Abstract
Urinary tract infections (UTI) are a major problem in human medicine for which better understanding of native immune defenses may reveal new pathways for therapeutic intervention. Tamm-Horsfall glycoprotein (THP), the most abundant urinary protein, interacts with bacteria including uropathogenic E. coli (UPEC) as well host immune cells. In addition to its well-studied functions to antagonize bacterial colonization, we hypothesize that THP serves a critical host defense function through innate immune modulation. Using isolated human neutrophils, we found that THP binds neutrophils and that this interaction reduces reactive oxygen species generation, chemotaxis, and killing of UPEC. We discovered that THP engages the inhibitory neutrophil receptor sialic acid-binding Ig-like lectin-9 (Siglec-9), and mouse functional ortholog Siglec-E, in a manner dependent on sialic acid on its N-glycan moieties. THP-null mice have significantly more neutrophils present in the urine compared to WT mice, both with and without the presence of inflammatory stimuli. These data support THP as an important negative regulator of neutrophil activation in the urinary tract, with dual functions to counteract bacterial colonization and suppress excessive inflammation within the urinary tract.
Introduction
Urinary tract infections (UTI) are a major medical burden in the United States, especially for women and the elderly. Uropathogenic strains of Escherichia coli (UPEC) are by far the most common etiologic agent of UTI, causing severe bladder infection (cystitis) and acute kidney infections (pyelonephritis)1. UTI onset frequently involves an underlying dysfunction of host defenses including pathogen recognition and antimicrobial factors, coupled with effective pathogen-specific virulence properties of invasiveness and immune resistance.
Tamm-Horsfall glycoprotein (THP, or uromodulin) is the most abundant protein in urine2 and is expressed exclusively in the thick ascending loop of Henle (TAL) of the kidney. By weight this ∼85 kDa protein is composed 30% of glycans, consisting of N-linked high-mannose sequences and di-, tri-, and tetra-antennary complex-type N-glycans that are sialylated (including the Sda-determinant), fucosylated, or sulfated3. O-linked glycan chains have also been reported on THP4.
THP plays a role in antagonizing UPEC colonization of the urinary tract along with less well-studied immunomodulatory effects. THP directly binds S-fimbriated and type 1 fimbriated UPEC5 without exerting direct bactericidal or bacteriostatic activity. High mannose glycans of THP interact with UPEC fimbrial tip protein FimH, preventing the bacterium from binding the uroepithelial receptors uroplakin Ia and Ib thus limiting colonization of the bladder6. THP also inhibits fimbriated UPEC adherence to cultured renal epithelial cells7, and the Sda determinant present on its N-glycans prevents UPEC colonization of renal epithelium8.
An independent discovery of “uromodulin” in 1985 by Muchmore and Decker, subsequently recognized to represent THP, described a role in suppression of T cell proliferation9. Since then, focused studies on the interactions between purified THP and host immune cells and proteins in vitro have yielded a mixed array of pro-inflammatory and anti-inflammatory phenotypes. For example, dependent on the model system chosen, THP has been reported to suppress10 or enhance11 neutrophil phagocytosis, and block NF-κB activation and cytokine release from kidney cells12 while stimulating cytokine production from monocytes13. In 2004, two independently derived THP knockout (THP -/-) mouse lines were generated and demonstrate a consistent protective effect of THP on the bladder during UTI14,15. Recently, it has been suggested that THP may regulate bone marrow granulopoiesis, with THP deficiency promoting systemic neutrophilia16.
In this study, we examined new mechanistic aspects of THP modulation of the host immune response using neutrophils, the first innate immune responders to UTI pathogens. THP exerted a suppressive effect on neutrophil activation including reduced chemotaxis, ROS production and bactericidal activity against UPEC. Blunting of neutrophil activation was secondary to THP engagement of the inhibitory neutrophil receptor sialic acid-binding Ig-like lectin-9 (Siglec-9), an interaction that depended on terminal sialic acids on the THP glycoprotein. THP thus has dual functions during UTI – its documented ability to directly bind and interfere with colonization potential of the global pathogen UPEC, coupled to Siglec-9 mediated counter-regulation of neutrophil activation to mitigate against excessive inflammation and host tissue damage.
Results
THP binds neutrophils and suppresses their activation and function
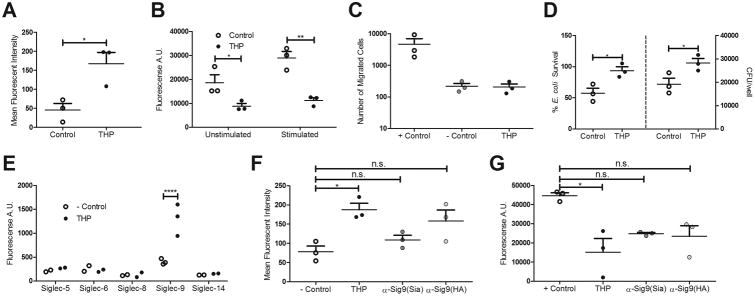
THP -/- mice have increased urine and bladder burdens upon challenge with various pathogens including UPEC14,15. We hypothesize host inflammatory responses are altered in the absence of THP and examined the impact of THP on neutrophils, the first immune cell responders to UTI. THP binds neutrophils and alters phagocytosis at physiologic concentrations found in urine and plasma10,11,17, but there is currently no firm mechanistic basis regarding the molecular events governing THP and neutrophil interactions. We incubated isolated human neutrophils with a physiologic urinary concentration of purified THP (50 μg/mL) for 30 min prior to assessing function. Neutrophils pre-treated with THP were stained with a FITC-labeled, mouse anti-human THP antibody and analyzed via flow cytometry, revealing a strong fluorescent signal indicative of THP binding (Fig. 1A). THP pre-treatment markedly reduced reactive oxygen species (ROS) production following stimulation with phorbol 12-myristate 13-acetate (PMA), a potent activator of protein kinase C (Fig. 1B). Using a Transwell™ cell migration model, THP exposure strongly inhibited neutrophil chemotaxis in response to the chemoattractant fMLP, reducing migrated cells to the background number seen in the absence of fMLP (- Control, Fig. 1C). Finally, THP pre-treatment inhibited neutrophil killing of UPEC (Fig. 1D). THP exerted these inhibitory effects without altering neutrophil viability under our experimental conditions (30 min treatment), and by 90 min demonstrated a protective effect as determined by propidium iodide uptake (Supp. Fig. 1A). THP did not show direct antimicrobial activity against UPEC in bacterial medium (LB broth) or eukaryotic medium (RPMI 1640) (Supp. Fig. 1B).
Figure 1. THP suppresses neutrophil function through engagement of Siglec-9.
(A) Mean fluorescent intensity of neutrophils pretreated with THP (50 μg/mL) or not (control), stained with FITC mouse anti-human THP antibody. (B) ROS production of neutrophils pretreated with THP or not (control), with or without PMA stimulation. (C) Transwell migration of neutrophils, pretreated with THP or not (+ control), in response to chemoattractant fMLP. Background neutrophil migration was recorded in the absence of fMLP (- control). (D) Percent survival (left) and recovered CFU (right) of UPEC UTI89 after 30 min of exposure to neutrophils pretreated with THP or not (control). (E)Plate-based binding assays of immobilized THP with human Siglec-Fcs and visualized with PE anti-human IgG Fc antibody. (F) Mean fluorescent intensity (F) or ROS production (G) of neutrophils pretreated with mouse anti-human Siglec-9 (sialic acid blocking, Sia) or anti-human Siglec-9 (Hyaluronic acid blocking, HA) antibodies, and treated with THP or not (control). Data represent the mean of two independent experiments performed in technical triplicate with combined results, n = 2/group (E), or three independent experiments performed in technical triplicate with the mean and SEM of combined results, n = 3/group (all other panels). Data was analyzed using Student's unpaired two-tailed t-test (A,D), two-way ANOVA with Bonferroni's multiple comparisons post-test (B, E), or one-way ANOVA with Bonferroni's multiple comparisons post-test (C, F-G). *P < 0.05, **P < 0.01, and ****P < 0.0001 represent statistical significance, or n.s. represents non-significant (P > 0.05).
THP engages the inhibitory neutrophil receptor Siglec-9
Previous work showed that THP interaction with the lymphocyte cell surface depended on N-glycan modifications18 and that THP engaged an unknown surface glycan-binding receptor on human neutrophils in a manner out-competed by exogenous sialic acid17. These clues, combined with our results revealing a consistent THP-mediated suppression of neutrophil function, led us to examine if THP interacts with sialic acid-binding Ig-like lectins (Siglecs). CD33-related Siglecs are inhibitory cell surface receptors present on multiple immune cell types that recognize sialic acids as“self-associated molecular patterns” and limit cell activation by recruitment of inhibitory SHP family phosphatases to their intracellular domains19. Using a plate-based assay, we examined binding of immobilized human THP to multiple chimeric human Siglec-Fc proteins, containing the extra-cellular domains of the molecules. THP specifically bound human Siglec-9, but not Siglec-5, Siglec-6, Siglec-8, or Siglec-14 (Fig. 1E). Siglec-9 is highly expressed on neutrophils (where it is the most abundant Siglec) and monocytes, with weaker expression on subsets of B cells, T cells, and NK cells20. THP/Siglec-9 interactions were confirmed by flow cytometry:an anti-Siglec-9 antibody, α-Sig9(Sia), which prevents Siglec-9/sialic acid interactions blocked THP/neutrophil binding, while another anti-Siglec-9 antibody, α-Sig9(HA), that prevents Siglec-9/hyaluronic acid but not sialic acid interactions21 blocked THP/neutrophil binding to a lesser degree (Fig. 1F). These results demonstrate Siglec-9 serves as the primary neutrophil receptor for THP, and points toward THP terminal sialic acids as key constituents of this interaction. Siglec-9 engagement of sialic acid ligands can suppress neutrophil oxidative burst21, and indeed, we observed a partial reversal of THP-mediated suppression of ROS in neutrophils exposed to α-Sig9(Sia) or α-Sig9(HA) prior to THP treatment (Fig. 1G).
THP interactions with Siglec-9 are sialic acid-dependent
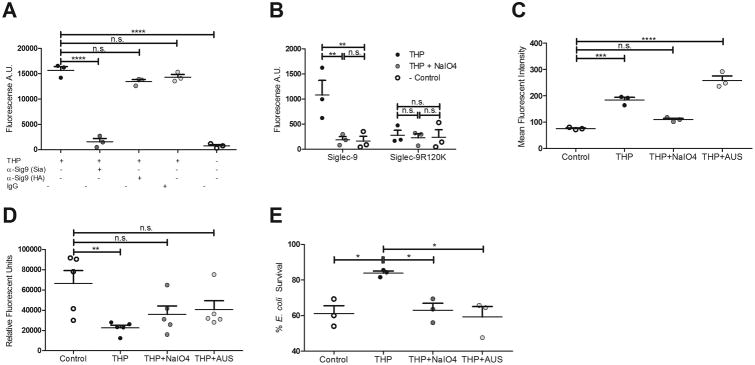
Siglec-9 ligands are typically glycans on host or bacterial cell surface glycoproteins and glycolipids containing sialic acid, although host and bacterial hyaluronan has recently been shown to bind human Siglec-9 as well21. Siglec-9 binds the glycan sequence Neu5Acα2-3Galβ1-4GlcNAc present on host cell surfaces and the exopolysaccharide capsule of the bacterium group B Streptococcus22, and this same sequence is present on THP N-glycans as a terminal structure and as a portion of the Sda determinant Neu5Acα2-3(GalNAcβ1-4)Galβ1-4GlcNAc. We analyzed the contribution of THP terminal sialic acids, including the Sda determinant, to binding of Siglec-9-Fc chimera immobilized on a microtiter plate. Binding of Siglec-9 by soluble THP was blocked ∼90% by α-Sig9(Sia) prior to THP treatment, but only minimally impacted with α-Sig9 (HA) (<15%) (Fig. 2A). When the arginine residue at position 120 of Siglec-9, critical for ligand binding of NeuAcα2-3Galβ1-4GlcNAc, was mutated to lysine (Siglec-9-R120K), THP binding was reduced to background levels (Fig. 2B). Furthermore, mild periodate oxidation using NaIO4 of the side chains of THP sialic acids followed by aldehyde quenching with MTSC, completely abrogated THP binding to Siglec-9 (Fig. 2B). Using flow cytometry, we found NaIO4-treated THP did not bind primary human neutrophils (Fig. 2C). THP treatment with Arthrobacter ureafaciens sialidase (AUS)did not significantly alter neutrophil binding, but this may be the result of compensatory neutrophil binding to newly exposed underlying glycans (e.g. galactose) via alternate receptors (Fig. 2C). Lastly, we observed that the inhibitory activity of THP on neutrophil ROS production and killing of UTI89 was partially or completely abolished with modification of THP terminal sialic acid through mild periodate oxidation, or enzymatic removal of sialic acid with sialidase treatment (Fig. 2D-E, Supp. Table 1). Treatment of THP with β-hexosaminidase from Canavalia ensiformis (jack bean) to remove GalNAcβ1-4, a terminal structure on the Sda determinant, did not alter binding to Siglec-9-Fc, nor was there an additive reduction of binding when combined with AUS treatment (Supp. Fig. 1C). Activity of β-hexosaminidase and AUS were confirmed under the assay conditions (data not shown).
Figure 2. THP engagement of Siglec-9 and neutrophil suppression requires sialic acid.
(A) Plate-based binding assay of immobilized human Siglec-9-Fc, blocked with anti-Siglec-9 (sialic acid blocking, Sia), anti-Siglec-9 (Hyaluronic acid blocking, HA), or human IgG, and incubated with soluble THP. (B) Plate-based binding assay of immobilized mock-treated THP, NaIO4-treated THP, or no THP (Control), with human Siglec-9-Fc or Siglec-9 R120K-Fc mutant and visualized with PE anti-human IgG Fc antibody. (C) Mean fluorescent intensity of neutrophils treated with THP, sialidase-treated THP, or no THP (Control), stained with FITC mouse anti-human THP antibody. (D) ROS production of neutrophils treated with THP, NaIO4-treated THP, sialidase-treated THP, or no THP (Control), and stimulated with PMA. (E) Percent survival (left) or total CFU (right) of E. coli UTI89 after 30 min of exposure to neutrophils pretreated with THP, NaIO4-treated THP, sialidase-treated THP, or no THP (Control). Data represent the mean and SEM of three (or five in panel D) independent experiments performed in technical triplicate with combined results shown, n = 3/group (A-C, E) or n = 5/group (D). Data was analyzed using one-way ANOVA with Bonferroni's multiple comparisons post-test (A, C-E) or two-way ANOVA with Bonferroni's multiple comparisons post-test (B). *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 represent statistical significance, or n.s. represents non-significant (P > 0.05).
THP regulates neutrophil populations in the urinary tract
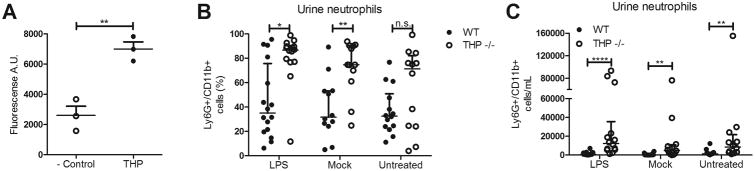
To assess the impact of THP modulation of neutrophils in vivo, we first confirmed that purified mouse THP bound mouse Siglec-E, the murine functional ortholog of Siglec-9 (Fig. 3A). Similar to Siglec-9, murine Siglec-E is expressed on neutrophils and peritoneal macrophages, as well as subsets of NK cells and dendritic cells23. Indirect bacterial antagonism of THP has been documented previously in prevention of bladder colonization6-8, although THP does not exert direct bactericidal/static activity, a finding confirmed in this study (Supp. Fig. 1B). Because of the inherent antibacterial properties of THP in WT mice, we examined neutrophil responses to a static bacterial stimulus, purified LPS, to avoid the confounding factor of increased bacterial numbers in THP -/- urinary tract over the experimental time course. Mice received a trans-urethral dose of purified LPS, and 24 h later, urine and bladders were collected. Flow cytometry analysis revealed a higher percentage of neutrophils (% Ly6G+/CD11b+ of gated cells) in urine of THP -/- mice in all groups; LPS treated, mock treated, and untreated (not significant) (Fig. 3B). Additionally, total neutrophil numbers per mL of urine were significantly increased THP -/- mice in all groups (Fig. 3C).
Figure 3. THP binds mouse Siglec-E, and regulates urinary neutrophil populations in mice.
(A)Plate-based binding assay of immobilized mouse THP with mouse Siglec-E-Fc and visualized with PE anti-human IgG Fc antibody. Percent (B) or total (C) Ly6G+/CD11b+ cells in mouse urine collected 24 h post-treatment with LPS, and quantified via flow cytometry. Experiments were performed independently three times, and combined results are shown. Data represent the mean and SEM of three independent experiments performed in technical triplicate, n = 3/group (A). Circles represent individual mice and lines represent the median and IQ ranges of each group, n = 12-16/group (B, C). Data were analyzed using Mann-Whitney test, two-tailed (B,C), or using Student's unpaired two-tailed t-test (A). *P < 0.05, **P < 0.01, and ****P < 0.0001 represent statistical significance, or n.s. represents non-significant (P > 0.05).
Discussion
Although several earlier studies described THP binding to human neutrophils10,11,17, ours is the first to identify the primary receptor as Siglec-9, in a manner where functional THP-neutrophil interactions depend upon the terminal sialylated N-glycan structures of the abundant urinary glycoprotein. The significance of THP glycan moieties for biological activity has also been observed in interaction with other immune system components. THP glycans are required for direct interactions with IL-1 and T cell immunosuppression can also be attributed to its purified N-glycan portion, in the absence of intact protein24. However, this glycan-mediated lymphocyte immunosuppression is enhanced by stepwise enzymatic removal of sialic acids and underlying beta-galactose residues, and thus localized to the central core structure of the THP N-glycan18. In contrast, we observed removal or side chain truncation of the terminal sialic acid on the THP N-glycan eliminated Siglec-9 binding and reversed neutrophil suppression, consistent with a reported finding in which exogenous sialic acid outcompeted THP binding to neutrophils17.
Changes in THP sialylation appear to be important in various disease associations. Although total levels of THP are unchanged, patients with interstitial cystitis have only half of the THP sialic acid content of healthy controls25. Similarly, mass spectrometry of urine THP from kidney stone patients revealed >20% less sialic acid content than healthy controls26. Patients with type I diabetes are more susceptible to UTI, have more glucose and less sialic acid in their THP glycans than healthy controls27, and may exhibit reduced THP urinary excretion rates28. Although reduced THP N-acetylgalactosamine (GalNAc), another component of the Sda antigen, has also been reported in patients with UTI29, terminal GalNAcβ1-4 did not contribute to THP/Siglec-9 interactions (Supp. Fig. 1C).
Allelic variants of THP correlate both with pathogen diversity and frequency of antibiotic-resistant UTI, with the UMOD ancestral allele, which drives higher urinary THP, retained at higher frequency because of its protective effect against UTIs30. Furthermore, these authors observed an inverse correlation between THP levels and leukocytes in human urine; an effect that we corroborated experimentally in vivo (Fig. 3). Neutrophils pretreated with THP showed reduced UPEC killing, but this inhibitory phenotype was abrogated when THP sialic acid was modified or removed (Fig. 2). Thus THP-mediated neutrophil suppression comes with a cost – a diminished neutrophil contribution to bacterial clearance. However, since very few immune cells are typically found in healthy urine, and THP directly binds UPEC to impede bladder colonization6-8, we hypothesize a dual role to limit inflammatory responses and potential damage to the vulnerable kidney is advantageous.
Collectively, our data demonstrate that THP can bind neutrophil Siglec-9 to control excessive neutrophil inflammatory responses. This interaction requires the sialic acid present on THP, and varying glycosylation patterns of THP may explain susceptibility of certain individuals to recurrent UTI. Future work seeks to examine THP-Siglec signaling during UTI as a potential therapeutic target to prevent or treat acute and chronic UPEC UTI.
Methods
Bacterial strains and growth conditions
Wild-type UPEC strain UTI89 (O18:K1:H7)31 was incubated overnight to stationary phase at 37°C with shaking in Luria-Bertani (LB) broth, and overnight cultures were diluted 1:30 in fresh LB broth, and incubated shaking at 37°C until mid-log phase (OD600nm = 0.4).
Primary neutrophil isolation
With approval from UCSD IRB/HRPP, protocol# 070278X, human venous blood was obtained from 12 healthy volunteers under informed consent, with heparin used as an anticoagulant. Neutrophils were isolated using PolymorphPrep™ (Axis-Shield, Dundee, Scotland) to create a density gradient by centrifugation according to manufacturer's instructions.
Tamm-Horsfall glycoprotein purification and modification
Purified human pooled Tamm-Horsfall glycoprotein (THP) was purchased from BBI Solutions, Cardiff, UK (Catalog number: P135-1). Mouse THP was purified from pooled mouse urine as described originally by Tamm and Horsfall using salt precipitation2 with several adaptions. Pooled mouse urine was diluted with an equal volume of chilled distilled H2O, and subsequent precipitation and desalting steps were carried out at 4°C. THP was precipitated by adding NaCl2 to a final concentration of 0.58M, mixed, and precipitate allowed to settle overnight. The precipitate was pelleted at 3220 × g for 10 min, supernatant discarded, and pellet washed with fresh, chilled 0.58M NaCl2. Sample was vortexed, precipitate again allowed to settle overnight, and pelleted as in the previous step. Pellet was resuspended in 3 volumes of distilled H2O, and desalted using a 50 kDa Amicon Ultra-15 column (EMD Millipore, Billerica, MA), and a minimum of 3× buffer exchanges with distilled H2O. After desalting and concentrating to 1mL, the sample was centrifuged at 9,300 × g for 30 min, and any pelleted impurities were discarded. Mouse THP was quantified via BCA assay (Pierce, Rockford, IL). A single band (molecular weight ∼85 kDa) was visualized with SDS-PAGE after staining with InstantBlue (Expedeon Inc., San Diego, CA) and positively identified via western blot following incubation with a goat anti-THP polyclonal antibody (Cat# sc-19554, Santa Cruz Biotechnology, Dallas, TX).
To remove or modify the sialic acid on THP for binding assays, human THP was exposed to either sialidase treatment or mild periodate oxidation. For sialidase treatment, THP (100μg/mL) was incubated with 100 mU/mL of sialidase purified from Arthrobacter ureafaciens (AUS, Sigma Aldrich, St. Louis, MO) in 1× DPBS (pH = 7.0) for 1 hour at 37°C. Sialidase activity was confirmed using a Neuraminidase Assay Kit (abcam, ab138888). Selective periodate oxidation of THP sialic acid-containing glycans was accomplished by incubating purified THP (200μg/mL) with fresh 2mM NaIO4 (Sigma Aldrich) on ice for 20 min to generate aldehydes at the C7 or C8 position of sialic acid. To stop the reaction, NaIO4 was removed by transferring sample to a 30kDa Microcon column (EMD Millipore), centrifuged for 15 min at 14,000 × g at 4°C, and washed 3× with ice cold 1× DPBS. Sialic acid aldehydes were then quenched with 4-methyl-3-thiosemicarbazide (MTSC, Sigma Aldrich) as performed previously32. Subsequent treatment with FITC-thiosemicarbazide verified all aldehydes were fully quenched under assay conditions (data not shown).
Neutrophil flow cytometry
Freshly isolated human neutrophils (6 × 105 cells/mL) were incubated with THP (50μg/mL) in HBSS with calcium and magnesium for 15 min on ice. Where indicated, neutrophils were incubated with 1μg/mL mouse anti-human Siglec-9 monoclonal antibody [α-Sig9(Sia), Cat# MAB1139, R&D Systems, Minneapolis, MN] or mouse anti-human Siglec-9 monoclonal antibody [α-Sig9(HA), Cat# 624084, BD Pharmingen] for 15 min on ice prior to incubation with THP. Non-bound THP was removed by centrifuging at 200 × g for 5 min and washing once with HBSS. Cells were then incubated with 1μg/mL FITC mouse anti-human THP antibody (Cat# AM31843FC-N, Acris, Rockville, MD) for 15 min on ice. Cells incubated with the anti-human THP antibody only were used as a negative control. Cells were washed once with HBSS and run on BD FACSCalibur (BD Biosciences, San Jose, CA). Data was analyzed using CellQuest Pro v.6 software (BD Biosciences).
Neutrophil killing assay
Neutrophils were diluted to 2×106 cells/mL in RPMI 1640 (Gibco, Cat#11875-093), treated with THP (50μg/mL) and incubated at 37°C in 5% CO2 for 30 min. Untreated neutrophils were used as a control. Neutrophils were seeded at 2×105 cells/well in a tissue cultured-treated 96-well plate. UTI89 diluted in RPMI 1640 was added to neutrophils at a multiplicity of infection of 1:1 (UTI89-to-neutrophil ratio). Control wells without neutrophils were used to determine baseline bacterial counts at the assay endpoint. Plates were centrifuged at 300 × g for 5 min to facilitate bacterial contact with neutrophils and incubated at 37°C in 5% CO2 for 30 min. Samples were lysed, serially diluted, and then plated on LB agar for enumeration of surviving UTI89 CFU. Percent survival of UTI89 was calculated as [(CFU per experimental well)/(CFU per control well)] × 100.
ROS production assay
Neutrophils were stained in HBSS without calcium and magnesium containing 20 μM 2′, 7′-dichlorofluorescein diacetate (Sigma Aldrich) and were incubated with THP (50 μg/mL) for 30 min at 37°C with 5% CO2. Where indicated, neutrophils were incubated with 1μg/mL mouse anti-human Siglec-9 monoclonal antibody α-Sig9(Sia), or mouse anti-human Siglec-9 monoclonal antibody α-Sig9(HA) for 15 min prior to incubation with THP. Cells were then added to a 96-well plate (5 × 105 cells/well) and mixed at a 1:1 ratio with 25 nM PMA (Sigma Aldrich) to stimulate ROS release. Plates were incubated at 37°C with 5% CO2 for 30 min in the dark. Fluorescence intensity (485nm excitation, 530nm emission) was measured in a SpectraMAX Gemini EM fluorescence plate reader (Molecular Devices, Sunnyvale, CA).
Transwell chemotaxis assay
Neutrophils (2 × 106 cells/mL) were incubated with THP (50 μg/mL) for 30 min at 37°C with 5% CO2. Cells were seeded in 6-mm transwell permeable supports (3-μm pore size; Corning Inc., Corning NY) placed in 24-well plates. Lower chambers contained either HBSS alone, or 100 nM of the chemoattractant fMLP (Sigma Aldrich). Following a 45-min incubation at 37°C with 5% CO2, inserts were removed, cells suspended with gentle pipetting, and 5 mM EDTA was added. Samples were immediately run on BD FACSCalibur (BD Biosciences), gated based on forward and side scatter profiles of input (isolated neutrophils), and data analyzed using FlowJo v10.2 software (FlowJo LLC, Ashland, OR).
Siglec-Fc binding assay
Chimeric recombinant Siglec-Fc fusion proteins of human Siglec-5, Siglec-6, Siglec-8, Siglec-9 (wild-type and R120K), Siglec-14, or mouse Siglec-E extracellular domains and a human IgG Fc tail were generated as described previously33. For all binding assays except those using anti-Siglec-9 antibodies, THP (10 μg/well) was immobilized on high affinity binding microtiter plates (Corning, Catalog number 3361) in 1× DPBS overnight at 4°C. Uncoated wells were used as negative controls and subjected to all subsequent steps. Wells were blocked for 1 hour with 1% BSA, incubated with indicated Siglec-Fc constructs (1 μg/well) for 1 h, and wells were then incubated with 0.5 μg/well PE anti-human-IgG Fc antibody (Clone HP6017, Cat# 409304, BioLegend, San Diego, CA). Fluorescence intensity (546nm excitation, 578nm emission) was measured on a SpectraMAX Gemini EM fluorescence plate reader.
For binding assays using anti-Siglec-9 antibodies, Protein A-coated microtiter plates (Cat# 15155, Pierce) were incubated with Siglec-9-Fc (1 μg/well) in 1X DPBS overnight at 4°C. Wells were blocked for 1 hour with 1% BSA, incubated for 1 hour with 10 μg/mL mouse anti-human Siglec-9 monoclonal antibody α-Sig9(Sia), mouse anti-human Siglec-9 monoclonal antibody α-Sig9(HA), recombinant human IgG (non-specific control, Bio-Rad), or 1% BSA only (for positive and negative controls). Wells were subsequently incubated with THP (10 μg/well) in 1% BSA with PBS at room temperature for 1 h, followed by 0.5 μg/well FITC mouse anti-human THP antibody in 1% BSA with PBS for 30 min. Fluorescence intensity (494nm excitation, 520nm emission) was measured on a SpectraMAX Gemini EM fluorescence plate reader.
Mouse LPS cystitis model
All studies involving animals were reviewed and approved by the University of California San Diego Animal Care and Use Committee, and performed using accepted veterinary standards. THP +/+ (WT) and THP -/- mouse breeding pairs, described previously15, were a generous gift from the Kumar lab, and bred and maintained at UCSD. All animals used in this study were female aged 2-4 months. Mice were allowed to eat and drink ad libitum. All efforts were made to minimize suffering of animals employed in this study.
To induce cystitis with purified E. coli LPS (Sigma Aldrich) was suspended at 1 mg/mL in molecular grade water. LPS was diluted to 100μg/mL in tissue culture grade sterile Dulbecco's PBS and 50 μl was introduced into the bladder through transurethral insertion of a 30g ½ in. hypodermic needle catheter encased in an UV-sterilized polyethylene tube (inner dimension 0.28 mm, outer dimension 0.61 mm, Catalog #598321, Harvard Apparatus) into an isoflurane-anesthetized mouse. The bladder was voided of urine prior to LPS introduction. Mock-treated animals received 50 μl of sterile Dulbecco's PBS via catheter. Urine was collected 24 h post-treatment.
Flow cytometry of urine
Urine samples were passed through a 40-μm filter, and cells were washed in PBS, and blocked with 2% FBS for 15 min on ice. Staining of surface markers was performed in 2% FBS using 0.5 μg/mL anti-CD11b-FITC (Clone M1/70, Cat#553310, BD Pharmingen) and anti-Ly6G-APC (Clone 1A8, Cat# 127614, BioLegend) for 30 min on ice. Samples were gated on unstained cells and positive signals determined using single-stain controls. Samples were run on BD FACSCantoII (BD Biosciences) and data analyzed data analyzed using FlowJo v10.2 software (FlowJo LLC).
Statistical analyses
All in vitro experiments were performed in technical triplicates, and repeated in at least three independent experiments. Statistical analyses were performed on the means of independent experiments. All neutrophil assays were performed with at least 3 biological (donor) replicates. All in vivo experiments were performed using at least 4 mice per group, and repeated in three independent experiments with results combined prior to statistical analyses. For in vitro experiments, sample size to ensure adequate power to detect effects was based on prior similar studies performed by our group and others. For in vivo experiments, sample size was estimated using the Power/Sample Size Calculator provided by the University of British Columbia Department of Statistics (https://www.stat.ubc.ca/∼rollin/stats/ssize/n2.html). Animals were not randomized or blinded prior to experiments. Statistical analyses were conducted using GraphPad Prism, version 5.04 (GraphPad Software Inc., La Jolla, CA). For in vitro experiments, all data was assumed parametric and statistical analyses performed include Student's unpaired two-tailed t-test, one-way ANOVA with Bonferroni's multiple comparisons post-test, or two-way ANOVA with Bonferroni's multiple comparisons post-test as indicated in figure legends. For in vivo experiments, statistical analyses performed include non-parametric two-tailed Mann-Whitney test as indicated in figure legends. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 represent statistical significance, or n.s. represents non-significant (P > 0.05).
Supplementary Material
(A) Percent cells positive for propidium iodide of neutrophils pretreated with THP for time given, or not (control), and quantified via flow cytometry. (B) Overnight growth of UPEC UTI89 incubated with given concentrations of THP in either LB or RPMI 1640 supplemented with 10% LB. (C) Plate-based binding assay of immobilized THP with human Siglec-9-Fc and visualized with PE anti-human IgG Fc antibody. THP was pretreated with β hexosaminidase from Canavalia ensiformis (500 mU/mL, β-Hex) or Arthrobacter ureafaciens sialidase (100 mU/mL, AUS), or both for 1 h. An anti-Siglec-9 antibody was used as a positive control. Data represent the means of two independent experiments performed in technical triplicate with results combined, n = 2/group.
Acknowledgments
We thank Michael Florio for breeding and maintaining the mice along with the UCSD vivarium staff. Studies were supported by NIH/NHLBI Program of Excellence in Glycosciences (P01 HL107150 to VN and AV), and by NIH/NHLBI grant HL125352 (VN). KAP was supported through a postdoctoral fellowship from University of California Chancellor's Postdoctoral Fellowship Program and AC through a postdoctoral fellowship from the A. P. Giannini Foundation. We are grateful to Ross Corriden for assistance with neutrophil studies.
Footnotes
Conflict of Interest: The authors have declared that no conflict of interest exists.
References
- 1.McLellan LK, Hunstad DA. Urinary tract infection: pathogenesis and outlook. Trends Mol Med. 2016 doi: 10.1016/j.molmed.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tamm I, Horsfall FL., Jr Characterization and separation of an inhibitor of viral hemagglutination present in urine. Proc Soc Exp Biol Med. 1950;74:106–108. [PubMed] [Google Scholar]
- 3.van Rooijen JJ, Voskamp AF, Kamerling JP, Vliegenthart JF. Glycosylation sites and site-specific glycosylation in human Tamm-Horsfall glycoprotein. Glycobiology. 1999;9:21–30. doi: 10.1093/glycob/9.1.21. [DOI] [PubMed] [Google Scholar]
- 4.Easton RL, Patankar MS, Clark GF, Morris HR, Dell A. Pregnancy-associated changes in the glycosylation of tamm-horsfall glycoprotein. Expression of sialyl Lewis(x) sequences on core 2 type O-glycans derived from uromodulin. J Biol Chem. 2000;275:21928–21938. doi: 10.1074/jbc.M001534200. [DOI] [PubMed] [Google Scholar]
- 5.Parkkinen J, Virkola R, Korhonen TK. Identification of factors in human urine that inhibit the binding of Escherichia coli adhesins. Infect Immun. 1988;56:2623–2630. doi: 10.1128/iai.56.10.2623-2630.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pak J, Pu Y, Zhang ZT, Hasty DL, Wu XR. Tamm-Horsfall protein binds to type 1 fimbriated Escherichia coli and prevents E. coli from binding to uroplakin Ia and Ib receptors. J Biol Chem. 2001;276:9924–9930. doi: 10.1074/jbc.M008610200. [DOI] [PubMed] [Google Scholar]
- 7.Leeker A, Kreft B, Sandmann J, Bates J, Wasenauer G, Muller H, et al. Tamm-Horsfall protein inhibits binding of S- and P-fimbriated Escherichia coli to human renal tubular epithelial cells. Exp Nephrol. 1997;5:38–46. [PubMed] [Google Scholar]
- 8.Serafini-Cessi F, Monti A, Cavallone D. N-Glycans carried by Tamm-Horsfall glycoprotein have a crucial role in the defense against urinary tract diseases. Glycoconj J. 2005;22:383–394. doi: 10.1007/s10719-005-2142-z. [DOI] [PubMed] [Google Scholar]
- 9.Muchmore AV, Decker JM. Uromodulin: a unique 85-kilodalton immunosuppressive glycoprotein isolated from urine of pregnant women. Science. 1985;229:479–481. doi: 10.1126/science.2409603. [DOI] [PubMed] [Google Scholar]
- 10.Kuriyama SM, Silverblatt FJ. Effect of Tamm-Horsfall urinary glycoprotein on phagocytosis and killing of type I-fimbriated Escherichia coli. Infect Immun. 1986;51:193–198. doi: 10.1128/iai.51.1.193-198.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siao SC, Li KJ, Hsieh SC, Wu CH, Lu MC, Tsai CY, et al. Tamm-Horsfall glycoprotein enhances PMN phagocytosis by binding to cell surface-expressed lactoferrin and cathepsin G that activates MAP kinase pathway. Molecules. 2011;16:2119–2134. doi: 10.3390/molecules16032119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dinour D, Ganon L, Nomy LI, Ron R, Holtzman EJ. Wild-type uromodulin prevents NFkB activation in kidney cells, while mutant uromodulin, causing FJHU nephropathy, does not. J Nephrol. 2014;27:257–264. doi: 10.1007/s40620-014-0079-7. [DOI] [PubMed] [Google Scholar]
- 13.Darisipudi MN, Thomasova D, Mulay SR, Brech D, Noessner E, Liapis H, et al. Uromodulin triggers IL-1β-dependent innate immunity via the NLRP3 inflammasome. J Am Soc Nephrol. 2012;23:1783–1789. doi: 10.1681/ASN.2012040338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mo L, Zhu XH, Huang HY, Shapiro E, Hasty DL, Wu XR. Ablation of the Tamm-Horsfall protein gene increases susceptibility of mice to bladder colonization by type 1-fimbriated Escherichia coli. Am J Physiol Renal Physiol. 2004;286:F795–802. doi: 10.1152/ajprenal.00357.2003. [DOI] [PubMed] [Google Scholar]
- 15.Bates JM, Raffi HM, Prasadan K, Mascarenhas R, Laszik Z, Maeda N, et al. Tamm-Horsfall protein knockout mice are more prone to urinary tract infection: rapid communication. Kidney Int. 2004;65:791–797. doi: 10.1111/j.1523-1755.2004.00452.x. [DOI] [PubMed] [Google Scholar]
- 16.Micanovic R, Chitteti BR, Dagher PC, Srour EF, Khan S, Hato T, et al. Tamm-Horsfall protein regulates granulopoiesis and systemic neutrophil homeostasis. J Am Soc Nephrol. 2015;26:2172–2182. doi: 10.1681/ASN.2014070664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas DB, Davies M, Peters JR, Williams JD. Tamm Horsfall protein binds to a single class of carbohydrate specific receptors on human neutrophils. Kidney Int. 1993;44:423–429. doi: 10.1038/ki.1993.260. [DOI] [PubMed] [Google Scholar]
- 18.Dall'Olio F, Chiricolo M, Malagolini N, Franceschi C, Serafini-Cessi F. Immunosuppressive activity of Tamm-Horsfall glycoprotein oligosaccharides: effect of removal of outer sugars and conjugation with a protein carrier. Cell Immunol. 1991;137:303–315. doi: 10.1016/0008-8749(91)90081-l. [DOI] [PubMed] [Google Scholar]
- 19.Crocker PR, Varki A. Siglecs in the immune system. Immunology. 2001;103:137–145. doi: 10.1046/j.0019-2805.2001.01241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang JQ, Nicoll G, Jones C, Crocker PR. Siglec-9, a novel sialic acid binding member of the immunoglobulin superfamily expressed broadly on human blood leukocytes. J Biol Chem. 2000;275:22121–22126. doi: 10.1074/jbc.M002788200. [DOI] [PubMed] [Google Scholar]
- 21.Secundino I, Lizcano A, Roupe KM, Wang X, Cole JN, Olson J, et al. Host and pathogen hyaluronan signal through human siglec-9 to suppress neutrophil activation. J Mol Med (Berl) 2016;94:219–233. doi: 10.1007/s00109-015-1341-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carlin AF, Uchiyama S, Chang YC, Lewis AL, Nizet V, Varki A. Molecular mimicry of host sialylated glycans allows a bacterial pathogen to engage neutrophil Siglec-9 and dampen the innate immune response. Blood. 2009;113:3333–3336. doi: 10.1182/blood-2008-11-187302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang JQ, Biedermann B, Nitschke L, Crocker PR. The murine inhibitory receptor mSiglec-E is expressed broadly on cells of the innate immune system whereas mSiglec-F is restricted to eosinophils. Eur J Immunol. 2004;34:1175–1184. doi: 10.1002/eji.200324723. [DOI] [PubMed] [Google Scholar]
- 24.Muchmore AV, Shifrin S, Decker JM. In vitro evidence that carbohydrate moieties derived from uromodulin, an 85,000 dalton immunosuppressive glycoprotein isolated from human pregnancy urine, are immunosuppressive in the absence of intact protein. J Immunol. 1987;138:2547–2553. [PubMed] [Google Scholar]
- 25.Parsons CL, Stein P, Zupkas P, Chenoweth M, Argade SP, Proctor JG, et al. Defective Tamm-Horsfall protein in patients with interstitial cystitis. J Urol. 2007;178:2665–2670. doi: 10.1016/j.juro.2007.07.125. [DOI] [PubMed] [Google Scholar]
- 26.Argade S, Chen T, Shaw T, Berecz Z, Shi W, Choudhury B, et al. An evaluation of Tamm-Horsfall protein glycans in kidney stone formers using novel techniques. Urolithiasis. 2015;43:303–312. doi: 10.1007/s00240-015-0775-3. [DOI] [PubMed] [Google Scholar]
- 27.Rambausek M, Dulawa J, Jann K, Ritz E. Tamm Horsfall glycoprotein in diabetes mellitus: abnormal chemical composition and colloid stability. Eur J Clin Invest. 1988;18:237–242. doi: 10.1111/j.1365-2362.1988.tb01252.x. [DOI] [PubMed] [Google Scholar]
- 28.Torffvit O, Agardh CD. Tubular secretion of Tamm-Horsfall protein is decreased in type 1 (insulin-dependent) diabetic patients with diabetic nephropathy. Nephron. 1993;65:227–231. doi: 10.1159/000187479. [DOI] [PubMed] [Google Scholar]
- 29.Olczak T, Olczak M, Kubicz A, Dulawa J, Kokot F. Composition of the sugar moiety of Tamm-Horsfall protein in patients with urinary diseases. Int J Clin Lab Res. 1999;29:68–74. doi: 10.1007/s005990050066. [DOI] [PubMed] [Google Scholar]
- 30.Ghirotto S, Tassi F, Barbujani G, Pattini L, Hayward C, Vollenweider P, et al. The uromodulin gene locus shows evidence of pathogen adaptation through human evolution. J Am Soc Nephrol. 2016;27:2983–2996. doi: 10.1681/ASN.2015070830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mulvey MA, Schilling JD, Hultgren SJ. Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect Immun. 2001;69:4572–4579. doi: 10.1128/IAI.69.7.4572-4579.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lizcano A, Secundino I, Dohrmann S, Corriden R, Rohena C, Diaz S, et al. Erythrocyte sialoglycoproteins engage Siglec-9 on neutrophils to suppress activation. Blood. 2017 doi: 10.1182/blood-2016-11-751636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Angata T, Varki A. Cloning, characterization, and phylogenetic analysis of siglec-9, a new member of the CD33-related group of siglecs. Evidence for co-evolution with sialic acid synthesis pathways. J Biol Chem. 2000;275:22127–22135. doi: 10.1074/jbc.M002775200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Percent cells positive for propidium iodide of neutrophils pretreated with THP for time given, or not (control), and quantified via flow cytometry. (B) Overnight growth of UPEC UTI89 incubated with given concentrations of THP in either LB or RPMI 1640 supplemented with 10% LB. (C) Plate-based binding assay of immobilized THP with human Siglec-9-Fc and visualized with PE anti-human IgG Fc antibody. THP was pretreated with β hexosaminidase from Canavalia ensiformis (500 mU/mL, β-Hex) or Arthrobacter ureafaciens sialidase (100 mU/mL, AUS), or both for 1 h. An anti-Siglec-9 antibody was used as a positive control. Data represent the means of two independent experiments performed in technical triplicate with results combined, n = 2/group.