Abstract
Objective
To investigate the association between intraperitoneal (IP) disease dissemination patterns, residual disease (RD), surgical complexity, and molecular subtypes in advanced high-grade serous ovarian cancer (HGSOC).
Methods
741 patients with operable stage III–IV HGSOC undergoing primary debulking surgery at Mayo Clinic from 1994–2011 were categorized into four mutually exclusive IP disease dissemination patterns: upper abdominal (60%), miliary (16%), lower abdominal (15%), and pelvic (9%). Surgical complexity was classified as high, intermediate, or low; RD status was defined as 0, 0.1–0.5, 0.6–1.0, or >1 cm; molecular subtype assignments were derived from expression profiling of tumors from 334 patients.
Results
Patients with either miliary or upper abdominal dissemination patterns were less likely to achieve RD0 compared to patients with pelvic and lower abdominal dissemination patterns (25% vs. 9% and 62%, each P<0.001) despite higher surgical complexity (39% vs. 6% and 20%, each P<0.001). Among the subset with molecular subtype data, patients with mesenchymal subtype of tumors were more likely to have upper abdominal or miliary dissemination patterns compared to patients with differentiated, proliferative, or immunoreactive subtypes (90% vs. 77%, 70%, 69%, respectively, P<0.05).
Conclusions
IP disease dissemination patterns are associated with RD, surgical complexity, and tumor molecular subtypes. Patients with upper abdominal or miliary dissemination patterns are more likely to have mesenchymal HGSOC and in turn achieve lower rates of complete resection. This provides a plausible model for how the biologic behavior of molecular subtypes is manifest in disease and oncologic outcomes.
1. INTRODUCTION
High-grade serous ovarian cancer (HGSOC) is the most common histology of epithelial ovarian cancer (EOC) [1]. Most diagnoses are made in patients with advanced stage disease, and treatment is usually aggressive primary debulking surgery (PDS) followed by platinum-based adjuvant chemotherapy [2–4]. Patients without visible residual disease (RD0) after PDS have the longest overall survival (OS). If RD0 is not obtained, patients with RD ≤1 cm (i.e. optimal debulking) have a significantly longer OS than patients with RD >1 cm (suboptimal debulking) [5, 6]. Therefore, the goal of PDS is to achieve the lowest gross RD when RD0 debulking is not feasible.
Several studies have consistently reported the impact of disease distribution on surgical outcomes in advanced stage disease, irrespective of RD. Disease in the upper abdomen is more difficult to resect and is associated with a higher number of procedures to achieve RD ≤1 cm [7–10]. Patients with upper abdominal disease are more likely to have shorter progression-free survival (PFS) and OS [9]. These findings raise the possibility that the association between survival outcomes and disease spread may be at least partially explained by differences in RD.
HGSOC molecular subtypes have been independently confirmed by multiple studies [11–13]. Our previous studies [13] demonstrated resectability of disease differs by molecular subtype. HGSOC patients with the mesenchymal (mesenchymal) subtype had the lowest RD0 rates and required more complex surgery to achieve RD ≤1 cm compared to patients with other subtypes of tumors. Patients with mesenchymal subtype had the shortest OS even after adjusting for age, stage, and grade [11, 13]. Despite a significant association between surgical and clinical outcomes across molecular subtypes, it is unclear as to how the molecular subtype affects the behavior of disease. To date, no studies have investigated the influence of molecular subtypes on patterns of disease dissemination in HGSOC. This is partly due to a lack of detailed operative findings and surgical outcomes in existing databases. This knowledge is important to better understand the manifestation of molecular subtypes on disease phenotype and to discern the factors underlying the prognostic significance of molecular subtypes [11, 13]. If molecular subtypes are associated with intraperitoneal (IP) disease dissemination patterns, preoperative molecular profiling may serve as an adjunct to radiologic imaging and intraoperative laparoscopic scoring systems to assist gynecologic oncologists in determining extent of disease and assessing the probability of optimal PDS. Using molecular subtypes to predict dissemination patterns and lack of resectability of disease may have implications in triaging patients to neoadjuvant chemotherapy (NACT) vs. PDS. Given the biologic differences between molecular subtypes [12], we hypothesize that different subtypes of HGSOC may in part contribute to various types of IP disease dissemination patterns, resectability of disease, and in turn oncological outcomes.
Our objective was to investigate the relationship between IP disease dissemination patterns, RD, surgical complexity, and tumor molecular subtypes in advanced HGSOC. First, we hypothesized that more aggressive and diffuse IP disease dissemination patterns are associated with increased RD and surgical complexity. Second, we hypothesized that IP disease dissemination patterns vary by HGSOC molecular subtype. As the survival differences among molecular subtypes may be driven by relative lack of resectability, our two-pronged hypothesis provides a plausible model for how the biologic behavior of molecular subtypes is manifested in disease spread and influences oncologic outcomes.
2. METHODS
The Mayo Clinic Institutional Review Board approved this single institution, retrospective study. Perioperative patient characteristics and surgical outcome variables were collected from prospectively maintained databases of patients undergoing PDS from 1994 – 2011. Inclusion criteria were high-grade serous or mixed high-grade (grade 2–4) serous histology, ovarian, fallopian, or primary peritoneal cancer, and operable stages III–IV. Patients with stage I–II disease, borderline tumors, those who were treated with NACT, and those without research consent were excluded.
We referenced previously described disease distributions [7–10], and the consensus among all gynecologic oncologists in the Division of Gynecologic Surgery at Mayo Clinic was to divide IP disease dissemination patterns in patients with stage III and operative stage IV HGSOC into four categories: pelvic disease, lower abdominal disease, upper abdominal disease, and miliary disease. Since patients with stage I–II disease were excluded, pelvic disease represented gross adnexal disease microscopic extrapelvic disease and/or lymphadenopathy. A gynecologic oncology physician (DT) reviewed the operative reports and assigned each patient to one of four mutually exclusive dissemination patterns as defined in Table 1. Inter-observer variation in the pattern assignment was assessed by having another gynecologic oncology physician (SW) independently review 89 randomly-selected operative reports.
Table 1.
Intraperitoneal disease dissemination definitions for stage III and operable stage IV disease.
| Category | Definition |
|---|---|
| Pelvic disease | Tumor present in the adnexa with or without bulky pelvic and/or paraaortic lymph nodes |
| Lower abdominal disease | Tumor present in the abdomen (including omentum) and pelvis but sparing the diaphragm, liver, pancreas, stomach, lesser sac, mesenteric root, spleen, and lesser curvature of the stomach |
| Upper abdominal disease | Tumor present in or on the diaphragm, liver, pancreas, stomach, lesser sac, mesenteric root, spleen, and lesser curvature of the stomach |
| Miliary disease | Diffuse abdominal and pelvic tumor studding (<1 cm in greatest dimension) with or without omental caking in the absence of bulky pelvic and abdominal disease |
Four RD groups were defined, RD0, RD 0.1–0.5 cm, RD 0.6–1.0 cm, RD >1 cm, based on the largest residual tumor diameter. Surgical complexity was assigned using previously published methods and classified as low, intermediate, or high complexity surgery [14]. Since patients with miliary disease often have disease in the upper abdomen, we combined upper abdominal and miliary disease into one disease spread for the statistical comparisons to account for the potential overlap between the two IP disease dissemination patterns. Gene expression profiles of the primary tumor were measured using Agilent Whole Human Genome 4×44K Expression Arrays. Expression data normalization and molecular subtype assignment were done as described in past publications [11, 13]. Patients with molecular profiling data available were assigned to one of four HGSOC molecular subtypes: mesenchymal, immunoreactive, proliferative, or differentiated.
Associations between molecular subtype, IP disease dissemination pattern, RD, and surgical complexity, and were quantified using odds ratios (OR) and corresponding 95% confidence intervals (CI) estimated from univariate and multivariable logistic regression models. All calculated P values were two-sided and P values <0.05 were considered statistically significant. SAS version 9.3 package (SAS Institute, Inc.; Cary, NC) was used for the analysis.
3. RESULTS
During the study period, 741 patients met inclusion criteria; their perioperative characteristics are summarized in Table 2.
Table 2.
Patient perioperative characteristics, overall and by intraperitoneal dissemination patterns.
| Characteristic | Intraperitoneal disease dissemination pattern | Total (N=741) |
|||
|---|---|---|---|---|---|
| Pelvic (N=67) |
Lower abdominal (N=111) |
Upper
abdominal (N=445) |
Miliary (N =118) |
||
| Age at surgery (years), mean (SD) | 62.7 (12.2) | 64.8 (12.3) | 64.2 (11.1) | 66.3 (9.8) | 64.5 (11.3) |
| Body mass index (kg/m2), mean (SD) | 28.6 (6.0) | 28.7 (6.4) | 28.3 (6.6) | 27.6 (6.6) | 28.3 (6.5) |
| ASA score, N (%) | |||||
| <3 | 41 (62.1) | 57 (51.4) | 223 (50.1) | 53 (44.9) | 374 (50.5) |
| ≥3 | 25 (37.9) | 54 (48.6) | 222 (49.9) | 65 (55.1) | 366 (49.5) |
| Unknown | 1 | – | – | – | 1 |
| Primary organ site, N (%) | |||||
| Ovarian | 55 (82.1) | 84 (75.7) | 364 (82.0) | 32 (27.1) | 535 (72.3) |
| Peritoneum | 4 (6.0) | 22 (19.8) | 58 (13.1) | 82 (69.5) | 166 (22.4) |
| Fallopian tube | 8 (11.9) | 5 (4.5) | 22 (5.0) | 4 (3.4) | 39 (5.3) |
| Unknown | – | – | 1 | – | 1 |
| Stage, N (%) | |||||
| IIIA/B | 21 (31.3) | 9 (8.1) | 12 (2.7) | 4 (3.4) | 46 (6.2) |
| IIIC | 41 (61.2) | 88 (79.3) | 312 (70.1) | 92 (78.0) | 533 (71.9) |
| IV | 5 (7.5) | 14 (12.6) | 121 (27.2) | 22 (18.6) | 162 (21.9) |
| Grade, N (%) | |||||
| 2 | 3 (4.5) | 2 (1.8) | 11 (2.5) | 2 (1.7) | 18 (2.4) |
| 3 | 55 (82.1) | 100 (90.1) | 361 (81.1) | 110 (93.2) | 626 (84.5) |
| 4 | 9 (13.4) | 9 (8.1) | 73 (16.4) | 6 (5.1) | 97 (13.1) |
| Histology, N (%) | |||||
| Pure serous | 60 (89.6) | 100 (90.1) | 423 (95.1) | 115 (97.5) | 698 (94.2) |
| Mixed serous | 7 (10.4) | 11 (9.9) | 22 (4.9) | 3 (2.5) | 43 (5.8) |
| Ascites, N (%) | |||||
| No | 46 (74.2) | 44 (42.3) | 111 (27.1) | 25 (21.4) | 226 (32.6) |
| Yes | 16 (25.8) | 60 (57.7) | 299 (72.9) | 92 (78.6) | 467 (67.4) |
| Unknown | 5 | 7 | 35 | 1 | 48 |
Abbreviations: ASA, American Society of Anesthesiologists; SD, standard deviation
We began by investigating the relationship between IP disease dissemination patterns, surgical complexity, and RD (Figure 1). Intraperitoneal disease dissemination patterns were assigned as follows: pelvic disease (9%), lower abdominal (15%), upper abdominal (60%), and miliary (16%); inter-observer variability was 91% (81/89) with substantial concordance (kappa K=0.78, 95% CI 0.64–0.92). Not surprisingly, patients with pelvic disease had the highest RD0 rate (91%), followed by patients with lower abdominal disease (62% RD0), those with upper abdominal disease (28% RD0), and those with miliary disease (18% RD0). Patients with either miliary or upper abdominal disease were less likely to achieve RD0 compared to patients with pelvic disease (25% vs. 91%, OR 0.03, 95% CI 0.01–0.08, P<0.001) or lower abdominal disease (25% vs. 62%, OR 0.21, 95% CI 0.14–0.33, P<0.001) (Figure 2). Further, patients with either miliary or upper abdominal disease were more likely to be suboptimally debulked compared to patients with pelvic disease (17% vs. 4%, OR 4.37, 95% CI 1.34–14.20, P=0.01) or lower abdominal disease (17% vs. 9%, OR 2.05, 95% CI 1.03–4.07, P=0.04) (Figure 2). Regarding the extent of surgery necessary to achieve lowest RD, those with either upper abdominal or miliary disease were more likely to have had high complexity surgery compared to patients with pelvic disease (39% vs. 6%, OR 9.94, 95% CI 3.57–27.71, P<0.001) or lower abdominal disease (39% vs. 20%, OR 2.60, 95% CI 1.58–4.26, P<0.001) (Figure 3). These results demonstrate that IP disease dissemination patterns, specifically upper abdominal and miliary types, require higher complexity surgery for resection and despite this higher effort, are still more likely to have higher amounts of RD.
Figure 1.
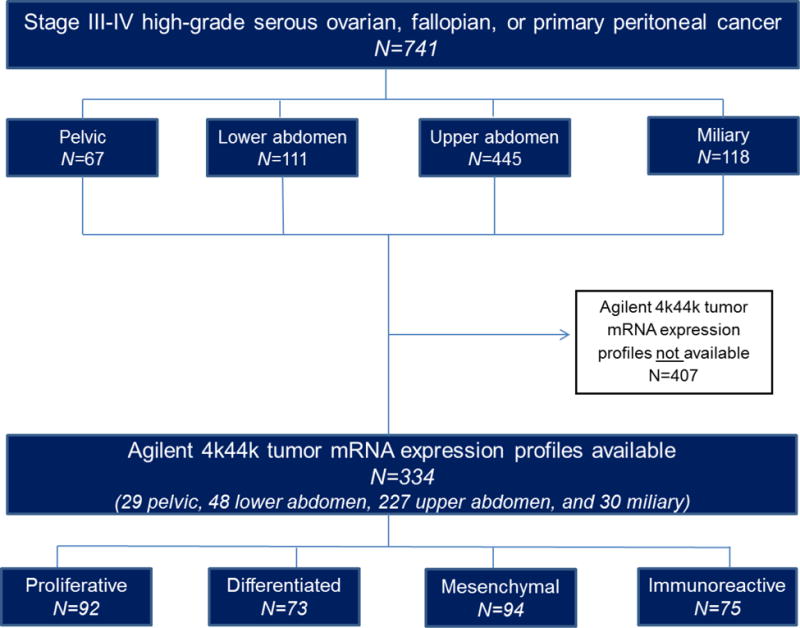
Study design.
Figure 2.
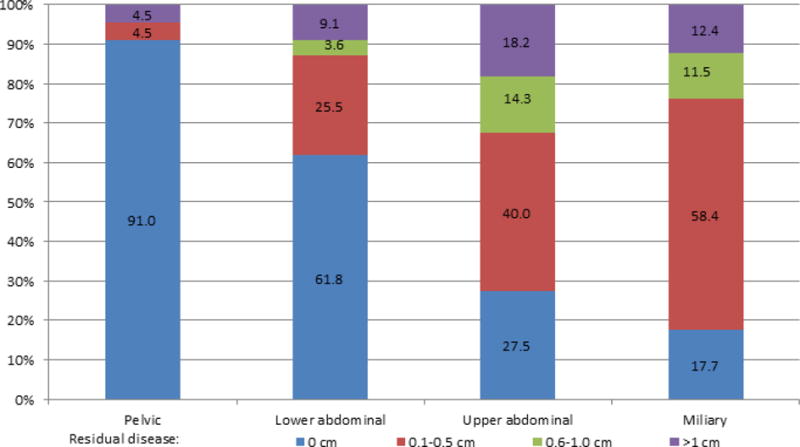
Residual disease status by intraperitoneal disease dissemination patterns, among 741 patients with high-grade serous ovarian cancer. Percentages based on the number of patients in each pattern group with information on residual disease (pelvic, N=67; lower abdominal, N=110 of 111; upper abdominal, N=440 of 445, miliary, N=113 of 118).
Figure 3.
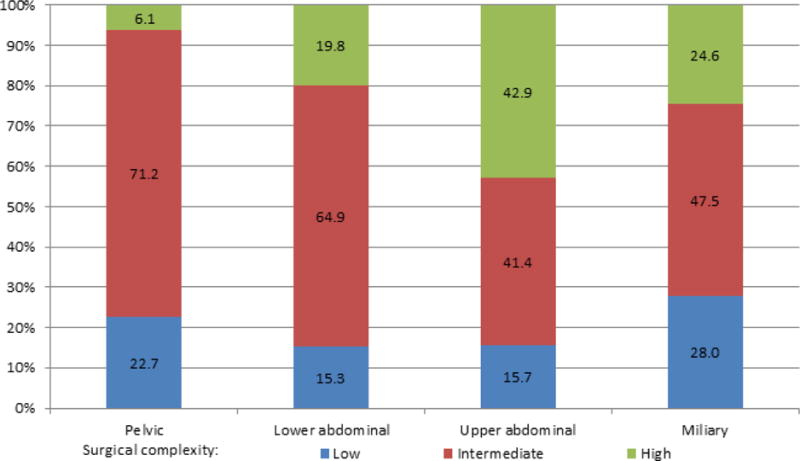
Surgical complexity by intraperitoneal disease dissemination patterns, among 741 patients with high-grade serous ovarian cancer. Percentages based on the number of patients in each pattern group with information on surgical complexity (pelvic, N=66 of 67; lower abdominal, N=111; upper abdominal, N=445, miliary, N=118).
We next investigated the relationship between tumor molecular subtypes and IP disease dissemination patterns in the subset of patient having molecular characterization available. Of the 741 patients with stage III and operable stage IV disease, 334 had tumor mRNA expression profiles available. Within this subset, IP disease dissemination patterns were similar to the larger overall cohort, with most patients having upper abdominal disease (68%). Patients with differentiated, proliferative, or immunoreactive subtypes were less likely to have upper abdominal or miliary disease compared to patients with mesenchymal subtype (77%, 70%, 69% vs. 90%, respectively, P<0.05). Nearly all patients (90%) with mesenchymal subtype had either upper abdominal or miliary disease (Table 3).
Table 3.
Association between HGSOC molecular subtype and presence of upper abdominal disease or miliary disease among 334 high-grade serous ovarian cancer patients with molecular profiling available.
| HGSOC molecular subtype | No. (%) with upper abdominal disease or miliary disease | Odds ratio (95% CI) | P |
|---|---|---|---|
| Mesenchymal (N=94) | 85 (90.4%) | Reference | |
| Differentiated (N=73) | 56 (76.7%) | 0.35 (0.15–0.84) | 0.02 |
| Proliferative (N=92) | 64 (69.6%) | 0.24 (0.11–0.55) | <0.001 |
| Immunoreactive (N=75) | 52 (69.3%) | 0.24 (0.10–0.56) | <0.001 |
Abbreviations: CI, confidence interval
Given the differing rates of upper abdominal or miliary IP disease spread between mesenchymal and all other subtypes, we combined proliferative, differentiated, and immunoreactive subtypes into one category (non-mesenchymal) to investigate the impact of mesenchymal on RD0 rates (due to the clear survival benefit of a complete resection). Patients with mesenchymal subtype were significantly less likely to be resected to RD0 compared to non-mesenchymal subtypes (13% vs. 38%, OR 0.25, 95% CI 0.13–0.49, P<0.001) (Supplementary Figure 1). Even after adjusting for IP disease dissemination pattern (pelvic vs. lower abdominal vs. upper abdominal or miliary) the association between mesenchymal subtype and RD0 resectability of disease remained significant (adjusted OR 0.28, 95% CI 0.13–0.60, P<0.001). These results demonstrate that molecular subtype impacts both IP disease dissemination pattern and RD independently.
4. DISCUSSION
Our study investigated the associations among IP disease dissemination patterns, molecular subtypes, and RD in patients with advanced HGSOC. Our study uniquely includes detailed information on surgical findings and disease spread patterns. We observed that patients with the mesenchymal subtype of HGSOC were more likely to have upper abdominal or miliary disease when compared to patients with other subtypes (Table 3). Additionally, we demonstrated that patients with upper abdominal or miliary disease were less likely to be completely resected despite higher surgical complexity (Figures 2, 3). After adjusting for IP disease dissemination patterns, we further observed that patients with the mesenchymal subtype of HGSOC remained less likely to achieve a complete resection compared to non-mesenchymal subtypes. Collectively, these results demonstrate independent complex relationships among molecular subtypes, IP disease dissemination patterns, and resectability of disease.
We primarily utilized previously defined classification systems to divide IP disease dissemination patterns into four mutually exclusive categories [7–10]. However, we considered miliary disease as a separate category to uniquely capture patients with diffuse, non-bulky disease that is clinically challenging to resect to RD0. As previously reported, we confirm that upper abdominal disease is more difficult to completely resect despite higher surgical complexity (Figure 2, 3)[7–10]. Since many patients with miliary disease are likely to have disease in the upper abdomen, particularly the diaphragm, we reasoned that miliary disease would be as difficult to completely resect. In fact, our results importantly add that patients with miliary disease have rates of RD0 similar to those with upper abdominal disease. Taken together, patients with either upper abdominal or miliary disease represented the majority (90%, 412/460) of our patients with RD>0 cm. The similarity in surgical outcomes among patients with upper abdominal and miliary disease likely explains why both IP disease dissemination patterns have been associated with worse PFS and OS [7, 15].
The major strength of this study is the use of a large single institution surgical database with extremely detailed information on disease burden and resectability of disease. The surgical database is prospectively collected and describes location and size of disease at the beginning and end of PDS. To our knowledge, it is the most detailed surgical database of patients with molecular subtyping. The surgical complexity system we utilized has been used to demonstrate the benefits of aggressive surgical efforts to achieved RD0[2, 6], and the RD groups we chose consistently reflect progressive differences in survival outcomes[6, 16]. Detailed operative reports were used to assign patients to one of four mutually exclusive IP disease dissemination patterns. All assignments were performed by the same gynecologic oncologist (DT) and inter-observer variability with another gynecologist oncologist (SW) was strongly concordant. Our large sample of patients was limited to patients with HGSOC because it’s the most common subtype of EOC and the only subtype with molecularly profiled validation cohorts. Molecular profiling was performed as previously described and our technique has been validated in public cohorts [11, 13].
Weaknesses of this study include the retrospective design, particularly the use of operative reports to define IP disease dissemination patterns. Despite the detail of most operative reports used to group IP disease dissemination, the relative bulk of disease at each site was sometimes unclear and analyses were limited to either presence or absences of disease in the organs listed in Table 1. Additionally, it was difficult to retrospectively differentiating bulky (upper abdominal IP disease dissemination) from non-bulky (miliary IP disease dissemination) upper abdominal disease. Despite substantial concordance between the two surgeons that performed the data abstraction, seven of eight disagreements were between miliary versus upper abdominal IP disease dissemination patterns but all discordances were resolved after a joint discussion and review of the operative reports. In total the number of patients in our entire cohort with miliary disease was small and these patients were more likely to have large volume ascites and the diagnosis of primary peritoneal carcinoma. Miliary disease has been associated with worse survival outcomes [15] and 50% of our patients with miliary disease had mesenchymal molecular profiles. Based on all these factors and our report that miliary disease is less likely to achieve RD0 despite higher surgical complexity, we believe we objectively captured the majority of patients with miliary disease. Furthermore, we have stressed the similarity in poorer surgical outcomes between upper abdominal and miliary disease and grouped these two categories together to collectively emphasize the higher likelihood of patients with upper abdominal or miliary disease having a mesenchymal molecular subtype. Future studies should consider prospectively documenting miliary disease patterns, as recommended by the NCNN, and focusing on more recent surgeries to reflect current surgical practice; e.g. suboptimal RD rate in our institution has progressively decreased over time[6]
In summary, molecular subtypes were associated with IP disease dissemination patterns and surgical outcomes in HGSOC. The mesenchymal subtype was independently associated with the presence of upper abdominal or miliary disease and lower rates of complete resection; both associations at least partially explain the poorer OS associated with the mesenchymal subtype. Resecting upper abdominal or miliary disease is more likely to require radical procedures. Thus, preoperative molecular profiling from CT or ultrasound guided tumor biopsies or cytology from paracentesis specimens may serve as an adjunct to radiologic imaging and intraoperative laparoscopic scoring systems to assist gynecologic oncologists determine resectability of disease, assist in preparing for a complex PDS, or in referring the patient to an expert center to optimize intra and postoperative care. Since survival outcomes improve across all molecular subtypes as RD progressively decreases[13], it is critical to identify which subgroup of patients with a particular molecular subtype are least likely to achieve an optimal PDS and require complex perioperative care. Future studies should focus on the role of molecular subtype as biomarker to predict RD, particularly in patients at high-risk of suboptimal PDS, to assist gynecologic oncologists in the counseling on PDS vs. NACT in patients with newly-diagnosed advanced stage disease.
Supplementary Material
HIGHLIGHTS.
Patients with upper abdominal and miliary disease have similar RD0 rates
IP disease dissemination patterns are associated with molecular subtypes
>90% of patients with MES tumors have either upper abdominal or miliary disease
Patients with MES tumor subtype were significantly less likely to achieve RD0
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
None of the authors have any conflicts of interest to declare.
References
- 1.Howlader N, NA, Krapcho M, Miller D, Bishop K, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA. SEER Cancer Statistics Review, 1975–2013. National Cancer Institute; Bethesda, MD: http://seer.cancer.gov/csr/1975_2013/, based on November 2015 SEER data submission, posted to the SEER web site, April 2016. [Google Scholar]
- 2.Aletti GD, et al. Aggressive surgical effort and improved survival in advanced-stage ovarian cancer. Obstetrics and gynecology. 2006;107(1):77–85. doi: 10.1097/01.AOG.0000192407.04428.bb. [DOI] [PubMed] [Google Scholar]
- 3.Heintz AP, et al. Carcinoma of the ovary. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006;95(Suppl 1):S161–92. doi: 10.1016/S0020-7292(06)60033-7. [DOI] [PubMed] [Google Scholar]
- 4.Ozols RF, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21(17):3194–200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- 5.Sioulas VD, et al. Optimal primary management of bulky stage IIIC ovarian, fallopian tube and peritoneal carcinoma: Are the only options complete gross resection at primary debulking surgery or neoadjuvant chemotherapy? Gynecologic oncology. 2017;145(1):15–20. doi: 10.1016/j.ygyno.2017.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wallace S, et al. Efforts at maximal cytoreduction improve survival in ovarian cancer patients, even when complete gross resection is not feasible. Gynecol Oncol. 2017;145(1):21–26. doi: 10.1016/j.ygyno.2017.01.029. [DOI] [PubMed] [Google Scholar]
- 7.Aletti GD, et al. Identification of patient groups at highest risk from traditional approach to ovarian cancer treatment. Gynecologic oncology. 2011;120(1):23–8. doi: 10.1016/j.ygyno.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Hamilton CA, et al. The impact of disease distribution on survival in patients with stage III epithelial ovarian cancer cytoreduced to microscopic residual: a Gynecologic Oncology Group study. Gynecologic oncology. 2011;122(3):521–6. doi: 10.1016/j.ygyno.2011.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horowitz NS, et al. Does aggressive surgery improve outcomes? Interaction between preoperative disease burden and complex surgery in patients with advanced-stage ovarian cancer: an analysis of GOG 182. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2015;33(8):937–43. doi: 10.1200/JCO.2014.56.3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sehouli J, et al. Intra-abdominal tumor dissemination pattern and surgical outcome in 214 patients with primary ovarian cancer. Journal of surgical oncology. 2009;99(7):424–7. doi: 10.1002/jso.21288. [DOI] [PubMed] [Google Scholar]
- 11.Konecny GE, et al. Prognostic and therapeutic relevance of molecular subtypes in high-grade serous ovarian cancer. Journal of the National Cancer Institute. 2014;106(10) doi: 10.1093/jnci/dju249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tothill RW, et al. Novel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcome. Clinical cancer research: an official journal of the American Association for Cancer Research. 2008;14(16):5198–208. doi: 10.1158/1078-0432.CCR-08-0196. [DOI] [PubMed] [Google Scholar]
- 13.Wang C, et al. Pooled clustering of high-grade serous ovarian cancer gene expression leads to novel consensus subtypes associated with survival and surgical outcomes. Clin Cancer Res. 2017 doi: 10.1158/1078-0432.CCR-17-0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aletti GD, et al. Relationship among surgical complexity, short-term morbidity, and overall survival in primary surgery for advanced ovarian cancer. American journal of obstetrics and gynecology. 2007;197(6):676 e1–7. doi: 10.1016/j.ajog.2007.10.495. [DOI] [PubMed] [Google Scholar]
- 15.Eng KH, et al. Prognostic value of miliary versus non-miliary sub-staging in advanced ovarian cancer. Gynecologic oncology. 2017 doi: 10.1016/j.ygyno.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chi DS, et al. What is the optimal goal of primary cytoreductive surgery for bulky stage IIIC epithelial ovarian carcinoma (EOC)? Gynecol Oncol. 2006;103(2):559–64. doi: 10.1016/j.ygyno.2006.03.051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


