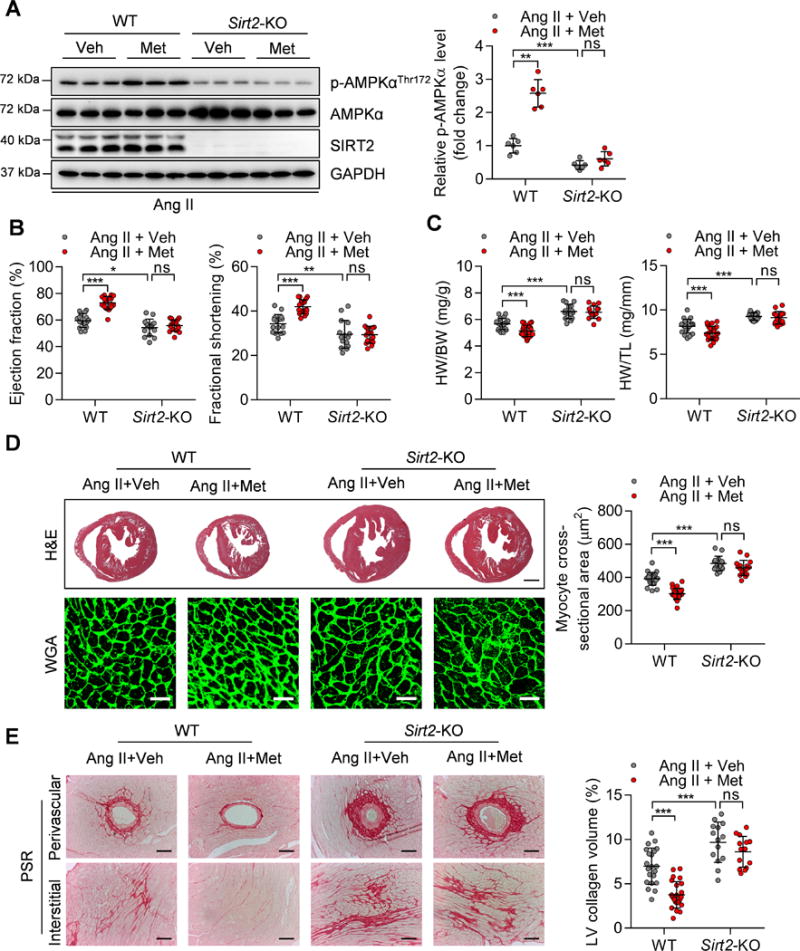
(A) Left: Representative western blotting showing the phosphorylation of AMPK at Thr172 in the hearts of WT and Sirt2-KO mice treated with Ang II (1.3 mg/kg/day) and metformin (200 mg/kg/day). Veh: Vehicle; Met: Metformin. Right: Quantification of phosphorylated AMPK levels (n=6; **P<0.01, ***P<0.001, ns: not significant).
(B) Ejection fraction and fractional shortening of WT and Sirt2-KO mice treated with Ang II and metformin (n=15~17; *P<0.05, **P<0.01, ***P<0.001, ns: not significant).
(C) Ratios of heart weight-to-body weight (HW/BW) or heart weight-to-tibia length (HW/TL) in WT and Sirt2-KO mice treated with Ang II and metformin (n=15~24; ***P<0.001, ns: not significant).
(D) Left: Hematoxylin-eosin (H&E, scale bar=1 mm) staining and wheat germ agglutinin (WGA, scale bar=30 μm) staining were performed to determine the hypertrophic growth of the hearts in WT and Sirt2-KO mice treated with Ang II and metformin. Right: Quantification of cardiomyocyte size of the hearts in WT and Sirt2-KO mice treated with Ang II and metformin (n=14~24; ***P<0.001, ns: not significant).
(E) Left: Picrosirius red (PSR, scale bar=50 μm) staining was performed to determine cardiac fibrosis of the hearts from WT and Sirt2-KO mice treated with Ang II and metformin. Right: Quantification of cardiac fibrosis of the hearts in WT and Sirt2-KO mice treated with Ang II and metformin (n=14~24; ***P<0.001, ns: not significant).
This surprising result prompted us to analyze the effects of metformin on cardiac function and pathological remodeling in WT and Sirt2-KO mice. Metformin treatment rescued the ejection fraction and fractional shortening of hypertrophic hearts in WT mice infused with Ang II (Figure 8B). In addition, the histochemical analysis showed that normalized heart weight, cardiomyocyte size, and cardiac fibrosis were reduced by metformin treatment in WT mice infused with Ang II (Figure 8C–E). However, the cardioprotective functions of metformin did not rely on its effect on heart rate and blood pressure, since metformin did not affect heart rate or blood pressure in WT mice infused with Ang II (Supplementary Figure 12A–B). Notably, in Sirt2-KO mice infused with Ang II, metformin treatment did not rescue ejection fraction and fractional shortening (Figure 8B). The inhibitory effects of metformin on cardiac hypertrophy and fibrosis were also blunted by Sirt2-KO (Figure 8C–E).
These results indicate that SIRT2 is essential for metformin-mediated activation of AMPK signaling and that SIRT2 contributes to the cardioprotective effects of metformin.
Discussion
In the present study, we identified SIRT2 as a cardioprotective deacetylase in pathological cardiac hypertrophy. SIRT2 expression was decreased in hypertrophic hearts induced by aging and Ang II. Sirt2 deficiency promoted aging-related and Ang II-induced cardiac hypertrophy and led to a decline in cardiac function. Cardiac-specific SIRT2 transgene inhibited Ang II-induced cardiac hypertrophy and fibrosis and rescued cardiac function. The mechanistic study demonstrated that SIRT2 activated AMPK signaling by deacetylating and activating its upstream kinase LKB1, which contributed to the effects of SIRT2 on cardiac hypertrophy. In addition, Sirt2-KO repressed metformin-induced AMPK activation and blunted metformin-mediated cardioprotective functions.
Aging is one of the key risk factors for cardiac hypertrophy.8 The Sirtuins modulate lifespan in species ranging from yeast to mammals.14 In mammalian, the Sirtuin family has seven members with diverse cellular locations. Previous works from our group and others have reported the functions of Sirtuins (excluding SIRT2) in pathological cardiac hypertrophy. Although the roles of SIRT1 in pathological hypertrophy are still far from conclusive, all other Sirtuins (excluding SIRT4) are cardioprotective in cardiac hypertrophy.18, 19, 30–33 However, the roles of the Sirtuins in aging-related cardiac hypertrophy remain largely unknown. Genetical knockout of Sirt1, Sirt6 or Sirt7 leads to developmental defects, premature and reduction in lifespan (ranging from a usual survival of days [SIRT1] to weeks [SIRT6] or months [SIRT7]) in mice.33–35 These facts make it difficult to investigate the functions of endogenous SIRT1/6/7 in aging-dependent cardiac hypertrophy. We found that Sirt2-KO mice developed normally and could live up to two years. Notably, these mice displayed more severe aging features (e.g. hair browning) compared with wild-type mice at the late stage of life (24-month-old). Interestingly, we observed that Sirt2 deficiency caused decreases in the ejection fraction and fractional shortening of aged mice. Importantly, Sirt2-KO promoted aging-related increases in cardiomyocyte size and myocardial fibrosis. These findings suggest that Sirt2 deficiency facilitates aging-dependent cardiac hypertrophy. The concentration of Ang II is elevated in aged rodent hearts, and the activation of the renin-angiotensin system contributes to aging-dependent cardiac hypertrophy.22 Ang II supplement could recur the development of cardiac hypertrophy observed in aged mice.18 Sirt2-KO aggravated Ang II-induced cardiac hypertrophy and fibrosis and decreased ejection fraction and fractional shortening. Notably, SIRT2 protein levels and SIRT2 enzymatic activity levels were reduced in hypertrophic hearts. Therefore, loss of Sirt2 may be a mechanism that contributes to aging-related cardiac hypertrophy.
The mechanisms by which the Sirtuins participate in the pathogenesis of cardiac hypertrophy is much different from each other, which may be mainly due to the difference in subcellular locations and primary enzymatic activities. SIRT1 is the most extensively studied among the sirtuins in cardiac diseases. Low to moderate overexpression of SIRT1 in transgenic mouse hearts represses oxidative stress and attenuates stress-induced cardiac hypertrophy,19 whereas some studies reported that SIRT1 promotes the transition from hypertrophy to heart failure by promoting mitochondrial dysfunction or activating the Akt signaling.36, 37 In the mitochondria, SIRT3 participates in pathological hypertrophy by activating forkhead box O3a (FoxO3a) and manganese-dependent superoxide dismutase (MnSOD) to repress oxidative stress whereas SIRT4 represses SIRT3-MnSOD pathway and promotes stress-induced cardiac hypertrophy.18, 30, 38 Mice with Sirt5 deficiency develop hypertrophic cardiomyopathy within nine months partially due to metabolic defect.32 In the nucleus, SIRT6 regulates insulin-like growth factor (IGF)-Akt signaling and represses stress-induced cardiac hypertrophy while Sirt7 knockout leads to apoptosis and hypertrophy of cardiomyocytes.31, 33 Differently, SIRT2 is predominantly located in the cytosol and deacetylates LKB1 to activate AMPK and subsequently repress pathological cardiac hypertrophy. Sirt2 deficiency reduced AMPK phosphorylation but did not alter SIRT1 expression or IGF1R phosphorylation in aged hearts. SIRT2-mediated activation of AMPK signaling was confirmed in the hearts of Ang II-treated Sirt2-KO mice and NRCMs with SIRT2 inhibition. In contrast, SIRT2 overexpression in hearts and NRCMs could activate AMPK signaling. Significantly, SIRT2-mediated AMPK activation contributed to the inhibitory effects of SIRT2 on Ang II-induced cardiomyocyte hypertrophy. AMPK activation is impaired in aged murine hearts compared with young murine hearts,39 and AMPK deficiency promoted aging-related cardiac hypertrophy.12 Our results showed that the SIRT2 expression was decreased in aged and Ang II-induced hypertrophic hearts. Therefore, the repressed AMPK activation in aged hearts may be due to the reduced SIRT2 protein levels. AMPK activation is a core contributor to metformin-mediated cardioprotective functions.13 Notably, metformin-mediated activation of AMPK in hypertrophic hearts was also repressed by Sirt2 deficiency. These findings support the notion that SIRT2 is essential for AMPK activation in myocardial tissues.
When activated by phosphorylation, LKB1 translocates from the nucleus to the cytoplasm and phosphorylates its substrates such as AMPK.9 LKB1 is the major upstream kinase of AMPK and LKB1 mediates AMPKα2 phosphorylation in the cardiac tissues.9 Cardiomyocyte-specific knockout of Lkb1 would cause cardiac hypertrophy through inhibition of AMPK signaling and activation of mTOR signaling in mice.40 However, the upstream regulators of LKB1 in the cardiac tissues are largely unknown. Our findings demonstrate that SIRT2 serves as an upstream activator of LKB1. Sirt2 deficiency reduced LKB1 phosphorylation in aged and Ang II-induced hypertrophic hearts, whereas SIRT2 overexpression increased LKB1 phosphorylation in hypertrophic hearts. Additionally, SIRT2 also promoted the translocation of LKB1 from the nucleus to the cytoplasm, where it cooperates with its partners to phosphorylate AMPK at Thr172. SIRT2-mediated LKB1 activation relies on LKB1 deacetylation. LKB1 could interact with SIRT2, which made it possible for SIRT2 to deacetylate LKB1 in cardiomyocytes and myocardial tissues. SIRT2 deacetylates LKB1 at lysine 48, which is critical for LKB1 phosphorylation and translocation.29 Mutation of LKB1 at lysine 48 to arginine 48 activated LKB1 and its downstream substrate AMPK and blocked the effect of SIRT2 on LKB1 and AMPK activation. Notably, LKB1 contributes to SIRT2-mediated inhibition of cardiac hypertrophy. Although the Sirtuins share some substrates, their affinity and deacetylation efficiency are much different. Indeed, SIRT1 was also reported to deacetylate LKB1 and activate AMPK in cells.29 However, a later report using in vitro acetylome peptide microarray revealed that the deacetylation efficiency of SIRT1 at lysine 48 (and some other lysine sites) of LKB1 is much lower than that of SIRT2.41 Additionally, an in vitro deacetylation assay also showed that SIRT1 seemed not to deacetylate LKB1 peptide containing acetylated lysine 48.42 SIRT1 may regulate AMPK by other mechanisms. For instance, SIRT1 regulates secretion of adipokines such as adiponectin,43 which activates AMPK through a CaMKKβ-dependent manner.44 Our findings support that SIRT2 may be the primary direct deacetylase for LKB1 and that SIRT2 activates AMPK depending upon LKB1 but not CaMKKβ. In this regard, SIRT1 and SIRT2 may activate AMPK through different mechanisms.
In addition to the LKB1-AMPK signaling, some other mechanisms may also contribute to SIRT2-mediated cardioprotective functions. For instance, SIRT2 exerts effects on microtubule stabilization via Tubulin deacetylation and on oxidative stress via Foxo3a signaling. Both mechanisms are involved in hypertrophy pathogenesis.45, 46 Some other targets of SIRT2, such as Foxo1 and PGC1-α, are also known regulators for cardiac hypertrophy.11, 17 Interestingly, most of these factors (Tubulin, Foxo1, Foxo3a, and PGC1α) are also downstream of the LKB1-AMPK signaling.11, 45 Therefore, activation of the LKB1-AMPK signaling may be a core mechanism underlying SIRT2-mediated cardioprotective functions.
Metformin is a first-line antidiabetic drug, which also reduces mortality and readmission in patients with heart failure.47 AMPK is the major target of metformin. AMPK activation by metformin represses cardiac remodeling and rescues cardiac function in aging, and stress-induced hypertrophy and ischemia-induced cardiac injury in rodents and dogs.12, 48–50 Metformin activates AMPK through LKB1-dependent mechanisms.13 Sirt2 deficiency repressed metformin-mediated AMPK activation, which may be due to repressed LKB1 activation in Sirt2-deficient hearts. Currently, clinical trials studying the therapeutic effects of metformin on longevity (ClinicalTrials.gov Identifier: NCT02432287) and ventricular hypertrophy (ClinicalTrials.gov Identifier: NCT02226510; NCT01879293) are ongoing. Our results showed that SIRT2 expression was decreased in hypertrophic hearts and that the cardioprotective effects of metformin were significantly abolished in Sirt2-KO mice. Therefore, it is advised that SIRT2 expression should be considered in future trials of metformin therapies in patients with cardiac hypertrophy and heart failure.
In conclusion, we identify SIRT2 as a cardioprotective deacetylase. SIRT2 prevents aging-dependent and Ang II-induced pathological hypertrophy by maintaining cardiac LKB1-AMPK signaling. These findings improve our understanding of aging-dependent and stress-induced pathological cardiac hypertrophy and indicate that SIRT2 is a potential target for therapeutic interventions for pathological cardiac hypertrophy.
Supplementary Material
Clinical Perspective.
What is new?
For the first time, we demonstrate that SIRT2 protein level and activity are reduced during pathological cardiac hypertrophy, and SIRT2 deficiency promotes aging- and Ang II-induced pathological cardiac hypertrophy, whereas SIRT2 overexpression represses pathological cardiac hypertrophy.
SIRT2 deacetylates LKB1 at lysine 48 to activate the LKB1-AMPK signaling and prevents against hypertrophy of cardiomyocytes.
SIRT2 is critically involved in metformin-mediated activation of AMPK and cardioprotective effects.
What are the clinical implications?
We identify SIRT2 as a novel target for treatment of pathological cardiac hypertrophy induced by aging and other pathological stress.
Our study suggests that SIRT2 may be essential for the beneficial effects of metformin against pathological cardiac hypertrophy.
Acknowledgments
The authors thank Dr. Sheng-Cai Lin (Xiamen University) for providing LKB1 expression plasmids, and Dr. Hengyi Xiao (Sichuan University) for providing AMPK expression plasmids.
Sources of funding
This work was supported by grants from the National Natural Science Foundation of China (nos. 81422002, 91339201, 91639304, and 31571193), the National Science and Technology Support Project (2014BAI02B01, 2015BAI08B01), the National Youth Top-notch Talent Support Program and the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (CIFMS, 2016-I2M-1-015, 2016-I2M-1-016, 2016-I2M-1-011). Dr. M.H. Zou is an eminent scholar of Georgia Research Alliance and he is supported by the grants NHLBI (HL080499, HL089920).
Footnotes
Twitter handle for Xiaoqiang Tang: @tang_xiaoqiang
Disclosures
None.
References
- 1.Driver JA, Djousse L, Logroscino G, Gaziano JM, Kurth T. Incidence of cardiovascular disease and cancer in advanced age: prospective cohort study. BMJ. 2008;337:a2467. doi: 10.1136/bmj.a2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braunwald E. The war against heart failure: the Lancet lecture. Lancet. 2015;385:812–824. doi: 10.1016/S0140-6736(14)61889-4. [DOI] [PubMed] [Google Scholar]
- 3.Dolinsky VW, Morton JS, Oka T, Robillard-Frayne I, Bagdan M, Lopaschuk GD, Des Rosiers C, Walsh K, Davidge ST, Dyck JRB. Calorie restriction prevents hypertension and cardiac hypertrophy in the spontaneously hypertensive rat. Hypertension. 2010;56:412–421. doi: 10.1161/HYPERTENSIONAHA.110.154732. [DOI] [PubMed] [Google Scholar]
- 4.Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–4. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fontana L, Klein S. Aging, adiposity, and calorie restriction. JAMA. 2007;297:986–94. doi: 10.1001/jama.297.9.986. [DOI] [PubMed] [Google Scholar]
- 6.Schiattarella GG, Hill JA. Inhibition of hypertrophy is a good therapeutic strategy in ventricular pressure overload. Circulation. 2015;131:1435–47. doi: 10.1161/CIRCULATIONAHA.115.013894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doenst T, Nguyen TD, Abel ED. Cardiac metabolism in heart failure: implications beyond ATP production. Circ Res. 2013;113:709–24. doi: 10.1161/CIRCRESAHA.113.300376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shirakabe A, Ikeda Y, Sciarretta S, Zablocki DK, Sadoshima J. Aging and autophagy in the heart. Circ Res. 2016;118:1563–1576. doi: 10.1161/CIRCRESAHA.116.307474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zaha VG, Young LH. AMP-activated protein kinase regulation and biological actions in the heart. Circ Res. 2012;111:800–14. doi: 10.1161/CIRCRESAHA.111.255505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dasgupta B, Chhipa RR. Evolving lessons on the complex role of AMPK in normal physiology and cancer. Trends Pharmacol Sci. 2016;37:192–206. doi: 10.1016/j.tips.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim TT, Dyck JR. Is AMPK the savior of the failing heart? Trends Endocrinol Metab. 2015;26:40–8. doi: 10.1016/j.tem.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Turdi S, Fan X, Li J, Zhao J, Huff AF, Du M, Ren J. AMP-activated protein kinase deficiency exacerbates aging-induced myocardial contractile dysfunction. Aging Cell. 2010;9:592–606. doi: 10.1111/j.1474-9726.2010.00586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foretz M, Guigas B, Bertrand L, Pollak M, Viollet B. Metformin: from mechanisms of action to therapies. Cell Metab. 2014;20:953–66. doi: 10.1016/j.cmet.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 14.Bonkowski MS, Sinclair DA. Slowing ageing by design: the rise of NAD+ and sirtuin-activating compounds. Nat Rev Mol Cell Biol. 2016;17:679–690. doi: 10.1038/nrm.2016.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shah MS, Brownlee M. Molecular and cellular mechanisms of cardiovascular disorders in diabetes. Circ Res. 2016;118:1808–1829. doi: 10.1161/CIRCRESAHA.116.306923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winnik S, Auwerx J, Sinclair DA, Matter CM. Protective effects of sirtuins in cardiovascular diseases: from bench to bedside. Eur Heart J. 2015;36:3404–3412. doi: 10.1093/eurheartj/ehv290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gomes P, Outeiro TF, Cavadas C. Emerging role of Sirtuin 2 in the regulation of mammalian metabolism. Trends Pharmacol Sci. 2015;36:756–68. doi: 10.1016/j.tips.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Luo YX, Tang X, An XZ, Xie XM, Chen XF, Zhao X, Hao DL, Chen HZ, Liu DP. Sirt4 accelerates Ang II-induced pathological cardiac hypertrophy by inhibiting manganese superoxide dismutase activity. Eur Heart J. 2017;38:1389–1398. doi: 10.1093/eurheartj/ehw138. [DOI] [PubMed] [Google Scholar]
- 19.Alcendor RR, Gao S, Zhai P, Zablocki D, Holle E, Yu X, Tian B, Wagner T, Vatner SF, Sadoshima J. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res. 2007;100:1512–21. doi: 10.1161/01.RES.0000267723.65696.4a. [DOI] [PubMed] [Google Scholar]
- 20.Flynn JM, O’Leary MN, Zambataro CA, Academia EC, Presley MP, Garrett BJ, Zykovich A, Mooney SD, Strong R, Rosen CJ, Kapahi P, Nelson MD, Kennedy BK, Melov S. Late-life rapamycin treatment reverses age-related heart dysfunction. Aging Cell. 2013;12:851–62. doi: 10.1111/acel.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ock S, Lee WS, Ahn J, Kim HM, Kang H, Kim H-S, Jo D, Abel ED, Lee TJ, Kim J. Deletion of IGF-1 receptors in cardiomyocytes attenuates cardiac aging in male mice. Endocrinology. 2016;157:336–345. doi: 10.1210/en.2015-1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Conti S, Cassis P, Benigni A. Aging and the renin-angiotensin system. Hypertension. 2012;60:878–883. doi: 10.1161/HYPERTENSIONAHA.110.155895. [DOI] [PubMed] [Google Scholar]
- 23.North BJ, Marshall BL, Borra MT, Denu JM, Verdin E. The human Sir2 ortholog, SIRT2, iIs an NAD+-dependent tubulin deacetylase. Mol Cell. 2003;11:437–444. doi: 10.1016/s1097-2765(03)00038-8. [DOI] [PubMed] [Google Scholar]
- 24.Choi YH, Kim H, Lee SH, Jin YH, Lee KY. Src regulates the activity of SIRT2. Biochem Biophys Res Commun. 2014;450:1120–5. doi: 10.1016/j.bbrc.2014.06.117. [DOI] [PubMed] [Google Scholar]
- 25.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 26.Outeiro TF, Kontopoulos E, Altmann SM, Kufareva I, Strathearn KE, Amore AM, Volk CB, Maxwell MM, Rochet JC, McLean PJ, Young AB, Abagyan R, Feany MB, Hyman BT, Kazantsev AG. Sirtuin 2 inhibitors rescue alpha-synuclein-mediated toxicity in models of Parkinson’s disease. Science. 2007;317:516–9. doi: 10.1126/science.1143780. [DOI] [PubMed] [Google Scholar]
- 27.Lin Y-y, Kiihl S, Suhail Y, Liu S-Y, Chou Y-h, Kuang Z, Lu J-y, Khor CN, Lin C-L, Bader JS, Irizarry R, Boeke JD. Functional dissection of lysine deacetylases reveals that HDAC1 and p300 regulate AMPK. Nature. 2012;482:251–255. doi: 10.1038/nature10804. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Tiainen M, Ylikorkala A, Mäkelä TP. Growth suppression by Lkb1 is mediated by a G1 cell cycle arrest. Proc Natl Acad Sci U S A. 1999;96:9248–9251. doi: 10.1073/pnas.96.16.9248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lan F, Cacicedo JM, Ruderman N, Ido Y. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. J Biol Chem. 2008;283:27628–35. doi: 10.1074/jbc.M805711200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sundaresan NR, Gupta M, Kim G, Rajamohan SB, Isbatan A, Gupta MP. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J Clin Invest. 2009;119:2758–2771. doi: 10.1172/JCI39162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sundaresan NR, Vasudevan P, Zhong L, Kim G, Samant S, Parekh V, Pillai VB, Ravindra PV, Gupta M, Jeevanandam V, Cunningham JM, Deng CX, Lombard DB, Mostoslavsky R, Gupta MP. The sirtuin SIRT6 blocks IGF-Akt signaling and development of cardiac hypertrophy by targeting c-Jun. Nat Med. 2012;18:1643–50. doi: 10.1038/nm.2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sadhukhan S, Liu X, Ryu D, Nelson OD, Stupinski JA, Li Z, Chen W, Zhang S, Weiss RS, Locasale JW, Auwerx J, Lin H. Metabolomics-assisted proteomics identifies succinylation and SIRT5 as important regulators of cardiac function. Proc Natl Acad Sci U S A. 2016;113:4320–4325. doi: 10.1073/pnas.1519858113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vakhrusheva O, Smolka C, Gajawada P, Kostin S, Boettger T, Kubin T, Braun T, Bober E. Sirt7 increases stress resistance of cardiomyocytes and prevents apoptosis and inflammatory cardiomyopathy in mice. Circ Res. 2008;102:703–710. doi: 10.1161/CIRCRESAHA.107.164558. [DOI] [PubMed] [Google Scholar]
- 34.Cheng HL, Mostoslavsky R, Saito S, Manis JP, Gu Y, Patel P, Bronson R, Appella E, Alt FW, Chua KF. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc Natl Acad Sci U S A. 2003;100:10794–9. doi: 10.1073/pnas.1934713100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mostoslavsky R, Chua KF, Lombard DB, Pang WW, Fischer MR, Gellon L, Liu P, Mostoslavsky G, Franco S, Murphy MM, Mills KD, Patel P, Hsu JT, Hong AL, Ford E, Cheng HL, Kennedy C, Nunez N, Bronson R, Frendewey D, Auerbach W, Valenzuela D, Karow M, Hottiger MO, Hursting S, Barrett JC, Guarente L, Mulligan R, Demple B, Yancopoulos GD, Alt FW. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell. 2006;124:315–29. doi: 10.1016/j.cell.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 36.Sundaresan NR, Pillai VB, Wolfgeher D, Samant S, Vasudevan P, Parekh V, Raghuraman H, Cunningham JM, Gupta M, Gupta MP. The deacetylase SIRT1 promotes membrane localization and activation of Akt and PDK1 during tumorigenesis and cardiac hypertrophy. Sci Signal. 2011;4:ra46. doi: 10.1126/scisignal.2001465. [DOI] [PubMed] [Google Scholar]
- 37.Oka S, Alcendor R, Zhai P, Park JY, Shao D, Cho J, Yamamoto T, Tian B, Sadoshima J. PPARalpha-Sirt1 complex mediates cardiac hypertrophy and failure through suppression of the ERR transcriptional pathway. Cell Metab. 2011;14:598–611. doi: 10.1016/j.cmet.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang X, Chen X-F, Chen H-Z, Liu D-P. Mitochondrial Sirtuins in cardiometabolic diseases. Clin Sci. 2017;131:2063–2078. doi: 10.1042/CS20160685. [DOI] [PubMed] [Google Scholar]
- 39.Ma H, Wang J, Thomas DP, Tong C, Leng L, Wang W, Merk M, Zierow S, Bernhagen J, Ren J, Bucala R, Li J. Impaired macrophage migration inhibitory factor-AMP-activated protein kinase activation and ischemic recovery in the senescent heart. Circulation. 2010;122:282–92. doi: 10.1161/CIRCULATIONAHA.110.953208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ikeda Y, Sato K, Pimentel DR, Sam F, Shaw RJ, Dyck JR, Walsh K. Cardiac-specific deletion of LKB1 leads to hypertrophy and dysfunction. J Biol Chem. 2009;284:35839–49. doi: 10.1074/jbc.M109.057273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rauh D, Fischer F, Gertz M, Lakshminarasimhan M, Bergbrede T, Aladini F, Kambach C, Becker CF, Zerweck J, Schutkowski M, Steegborn C. An acetylome peptide microarray reveals specificities and deacetylation substrates for all human sirtuin isoforms. Nat Commun. 2013;4:2327. doi: 10.1038/ncomms3327. [DOI] [PubMed] [Google Scholar]
- 42.Bai B, Man AW, Yang K, Guo Y, Xu C, Tse HF, Han W, Bloksgaard M, De Mey JG, Vanhoutte PM, Xu A, Wang Y. Endothelial SIRT1 prevents adverse arterial remodeling by facilitating HERC2-mediated degradation of acetylated LKB1. Oncotarget. 2016;7:39065–39081. doi: 10.18632/oncotarget.9687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Banks AS, Kon N, Knight C, Matsumoto M, Gutierrez-Juarez R, Rossetti L, Gu W, Accili D. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab. 2008;8:333–41. doi: 10.1016/j.cmet.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iwabu M, Yamauchi T, Okada-Iwabu M, Sato K, Nakagawa T, Funata M, Yamaguchi M, Namiki S, Nakayama R, Tabata M, Ogata H, Kubota N, Takamoto I, Hayashi YK, Yamauchi N, Waki H, Fukayama M, Nishino I, Tokuyama K, Ueki K, Oike Y, Ishii S, Hirose K, Shimizu T, Touhara K, Kadowaki T. Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca(2+) and AMPK/SIRT1. Nature. 2010;464:1313–9. doi: 10.1038/nature08991. [DOI] [PubMed] [Google Scholar]
- 45.Fassett JT, Hu X, Xu X, Lu Z, Zhang P, Chen Y, Bache RJ. AMPK attenuates microtubule proliferation in cardiac hypertrophy. Am J Physiol Heart Circ Physiol. 2013;304:H749–H758. doi: 10.1152/ajpheart.00935.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mudd JO, Kass DA. Tackling heart failure in the twenty-first century. Nature. 2008;451:919–28. doi: 10.1038/nature06798. [DOI] [PubMed] [Google Scholar]
- 47.Crowley MJ, Diamantidis CJ, McDuffie JR, Cameron CB, Stanifer JW, Mock CK, Wang X, Tang S, Nagi A, Kosinski AS, Williams JW., Jr Clinical outcomes of metformin use in populations with chronic kidney disease, congestive heart failure, or chronic liver disease: A systematic review. Ann Intern Med. 2017;166:191–200. doi: 10.7326/M16-1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sasaki H, Asanuma H, Fujita M, Takahama H, Wakeno M, Ito S, Ogai A, Asakura M, Kim J, Minamino T, Takashima S, Sanada S, Sugimachi M, Komamura K, Mochizuki N, Kitakaze M. Metformin prevents progression of heart failure in dogs: role of AMP-activated protein kinase. Circulation. 2009;119:2568–77. doi: 10.1161/CIRCULATIONAHA.108.798561. [DOI] [PubMed] [Google Scholar]
- 49.Gundewar S, Calvert JW, Jha S, Toedt-Pingel I, Yong Ji S, Nunez D, Ramachandran A, Anaya-Cisneros M, Tian R, Lefer DJ. Activation of AMP-activated protein kinase by metformin improves left ventricular function and survival in heart failure. Circ Res. 2009;104:403–411. doi: 10.1161/CIRCRESAHA.108.190918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cittadini A, Napoli R, Monti MG, Rea D, Longobardi S, Netti PA, Walser M, Sama M, Aimaretti G, Isgaard J, Sacca L. Metformin prevents the development of chronic heart failure in the SHHF rat model. Diabetes. 2012;61:944–53. doi: 10.2337/db11-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


