Abstract
The structurally related neuropeptides vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase-activating polypeptide (PACAP) have been implicated in stress regulation and learning and memory. Several bodies of research have shown the impact of the PACAP specific receptor PAC1 on fear memory, but the roles of other PACAP receptors in regulating fear stress responses remain to be elucidated. Here we aimed to investigate the effects of genetic deletion of VIPR2 encoding the VPAC2 receptor, which binds both VIP and PACAP, on fear-related memory and on dendritic morphology in the brain regions of the fear circuitry. Male VPAC2 receptor knockout (VPAC2-KO) and littermate wild-type control mice were subjected to Pavlovian fear conditioning paradigm. VPAC2-KO mice displayed normal acquisition of fear conditioning, contextual and cued fear memory, but impaired extinction of cued fear memory. Morphological analyses revealed reductions in cell body size and total branch number and length of apical and basal dendrites of prelimbic cortex neurons in VPAC2-KO mice. In addition, Sholl analysis indicated that the amount of dendritic material distal to the soma was decreased, while proximal dendritic material was increased. In the infralimbic cortex, the amount of apical dendritic material proximal to the soma was increased in VPAC2-KO mice, while other indices of axonal morphology did not differ. Finally, there were no differences in dendritic morphology in basolateral amygdala neurons between genotypes. These findings suggest that the VPAC2 receptor plays an important role in the fear extinction processes and the regulation of the dendritic morphology in the prelimbic and infralimbic cortices.
Keywords: VPAC2 receptor (VIPR2), fear conditioning, extinction, dendritic morphology, prelimbic and infralimbic cortices
1. Introduction
Fear represents a natural emotion based on a perception of a threat. However, dysfunction in the fear system can produce inappropriate and excessive fear responses that lead to psychiatric diseases, such as anxiety disorders (Dymond, Dunsmoor, Vervliet, Roche, & Hermans, 2015), post-traumatic stress disorder (PTSD) (Maren, Phan, & Liberzon, 2013), autism spectrum disorder (Evans, Canavera, Kleinpeter, Maccubbin, & Taga, 2005; Gillott, Furniss, & Walter, 2001), and schizophrenia (Holt et al., 2009; Holt, Coombs, Zeidan, Goff, & Milad, 2012). Pavlovian fear conditioning in rodents has been widely used to understand mechanisms that underlie distinct components of learning and memory (Fanselow & Wassum, 2015; Herry & Johansen, 2014, Sanders, Wiltgen, & Fanselow, 2003; Tovote, Fadok, & Lüthi, 2015). The study of fear behavior in rodents can provide a valuable platform to study how various gene mutations linked to psychiatric diseases may lead to deficits in cognition. Importantly, many of the implicated genes encode proteins believed to play common, yet incompletely understood roles in the development of neural circuits and in synapse formation, maintenance and plasticity. Therefore, identification and investigation of these processes and the mechanisms that control them may eventually provide new opportunities for early identification and therapeutic intervention for these diseases.
Pituitary adenylate cyclase-activating polypeptide (PACAP) is a member of the secretin/glucagon/vasoactive intestinal peptide (VIP) family and it functions as a hypothalamic hormone, neurotransmitter, neuromodulator, and neuroprotective/regeneration factor (Falluel-Morel et al., 2007; Harmar et al., 2012; Hashimoto et al., 2011; Tan & Waschek 2011; Vaudry et al., 2009; Waschek, 1996, 2002). The diverse activities of PACAP are mediated by G protein-coupled receptors, including a PACAP-specific receptor (PAC1) and two other receptors (VPAC1 and VPAC2), shared with the VIP (Hashimoto, Shintani, & Baba, 2006; Waschek, 2013). PACAP, VIP, and their receptors are widely distributed in the central nervous system, with high expression levels shown within the brain regions of the fear circuitry including the amygdala, prefrontal cortex, and hippocampus (Condro et al., 2016; Hashimoto et al., 1996; Joo et al., 2004; Kalló, Kalamatianos, Piggins, & Coen, 2004; Marzagalli et al., 2016; Sheward, Lutz, & Harmar, 1995; Vaudry et al., 2009; Vertongen, Schiffmann, Gourlet, & Robberecht, 1997). The VPAC2 receptor gene was linked in large genetic studies to schizophrenia in 2011 (Levinson et al., 2011; Vacic et al., 2011), whereas the PACAP–PAC1 pathway in the same year was linked to PTSD diagnosis and symptom severity in females (Ressler et al., 2011; Wang et al., 2013). The PAC1 receptor gene polymorphism has been found to impact conditioned fear behaviors in humans via its effects on the neural circuitry that regulates fear responses (Pohlack et al., 2015; Stevens et al., 2014). In rodent models, mutant mouse strains harboring a global loss or a forebrain-specific inactivation of the PAC1 receptor showed deficits of contextual, but not cued, fear memory (Otto et al., 2011), whereas mice lacking the ligand (PACAP) showed impairment of contextual fear memory while fear acquisition was intact (Takuma et al., 2014b). Other studies suggest that other PACAP receptors, including VPAC1 and VPAC2 might also be critically involved in fear behaviors. For example, VIP knockout mice exhibited impaired recall of contextual fear conditioning (Chaudhury, Loh, Dragich, Hagopian, & Colwell, 2008) and intracerebroventricular (icv) injection of VIP facilitated extinction of active and passive avoidance behavior (Cottrell, Veldhuis, Rostene, & de Kloet, 1984). Yet other studies seem to specifically implicate a specific involvement of the VPAC1 receptor. In this regard, PACAP was shown to facilitate neurotransmission at excitatory glutamatergic projections from the basolateral nucleus to the lateral division of the central nucleus of the amygdala, a projection known to be implicated in responses to innately aversive stimuli (Tye et al., 2011), through activation of the VPAC1 receptor (Cho et al., 2012). Finally, icv injection of PACAP to rats just prior to conditioning reduced freezing in contextual fear memory tests at 1 and 7 days, but enhanced freezing at 14 days, although the responsible receptor subtypes were not examined (Meloni, Venkataraman, Donahue, & Carlezon, 2016). However, the role of VPAC2 receptors in fear responses and the associated neural circuitry remain to be understood.
Here we aimed to investigate the roles of the VPAC2 receptor in fear acquisition, contextual and cued fear memory, and cued fear extinction using VPAC2 receptor knockout (VPAC2-KO) mice. We observed that VPAC2-KO mice exhibited a deficit of fear extinction, whereas fear learning processes were intact. We thus subsequently analyzed the dendritic morphology in the prelimbic (PrL) and infralimbic (IL) cortex and basolateral amygdala (BLA), the critical sites of plasticity for fear extinction (Izquierdo, Wellman, & Holmes, 2006; Maroun et al., 2013; Moench, Maroun, Kavushansky, & Wellman, 2015; Orsini & Maren, 2012; Quirk & Mueller, 2008; Wellman et al., 2007).
2. Materials and methods
2.1. Animals
All animal studies were approved by the Animal Research Committee at University of California, Los Angeles (UCLA) and the Animal Care and Use Committee of the Graduate School of Pharmaceutical Sciences, Osaka University. All experimental procedures were conducted in accordance with the guidelines of the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996). Every effort was made to minimize animal suffering, and to reduce the number of animals used. The generation of VPAC2-KO mice was described previously (Harmar et al., 2002). VPAC2 receptor null and littermate wild-type control mice obtained by interbreeding C57BL/6 serially-backcrossed VPAC2 receptor heterozygous mice were used and compared in all studies. Mice were housed in clear cages in groups of 3–5 animals under controlled environmental conditions (12-h light-dark cycle, lights on at 0700 h; food and water ad libitum). All experiments were conducted on 3−4 months-old male mice between 10 AM and 4 PM during the lights-on phase. Experimenters were blinded to the genotype during testing. All behavioral tests were performed in the UCLA behavioral test core.
2.2. Fear conditioning test
All behavioral testing was performed using four identical fear conditioning chambers (30 cm × 24 cm × 21 cm, Med-Associates, Inc. St. Albans, VT), equipped with a MedAssociates VideoFreeze system as previously reported (Ago et al., 2015). Tone conditioning on Day 1 in the conditioning chamber (Context A) consisted of a 150-s baseline period followed by five (Fig. 1) or three (Fig. 2) tones (30 s, 2.8 kHz, 80 dB) paired with electric footshock (2 s, 0.5 mA) that began immediately after the offset of each tone presentation, with a 90-s inter-trial interval in between the termination of each shock and the onset of the following tone. Freezing was recorded during both the baseline period and each tone period. Twenty-four hours later (Day 2), mice were placed in the same conditioning chamber (Context A) for an 8-min context fear test. No stimuli were presented during this period. Freezing was recorded throughout the 8-min test. In the cued fear test on Day 3, mice were placed in the chamber with a dark roof-like triangular ceiling and grid floor covering (Context B) and allowed to explore the novel environment for 150 s, and then five or three tones (30 s, 2.8 kHz, 80 dB) were presented, each spaced by a 90-s inter-trial interval. Freezing was recorded during both the baseline period and each tone period. Data are expressed as the average of five or three tones. The extinction phase started in Context B 24 h following the cued fear test. After 3-min of exploration, the mice were exposed to 20 tones (30 s, 2.8 kHz, 80 dB) with a 5-s inter-trial interval each day for two consecutive days (Day 4 (extinction learning) and Day 5 (extinction recall)). Mice were removed from the chamber 3 min after the final cue presentation. Freezing was recorded during both the baseline period and each tone period. Data are expressed as four bins from the average for every five tones.
Figure 1.
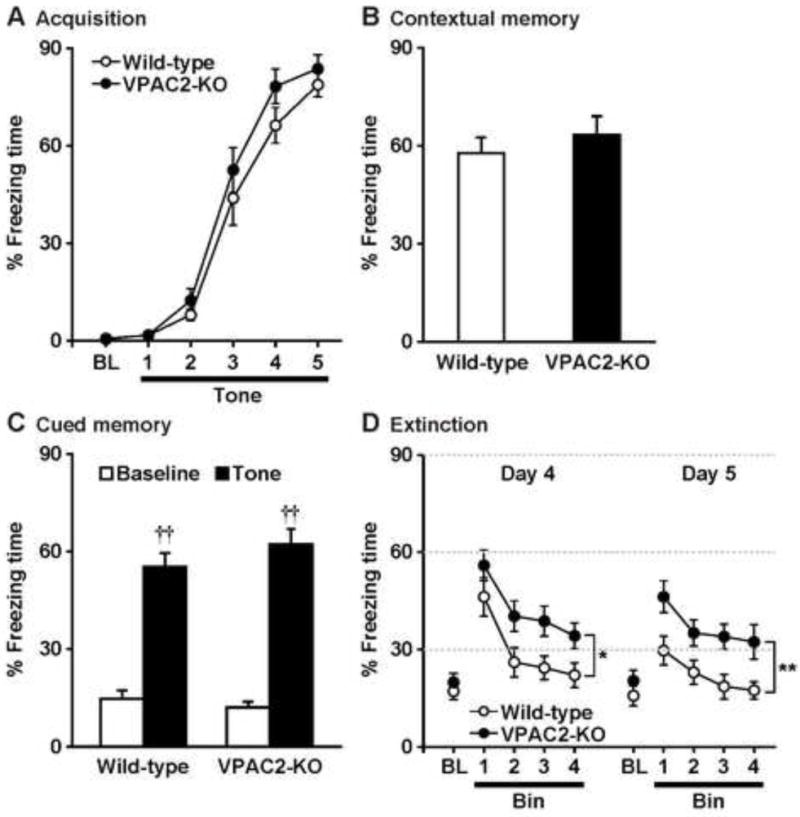
Freezing behaviors of VPAC2-KO mice in the fear conditioning test. On Day 1, mice were trained with 5 tone-shock pairings (A). Then, mice were subjected to the contextual fear memory test on Day 2 (B), cued fear memory test on Day 3 (C), and cued fear extinction paradigm on Days 4 and 5 (D). Results are expressed as the mean ± S.E.M. of 14 mice per group. BL: Baseline. ††P < 0.01, compared with the baseline. *P < 0.05, **P < 0.01, compared with wild-type.
Figure 2.
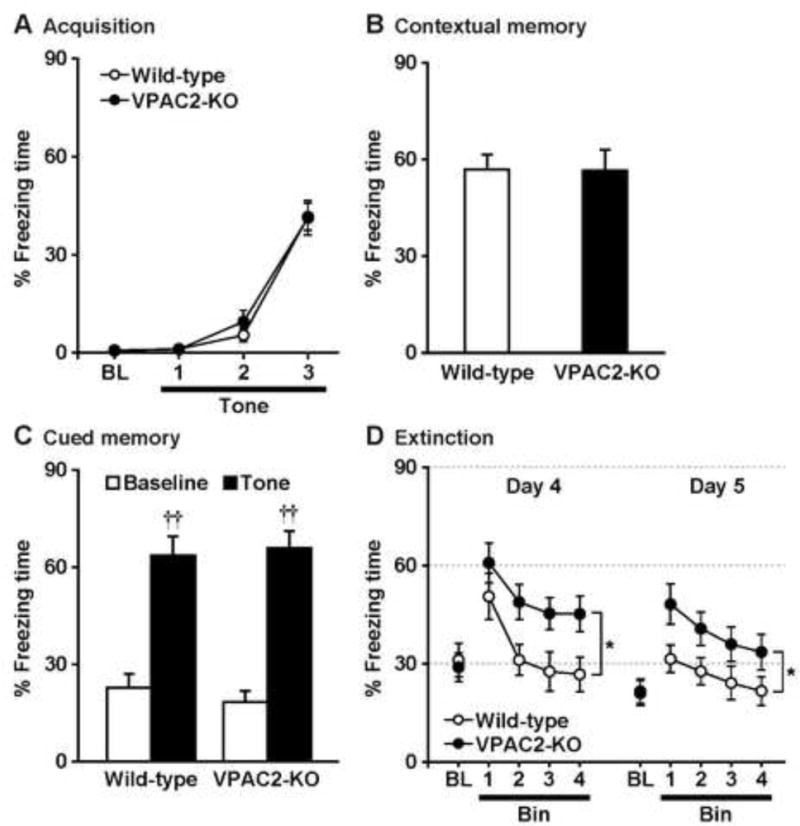
Defective fear extinction in low shock-conditioned VPAC2-KO mice. On Day 1, mice were trained with 3 tone-shock pairings (A). Then, mice were subjected to the contextual fear memory test on Day 2 (B), cued fear memory test on Day 3 (C), and cued fear extinction paradigm on Days 4 and 5 (D). Results are expressed as the mean ± S.E.M. of 12 mice per group. BL: Baseline. ††P < 0.01, compared with the baseline. *P < 0.05, compared with wild-type.
2.3. Histology and dendritic analyses
Dendritic morphological analysis was performed on fear conditioning- and test-naïve animals. Golgi-Cox impregnation was performed using the FD Rapid GolgiStain™ Kit (FD Neurotechnologies Inc., Ellicott, MD, USA) as previously described (Fujita et al., 2017; Hara et al., 2016; Takuma et al., 2014a). Briefly, mice were deeply anesthetized with isoflurane, and their brains were removed, rinsed with Milli-Q water, and immersed in the impregnation solution composed of potassium dichromate, mercuric chloride and potassium chromate. The brains were stored at room temperature for 2 weeks and then transferred and stored in the cryoprotectant solution for 5 days in the dark. The impregnated brains were embedded in 3.5% agarose gel and cut with a vibratome (VT1000S; Leica Microsystems) at room temperature. Coronal sections of 100 μm thickness were mounted on Gelatin-Coated Microscope Slides (FD Neurotechnologies Inc.), and dried naturally at room temperature in the dark for 24 h. After drying, the sections were rinsed with Milli-Q water, reacted in the working solution, and dehydrated with a 50%, 75%, 95%, and 100% graded ethanol series. Finally, the sections were defatted in xylene and coverslipped using Mount Quick (Daido Sangyo, Saitama, Japan). Digitized images from the PrL cortex (+2.0 through +1.7 mm with respect to bregma; Franklin & Paxinos, 1997) and IL cortex (+1.6 through +1.3 mm with respect to bregma) and BLA (−1.2 through −1.6 mm with respect to bregma) were obtained with an upright light microscope with a cooled CCD digital camera system (Axio Imager.M2/AxioCam MRc5; Carl Zeiss, Jena, Germany). A 20× lens was used to measure dendrites. Only fully impregnated neurons that displayed dendritic trees without obvious truncation and that were isolated from neighboring impregnated neurons were retained for analysis. Sixty pyramidal neurons with the soma in layer II/III were selected in the PrL cortex and thirty IL pyramidal neurons were sampled from all cortical layers from 3 mice per group (Cook & Wellman, 2004; Izquierdo, Wellman, & Holmes, 2006; Radley et al., 2004). For BLA, 8–10 pyramidal neurons and 15–17 stellate neurons were sampled from 3 mice per group (Lau, Bigio, Zelli, McEwen, & Nasca, 2017; Vyas, Mitra, Shankaranarayana Rao, & Chattarji, 2002). Morphology of apical and basal dendrites was quantified in three dimensions using Neurolucida neuron tracing system (MBF Bioscience, Williston, VT) with the experimenter blind to genotype. Cell body size and total length and branch number of apical and basal dendrites were compared across genotypes. To assess differences in the amount and location of dendritic material, Sholl analysis was performed using NeuroExplorer (MBF Bioscience).
2.4. Statistical analysis
All data are expressed as the mean ± standard error of the mean (S.E.M.). Data for fear acquisition, cued fear memory and fear extinction were analyzed using two-way analysis of variance (ANOVA) with genotype as the intersubject factor and repeated measures with tone exposure as the intrasubject factor, followed by the Tukey–Kramer post hoc test. Data for contextual test were analyzed by Student’s t-test. For dendritic morphology, cell body size, total number of dendrites and dendritic length were analyzed by Student’s t-test. For Sholl analysis, data were analyzed using two-way analysis of variance (ANOVA) with genotype as the intersubject factor and repeated measures with distance from soma as the intrasubject factor, followed by the Tukey–Kramer post hoc test. Statistical analyses were made using a software package StatView® 5.0 for Windows (SAS Institute, Cary, NC). A value of P < 0.05 was considered statistically significant.
3. Results
3.1. Fear behaviors in VPAC2-KO mice
Wild-type and VPAC2-KO C57BL/6 male mice obtained from the intercross of the heterozygous animals were used for the studies, and were performed at between 10 AM and 4 PM time of day to reduce any potential effects of parental genotype (Stack et al., 2008) or circadian timing (Harmar et al., 2002; Colwell et al., 2003), respectively. In the training phase of fear conditioning (Day 1), wild-type and VPAC2-KO mice were exposed to five trials of tone (conditioned stimulus), paired with an electric footshock (unconditioned stimulus) that began immediately after the offset of each tone presentation. During conditioning, both groups of mice showed similar levels of freezing (genotype, F1,26 = 2.3, P > 0.05; conditioning, F5,130 = 168.5, P < 0.0001; genotype × conditioning interaction, F5,130 = 0.7, P > 0.05) (Fig. 1A). After the conditioning day, we performed the contextual fear test on Day 2 (Fig. 1B) and the tone-cued fear test on Day 3 (Fig. 1C). There was no significant difference in contextual freezing responses under context A (P > 0.05) or tone-cued freezing responses under context B (genotype, F1,26 = 0.4, P > 0.05; tone, F1,26 = 186.8, P < 0.0001; genotype × tone interaction, F1,26 = 2.1, P > 0.05) between groups. We then exposed mice to repeated conditioned stimulus without unconditioned stimulus under context B on Day 4 (extinction learning; Fig. 1D). Both genotypes showed progressively decreased levels of freezing, but VPAC2-KO mice maintained persistently high levels of freezing compared with the wild-type mice. A repeated measures ANOVA revealed the significant main effects of the genotype (F1,26 = 5.7, P < 0.05) and repeated tone exposure (F3,78 = 23.8, P < 0.0001), but there was no significant interaction between the genotype and tone exposure (F3,78 = 0.3, P > 0.05). Next, 24 h after extinction learning was complete, mice were re-exposed to conditioned stimulus without unconditioned stimulus under context B on Day 5 (extinction recall; Fig. 1D). Cued tone stimulation still caused increases in freezing responses of both wild-type and VPAC2-KO mice, but VPAC2-KO mice showed high levels of freezing. Freezing behaviors of mice were gradually decreased and then reached a similar level as the baseline. There were significant main effects of the genotype (F1,26 = 10.3, P < 0.01) and repeated tone exposure (F3,78 = 7.6, P < 0.001), but there was no significant interaction between the genotype and tone exposure (F3,78 = 0.2, P > 0.05).
To eliminate the presence of a ceiling effect on the freezing response, we examined the performance of the two genotypes with a milder training protocol that consisted of 3 conditioned–unconditioned stimulus pairings with 0.5 mA shock (Fig. 2). The results were similar to those seen when stronger conditioning (above) was employed; VPAC2-KO mice showed normal conditioning (genotype, F1,22 = 0.3, P > 0.05; conditioning, F3,66 = 103.8, P < 0.0001; genotype × conditioning interaction, F3,66 = 0.3, P > 0.05), contextual fear memory (P > 0.05), cued fear memory (genotype, F1,22 = 0.03, P > 0.05; tone, F1,22 = 122.7, P < 0.0001; genotype × tone interaction, F1,22 = 0.7, P > 0.05), but impaired fear extinction on Day 4 (genotype, F1,22 = 5.1, P < 0.05; tone, F3,66 = 19.3, P < 0.0001; genotype × tone interaction, F3,66 = 0.8, P > 0.05) and Day 5 (genotype, F1,22 = 4.5, P < 0.05; tone, F3,66 = 7.9, P < 0.001; genotype × tone interaction, F3,66 = 0.4, P > 0.05).
3.2. Morphological analyses
Because medial prefrontal cortex including the PrL and IL cortex and BLA play an important role in the fear learning and extinction (Herry et al., 2010; Maren et al., 2013; Martel et al., 2012; Myers & Davis, 2002; Quirk & Mueller, 2008), we examined the dendritic morphology in these brain regions of VPAC2-KO mice. In the PrL cortex (Fig. 3), VPAC2-KO mice had smaller neuronal cell bodies than wild-type mice. In addition, reductions in the total branch number and length of apical and basal dendrites of PrL cortex neurons from VPAC2-KO mice were observed. Number of primary basal dendrites also decreased in VPAC2-KO mice. Sholl analysis revealed that apical dendritic material was increased proximal to the soma and decreased distal to the soma (genotype, F1,118 = 72.5, P < 0.0001; distance from soma, F9,1062 = 96.6, P < 0.0001; genotype × distance interaction, F9,1062 = 13.4, P < 0.0001). The amount of basal dendritic material proximal to the soma was decreased in VPAC2-KO mice (genotype, F1,118 = 54.9, P < 0.0001; distance from soma, F4,472 = 724.8, P < 0.0001; genotype × distance interaction, F4,472 = 19.9, P < 0.0001). In the IL cortex (Fig. 4), the amount of dendritic material proximal to the soma in apical, but not basal, dendrites was increased in VPAC2-KO mice (apical dendrites [genotype, F1,58 = 1.9, P > 0.05; distance from soma, F6,348 = 72.5, P < 0.0001; genotype × distance interaction, F6,348 = 2.4, P < 0.05]; basal dendrites [genotype, F1,58 = 0.01, P > 0.05; distance from soma, F6,348 = 234.8, P < 0.0001; genotype × distance interaction, F6,348 = 0.6, P > 0.05]). Cell body size and total branch number and length of apical and basal dendrites of IL cortex neurons were not altered in VPAC2-KO mice. On the other hand, there was no difference in cell body size or dendritic morphology in BLA pyramidal and stellate neurons between VPAC2-KO and wild-type mice (Fig. 5). For Sholl analysis, a repeated measures ANOVA revealed the significant main effect of the distance from soma (F5,75 = 128.6, P < 0.0001 for pyramidal; F5,150 = 248.5, P < 0.0001 for stellate), but not of the genotype (F1,15 = 2.3, P > 0.05 for pyramidal; F1,30 = 0.2, P > 0.05 for stellate), and there was no significant interaction between the genotype and distance (F5,75 = 0.9, P > 0.05 for pyramidal; F5,150 = 0.7, P > 0.05 for stellate).
Figure 3.
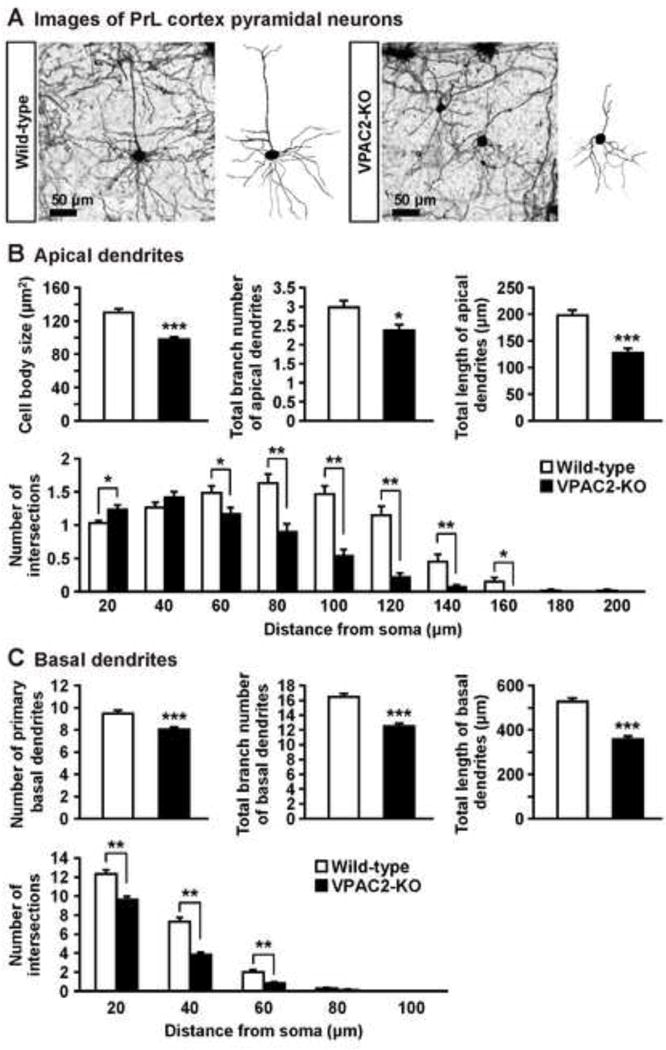
Dendritic morphology of PrL pyramidal neurons in VPAC2-KO mice. (A) Golgi-stained pyramidal neuron in PrL cortex and representative tracings of the dendrites of wild-type and VPAC2-KO mice. (B, C) Cell body size and total branch number and length of apical and basal dendrites are shown. The number of intersections of dendrites with 20 μm concentric spheres centered on the soma was measured by Sholl analysis. Results are expressed as the mean ± S.E.M. of 60 neurons from 3 mice per group. *P < 0.05, **P < 0.01, ***P < 0.001, compared with wild-type.
Figure 4.
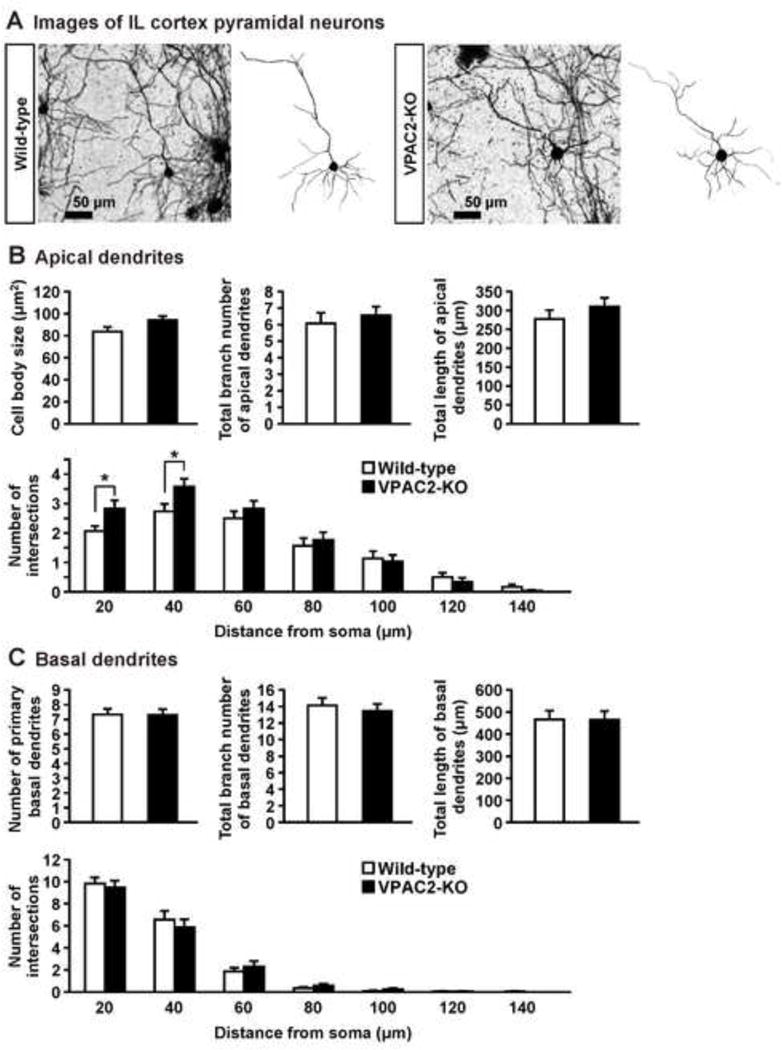
Dendritic morphology of IL pyramidal neurons in VPAC2-KO mice. (A) Golgi-stained pyramidal neuron in IL cortex and representative tracings of the dendrites of wild-type and VPAC2-KO mice. (B, C) Cell body size and total branch number and length of apical and basal dendrites are shown. The number of intersections of dendrites with 20 μm concentric spheres centered on the soma was measured by Sholl analysis. Results are expressed as the mean ± S.E.M. of 30 neurons from 3 mice per group. *P < 0.05, compared with wild-type.
Figure 5.
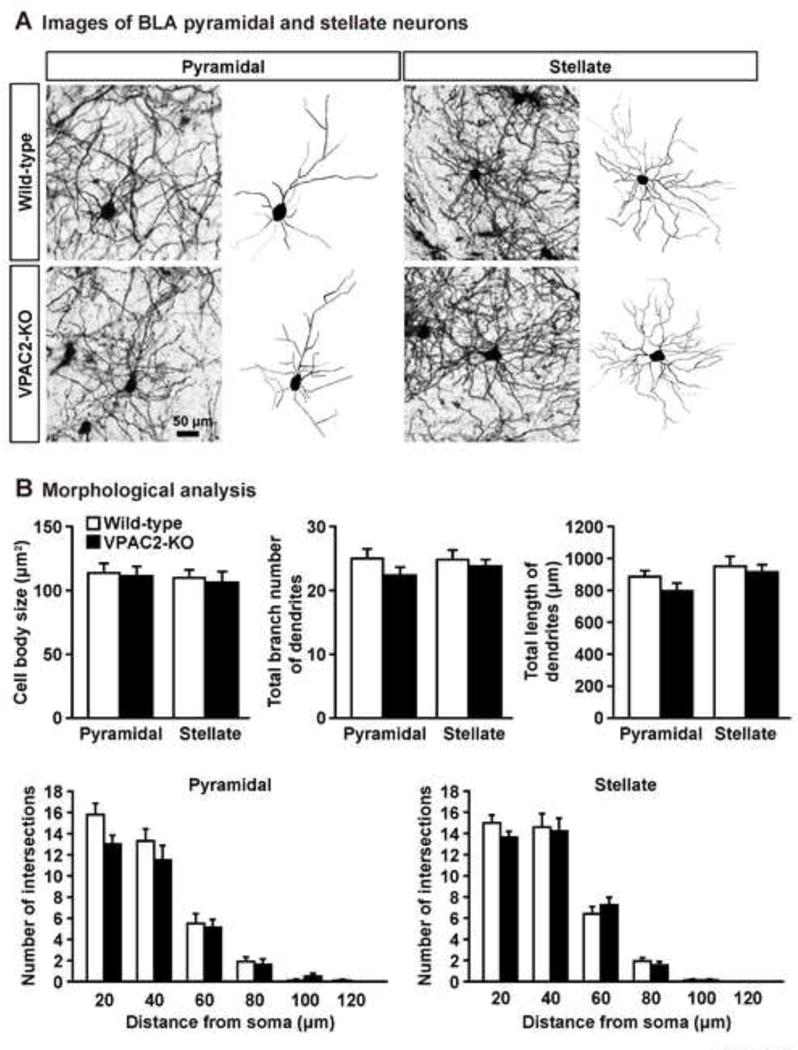
Dendritic morphology of BLA pyramidal and stellate neurons in VPAC2-KO mice. (A) Golgi-stained pyramidal and stellate neuron in BLA and representative tracings of the dendrites of wild-type and VPAC2-KO mice. (B) Cell body size and total branch number and length of dendrites are shown. The number of intersections of dendrites with 20 μm concentric spheres centered on the soma was measured by Sholl analysis. Results are expressed as the mean ± S.E.M. of 8–10 pyramidal neurons and 15–17 stellate neurons from 3 mice per group.
4. Discussion
Given that the VPAC2 receptors are abundant in brain regions of fear circuitry such as the prefrontal cortex, hippocampus, and the basolateral amygdala (Joo et al., 2004; Kalló, Kalamatianos, Piggins, & Coen, 2004; Marzagalli et al., 2016; Sheward, Lutz, & Harmar, 1995; Vertongen, Schiffmann, Gourlet, & Robberecht, 1997), the present study was designed to determine if fear behaviors such as fear acquisition, contextual and cued fear memory, and extinction are altered in VPAC2-KO mice. Previous studies have shown that the loss of PACAP, VIP, or the PAC1 receptor does not affect the acquisition of cued and/or contextual fear conditioning (Chaudhury, Loh, Dragich, Hagopian, & Colwell, 2008; Otto et al., 2011; Stack et al., 2008; Takuma et al., 2014b). Likewise, VPAC2-KO mice showed normal acquisition of fear conditioning. On the other hand, both PACAP and PAC1 receptor knockout mice are known to have deficits of contextual fear memory (Otto et al., 2011; Takuma et al., 2014b). Moreover, VIP knockout mice exhibit impaired recall of contextual fear conditioning 48, 72, 96, and 120 h, after fear conditioning, while early recall (at 24 h) was unaffected. (Chaudhury, Loh, Dragich, Hagopian, & Colwell, 2008). Thus, it seems possible that maintenance of contextual fear memory might be affected in VPAC2-KO mice, but the details are unknown. In any case, the findings suggest that neither the PAC1 nor VPAC2 receptor is critically involved in cued fear learning. The roles of the VPAC1 receptor in contextual and cued fear memories remain to be elucidated, although PACAP was reported to enhance excitatory synaptic transmission in the intra-amygdala circuit through VPAC1 receptors (Cho et al., 2012). An early study by Cottrell, Veldhuis, Rostene, & de Kloet (1984) showed that central administration of VIP facilitated extinction of active and passive avoidance behavior, although the distinct role of VIP receptors in that effect was not determined. In the present study, we found that extinction learning and retrieval were impaired in VPAC2-KO mice, suggesting that the VPAC2 receptor is involved most-specifically in the processes of fear extinction.
Numerous studies have tried to investigate the neural mechanisms underlying fear extinction. Converging evidence suggests that extinction of fear memory requires plasticity in both the medial prefrontal cortex and the amygdala. These brain areas are also deeply involved in mediating the effects of exposure to stress on memory. Differences in dendritic patterns and distribution are known to determine the functional properties of cortical neurons (Koch & Segev, 2000; Mainen & Sejnowski, 1996; Rall et al., 1992), and alterations in neuronal excitability are associated with changes in dendritic morphology (Gazzaley, Kay, & Benson, 2002; Monfils & Teskey, 2004; Monfils, VandenBerg, Kleim, & Teskey, 2004; Muller, Toni, & Buchs, 2000). Thus, the present study examined the dendritic morphology in the PrL and IL cortices and BLA. We observed the reductions in cell body size and total branch number and length of apical and basal dendrites of PrL cortex neurons in VPAC2-KO mice. In addition, Sholl analysis in apical dendrites revealed that the amount of dendritic material distal to the soma was decreased, while proximal dendritic material was increased. The amount of dendritic material proximal to the soma in apical dendrites of IL cortex neurons was also increased in VPAC2-KO mice but total branch number and length of apical and basal dendrites were not altered. There was no difference in dendritic morphology in BLA neurons between VPAC2-KO and wild-type mice. These findings suggest that loss of the VPAC2 receptor affects mainly the development of PrL pyramidal neurons, although the reason of this region-specific effect is unclear. Chronic stress or corticosterone treatment are known to impair fear extinction learning and/or subsequent recall of extinction memory (Baran, Armstrong, Niren, Hanna, & Conrad, 2009; Farrell, Sayed, Underwood, & Wellman, 2010; Garcia, Spennato, Nilsson-Todd, Moreau, & Deschaux, 2008; Gourley, Kedves, Olausson, & Taylor, 2009; Miracle, Brace, Huyck, Singler, & Wellman, 2006). Acute stress also impairs fear extinction (Akirav & Maroun, 2007; Maroun et al., 2013; Moench, Maroun, Kavushansky, & Wellman, 2015). Similar to our findings, chronic corticosterone administration reorganized apical, but not basal, arbors of layer II/III neurons in the PrL cortex, with an increase of 21% in dendritic material proximal to the soma, along with a decrease of up to 58% in dendritic material distal to the soma (Wellman, 2001). Chronic restraint stress also decreases the number and length of apical, but not basal, dendritic branches of pyramidal neurons of layer II/III in the PrL and anterior cingulate cortices (Cook & Wellman, 2004; Radley et al., 2004, 2006). Furthermore, Wellman et al. (2007) have reported that serotonin transporter knockout mice exhibited a selective deficit in extinction recall and that apical, but not basal, dendritic branch length of IL pyramidal neurons in serotonin transporter knockout mice was increased relative to wild-type mice, while pyramidal neurons in BLA had normal dendritic morphology. Conversely, it is reported that uncontrollable stress impairs fear extinction but it causes retraction of apical dendrites in IL, but not PrL, pyramidal neurons (Izquierdo, Wellman, & Holmes, 2006). These findings suggest that changes in dendritic morphology, especially for apical dendrites, in the PrL and IL cortices might be related to extinction deficits in VPAC2-KO mice.
Regarding the roles of PrL and IL cortices in fear extinction, it has been suggested that activity of IL neurons is correlated with and necessary for extinction retrieval, whereas PL activation is associated with fear learning and a resistance to induce fear extinction learning (Burgos-Robles, Vidal-Gonzalez, & Quirk, 2009; Do-Monte, Manzano-Nieves, Quiñones-Laracuente, Ramos-Medina, & Quirk, 2015; Giustino & Maren, 2015; Kim, Cho, Augustine, & Han., 2016; Kwapis, Jarome, & Helmstetter, 2014; Santini, Quirk, & Porter, 2008; Sierra-Mercado, Corcoran, Lebrón-Milad, & Quirk, 2006; Vidal-Gonzalez, Vidal-Gonzalez, Rauch, & Quirk, 2006). VPAC2-KO mice exhibited a specific deficit in fear extinction, so dendritic retraction in PrL cortex was an unexpected finding. On the other hand, there are contrasting reports showing that rats with PrL cortex lesions had an increased resistance to extinction (Morgan & LeDoux, 1995) and that inactivation of IL cortex neurons by microinfusion of the GABAA receptor agonist muscimol or the sodium channel blocker tetrodotoxin before extinction training facilitated extinction learning (Akirav, Raizel, & Maroun, 2006). Therefore, precisely how the loss of the VPAC2 receptor might alter the fear circuitry regarding the extinction deficits remains to be determined. Future studies on neuronal activities in the PrL and IL cortices and other brain regions such amygdala and hippocampus in VPAC2-KO mice are needed.
In conclusion, VPAC2-KO mice exhibited impaired extinction of cued fear memory, whereas they showed normal acquisition of fear conditioning and no differences in contextual and cued fear memory compared with wild-type mice. Loss of the VPAC2 receptor reorganized apical and basal dendritic arbors of PrL cortex neurons and apical, but not basal, dendritic arbors of IL cortex neurons. These results suggest that the VPAC2 receptor plays an important role in fear extinction and the regulation of the dendritic morphology in the prelimbic and infralimbic cortices.
Highlights.
VPAC2 receptor (VIPR2)-deficient mice showed normal acquisition of fear conditioning.
No differences in contextual and cued fear memory between groups.
VPAC2 receptor-deficient mice showed impaired extinction of cued fear memory.
Abnormal pyramidal dendritic morphology in prelimbic and infralimbic cortices.
Results suggest a critical role of VPAC2 receptors in fear extinction.
Acknowledgments
This study was supported in part by grants from National Institutes of Health; Grant Number: MH098506 (M.S.F. and J.A.W.), HD04612 (J.A.W.), and F32 MH107212-01A1 (A.K.R.), Simons Foundation (J.A.W.), the JSPS Program for Advancing Strategic International Networks to Accelerate the Circulation of Talented Researchers (S2603; H.H.), JSPS KAKENHI; Grant Numbers: JP16K08268 (Y.A.), JP16K08269 (A. H.-T.), JP26293020 (H.H.), JP17H03989 (H.H.), and the SRPBS and Brain/MINDS from AMED (H.H.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This paper is dedicated to the late Professor Anthony J. Harmar, who originally generated VPAC2-KO mice and greatly contributed to the understanding of the critical biological functions of the VPAC2 receptor and its signaling mechanisms.
Authorship contributions
Y. Ago performed the experiments, and analyzed the data. A. Hayata-Takano, T. Kawanai, R. Yamauchi, and S. Takeuchi contributed to the histology and dendritic morphological analysis. J. D. Cushman and A. K. Rajbhandari contributed to the behavioral experiments. Y. Ago, M. S. Fanselow, H. Hashimoto, and J. A. Waschek conceived the project and designed the experiments. Y. Ago and J. A. Waschek wrote the paper. J. A. Waschek coordinated and directed the project.
Conflicts of interest
The authors state no conflicts of interest.
References
- Ago Y, Condro MC, Tan YV, Ghiani CA, Colwell CS, Cushman JD, Fanselow MS, Hashimoto H, Waschek JA. Reductions in synaptic proteins and selective alteration of prepulse inhibition in male C57BL/6 mice after postnatal administration of a VIP receptor (VIPR2) agonist. Psychopharmacology (Berl) 2015;232:2181–2189. doi: 10.1007/s00213-014-3848-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akirav I, Maroun M. The role of the medial prefrontal cortex-amygdala circuit in stress effects on the extinction of fear. Neural Plasticity. 2007;2007:30873. doi: 10.1155/2007/30873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akirav I, Raizel H, Maroun M. Enhancement of conditioned fear extinction by infusion of the GABAA agonist muscimol into the rat prefrontal cortex and amygdala. European Journal of Neuroscience. 2006;23:758–764. doi: 10.1111/j.1460-9568.2006.04603.x. [DOI] [PubMed] [Google Scholar]
- Baran SE, Armstrong CE, Niren DC, Hanna JJ, Conrad CD. Chronic stress and sex differences on the recall of fear conditioning and extinction. Neurobiology of Learning and Memory. 2009;91:323–332. doi: 10.1016/j.nlm.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Quirk GJ. Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure. Journal of Neuroscience. 2009;29:8474–8482. doi: 10.1523/JNEUROSCI.0378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JH, Zushida K, Shumyatsky GP, Carlezon WA, Jr, Meloni EG, Bolshakov VY. Pituitary adenylate cyclase-activating polypeptide induces postsynaptically expressed potentiation in the intra-amygdala circuit. Journal of Neuroscience. 2012;32:14165–14177. doi: 10.1523/JNEUROSCI.1402-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury D, Loh DH, Dragich JM, Hagopian A, Colwell CS. Select cognitive deficits in vasoactive intestinal peptide deficient mice. BMC Neuroscience. 2008;9:63. doi: 10.1186/1471-2202-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell CS, Michel S, Itri J, Rodriguez W, Tam J, Lelievre V, Hu Z, Liu X, Waschek JA. Disrupted circadian rhythms in VIP- and PHI-deficient mice. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2003;285:R939–R949. doi: 10.1152/ajpregu.00200.2003. [DOI] [PubMed] [Google Scholar]
- Condro MC, Matynia A, Foster NN, Ago Y, Rajbhandari AK, Van C, Jayaram B, Parikh S, Diep AL, Nguyen E, May V, Dong HW, Waschek JA. High-resolution characterization of a PACAP-EGFP transgenic mouse model for mapping PACAP-expressing neurons. Journal of Comparative Neurology. 2016;524:3827–3848. doi: 10.1002/cne.24035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. Journal of Neurobiology. 2004;60:236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- Cottrell GA, Veldhuis HD, Rostene WH, de Kloet ER. Behavioral actions of vasoactive intestinal peptide (VIP) Neuropeptides. 1984;4:331–341. doi: 10.1016/0143-4179(84)90008-8. [DOI] [PubMed] [Google Scholar]
- Do-Monte FH, Manzano-Nieves G, Quiñones-Laracuente K, Ramos-Medina L, Quirk GJ. Revisiting the role of infralimbic cortex in fear extinction with optogenetics. Journal of Neuroscience. 2015;35:3607–3615. doi: 10.1523/JNEUROSCI.3137-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dymond S, Dunsmoor JE, Vervliet B, Roche B, Hermans D. Fear Generalization in Humans: Systematic Review and Implications for Anxiety Disorder Research. Behavior Therapy. 2015;46:561–582. doi: 10.1016/j.beth.2014.10.001. [DOI] [PubMed] [Google Scholar]
- Evans DW, Canavera K, Kleinpeter FL, Maccubbin E, Taga K. The fears, phobias and anxieties of children with autism spectrum disorders and Down syndrome: comparisons with developmentally and chronologically age matched children. Child Psychiatry and Human Development. 2005;36:3–26. doi: 10.1007/s10578-004-3619-x. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Wassum KM. The Origins and Organization of Vertebrate Pavlovian Conditioning. Cold Spring Harbor Perspectives in Biology. 2015;8:a021717. doi: 10.1101/cshperspect.a021717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell MR, Sayed JA, Underwood AR, Wellman CL. Lesion of infralimbic cortex occludes stress effects on retrieval of extinction but not fear conditioning. Neurobiology of Learning and Memory. 2010;94:240–246. doi: 10.1016/j.nlm.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Falluel-Morel A, Chafai M, Vaudry D, Basille M, Cazillis M, Aubert N, Louiset E, de Jouffrey S, Le Bigot JF, Fournier A, Gressens P, Rostène W, Vaudry H, Gonzalez BJ. The neuropeptide pituitary adenylate cyclase-activating polypeptide exerts anti-apoptotic and differentiating effects during neurogenesis: focus on cerebellar granule neurones and embryonic stem cells. Journal of Neuroendocrinology. 2007;19:321–327. doi: 10.1111/j.1365-2826.2007.01537.x. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. San Diego: Academic Press Inc.; 1997. [Google Scholar]
- Fujita Y, Masuda K, Bando M, Nakato R, Katou Y, Tanaka T, Nakayama M, Takao K, Miyakawa T, Tanaka T, Ago Y, Hashimoto H, Shirahige K, Yamashita T. Decreased cohesin in the brain leads to defective synapse development and anxiety-related behavior. Journal of Experimental Medicine. 2017;214:1431–1452. doi: 10.1084/jem.20161517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia R, Spennato G, Nilsson-Todd L, Moreau JL, Deschaux O. Hippocampal low-frequency stimulation and chronic mild stress similarly disrupt fear extinction memory in rats. Neurobiology of Learning and Memory. 2008;89:560–566. doi: 10.1016/j.nlm.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Kay S, Benson DL. Dendritic spine plasticity in hippocampus. Neuroscience. 2002;111:853–862. doi: 10.1016/s0306-4522(02)00021-0. [DOI] [PubMed] [Google Scholar]
- Gillott A, Furniss F, Walter A. Anxiety in high-functioning children with autism. Autism. 2001;5:277–286. doi: 10.1177/1362361301005003005. [DOI] [PubMed] [Google Scholar]
- Giustino TF, Maren S. The Role of the Medial Prefrontal Cortex in the Conditioning and Extinction of Fear. Frontiers in Behavioral Neuroscience. 2015;9:298. doi: 10.3389/fnbeh.2015.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Kedves AT, Olausson P, Taylor JR. A history of corticosterone exposure regulates fear extinction and cortical NR2B, GluR2/3, and BDNF. Neuropsychopharmacology. 2009;34:707–716. doi: 10.1038/npp.2008.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y, Ago Y, Taruta A, Katashiba K, Hasebe S, Takano E, Onaka Y, Hashimoto H, Matsuda T, Takuma K. Improvement by methylphenidate and atomoxetine of social interaction deficits and recognition memory impairment in a mouse model of valproic acid-induced autism. Autism Research. 2016;9:926–939. doi: 10.1002/aur.1596. [DOI] [PubMed] [Google Scholar]
- Harmar AJ, Fahrenkrug J, Gozes I, Laburthe M, May V, Pisegna JR, Vaudry D, Vaudry H, Waschek JA, Said SI. Pharmacology and functions of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide: IUPHAR review 1. British Journal of Pharmacology. 2012;166:4–17. doi: 10.1111/j.1476-5381.2012.01871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmar AJ, Marston HM, Shen S, Spratt C, West KM, Sheward WJ, Morrison CF, Dorin JR, Piggins HD, Reubi JC, Kelly JS, Maywood ES, Hastings MH. The VPAC2 receptor is essential for circadian function in the mouse suprachiasmatic nuclei. Cell. 2002;109:497–508. doi: 10.1016/s0092-8674(02)00736-5. [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Nogi H, Mori K, Ohishi H, Shigemoto R, Yamamoto K, Matsuda T, Mizuno N, Nagata S, Baba A. Distribution of the mRNA for a pituitary adenylate cyclase-activating polypeptide receptor in the rat brain: an in situ hybridization study. Journal of Comparative Neurology. 1996;371:567–577. doi: 10.1002/(SICI)1096-9861(19960805)371:4<567::AID-CNE6>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Shintani N, Baba A. New insights into the central PACAPergic system from the phenotypes in PACAP- and PACAP receptor-knockout mice. Annals of the New York Academy of Sciences. 2006;1070:75–89. doi: 10.1196/annals.1317.038. [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Shintani N, Tanida M, Hayata A, Hashimoto R, Baba A. PACAP is implicated in the stress axes. Current Pharmaceutical Design. 2011;17:985–989. doi: 10.2174/138161211795589382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C, Ferraguti F, Singewald N, Letzkus JJ, Ehrlich I, Lüthi A. Neuronal circuits of fear extinction. European Journal of Neuroscience. 2010;31:599–612. doi: 10.1111/j.1460-9568.2010.07101.x. [DOI] [PubMed] [Google Scholar]
- Herry C, Johansen JP. Encoding of fear learning and memory in distributed neuronal circuits. Nature Neuroscience. 2014;17:1644–1654. doi: 10.1038/nn.3869. [DOI] [PubMed] [Google Scholar]
- Holt DJ, Coombs G, Zeidan MA, Goff DC, Milad MR. Failure of neural responses to safety cues in schizophrenia. Archives of General Psychiatry. 2012;69:893–903. doi: 10.1001/archgenpsychiatry.2011.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt DJ, Lebron-Milad K, Milad MR, Rauch SL, Pitman RK, Orr SP, Cassidy BS, Walsh JP, Goff DC. Extinction memory is impaired in schizophrenia. Biological Psychiatry. 2009;65:455–463. doi: 10.1016/j.biopsych.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Wellman CL, Holmes A. Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. Journal of Neuroscience. 2006;26:5733–5738. doi: 10.1523/JNEUROSCI.0474-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo KM, Chung YH, Kim MK, Nam RH, Lee BL, Lee KH, Cha CI. Distribution of vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide receptors (VPAC1, VPAC2, and PAC1 receptor) in the rat brain. Journal of Comparative Neurology. 2004;476:388–413. doi: 10.1002/cne.20231. [DOI] [PubMed] [Google Scholar]
- Kalló I, Kalamatianos T, Piggins HD, Coen CW. Ageing and the diurnal expression of mRNAs for vasoactive intestinal peptide and for the VPAC2 and PAC1 receptors in the suprachiasmatic nucleus of male rats. Journal of Neuroendocrinology. 2004;16:758–766. doi: 10.1111/j.1365-2826.2004.01232.x. [DOI] [PubMed] [Google Scholar]
- Kim HS, Cho HY, Augustine GJ, Han JH. Selective Control of Fear Expression by Optogenetic Manipulation of Infralimbic Cortex after Extinction. Neuropsychopharmacology. 2016;41:1261–1273. doi: 10.1038/npp.2015.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C, Segev I. The role of single neurons in information processing. Nature Neuroscience. 2000;3(Suppl):1171–1177. doi: 10.1038/81444. [DOI] [PubMed] [Google Scholar]
- Kwapis JL, Jarome TJ, Helmstetter FJ. The role of the medial prefrontal cortex in trace fear extinction. Learning & Memory. 2014;22:39–46. doi: 10.1101/lm.036517.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau T, Bigio B, Zelli D, McEwen BS, Nasca C. Stress-induced structural plasticity of medial amygdala stellate neurons and rapid prevention by a candidate antidepressant. Molecular Psychiatry. 2017;22:227–234. doi: 10.1038/mp.2016.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson DF, Duan J, Oh S, Wang K, Sanders AR, Shi J, Zhang N, Mowry BJ, Olincy A, Amin F, Cloninger CR, Silverman JM, Buccola NG, Byerley WF, Black DW, Kendler KS, Freedman R, Dudbridge F, Pe’er I, Hakonarson H, Bergen SE, Fanous AH, Holmans PA, Gejman PV. Copy number variants in schizophrenia: confirmation of five previous findings and new evidence for 3q29 microdeletions and VIPR2 duplications. American Journal of Psychiatry. 2011;168:302–316. doi: 10.1176/appi.ajp.2010.10060876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainen ZF, Sejnowski TJ. Influence of dendritic structure on firing pattern in model neocortical neurons. Nature. 1996;382:363–366. doi: 10.1038/382363a0. [DOI] [PubMed] [Google Scholar]
- Maren S, Phan KL, Liberzon I. The contextual brain: Implications for fear conditioning, extinction and psychopathology. Nature Reviews Neuroscience. 2013;14:417–428. doi: 10.1038/nrn3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroun M, Ioannides PJ, Bergman KL, Kavushansky A, Holmes A, Wellman CL. Fear extinction deficits following acute stress associate with increased spine density and dendritic retraction in basolateral amygdala neurons. European Journal of Neuroscience. 2013;38:2611–2620. doi: 10.1111/ejn.12259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel G, Hevi C, Wong A, Zushida K, Uchida S, Shumyatsky GP. Murine GRPR and stathmin control in opposite directions both cued fear extinction and neural activities of the amygdala and prefrontal cortex. PLoS One. 2012;7:e30942. doi: 10.1371/journal.pone.0030942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzagalli R, Leggio GM, Bucolo C, Pricoco E, Keay KA, Cardile V, Castorina S, Salomone S, Drago F, Castorina A. Genetic blockade of the dopamine D3 receptor enhances hippocampal expression of PACAP and receptors and alters their cortical distribution. Neuroscience. 2016;316:279–295. doi: 10.1016/j.neuroscience.2015.12.034. [DOI] [PubMed] [Google Scholar]
- Meloni EG, Venkataraman A, Donahue RJ, Carlezon WA., Jr Bi-directional effects of pituitary adenylate cyclase-activating polypeptide (PACAP) on fear-related behavior and c-Fos expression after fear conditioning in rats. Psychoneuroendocrinology. 2016;64:12–21. doi: 10.1016/j.psyneuen.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miracle AD, Brace MF, Huyck KD, Singler SA, Wellman CL. Chronic stress impairs recall of extinction of conditioned fear. Neurobiology of Learning and Memory. 2006;85:213–218. doi: 10.1016/j.nlm.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Moench KM, Maroun M, Kavushansky A, Wellman C. Alterations in neuronal morphology in infralimbic cortex predict resistance to fear extinction following acute stress. Neurobiology of Stress. 2015;3:23–33. doi: 10.1016/j.ynstr.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monfils MH, Teskey GC. Induction of long-term depression is associated with decreased dendritic length and spine density in layers III and V of sensorimotor neocortex. Synapse. 2004;53:114–121. doi: 10.1002/syn.20039. [DOI] [PubMed] [Google Scholar]
- Monfils MH, VandenBerg PM, Kleim JA, Teskey GC. Long-term potentiation induces expanded movement representations and dendritic hypertrophy in layer V of rat sensorimotor neocortex. Cerebral Cortex. 2004;14:586–593. doi: 10.1093/cercor/bhh020. [DOI] [PubMed] [Google Scholar]
- Morgan MA, LeDoux JE. Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behavioral Neuroscience. 1995;109:681–688. doi: 10.1037//0735-7044.109.4.681. [DOI] [PubMed] [Google Scholar]
- Muller D, Toni N, Buchs PA. Spine changes associated with long-term potentiation. Hippocampus. 2000;10:596–604. doi: 10.1002/1098-1063(2000)10:5<596::AID-HIPO10>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M. Behavioral and neural analysis of extinction. Neuron. 2002;36:567–584. doi: 10.1016/s0896-6273(02)01064-4. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. National Academy Press; Washington, DC: 1996. [Google Scholar]
- Orsini CA, Maren S. Neural and cellular mechanisms of fear and extinction memory formation. Neuroscience and Biobehavioral Reviews. 2012;36:1773–1802. doi: 10.1016/j.neubiorev.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto C, Kovalchuk Y, Wolfer DP, Gass P, Martin M, Zuschratter W, Gröne HJ, Kellendonk C, Tronche F, Maldonado R, Lipp HP, Konnerth A, Schütz G. Impairment of mossy fiber long-term potentiation and associative learning in pituitary adenylate cyclase activating polypeptide type I receptor-deficient mice. Journal of Neuroscience. 2011;21:5520–5527. doi: 10.1523/JNEUROSCI.21-15-05520.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlack ST, Nees F, Ruttorf M, Cacciaglia R, Winkelmann T, Schad LR, Witt SH, Rietschel M, Flor H. Neural Mechanism of a Sex-Specific Risk Variant for Posttraumatic Stress Disorder in the Type I Receptor of the Pituitary Adenylate Cyclase Activating Polypeptide. Biological Psychiatry. 2015;78:840–847. doi: 10.1016/j.biopsych.2014.12.018. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR, McEwen BS, Morrison JH. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cerebral Cortex. 2006;16:313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, McEwen BS, Morrison JH. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Rall W, Burke RE, Holmes WR, Jack JJ, Redman SJ, Segev I. Matching dendritic neuron models to experimental data. Physiological Reviews. 1992;72(4 Suppl):S159–186. doi: 10.1152/physrev.1992.72.suppl_4.S159. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, Norrholm SD, Kilaru V, Smith AK, Myers AJ, Ramirez M, Engel A, Hammack SE, Toufexis D, Braas KM, Binder EB, May V. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature. 2011;470:492–497. doi: 10.1038/nature09856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders MJ, Wiltgen BJ, Fanselow MS. The place of the hippocampus in fear conditioning. European Journal of Pharmacology. 2003;463:217–223. doi: 10.1016/s0014-2999(03)01283-4. [DOI] [PubMed] [Google Scholar]
- Santini E, Quirk GJ, Porter JT. Fear conditioning and extinction differentially modify the intrinsic excitability of infralimbic neurons. Journal of Neuroscience. 2008;28:4028–4036. doi: 10.1523/JNEUROSCI.2623-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheward WJ, Lutz EM, Harmar AJ. The distribution of vasoactive intestinal peptide2 receptor messenger RNA in the rat brain and pituitary gland as assessed by in situ hybridization. Neuroscience. 1995;67:409–418. doi: 10.1016/0306-4522(95)00048-n. [DOI] [PubMed] [Google Scholar]
- Sierra-Mercado D, Jr, Corcoran KA, Lebrón-Milad K, Quirk GJ. Inactivation of the ventromedial prefrontal cortex reduces expression of conditioned fear and impairs subsequent recall of extinction. European Journal of Neuroscience. 2006;24:1751–1758. doi: 10.1111/j.1460-9568.2006.05014.x. [DOI] [PubMed] [Google Scholar]
- Stack CM, Lim MA, Cuasay K, Stone MM, Seibert KM, Spivak-Pohis I, Crawley JN, Waschek JA, Hill JM. Deficits in social behavior and reversal learning are more prevalent in male offspring of VIP deficient female mice. Experimental Neurology. 2008;211:67–84. doi: 10.1016/j.expneurol.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JS, Almli LM, Fani N, Gutman DA, Bradley B, Norrholm SD, Reiser E, Ely TD, Dhanani R, Glover EM, Jovanovic T, Ressler KJ. PACAP receptor gene polymorphism impacts fear responses in the amygdala and hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:3158–3163. doi: 10.1073/pnas.1318954111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takuma K, Hara Y, Kataoka S, Kawanai T, Maeda Y, Watanabe R, Takano E, Hayata-Takano A, Hashimoto H, Ago Y, Matsuda T. Chronic treatment with valproic acid or sodium butyrate attenuates novel object recognition deficits and hippocampal dendritic spine loss in a mouse model of autism. Pharmacology, Biochemistry, and Behavior. 2014a;126:43–49. doi: 10.1016/j.pbb.2014.08.013. [DOI] [PubMed] [Google Scholar]
- Takuma K, Maeda Y, Ago Y, Ishihama T, Takemoto K, Nakagawa A, Shintani N, Hashimoto H, Baba A, Matsuda T. An enriched environment ameliorates memory impairments in PACAP-deficient mice. Behavioural Brain Research. 2014b;272:269–278. doi: 10.1016/j.bbr.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Tan YV, Waschek JA. Targeting VIP and PACAP receptor signalling: new therapeutic strategies in multiple sclerosis. ASN Neuro. 2011;3:e00065. doi: 10.1042/AN20110024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovote P, Fadok JP, Lüthi A. Neuronal circuits for fear and anxiety. Nature Reviews Neuroscience. 2015;16:317–331. doi: 10.1038/nrn3945. [DOI] [PubMed] [Google Scholar]
- Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, Thompson KR, Gradinaru V, Ramakrishnan C, Deisseroth K. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471:358–362. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacic V, McCarthy S, Malhotra D, Murray F, Chou HH, Peoples A, Makarov V, Yoon S, Bhandari A, Corominas R, Iakoucheva LM, Krastoshevsky O, Krause V, Larach-Walters V, Welsh DK, Craig D, Kelsoe JR, Gershon ES, Leal SM, Dell Aquila M, Morris DW, Gill M, Corvin A, Insel PA, McClellan J, King MC, Karayiorgou M, Levy DL, DeLisi LE, Sebat J. Duplications of the neuropeptide receptor gene VIPR2 confer significant risk for schizophrenia. Nature. 2011;471:499–503. doi: 10.1038/nature09884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudry D, Falluel-Morel A, Bourgault S, Basille M, Burel D, Wurtz O, Fournier A, Chow BK, Hashimoto H, Galas L, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacological Reviews. 2009;61:283–357. doi: 10.1124/pr.109.001370. [DOI] [PubMed] [Google Scholar]
- Vertongen P, Schiffmann SN, Gourlet P, Robberecht P. Autoradiographic visualization of the receptor subclasses for vasoactive intestinal polypeptide (VIP) in rat brain. Peptides. 1997;18:1547–1554. doi: 10.1016/s0196-9781(97)00229-5. [DOI] [PubMed] [Google Scholar]
- Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, Quirk GJ. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learning & Memory. 2006;13:728–733. doi: 10.1101/lm.306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. Journal of Neuroscience. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Cao C, Wang R, Qing Y, Zhang J, Zhang XY. PAC1 receptor (ADCYAP1R1) genotype is associated with PTSD’s emotional numbing symptoms in Chinese earthquake survivors. Journal of Affective Disorders. 2013;150:156–159. doi: 10.1016/j.jad.2013.01.010. [DOI] [PubMed] [Google Scholar]
- Waschek JA. VIP and PACAP receptor-mediated actions on cell proliferation and survival. Annals of the New York Academy of Sciences. 1996;805:290–300. doi: 10.1111/j.1749-6632.1996.tb17491.x. [DOI] [PubMed] [Google Scholar]
- Waschek JA. Multiple actions of pituitary adenylyl cyclase activating peptide in nervous system development and regeneration. Developmental Neuroscience. 2002;24:14–23. doi: 10.1159/000064942. [DOI] [PubMed] [Google Scholar]
- Waschek JA. VIP and PACAP: neuropeptide modulators of CNS inflammation, injury, and repair. British Journal of Pharmacology. 2013;169:512–523. doi: 10.1111/bph.12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman CL. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. Journal of Neurobiology. 2001;49:245–253. doi: 10.1002/neu.1079. [DOI] [PubMed] [Google Scholar]
- Wellman CL, Izquierdo A, Garrett JE, Martin KP, Carroll J, Millstein R, Lesch KP, Murphy DL, Holmes A. Impaired stress-coping and fear extinction and abnormal corticolimbic morphology in serotonin transporter knock-out mice. Journal of Neuroscience. 2007;27:684–691. doi: 10.1523/JNEUROSCI.4595-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]


