Dear Editor
The R183H mutation in the growth hormone gene (GH1) is a well-described genetic variant that causes autosomal dominant isolated growth hormone deficiency (IGHD) type II. Previous studies have demonstrated that individuals with this mutation have releasable growth hormone (GH) stores, but such release is severely impaired1. Hess et al. reported variable height deficits (−4.5 to −1.0 SDS), variable IGF-I concentrations (−2.9 to −0.8 SDS), and low but detectable, or even normal stimulated peak GH in several patients with the R183H mutation2. In contrast, IGHD type IB (caused by homozygous GH1 or GHRHR mutations) results in very low but measurable stimulated GH, while IGHD type IA (caused by homozygous deletions and nonsense mutations in GH1), results in complete absence of GH leading to a more severe phenotype, reflecting a spectrum of growth hormone deficiency (GHD).
Adult GHD (AGHD) causes a distinct phenotype with significant morbidity including increased fat mass, decreased muscle mass and exercise capacity, decreased bone mineral density (BMD), and decreased quality of life, in addition to an abnormal cardiovascular risk profile. However, the majority of studies supporting these findings were conducted in patients with hypopituitarism, which is the most common cause of AGHD. There is limited information regarding the clinical phenotype of patients with IGHD - especially related to the comorbidities seen in AGHD. As the R183H mutation leads to decreased GH secretion, but not a complete absence of GH, it is unknown what the long-term effects of this mutation are in adult carriers. To gain insights into this question, we performed a comprehensive clinical evaluation of a four-generation family with six members affected by the R183H mutation in GH1.
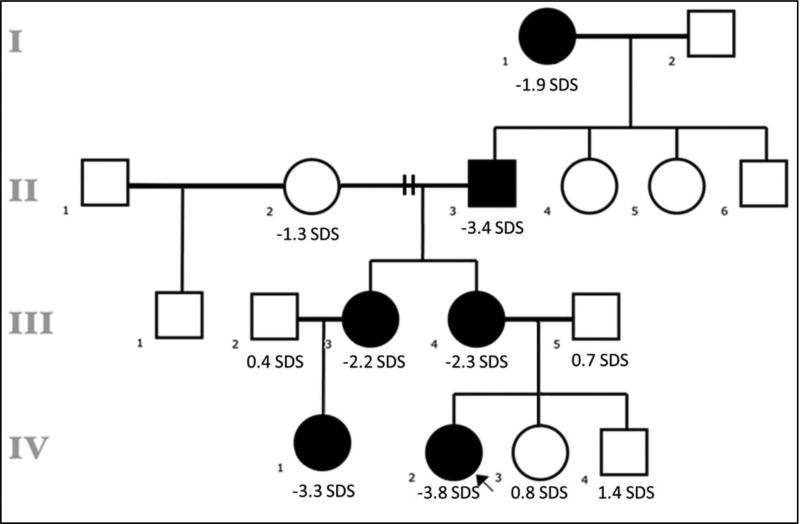
The proband (Subject IV.2) was referred to the endocrinology clinic at age 5 years for evaluation of proportional short stature (height SDS −3.0). Her initial workup revealed low IGF-I and low IGF binding protein-3 concerning for GHD. However, her GH stimulation test with arginine and clonidine revealed a normal peak GH concentration of 8.7 ng/mL (normal ≥5.2 using chemiluminescence assay). On further history we identified several family members with short stature that followed a dominant mode of inheritance (Figure 1). Via whole exome sequencing, we found that the affected individuals carried the heterozygous R183H mutation in GH1. The adults underwent a comprehensive clinical evaluation including GH stimulation test using glucagon, bone densitometry, and complete cardiovascular risk assessment (Supplementary Data). The affected children underwent GH stimulation testing using arginine and clonidine.
Figure 1.
Family pedigree with affected individuals indicated by black squares and circles. When known, the height SD score is reported.
The results are summarized in Table 1. We found that our patients had variable degrees of short stature and low-normal IGF-I concentrations. Interestingly, the patients had marked variability on the GH stimulation test; the affected children and one adult had delayed stimulated peak GH secretion, and two of the affected adults had absent responses. Hess et al. hypothesized that the variability in GH secretion could be explained by a mild dominant-negative effect of R183H-GH on the wild-type GH, and possibly due to compensation of the GH-insufficiency by additional genes2. Additionally, Zhu et al. proposed that the R183H mutation results in prolonged retention of GH molecules in the secretory granules affecting the rate of secretory granule release3. This mechanism could explain the delayed peak GH response observed in some of the patients from this family.
Table 1.
Anthropometric characteristics, laboratory measurements, BMD and body composition.
| IV.1 | IV.2 | III.3 | III.4 | I.1 | |
|---|---|---|---|---|---|
| Gender | Female | Female | Female | Female | Female |
| Age (years) | 6.6 | 5.9 | 30 | 33 | 73 |
| Weight (kg) | 19 | 12 | 72 | 96 | 43 |
| Height (cm) | 103 | 96 | 148 | 149 | 151 |
| Height Z-score | −3.3 | −3.8 | −2.3 | −2.2 | −1.9 |
| BMI (kg/m2) | 18 | 14 | 33 | 43 | 18 |
| Laboratory measurements | |||||
| IGF-I (µg/L) | 53.3 | 27.4 | 95 | 117 | 47 |
| IGF-I ref. range for age | 45 – 254 | 33–276 | 75 – 253 | 72 – 241 | 36 – 164 |
| IGFBP-3 (µg/L) | 2801 | 1260 | 4702 | 4222 | 2518 |
| IGFBP-3 ref. range for age | 1983–5461 | 2169–4790 | 2654 – 5982 | 2562–5783 | 1999 – 5543 |
| Peak GH (µg/L) | 5.2 | 8.7 | 0.5 | 0.4 | 9.3 |
| ALS (mg/L) | 20 | 15 | 6.5 | ||
| Total cholesterol (mmol/L) | 4.2 | 4.4 | 5.3 | ||
| LDL-C (mmol/L) | 2.2 | 2.8 | 3.6 | ||
| HDL-C (mmol/L) | 1.3 | 1.2 | 1.1 | ||
| Triglycerides (mmol/L) | 1.5 | 1.0 | 1.4 | ||
| Apolipoprotein B (g/L) | 0.88 | 0.94 | 1.1 | ||
| Glucose (mmol/L) | 5.1 | 6.5 | 4.3 | ||
| Insulin (pmol/L) | 75 | 144 | 13 | ||
| Hemoglobin A1c (%) | 4.8 | 5.5 | 4.8 | ||
| IL-6 (pg/mL) | 0.0 | 0.0 | 6.3 | ||
| CRP (µg/mL) | 1.2 | 1.8 | 0.4 | ||
| VW factor Ag (kIU/L) | 30 | 46 | 146 | ||
| BMD and body composition | |||||
| LS BMD T- score | 0 | 0.1 | −0.9 | ||
| Z-score | 0 | 0.1 | 1.4 | ||
| Hip BMD T-score | 0.7 | 0.9 | −2.3 | ||
| Z score | 0.8 | 1 | −0.3 | ||
| FA BMD T-score | −0.4 | −0.4 | −0.8 | ||
| Z-score | −0.2 | −0.2 | 1.6 | ||
| TB BMD T score | −0.6 | 0.5 | −1.8 | ||
| Z score | −0.7 | 0.3 | −0.2 | ||
| % Body fat (SDS for age) | 44 (1.4) | 50 (2.0) | 44 (0.8) |
Abbreviations: ALS, acid labile subunit. BMD, bone mineral density. LS, lumbar spine. TB, total body. FA, forearm. Normal reference ranges: ALS: 7–16 mg/L, total cholesterol: <5.2 mmol/L, LDL-C: <4.1 mmol/L, HDL-C: >1.0 mmol/L, triglycerides: <2.2 mmol/L, apoliprotein B: 0.6 – 1.17 g/L, glucose <5.5 mmol/L, insulin: <102 pmol/L, Hemoglobin A1C: <5.7%, IL-6: <7 pg/mL, CRP: <5 μg/mL, WV factor Ag: 50–100 kIU/L.
A thorough cardiovascular risk assessment including echocardiography and noninvasive arterial imaging for carotid thickness and arterial stiffness (pulse wave velocity, augmentation index and brachial flow-mediated dilation) did not suggest increased cardiovascular risk among the adult patients in this family with IGHD secondary to the R183H mutation (Supplemental Table 1). In addition, all three adult patients had normal biochemical parameters including lipid profile, apolipoprotein B, and inflammatory markers. One individual with obesity was found to have evidence of insulin resistance but it is difficult to ascribe this to her GHD given that the other two adult subjects had normal glucose metabolism. Similarly, previous studies have shown that patients with IGHD type IB due to GHRHR mutations have normal echocardiographic parameters and normal carotid thickness with no evidence of accelerated atherosclerosis4 and not surprisingly, normal longevity5. However, these patients exhibit increased total and LDL cholesterol, in addition to increased inflammatory markers which is not present in our patients. Nonetheless, using a DXA scan, we found that the two younger adult patients from our family had central obesity and increased fat mass, comparable to previous reports in IGHD type IB6. However, the oldest individual had a normal BMI and body composition suggesting that the findings on the younger patients could be at least partially due to lifestyle choices. Lastly, we found that all the patients had normal bone mineral density (BMD) compared to age- and sex-matched controls. Likewise, patients with IGHD type IB have normal volumetric BMD but they have a high prevalence of hip joint problems and genu valgum7 which was not present in our patients.
In summary, this is the first study describing in detail the adult phenotype of a family with several members affected by familial IGHD type II. We acknowledge that it is difficult to draw definitive generalizable conclusions from a single family study. However, the detailed investigation of this family with a known pathogenic GH1 mutation does provide some important insights into the potential effects of this mutation on adult manifestations of IGHD. While there are many factors that can affect co-morbidities such as hyperlipidemia, decreased BMD, and increased cardiovascular risk, it is notable that our patients do not consistently manifest any of these co-morbidities, even in the elderly great-grandmother. This suggests that in this family, while the R183H mutation has significant effects on childhood linear growth, there is sufficient residual GH effect to meet the metabolic needs of adults carrying this mutation. Combining our present results with those from earlier studies, we conclude that familial IGHD results in different phenotypes leading to diverse outcomes, where IGHD type IA causes the most severe phenotype with undetectable GH concentrations resulting in severe short stature and reduced longevity8; followed by IGHD type IB, which results in short stature with some metabolic abnormalities; and lastly, the least severe phenotype, IGHD type II. The R183H mutation in GH1 results in partial GHD that appears to have a milder presentation when compared to other forms of IGHD. Therefore, GH therapy may not be required for the adult patients that participated in this study. In addition, patients with IGHD type II can have normal stimulated GH concentrations that can result in inappropriate diagnosis and treatment, thus pointing out the limitations of current growth hormone stimulation tests and the potential benefits of genetic testing. Furthermore, our findings support the need for additional research into the manifestations of adult GHD in other patients with the R183H GH1 mutation and other genetic etiologies of GHD.
Supplementary Material
Acknowledgments
This work was supported by grant K23HD07335 (to A.D.) from the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health. Additional support was provided by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant 1UL1 TR001425. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflict of Interest Statement: The authors have nothing to disclose.
References
- 1.Deladoey J, Stocker P, Mullis PE. Autosomal dominant GH deficiency due to an Arg183His GH-1 gene mutation: clinical and molecular evidence of impaired regulated GH secretion. The Journal of clinical endocrinology and metabolism. 2001 Aug;86(8):3941–3947. doi: 10.1210/jcem.86.8.7723. [DOI] [PubMed] [Google Scholar]
- 2.Hess O, Hujeirat Y, Wajnrajch MP, et al. Variable phenotypes in familial isolated growth hormone deficiency caused by a G6664A mutation in the GH-1 gene. The Journal of clinical endocrinology and metabolism. 2007 Nov;92(11):4387–4393. doi: 10.1210/jc.2007-0684. [DOI] [PubMed] [Google Scholar]
- 3.Zhu YL, Conway-Campbell B, Waters MJ, Dannies PS. Prolonged retention after aggregation into secretory granules of human R183H-growth hormone (GH), a mutant that causes autosomal dominant GH deficiency type II. Endocrinology. 2002 Nov;143(11):4243–4248. doi: 10.1210/en.2002-220575. [DOI] [PubMed] [Google Scholar]
- 4.Menezes Oliveira JL, Marques-Santos C, Barreto-Filho JA, et al. Lack of evidence of premature atherosclerosis in untreated severe isolated growth hormone (GH) deficiency due to a GH-releasing hormone receptor mutation. The Journal of clinical endocrinology and metabolism. 2006 Jun;91(6):2093–2099. doi: 10.1210/jc.2005-2571. [DOI] [PubMed] [Google Scholar]
- 5.Aguiar-Oliveira MH, Oliveira FT, Pereira RM, et al. Longevity in untreated congenital growth hormone deficiency due to a homozygous mutation in the GHRH receptor gene. The Journal of clinical endocrinology and metabolism. 2010 Feb;95(2):714–721. doi: 10.1210/jc.2009-1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barreto-Filho JA, Alcantara MR, Salvatori R, et al. Familial isolated growth hormone deficiency is associated with increased systolic blood pressure, central obesity, and dyslipidemia. The Journal of clinical endocrinology and metabolism. 2002 May;87(5):2018–2023. doi: 10.1210/jcem.87.5.8474. [DOI] [PubMed] [Google Scholar]
- 7.Epitacio-Pereira CC, Silva GM, Salvatori R, et al. Isolated GH deficiency due to a GHRH receptor mutation causes hip joint problems and genu valgum, and reduces size but not density of trabecular and mixed bone. The Journal of clinical endocrinology and metabolism. 2013 Nov;98(11):E1710–1715. doi: 10.1210/jc.2013-2349. [DOI] [PubMed] [Google Scholar]
- 8.Besson A, Salemi S, Gallati S, et al. Reduced longevity in untreated patients with isolated growth hormone deficiency. The Journal of clinical endocrinology and metabolism. 2003 Aug;88(8):3664–3667. doi: 10.1210/jc.2002-021938. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.