Abstract
Background
Studies of antigen presentation in retina using mice that expressed green fluorescent protein (GFP) from a transgenic CD11c promoter found that retinal GFPhi cells possessed antigen presentation function. Subsequent studies found that these high GFPhi cells preferentially localized to sites of retinal injury, consistent with their APC function. Interest in the roles of macrophages in degenerative CNS diseases led us to study the GFPhi cells in a retinal model of neurodegeneration. We asked if apoptotic cone photoreceptor cell death in Rpe65−/− knockout mice induced the GFPhi cells, explored their relationship to resident microglia (MG), and tested their role in cone survival.
Methods
Rpe65−/− mice were bred to CD11cGFP mice on the B6/J background. CD11cGFPRpe65−/− mice were also backcrossed to CX3CR1YFP-creERROSADTA mice so that CX3CR1+ mononuclear cells could be depleted by Tamoxifen. Retinas were analyzed by immunohistochemistry, confocal microscopy, fluorescence fundoscopy and flow cytometry.
Results
Elevated numbers of GFPhi cells were concentrated in photoreceptor cell layers of CD11cGFPRpe65−/− mice coinciding with the peak of cone death at 2 to 4 weeks of age, and persisted for at least 14 months. After the initial wave of cone loss, a slow progressive loss of cones was found that continued to retain GFPhi cells in the outer retina. Sustained, four-week Tamoxifen depletions of the GFPhi cells and MG in Rpe65−/− mice from day 13 to day 41, and from day 390 to day 420 promoted a small increase in cone survival. We found no evidence that the GFPhi cells were recruited from the circulation; all data pointed to a MG origin. MG and GFPhi cells were well segregated in the dystrophic retina; GFPhi cells were foremost in the photoreceptor cell layer, while MG were concentrated in the inner retina.
Conclusions
The expression of GFP on a subset of retinal mononuclear cells in CD11cGFP mice identifies a distinct population of cells performing functions previously attributed to MG. Although GFPhi cells dominated the macrophage response to cone death in the photoreceptor cell layer, their ablation led to only an incremental increase in cone survival. The ability to identify, ablate, and isolate these cells will facilitate analysis of this activated, antigen-presenting subset of MG.
Keywords: microglia, macrophage, dendritic cell, neurodegeneration, retina, RPE65, photoreceptors
Introduction
Rod and cone photoreceptors are the two neural retina cell types that mediate phototransduction, the basis for vision. The visual cycle is a series of enzymatic reactions among photoreceptors, the retinal pigment epithelium (RPE), and the Müller glia to metabolize Vitamin A derivatives (retinoids) for phototransduction (Fig. 1A). The essential product is the 11-cis form of the retinal aldehyde (RAL), a chromophore that binds opsin G-protein coupled receptors within the outer segment (OS) of photoreceptors to form photopigment. The RPE65 protein is highly expressed in RPE and cone OS [1–4], and is essential for the eye to metabolize retinoids [5, 6]. Mutation of the human Rpe65 gene is the underlying defect in Type 2 Leber congenital amaurosis (LCA2), leading to extensive cone loss within the first year of life [7, 8]. In the Rpe65−/− mouse, production of 11-cis RAL within the eye was undetectable, and a wave of cone death occurred within the first month of life [9]. Treating Rpe65−/− mouse pups with exogenous 11-cis RAL immediately after birth rescued cones, reaffirming that an active visual cycle is essential for cone health [10].
Figure 1. Retinoid visual cycle and cone death.

(A) Schematic diagram depicts trafficking of retinoids between cell types in mammalian retina. Retinoids destined for photo-transduction are synthesized by retinal pigment epithelium (RPE) and Müller glia, and then trafficked to photoreceptors (black arrows). Spent retinoids from phototransduction events are trafficked back to the RPE and Müller cells for recycling (grey arrows). RPE65 protein is localized within the RPE and cone outer segments to support the retinoid visual cycle. (B) Cone survival at P28 was determined from cone densities in retinal flatmounts by counting S-opsin+ cells from dorsal and ventral regions adjacent to the optic nerve head. CD11cGFPRpe65+/+ mice (black bars) exhibited significantly more (P<0.05 indicated by *; error bars show SEM) cone survival at P28 compared to age-matched CD11cGFPRpe65−/− mice (grey bars). (C) Cone stress in P21 CD11cGFPRpe65−/− retina leads to characteristic mislocalization of cone opsin to the OPL. Scale bar = 15 μm. Blue, DAPI; Red, S-opsin.
Our previous studies of retinal mononuclear cells began with the search for cells in the retina with antigen presenting cell function (APC) that might support the T cell responses associated with retinal autoimmunity. The study of APC’s in retina was facilitated by use of transgenic mice whose dendritic cells (DCs) express a chimeric protein containing green fluorescent protein (GFP) using a transgenic CD11c promoter (CD11cGFP) [11]. We previously showed that the level of CD11c (ITGAX protein) from the endogenous promoter in retinal macrophages from CD11cGFP mice does not correlate with the expression of DTR/GFP from the transgenic promoter [12], and led us to describe the cells as high GFP-expressing (GFPhi), rather than CD11chi. We found that the GFPhi cells in retina had APC function based on in vivo and in vitro studies revealing that antigen-specific retinal T cell responses in the retina were dependent on antigen processing and presentation by local APC that could be visualized by their expression of GFP in CD11cGFP mice [13–15]. Conversely, low GFP-expressing cells (GFPlo) cells bearing markers of microglia (MG) lacked APC function for naïve, antigen-specific CD4 T cells [14]. In studies designed to examine the influence of the retinal environment on the APC function of GFPhi cells, an optic nerve crush (ONC) was performed. This injury to the axons of the retinal ganglion cells (RGC) stimulated an increase in the number of retinal GFPhi cells, promoted their close association with the retinal ganglion cells (RGC) and nerve fibers, and showed that they engulfed the RGC post-ONC [16]. With respect to their APC function, the ONC reduced production of regulatory T cells (Tregs) by the retinal GFPhi cells and increased the number of effector T cells in the retina. Another test of the significance of the GFP reporter in the CD11c-DTR/GFP mice was done by crossing them onto MyD88 and TRIF double knockout mice [16]. When crossed to TRIF/MyD88 deficient mice, the appearance of GFPhi cells was dramatically reduced post-ONC. The absence of GFPhi cells led to the phagocytosis of RGC debris by GFPlo MG. Depletion of GFPhi cells by diphtheria toxin (DTx) ablation was followed by GFPlo MG replacing GFPhi cells in close contact with injured neurons.
We also demonstrated in CD11cGFP mice that GFPhi cells also appeared at sites of stress associated with light-induced photoreceptor injury [12]. The Saban lab has also observed GFPhi cells in the retina of this particular strain of CD11c-GFP reporter mice [17]. Studies to further test the hypothesis that GFPhi cells preferentially respond to stressed or injured cells led to this study of Rpe65−/− mice, in which a defect in retinoid metabolism leads to apoptotic death of the small population of cones found in murine retina. The question of their effect on the survival of stressed cones was also of interest. To this end, Rpe65−/− mice were bred onto the CD11cGFP background to generate a mouse model that exhibited RPE65 dysfunction and GFP expression from a CD11c promoter (CD11cGFPRpe65−/− mice). In addition, CD11cGFPRpe65−/− mice were backcrossed to CX3CR1YFP-creERROSADTA mice so that all retinal microglial-like cells could be tracked and ablated.
Our results showed that GFPhi cells were preferentially attracted to stressed and dying cones in the outer retina of CD11cGFPRpe65−/− mice, while the GFPlo MG remained in the inner retina. We found no evidence of mononuclear cell recruitment from the circulation in the Rpe65−/− mouse model, which has minimal inflammation. The GFPhi cells represented the fraction of MG that responded to the stimulus provided by cone degeneration, and allowed their distinction from MG. Ablation of CX3CR1+ cells by Tamoxifen (TAM) also ablated GFPhi cells associated with the cones, and gave a small, but significant increase in the number of surviving cones. Use of this reporter will enable more specific studies of the pathways to MG activation and APC function in the retina. In the absence of this GFP reporter, all resting and activated retinal macrophages appeared as MG. Previous studies lack the ability to make this distinction, which has limited specific analysis of the responding cells. While GFPhi cells possess the functions, and many of the markers of CD11b+ DCs, their origin in retinas with minimal inflammation suggests they are an activation state of MG that supports immune surveillance without loss of immune privilege.
Materials and Methods
Animals
Rpe65−/− mice were a generous gift of T.M. Redmond (National Eye Institute, National Institutes of Health, Bethesda, MD) [18]. CD11cGFP mice have been previously described and express GFP as a chimeric cell surface protein comprised of the diphtheria toxin receptor (DTR) and GFP, under control of a transgenic CD11c promoter [11]. The Rpe65−/− mice and CD11cGFP mice were bred to produce the CD11cGFPRpe65−/− mice used for this study, and genotypes were confirmed by PCR (data not shown). CD11cGFPRpe65−/− mice were crossed with CX3CR1YFP-creERROSADTA mice so that MG and GFPhi cells could be depleted with TAM treatment and distinguished by expression of YFP in all mononuclear cells, and expression of YFP in the GFPhi cells. Mice were confirmed to be rd8-negative [19]. All mice were reared under cyclic light under specific pathogen-free conditions. Mice were sacrificed by CO2 exposure.
Ethics approval
All experiments were performed in accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research. The protocol was approved by the University of Minnesota Institutional Animal Care and Use Committee (IACUC).
Tamoxifen
TAM was dissolved in highly refined olive oil (Sigma, #O1514) up to a concentration of 40 mg/mL and given IP at a dose of 2 mg in a volume of 50 μL oil or 3 mg in 75μl.
Flow cytometry
For flow cytometry on retinal cells, mice were euthanized, perfused, and the retinas removed as previously described [12]. Briefly, retinas were suspended in a solution of 0.5 mg/ml Liberase/TM (Roche) and 0.1% DNase in DPBS. The retinal cell suspension was gently homogenized by trituration, and fluorescent-labeled antibodies (BD Biosciences or eBioscience) and viability dye were added to the cell suspensions, 0.25 to 2.0 μl/106 cells, and incubated on ice for 30 min. The cells were washed, resuspended in FACS buffer (PBS with 2% FCS and 0.02% sodium azide), followed by filtration through a 35 μM cell strainer and suspended in FACS buffer, and analyzed on FACS Canto or LSRII flow cytometers (BD Biosciences). Samples containing eGFP and eYFP reporters were excited using a 488 nm laser and detected using 550/30 (eYFP) and 510/21 (eGFP) bandpass filters separated by a 525 nm longpass mirror [20]. Fluorescent proteins and other fluorophores were carefully compensated, using FMO (fluorescence minus one) controls: B6 only, eGFP only, eYFP only, and finally both eGFP and eYFP. Data analysis was done with FlowJo (Tree Star) software. Data collected from a single retina comprised a single sample.
Cone density measurements
Retina flatmounts were prepared and analyzed for cone density according to previously published protocol [10]. Briefly, after removing the cornea, the retina-lens complex was separated from the RPE-choroid layer and fixed in 4% paraformaldehyde (PFA) in 0.1 M phosphate-buffered saline (PBS) for 10 min at room temperature (RT). After washing with PBS (3X, 20 min, 4ºC), retinas were incubated with affinity-purified anti-S-opsin antibody (1:1000 dilution; N20 S-opsin antibody; Santa Cruz Laboratories, Santa Cruz, CA) in a humidified chamber overnight at 4ºC. Retinas were washed with PBS (3X, 20 min, 4ºC), flattened by relaxing cuts, mounted flat on a slide, and coverslipped after application of Fluoromount-G (Southern Biotech, Birmingham, AL). Samples were analyzed by fluorescence microscopy. Cones were counted within two fields of view, immediately dorsal and ventral to the optic nerve head, at 20X magnification along the dorsal-ventral axis. Cone densities are expressed as cones/mm2.
Histology
For immunofluorescence analysis of cryosections, eyes were cryoprotected, embedded, frozen, and sectioned at 10 – 40 μm thickness. Sections were incubated as previously described [21] with affinity-purified anti-S-opsin antibody as described above in a humidified chamber overnight at 4ºC. After washing with PBS (1X, 10 min, RT), sections were incubated with solution containing AlexaFluor594 (AF594)-conjugated secondary antibody (Molecular Probes, Eugene, OR) and 4′,6-diamidino-2-phenylindole (DAPI, 1:3000 dilution; Invitrogen, Carlsbad, CA) for 2 h in RT, washed with PBS (2X, 10 min, RT), and coverslip-mounted with Fluoromount-G for analysis. Confocal microscopy was done on an Olympus FV 1000 using conventional three color staining for GFP, CD11b/AF594, and S-opsin/AF350 with appropriate filters. YFP excitation was done with a 514 nm laser. For retinal flatmounts, eyes were fixed in 4% PFA for 5 min, the retinas were dissected off and fixed in 4% PF for an additional 5 min. The retinas were then washed in PBS for 3 times and then blocked with 10% normal donkey serum for 1 h. Subsequently, retinas were washed with PBS and then incubated with primary antibody cocktail for CD11b (1:100 dilution; BD Biosciences, San Jose, CA). Afterwards, retinas were washed 6 times in PBS and then incubated with secondary antibody cocktail containing AF594 and AF350 (Molecular Probes) for 3 h, washed again 6 times with PBS, and then coverslipped. Some samples were mounted with DAPI (Immu-Mount, Vectashield, Burlingame, CA).
Apoptosis assay, TUNEL
After enucleating the eyes, a small incision was made into the cornea for faster penetration of the fixative, and the tissue was submerged in 4% PFA for 4 h. After rinsing the tissue, the whole eye was cryoprotected in a gradient of sucrose (15%, 20%, and 30%) and embedded in OCT. 40 μm sections of the central retina were permeabilized with a 0.1% Triton X-100 and 0.1% sodium citrate solution for 5 min at 4ºC, washed 4X for 5 min each, and incubated for 2 h at 37ºC with the TUNEL reaction solution following the manufacturer instruction. Following the TUNEL assay, sections were rinsed and blocked with 10% normal donkey serum and incubated overnight with primary S-opsin antibody. Corresponding secondary antibody (Alexa Fluor-488) was applied the next day and incubated for 3 h. To confirm specificity of the primary antibodies, control samples in which the primary antibody was omitted were included.
PCR
PCR methods have been previously described [22]. Briefly, tail snip biopsies were obtained from mouse pups using DNeasy Tissue Kit (Qiagen). Three specific oligonucleotide primers for Rpe65 were used. Oligo(A) (5′ GGG AAC TTC CTG ACT AGG GGA GG-3′) is a reverse primer for the PGK-neo gene (reversed in the mutant allele); oligo(B) (5′-GAT GTG GGC CAG GGC TCT T-FG AAG-3′) and oligo(C) (5′-CCC AAT AGT CTA GTA ATC ACA GAT G-3′) are forward and reverse primers from exon 3 and intron C of the Rpe65 gene, respectively. PCR products were run on a QIAxcel Advanced capillary gel electrophoresis system. An RPE65 mutant mouse results in a 459bp PCR product and an RPE normal mouse gives a 546 bp product, while a heterozygous animal gives both.
Statistical analysis
Two-way ANOVA, SAS, version 9.1, statistical software (SAS Institute, Inc., Cary, NC) was used to test for differences in cone density evaluation by animal group and retinal region with post-hoc pair-wise t tests used to compare individual groups. A two-sided P value of < 0.05 was considered significant. Analysis of misplaced cone opsin expression in the outer plexiform layer (OPL) was done by one-way ANOVA with Tukey HSD post-hoc analysis.
Results
Cone death in the Rpe65−/− retina
Analysis of retinal flatmounts showed a significant loss of cones in CD11cGFPRpe65−/− mice by post-natal day 28 (P28) compared to age-matched CD11cGFPRpe65+/+ mice, which is similar to previously published data (Fig. 1B) [9, 10, 21]. Cones from Rpe65−/− mice have been shown to exhibit early mistrafficking of cone OS proteins (Fig. 1C) [9, 10, 21]. This is hypothesized to disrupt the normal intracellular function of the cone, leading to ER stress and release of multiple apoptotic cell signals that result in a wave of cone death from P14 to P28 [23]. The mistrafficking of OS proteins in stressed cones, including cone opsin, is readily visualized by immunofluorescence as short-wavelength (S-) cone opsin staining in the OPL (Fig. 1C).
Properties and origin of the GFPhi cells in CD11cGFPRpe65−/− retina
The CD11cGFPRpe65−/− mice allowed us to track the GFPhi immune cells and differentiate them from GFPlo MG. GFPhi cells found in the CD11cGFPRpe65−/− retina were tested for expression of molecules often found on MG, including Iba-1 (Fig. 2A) and MHC-II (Fig. 2B). Comparison by flow cytometry showed that the GFPhi cells exhibited the reduced expression level of CD45 that is associated with MG, high levels of CD11b, and similar levels of CX3CR1YFP expression (Fig. 2C). Elsewhere we showed similar levels of F4/80 expression by GFPhi and GFPlo cells [16]. The marker that easily identifies the GFPhi cells from retina as the injury-responsive and antigen presenting cells is their GFP expression.
Figure 2. Phenotype of the retinal GFPhi cells.
(A) GFPhi cells from the OPL of P28 stressed retina were Iba-1+ (red) and CD11b+ (blue). (B) Some GFPhi cells in the IPL of naïve retina expressed MHC-II (red). (C) Flow cytometry of CD45med GFPlo and GFPhi cells from CX3CR1YFP-creER ROSADTACD11cGFPRpe65−/− retinas and controls. Cells in the CD45hi gate (red) were not included in the panels on the right. (D) Changes in GFPhi cells in retina of CD11cGFP retina after a single 5 ng injection of DTx into the anterior chamber. Unt - untreated. (E) TAM ablation of Cx3CR1+ cells in the retina of CX3CR1YFP-creER ROSADTACD11cGFP mice leads to prolonged loss of GFPhi cells. CD11b+ mononuclear cells were CD11bhiCD45medLy6G−; GFPlo cells were CD11bhi Ly6G−CD45medCX3CR1-YFPhiCD11c-GFPlo; GFPhi cells were CD11bhiLy6G−CD45medCX3CR1-YFPhiCD11c-GFPhi.
MG derived from circulating yolk sac macrophages first appear in the CNS at E9.5 [24], and the retina is well populated with MG prior to the onset of cone loss in Rpe65−/− retina. Several experiments indicated a MG origin of retinal GFPhi cells in response to cone loss in CD11cGFPRpe65−/− mice. Depletion of GFPhi cells via DTx administration into the anterior segment of the eye, avoiding contact with the retina, shows rapid loss of GFPhi cells with no effect on the GFPlo MG (Fig. 2D). The GFPhi cells rapidly recovered four days later, returning to background levels by day 7. Conversely, ablation of MG and GFPhi cells shows very little recovery of GFPhi cells 8 days later (Fig. 2E). Together, the results were consistent with a MG origin of the GFPhi cells.
Retinal GFPhi cells in CD11cGFPRpe65−/− mice prior to cone degeneration
Studies to determine the onset of the mononuclear cell response to cone degeneration showed that a substantial population of GFPhi cells was already present in the retina at P7 (Fig. 3B&C), prior to the onset of cone loss in Rpe65−/− retina. GFPhi cells were first detected at E18, corresponding to the increase in apoptotic cells in the inner neuroblastic layer [25], and increased substantially by P7, regardless of the presence or absence of RPE65 (Fig. 3C). By P16, there was a rapid decline in TUNEL+ cells in the INL in both CD11cGFPRpe65−/− and control mice; however, there was a significant increase in TUNEL+ cells in the ONL of CD11cGFPRpe65−/− mice compared to control mice. In Rpe65+/+ retina, the GFPhi cells in the INL declined by P14 (Fig. 3C; p<0.05), consistent with their activation and association with clearing inner retinal neurons undergoing apoptosis at that time [26]. Onset of cone degeneration in the Rpe65−/− mice, based on the first appearance of TUNEL+ cells in the ONL at P13 [27], overlapped with the end of developmental remodeling in the INL and associated apoptosis in the INL (Fig. 3A). Loss of cones in the first wave of cone death from P14 to P28 followed quickly, with a peak of TUNEL+ cells in the ONL at P16, and elevated numbers of GFPhi cells at P14, relative to the RPE65-sufficient controls.
Figure 3. Early appearance of GFPhi cells in the retina.
(A) Counts of TUNEL+ cells in the nuclear layers of the retina of control (CD11cGFPRpe65+/+) and CD11cGFPRpe65−/− mice. * indicates P < 0.05. (B) Image acquired from the inner retina at P7. Retinal flatmounts prepared from CD11cGFPRpe65−/− mice. (C) CD11cGFPRpe65−/− and control retinas showed substantial increases in GFPhi and GFPlo cells from E17 to P7 by flow cytometry. Total mononuclear cell counts included CD11bhiCD45medLy6G− cells. Counts in adult B6 wt control mice were also shown.
Increased numbers of GFPhi cells appear in Rpe65−/− retina during the peak of cone loss
Flow cytometric analyses of retinas from CD11cGFPRpe65−/− and CD11cGFPRpe65+/+ were done to compare the numbers of GFPhi cells and GFPlo MG (Fig. 4) in the response to the peak of cone degeneration. Representative flow cytometric analyses of retina showed a substantial increase in CD11b+GFPhi cells at P14 and P28 in CD11cGFPRpe65−/− retinas (Fig. 4A), which was confirmed by analyses of results from multiple retinas from P14, P21, and P28 (Fig. 4B). The increase in GFPhi cells correlated with the appearance of TUNEL+ cells in the outer nuclear layer (ONL) at P14 and P21. Results from heterozygous mice (CD11cGFPRpe65+/−) were indistinguishable from CD11cGFPRpe65+/+ retinas in these assays (data not shown). Substantial TUNEL staining was observed in the ONL at P14 and P21 (Fig. 4B), but not in the INL. As previously shown for apoptotic retinal ganglion cells (RGC) [12, 28], neuron death attracted GFPhi cells, which removed injured neurons by phagocytosis [16].
Figure 4. CD11b+GFPhi cells increased in CD11cGFPRpe65−/− retinas.

(A) Representative flow cytometry of individual retinas at 2 and 4 wks of age. CD11cGFPRpe65−/− retinas contained more GFPhi cells per retina than CD11cGFPRpe65+/+ mice. (B) Summary of flow cytometry data showed that GFPhi cells were present in significantly higher numbers, relative to GFPlo MG, from P14 to P28. (C) Analysis by TUNEL staining showed that TUNEL+ cells peaked at P14 in the ONL and declined, while few TUNEL+ cells were found in the INL at P14 or later. * indicates P < 0.05. N = number of samples.
GFPhi cells localize to the photoreceptor cell layers in Rpe65−/− retina
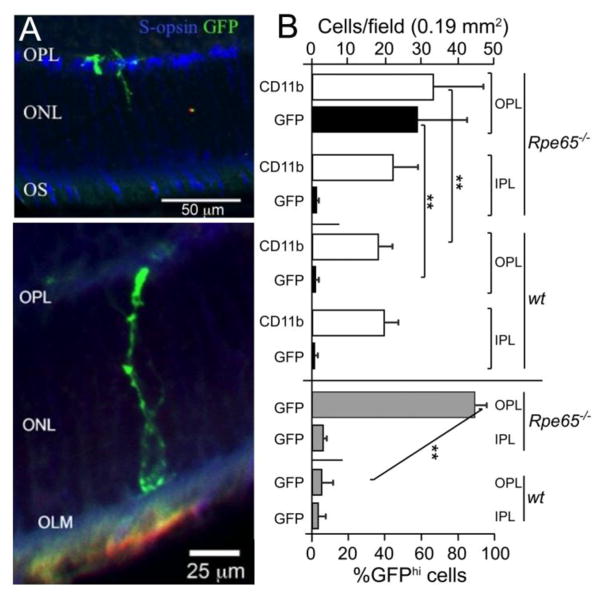
Fluorescence microscopy of sections and flatmounts was performed to localize the GFPhi and GFPlo cells in normal and Rpe65−/− retina. Retina cross-sections revealed that the GFPhi cells were prominent in the OPL, and extended processes into the ONL and outer retina (Fig. 5A). Confocal microscopy of retinal flatmounts from P21 CD11cGFPRpe65−/− mice showed that few GFPhi cells were observed within the inner plexiform layer (IPL) (Fig. 5B and 6). A smaller population of GFPhi cells was found in the inner segments (IS) and OS of cones (Fig. 6). The white arrowheads on the confocal stacks identify three clusters that extend from the OPL to the IS/OS (Fig. 6). GFPhi cells were rare in the outer retina of normal CD11cGFPRpe65+/+ mice (data not shown). Retinas of Rpe65−/− mice demonstrated a remarkable segregation of MG and GFPhi cells in retina (Fig. 6); GFPlo MG dominated in the IPL, which lacks photoreceptors, while GFPhi cells dominated in the OPL, ONL, and IS/OS, the layers containing mislocalized cone opsins (Fig. 5B). Note that all retinal macrophages express CD11b, which was detected in all layers.
Figure 5. GFPhi cells were concentrated in the outer retinal layers.
(A) Confocal microscopy of retinal sections from P21 CD11cGFPRpe65−/− mice show GFPhi cells extending from the OPL to the OLM. Blue, S-opsin; Red, CD11b; Green, GFP. (B) Counts of fields from the OPL compared to the IPL showed that the GFPhi cells were highly concentrated in the OPL. Mean ± SD; **, p<0.01
Figure 6. GFPhi cells dominate the response in the outer retina.
Confocal microscopy of retinal flatmounts from P21 CD11cGFPRpe65−/− mice showed localization of GFPhi cells throughout the outer retina. The IPL exhibited very few GFPhi cells at P21. White arrow heads on the stacks in the OPL, ONL, and IS/OS track three GFPhi cells and their processes that extended continuously from the OPL to the IS/OS. Blue, S-opsin; Red, CD11b; Green, GFP.
Elevated numbers of GFPhi cells persisted after the early wave of cone apoptosis
Although P14 through P28 was found to contain the peak of cone death in the Rpe65−/− retina [9, 10, 29], we asked if the GFPhi cells remained elevated afterwards. Retinas were analyzed by flow cytometry from 2 weeks through 40 weeks of age (Fig. 7). Total mononuclear cells, identified as live CD11bhiCD45medLy6G- cells, were increased early, but returned to near-normal levels by 10 weeks of age (Fig. 7A). At all times from 2 to 40 weeks of age, CD11cGFPRpe65−/− retinas exhibited a significantly greater proportion of GFPhi cells than CD11cGFPRpe65+/+ retinas (Fig. 7B), long after the initial peak of cone apoptosis. GFPlo cell numbers showed relatively little change when compared to normal wt B6J controls (Fig. 7C).
Figure 7. GFPhi cells in Rpe65−/− retina remained elevated, relative to Rpe65+/+ retina, up to 40 wks of age.
(A) The total number of mononuclear cells in retinas of CD11cGFPRpe65−/− and CD11cGFPRpe65+/+mice compared to retinas from adult B6J mice. (B) The proportion of GFPhi cells in the knockout retinas remained significantly elevated at all time points. (C) The retinal GFPlo cells of RPE65 sufficient mice remained constant after the elevation during early retinal remodeling at P14. The number of samples at each time point are shown on panel A. The number of samples for each point are given on panel A. Data analyzed by ANOVA with Tukey HSD post-test. At every time point in panel B, the GFPhi cells in KO mice were significantly elevated relative to their numbers in wt retina.
Mislocalization of cone opsin persisted after the early wave of cone apoptosis
TUNEL staining from 10 to 40 weeks of age found few apoptotic cells within the ONL and INL in either CD11cGFPRpe65−/− or control mice (data not shown). However, retinas harvested at 15 weeks of age from CD11cGFPRpe65−/− mice were analyzed by fluorescence microscopy and revealed the presence of mislocalized cone opsin in the outer plexiform layer (OPL) along with numerous GFPhi cells, some of which extended into the outer nuclear layer (ONL), and cone inner segments (IS) and outer segments (OS) (Fig. 8A). This is well illustrated by a side view of the confocal stack from the same field showing that GFPhi and GFPlo cells span the photoreceptor cell layers from OPL to IS/OS (Fig. 8B). Examination of the inner plexiform layer (IPL) showed that GFPhi cells were absent, but were numerous in the OPL, indicating that they had been attracted to the sites of cone stress (Fig. 8C). Although the rate of cone loss was diminished, remaining cones were stressed as shown by S-cone opsin staining in the OPL, and attracted GFPhi cells.
Figure 8. GFPhi cells persisted in the outer retina of adult Rpe65−/− mice at P105.
Confocal images acquired from CD11cGFPRpe65−/− retinas at 15 weeks of age. (A) Mislocalization of cone opsin was still observed at this time, and GFPhi cells were associated with regions containing these cones. (B) Side view of the confocal stack from (A). (C) The IPL remained devoid of GFPhi cells at this age, but they were abundant in the OPL. Scale bars, A = 50 μm; B = 50 μm; C = 75 μm. All panels show staining for S-opsin, blue; CD11b, red; and expression of GFP, green.
Although cone loss in the Rpe65−/− mice was thought to be an acute process, flow cytometric analysis of aging mice showed the persistence of GFPhi cells. CD11cGFPRpe65−/− retinas were examined to detect mislocalized cone opsin, to determine if the association of GFPhi cells with the photoreceptor layer persisted at 33 weeks of age, and if the MG and GFPhi cells were still segregated. The presence of GFPhi cells and mislocalized cone opsin were found in the OPL at 33 weeks, and GFPhi cells were also found in the IS/OS (Fig. 9). The survival of S-opsin-expressing cone cells at this late time point was unexpected, given their rapid loss in the first month. However, stressed cones remained in sufficient numbers to sustain the attraction of GFPhi cells to the outer retina. The segregation of GFPhi cells in the photoreceptor cell layer, with the MG in the inner retina, was sustained.
Figure 9. Segregation of GFPhi cells in outer retina of Rpe65−/− mice persists at 33 weeks.
Confocal images acquired from CD11cGFPRpe65−/− retinas at 33 weeks of age. RPE65 wt controls were added to show the normal OPL staining in CD11cGFP retina (far right panels). Blue, S-opsin; Red, CD11b; Green, GFP. Scale bar = 50 μm. The green/blue spot on the right side of the IS/OS picture appears to be an artifact on close examination.
Role of CX3CR1+ GFPhi cells in cone survival
We investigated the functional role of retinal mononuclear cells, both MG and GFPhi cells, in LCA2-associated cone death using the CX3CR1YFP-creER ROSADTACD11cGFPRpe65−/− mice. TAM ablation of CX3CR1+ cells in the retina was very effective, depleting GFPhi cells (Fig. 10A&B; Fig. 2E) as well as GFPlo MG (Fig. 10C & Fig. 2E). Breeding was done to provide mice with or without the floxed diphtheria toxin A (DTA) subunit. In this way, all mice were treated with TAM, but only flox+ mice were ablated. Ideally, the GFPhi cells would have been ablated by treatment with diphtheria toxin (DTx), but these cells are rapidly replaced if MG are present (Fig. 2D), even in the absence of recruitment from the circulation. Sustained depletion with DTx is not feasible since the transgenic CD11c reporter/promoter construct is known to drive ectopic DTR expression in a non-immune cell site sensitive to DTx, making repeated systemic administration of DTx lethal within 8 days [11]. However, as seen in Figures 5, 6, 8 and 9, the GFPhi cells and MG segregated into outer and inner retina, respectively, in this model of cone degeneration. Since the GFPhi cells are also CX3CR1+, they were initially ablated together with the GFPlo MG following TAM treatment. This approach served two purposes: it eliminated GFPhi cells, and their immediate precursor, the MG, to give efficient ablation of the GFPhi cells in the outer retina that was maintained by single TAM treatments at 7–10 day intervals (Fig. 10D). Since the GFPhi cells fail to repopulate in the absence of MG (Fig. 2), photoreceptor cell layers remained devoid of GFPhi cells throughout the 4–week depletion protocol, maintained by weekly TAM treatments. Cone survival was analyzed at P42, past the peak of cone apoptosis, using this ablation protocol.
Figure 10. TAM depletion of CX3CR1+GFPhi cells and microglia.
Animals were treated with TAM at P13, and at 7–10 d intervals thereafter for a total 3 to 4 treatments. (A, B) In vivo fluorescence fundus photography was performed at age P34, showing GFPhi cell depletion in CX3CR1YFP-creERROSADTACD11cGFPRpe65−/− mice treated with TAM (B) compared to those not treated with TAM (A). (C) Flow cytometric analysis of TAM depletion of CX3CR1+YFP+CD11b+ retinal macrophages 7 days post-TAM. (D) TAM was administered to all mice, but ablation was limited to floxed DTA mice. Flatmounts in D were prepared from retinas harvested for S-cone counts and show that ablation of GFPhi cells was efficient and limited to flox+ retinas at P41. GFP - green; S-opsin - red.
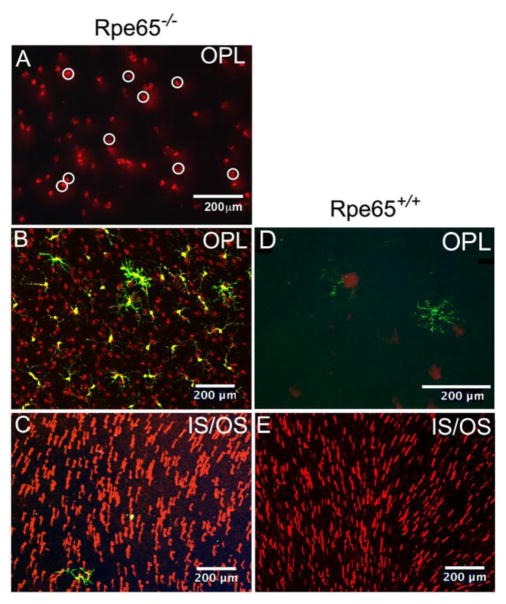
To assess survival of stressed cones, mislocalized cone opsin in the OPL (Fig. 11) was counted in P41 retinal flatmounts (note examples, Fig. 11A). Representative samples of cone staining and GFPhi and/or YFPhi cells in the OPL of Rpe65−/− retina (Fig. 11B) as compared to Rpe65+/+ retina (Fig. 11D) are shown. In both cases, the IS and OS also contained cone S-opsin (Fig. 11C and 11E).
Figure 11. Mislocalized cone opsin within the OPL was used to quantify the number of stressed, surviving cones within the retina.
(A) Examples of cone opsin staining in the OPL of an Rpe65−/− retina were marked with white circles. The circles were then counted by ImageJ. (B, C) Staining of the same retinal field of a retina at different depths, OPL (B) and IS/OS (C), from a CX3CR1YFP-creERROSADTACD11cGFPRpe65−/− retina that was not TAM depleted. (D, E) Panel E shows an area of a CD11cGFPRpe65+/+ mouse retina that was well-populated with cones, and the absence of cone staining in the overlying OPL (E). This Rpe65+/+ mouse was TAM treated, but lacked the YFP-creER transgenes, so there were no YFPhi cells and no depletion by TAM; a GFPhi cell is present in the OPL (D). Panel D was enlarged to better reveal the MG-like dendritiform morphology of the GFPhi cells in this quiescent retina. Mice were sacrificed at P41 to analyze cone survival. Yellow, YFP; Green, GFP; Red, cone S-opsin.
Cell counting strategy for S-opsin+ cones in wt and KO retina
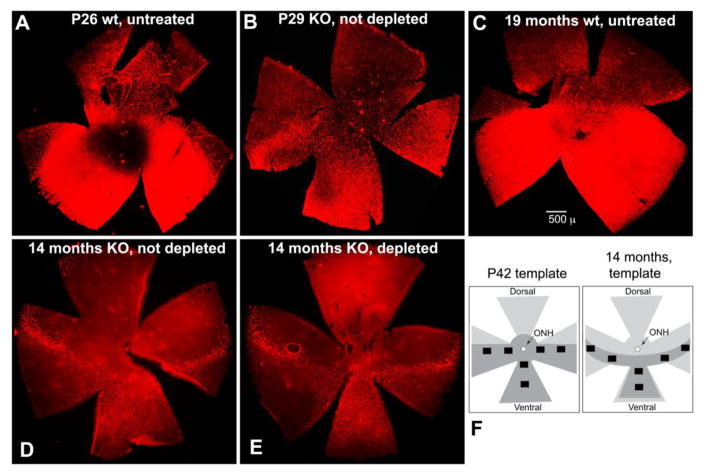
S-opsin stained cones (S-cones) are known to be present in a gradient from low density in dorsal retina to high density in ventral retina in normal young B6J mice (Fig. 12A); this pattern was sustained in mice at 19 months of age (Fig. 12C) [30, 31]. Significant loss of S-cones in Rpe65−/− mice was observed to occur across the retina at P29, following the peak of the initial cone loss (Fig. 12B). Accordingly, counts of surviving cones after the first wave of cone loss were done in the 6-field pattern shown on the P42 template (Fig. 12F). Although aging wt retina was still well-populated with S-cones at 19 months of age (Fig. 12C), progressive loss of S-cones continued, as shown by retinas sampled at 14 months (Fig. 12 D&E). The dorsal petal of Rpe65−/− retina was nearly devoid of S-cones, and the ventral petal was clearly diminished (Fig. 12D&E). Depletion of macrophages did not alter the topography of cone loss (Fig. 12D&E). A narrow band of surviving cones was found across the nasal-temporal petals, as reported by others [32]. This topography suggested a different counting pattern would be necessary for counts at P42 versus counts at 14 months, as shown in the template for 14 months of age (Fig. 12F). Six fields from the indicated areas were counted for each retina.
Figure 12. Topography of cone loss, and counting templates for cones.
(A–E) Low power inspection of flatmounted retinas prepared as shown in Fig. 11 were used to observe the presence and pattern of S-cone loss and to identify regions for counting. (A) P26 wt; (B) P29 Rpe65−/−; (C) wt at 19 months of age; (D) Rpe65−/− not depleted; (E) Rpe65−/− depleted by TAM treatment. (F) Templates for counting, in retinas of mice at P42 and 14 months of age, total vs stressed cones in the IS/OS and OPL layers, respectively. Dark grey shading indicates fields with most surviving cones. Counting fields are shown by black boxes.
Ablation of retinal macrophages reveals a small difference in cone survival
Retinas of young adult wt mice (P40–P70), with or without TAM treatment, contained a large number of healthy cones and very few stressed cones (0.6%) (Fig. 13A). At age P42, the number of events in the Rpe65−/− OPL did not differ between retinas that were TAM-depleted or not (Fig. 13B). The same was true for S-opsin+ events in the IS/OS at P42. At 14 months of age the number of S-opsin+ cones detected in the IS/OS of TAM-depleted retinas was greater than those detected by S-opsin staining in the OPL of retinas that were not depleted by TAM. Comparison of the fraction of stressed cones as a percent of total cones showed that there was a smaller fraction in depleted retinas at P42 (Fig. 13C). Comparison of the numbers of healthy cones (IS/OS counts - OPL counts) indicated better survival at P42 and 14 months in macrophage-depleted retinas (Fig. 13D).
Figure 13. Counts of cone opsin staining in the OPL and outer retina at P42 and 14 months of age after sustained depletion of CX3CR1+ cells.
(A–D) Counts of S-opsin-stained cone photoreceptor cells in Rpe65−/−CX3CR1YFPcreERROSADTACD11cGFP retinas from mice treated with TAM for one month prior to harvest are shown. All mice were treated with TAM, but mice lacking the floxed DTA transgene do not deplete. Half of the mice expressed ROSADTA so that their CX3CR1+ cells were depleted. The other mice lacked ROSADTA and were not depleted. Controls (Rpe65+/+ wt) are also shown. Mean ± SEM. ANOVA with Tukey HSD post-analysis. * indicates P<0.05; ** indicates P<0.01.
Discussion
To our knowledge, this is the first study to show the appearance of GFPhi cells in the CD11cGFP mice to the outer retina in an animal model of photoreceptor cell degeneration, and lends further clarification to the innate immune mechanisms that accompany degeneration of cones in clinical diseases such as LCA2. In the CD11cGFPRpe65−/− retina, cone loss leads to the appearance of GFP-expressing cells in which the GFPhi cells were concentrated in the photoreceptor cell layers while the GFPlo cells, MG, were found in the inner retina. This segregation was striking and suggested a division of labor for these two populations. This hypothesis is supported by previous studies from our group showing that the GFPhi cells migrated to the RGC/nerve fiber layer of the inner retina following experimentally-induced ONC [12, 15]. The rest of the retina was populated by GFPlo MG. Together, these findings suggested that the expression of GFP identified a subset of mononuclear cells that were responding to stress or injury. In the absence of this transgene, the responding cells would not be easily distinguished from the rest of the MG, and it may prove to be a very useful marker for studies of the responding cells.
MG were found to be the likely origin of the GFPhi cells in this model. We showed that ablation of MG prevented repopulation by GFPhi cells, even though GFPhi cells rapidly recovered from ablation if MG were present. In a different model of retinal neurodegeneration, using parabiotic mice, we found that only 0.5% of 2000-plus responding macrophages in retina were recruited from the circulation (manuscript in preparation). Elsewhere, using a unilateral optic nerve crush in the CD11cGFP mice, we showed that a small peak of GFPhi cells appeared in the contralateral, uninjured retina with the same time course as their appearance in the injured, ipsilateral retina [12]. Together, the data supports a MG origin in models of limited stress or injury to the retina.
We have shown elsewhere that these GFPhi cells are the APCs of the retina, based on the functional criteria that they process antigen for presentation in MHC-II, and present that cognate antigen to naïve, antigen-specific CD4 T cells [13–15]. These are classical criteria for CD11b+ myeloid dendritic cells [33]. However, our evidence points to MG as their origin. Since the origin of MG is known [24] and distinct from that of cDCs [34], the GFPhi cells may be best described as antigen-presenting macrophages. Their macrophage function was demonstrated by their ability to phagocytose dying RGCs [16]. In preliminary studies using models with much more severe retinal injury or inflammation than found in the Rpe65−/− mice, we have found that the GFPhi cells can be recruited from the circulation.
The peak in GFPhi cell numbers in Rpe65−/− retina correlated with the early wave in cone apoptosis from P14 to P28. While the number of GFPhi cells declined after P28 in the CD11cGFPRpe65−/− retina, their numbers remained elevated compared to the control animals through at least 40 weeks of age, suggesting ongoing stimuli. Cone loss was found to continue at a low rate to at least 14 months of age. Another potential source for the ongoing signals that attract GFPhi cells to the photoreceptor layer is the slow and prolonged death of rods, a key clinical feature of LCA2 that has also been shown [18, 35].
There is recent data to suggest that retinal MG and macrophages are pathologically involved in rod photoreceptor degeneration, beyond simple clearing of debris. Small molecule inhibitors of inflammation and apoptosis, including minocycline, have been found to attenuate photoreceptor loss in rd10 mice [36, 37]. Other molecules with neuroprotective properties in light damage or models of retinal degeneration were found, including Norgestrel [38, 39] and TAM [40]. The TAM-mediated protection was found in mice lacking creER-linked ablation of CX3CR1+ cells, suggesting that TAM has neuroprotective properties that inhibit of macrophage activation [40]. MG/macrophage involvement in rod photoreceptor degeneration in the rd10 mouse model for retinitis pigmentosa was found to be mediated by primary phagocytosis of living rod photoreceptor cells through a CX3CL1-CX3CR1 mediated signaling pathway [37, 41]. Since the retinal degeneration models cited above yield substantial retinal damage, the potential for participation of monocyte-macrophages recruited from the circulation is present, and experiments to rule out their contributions were not done. Since there was no differentiation in those studies between MG and the population we have identified as GFPhi cells detectable in the CD11cGFP mice, it is unclear if functions attributed to MG were instead being mediated by the GFPhi cells we have described. The extensive infiltration of retinal macrophages into the subretinal space found by Zabel et al [41] in the rd10 model were not recapitulated in our study of the Rpe65−/− mice; relatively few GFPhi cells were found in the OS, and only rare cells were found on the RPE. An important difference in the models is that cones are greatly outnumbered by rods, 1.8 × 105 cones versus 6.4 × 106 rods [42], so that rod degeneration may have a much larger impact on retinal homeostasis. Accordingly, we would not expect to see the monocytic recruitment others have seen in these massive rod degenerations, raising the possibility that recruited macrophages played a significant role in promoting loss of rod photoreceptor cells in the rd10 mice.
Our results from TAM-treated CX3CR1YFP-creERROSADTARpe65−/−CD11cGFP mice indicate that the presence of these subsets of retinal macrophages within the outer retina may not be pro-apoptotic to the degree seen in the rd10 models. TAM-treated and control retinas were flatmounted and analyzed at P41 to look for surviving cones expressing misplaced cone opsin, since peak TUNEL+ staining in the ONL was observed between P14 to P21 and greatly decreased by P41. The mistrafficked opsin in the OPL indicated that these cones were “sick” [9, 10, 21]; however, a slightly greater proportion of stressed cones survived up to P41 when DCs were not depleted. Conversely, the increased proportion of cones identified by OS staining, especially at 14 months of age suggested that a small increase in healthy cones was present in the absence of retinal macrophages. Interpretation of these results is informed by two factors. First, despite detection of significant differences, the small effects may have limited biological significance. Second, the effect of macrophage depletion in this model did not show the more potent effects seen in models with more extensive loss of photoreceptors. This outcome may reflect the absence of macrophage recruitment from the circulation in the Rpe65−/− model, and the possibility that recruited mononuclear cells are more pathogenic than resident macrophages.
The result is interesting in light of recent findings that implicate activated MG in the induction of neurotoxic A1 astrocytes [43]. While astrocytes are found in the inner retina, separate from the photoreceptor cells, another type of macroglia, the Müller glia, are numerous and in direct contact with photoreceptors; perhaps they play a similar role in the retina. The mechanism(s) by which retinal macrophages recognize stressed or apoptotic photoreceptor cells is unknown, recent results in brain point to MG expression of Mer as a required component in clearance of apoptotic neurons [44]. These data highlight the multiple roles that retinal mononuclear cells may exhibit, including clearing dead and dying cells, or sustaining the survival of cells, or inducing a neurotoxic response.
Unlike our findings, and those of the Saban lab [17], some others have failed to find the GFP-expressing cells in retina using what was claimed to be the same strain as the CD11cGFP mice we report [45]. As shown in the many photographs and flow cytometric analyses in this report, GFP-expressing cells were readily found, without use of anti-GFP antibodies. The CD11c-eYFP+ cells described by Dando et al [45] differ from the CD11c-GFPhi cells we report here. Many more CD11c-eYFP+ cells (extrapolate to approximately 1500/retina) than CD11c-GFPhi cells (approximately 200/retina) were found in retina. CD11c-eYFP+ cells were also found in the subretinal space (approximately 150/retina) while CD11c-GFPhi cells are rare in the subretinal space. The number of CD11c-eYFP+ cells found in rd8/rd8 retinas were similar to wt, while the number of CD11c-GFPhi cells increase several fold in rd8/rd8 retina (unpublished). A likely explanation for the differences between the CD11c-DTR/GFP mice and the CD11c-eYFP mice is that the promoter constructs may differ, and/or their insertion into the genome is different, leading to differing expression patterns of their reporters. Finally, there is only one meaningful assessment of antigen presentation function - the processing of an antigen into peptides, their occupancy of class II MHC, and presentation to naïve, antigen-specific CD4 T cells. We have shown elsewhere that the CD11c-GFPhi cells from retina meet this criterion [14].
Conclusions
The expression of GFP on retinal mononuclear cells in CD11cGFP mice identifies cells performing functions, especially responses to stress and injury, previously attributed to MG. Retinal stress in CD11cGFP mice that did not lead to mononuclear cell recruitment from the circulation, as in this model of Rpe65−/− cone degeneration, induced these cells that appear to be an activated form of MG. Although they dominated the cellular response to cone death in the photoreceptor cell layer in Rpe65−/− mice, their ablation led to only an incremental increase in cone survival. Use of these reporter mice will enable more specific studies of the stimuli and pathways to MG activation and APC function in the retina. The ability to readily identify, ablate, and isolate these cells will facilitate analysis of this activated, antigen-presenting subset of MG.
Highlights.
Retinal macrophages expressing GFP from a transgenic CD11c promoter concentrated in elevated numbers in the retinal photoreceptor cell layer of RPE65 knockout mice, a murine model of Leber congenital amaurosis in which cone photoreceptors begin to die at 2 – 3 weeks of age.
Elevated numbers of GFPhi cells remained in the outer retinas of knockout mice for more than a year, consistent with the continued loss of cones.
Generation of the GFPhi cells was found to be dependent on microglia.
Using mice in which CX3CR1+ macrophages/microglia, including the GFPhi cells, were susceptible to tamoxifen-induced death, month-long depletion regimens of macrophages lead to a modest increase in S-cone survival when tested during the acute phase of cone loss between days 7 to 41, and subsequent loss between days 390 to 420.
The expression of GFP on retinal mononuclear cells in CD11cGFP mice identifies cells performing functions previously attributed to microglia. The ability to identify, ablate, and isolate these cells will facilitate analysis of this activated, antigen-presenting subset of MG.
Acknowledgments
Funding
This study was supported by the Minnesota Lions Clubs, the VitreoRetinal Surgery Foundation Research Award (PT), Research to Prevent Blindness, the Wallin Neuroscience Discovery Fund (DG), NIH/NEI R01 EY021003 (DG) and NIH/NEI R01 EY025209 (DG).
The authors thank Heidi Roehrich for assistance with histology. The authors thank Scott McPherson, Ph.D., University of Minnesota, for his assistance in editing this manuscript. We thank Daniel Saban, Ph.D., Duke University, and Zsolt Ablonczy, Ph.D., Medical University of South Carolina, for critiques of this manuscript.
Abbreviations
- DC
Dendritic cell
- E
Embryonic day
- GFP
Green fluorescent protein
- INL
Inner nuclear layer
- IPL
Inner plexiform layer
- IS
Inner segment
- LCA2
Type 2 Leber congenital amaurosis
- ONL
Outer nuclear layer
- OPL
Outer plexiform layer
- OS
Outer segment
- P
Postnatal day
- PBS
Phosphate buffered saline
- PFA
Paraformaldehyde
- RAL
Retinaldehyde
- RGC
Retinal ganglion cell
- RPE
Retinal pigment epithelium
- RT
Room temperature
- TAM
Tamoxifen
Footnotes
Consent for publication
Not applicable
Availability of data and material
Data are available on request. Contact corresponding author.
Ethics approval
All experiments were performed in accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research and the University of Minnesota institutional animal care and use guidelines.
Conflict of interest
None
Author’s contributions
PT conducted experiments, analyzed data, wrote manuscript, edited manuscript. MP conducted experiments, analyzed data. NH conducted experiments, analyzed data. DG analyzed data, wrote manuscript, edited manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tang PH, Buhusi MC, Ma JX, Crouch RK. RPE65 is present in human green/red cones and promotes photopigment regeneration in an in vitro cone cell model. J Neurosci. 2011;31:18618–26. doi: 10.1523/JNEUROSCI.4265-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang PH, Wheless L, Crouch RK. Regeneration of photopigment is enhanced in mouse cone photoreceptors expressing RPE65 protein. J Neurosci. 2011;31:10403–11. doi: 10.1523/JNEUROSCI.0182-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Znoiko SL, Crouch RK, Moiseyev G, Ma JX. Identification of the RPE65 protein in mammalian cone photoreceptors. Invest Ophthalmol Vis Sci. 2002;43:1604–9. [PubMed] [Google Scholar]
- 4.Ma JX, Xu L, Othersen DK, Redmond TM, Crouch RK. Cloning and localization of RPE65 mRNA in salamander cone photoreceptor cells. Biochim Biophys Acta. 1998;1443:255–61. doi: 10.1016/s0167-4781(98)00221-8. [DOI] [PubMed] [Google Scholar]
- 5.Tang PH, Kono M, Koutalos Y, Ablonczy Z, Crouch RK. New insights into retinoid metabolism and cycling within the retina. Prog Retin Eye Res. 2012;32:48–63. doi: 10.1016/j.preteyeres.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moiseyev G, Takahasi Y, Chen Y, Gentleman S, Redmond TM, Crouch RK, et al. RPE65 is the isomerohydrolase in the retinoid visual cycle. Proc Natl Acad Sci U S A. 2005;102:12413–8. doi: 10.1073/pnas.0503460102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Travis GH, Golczak M, Moise AR, Palczewski K. Diseases caused by defects in the visual cycle: retinoids as potential therapeutic agents. Annu Rev Pharmocol Toxicol. 2007;47:1–44. doi: 10.1146/annurev.pharmtox.47.120505.105225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.den-Hollander AI, Roepman R, Koenekoop RK, Cremers FP. Leber congenital amaurosis: genes, proteins and disease mechanisms. Prog Retin Eye Res. 2008;27:391–419. doi: 10.1016/j.preteyeres.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Fan J, Rohrer B, Frederick JM, Baehr W, Crouch RK. RPE65−/− and LRAT−/− mice: comparable models of Leber Congenital Amaurosis. Invest Ophthalmol Vis Sci. 2008;49:2384–9. doi: 10.1167/iovs.08-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang PH, Fan J, Goletz PW, Wheless L, Crouch RK. Effective and sustained delivery of hydrophobic retinoids to photoreceptors. Invest Ophthalmol Vis Sci. 2010;51:5958–64. doi: 10.1167/iovs.10-5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, et al. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–20. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lehmann U, Heuss ND, McPherson SW, Roehrich H, Gregerson DS. Dendritic cells are early responders to retinal injury. Neurobiol Dis. 2010;40:177–84. doi: 10.1016/j.nbd.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McPherson SW, Heuss ND, Gregerson DS. Local “on-demand” generation and function of antigen-specific foxp3+ regulatory T cells. J Immunol. 2013;190:4971–81. doi: 10.4049/jimmunol.1202625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McPherson SW, Heuss ND, Pierson MJ, Gregerson DS. Retinal antigen-specific regulatory T cells protect against spontaneous and induced autoimmunity and require local dendritic cells. J Neuroinflammation. 2014;11:205. doi: 10.1186/s12974-014-0205-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heuss ND, Lehmann U, Norbury CC, McPherson SW, Gregerson DS. Local activation of dendritic cells alters the pathogenesis of autoimmune disease in the retina. J Immunol. 2012;188:1191–200. doi: 10.4049/jimmunol.1101621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heuss ND, Pierson MJ, Montaniel K, McPherson SW, Lehmann U, Hussong SA, et al. Retinal dendritic cell recruitment, but not function, was inhibited in MyD88 and TRIF deficient mice. J Neuroinflammation. 2014;11:143. doi: 10.1186/s12974-014-0143-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Koren EG, Mathew R, Saban DR. Fate mapping reveals that microglia and recruited monocyte-derived macrophages are definitively distinguishable by phenotype in the retina. Sci Rep. 2016;6:20636. doi: 10.1038/srep20636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Redmond TM, Yu S, Lee E, Bok D, Hamasaki D, Chen N, et al. RPE65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat Genet. 1998;20:344–51. doi: 10.1038/3813. [DOI] [PubMed] [Google Scholar]
- 19.Mattapallil MJ, Wawrousek EF, Chan CC, Zhao H, Roychoudhury J, Ferguson TA, et al. The Rd8 mutation of the Crb1 gene is present in vendor lines of C57BL/6N mice and embryonic stem cells, and confounds ocular induced mutant phenotypes. Invest Ophthalmol Vis Sci. 2012;53:2921–7. doi: 10.1167/iovs.12-9662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Telford WG, Hawley T, Subach F, Verkhusha V, Hawley RG. Flow cytometry of fluorescent proteins. Methods. 2012;57:318–30. doi: 10.1016/j.ymeth.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Rohrer B, Lohr HR, Humphries P, Redmond TM, Seeliger MW, Crouch RK. Cone opsin mislocalization in RPE65−/− mice: a defect that can be corrected by 11-cis retinal. Invest Ophthalmol Vis Sci. 2005;46:3876–82. doi: 10.1167/iovs.05-0533. [DOI] [PubMed] [Google Scholar]
- 22.Redmond TM, Hamel CP. Genetic analysis of RPE65: from human disease to mouse model. Methods in enzymology. 2000;316:705–24. doi: 10.1016/s0076-6879(00)16758-8. [DOI] [PubMed] [Google Scholar]
- 23.Zhang T, Zhang N, Baehr W, Fu Y. Cone opsin determines the time course of cone photoreceptor degeneration in Leber congenital amaurosis. Proc Natl Acad Sci U S A. 2011;108:8879–84. doi: 10.1073/pnas.1017127108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–5. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pequignot MO, Provost AC, Salle S, Taupin P, Sainton KM, Marchant D, et al. Major role of BAX in apoptosis during retinal development and in establishment of a functional postnatal retina. Dev Dyn. 2003;228:231–8. doi: 10.1002/dvdy.10376. [DOI] [PubMed] [Google Scholar]
- 26.Braunger BM, Demmer C, Tamm ER. Programmed cell death during retinal development of the mouse eye. Adv Exp Med Biol. 2014;801:9–13. doi: 10.1007/978-1-4614-3209-8_2. [DOI] [PubMed] [Google Scholar]
- 27.Métrailler S, Schorderet DF, Cottet S. Early apoptosis of rod photoreceptors in Rpe65(−/−) mice is associated with the upregulated expression of lysosomal-mediated autophagic genes. Exp Eye Res. 2012;96:70–81. doi: 10.1016/j.exer.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 28.Sadun F, Pece A, Brancato R. Fluorescein and indocyanine green angiography in arteritic anterior ischaemic optic neuropathy. Br J Ophthalmol. 1998;82:1344–5. doi: 10.1136/bjo.82.11.1339h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang H, Fan J, Li S, Karan S, Rohrer B, Palczewski K, et al. Trafficking of membrane-associated proteins to cone photoreceptor outer segments requires the chromophore 11-cis-retinal. J Neurosci. 2008;28:4008–14. doi: 10.1523/JNEUROSCI.0317-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Applebury ML, Antoch MP, Baxter LC, Chun LL, Falk JD, Farhangfar F, et al. The murine cone photoreceptor: a single cone type expresses both S and M opsins with retinal spatial patterning. Neuron. 2000;27:513–23. doi: 10.1016/s0896-6273(00)00062-3. [DOI] [PubMed] [Google Scholar]
- 31.Ortin-Martinez A, Nadal-Nicolas FM, Jimenez-Lopez M, Alburquerque-Bejar JJ, Nieto-Lopez L, Garcia-Ayuso D, et al. Number and distribution of mouse retinal cone photoreceptors: differences between an albino (Swiss) and a pigmented (C57/BL6) strain. PLoS One. 2014;9:e102392. doi: 10.1371/journal.pone.0102392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rohlich P, van Veen T, Szel A. Two different visual pigments in one retinal cone cell. Neuron. 1994;13:1159–66. doi: 10.1016/0896-6273(94)90053-1. [DOI] [PubMed] [Google Scholar]
- 33.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–96. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 34.Guilliams M, Ginhoux F, Jakubzick C, Naik SH, Onai N, Schraml BU, et al. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat Rev Immunol. 2014;14:571–8. doi: 10.1038/nri3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamann S, Schorderet DF, Cottet S. Bax-induced apoptosis in Leber’s congenital amaurosis: a dual role in rod and cone degeneration. PLoS One. 2009;4:e6616. doi: 10.1371/journal.pone.0006616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peng B, Xiao J, Wang K, So KF, Tipoe GL, Lin B. Suppression of microglial activation is neuroprotective in a mouse model of human retinitis pigmentosa. J Neurosci. 2014;34:8139–50. doi: 10.1523/JNEUROSCI.5200-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao L, Zabel MK, Wang X, Ma W, Shah P, Fariss RN, et al. Microglial phagocytosis of living photoreceptors contributes to inherited retinal degeneration. EMBO Mol Med. 2015;7:1179–97. doi: 10.15252/emmm.201505298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roche SL, Wyse-Jackson AC, Gomez-Vicente V, Lax P, Ruiz-Lopez AM, Byrne AM, et al. Progesterone Attenuates Microglial-Driven Retinal Degeneration and Stimulates Protective Fractalkine-CX3CR1 Signaling. PLoS One. 2016;11:e0165197. doi: 10.1371/journal.pone.0165197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roche SL, Wyse-Jackson AC, Ruiz-Lopez AM, Byrne AM, Cotter TG. Fractalkine-CX3CR1 signaling is critical for progesterone-mediated neuroprotection in the retina. Sci Rep. 2017;7:43067. doi: 10.1038/srep43067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang X, Zhao L, Zhang Y, Ma W, Gonzalez SR, Fan J, et al. Tamoxifen Provides Structural and Functional Rescue in Murine Models of Photoreceptor Degeneration. J Neurosci. 2017;37:3294–310. doi: 10.1523/JNEUROSCI.2717-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zabel MK, Zhao L, Zhang Y, Gonzalez SR, Ma W, Wang X, et al. Microglial phagocytosis and activation underlying photoreceptor degeneration is regulated by CX3CL1-CX3CR1 signaling in a mouse model of retinitis pigmentosa. Glia. 2016;64:1479–91. doi: 10.1002/glia.23016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jeon CJ, Strettoi E, Masland RH. The major cell populations of the mouse retina. J Neurosci. 1998;18:8936–46. doi: 10.1523/JNEUROSCI.18-21-08936.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017 doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fourgeaud L, Traves PG, Tufail Y, Leal-Bailey H, Lew ED, Burrola PG, et al. TAM receptors regulate multiple features of microglial physiology. Nature. 2016;5324:240. doi: 10.1038/nature17630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dando SJ, Naranjo Golborne C, Chinnery HR, Ruitenberg MJ, McMenamin PG. A case of mistaken identity: CD11c-eYFP(+) cells in the normal mouse brain parenchyma and neural retina display the phenotype of microglia, not dendritic cells. Glia. 2016;6449:1331. doi: 10.1002/glia.23005. [DOI] [PubMed] [Google Scholar]