Summary
Objective
Correct subtyping of primary aldosteronism (PA) is essential for good surgical outcomes. Adrenal vein sampling (AVS) and/or computed tomography (CT) are used for PA sub-classification. Clinical and/or biochemical improvement after surgery, however, is not always achieved in patients with presumed unilateral PA. We aimed to identify the pitfalls in PA sub-classification leading to surgical treatment failures.
Patients and Design
We retrospectively studied 208 patients who underwent adrenal vein sampling (AVS) for PA sub-classification in a tertiary referral centre, between January 2009 and August 2016. Simultaneous bilateral AVS was performed before and after cosyntropin administration. We implemented immunohistochemistry for aldosterone synthase (CYP11B2) and 17α-hydroxylase/17,20 lyase (CYP17A1) in adrenal glands resected from patients without improvement of PA after surgical treatment and from those with limitations in AVS interpretation.
Results
Of 55 patients who underwent adrenalectomy, three (5.5%) had no improvement of PA. All three patients underwent partial adrenalectomy to remove a CT-detected nodule present on the same side with AVS lateralization. Immunohistochemistry revealed a CYP11B2-negative nodule in both cases available. All patients who underwent total adrenalectomy based on AVS lateralization benefitted from surgery, including three patients with unilateral unsuccessful AVS and aldosterone suppression in the catheterized side vs. inferior vena cava.
Conclusions
Radiographically identified adrenal nodules are not always a source of PA, even when ipsilateral with AVS lateralization. These data caution against reliance on imaging findings, either alone or in conjunction with AVS, to guide surgery for PA.
Keywords: primary aldosteronism, aldosterone producing adenoma, adrenal vein sampling, CYP11B2
Introduction
Primary aldosteronism (PA) is the most common identifiable form of secondary hypertension1. PA is highly prevalent among patients with resistant hypertension and is diagnosed in over 10% of the patients referred to specialized centres2–4. PA is associated with increased cardiovascular morbidity and mortality as compared with equivalent degrees of essential hypertension5–7, and hence early recognition and treatment of PA is imperative8. PA is traditionally sub-classified as either bilateral hyperaldosteronism (BHA) or aldosterone producing adenomas (APA)1, although some patients have features of both subtypes. Correct sub-classification of PA is essential, as patients with unilateral PA can benefit from surgical treatment. Immunostaining for aldosterone synthase (CYP11B2), the key enzyme in aldosterone production, is not widely available; consequently, when postoperative clinical assessment demonstrates surgical cure of hyperaldosteronism, it is assumed that a histologically identified macroscopic adrenal tumor was the primary source of aldosterone. Since the development of highly specific CYP11B2 antibodies, it has been demonstrated that the sub-classification of PA spans a continuum, ranging from single or multiple APA, to small aldosterone producing cell clusters (APCC), to zona glomerulosa hyperplasia9–12. The variable immunohistochemistry findings in resected adrenals from patients with PA underscore the limitations of adrenal imaging in identifying the source(s) of aldosterone excess.
Adrenal vein sampling (AVS) is considered the most accurate method for determining whether one or both adrenals produce excess aldosterone8. Aside from patients younger than 35 years with unequivocal PA13,14, cross-sectional adrenal imaging findings are frequently discordant with the aldosterone source as lateralized by AVS15–19. Nevertheless, resolution of PA after unilateral adrenalectomy is not always achieved, even if the decision is based on AVS lateralization9,13,20, in part because AVS technique and interpretation vary widely between centres21. Moreover, AVS results might be influenced by several antihypertensive agents and by autonomous adrenal cortisol synthesis1,8. For these reasons, the supremacy of AVS in PA sub-classification has been questioned20.
Several studies have focused on outcomes after adrenalectomy in PA, but the emphasis has been on success rates rather than on causes of treatment failure. While clinical improvement is achieved in the majority of PA cases treated surgically9,13,22–24, data regarding contributors to surgical treatment failure have been scarce. In the present study, we aimed to identify the pitfalls in PA sub-classification, leading to absence of PA improvement after surgical treatment.
Patients and Methods
Study participants
In our centre, all patients with PA considered for surgical treatment undergo AVS. We studied patients who underwent AVS at the University of Michigan between January 1st, 2009 and August 31st, 2016. Patient demographics, laboratory results, computed tomography (CT) imaging, AVS data, pathology reports, and postoperative follow-up records were retrospectively reviewed. The antihypertensive medications were converted to a standardized daily defined dose (DDD) according to the WHO ATC/DDD Index25. Immunohistochemistry studies were conducted on paraffin-embedded adrenal tissues in a subset of patients, as detailed below. All studies were conducted under University of Michigan Internal Review Board (IRB) approved protocols. A waiver of consent was granted for the retrospective studies; adrenal tissue experiments were conducted after obtaining written informed consent from all participants.
Clinical assessment
Plasma aldosterone concentration (PAC) and plasma renin activity (PRA) were measured for case detection of PA, and screening was positive in 164/170 (96.5%) of patients, based on a PAC (ng/L)/PRA (μg/L/h) ratio (ARR) ≥ 200, PAC >100 ng/L and PRA <1 μg/L/h. In 125/170 (73.5%) patients, the diagnosis was confirmed based on oral sodium loading test (followed by a 24 h urinary aldosterone >12 μg), saline infusion test (PAC >100 ng/L at 4 hours), or a suppressed PRA (<1.0 μg/L/h) with spontaneous hypokalaemia and PAC >200 ng/L8. Six patients with a high clinical index of suspicion did not meet ARR screening criteria but were included due to inability to discontinue interfering medications. PAC was measured by a competitive chemiluminescent immunoassay on the DiaSorin Liaison XL analyzer; the coefficient of variability (CV) was 8.9–9.4 %. PRA was determined using a radioimmunoassay for angiotensin I manufactured by DiaSorin, with a CV between 13.0% (at 2.3 μg/L/h) and 8.6% (at 5.8 μg/L/h). Serum cortisol was measured by a competitive chemiluminescent immunoassay on a Siemens ADVIA Centaur analyzer, with CV of 5.1–7.1%. Urinary aldosterone was measured by liquid chromatography-tandem mass spectrometry (LC-MS/MS) at Mayo Medical Laboratories.
Because patients with unilateral dominance of PA might still have milder autonomous aldosterone production from the contralateral side, we focused on patients with lack of PA improvement after surgery, rather than on PA cure. Absence of PA improvement was defined as: postoperative PRA <1 μg/L/h and PAC fall by less than 50% as compared with the pre-operative value.
AVS
AVS was performed by one of two experienced interventional radiologists at the University of Michigan. Samples were obtained simultaneously from the inferior vena cava (IVC) and both adrenal veins (AV) before and 10–30 minutes after 0.25 mg cosyntropin administration (minimum three time points). Cosyntropin was injected as a 0.125 mg bolus followed by continuous infusion (0.75 mg/h prior to November 2014, and 0.125 mg/h thereafter). AV catheterization was considered successful when the selectivity index (SI), defined by the AV/IVC cortisol concentrations, was ≥2 prior to and ≥5 after cosyntropin administration, respectively. The lateralization index (LI), defined as the aldosterone/cortisol ratio between the two AVs, and contralateral index (CI), defined as (aldosterone/cortisol)non-dominant AV / (aldosterone/cortisol)IVC were used to assess lateralization. Unilateral PA was diagnosed if the LI was ≥2 before and ≥4 after cosyntropin administration, respectively. A CI <1 defined contralateral suppression.
Immunohistochemistry
We assessed the aldosterone synthetic capacity of the surgically removed adrenal tissue in patients who failed surgical treatment and in patients with unsuccessful access of one AV. Localization of CYP11B2 and 17α-hydroxylase/17,20 lyase (CYP17A1, an enzyme involved in glucocorticoid and androgen synthesis) expressing cells was assessed by immunohistochemistry, using corresponding anti-human antibodies (anti-CYP11B2, mouse; 1:1500, Millipore, Billerica, MA, USA, #MABS1251; anti-CYP17A1, rabbit; 1:1000, kindly provided by Dr. Michael R. Waterman, Vanderbilt University School of Medicine, Nashville, TN, USA), as previously described10,26.
Statistical analysis
Statistical differences in measured parameters between groups were evaluated using the Mann-Whitney U test. A p value <0.05 was considered significant.
Results
Demographic and diagnostic data
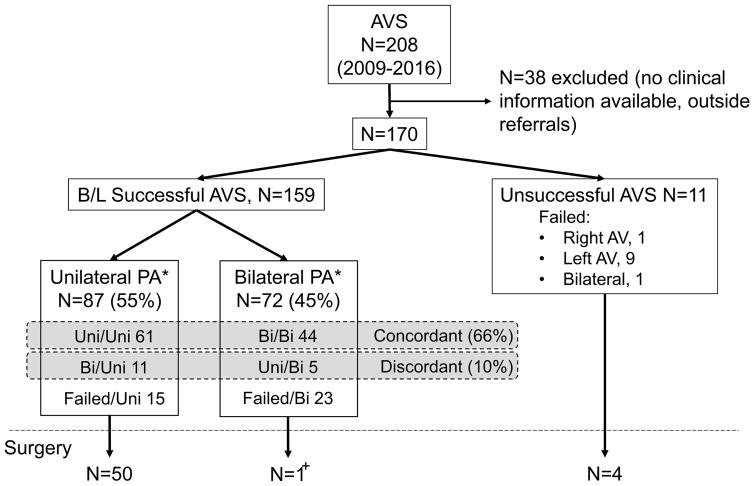
Between January 2009 and August 2016, 208 patients underwent AVS in our centre. Of these, 38 patients referred directly to AVS, without clinical records available for review, were excluded (Figure 1). Of the remaining 170 patients, 101 (59%) were men. The median age of all participants was 54 (range, 30–79). AVS was considered successful (based on SI criteria defined in Methods) in 122 (72%) and 159 patients (94%) before and after cosyntropin administration, respectively (Figure 1). Unilateral PA was diagnosed in 66/122 (54%) and 87/159 (55%) patients before and after cosyntropin administration, respectively. Contralateral suppression was present in 50/66 (75%) patients and 82/87 (94%) patients with unilateral PA before and after cosyntropin administration, respectively. In total, 16 of the 159 (10%) successfully catheterized patients had discordant lateralization before vs. after cosyntropin administration. In five patients, unilateral PA was apparent only before cosyntropin, while 11 other patients lateralized only after cosyntropin administration (Figure 1). No patients had opposite lateralization based on pre- vs. post-cosyntropin LI.
Figure 1.
Adrenal vein sampling (AVS) data.
Adrenal vein catheterization was considered successful when selectivity index (SI) was ≥2 before and ≥5 after cosyntropin administration.
Unilateral PA was diagnosed if lateralized index (LI) was ≥2 before and ≥4 after cosyntropin administration. B/L, bilateral.
*, Classification based on post-cosyntropin data. Uni, unilateral; Bi, bilateral; Failed, failure of adrenal vein catheterization.
+, In this patient, AVS data suggested clear lateralization and contralateral suppression prior to cosyntropin stimulation, but bilateral PA after cosyntropin.
Because catheterization is less often successful without cosyntropin administration, AVS data after cosytropin administration have been preferentially used for clinical decisions in our centre, and PA will be further referred to as unilateral or bilateral based on these results, unless otherwise specified. In unilateral PA, median PAC (330 ng/L vs. 210 ng/L, p <0.001) and ARR (1970 vs. 870, p <0.001) were higher, and PRA was lower (0.1 μg/L/h vs. 0.3 μg/L/h, p=0.007) than in bilateral PA (Table 1). Hypokalaemia was more frequent in patients with unilateral PA (97%) than in those with bilateral PA (75%).
Table 1.
Clinical and biochemical characteristics of study participants
| Unilateral PA N=87 |
Bilateral PA N=72 |
|
|---|---|---|
| Sex(M/F) | 55 (63%) /32 (37%) | 40 (56%) /32 (44%) |
| Age | 53 (30–78) | 56 (31–79) |
| BMI | 34 (20–54) | 33 (23–56) |
| Hypokalaemia (%) | 97% | 75% |
| No. of antihypertensive agents (0/1/2/3/4/5+) | 1/4/19/11/8/44 | 2/10/9/17/18/16 |
| Basal PAC (ng/L)* | 330+ [210 – 460] | 220 [160 – 290] |
| Basal PRA (μg/L/h)* | 0.1++ [0.1 – 0.4] | 0.3 [0.1 – 0.6] |
Classification based on post-cosyntropin data. BMI, body mass index;
Data are expressed as medians [interquartile range];
p <0.001 vs. bilateral PA;
p <0.01 vs. bilateral PA;
PAC, plasma aldosterone concentration; PRA, plasma renin activity.
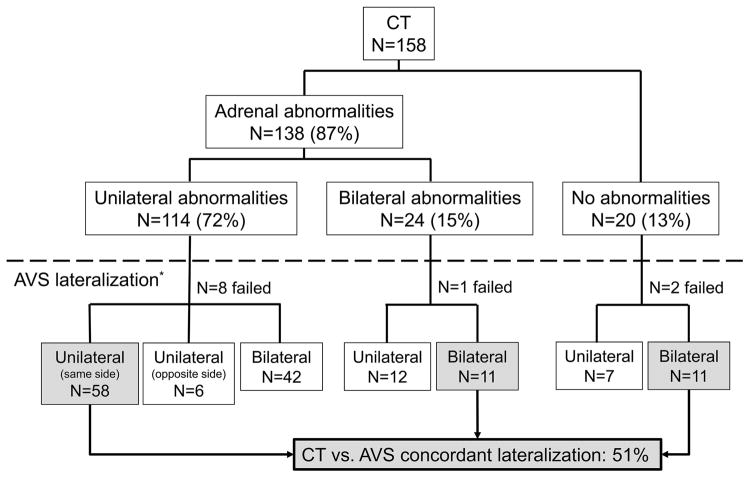
Adrenal CT was performed in 158 patients (Figure 2). Unilateral adrenal abnormalities were diagnosed in 114/158 (72%) patients: single nodule in 93 patients; multiple nodules in two patients; nodules and underlying hyperplasia in four patients; unilateral thickening alone in 15 patients. Bilateral adrenal nodules and/or hyperplasia were observed in 24 patients (15%). CT findings coincided with the lateralization determined by AVS in 80/158 (51%) patients (Figure 2). Of 117 patients with adrenal nodules, 51 patients were evaluated for autonomous cortisol synthesis; five patients had a cortisol >50 nmol/l after 1 mg dexamethasone suppression test. Of these five patients, two lateralized on the same side with the nodule, suggesting cortisol co-secretion from the ipsilateral side; two had bilateral PA, and in one patient AVS was unsuccessful.
Figure 2.
Comparison between CT findings and AVS lateralization.
Postsurgical outcomes
In total, 55 (32%) patients underwent unilateral adrenalectomy, either total (52) or partial (3). Of the remaining 37 patients with unilateral PA, 24 were subsequently managed at other institutions, and in the other 13 patients surgery was postponed for various reasons, including physician’s recommendations or personal preference. Postoperative follow up ranged from 5 days to 20 months (median 20 days). Postoperative clinical assessment and information regarding antihypertensive medications was available in all but two patients, while hormonal follow up was performed in 38 (69%) patients.
Of 55 patients treated surgically, three had no improvement of PA (Table 2). All three patients demonstrated lateralization of PA based on AVS. CT showed one adrenal nodule on the dominant side in all three patients; an additional subcentimetre nodule and lentiform thickening of the contralateral gland was observed in one patient. Intraoperative ultrasound was performed in two of the patients and detected a single nodule on the side with AVS lateralization. All three patients underwent partial adrenalectomy, removing the area of the gland containing the nodule identified on imaging. The clinical pathology report noted an adrenocortical adenoma in all three cases.
Table 2.
Characteristics of patients without clinical benefit after surgery
| PAC (ng/L) | PRA (μg/L/h) | ARR | HC | AVS LI (Dominant Side) | CI | CT findings | Surgery | Pathology | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||||
| PreOp | PostOp | Pre Op | PostOp | Pre Op | Post Op | Before ACTH | After ACTH | ||||||
| Case 1 | 417 | 466 | 0.5 | <0.1 | 834 | 4660 | Negative | 4.5 (R) | 4.3 (R) | 2.6 | 1.9 cm R nodule; 0.9 cm L nodule and diffuse thickening | R nodule resection | ACA |
| Case 2 | 382 | 203 | 0.1 | <0.1 | 3820 | 2030 | - | 4.5 (L) | 4.4 (L) | 0.3 | 1.3 cm L nodule | L nodule resection | ACA |
| Case 3 | 269 | 336 | 0.1 | 0.1 | 2690 | 3360 | Negative | Failed | 5.9 (L) | 0.1 | 1.2 cm L nodule | L nodule resection | ACA |
PAC, plasma aldosterone concentration; PRA, plasma renin activity; ARR, PAC/PRA ratio; PreOp, before surgery; PostOp, after surgery; HC, hypercortisolism; -, not tested; AVS, adrenal vein sampling; LI, lateralized index; CI, contralateral index, after cosyntropin; R, right; L, left; ACA, adrenocortical adenoma.
Adrenalectomy was performed in four patients in whom AVS had failed on one side, three with an aldosterone/cortisol ratio lower in the successfully catheterized side than in IVC. All four patients experienced resolution of PA and a decline in the ATC/DDD index.
Immunohistochemistry
To understand the pathophysiology of poor clinical outcomes after adrenalectomy, as well as successful outcomes in cases with incomplete AVS data, we performed immunohistochemical staining for CYP11B2 on the adrenal glands from patients who failed surgical treatment (two patients; for the third, pathology specimen was not available) and patients in whom AVS failed unilaterally (four patients).
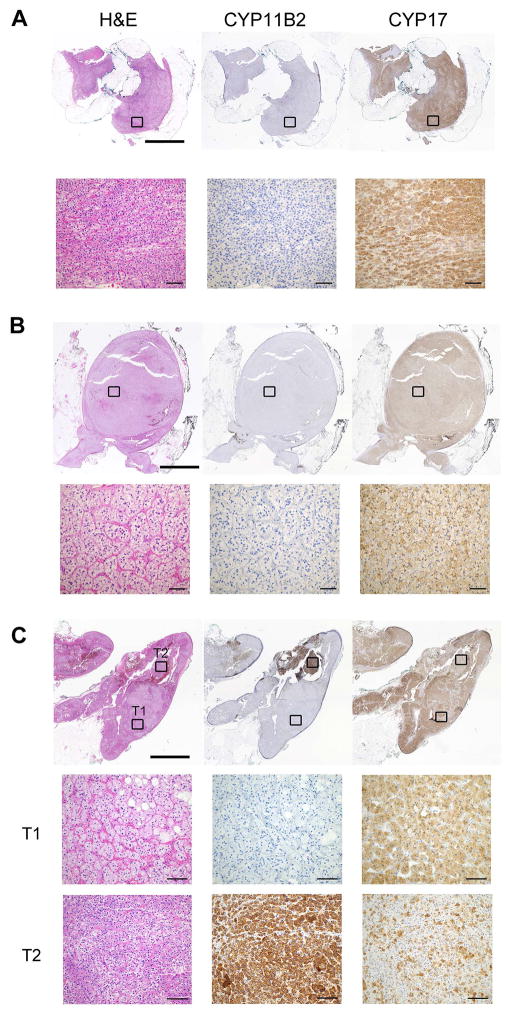
In the two patients with available pathology specimens who had failed surgical treatment, the cortical adenomas removed were negative for CYP11B2, while the entire tissue was diffusely positive for CYP17A1 (Figure 3, A and B, Cases 2 and 3 in Table 2). These results demonstrated that despite AVS lateralization, the largest nodule was not the cause of PA in these patients. One of these patients underwent a second AVS, which again demonstrated lateralization of PA to the partial adrenalectomy side. CT –guided radiofrequency ablation of the remnant adrenal tissue was performed, with subsequent resolution of PA (PAC fell from 336 ng/L to 56 ng/L), decline of ATC/DDD index and resolution of hypokalaemia.
Figure 3.
Histopathological findings of selected adrenal tissue
A and B: Adrenal nodules resected from patients who underwent selective resection of a CT-detected nodule (Cases 2 and 3, Table 2). Both nodules demonstrated no CYP11B2 expression, while CYP17A1 was positive in the nodules. C. Adrenal tissue resected from a patient who had unilaterally unsuccessful AVS. The CT-detected nodule was negative for CYP11B2, while another smaller nodule had CYP11B2 expression.
Of the four patients with unilateral AVS failure who underwent unilateral adrenalectomy, CYP11B2 staining localized to the macroscopic nodule in three. In the fourth patient, however, the CT-detected nodule was negative for CYP11B2 and positive for CYP17A1, while another, smaller, nodular structure showed positive CYP11B2 and weak CYP17A1 expression, suggesting that the source of excess aldosterone production was the smaller nodule rather than the one identified on CT (Figure 3C).
Discussion
AVS has been widely accepted as the “gold standard” for lateralization of PA8,16,27. The basis for recommending AVS in all patients with PA older than 35 who might consider surgery8 has emerged from numerous studies demonstrating poor correlation of AVS with cross-sectional imaging13,14,16. Previous studies have largely focused on success rates after adrenalectomy, but the cases with absent clinical benefit after surgery have been poorly studied. Published data suggest that both CT and AVS are imperfect tools for PA sub-classification13,20. In the present study, we sought to identify parameters associated with poor outcomes after surgical treatment in PA patients guided with both AVS and CT data.
Of all surgically treated cases, the only ones without PA improvement were three patients who underwent partial adrenalectomy to remove an adrenal nodule demonstrated by CT imaging on the ipsilateral side with AVS lateralization. Staining for CYP11B2 was negative in both patients with available pathology specimens, emphasizing that a prominent nodule is not always the source of aldosterone, even when present on the ipsilateral side with AVS lateralization. One of the patients experienced PA resolution after radiofrequency ablation of the remnant adrenal gland, consistent with the AVS results. Failure of PA resolution has been previously reported in 2/29 patients with partial adrenalectomy24, although other groups reported no difference in clinical outcomes between patients who underwent total or partial adrenalectomy23,28. Using CYP11B2 immunostaining, we further show that in some patients with unilateral PA, the source of aldosterone excess includes neoplastic cells outside of a dominant nodule, as other investigators have reported9,29. Moreover, adrenal CT abnormalities can be contralateral to the side suggested by AVS as the source of autonomous aldosterone production13,16. Taken together, these results suggest that while macroscopic APAs are more common than other types of unilateral PA, surgical planning cannot be reliably based on CT findings.
Next, we investigated potential sources of error in AVS interpretation. AVS technique and criteria for successful catheterization and lateralization vary widely among centres21,30,31. In our institution, samples are routinely obtained both before and after cosyntropin stimulation. The rate of successful catheterization in our institution using the stated SI criteria is considerably higher when cosyntropin is used (94% vs 72%), even in the hands of experienced interventional radiologists, in agreement with the experiences reported by other centres32,33. The proportion of uni- vs. bilateral PA was comparable before and after cosyntropin administration, although 10% of AVS studies yielded discrepant results. In total, 6/16 patients with discordant pre- vs. post-cosyntropin AVS lateralization had adrenalectomy: one who lateralized only before and five who lateralized only after cosyntropin. Of these, only one patient – whose surgery was a partial adrenalectomy – did not have improvement of PA after surgery. In a recent study of 175 patients who underwent AVS at another tertiary referral centre, the lateralization index was discordant in 28% of cases, the majority of which lateralized only prior to cosyntropin administration32. Taken together, these data suggest that lateralization under any AVS protocol is a strong predictor of clinical improvement after surgery, even if lateralization is not present under all tested conditions.
Adrenalectomy was performed in three patients with unilateral AVS failure but who demonstrated suppression of aldosterone production in the successfully catheterized side as compared to IVC. All three patients experienced resolution of PA and reduction of their antihypertensive regimen. These outcomes suggest that unilateral aldosterone suppression alone is a strong indicator of contralateral dominance in PA and that AV/IVC aldosterone suppression can be utilized as a decision tool when AVS fails unilaterally. Our results substantiate previous reports of favourable outcomes in patients with unilateral PA and contralateral suppression34–36. As was previously observed37, we found that contralateral suppression was more frequent post- as compared with pre- cosyntropin administration. These results, along with the favourable surgical outcomes in such patients35–37, suggest that cosyntropin does not often disproportionately stimulate aldosterone production from the contralateral adrenal in unilateral PA. Nonetheless, this aspect requires further elucidation by both in vitro experiments and prospective clinical trials.
While several caveats in AVS technique and interpretation13,15,21,38,39 require further clarification, we have not identified any cases with absent clinical benefit after AVS-guided total adrenalectomy. The superiority of AVS in PA has been recently questioned by Dekkers and colleagues, who randomly assigned 200 patients with PA to undergo sub-classification by either CT or AVS20. At one year follow up, there was no statistical difference in the antihypertensive treatment intensity between the two groups, despite the fact that the agreement between AVS and CT findings was only 50%. Of the 92 patients (46 in each group) who underwent surgery, PA was persistent in nine (20%) versus five (11%) patients with CT- or AVS-guided surgery, respectively. These failure rates are much higher than in other reports11,13,22. Nonetheless, the study was underpowered to detect a difference in failure rates after AVS or CT-guide adrenalectomy. Furthermore, MRA use was permitted postoperatively, allowing blood pressure control in uncured patients from both groups40.
Similar to previous reports, the concordance between AVS and CT findings in our study was only 53% and highest in cases with single adrenal nodules. Moreover, unilateral PA was diagnosed by AVS in 35% of patients with no detectable adrenal abnormalities by CT, and all of these patients benefited clinically from adrenalectomy. These results reinforce that, in contrast with cases of hypercortisolism, autonomous aldosterone synthesis from a small group of highly efficient cells might be sufficient to cause disease. Multiple adrenocortical micronodules or diffuse hyperplasia of the zona glomerulosa has been described in such patients12. Taken together, these results suggest that over a third of patients with PA could be denied the opportunity of surgical treatment if subtyping was based on cross-imaging studies.
In summary, we explored the reasons behind surgical failure and the pitfalls of the commonly used PA sub-classification tools, CT and AVS. Among the strengths of our study are the large numbers of patients captured within a tertiary referral adrenal centre and the routine collection of serum both before and after cosyntropin administration during AVS. Most importantly, implementing CYP11B2 immunostaining, we demonstrated that autonomous aldosterone synthesis does not always correspond with radiological findings, even when ipsilateral AVS lateralization is present. Consequently, our data caution against reliance on cross-sectional imaging as a criterion for PA lateralization or guide for adrenalectomy. The limitations to our data include the retrospective design and the number of patients who did not undergo surgery. In addition, postsurgical evaluation was heterogeneous and relatively limited, making it unfeasible to assess a range of outcomes. Carefully planned prospective studies, incorporating uniform criteria for postoperative follow up, remain necessary to clarify the optimal approach to PA lateralization in cases amenable to surgical treatment.
Acknowledgments
AFT was supported by grant 1K08DK109116; JBB was supported by grant 5K23HL128909; RJA and WER were supported by grant R21DK103183. Research reported in this publication was supported by the National Cancer Institutes of Health under award number P30CA046592.
We thank Kristina Fields for technical support with immunohistochemistry; Michelle Vinco for assistance with pathology blocks procurement; Carole Ramm and David Madrigal for assistance with participants consent and IRB regulations; Don Giancherio for clinical laboratory assistance; Ken Cho for establishing AVS at our centre and for performing the initial studies; and all study participants.
Footnotes
Conflict of interest disclosure: The authors have no conflict of interest in relation with this manuscript.
References
- 1.Rossi GP, Auchus RJ, Brown M, et al. An expert consensus statement on use of adrenal vein sampling for the subtyping of primary aldosteronism. Hypertension. 2014 Jan;63(1):151–160. doi: 10.1161/HYPERTENSIONAHA.113.02097. [DOI] [PubMed] [Google Scholar]
- 2.Rossi GP, Bernini G, Caliumi C, et al. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol. 2006 Dec 5;48(11):2293–2300. doi: 10.1016/j.jacc.2006.07.059. [DOI] [PubMed] [Google Scholar]
- 3.Douma S, Petidis K, Doumas M, et al. Prevalence of primary hyperaldosteronism in resistant hypertension: a retrospective observational study. Lancet. 2008 Jun 7;371(9628):1921–1926. doi: 10.1016/S0140-6736(08)60834-X. [DOI] [PubMed] [Google Scholar]
- 4.Kayser SC, Dekkers T, Groenewoud HJ, et al. Study Heterogeneity and Estimation of Prevalence of Primary Aldosteronism: A Systematic Review and Meta-Regression Analysis. The Journal of clinical endocrinology and metabolism. 2016 Jul;101(7):2826–2835. doi: 10.1210/jc.2016-1472. [DOI] [PubMed] [Google Scholar]
- 5.Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. Journal of the American College of Cardiology. 2005 Apr 19;45(8):1243–1248. doi: 10.1016/j.jacc.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 6.Stowasser M, Sharman J, Leano R, et al. Evidence for abnormal left ventricular structure and function in normotensive individuals with familial hyperaldosteronism type I. The Journal of clinical endocrinology and metabolism. 2005 Sep;90(9):5070–5076. doi: 10.1210/jc.2005-0681. [DOI] [PubMed] [Google Scholar]
- 7.Catena C, Colussi G, Nadalini E, et al. Cardiovascular outcomes in patients with primary aldosteronism after treatment. Archives of internal medicine. 2008 Jan 14;168(1):80–85. doi: 10.1001/archinternmed.2007.33. [DOI] [PubMed] [Google Scholar]
- 8.Funder JW, Carey RM, Mantero F, et al. The Management of Primary Aldosteronism: Case Detection, Diagnosis, and Treatment: An Endocrine Society Clinical Practice Guideline. The Journal of clinical endocrinology and metabolism. 2016 May;101(5):1889–1916. doi: 10.1210/jc.2015-4061. [DOI] [PubMed] [Google Scholar]
- 9.Dekkers T, ter Meer M, Lenders JW, et al. Adrenal nodularity and somatic mutations in primary aldosteronism: one node is the culprit? The Journal of clinical endocrinology and metabolism. 2014 Jul;99(7):E1341–1351. doi: 10.1210/jc.2013-4255. [DOI] [PubMed] [Google Scholar]
- 10.Nanba K, Chen AX, Omata K, et al. Molecular Heterogeneity in Aldosterone-Producing Adenomas. J Clin Endocrinol Metab. 2016 Mar;101(3):999–1007. doi: 10.1210/jc.2015-3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monticone S, Castellano I, Versace K, et al. Immunohistochemical, genetic and clinical characterization of sporadic aldosterone-producing adenomas. Molecular and cellular endocrinology. 2015 Aug 15;411:146–154. doi: 10.1016/j.mce.2015.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamazaki Y, Nakamura Y, Omata K, et al. Histopathological Classification of Cross-Sectional Image-Negative Hyperaldosteronism. The Journal of clinical endocrinology and metabolism. 2017 Apr 01;102(4):1182–1192. doi: 10.1210/jc.2016-2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim V, Guo Q, Grant CS, et al. Accuracy of adrenal imaging and adrenal venous sampling in predicting surgical cure of primary aldosteronism. The Journal of clinical endocrinology and metabolism. 2014 Aug;99(8):2712–2719. doi: 10.1210/jc.2013-4146. [DOI] [PubMed] [Google Scholar]
- 14.Zhu L, Zhang Y, Zhang H, et al. Comparison between adrenal venous sampling and computed tomography in the diagnosis of primary aldosteronism and in the guidance of adrenalectomy. Medicine. 2016 Sep;95(39):e4986. doi: 10.1097/MD.0000000000004986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magill SB, Raff H, Shaker JL, et al. Comparison of adrenal vein sampling and computed tomography in the differentiation of primary aldosteronism. The Journal of clinical endocrinology and metabolism. 2001 Mar;86(3):1066–1071. doi: 10.1210/jcem.86.3.7282. [DOI] [PubMed] [Google Scholar]
- 16.Young WF, Stanson AW, Thompson GB, Grant CS, Farley DR, van Heerden JA. Role for adrenal venous sampling in primary aldosteronism. Surgery. 2004 Dec;136(6):1227–1235. doi: 10.1016/j.surg.2004.06.051. [DOI] [PubMed] [Google Scholar]
- 17.Kempers MJ, Lenders JW, van Outheusden L, et al. Systematic review: diagnostic procedures to differentiate unilateral from bilateral adrenal abnormality in primary aldosteronism. Annals of internal medicine. 2009 Sep 1;151(5):329–337. doi: 10.7326/0003-4819-151-5-200909010-00007. [DOI] [PubMed] [Google Scholar]
- 18.Nwariaku FE, Miller BS, Auchus R, et al. Primary hyperaldosteronism: effect of adrenal vein sampling on surgical outcome. Archives of surgery. 2006 May;141(5):497–502. doi: 10.1001/archsurg.141.5.497. discussion 502–493. [DOI] [PubMed] [Google Scholar]
- 19.White ML, Gauger PG, Doherty GM, et al. The role of radiologic studies in the evaluation and management of primary hyperaldosteronism. Surgery. 2008 Dec;144(6):926–933. doi: 10.1016/j.surg.2008.07.025. discussion 933. [DOI] [PubMed] [Google Scholar]
- 20.Dekkers T, Prejbisz A, Kool LJ, et al. Adrenal vein sampling versus CT scan to determine treatment in primary aldosteronism: an outcome-based randomised diagnostic trial. The lancet. Diabetes & endocrinology. 2016 Sep;4(9):739–746. doi: 10.1016/S2213-8587(16)30100-0. [DOI] [PubMed] [Google Scholar]
- 21.Rossi GP, Barisa M, Allolio B, et al. The Adrenal Vein Sampling International Study (AVIS) for identifying the major subtypes of primary aldosteronism. The Journal of clinical endocrinology and metabolism. 2012 May;97(5):1606–1614. doi: 10.1210/jc.2011-2830. [DOI] [PubMed] [Google Scholar]
- 22.Volpe C, Hamberger B, Hoog A, et al. Primary aldosteronism: functional histopathology and long-term follow-up after unilateral adrenalectomy. Clinical endocrinology. 2015 May;82(5):639–647. doi: 10.1111/cen.12645. [DOI] [PubMed] [Google Scholar]
- 23.Fu B, Zhang X, Wang GX, et al. Long-term results of a prospective, randomized trial comparing retroperitoneoscopic partial versus total adrenalectomy for aldosterone producing adenoma. The Journal of urology. 2011 May;185(5):1578–1582. doi: 10.1016/j.juro.2010.12.051. [DOI] [PubMed] [Google Scholar]
- 24.Ishidoya S, Ito A, Sakai K, et al. Laparoscopic partial versus total adrenalectomy for aldosterone producing adenoma. The Journal of urology. 2005 Jul;174(1):40–43. doi: 10.1097/01.ju.0000162045.68387.c3. [DOI] [PubMed] [Google Scholar]
- 25.Vander Stichele RH, Elseviers MM, Ferech M, Blot S, Goossens H. European surveillance of antimicrobial consumption (ESAC): data collection performance and methodological approach. Br J Clin Pharmacol. 2004 Oct;58(4):419–428. doi: 10.1111/j.1365-2125.2004.02164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gomez-Sanchez CE, Qi X, Velarde-Miranda C, et al. Development of monoclonal antibodies against human CYP11B1 and CYP11B2. Mol Cell Endocrinol. 2014 Mar 5;383(1–2):111–117. doi: 10.1016/j.mce.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mulatero P, Bertello C, Rossato D, et al. Roles of clinical criteria, computed tomography scan, and adrenal vein sampling in differential diagnosis of primary aldosteronism subtypes. J Clin Endocrinol Metab. 2008 Apr;93(4):1366–1371. doi: 10.1210/jc.2007-2055. [DOI] [PubMed] [Google Scholar]
- 28.Chen SF, Chueh SC, Wang SM, et al. Clinical outcomes in patients undergoing laparoscopic adrenalectomy for unilateral aldosterone producing adenoma: partial versus total adrenalectomy. Journal of endourology. 2014 Sep;28(9):1103–1106. doi: 10.1089/end.2014.0102. [DOI] [PubMed] [Google Scholar]
- 29.Nanba K, Tsuiki M, Sawai K, et al. Histopathological diagnosis of primary aldosteronism using CYP11B2 immunohistochemistry. The Journal of clinical endocrinology and metabolism. 2013 Apr;98(4):1567–1574. doi: 10.1210/jc.2012-3726. [DOI] [PubMed] [Google Scholar]
- 30.Rossi GP, Pitter G, Bernante P, Motta R, Feltrin G, Miotto D. Adrenal vein sampling for primary aldosteronism: the assessment of selectivity and lateralization of aldosterone excess baseline and after adrenocorticotropic hormone (ACTH) stimulation. Journal of hypertension. 2008 May;26(5):989–997. doi: 10.1097/HJH.0b013e3282f9e66a. [DOI] [PubMed] [Google Scholar]
- 31.Mulatero P, Bertello C, Sukor N, et al. Impact of different diagnostic criteria during adrenal vein sampling on reproducibility of subtype diagnosis in patients with primary aldosteronism. Hypertension. 2010 Mar;55(3):667–673. doi: 10.1161/HYPERTENSIONAHA.109.146613. [DOI] [PubMed] [Google Scholar]
- 32.El Ghorayeb N, Mazzuco TL, Bourdeau I, et al. Basal and Post-ACTH Aldosterone and Its Ratios Are Useful During Adrenal Vein Sampling in Primary Aldosteronism. The Journal of clinical endocrinology and metabolism. 2016 Apr;101(4):1826–1835. doi: 10.1210/jc.2015-3915. [DOI] [PubMed] [Google Scholar]
- 33.Wolley M, Ahmed A, Gordon R, Stowasser M. Os 35-08 Does Adrenocorticotropic Hormone (Acth) Administration Improve the Diagnostic Performance of Adrenal Vein Sampling for Primary Aldosteronism? Journal of hypertension. 2016 Sep;34(Suppl 1) ISH 2016 Abstract Book:e401. [Google Scholar]
- 34.Kline GA, Chin A, So B, Harvey A, Pasieka JL. Defining contralateral adrenal suppression in primary aldosteronism: implications for diagnosis and outcome. Clin Endocrinol (Oxf) 2015 Jul;83(1):20–27. doi: 10.1111/cen.12669. [DOI] [PubMed] [Google Scholar]
- 35.Umakoshi H, Tanase-Nakao K, Wada N, et al. Importance of contralateral aldosterone suppression during adrenal vein sampling in the subtype evaluation of primary aldosteronism. Clinical endocrinology. 2015 Oct;83(4):462–467. doi: 10.1111/cen.12761. [DOI] [PubMed] [Google Scholar]
- 36.Wolley MJ, Gordon RD, Ahmed AH, Stowasser M. Does contralateral suppression at adrenal venous sampling predict outcome following unilateral adrenalectomy for primary aldosteronism? A retrospective study. The Journal of clinical endocrinology and metabolism. 2015 Apr;100(4):1477–1484. doi: 10.1210/jc.2014-3676. [DOI] [PubMed] [Google Scholar]
- 37.Monticone S, Satoh F, Viola A, et al. 9b.01: Clinical Significance of Contralateral Adrenal Suppression during Adrenal Vein Sampling in Primary Aldosteronism. Journal of hypertension. 2015 Jun;33( Suppl 1):e120. [Google Scholar]
- 38.Rossi GP, Ganzaroli C, Miotto D, et al. Dynamic testing with high-dose adrenocorticotrophic hormone does not improve lateralization of aldosterone oversecretion in primary aldosteronism patients. J Hypertens. 2006 Feb;24(2):371–379. doi: 10.1097/01.hjh.0000202818.10459.96. [DOI] [PubMed] [Google Scholar]
- 39.Monticone S, Satoh F, Giacchetti G, et al. Effect of adrenocorticotropic hormone stimulation during adrenal vein sampling in primary aldosteronism. Hypertension. 2012 Apr;59(4):840–846. doi: 10.1161/HYPERTENSIONAHA.111.189548. [DOI] [PubMed] [Google Scholar]
- 40.Ghose RP, Hall PM, Bravo EL. Medical management of aldosterone-producing adenomas. Annals of internal medicine. 1999 Jul 20;131(2):105–108. doi: 10.7326/0003-4819-131-2-199907200-00005. [DOI] [PubMed] [Google Scholar]