Abstract
Bioorthogonal fluorogenic reactions serve as enabling tools in research and biotechnology. Herein we describe fluorogenic conjugations of semicarbazide with courmarin derivatives that incorporate a 2-acetylphenylboronic acid motif. These designed courmarins rapidly conjugate with semicarbazide to give diazaborine products with significantly enhanced fluorescence. To demonstrate potential applications of this fluorogenic reaction, we synthesized a semicarbazide-presenting amino acid D-Dap-Scz, which readily incorporates into the cell wall of Staphalococcus aureus and serves as a handle for conjugation with the coumarins. The fluorogenic conjugation of the coumarins to cell surface semicarbazide enables facile visualization of D-Dap-Scz treated bacteria.
Graphical abstract
Molecular probes that turn on fluorescence in response to a target are of great utility in biological research and medical applications.1–6 Such probes have been successfully developed for various metal ions and small molecule messengers, which can elicit a fluorescence increase through coordination or conjugation with a properly designed probe. Fluorogenic substrates have also been used for the study of various enzymes, which can catalyse formation of a fluorescent product.7 In contrast, it had been challenging to develop specific, fluorogenic probes for biomolecules with no catalytic function.
The recent development of bioorthogonal conjugation chemistry has made it possible to fluorescently label biomolecules with or without enzymatic activities.8–11 Typically a small reactive handle is introduced into the target molecule through natural or engineered metabolic processes. Then, a properly derivatized fluorophore can be conjugated to the reactive handle in a bioorthogonal manner. This strategy has found numerous applications in tracking specific proteins, as well as carbohydrate metabolism in live cells. However, a limitation of this approach lies in the need of excess labelling reagents, which often give rise to high background fluorescence. Bioorthogonal conjugations that are fluorogenic12 would overcome this limitation and greatly expand the scope of applications in biology.
We recently reported a novel bioorthogonal conjugation reaction in which semicarbazide reacts with 2-acetylphenyl boronic acid (2-APBA) to give a diazaborine (Figure 1).13 This reaction is fast (>103 M−1s−1) under physiological conditions, encountering little interference by serum or cell lysates. Importantly, little toxicity is observed towards mammalian or bacterial cells. Herein we report a fluorogenic version of the diazaborine conjugation chemistry, which is made possible with quenched coumarin probes (Figure 1). The potential for biological applications of this fluorogenic reaction is demonstrated by the convenient and specific visualization of semicarbazide-presenting bacterial cells.
Fig 1.
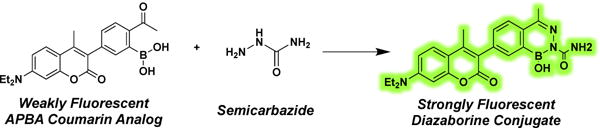
Schematic of the reaction of a coumarin derivative (AB21) with semicarbazide to generate a highly fluorescent diazaborine conjugate.
To create fluorogenic, diazaborine-forming probes, we synthesized several coumarin derivatives (AB21-24) that either carry a 2-APBA motif or its structural analogues at the C3 position (Figure 2a). AB21 and AB22 are expected to conjugate with semicarbazide to form diazaborines, while AB23 and AB24 are designed to serve as negative controls. The coumarin core structure has been widely used in the design of fluorescent molecules as its fluorescence can be easily tuned with electron donating and withdrawing groups.14–18 The synthetic details of the coumarins are given in the Supporting Information. With the compounds in hand, we first characterized their absorption and fluorescence properties. The results show that all four coumarin derivatives exhibit similar absorption profiles with absorption maxima around 395 nm (Figure 2b). Interestingly, the fluorescence properties of AB21-4 were found to differ dramatically (Figure 2c): while AB24 fluoresces strongly, AB21 and AB22 give minimal fluorescence and AB23 lies in between. The varying fluorescence intensities correlate well with the electron withdrawing capabilities of the C3 substituents: the 2-APBA motif of AB21 and AB22 is the most electron deficient and the phenol substituent of AB24 is electron rich.
Fig 2. Coumarin derivatives display variable fluorescence properties.
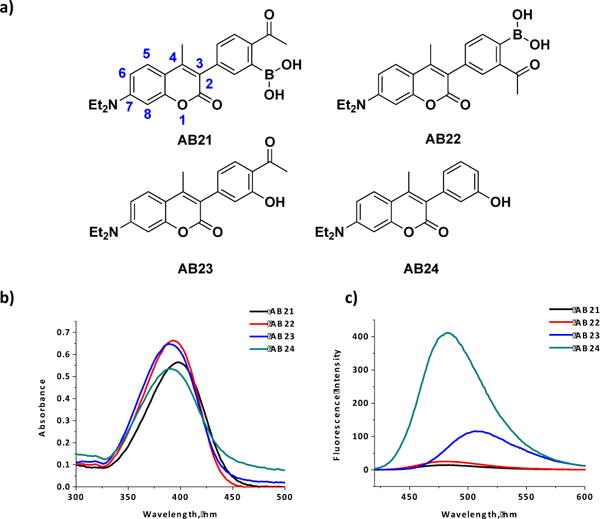
(a) Structures of the coumarin derivatives AB21, AB22, AB23 and AB24. Numbering of the coumarin core structure is shown on AB21. (b) Coumarin derivatives displaying similar absorbance properties (50 μM, 1 cm cuvette, 1X PBS Buffer, pH 7.4).(c) Fluorescence emission of the coumarin derivatives showing quenched fluorescence of AB21 and AB22 (10 μM, 1X PBS Buffer, pH 7.4, λex= 392 nm (AB22/24), 397 nm (AB21) and 402 nm (AB23)).
The quenching effect of 2-APBA suggests that AB21 and AB22 can display turn-on fluorescence upon chemical transformations, such as the diazaborine formation with semicarbazide. To test this hypothesis, the coumarin derivatives were first examined for their ability to conjugate with semicarbazide. As shown by the LC-MS results (Figure 3a), 30 min incubation of AB21 or AB22 with semicarbazide (200 μM each) afforded quantitative conversion to the corresponding diazaborine products. Consistent with our previous report,13 the conjugation reaction readily proceeds even in the presence of 20% blood serum, although a slight decrease of conjugation yield was observed (Figure S1). To determine the postulated fluorogenic property of AB21/AB22, the conjugation products were diluted 20x to 10 μM for fluorescence measurement (Figure 3b). Satisfyingly, both coumarin derivatives displayed significant fluorescence increase: semicarbazide conjugation of AB21 elicited a 5 fold increase in fluorescence emission, while AB22 gave a fluorescence enhancement of 7 times. For comparison, AB23 and AB24 showed no change in fluorescence emission when mixed with semicarbazide, consistent with their inability react with semicarbazide (Figure S2). The fluorogenic properties of AB21 and AB22 allowed facile quantification of the conjugation kinetics, yielding rate constants of 144 M−1·s−1 and 177 M−1·s−1 for AB21 and AB22 respectively. Although slightly slower than that of 2-APBA alone (Figure S3),13 the diazaborine formation of AB21/22 is still remarkably fast with the reaction reaching completion in just a few minutes with low micromolar concentrations of reactants.
Fig 3. Diazaborine formation of AB21/22 with semicarbazide induces an increase in fluorescence.
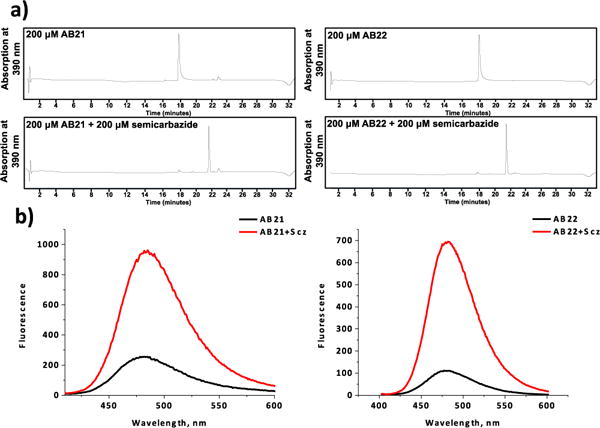
(a) LC traces showing clean and complete conjugation of AB21/22 (200 μM) to semicarbazide (200 μM) in 30 minutes. The identity of the peaks were confirmed with mass-spec data. (b) Conjugation to semicarbazide leading to much enhanced fluorescence of AB21 and AB22 (10 μM, 1x PBS Buffer, pH 7.4, λex= 392 nm (AB22) and 397 nm (AB21)).
The fast and fluorogenic conjugation of AB21/22 with semicarbazide makes them appealing for biological applications. We set out to probe the potential of this fluorogenic conjugation for labeling bacterial pathogens. Specific labeling and detection of pathogenic bacteria in complex biological milieu is highly desirable towards quick diagnosis and effective treatment of bacterial infections. A recently developed and powerful approach to bacterial labeling relies on peptidoglycan remodelling with D-amino acids. The work of several groups including our own11 has revealed a number of unnatural amino acids that can be incorporated into various bacterial species through this mechanism.19–23 We envisioned that fluorogenic diazaborine formation of AB21/AB22 can be implemented for bacterial cell labeling via a semicarbazide-presenting D-amino acid (Figure 4a). Toward this end, we have synthesized the unnatural amino acid D-Dap-Scz, which was obtained by conjugating a semicarbazide derivative to Boc-D-Dap-OH through formation of an amide bond (Figure S4). With the amino acid synthesized, we tested the conjugation of D-Dap-Scz to AB21 and AB22 (Figure S5), as well as the fluorescence increase upon conjugation (Figure S6), both of which agreed with the results obtained for semicarbazide (Figure 3).
Fig 4. APBA coumarin derivatives efficiently label S. aureus membranes without washing.
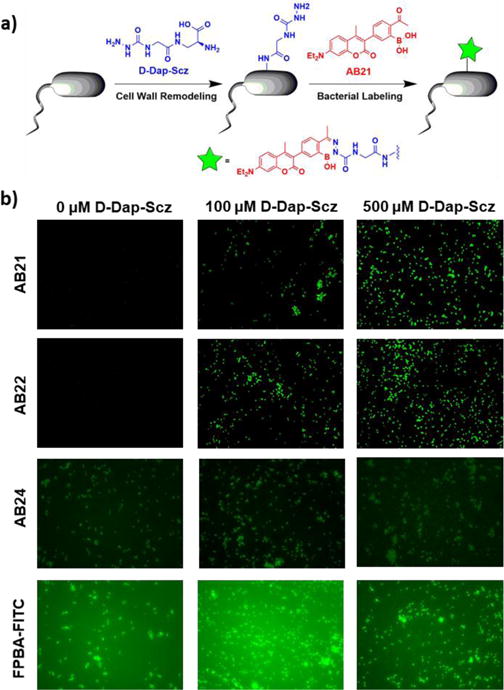
(a) Schematic two-step labelling process involving 1) D-Dap-semicarbazide incorporation into peptidoglycan followed by 2) APBA coumarin conjugation to form a stable diazaborine. (b) S. aureus incubated with 0 μM, 100 μM or 500 μM D-Dap-Semicarbazide for four hours followed by 100 μM AB21, AB22, AB24 or FPBA-FITC incubation for 2 hours. AB21 and AB22 showed dose dependent bacterial membrane labelling, whereas AB24 displayed no fluorescence labelling and FPBA-FITC gave significant background staining.
Encouraged by these results, we then examined the ability of D-Dap-Scz to incorporate into the cell walls of S. aureus, which is one of the most prevalent bacterial pathogens. Specifically, we incubated the bacteria with various concentrations of D-Dap-Scz (5–500 μM) for varied time period (0.5–4 hours).
The amount of D-Dap-Scz incorporation into peptidoglycan was assessed by using a fluorophore-labelled 2-formylphenylboronic acid (FPBA-FITC, Figure S7). 2-FPBA is the aldehyde analogue of 2-APBA, also known to quickly and quantitatively conjugate with semicarbazide to form diazaborines.13 The FPBA-FITC treated cells were analysed using flow cytometry to quantify the mean fluorescence intensity of the bacterial cells (Figure S8). The results show that the extent of bacterial incorporation of D-Dap-Scz depends on both concentration and incubation time. Efficient D-Dap-Scz incorporation was observed after 4 hours of incubation with D-Dap-Scz at 100 μM concentration or higher (Figure S8). Specifically, with 4 hours of incubation, FBPA-FITC afforded proportionally strong fluorescence staining of the cells treated with D-Dap-Scz at 100 and 500 μM respectively. These conditions were then adopted to examine the fluorogenic conjugation of the coumarin derivatives on live bacterial cell surface. Briefly, S. aureus cells were treated with 0, 100, and 500 μM of D-Dap-Scz in LB media for 4 hours, then the cells were stained with AB21 and AB22. FPBA-FITC and AB24 were tested in parallel as non-fluorogenic controls. Although, with wash, FPBA-FITC allowed facile visualization of the D-Dap-Scz treated cells (Figure S8), strong background fluorescence was observed without the washing step (Figure 4b). Even worse, FPBA-FITC afforded significant staining of the cells without D-Dap-Scz treatment. Similarly, background fluorescence and nonspecific cell staining were observed for AB24 as well under the no-wash conditions, although AB24 is not as bright as FPBA-FITC. In sharp contrast, little background fluorescence was observed with the AB21/22 stained cells under the same setting. Comparing S. aureus incubated with no D-Dap-Scz (0 μM) versus 100 μM and 500 μM D-Dap-Scz, it is apparent that AB21 and AB22 elicited bright fluorescence staining only in the presence of D-Dap-Scz (Figure 4b). The D-Dap-Scz-dependent bacterial staining by AB21 and AB22 is presumably due to their fluorogenic conjugation with D-Dap-Scz to form diazaborines. Although it is difficult to determine whether AB21 and AB22 afford non-specific bacterial binding, their quenched fluorescence allows them to only fluorescently reveal bacterial cells that incorporate semicarbazide functionalities.
In summary, we have designed and synthesized two fluorogenic coumarin derivatives (AB21 and AB22) that can conjugate with semicarbazide to give fluorescent diazaborines. The fluorogenic diazaborine formation proceeds under physiologic conditions, even in the presence of blood serum. We further demonstrate the utility of this fluorogenic reaction by devising a semicarbazide-presenting amino acid D-Dap-Scz, which incorporates into S. aureus via peptidoglycan remodelling. Owing to the fluorogenic diazaborine formation, S. aureus cells displaying semicarbazide can be fluorescently labelled with AB21 or AB22 with much improved signal to background ratios in comparison to the non-fluorogenic controls. To the best of our knowledge, the work described herein presents the first example of bacterial cell wall remodelling with semicarbazide, an α-nucleophile with little toxicity for both bacterial and mammalian cells.13 Furthermore, this contribution describes the first fluorogenic versions of the bioorthogonal conjugation of semicarbazide to form diazaborines, which we showed to be fast and highly biocompatible. We note that Gillingham and co-workers recently reported a similar fluorogenic diazaborine formation with ortho-hydroxy phenylhydrazine as reactants.24 In comparison, semicarbazide enjoys greater stability and lack of toxicity in biological applications.11 Overall, we believe the efficient bacterial labeling and the fluorogenic properties will make the semicarbazide-AB21/22 combination powerful tools for studying bacterial virulence in vitro and possibly in animal models.
Supplementary Material
Acknowledgments
This work is supported by the National Institutes of Health via grant GM102735 to JG.
Footnotes
Electronic Supplementary Information (ESI) available: details of small molecule synthesis and characterization, experimental details additional data of bacterial cell labeling. See DOI: 10.1039/x0xx00000x
References
- 1.Chan J, Dodani SC, Chang CJ. Nat Chem. 2012;4:973. doi: 10.1038/nchem.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jun ME, Roy B, Ahn KH. Chem Commun. 2011;47:7583. doi: 10.1039/c1cc00014d. [DOI] [PubMed] [Google Scholar]
- 3.Aron AT, Ramos-Torres KM, Cotruvo JA, Jr, Chang CJ. Acc Chem Res. 2015;48:2434. doi: 10.1021/acs.accounts.5b00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devaraj NK, Hilderbrand S, Upadhyay R, Mazitschek R, Weissleder R. Angew Chem Int Ed. 2010;49:2869. doi: 10.1002/anie.200906120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pitter DRG, Wigenius J, Brown AS, Baker JD, Westerlund F, Wilson JN. Org Lett. 2013;15:1330. doi: 10.1021/ol400268t. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y, Miao K, Dunham NP, Liu H, Fares M, Boal AK, Li X, Zhang X. Biochemistry. 2017;56:1585. doi: 10.1021/acs.biochem.7b00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Razgulin A, Ma N, Rao J. Chem Soc Rev. 2011;40:4186. doi: 10.1039/c1cs15035a. [DOI] [PubMed] [Google Scholar]
- 8.Prescher JA, Bertozzi CR. Nat Chem Biol. 2005;1:13. doi: 10.1038/nchembio0605-13. [DOI] [PubMed] [Google Scholar]
- 9.Bertozzi CR. Acc Chem Res. 2011;44:651. doi: 10.1021/ar200193f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahdavi A, Szychowski J, Ngo JT, Sweredoski MJ, Graham RLJ, Hess S, Schneewind O, Mazmanian SK, Tirrell DA. Proc Nat Acad Sci. 2014;111:433. doi: 10.1073/pnas.1301740111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du S, Wang D, Lee JS, Peng B, Ge J, Yao SQ. Chem Comm. 2017;53:8443. doi: 10.1039/c7cc04536k. [DOI] [PubMed] [Google Scholar]
- 12.Shieh P, Bertozzi CR. Org Biomol Chem. 2014;12:9307. doi: 10.1039/c4ob01632g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bandyopadhyay A, Cambray S, Gao J. J Am Chem Soc. 2017;139:871. doi: 10.1021/jacs.6b11115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sivakumar K, Xie F, Cash BM, Long S, Barnhill HN, Wang Q. Org Lett. 2004;6:4603. doi: 10.1021/ol047955x. [DOI] [PubMed] [Google Scholar]
- 15.Tran TM, Alan Y, Glass TE. Chem Commun. 2015;51:7915. doi: 10.1039/c5cc00415b. [DOI] [PubMed] [Google Scholar]
- 16.Li J, Zhang CF, Yang SH, Yang WC, Yang GF. Anal Chem. 2014;86:3037. doi: 10.1021/ac403885n. [DOI] [PubMed] [Google Scholar]
- 17.Wang J, Xie J, Schultz PG. J Am Chem Soc. 2006;128:8738. doi: 10.1021/ja062666k. [DOI] [PubMed] [Google Scholar]
- 18.Jiang X, Yu Y, Chen J, Zhao M, Chen H, Song X, Matzuk AJ, Carroll SL, Tan X, Sizovs A, Cheng N, Wang MC, Wang J. ACS Chem Biol. 2015;10:864. doi: 10.1021/cb500986w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuru E, Hughes HV, Brown PJ, Hall E, Tekkam S, Cava F, de Pedro MA, Brun YV, VanNieuwenhze MS. Angew Chem Int Ed. 2012;51:12519. doi: 10.1002/anie.201206749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siegrist MS, Whiteside S, Jewett JC, Aditham A, Cava F, Bertozzi CR. ACS Chem Biol. 2013;8:500. doi: 10.1021/cb3004995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lebar MD, May JM, Meeske AJ, Leiman SA, Lupoli TJ, Tsukamoto H, Losick R, Rudner DZ, Walker S, Kahne D. J Am Chem Soc. 2014;136:10874. doi: 10.1021/ja505668f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pidgeon SE, Fura JM, Leon W, Birabaharan M, Vezenov D, Pires MM. Angew Chem Int Ed. 2015;54:6158. doi: 10.1002/anie.201409927. [DOI] [PubMed] [Google Scholar]
- 23.Sherratt AR, Chigrinova M, MacKenzie DA, Rastogi NK, Ouattara MT, Pezacki AT, Pezacki JP. Bioconjug Chem. 2016;27:1222. doi: 10.1021/acs.bioconjchem.6b00063. [DOI] [PubMed] [Google Scholar]
- 24.Stress CJ, Schmidt PJ, Gillingham DG. Org Biomol Chem. 2016;14:5529. doi: 10.1039/c6ob00168h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


