Abstract
Purpose
To determine the incidence, predictors and clinical outcomes of post-contrast acute kidney injury (PC-AKI) following renal artery stent placement for atherosclerotic renal artery stenosis.
Materials and Methods
This retrospective study reviewed 1,052 patients who underwent renal artery stent placement for atherosclerotic renal artery stenosis. In total, 437 patients with follow-up were included in this study. Mean age was 73.6 ± 8.3 years. Post-contrast acute kidney injury was defined as an absolute serum creatinine increase ≥0.3 mg/dL or percentage increase in serum creatinine ≥50% within 48 hours of intervention. Logistic regression analysis was performed to identify risk factors for PC-AKI. The cumulative proportion of patients who died or went on to hemodialysis was determined using Kaplan-Meier survival analysis.
Results
Mean follow-up was 71.1±68.4 months. Twenty-six patients (5.9%) developed PC-AKI. Patients who developed PC-AKI had significantly higher levels of baseline proteinuria compared to those who did not (Odds ratio 1.38; 95% CI 1.11–1.72; P=0.004). Prehydration, chronic kidney disease stage, baseline GFR, statin medications, contrast volume and iodine load were not associated with higher rates of PC-AKI. Dialysis-free survival and mortality rates were not significantly different between patients with and without PC-AKI (P=0.50 and P=0.17, respectively).
Conclusion
Elevated baseline proteinuria was the only predictor for PC-AKI in patients undergoing renal artery stent placement. Patients who developed PC-AKI were not at greater risk for hemodialysis or death.
Introduction
Post-contrast acute kidney injury (PC-AKI) describes the acute decline of renal function after exposure to iodinated contrast material in the absence of another etiology (1). Studies have implicated iodinated contrast as one of the most common culprits of iatrogenic renal injury (2, 3). Furthermore, PC-AKI may be associated with increased risk for hemodialysis, mortality, longer hospital admissions and greater healthcare costs (4–6). However, other studies have questioned these implications by asserting a lack of causal evidence between contrast and renal injury as well as an overestimation of PC-AKI in the presence of physiologic creatinine fluctuations (7–10).
The majority of data on PC-AKI following intra-arterial contrast administration is based on cardiac angiography (8). The incidence of PC-AKI from intra-arterial contrast administration may range from 2% to 45% (11–14). In part, this wide range is a result of the variable definitions used for PC-AKI, many of which do not follow the definition adopted by the American College of Radiology (15). Limited studies have investigated PC-AKI in the setting of renal intervention despite the fact that these patients often have some degree of compromised renal function and may be at higher risk for acute kidney injury (16). Given this lack of data, the current study aimed to elucidate the incidence, predictors and outcomes of PC-AKI after renal artery stent implantation for atherosclerotic renal artery stenosis.
Materials and Methods
Patient population
Institutional review board approval was obtained for this Health Insurance Portability and Accountability Act compliant study. This was a retrospective, longitudinal follow-up study. Clinical data was reviewed for all patients who underwent renal artery stent implantation for atherosclerotic renal artery stenosis from January 1996 to June 2009 with follow-up to December 2015. In total, 1,052 patients underwent renal artery stent placement. Of these, 299 patients were excluded for incomplete baseline clinical data, 219 patients were excluded for inadequate information regarding iodinated contrast type or dose, and 97 patients excluded because they were lost to follow-up. The remaining 437 patients were included in the study; all of whom underwent renal artery stent implantation with iodinated contrast. Gadolinium and/or CO2 were not used as contrast agents in these patients. Mean age of the study cohort was 73.6 ± 8.3 years. Baseline patient characteristics are summarized in Table 1.
Table 1.
Baseline characteristics of patients who underwent renal artery stent placement.
| CIN (N=26) | No CIN (N=411) | Total (N=437) | P | |
|---|---|---|---|---|
| Gender, n (%) | ||||
| Female | 16(61.5) | 195(47.4) | 211(55.8) | 0.16 |
| Male | 10(38.5) | 216(52.6) | 226(51.7) | |
| Age, mean (SD), years | 75.7(6.8) | 72.9(8.9) | 0.12 | |
| Bilateral renal artery stenosis, n (%) | 2(7.7) | 66(16.1) | 68(55.6) | 0.25 |
| GFR, mean (SD) | 49.6(27.5) | 46.0(15.9) | 46.3(16.8) | 0.31 |
| Proteinuria, mean (SD), (mg/24hr) | 1103(2386.1) | 376.9(854.3) | 419.5(1018.9) | <0.001 |
| CKD stage, n (%) | ||||
| 1/2 | 3(11.5) | 69(16.8) | 72(16.5) | 0.47 |
| 3A | 11(42.3) | 142(34.5) | 153(35.0) | 0.42 |
| 3B | 9(34.6) | 142(34.5) | 151(34.6) | 0.99 |
| 4 | 2(7.7) | 55(13.4) | 57(13.0) | 0.40 |
| 5 | 1(3.8) | 3(0.7) | 4(0.9) | 0.11 |
| Current smoker, n (%) | 5(19.2) | 76(18.5) | 81(18.5) | 0.93 |
| Diabetes, n (%) | 12(46.2) | 126(30.7) | 138(31.6) | 0.10 |
| Coronary artery disease, n (%) | 18(69.2) | 246(59.8) | 264(60.4) | 0.34 |
| Hyperlipidemia, n (%) | 20(76.9) | 333(81.0) | 353(80.8) | 0.61 |
| Hypertension, n (%) | 26(100.0) | 403(98.1) | 429(98.2) | 0.47 |
Procedure
Patients were referred to the Intervention Radiology Division for renal artery revascularization if Doppler ultrasound demonstrated a peak systolic velocity >180 cm/s or a renal-to-aortic ratio >3.5 or if the stenosis exceeded 50% of the luminal diameter on computed tomography angiography or conventional angiography. Renal artery stent placement was performed by three board-certified interventional radiologists. Antihypertensive and statin medications were continued until the day of the procedure in all patients. Intravascular access was achieved via the common femoral artery. Upon obtaining access, the renal arteries were selected and angiograms were performed. Identified stenoses were treated with balloon-mounted bare-metal stents. None of the patients received embolic protection.
A variety of contrast media were used including low-osmolar agents, Iohexol (Omnipaque; GE Healthcare, Chicago, IL, USA) and iopamidol (Isovue; Bracco Diagnostics, Milan, Italy), as well as iso-osmolar, iodixanol (Visipaque; GE Healthcare). Seventy-eight percent of patients with CKD stage 3B or greater (Glomerular filtration rate (GFR) ≤44 mL/min/1.73 m2) were admitted within 24 hours of the procedure and pretreated with intravenous isotonic fluids at a rate of 1 mL/kg/hr. Other potential renoprotective treatments such as bicarbonate and N-acetylcysteine (NAC) were not recorded for this study.
Measured outcomes
The primary outcome was the incidence of PC-AKI within 48 hours of renal stent placement. Secondary endpoints included time to hemodialysis and death. Estimated glomerular filtration rate (eGFR), 24-hour proteinuria, medications and comorbidities including chronic kidney disease (CKD), cardiac disease, metabolic disease, and smoking history were analyzed as possible contributing factors for PC-AKI. Urine protein levels were obtained within one month of the procedure. Information on the need for dialysis was obtained by querying the USRDS database. Mortality information was obtained by querying the death data in the US Social Security Death Index and health system medical record.
Glomerular filtration rate (GFR) was calculated based on preintervention serum creatinine levels and patient demographic information using the Modification of Diet in Renal Disease equation (17). Chronic kidney disease stages were determined based on the Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group classification (18).
Post-contrast acute kidney injury was determined using the Acute Kidney Injury Network (AKIN) criteria, which defines stage 1 PC-AKI as an absolute serum creatinine increase ≥0.3 mg/dL or a relative increase in serum creatinine ≥50% within 48 hours of intervention (19). Although a reduction in urine output ≤0.5 mL/kg/hr for at least 6 hours within 48 hours of a nephrotoxic event is also considered criteria for acute kidney injury, this metric was not routinely recorded during or after intervention and was excluded from analysis.
Statistical analysis
Statistical analysis was performed with SAS version 9.4 (SAS Institute, Cary, NC). Comparisons were made between normally distributed continuous variables with the Student paired t-test. Categorical variables were compared using the Pearson chi-squared test. Logistic regression analysis was performed to identify risk factors associated with PC-AKI after renal artery stent placement. Statistical significance was defined as p<0.05. The cumulative proportion of patients surviving with and without hemodialysis as well as mortality w determined using Kaplan-Meier survival analysis.
Results
A total of 437 patients fulfilled the inclusion criteria and were analyzed. Mean follow-up was 71.1±68.4 months. Bilateral stents were placed in 98 (22.4%) individuals. Average stent diameter was 6.0±0.9 mm. Twenty-six (5.9%) patients developed PC-AKI consistent with at least AKIN stage 1 renal injury. None of the patients required hemodialysis for immediate management of PC-AKI. No major postprocedural complications requiring intervention or prolonged hospitalization occurred.
Predictors for post-contrast acute kidney injury
Risk factors associated with PC-AKI were determined with logistic regression analysis (Table 2). The only significant risk factor was the severity of baseline 24-hr proteinuria, where patients with PC-AKI had higher levels of urine protein (Odds ratio 1.38; 95% CI 1.11–1.72; P=0.004). Baseline creatinine, eGFR and CKD stage were not significantly associated with PC-AKI (P=0.72, P=0.29 and P=0.12–0.97, respectively).
Table 2.
Logistic regression analysis for predictors of post-contrast acute kidney injury following renal artery stent placement.
| N | Odds Ratio | Lower 95% CI | Upper 95% CI | P | |
|---|---|---|---|---|---|
| Female sex | 211 | 1.77 | 0.79 | 4.00 | 0.17 |
| Proteinuria† | 1.38 | 1.11 | 1.72 | 0.004 | |
| Creatinine | 1.12 | 0.06 | 2.10 | 0.72 | |
| eGFR | 1.01 | 0.99 | 1.03 | 0.29 | |
| CKD stage | |||||
| 1/2 | 72 | 0.65 | 0.19 | 2.21 | 0.49 |
| 3A | 153 | 1.78 | 0.48 | 6.59 | 0.97 |
| 3B | 151 | 1.46 | 0.38 | 5.56 | 0.65 |
| 4 | 57 | 0.84 | 0.14 | 5.18 | 0.25 |
| 5 | 4 | 7.67 | 0.61 | 97.4 | 0.12 |
| Statin medication | 351 | 1.03 | 0.38 | 2.82 | 0.95 |
| Antihypertensive medication | |||||
| ACEI/ARB | 385 | 3.54 | 0.47 | 26.7 | 0.22 |
| Calcium channel blocker | 350 | 1.97 | 0.58 | 6.72 | 0.28 |
| Prehydration | 165 | 0.59 | 0.27 | 1.30 | 0.19 |
| Contrast volume | 1.00 | 0.99 | 1.01 | 0.17 | |
| Total iodine mass | 1.01 | 0.99 | 1.03 | 0.15 | |
| Stent diameter | 0.90 | 0.59 | 1.40 | 0.65 | |
| Bilateral intervention | 68 | 0.44 | 0.10 | 1.89 | 0.27 |
| Current smoker | 81 | 0.99 | 0.36 | 2.73 | 0.99 |
| Diabetes | 138 | 1.93 | 0.87 | 4.30 | 0.11 |
| Coronary artery disease | 264 | 1.47 | 0.63 | 3.47 | 0.38 |
| Hypertension | 429 | 0.99 | 0.05 | 21.57 | 0.99 |
| Hyperlipidemia | 353 | 0.75 | 0.29` | 1.93 | 0.55 |
Odds ratios for continuous variables are all per 1 unit increase unless otherwise specified
per 1,000 unit increase
eGFR=estimated glomerular filtration rate; CKD=chronic kidney disease; ACEI=angiotensin-converting-enzyme inhibitor; ARB=angiotensin receptor blocker; CI=confidence interval.
One hundred and sixty-six (38%) patients were admitted within 24 hours prior to intervention for intravenous hydration. These patients did not have lower rates of PC-AKI (P=0.189). No increased risk for PC-AKI was observed based on stent diameter (P=0.65), bilateral intervention (P=0.27), or medication including statins (P=0.95), calcium channel blockers (P=0.28), and ACEI/ARB (P=0.22).
Mean iodinated contrast volume was 135.2±72.2 mL. The iodine load was calculated to account for differences in iodine concentration among contrast agents. This parameter was calculated for each procedure by multiplying the contrast concentration by the volume of contrast delivered. Mean iodine load was 42.5±22.5 g. Neither contrast volume nor iodine load were associated with PC-AKI (P=0.17 and P=0.15, respectively).
Outcomes after post-contrast acute kidney injury
The Kaplan-Meier survival analyses for the cumulative incidence of hemodialysis and death are demonstrated in Figure 1. Median time to hemodialysis among patients with PC-AKI was 43.7±31.8 months compared to 46.4±43.6 months among those without PC-AKI. Patients who developed PC-AKI did not have significantly greater risk for long-term hemodialysis (Hazard ratio [HR] 1.16; 95% CI 0.75–1.81; P=0.50). Two hundred and nineteen patients died within the follow-up period. Mortality rates were not significantly different between the two groups (HR 1.44; 95% CI 0.85–2.44; P=0.17).
Figure 1.
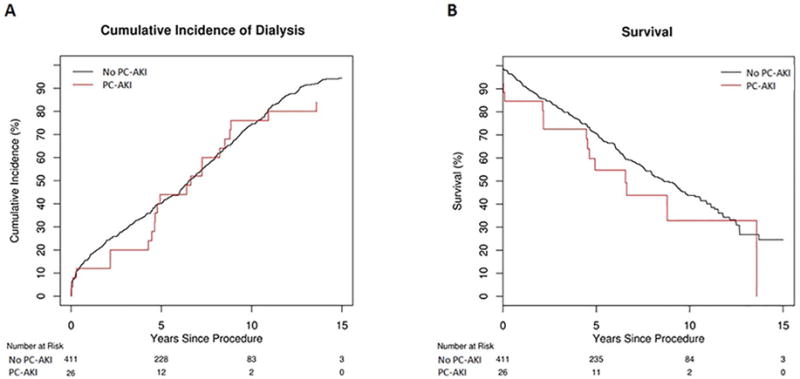
(A) Kaplan-Meier hemodialysis-free survival curves for patients with and without PC-AKI. (B) Kaplan-Meier overall survival curves for 437 patients with and without PC-AKI.
Discussion
The incidence and clinical significance of PC-AKI is controversial. In this large, single-institution, retrospective study, 5.9% of patients who underwent renal artery stent placement developed PC-AKI. A systematic review of patients who underwent peripheral angiography and endovascular therapy by Prasad et al demonstrated a median PC-AKI incidence rate of 10% (range: 0–45%) among 10,316 peripheral procedures with a median volume of contrast of 138.2 mL (range: 49.0–240.5 mL) (14). The incidence of PC-AKI in the present study was at the lower end of this range despite higher mean contrast volume. Peng et al reported a PC-AKI rate of 17.3% in patients (n=150) who underwent renal artery intervention (16). In their study, PC-AKI was defined as a relative increase in serum creatinine by ≥25% or an absolute increase in serum creatinine by ≥0.5 mg/dL within 72 hours. The use of less stringent parameters for defining PC-AKI with regard to time and relative creatinine change compared to the current study may have contributed to the higher incidence rate.
Patients with PC-AKI had higher baseline 24-hour proteinuria than patients who did not develop PC-AKI. In 2010, a large cohort study with 920,985 adults reported that the risk of acute kidney injury increased substantially with the presence and severity of proteinuria (20). Subsequent studies implicated proteinuria as a risk factor of AKI after intra-arterial contrast administration in the settings of percutaneous coronary intervention and stroke intervention (21–23). Piskinpasa et al reported that PC-AKI was significantly higher when proteinuria exceeded 1 g/day (24). The authors of the Cardiovascular Outcomes in Renal Atherosclerotic Lesions (CORAL) trial reported that patients had significantly better event-free survival, cardiovascular disease-related death, progressive renal insufficiency, and overall survival when the albumin/creatinine ratio was ≤ 22.5 mg/g (25). The cutoff of 22.5 mg/g was established by taking median ratio of the study population, which consisted of 826 patients. Further investigation with larger populations is needed to establish a more discrete threshold level of proteinuria that predicts the risk for developing PC-AKI. While proteinuria may be important for assessing renal function and PC-AKI risk, accurate urine protein evaluation, performed over 24 hours, is time intensive. Therefore, using the albumin/creatinine ratio, which relies on spot albumin, may be more clinically feasible.
The mechanism linking proteinuria to PC-AKI is unclear. Proteinuria is an etiology for tubulointerstitial damage though upregulation of inflammatory mediators such as endothelin-1, NF-κB and monocyte chemoattractant protein-1 (26). Greater levels of proteinuria may represent more advanced renal compromise with less functional reserve to withstand additional insults from nephrotoxic chemicals including iodinated contrast.
Patients taking statin medications did not have lower rates of PC-AKI. Some studies have demonstrated that statins lowered PC-AKI risk (27–29). For example, a placebo-controlled, double-blind clinical trial with 220 subjects found 80 mg of atorvastatin combined with NAC and standard intravenous hydration decreased the risk of renal injury within 24 hours of percutaneous coronary intervention compared to a combination of placebo, NAC and hydration. However, the rate of acute kidney injury was not statistically different at 48 hours (30). Other studies have not found an association between statins and kidney injury reduction (31–33). The value of NAC for PC-AKI prevention is controversial and studies have suggested NAC may reduce creatinine without actually preventing renal injury (19). Thus, NAC was not investigated in this study.
Contrast volume and iodine load were not observed risk factors for PC-AKI. Prior studies have reported a positive correlation between contrast volume and PC-AKI (34, 35). There are limited data on the effect of iodine load on PC-AKI incidence (36). Iodine load is a derivative of contrast volume and iodine concentration and may have a more direct effect on PC-AKI than volume alone. Iodine concentration influences contrast viscosity, which negatively correlates with renal oxygenation and potentially increases the risk for kidney injury (37). Additional previously reported risk factors including advanced chronic kidney disease and diabetes were not associated with increased rates of PC-AKI in this study (38, 39).
Long-term mortality may be increased by PC-AKI, especially in patients with advanced CKD (15, 40, 41). Patients in this study who developed renal injury within 48 hours of intervention did not have an increased risk for hemodialysis or mortality. These findings parallel those reported by McDonald et al who reported intravenous contrast material was not associated with increased risk of hemodialysis and mortality among 6,902 subjects (42).
This study was limited by its nonrandomized retrospective design. As a result, follow-up did not occur at regular intervals and medical therapy was not standardized. In many patients, multiple contrast agents with different osmotic concentrations were administered, undermining the feasibility of a subgroup analysis on the effects of contrast osmolarity. However, only low-osmolar or iso-osmolar contrast media were used in this study and the efficacy of iso-osmolar agents compared to that of low-osmolar agents in reducing AKI has been debated (43, 44). Other clinical factors such as potentially nephrotoxic medications may impact renal function, but were not measured in this study. The contribution of atherosclerotic emboli to renal injury remains indeterminant, but embolic protection, which was not used in this study, was not a confounding variable.
The incidence of PC-AKI in this study was at the lower end of the reported range for intra-arterially administered contrast. Proteinuria was a significant predictor for PC-AKI and should be assessed prior to renal artery endovascular intervention when clinically feasible. PC-AKI did not have a significant impact on hemodialysis or mortality in this study.
Acknowledgments
This work was funded by National Institutes of Health Grant HL098967 (S.M.) from the National Heart, Lung, and Blood Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have none.
This material was presented at the 2017 SIR Annual Scientific Meeting.
References
- 1.Morcos SK. Prevention of contrast media nephrotoxicity—the story so far. Clin Radiol. 2004;59(5):381–9. doi: 10.1016/j.crad.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Nash K, Hafeez A, Hou S. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39(5):930–6. doi: 10.1053/ajkd.2002.32766. [DOI] [PubMed] [Google Scholar]
- 3.Lipinski MJ, Satler LF. Contrast-induced nephropathy and peripheral intervention: Who’s keeping track? Catheter Cardiovasc Interv. 2016;88(2):274–5. doi: 10.1002/ccd.26699. [DOI] [PubMed] [Google Scholar]
- 4.Goldenberg I, Matetzky S. Nephropathy induced by contrast media: pathogenesis, risk factors and preventive strategies. CMAJ. 2005;172(11):1461–71. doi: 10.1503/cmaj.1040847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rear R, Bell RM, Hausenloy DJ. Contrast-induced nephropathy following angiography and cardiac interventions. Heart. 2016;102(8):638–48. doi: 10.1136/heartjnl-2014-306962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sigterman TA, Krasznai AG, Snoeijs MG, Heijboer R, Schurink GWH, Bouwman LH. Contrast Induced Nephropathy and Long-term Renal Decline After Percutaneous Transluminal Angioplasty for Symptomatic Peripheral Arterial Disease. Eur J Vasc Endovasc Surg. 2016;51(3):386–93. doi: 10.1016/j.ejvs.2015.08.023. [DOI] [PubMed] [Google Scholar]
- 7.Katzberg RW, Newhouse JH. Intravenous Contrast Medium–induced Nephrotoxicity: Is the Medical Risk Really as Great as We Have Come to Believe? Radiology. 2010;256(1):21–8. doi: 10.1148/radiol.10092000. [DOI] [PubMed] [Google Scholar]
- 8.McDonald RJ, McDonald JS, Newhouse JH, Davenport MS. Controversies in Contrast Material–induced Acute Kidney Injury: Closing in on the Truth? Radiology. 2015;277(3):627–32. doi: 10.1148/radiol.2015151486. [DOI] [PubMed] [Google Scholar]
- 9.Palevsky PM. Defining Contrast-Induced Nephropathy. Clin J Am Soc Nephrol. 2009;4(7):1151–3. doi: 10.2215/CJN.03410509. [DOI] [PubMed] [Google Scholar]
- 10.Tong GE, Kumar S, Chong KC, Shah N, Wong MJ, Zimmet JM, et al. Risk of contrast-induced nephropathy for patients receiving intravenous vs. intra-arterial iodixanol administration. Abdom Radiol (NY) 2016;41(1):91–9. doi: 10.1007/s00261-015-0611-9. [DOI] [PubMed] [Google Scholar]
- 11.Trani C, Porto I, Tommasino A, Giammarinaro M, Burzotta F, Niccoli G, et al. Baseline inflammatory status and long-term changes in renal function after percutaneous renal artery stenting: A prospective study. Int J Cardiol. 2013;167(3):1006–11. doi: 10.1016/j.ijcard.2012.03.078. [DOI] [PubMed] [Google Scholar]
- 12.McCullough P. Outcomes of contrast-induced nephropathy: experience in patients undergoing cardiovascular intervention. Catheter Cardiovasc Interv. 2006;67(3):335–43. doi: 10.1002/ccd.20658. [DOI] [PubMed] [Google Scholar]
- 13.Assareh A, Yazdankhah S, Majidi S, Nasehi N, Beladi Mousavi SS. Contrast induced nephropathy among patients with normal renal function undergoing coronary angiography. J Renal Inj Prev. 2016;5(1):21–4. doi: 10.15171/jrip.2016.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prasad A, Ortiz-Lopez C, Khan A, Levin D, Kaye DM. Acute kidney injury following peripheral angiography and endovascular therapy: A systematic review of the literature. Catheter Cardiovasc Interv. 2016;88(2):264–73. doi: 10.1002/ccd.26466. [DOI] [PubMed] [Google Scholar]
- 15.Harjai KJ, Raizada A, Shenoy C, Sattur S, Orshaw P, Yaeger K, et al. A Comparison of Contemporary Definitions of Contrast Nephropathy in Patients Undergoing Percutaneous Coronary Intervention and a Proposal for a Novel Nephropathy Grading System. Am J Cardiol. 2008;101(6):812–9. doi: 10.1016/j.amjcard.2007.10.051. [DOI] [PubMed] [Google Scholar]
- 16.Peng M, Jiang X-j, Dong H, Zou Y-b, Song L, Zhang H-m, et al. A Comparison of Nephrotoxicity of Contrast Medium in Elderly Patients who Underwent Renal or Peripheral Arterial Vascular Intervention. Intern Med. 2016;55(1):9–14. doi: 10.2169/internalmedicine.55.5321. [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Bosch JP, Lewis J, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Annals of Internal Medicine. 1999;130(6):461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 18.Abboud O, Adler S, Bertram K, Garabed E, Norbert L, Wheeler D. Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Journal of the International Society of Nephrology KDIGO. 2012:5–119. [Google Scholar]
- 19.ACR Manual on Contrast Media Version 10.2. American College of Radiology; 2016. [cited Oct. 5, 2016. [Google Scholar]
- 20.James MT, Hemmelgarn BR, Wiebe N, Pannu N, Manns BJ, Klarenbach SW, et al. Glomerular filtration rate, proteinuria, and the incidence and consequences of acute kidney injury: a cohort study. Lancet. 2010;376 doi: 10.1016/S0140-6736(10)61271-8. [DOI] [PubMed] [Google Scholar]
- 21.Saito Y, Watanabe M, Aonuma K, Hirayama A, Tamaki N, Tsutsui H, et al. Proteinuria and Reduced Estimated Glomerular Filtration Rate Are Independent Risk Factors for Contrast-Induced Nephropathy After Cardiac Catheterization. Circ J. 2015;79(7):1624–30. doi: 10.1253/circj.CJ-14-1345. [DOI] [PubMed] [Google Scholar]
- 22.Clark JJ, Wong LL, Lurie F, Kamitaki BK. Proteinuria as a Predictor of Renal Dysfunction in Trauma Patients Receiving Intravenous Contrast. Am Surg. 2011;77(9):1194–200. [PubMed] [Google Scholar]
- 23.Tao Y, Dong W, Li Z, Chen Y, Liang H, Li R, et al. Proteinuria as an independent risk factor for contrast-induced acute kidney injury and mortality in patients with stroke undergoing cerebral angiography. Journal of NeuroInterventional Surgery. 2016 doi: 10.1136/neurintsurg-2016-012349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piskinpasa S, Altun B, Akoglu H, Yildirim T, Agbaht K, Yilmaz R, et al. An Uninvestigated Risk Factor for Contrast-Induced Nephropathy in Chronic Kidney Disease: Proteinuria. Renal Failure. 2013;35(1):62–5. doi: 10.3109/0886022X.2012.741646. [DOI] [PubMed] [Google Scholar]
- 25.Murphy TP, Cooper CJ, Pencina KM, D’Agostino R, Massaro J, Cutlip DE, et al. Relationship of Albuminuria and Renal Artery Stent Outcomes: Results From the CORAL Randomized Clinical Trial (Cardiovascular Outcomes With Renal Artery Lesions) Hypertension. 2016;68(5):1145–52. doi: 10.1161/HYPERTENSIONAHA.116.07744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abbate M, Zoja C, Remuzzi G. How Does Proteinuria Cause Progressive Renal Damage? J Am Soc Nephrol. 2006;17(11):2974–84. doi: 10.1681/ASN.2006040377. [DOI] [PubMed] [Google Scholar]
- 27.Gandhi S, Mosleh W, Abdel-Qadir H, Farkouh ME. Statins and Contrast-induced Acute Kidney Injury with Coronary Angiography. Am J Med. 2014;127(10):987–1000. doi: 10.1016/j.amjmed.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 28.Wu H, Li D, Fang M, Han H, Wang H. Meta-analysis of short-term high versus low doses of atorvastatin preventing contrast-induced acute kidney injury in patients undergoing coronary angiography/percutaneous coronary intervention. J Clin Pharmacol. 2015;55(2):123–31. doi: 10.1002/jcph.411. [DOI] [PubMed] [Google Scholar]
- 29.Shehata M, Hamza M. Impact of High Loading Dose of Atorvastatin in Diabetic Patients with Renal Dysfunction Undergoing Elective Percutaneous Coronary Intervention: A Randomized Controlled Trial. Cardiovasc Ther. 2015;33(2):35–41. doi: 10.1111/1755-5922.12108. [DOI] [PubMed] [Google Scholar]
- 30.Khosravi A, Dolatkhah M, Hashemi HS, Rostami Z. Preventive Effect of Atorvastatin (80 mg) on Contrast-Induced Nephropathy After Angiography in High-Risk Patients: Double-Blind Randomized Clinical Trial. Nephrourol Mon. 2016;8(3):e29574. doi: 10.5812/numonthly.29574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jo S-H, Hahn J-Y, Lee SY, Kim H-J, Song YB, Choi J-H, et al. High-dose atorvastatin for preventing contrast-induced nephropathy in primary percutaneous coronary intervention. J Cardiovasc Med. 2015;16(3):213–9. doi: 10.2459/JCM.0000000000000157. [DOI] [PubMed] [Google Scholar]
- 32.Toso A, Leoncini M, Maioli M, Gallopin M, Tedeschi D, Amato M, et al. Short-term high-dose atorvastatin for periprocedural myocardial infarction prevention in patients with renal dysfunction. J Cardiovasc Med (Hagerstown) 2011;12(5):318–21. doi: 10.2459/JCM.0b013e328341024f. [DOI] [PubMed] [Google Scholar]
- 33.Kandula P, Shah R, Singh N, Markwell SJ, Bhensdadia N, Navaneethan SD. Statins for prevention of contrast-induced nephropathy in patients undergoing non-emergent percutaneous coronary intervention. Nephrology. 2010;15(2):165–70. doi: 10.1111/j.1440-1797.2009.01204.x. [DOI] [PubMed] [Google Scholar]
- 34.Christakopoulous GE, Karmpaliotis D, Alaswad K, Yeh RW, Jaffer FA, Wyman R, et al. Contrast Utilization During Chronic Total Occlusion Percutaneous Coronary Intervention: Insights From a Contemporary Multicenter Registry. J Invasive Cardiol. 2016;28(9):288–94. [PMC free article] [PubMed] [Google Scholar]
- 35.Bhatt S, Rajpal N, Rathi V, Avasthi R. Contrast Induced Nephropathy with Intravenous Iodinated Contrast Media in Routine Diagnostic Imaging: An Initial Experience in a Tertiary Care Hospital. Radiol Res Pract. 2016;2016:8792984. doi: 10.1155/2016/8792984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jens S, Schreuder SM, De Boo DW, van Dijk LC, van Overhagen H, Bipat S, et al. Lowering iodinated contrast concentration in infrainguinal endovascular interventions: a three-armed randomized controlled non-inferiority trial. Eur Radiol. 2016;26:2446–54. doi: 10.1007/s00330-015-4109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li L-P, Franklin T, Du H, Papadopoulou-Rosenzweig M, Carbray J, Solomon R, et al. Intrarenal oxygenation by blood oxygenation level-dependent MRI in contrast nephropathy model: Effect of the viscosity and dose. J Magn Reson Imaging. 2012;36(5):1162–7. doi: 10.1002/jmri.23747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaul A. Contrast-induced acute kidney injury. Clinical Queries: Nephrology. 2012;1(1):34–41. [Google Scholar]
- 39.Seeliger E, Sendeski M, Rihal CS, Persson PB. Contrast-induced kidney injury: mechanisms, risk factors, and prevention. European Heart Journal. 2012;33(16):2007–15. doi: 10.1093/eurheartj/ehr494. [DOI] [PubMed] [Google Scholar]
- 40.Abe M, Morimoto T, Akao M, Furukawa Y, Nakagawa Y, Shizuta S, et al. Relation of Contrast-Induced Nephropathy to Long-Term Mortality After Percutaneous Coronary Intervention. Am J Cardiol. 2014;114(3):362–8. doi: 10.1016/j.amjcard.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 41.Barbieri L, Verdoia M, Marino P, Suryapranata H, De Luca G. Contrast volume to creatinine clearance ratio for the prediction of contrast-induced nephropathy in patients undergoing coronary angiography or percutaneous intervention. Eur J Prev Cardiol. 2016;23(9):931–7. doi: 10.1177/2047487315614493. [DOI] [PubMed] [Google Scholar]
- 42.McDonald JS, McDonald RJ, Lieske JC, Carter RE, Katzberg RW, Williamson EE, et al. Risk of Acute Kidney Injury, Dialysis, and Mortality in Patients With Chronic Kidney Disease After Intravenous Contrast Material Exposure. Mayo Clin Proc. 2015;90(8):1046–53. doi: 10.1016/j.mayocp.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McDonald RJ, McDonald JS, Bida JP, Carter RE, Fleming CJ, Misra S, et al. Intravenous Contrast Material-induced Nephropathy: Causal or Coincident Phenomenon? Radiology. 2016;278(1):306. doi: 10.1148/radiol.2015154044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rundback JH, Nahl D, Yoo V. Contrast-induced nephropathy. Journal of Vascular Surgery. 2011;54(2):575–9. doi: 10.1016/j.jvs.2011.04.047. [DOI] [PubMed] [Google Scholar]


