Abstract
Background
Coronary artery calcium (CAC) is an established predictor of future major adverse atherosclerotic cardiovascular events in asymptomatic individuals. However limited data exist as to how CAC compares to functional testing (FT) in estimating prognosis in symptomatic patients.
Methods
In the Prospective Multicenter Imaging Study for Evaluation of Chest Pain (PROMISE) trial, patients with stable chest pain (or dyspnea) and intermediate pre-test probability for obstructive coronary artery disease (CAD) were randomized to FT (exercise electrocardiography, nuclear stress, or stress echocardiography) or anatomic testing. We evaluated those who underwent CAC testing as part of the anatomic evaluation (n=4,209) and compared to results of FT (n=4,602). We stratified CAC and FT results as normal or mildly, moderately or severely abnormal (for CAC: 0, 1–99 Agatston Score [AS], 100–400 AS and >400 AS, respectively; for FT: normal, mild=late positive treadmill, moderate=early positive treadmill or single-vessel ischemia and severe=large ischemic region abnormality). The primary endpoint was all-cause death, myocardial infarction or unstable angina hospitalization over a median follow-up of 26.1 months. Cox regression models were used to calculate hazard ratios and C-statistic to determine predictive and discriminatory value.
Results
Overall, the distribution of normal or mildly, moderately or severely abnormal test results was significantly different between FT and CAC (FT = normal 3588 [78.0%], mild 432 [9.4%], moderate 217 [4.7%], severe 365 [7.9%]; CAC = normal 1,457 [34.6%], mild 1340 [31.8%], moderate 772 [18.3%], severe 640 [15.2%], p <0.0001). Moderate and severe abnormalities in both arms robustly predicted events (moderate: CAC HR 3.14, 95% CI 1.81–5.44 and FT HR 2.65, 95% CI 1.46–4.83; severe: CAC HR 3.56, 95% CI 1.99–6.36 and FT HR 3.88, 95% CI 2.58–5.85. In the CAC arm, the majority of events (n=112/133; 84%) occurred in patients with any positive CAC test (score >0) whereas less than half of events occurred in patients with mild, moderate or severely abnormal FT (n=57/132; 43%) (p<0.001). In contrast, any abnormality on FT was significantly more specific for predicting events (78.6% for FT vs 35.2% for CAC, p<0.001). Overall discriminatory ability in predicting the primary endpoint of mortality, nonfatal myocardial infarction, and unstable angina hospitalization was similar and fair for both CAC and FT (c-statistic, 0.67 vs. 0.64). Coronary computed tomographic angiography provided significantly better prognostic information compared to FT and CAC testing (C-index: 0.72).
Conclusion
Among stable outpatients presenting with suspected CAD, most patients experiencing clinical events have measurable CAC at baseline while less than half have any abnormalities on FT. However, an abnormal FT was more specific for cardiovascular events, leading to overall similarly modest discriminatory abilities of both tests.
Clinical Trial Registration
URL: https://clinicaltrials.gov; Unique Identifier: NCT01174550
Keywords: coronary artery disease, diagnostic testing, prognosis, coronary artery calcium
INTRODUCTION
Functional testing (FT) for patients with chest pain has been a preferred method for evaluation of coronary artery disease (CAD) during decades. Current guidelines recommend FT to risk stratify and identify patients with ischemia prior to invasive coronary angiography (ICA). However, as lower probability patients are being referred, the prognostic and diagnostic accuracy of FT has declined.1,2,3 Rozanski et al demonstrated that the prevalence of abnormal single photon emission computed tomography studies declined from 41% to only 9% over two decades.4 A lower pre-test probability being tested may have led to poorer performance of FT, especially for diagnosis of obstructive CAD. Currently, a majority of patients who undergo ICA after FT have non-obstructive CAD or normal coronary arteries, confirming a low diagnostic accuracy in current clinical practice.5 Of the patients enrolled in the National Cardiovascular Data Registry who had positive stress tests, 59% were found to have normal or non-obstructive disease at the time of ICA, slightly better than the 65% negative rate of those receiving ICA without stress testing prior.6 In another National Cardiovascular Data Registry analysis of 302,651 single photon emission computed tomography studies, only 134,670 (44.4%) had obstructive disease at ICA.5 Exercise treadmill testing, stress echocardiography and magnetic resonance imaging also yielded very low (44–45%) rates of obstructive CAD at time of ICA. This performance of FT in current practice calls for evaluation of alternative strategies for the initial work-up of patients who are presently at lower risk for myocardial ischemia and who have very low event rates given contemporary care.7 These alternative strategies could also include no testing.
Coronary artery calcium (CAC) is low cost (typically <$100) and low radiation (<1 milliSievert) test, which allows direct visualization of coronary atherosclerosis without needles, contrast or injection.8 According to the most recent American Heart Association (AHA) 9 and American College of Cardiology Foundation (ACCF)/AHA10 guidelines, CAC has Class IIA and IIB recommendations for assessing risk in intermediate and low-to-intermediate asymptomatic patients, respectively, and in guiding management of hyperlipidemia.11 Studies also indicate that CAC may accurately risk stratify both low risk stable patients with new onset chest pain 12,13 and those presenting to the emergency department with acute chest pain, and has a Class IIB recommendation for use in symptomatic individuals.9,14 It should be noted that CAC does not test for obstructive disease or functional ischemia, but rather is a surrogate for coronary atherosclerotic plaque burden. A low plaque burden in symptomatic individuals has been shown to be associated with a low risk state, and has been used for symptomatic individuals to predict a low risk state with absent or minimal CAC. However, most studies of CAC in symptomatic patients were limited by a relatively small numbers of patients and limited follow-up. No large scale or randomized study has evaluated the utility or prognostic ability of CAC in stable CAD compared to FT.
The Prospective Multicenter Imaging Study for Evaluation of Chest Pain (PROMISE) trial has published on the prognostic implications of functional testing compared to coronary computed tomographic angiography (CTA).7,15 We have recently published a sub-study of PROMISE comparing coronary CTA vs FT.16 Coronary CTA, by visualizing non-obstructive CAD, identifies additional at-risk patients and imparts better prognostic and discriminatory information than FT. Here we present unique data evaluating prognostic data related to CAC in the PROMISE trial. We hypothesize that coronary atherosclerosis burden, as determined by CAC, will be a robust predictor of major adverse cardiovascular events (MACE). We sought to evaluate the comparative prognostic ability of FT to CAC in a large cohort of symptomatic low-intermediate risk patients.
METHODS
Study Design and Population
PROMISE (ClinicalTrials.gov # NCT01174550) is a pragmatic comparative effectiveness trial that enrolled 10,003 patients at 193 sites in North America with expertise in the fields of cardiology, primary care, radiology, and anesthesia and representing both community practices and academic medical centers. Details regarding the PROMISE study population, selection criteria, design, and primary results have been described elsewhere.7,15 The study participants were stable symptomatic outpatients without known CAD who were randomized to either anatomic or functional noninvasive cardiovascular testing for further evaluation. The methods for FT results classification and prognostic performance have recently been published by Hoffmann et al, in an analysis comparing FT to CTA.16
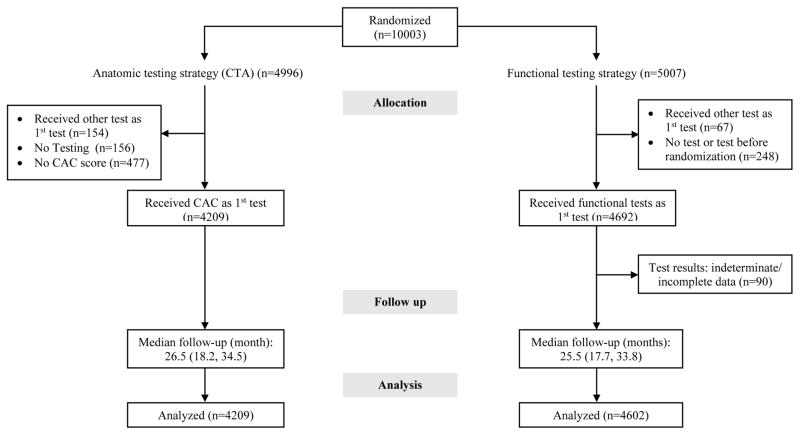
For this analysis we included patients who received the initial diagnostic test as randomized. We excluded subjects who received other tests as their first test, did not undergo any diagnostic test, or did not receive a non-contrast study as part of their CTA. Of the 4,996 patients who were randomized to the initial anatomical testing arm (CTA), 4,209 underwent CAC testing. This included those that received a non-contrast computed tomography (CT) only. Of the 4,996 who were randomized to anatomic imaging (CTA), 154 who received a different test other than CT first, 156 did not receive any testing despite randomization to anatomic imaging, and 477 did not receive CAC during the CTA. PROMISE was set up as a pragmatic study of CTA vs FT and CAC was not specified as a part of the scanning algorithm, but 90% of patients who underwent CTA had a CAC test as well and are included in this analysis.
For the FT group, we excluded patients whose test results could not be assigned to prespecified test strata due to indeterminate test results, including patients who underwent FT with exercise but achieved less than 75% of maximum predicted heart rate. The flow of patients is described in Figure 1.
Figure 1.
Inclusion criteria
Study Procedures
After providing written informed consent, participants were randomly assigned to either the anatomic or the FT group, with stratification according to study site and according to the choice, as indicated before randomization by the site clinician, of the intended FT if the patient were to be assigned to that study group.7 FT included exercise electrocardiogram (ECG), exercise or pharmacologic nuclear myocardial perfusion imaging, and exercise or pharmacologic stress echocardiography. CAC and/or CTA was performed with at least 64-slice multidetector computed tomographic technology. Not all patients received CAC as part of anatomic testing. This was a pragmatic study where the protocol required CTA testing, not specifying whether CAC needed to be done as part of that exam. Thus, each site was able to decide whether to include CAC or not at the time of scan. The information related to exclusion of patients, FT categories and all interpretations were based on site interpretations and have been described in detail previously.16
Diagnostic Test Results
We defined a positive CAC to be one that showed coronary calcium (Agatston score >0).17 An exercise ECG was considered positive if there were ST-segment changes consistent with ischemia during stress or early termination (<3 minutes) due to reproduction of symptoms, arrhythmia, and/or hypotension. A stress echocardiography or stress nuclear test was considered positive if there was any inducible ischemia in at least one coronary territory (anterior, inferior, or lateral) or early termination of exercise stress (<3 minutes) due to ST-segment changes consistent with ischemia, symptom reproduction, arrhythmia, and/or hypotension.
To further evaluate differences in test performance between functional and CAC testing, we stratified normal tests into (a) completely normal defined as CAC score =0, normal ECG, absence of symptoms during exercise, normal exercise duration, and normal imaging (absence of any findings suggesting myocardial abnormalities including fixed perfusion defects) and (b) mildly abnormal defined as presence of any abnormalities not representing obstructive CAD or myocardial ischemia (CAC score of 1–99); late positive treadmill, abnormal ECG, or fixed defects for functional imaging). A more detailed description of the classification of test results is provided in Table 1. The FT cut-off values have been previously published.16 We also chose to evaluate two different CAC cut-points of interest, a score of >400 and >300 for severe abnormality (as defined by ACC/AHA 2013 guidelines). We further evaluated the performance of scores of 0 (to define appropriate use criteria of zero, <10 (inclusive of zero) and >10 as prespecified points of interest clinically.
Table 1.
Prospective risk stratification of noninvasive imaging test results in the anatomical (coronary artery calcium [CAC]) and functional (exercise treadmill test, stress myocardial perfusion imaging [MPI], and stress echocardiography) testing arms of the study.
| Anatomical | Functional* | |||
|---|---|---|---|---|
| Test Strata | Coronary Artery Calcium | Exercise Treadmill Test | Stress MPI | Stress Echo |
| Severely abnormal |
Severe Calcification CAC Score >400 |
Ischemic ECG ST changes consistent with ischemia during stress + either severe ventricular arrhythmia OR Hypotension |
Large territory inducible Ischemia or mixed defect Septal/anterior/apical territory or other single territory with transient ischemic dilatation or 2 or more coronary territories with ischemia |
Large territory inducible Ischemia or mixed defect Wall motion abnormality or mixed abnormality (infarct and ischemia) Isolated Septal/anterior/apical or other single territory +↓EF <35 % during stress or 2 or more coronary territories |
| Moderately abnormal |
Moderate Calcification CAC Score 100–400 |
Early positive TM Failure to reach stage 2 (<3:00 min) with ST changes OR symptoms reproduced OR any arrhythmia or hypotension |
Inducible Ischemia or mixed defect Perfusion abnormality in one coronary territory (Lateral or Inferior/posterior) OR Normal imaging but Early positive TM Failure to reach stage 2 (<3:00 min) with ST changes OR symptoms reproduced OR any arrhythmia or hypotension |
Inducible Ischemia or mixed defect Wall motion abnormality or mixed abnormality (infarct and ischemia) in one coronary territory (Lateral or Inferior/posterior) OR Normal imaging but Early positive TM Failure to reach stage 2 (<3:00 min) with ST changes OR symptoms reproduced OR any arrhythmia or hypotension |
| Mildly abnormal |
Mild Calcification CAC Score 1–99 |
Late positive TM More than stage 2 (>3:00 min) but failure to finish protocol or target heart rate achieved due to ST changes OR symptoms reproduced OR any arrhythmia or hypotension |
Positive ECG Normal perfusion or fixed perfusion defect (Scar) OR Normal imaging but Late positive TM More than stage 2 (>3:00 min) but failure to finish protocol or target heart rate achieved due to ST changes OR symptoms reproduced OR any arrhythmia or hypotension |
Positive ECG but normal wall motion or resting wall motion abnormality without inducible ischemia OR Normal imaging but Late positive TM More than stage 2 (>3:00 min) but failure to finish protocol or target heart rate achieved due to ST changes OR symptoms reproduced OR any arrhythmia or hypotension |
| Normal | CAC Score Zero | Normal | Normal | Normal |
To standardize test reporting, site-reported test results were abstracted by a cardiology faculty or senior fellow physician using a prospectively designed protocol to deal with ambiguous test results, thereby standardizing interpretation of ambiguous test reports and harmonizing data across imaging modalities. CAD denotes coronary artery disease, ECG electrocardiography, LAD left anterior descending, and TM treadmill test.
-For secondary risk stratification, mildly abnormal was defined as <70% luminal narrowing.
As defined by Hoffmann et al. (2017), Ref 16
Cardiovascular Risk Factors
Patient demographics and traditional cardiovascular risk factors were assessed and documented in a standard fashion at the time of enrollment into the PROMISE trial.15
Study Endpoints
The primary endpoint was a composite of time to MACE including death from any cause, myocardial infarction, or hospitalization for unstable angina. The secondary endpoint was defined as a composite of cardiovascular death, myocardial infarction, or hospitalization for unstable angina, and the tertiary endpoint was a composite of cardiovascular death or myocardial infarction. An independent clinical events committee adjudicated all primary and secondary endpoint events in a blinded fashion on the basis of standard, prospectively determined definitions.7,15
Statistical Analysis
Descriptive statistics are presented as mean and standard deviation for continuous variables and absolute and relative frequencies for categorical variables. Cumulative event rates based on test results were computed for each testing strategy (CAC and FT) using the method of Kaplan and Meier.18 Cox proportional hazards regression models were used to compute hazard ratios and 95% confidence intervals regarding the relationship of test results to the time to the first clinical event (or censoring) for each composite endpoint.19 To appropriately account for heterogeneity among the subjects, analyses were adjusted for a prespecified set of baseline covariates, including age, sex, CAD risk equivalent (history of either diabetes mellitus, peripheral artery disease, or cerebrovascular disease), and the prespecification of the intended FT (if randomly assigned to the FT arm). Proportional-hazards assumption were tested on the basis of Schoenfeld residuals. To evaluate the predictive power of the Cox regression models we computed Harrell’s C (C-statistic).20,21 Analyses were performed for the primary, secondary, and tertiary endpoints. All P-values are 2-sided, and were considered significant if < 0.05. All analyses were performed using Stata, version 14.2 (StataCorp, College Station, Texas).
RESULTS
Of the patients included, 4,209 received CAC testing, and 4,602 received FT. The baseline demographics and risk factors are outlined in Table 2. There were no clinically meaningful differences in baseline patient demographics, cardiovascular risk, medication, or clinical presentation between CAC and FT patients (Table 2). Overall, patients were on average 61 years of age, slightly more than 50% were women, 78% were white, and the combined Diamond-Forrester and Coronary Artery Surgery Score was 53 in both groups. Approximately 51% were intermediate risk by Framingham Risk score (6–20%) and 73% presented with chest pain, of which 78% was described as atypical angina.
Table 2.
Characteristics of the Trial Participants at Baseline, According to Study Group.*
| Variable | Coronary Artery Calcium (N=4209) | Functional Testing (N=4602) | p-value |
|---|---|---|---|
| Demographics | |||
| Age (yrs) | 60.6 ± 8.2 | 61.0 ± 8.3 | 0.034 |
| Female sex | 2141 (50.9%) | 2458 (53.4%) | 0.018 |
| Racial or ethnic minority | 953 (22.8%) | 983 (21.5%) | 0.149 |
| Cardiac risk factors | |||
| BMI (kg/m2) | 30.4 ± 5.9 | 30.5 ± 6.1 | 0.503 |
| Hypertension | 2731 (64.9%) | 2999 (65.2%) | 0.788 |
| Diabetes | 878 (20.9%) | 999 (21.7%) | 0.335 |
| Dyslipidemia | 2865 (68.1%) | 3127 (67.9%) | 0.909 |
| Family history of premature CAD | 1389 (33.1%) | 1426 (31.1%) | 0.046 |
| Peripheral or cerebrovascular disease | 224 (5.3%) | 264 (5.7%) | 0.402 |
| CAD equivalent | 1037 (24.6%) | 1189 (25.8%) | 0.202 |
| History of heart failure | 160 (3.8%) | 176 (3.8%) | 0.956 |
| Metabolic syndrome | 1559 (37.0%) | 1763 (38.3%) | 0.226 |
| Current or past tobacco use | 2164 (51.4%) | 2367 (51.4%) | 1.000 |
| Sedentary lifestyle | 2005 (47.7%) | 2229 (48.5%) | 0.468 |
| History of depression | 802 (19.1%) | 992 (21.6%) | 0.004 |
| Risk factor burden and risk score | |||
| No risk factors | 106 (2.5%) | 130 (2.8%) | 0.391 |
| Risk factor burden | 2.4 ± 1.1 | 2.4 ± 1.1 | 0.669 |
| Combined Diamond-Forrester and Coronary Artery Surgery risk score | 53.5 ± 21.2 | 53.3 ± 21.2 | 0.651 |
| Framingham risk score | 0.265 | ||
| Low risk (<6%) | 269 (6.4%) | 325 (7.1%) | |
| Intermediate risk (6–20%) | 2164 (51.5%) | 2302 (50.1%) | |
| High risk (>20%) | 1768 (42.1%) | 1971 (42.9%) | |
| ASCVD pooled cohort risk prediction (2013) | 0.747 | ||
| Low risk (<7.5%) | 1331 (32.0%) | 1444 (31.7%) | |
| Elevated risk (>=7.5%) | 2829 (68.0%) | 3118 (68.3%) | |
| Relevant medications | |||
| Beta blocker | 1019 (25.2%) | 1095 (24.9%) | 0.706 |
| ACE or ARB | 1770 (43.8%) | 1952 (44.3%) | 0.661 |
| Statin | 1873 (46.4%) | 2008 (45.6%) | 0.471 |
| Aspirin | 1839 (45.5%) | 1941 (44.1%) | 0.174 |
| Clopidogrel | 54 (1.3%) | 69 (1.6%) | 0.414 |
| Prasugrel | 1 (<0.1%) | 1 (<0.1%) | 1.000 |
| Warfarin | 63 (1.6%) | 82 (1.9%) | 0.315 |
| Primary presenting symptom and anginal type | |||
| Chest pain | 3088 (73.4%) | 3299 (71.7%) | 0.077 |
| Dyspnea on exertion | 600 (14.3%) | 734 (16.0%) | 0.028 |
| Anginal type - site-reported | 0.975 | ||
| Typical | 470 (11.2%) | 521 (11.3%) | |
| Atypical | 3294 (78.3%) | 3595 (78.1%) | |
| Non-anginal | 445 (10.6%) | 486 (10.6%) | |
ACE indicates angiotensin-converting enzyme; ARB, angiotensin-receptor blocker; ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index; CAD, coronary artery disease; CTA, computed tomographic angiography.
Plus–minus values are means ± standard deviation.
Racial or ethnic minority group was self-reported, with the status of “minority” being defined by the patient.
Body-mass index is the weight in kilograms divided by the square of the height in meters.
A family history of premature CAD was defined as diagnosis of the disease in a male first-degree relative before 55 years of age or in a female first-degree relative before 65 years of age.
CAD risk equivalent was defined as diabetes, peripheral vascular disease, or cerebrovascular disease.
The metabolic syndrome was defined according to consensus criteria of the American Heart Association and the National Heart, Lung, and Blood Institute.
Sedentary lifestyle was defined by the patient as not participating in regular physical activities at least one time per week over the previous month.
Risk factors included hypertension, diabetes, dyslipidemia, family history of premature CAD, and tobacco use.
‡ Combined Diamond and Forrester and Coronary Artery Surgery Study risk scores range from 0 to 100, with higher scores indicating a greater likelihood of obstructive CAD.
The type of angina was reported by the study-site investigators.
Outcomes
During the median follow-up of 26.1 months (interquartile range: 18.0, 34.4), event rates (MACE) were similar in the CAC and FT arms: overall, 133/4,209 (3.2%) versus 132/4,602 (2.9%), p=0.69; as well as for hard cardiovascular events (myocardial infarction/cardiovascular death),: 53/4,209 (1.3%) versus 72/4,602 (1.6%), p=0.13 (Table 3).
Table 3.
Frequency of Abnormal and Normal Test Findings and Association With Clinical Events for CAC and Functional Testing.
| CAC Testing (N=4209) |
Functional Testing (N=4602) |
CAC Testing vs. FT | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Initial Test Results | Frequency n/N (%) |
Event Rate n/N (%) |
HR* (95% CI) | P-value | Frequency n/N (%) |
Event Rate n/N (%) |
HR* (95% CI) | P-value | P-value |
| All-cause death/MI/UA | 0.1214 | ||||||||
| Normal (CAC =0, FT = normal) | 1457/4209 (34.6) | 21/1457 (1.4) | 3588/4602 (78.0) | 75/3588 (2.1) | |||||
| Mild (CAC 1–99, FT = mild) | 1340/4209 (31.8) | 31/1340 (2.3) | 1.51 (0.86–2.65) | 0.147 | 432/4602 (9.4) | 9/432 (2.1) | 0.94 (0.47–1.89) | 0.867 | |
| Moderate (CAC 100–400, FT moderate) | 772/4209 (18.3) | 40/772 (5.2) | 3.14 (1.81–5.44) | <0.001 | 217/4602 (4.7) | 13/217 (6.0) | 2.65 (1.46–4.83) | 0.001 | |
| Severe (CAC >400, FT Severe) | 640/4209 (15.2) | 41/640 (6.4) | 3.56 (1.99–6.36) | <0.001 | 365/4602 (7.9) | 35/365 (9.6) | 3.88 (2.58–5.85) | <0.001 | |
| Cardiovascular death/MI/UA | 0.0948 | ||||||||
| Normal (CAC =0, FT = normal) | 1457/4209 (34.6) | 14/1457 (1.0) | 3588/4602 (78.0) | 56/3588 (1.6) | |||||
| Mild (CAC 1–99, FT = mild) | 1340/4209 (31.8) | 25/1340 (1.9) | 1.85 (0.96–3.58) | 0.068 | 432/4602 (9.4) | 8/432 (1.9) | 1.11 (0.53–2.33) | 0.783 | |
| Moderate (CAC 100–400, FT moderate) | 772/4209 (18.3) | 32/772 (4.2) | 3.85 (2.01–7.38) | <0.001 | 217/4602 (4.7) | 13/217 (6.0) | 3.50 (1.89–6.47) | <0.001 | |
| Severe (CAC >400, FT Severe) | 640/4209 (15.2) | 35/640 (5.5) | 4.72 (2.40–9.28) | <0.001 | 365/4602 (7.9) | 31/365 (8.5) | 4.59 (2.93–7.19) | <0.001 | |
| Cardiovascular death/MI | 0.1687 | ||||||||
| Normal (CAC =0, FT = normal) | 1457/4209 (34.6) | 9/1457 (0.6) | 3588/4602 (78.0) | 48/3588 (1.3) | |||||
| Mild (CAC 1–99, FT = mild) | 1340/4209 (31.8) | 17/1340 (1.3) | 1.77 (0.78–4.02) | 0.171 | 432/4602 (9.4) | 5/432 (1.2) | 0.81 (0.32–2.04) | 0.654 | |
| Moderate (CAC 100–400, FT moderate) | 772/4209 (18.3) | 14/772 (1.8) | 2.16 (0.90–5.16) | 0.084 | 217/4602 (4.7) | 5/217 (2.3) | 1.53 (0.60–3.90) | 0.368 | |
| Severe (CAC >400, FT Severe) | 640/4209 (15.2) | 13/640 (2.0) | 1.97 (0.78–5.02) | 0.153 | 365/4602 (7.9) | 14/365 (3.8) | 2.13 (1.16–3.91) | 0.014 | |
MI indicates myocardial infarction; UA, unstable angina.
Hazard ratios (HR) adjusted for age, sex, CAD risk equivalent (history of either diabetes mellitus, peripheral artery disease, or cerebrovascular disease), and the prespecification of the intended functional test (if randomly assigned to the functional testing arm).
An abnormal FT was significantly more specific for predicting events (78.6% for FT vs 35.2% for CAC, p<0.001) (Supplemental Table 1). Increasing the CAC cutpoint improves specificity, at an expense of sensitivity. A cutpoint of ≥100 increased specificity to 67%, while reducing sensitivity to 61%, and a CAC cutpoint of >400 revealed a specificity of 85%, while lowering sensitivity to 31%.
Testing and Outcomes
Increasing CAC scores were associated with increasing risk of MACE. A zero CAC score was associated with a very low event rate (21/1,457, 1.4%). Event rates (MACE) increased with increasing CAC scores, with scores 100–400 associated with a 5.2% event rate, increasing to 6.4% (41/640) in those with scores >400 (Table 3). A normal FT was associated with a 2.1% event rate (75/3588) increasing to 9.6% in those with severe abnormalities. Similar results were found if events were defined as cardiovascular death/myocardial infarction/unstable angina or cardiovascular death/myocardial infarction (Table 3).
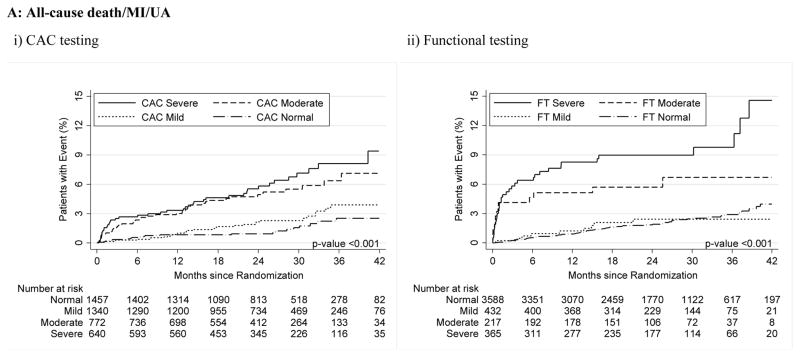
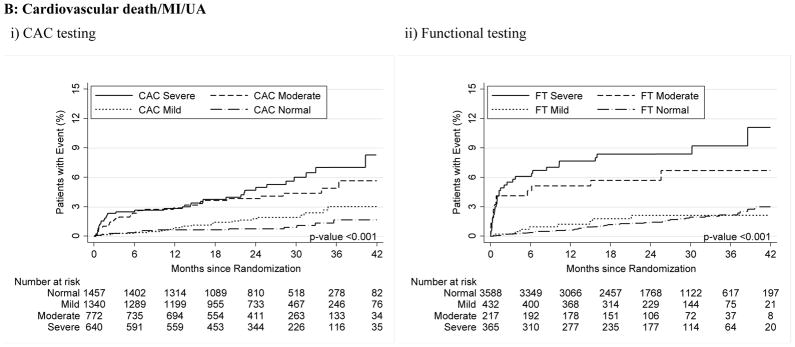
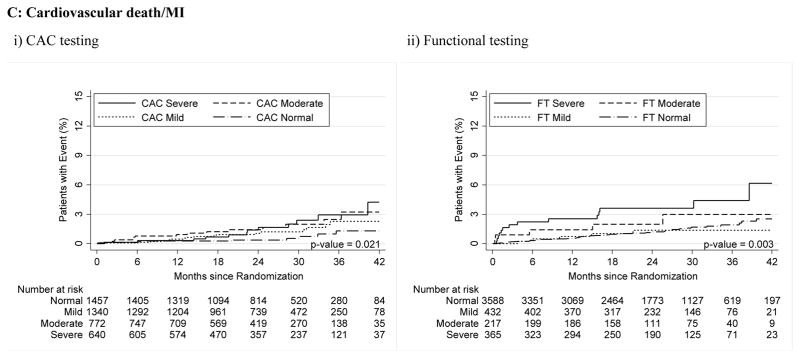
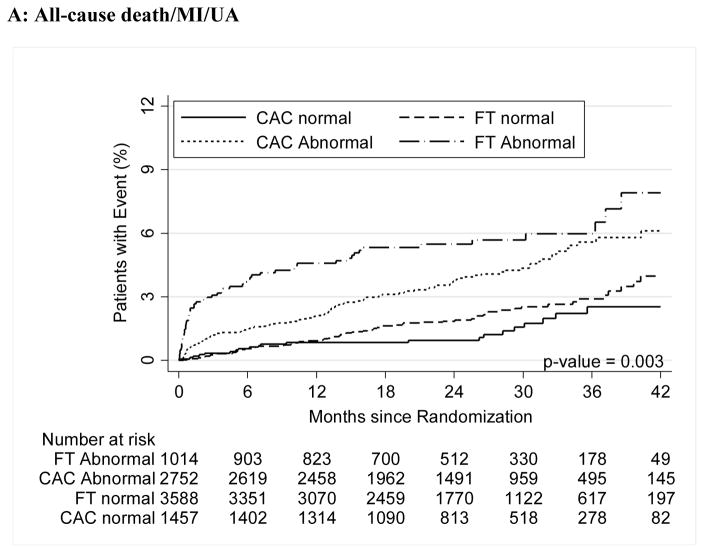
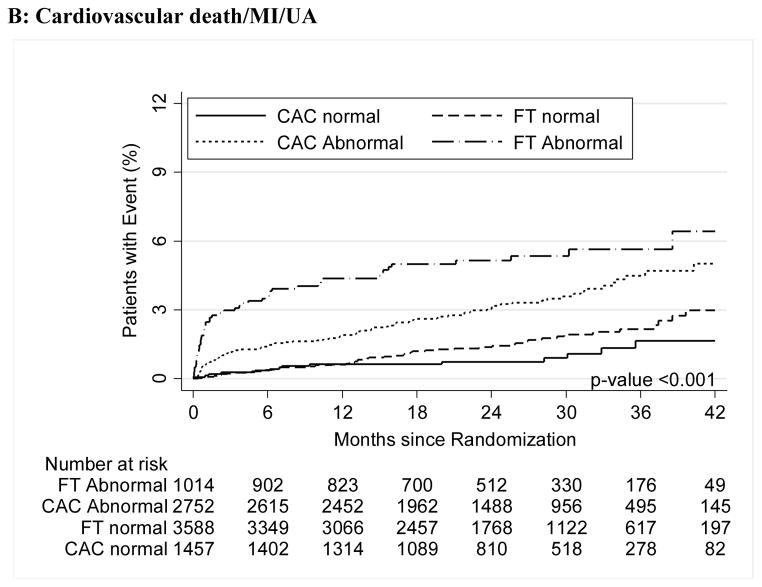
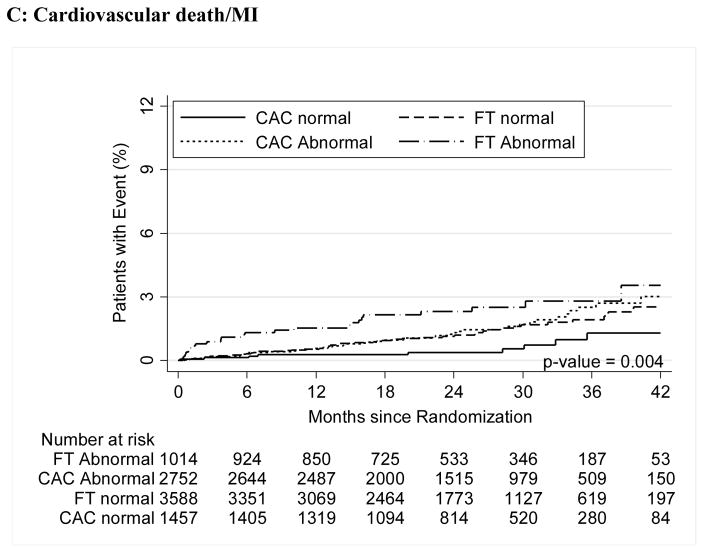
For both CAC and FT, severely abnormal test results were associated with a significantly increased relative risk for cardiovascular events for the primary endpoint compared to normal tests (HR, 3.56 [95% CI, 1.99–6.36] for CAC >400 vs. HR, 3.88 [95% CI, 2.58–5.85] for severe FT abnormality; p<0.001 for both) as well as for secondary endpoints and hard cardiovascular events (Table 3, Figure 2).
Figure 2.
A–C – Kaplan-Meier Survival Curves for CAC and Functional Testing for Normal, Mild, Moderate, and Severe Test Results in Symptomatic Persons in PROMISE
Distribution of Events based on CAC and FT abnormalities
The distribution of events between the groups were quite different between testing strategies. A positive CAC test (CAC>0) identified 112/133 total events (84.2%) and 44/53 hard events (83.0%), while a positive FT identified only 57/132 (43.2%) of total death, myocardial infarction and unstable angina and only 33.3% of hard cardiovascular events (myocardial infarction and cardiovascular death) (Table 3). Overall a negative FT had a low overall event rate (<1% annual cardiovascular event risk) and higher specificity for events than CAC. From a perspective of absolute risk, the majority of events did occur in patients with completely normal FT (75/132 [56.8%] for the primary endpoint and 48/72 [66.7%] for hard cardiovascular endpoints), while only 21/133 [15.8%] of events (and 9/53 [17.0%] of hard events) occurred in patients with normal CAC (score =0). Out of the 21 patients with CAC=0 who experienced a cardiovascular event, only two had severely abnormal CTA results (>70% stenosis). Of the remaining 19, 2 had moderately abnormal (50–70%), 6 mildly abnormal (<50% stenosis), and 11 normal coronary CTA results.
Discriminatory Ability
Based on this result, we further characterized the test results of patients who did not have an abnormal test defined as significant CAC (≥100) (n=2,797) or myocardial ischemia (n=4,020). CAC scanning identified 47.9% (n=1340/2797) of patients as having mild CAC (defined as score 1–99) and a minority of events occurred in these patients (23.3%, n=31/133) (Table 3). Combining all normal and mild CAC represented 39.1% of events (52/133). In contrast, only 10.7% (n=432/4020) of patients in the FT arm had mildly abnormal tests (defined as presence of any abnormalities not representing myocardial ischemia including late positive treadmill, abnormal ECG, or fixed defects; Table 1), and the majority of events (63.6%, n=84/132) occurred in those with mild or completely normal functional tests. Similar findings were seen for the endpoints of cardiovascular death/myocardial infarction/unstable angina and cardiovascular death and myocardial infarction (Table 3). CAC showed higher discriminatory value as compared to FT for the primary endpoint among patients without significant CAC or myocardial ischemia (C-statistic, 0.56 [95% CI, 0.49–0.63] vs. 0.49 [95% CI, 0.45–0.53]). Similar results were seen for the more specific cardiovascular endpoints of cardiovascular mortality, myocardial infarction, and unstable angina (C-statistic, 0.58 vs. 0.52) and cardiovascular mortality and myocardial infarction (C-statistic, 0.60 vs. 0.50).
Moderate and severe abnormalities in both arms robustly predicted events (Moderate: CAC HR 3.14; 95% CI 1.81–5.44 and FT HR 2.65; 1.46–4.83), Severe (CAC HR 3.56; 1.99–6.36 and FT HR 3.88; 2.58–5.85). Similar results were found for hard events (cardiovascular death/myocardial infarction).
Varying the cutpoints of CAC did not significantly alter the relationships with events (Supplemental Tables 2–3). Defining a CAC ≤10 as normal did not change the significant prediction of CAC>10 for cardiovascular events (HR 2.42 (1.56–3.76), p<0.001) (Table 4). There was no significant difference for any endpoint in stratifying by CAC = 0 or defining low risk as CAC <10. Similarly, defining severe CAC as >300 (as defined in the ACC/AHA risk assessment guidelines5) led to similar predictive power for events (HR 1.94 (1.32–2.86), p<0.001) (Table 4).
Table 4.
Alternative Cutpoints for CAC—Frequency of Test Findings and Association With Clinical Events for CAC Testing.
| CAC Testing (N=4209) |
||||
|---|---|---|---|---|
| Initial Test Results | Frequency n/N (%) |
Event Rate n/N (%) |
HR* (95% CI) | P-value |
| All-cause death/MI/UA | ||||
| CAC ≤10 | 1848/4209 (43.9) | 28/1848 (1.5) | ||
| CAC >10 | 2361/4209 (56.1) | 105/2361 (4.5) | 2.42 (1.56–3.76) | <0.001 |
| CAC ≤300 | 3418/4209 (81.2) | 84/3418 (2.5) | ||
| CAC >300 | 791/4209 (18.8) | 49/791 (6.2) | 1.94 (1.32–2.86) | 0.001 |
| Cardiovascular death/MI/UA | ||||
| CAC ≤10 | 1848/4209 (43.9) | 18/1848 (1.0) | ||
| CAC >10 | 2361/4209 (56.1) | 88/2361 (3.7) | 3.22 (1.89–5.47) | <0.001 |
| CAC ≤300 | 3418/4209 (81.2) | 66/3418 (1.9) | ||
| CAC >300 | 791/4209 (18.8) | 40/791 (5.1) | 2.02 (1.31–3.11) | 0.002 |
| Cardiovascular death/MI | ||||
| CAC ≤10 | 1848/4209 (43.9) | 12/1848 (0.7) | ||
| CAC >10 | 2361/4209 (56.1) | 41/2361 (1.7) | 1.99 (1.01–3.94) | 0.048 |
| CAC ≤300 | 3418/4209 (81.2) | 37/3418 (1.1) | ||
| CAC >300 | 791/4209 (18.8) | 16/791 (2.0) | 1.21 (0.64–2.29) | 0.557 |
Hazard ratios (HR) adjusted for age, sex, CAD risk equivalent (history of either diabetes mellitus, peripheral artery disease, or cerebrovascular disease)
Coronary Calcium compared to CT Angiography
When test findings were stratified as mildly, moderately, or severely abnormal, HRs for events as compared to normal tests increased proportionally for CTA and CAC testing, while HRs were higher for CTA (CTA: 2.94, 7.67, 10.13; all P<0.001; CAC: 1.51 [P=0.147], 3.14, 3.56 [both P<0.001]). In regards to obstructive disease, this study revealed that of those with zero CAC (n=1,457), only 22 had stenosis ≥50% (Table 5). Of those with CAC=0, only 7 (0.5%) had >70% stenosis, 15 had 50–70% (1.0%) and 241 (14.7%) had non-obstructive stenosis, and 1,177 (80.8%) had normal coronary arteries (zero stenosis) on coronary CTA.
Table 5.
CAC vs. CCTA results
| CAC results | CCTA results* | |||||
|---|---|---|---|---|---|---|
| Severe | Moderate | Mild | Normal | Indeterminate | Sum | |
| Severe (CAC >400) | 134 | 100 | 292 | 3 | 111 | 640 |
| Moderate (CAC 100–400) | 70 | 84 | 581 | 9 | 28 | 772 |
| Mild (CAC 1–99) | 27 | 49 | 1,112 | 133 | 19 | 1,340 |
| Normal (CAC =0) | 7 | 15 | 241 | 1,177 | 17 | 1,457 |
| Sum | 238 | 248 | 2,226 | 1,322 | 175 | 4,209 |
As defined by Hoffmann et al. (2017), Ref 16
Discussion
The optimal diagnostic evaluation of patients suspected of having obstructive CAD remains unclear. Given the low prevalence of CAD and excellent prognosis of symptomatic patients with contemporary care, we sought to evaluate whether CAC might provide more robust prognostic information in this cohort as it does in asymptomatic populations. The results of this study demonstrate that CAC can robustly predict events in symptomatic persons (HR 2.0–4.7) with similar results to FT. The study establishes, for the first time in a large prospective trial, the ability of CAC zero to predict very low rate of future events in symptomatic patients, and more importantly, the safety of a zero score to exclude future cardiovascular events.22 Similar to data in asymptomatic patients, this cohort demonstrates that a CAC=0 effectively excludes future cardiovascular events with <1% annual risk.
Several studies have been performed to evaluate CAC in symptomatic populations, but the data are limited by small sample sizes and paucity of events. Georgiou et al found prognostic value for CAC, but this study was limited by a relatively small number of events (n=30).23 Two other studies demonstrated significant prognostic value of CAC in symptomatic persons, but each cohort reported <23 hard events.24,25 While all three studies showed CAC to be predictive of cardiovascular events, the small numbers of hard events has limited the evidence of utility of CAC in symptomatic persons, as reflected in a IIb classification of guideline recommendations for use.,26
Although both CAC and FT had moderate discriminatory ability to predict future cardiovascular events, the respective distributions of events within the groups were quite different. A positive CAC test identified 112/133 total events (84%) and 44/53 hard events (83%), while a positive FT identified 57/132 (43%) of total death, myocardial infarction and unstable angina and only 33% of hard cardiovascular events (myocardial infarction and cardiovascular death) (Supplemental Table 4), resulting in a much higher sensitivity for future cardiovascular events with CAC as compared to FT (p<0.001).
In regards to obstructive CAD, a recently published study on 10,037 symptomatic patients who underwent concomitant coronary CTA and CAC scoring revealed that 84% of patients with zero CAC score had no CAD, 13% had non obstructive stenosis, and only 3.5% had ≥50% stenosis and 1.4% had ≥70% stenosis on coronary CTA.27 This study demonstrated a very high sensitivity for CAC of zero to rule out obstructive CAD. Overall, 18 studies demonstrated that the presence of any CAC had a pooled sensitivity of 98% for detection of significant CAD on ICA.28
CAC has also been shown to accurately risk stratify symptomatic patients presenting to the emergency department with acute chest pain. Intermediate risk patients with acute chest pain and no prior history of CAD commonly have a CAC score of zero with a very low subsequent risk of MACE or death/myocardial infarction. In these acute chest pain patients, a meta-analysis found that patients with CAC score of 0 have a significantly lower risk of future cardiovascular events as compared to those with a positive CAC and with a very low (<1%/year) rate of both MACE and hard cardiac events. In this setting, 60% of patients had a CAC of zero, much higher than in the present study, and were at very low risk and unlikely to benefit from hospital admission or further diagnostic testing.29 Absence of CAC had a negative predictive value of 99% for ruling out acute coronary syndrome in 4 studies.,29,28 This was further demonstrated in our cohort, where only 9 hard events occurred among 1457 negative (zero score) CAC scans, for an event rate of 0.6%. A normal functional test was associated with a 2-fold event rate, with 48 events occurring among 3,588 normal FT, event rate of 1.3% (figure 3, panel C).
Figure 3.
A–C: Kaplan-Meier Survival Curves for CAC and Functional Testing for Normal vs. Abnormal Test Results in Symptomatic Persons in PROMISE
While stress testing demonstrated a low sensitivity for cardiovascular events (48%), the specificity was significantly higher than for CAC testing, especially using a definition of CAC>0 as the threshold. CAC may have several advantages over FT. When compared to FT, CAC is a more rapid, simple test which has no contraindications, is performed on conventional CT systems, incurs little radiation exposure, is easily interpretable and relatively inexpensive.30 Given the low prevalence of obstructive CAD in the current populations, the optimal diagnostic strategy may be CAC as an initial test, followed by a second test. Thus the high sensitivity of CAC for cardiovascular events could potentially be used to rule out patients, and in those with positive CAC scans, a second test with high specificity could be used to determine those at risk of future cardiovascular events. FT is an ideal candidate for such a second test as it provides evidence of ischemia required to guide revascularization. Furthermore, in the PROMISE Trial, The CT strategy was associated with a higher proportion of patients newly initiated on aspirin (51% increase), statins (110% increase), and beta-blockers (52% greater), compared to FT (P<.0001 for each) and the patients reporting healthy eating was also higher after coronary CTA (p=0.002).31 The SCOT-Heart trial also reported more preventive therapies in the CTA arm.32
Alternatively, the current study, with a high sensitivity of CAC for cardiovascular events, also supports the sequential testing algorithm evaluated in the Calcium Imaging and Selective CT Angiography in Comparison to Functional Testing for Suspected Coronary Artery Disease (CRESCENT) Trial,33 a prospective randomized trial that used CAC as a first line test, advancing to CTA when CAC scores were 1–400. This study demonstrated that event-free survival was 96.7% for patients randomized to CT and 89.8% for patients randomized to FT (P <0.011). Furthermore, a tiered CT approach established diagnosis sooner than FT (P < 0.0001), resulting in lower downstream testing (25 vs. 53%, P <0.0001), and lower cumulative diagnostic costs (In Euros 369 vs. 440; P <0.0001). CRESCENT investigators concluded that “Incorporating the calcium scan into the diagnostic workup was safe and lowered diagnostic expenses and radiation exposure.” In this study, there was no significant difference in stratifying by CAC = 0 or defining low risk as CAC <10 (Tables 3 and 4). In PROMISE, 19 of 21 patients with zero CAC and cardiovascular events had <70% stenosis on coronary CTA, and hard event rates for CAC zero were far less than 1% annual risk. In the cardiovascular death/MI group, there were only 9 events among the 1,457 patients followed with CAC 0. In regards to obstructive disease, this study revealed that of those with zero CAC (n=1,457), only 7 (0.5%) had >70% stenosis on CTA (Supplemental Table 4). This very high sensitivity for obstructive disease and cardiovascular events supports use of a CAC first approach as was done in CRESCENT, using CAC to exclude further evaluation.
While CTA has been demonstrated to have higher discriminatory ability than both CAC and FT for CAD,16,34,35 it does require contrast, intravenous access and entails higher cost than both exercise testing and CAC. Hoffmann et al previously demonstrated CTA to have superior prognostic and discriminatory ability to FT.16 We found that anatomic assessment with coronary CTA provided significantly better prognostic information compared to CAC testing (C-index: 0.72 vs. 0.67). When test findings were stratified as mildly, moderately, or severely abnormal, HRs for events as compared to normal tests increased proportionally for CTA and CAC testing, while HRs were higher for CTA (CTA: 2.94, 7.67, 10.13; all P<0.001; CAC: 1.51 [P=0.147], 3.14, 3.56 [both P<0.001]). There was a moderate overlap of disease categories between CAC and CTA result groups. Patients with severe CAC had more severe stenosis and 98% of those with normal CAC had normal coronaries or non-obstructive disease (Table 5).
Limitations
This analysis represents a post-hoc evaluation of CAC testing, as the design of the PROMISE Trial was CTA versus FT.14 Furthermore, only 4,209 patients underwent CAC scanning, while 4,589 underwent CTA testing in PROMISE.16 The reason not all patients underwent CAC scanning with CTA is because this was set up as a pragmatic design, and the exact protocol for CTA was not pre-specified but left to the local imaging expert (radiologist or cardiologist). Further, it must be clear that tests in question (CAC versus FT) render different types of results: anatomic CAD vs FT. No quantitation of ischemia (ie – percent left ventricular ischemia) was performed in this study, as these are site read results and only a small minority of clinical sites clinically reported these variables. Because of its pragmatic, real world design, this study has inherent strengths and limitations of such reports. Future analysis should be done to look at quantitative measures of ischemia on stress imaging and compare to quantitative anatomic measures (ie – CT angiography stenosis or CAC).
Conclusion
Among stable patients presenting with suspected CAD, most events occur in patients who do not have inducible myocardial ischemia, as detected by FT. Conversely, CAC=0 can safely exclude future cardiovascular events in st symptomatic patients with suspected CAD. Most events occurred in patients with positive CAC scans and the discriminatory ability of CAC suggests that it may have a role in the initial evaluation of new onset stable chest pain. However, both approaches have strengths to detect future cardiovascular events in patients with stable CAD, and a combined tiered approach may be most prudent.
Supplementary Material
Clinical Perspective.
What is new?
This is the largest (N >8,800) comparison of the prognostic value of coronary artery calcium (CAC) with functional stress testing in patients with stable chest pain.
This study demonstrates that chest pain populations referred for testing have a low event rate and both tests have different strengths, with a high sensitivity for future cardiovascular events with CAC and a high specificity with functional testing.
What are the clinical implications?
This study provides comparative evidence on the relative prognostic value of functional testing and CAC in a large stable chest pain population
This may improve the use of this information to guide management of these patients.
A normal CAC score has a very low event rate, and may be used to avoid further cardiac testing in a stable chest pain population
An abnormal functional test result, including information on exercise, and symptoms, has moderate prognostic value.
Coronary computed tomography angiography provides better prognostic and discriminatory power than either CAC or functional testing.
Acknowledgments
FUNDING SOURCES: This project was supported by grants R01HL098237, R01HL098236, R01HL98305 and R01HL098235 from the National Heart, Lung, and Blood Institute (NHLBI). The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents. This paper does not necessarily represent the official views of NHLBI. Dr. Bittner was supported by NIH/NHLBI 5K24HL113128.
Footnotes
DISCLOSURES: Dr. Budoff reports receiving grants from the National Institutes of Health and General Electric outside of the submitted work. Dr. Ferencik reports receiving grant support from the American Heart Association. Dr. Douglas reports receiving grant support from HeartFlow and service on a data and safety monitoring board for GE HealthCare outside the submitted work. The other authors report no potential conflicts of interest. Dr. Hoffmann reports receiving grants from American College of Radiology Imaging Network and HeartFlow Inc. during the conduct of the study, and from Siemens Healthcare outside the submitted work.
References
- 1.SCOT-HEART Investigators. CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet. 2015;385:2383–2391. doi: 10.1016/S0140-6736(15)60291-4. [DOI] [PubMed] [Google Scholar]
- 2.Neglia D, Rovai D, Caselli C, Pietila M, Teresinska A, Aguade-Bruix S, Pizzi MN, Todiere G, Gimelli A, Schroeder S, Drosch T, Poddighe R, Casolo G, Anagnostopoulos C, Pugliese F, Rouzet F, Le Guludec D, Cappelli F, Valente S, Gensini GF, Zawaideh C, Capitanio S, Sambuceti G, Marsico F, Perrone Filardi P, Fernandez-Golfin C, Rincon LM, Graner FP, de Graaf MA, Fiechter M, Stehli J, Gaemperli O, Reyes E, Nkomo S, Maki M, Lorenzoni V, Turchetti G, Carpeggiani C, Marinelli M, Puzzuoli S, Mangione M, Marcheschi P, Mariani F, Giannessi D, Nekolla S, Lombardi M, Sicari R, Scholte AJ, Zamorano JL, Kaufmann PA, Underwood SR, Knuuti J. Detection of significant coronary artery disease by noninvasive anatomical and functional imaging. Circulation Cardiovascular imaging. 2015;8:e002179. doi: 10.1161/CIRCIMAGING.114.002179. [DOI] [PubMed] [Google Scholar]
- 3.Budoff MJ, Li D, Kazerooni EA, Thomas GS, Mieres JH, Shaw LJ. Diagnostic Accuracy of Noninvasive 64-row Computed Tomographic Coronary Angiography (CCTA) Compared with Myocardial Perfusion Imaging (MPI): The PICTURE Study, A Prospective Multicenter Trial. Acad Radiol. 2017;24:22–29. doi: 10.1016/j.acra.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Rozanski A, Gransar H, Hayes SW, Min J, Friedman JD, Thomson LE, Berman DS. Temporal trends in the frequency of inducible myocardial ischemia during cardiac stress testing: 1991 to 2009. J Am Coll Cardiol. 2013;61:1054–1065. doi: 10.1016/j.jacc.2012.11.056. [DOI] [PubMed] [Google Scholar]
- 5.Patel MR, Dai D, Hernandez AF, Douglas PS, Messenger J, Garratt KN, Maddox TM, Peterson ED, Roe MT. Prevalence and predictors of nonobstructive coronary artery disease identified with coronary angiography in contemporary clinical practice. Am Heart J. 2014;167:846–852. doi: 10.1016/j.ahj.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Patel MR, Peterson ED, Dai D, Brennan JM, Redberg RF, Anderson HV, Brindis RG, Douglas PS. Low diagnostic yield of elective coronary angiography. N Engl J Med. 2010;362:886–95. doi: 10.1056/NEJMoa0907272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Douglas PS, Hoffmann U, Patel MR, Mark DB, Al-Khalidi HR, Cavanaugh B, Cole J, Dolor RJ, Fordyce CB, Huang M, Khan MA, Kosinski AS, Krucoff MW, Malhotra V, Picard MH, Udelson JE, Velazquez EJ, Yow E, Cooper LS, Lee KL PROMISE Investigators. Outcomes of Anatomical versus Functional Testing for Coronary Artery Disease. N Engl J Med. 2015;372:1291–1300. doi: 10.1056/NEJMoa1415516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Budoff MJ, Shaw LJ, Liu ST, Weinstein SR, Mosler TP, Tseng PH, Flores FR, Callister TQ, Raggi P, Berman DS. Long-term prognosis associated with coronary calcification: Observations from a registry of 25,253 patients. J Am Coll Cardiol. 2007;49:1860–1870. doi: 10.1016/j.jacc.2006.10.079. [DOI] [PubMed] [Google Scholar]
- 9.Budoff MJ, Achenbach S, Blumenthal RS, Carr JJ, Goldin JG, Greenland P, Guerci AD, Lima JAC, Rader DJ, Rubin GD, Shaw LJ, Wiegers SE. Assessment of Coronary Artery Disease by Cardiac Computed Tomography, A Scientific Statement From the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation. 2006;114:1761–91. doi: 10.1161/CIRCULATIONAHA.106.178458. [DOI] [PubMed] [Google Scholar]
- 10.Goff DC, Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC, Jr, Sorlie P, Stone NJ, Wilson PW, Jordan HS, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC, Jr, Tomaselli GF American College of Cardiology/American Heart Association Task Force on Practice G. 2013 acc/aha guideline on the assessment of cardiovascular risk: A report of the american college of cardiology/american heart association task force on practice guidelines. Circulation. 2014;129:S49–73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 11.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Jr, Watson K, Wilson PW, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC, Jr, Tomaselli GF American College of Cardiology/American Heart Association Task Force on Practice G. 2013 acc/aha guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the american college of cardiology/american heart association task force on practice guidelines. Circulation. 2014;129:S1–45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 12.Mouden M, Timmer JR, Reiffers S, Oostdijk AH, Knollema S, Ottervanger JP, Jager PL. Coronary artery calcium scoring to exclude flow-limiting coronary artery disease in symptomatic stable patients at low or intermediate risk. Radiology. 2013;269:77–83. doi: 10.1148/radiol.13122529. [DOI] [PubMed] [Google Scholar]
- 13.Villines TC, Hulten EA, Shaw LJ, Goyal M, Dunning A, Achenbach S, Al-Mallah M, Berman DS, Budoff MJ, Cademartiri F, Callister TQ, Chang HJ, Cheng VY, Chinnaiyan K, Chow BJ, Delago A, Hadamitzky M, Hausleiter J, Kaufmann P, Lin FY, Maffei E, Raff GL, Min JK Investigators CR. Prevalence and severity of coronary artery disease and adverse events among symptomatic patients with coronary artery calcification scores of zero undergoing coronary computed tomography angiography: Results from the confirm (coronary ct angiography evaluation for clinical outcomes: An international multicenter) registry. Journal of the American College of Cardiology. 2011;58:2533–2540. doi: 10.1016/j.jacc.2011.10.851. [DOI] [PubMed] [Google Scholar]
- 14.Nabi F, Chang SM, Pratt CM, Paranilam J, Peterson LE, Frias ME, Mahmarian JJ. Coronary artery calcium scoring in the emergency department: Identifying which patients with chest pain can be safely discharged home. Annals of emergency medicine. 2010;56:220–229. doi: 10.1016/j.annemergmed.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 15.Douglas PS, Hoffmann U, Lee KL, Mark DB, Al-Khalidi HR, Anstrom K, Dolor RJ, Kosinski A, Krucoff MW, Mudrick DW, Patel MR, Picard MH, Udelson JE, Velazquez EJ, Cooper L PROMISE investigators. PROspective Multicenter Imaging Study for Evaluation of chest pain: rationale and design of the PROMISE trial. Am Heart J. 2014;167:796–803. doi: 10.1016/j.ahj.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffmann U, Ferencik M, Udelson JE, Picard MH, Truong QA, Patel MR, Huang M, Pencina MJ, Mark DB, Heitner JF, Fordyce CB, Pellikka PA, Tardif JC, Budoff MJ, Nahhas G, Chow BJ, Kosinski AS, Lee KL, Douglas PS PROMISE Investigators. Prognostic Value of Noninvasive Cardiovascular Testing in Patients with Stable Chest Pain: Insights from the PROMISE Trial. Circulation. 2017;135:2320–2332. doi: 10.1161/CIRCULATIONAHA.116.024360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicoll R, Wiklund U, Zhao Y, Diederichsen A, Mickley H, Ovrehus K, Zamorano P, Gueret P, Schmermund A, Maffei E, Cademartiri F, Budoff M, Henein M. The coronary calcium score is a more accurate predictor of significant coronary stenosis than conventional risk factors in symptomatic patients: Euro-CCAD study. Int J Cardiol. 2016;207:13–19. doi: 10.1016/j.ijcard.2016.01.056. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 19.Cox DR. Regression models and life-tables. J R Stat Soc. 1972;34:187–220. [Google Scholar]
- 20.Harrell FE, Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. JAMA. 1982;247:2543–2546. [PubMed] [Google Scholar]
- 21.Harrell FE, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Statistics in medicine. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 22.Greenland P, Bonow RO, Brundage BH, Budoff MJ, Eisenberg MJ, Grundy SM, Lauer MS, Post WS, Raggi P, Redberg RF, Rodgers GP, Shaw LJ, Taylor AJ, Weintraub WS, Harrington RA, Abrams J, Anderson JL, Bates ER, Grines CL, Hlatky MA, Lichtenberg RC, Lindner JR, Pohost GM, Schofield RS, Shubrooks SJ, Jr, Stein JH, Tracy CM, Vogel RA, Wesley DJ. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) Circulation. 2007;115:402–26. doi: 10.1161/CIRCULATIONAHA..107.181425. [DOI] [PubMed] [Google Scholar]
- 23.Georgiou D, Budoff MJ, Kaufer E, Kennedy JM, Lu B, Brundage BH. Screening patients with chest pain in the emergency department using electron beam tomography: a follow-up study. J Am Coll Cardiol. 2001;38:105–10. doi: 10.1016/s0735-1097(01)01364-x. [DOI] [PubMed] [Google Scholar]
- 24.Keelan PC, Bielak LF, Ashai K, Jamjoum LS, Denktas AE, Rumberger JA, Sheedy PF, Peyser PA, Schwartz RS. Long-term prognostic value of coronary calcification detected by electron-beam computed tomography in patients undergoing coronary angiography. Circulation. 2001;104:412–7. doi: 10.1161/hc2901.093112. [DOI] [PubMed] [Google Scholar]
- 25.Detrano R, Hsiai T, Wang S, Puentes G, Fallavolita J, Shields P, Stanford W, Wolfkiel C, Georgiou D, Budoff M, Reed J. Prognostic value of coronary calcification and angiographic stenoses in patients undergoing coronary angiography. J Am Coll Cardiol. 1996;27:285–90. doi: 10.1016/0735-1097(95)00460-2. [DOI] [PubMed] [Google Scholar]
- 26.Lauer MS, Topol EJ. Clinical trials--multiple treatments, multiple end points, and multiple lessons. JAMA. 2003;289:2575–7. doi: 10.1001/jama.289.19.2575. [DOI] [PubMed] [Google Scholar]
- 27.Villines TC, Hulten EA, Shaw LJ, Goyal M, Dunning A, Achenbach S, Al-Mallah M, Berman DS, Budoff MJ, Cademartiri F, Callister TQ, Chang HJ, Cheng VY, Chinnaiyan K, Chow BJ, Delago A, Hadamitzky M, Hausleiter J, Kaufmann P, Lin FY, Maffei E, Raff GL, Min JK CONFIRM Registry Investigators. Prevalence and severity of coronary artery disease and adverse events among symptomatic patients with coronary artery calcification scores of zero undergoing coronary computed tomography angiography: results from the CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter) registry. J Am Coll Cardiol. 2011;58:2533–40. doi: 10.1016/j.jacc.2011.10.851. [DOI] [PubMed] [Google Scholar]
- 28.Sarwar A, Shaw LJ, Shapiro MD, Blankstein R, Hoffmann U, Cury RC, Abbara S, Brady TJ, Budoff MJ, Blumenthal RS, Nasir K. Diagnostic and prognostic value of absence of coronary artery calcification. JACC Cardiovascular Imaging. 2009;2:675–88. doi: 10.1016/j.jcmg.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 29.Pursnani A, Chou ET, Zakroysky P, Deaño RC, Mamuya WS, Woodard PK, Nagurney JT, Fleg JL, Lee H, Schoenfeld D, Udelson JE, Hoffmann U, Truong QA. Use of coronary artery calcium scanning beyond coronary computed tomographic angiography in the emergency department evaluation for acute chest pain: the ROMICAT II trial. Circ Cardiovasc Imaging. 2015;8:e002225. doi: 10.1161/CIRCIMAGING.114.002225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blaha MJ, Cainzos-Achirica M, Greenland P, McEvoy JW, Blankstein R, Budoff MJ, Dardari Z, Sibley CT, Burke GL, Kronmal RA, Szklo M, Blumenthal RS, Nasir K. Role of Coronary Artery Calcium Score of Zero and Other Negative Risk Markers for Cardiovascular Disease: The Multi-Ethnic Study Of Atherosclerosis (MESA) Circulation. 2016;133:849–58. doi: 10.1161/CIRCULATIONAHA.115.018524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ladapo JA, Hoffmann U, Lee KL, Coles A, Huang M, Mark DB, Dolor RJ, Pelberg RA, Budoff M, Sigurdsson G, Severance HW, Douglas PS. Changes in Medical Therapy and Lifestyle After Anatomical or Functional Testing for Coronary Artery Disease. J Am Heart Assoc. 2016;5:e003807. doi: 10.1161/JAHA.116.003807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams MC, Hunter A, Shah AS, et al. Use of Coronary Computed Tomographic Angiography to Guide Management of Patients with coronary disease. J Am Coll Cardiol. 2016;67:1759–68. doi: 10.1016/j.jacc.2016.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lubbers M, Dedic A, Coenen A, Galema T, Akkerhuis J, Bruning T, Krenning B, Musters P, Ouhlous M, Liem A, Niezen A, Hunink M, de Feijter P, Nieman K. Calcium imaging and selective computed tomography angiography in comparison to functional testing for suspected coronary artery disease: the multicentre, randomized CRESCENT trial. Eur Heart J. 2016;37:1232–1243. doi: 10.1093/eurheartj/ehv700. [DOI] [PubMed] [Google Scholar]
- 34.Hou ZH, Lu B, Gao Y, Jiang SL, Wang Y, Li W, Budoff MJ. Prognostic value of coronary CT angiography and calcium score for major adverse cardiac events in outpatients. JACC Cardiovasc Imaging. 2012;5:990–9. doi: 10.1016/j.jcmg.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 35.Bittencourt MS, Hulten EA, Veeranna V, Blankstein R. Coronary computed tomography angiography in the evaluation of chest pain of suspected cardiac origin. Circulation. 2016;133:1963–1968. doi: 10.1161/CIRCULATIONAHA.116.017593. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.