Abstract
OBJECTIVE
The purpose of this article is to report our intermediate to long-term outcomes with image-guided percutaneous hepatic tumor cryoablation and to evaluate its technical success, technique efficacy, local tumor progression, and adverse event rate.
MATERIALS AND METHODS
Between 1998 and 2014, 299 hepatic tumors (243 metastases and 56 primary tumors; mean diameter, 2.5 cm; median diameter, 2.2 cm; range, 0.3–7.8 cm) in 186 patients (95 women; mean age, 60.9 years; range, 29–88 years) underwent cryoablation during 236 procedures using CT (n = 126), MRI (n = 100), or PET/CT (n = 10) guidance. Technical success, technique efficacy at 3 months, local tumor progression (mean follow-up, 2.5 years; range, 2 months to 14.6 years), and adverse event rates were calculated.
RESULTS
The technical success rate was 94.6% (279/295). The technique efficacy rate was 89.5% (231/258) and was greater for tumors smaller than 4 cm (93.4%; 213/228) than for larger tumors (60.0%; 18/30) (p < 0.0001). Local tumor progression occurred in 23.3% (60/258) of tumors and was significantly more common after the treatment of tumors 4 cm or larger (63.3%; 19/30) compared with smaller tumors (18.0%; 41/228) (p < 0.0001). Adverse events followed 33.8% (80/236) of procedures and were grade 3–5 in 10.6% (25/236) of cases. Grade 3 or greater adverse events more commonly followed the treatment of larger tumors (19.5%; 8/41) compared with smaller tumors (8.7%; 17/195) (p = 0.04).
CONCLUSION
Image-guided percutaneous cryoablation of hepatic tumors is efficacious; however, tumors smaller than 4 cm are more likely to be treated successfully and without an adverse event.
Keywords: cryoablation, hepatocellular carcinoma, liver, metastases
The liver is a common site of primary and metastatic tumors [1]. In the United States, metastatic disease predominates, with tumors most frequently arising from the colon, breast, lung, and pancreas [2]. Because hepatic metastases are a common cause of mortality, there is a significant survival benefit from surgical metastasectomy, particularly for colon cancer metastases [3, 4]. However, many hepatic metastases (and primary tumors) are either too large or too numerous to be removed surgically [5]. In addition, many patients have multiple medical comorbidities that render surgical resection contraindicated [6]. Image-guided percutaneous tumor ablation is therefore a rapidly growing alternative for those patients who are not surgical candidates [7, 8].
The safety and efficacy of image-guided percutaneous ablations using heat-based agents for both hepatocellular carcinoma (HCC) and hepatic metastases have been shown [8–11]. Indeed, the results of radio-frequency ablation (RFA) of hepatic metastases from colorectal cancer compare favorably with those of surgical metastasectomy [9]. However, there is a relative paucity of data regarding percutaneous hepatic tumor cryoablation as an alternative to RFA or microwave ablation, particularly when the latter two options may not be feasible because of adjacent critical structures [12–19]. Some have postulated that there may be a reluctance to treat hepatic tumors with cryoablation, perhaps because of concerns for bleeding, liver fracture, and cryoshock that were observed many years ago during open cryo-surgery [20]. However, these severe complications typically occurred after cryoablation of large tumors and involved the placement of applicators as large as 9 mm in diameter, much larger than those currently used percutaneously [21]. A single prospective randomized clinical trial compared percutaneous cryoablation to RFA for the treatment of HCC, and an equal overall 5-year survival rate was found [22]. However, that study included only patients with HCC, and all procedures were performed with CT guidance. Another recent study included metastases, as well as primary liver tumors, but used CT alone to guide all but one procedure [19]. Therefore, more data are needed to understand the role of cryoablation in the treatment of all hepatic tumors. We report our intermediate to long-term outcomes with image-guided percutaneous hepatic tumor cryoablation and evaluate its technical success, technique efficacy, local tumor progression, and adverse event rate.
Materials and Methods
Study Cohort
This retrospective cohort study was conducted after approval by the institutional review board of Brigham and Women’s Hospital; the need for written informed consent was waived. Our interventional radiology database was used to identify all image-guided percutaneous cryoablation procedures during which hepatic tumors were treated between November 1998, when the institution’s tumor ablation program began, and August 2014; the end date of the study period was chosen to allow adequate follow-up. Although cryoablation was often selected in lieu of heat-based techniques to treat tumors adjacent to critical structures, the retrospective nature of this study precluded meaningful analysis of selection criteria for cryoablation in comparison with other ablation technologies. In general, cryoablation was selected to treat tumors that were in close proximity to the gallbladder, diaphragm, major bile ducts, chest wall, bowel, or heart and when intraprocedural monitoring of the ice ball was desired. All patients were referred for ablation by their primary internist, oncologist, or surgeon. Reasons for not performing surgery included multiplicity or location of hepatic tumors, concomitant distant metastatic disease, medical comorbidities, limited hepatic reserve secondary to prior surgery, or patient refusal. During the study period, 299 tumors in 186 patients (95 women and 91 men; age range, 29–88 years; mean age, 60.9 years; range, 29–88 years) were treated during 236 procedures. The intent of cryoablation was local cure except for four tumors that were large or inseparable from adjacent critical structures, where the intent was de-bulking (n = 3) or palliation of symptoms (n = 1). All ablations were performed in a single session. Of 299 tumors in 186 patients, 127 (42.4%) have been reported previously [23–26]. Each of these prior reports included a smaller number of patients, examined individual distinct aspects of hepatic cryoablation, and did not address the metrics, endpoints, and purpose evaluated in this study.
Tumor Characteristics
Two hundred ninety-nine tumors (mean diameter, 2.5 cm; median diameter, 2.2 cm; range, 0.3–7.8 cm) were treated. Tumor size categories included tumors smaller than 1 cm (n = 9), 1–1.9 cm (n = 103), 2–2.9 cm (n = 91), 3–3.9 cm (n = 53), 4–4.9 cm (n = 31), and 5–7.8 cm (n = 12). Of 299 tumors, 243 were metastases and 56 were primary liver neoplasms (50 HCCs and six cholangiocarcinomas). The metastases were from colon carcinoma (n = 68), ovarian carcinoma (n = 30), gastrointestinal stromal tumor (n = 21), breast carcinoma (n = 16), kidney cancer (n = 13), lung cancer (n = 12), leiomyosarcoma (n = 12), esophageal cancer (n = 11), pancreatic neuroendocrine tumor (n = 9), adenoid cystic carcinoma (n = 4), adrenal cortical carcinoma (n = 4), urothelial carcinoma (n = 3) carcinoid tumor (n= 2), thymoma ( n= 2), mesothelioma (n= 2), melanoma ( n= 2), and others ( n= 32).
Cryoablation Technique
All cryoablations were performed with a system using argon gas for cooling and helium for active thawing (Cryohit, Galil Medical). Procedures were performed by one of seven staff radiologists whose experience with tumor ablation ranged from 1 to 16 years depending on the study time point. Ablations were performed with general anesthesia (n= 177) or IV conscious sedation ( n= 59) and used CT (n = 126), MRI (n = 100), or PET/CT (n= 10) guidance. Almost all procedures were performed with the assistance of a trainee. From 1998 to 2006, procedures (n = 65) were performed with 13-gauge applicators. Subsequently, procedures (n = 171) were performed with only 17-gauge applicators. A protocol consisting of a 15-minute freeze, 10-minute passive thaw, and a 15-minute freeze was used. Operators could deviate from the standard protocol and adjust the length of freezing or the percentage of cooling applied to each applicator on the basis of findings on intraprocedural images. Intraprocedural monitoring of the ice ball facilitated the treatment objective of achieving a minimum tumor ablation margin of 5–10 mm. When necessary, either another applicator was placed or the freezing cycle was lengthened [27]. Also, if the ice ball edge encroached on an adjacent critical structure, the power to that particular applicator was decreased. Mean applicator density (number of applicators used per tumor divided by tumor diameter) was 1.41 applicators per centimeters of tumor diameter (range, 0.36–6.6 applicators/cm). In addition to monitoring ice ball growth with imaging during cryoablation procedures, ancillary maneuvers were performed in 27 procedures to displace adjacent critical structures (22 hydrodissections, four artificial pneumothoraces, and one external hand compression to displace bowel). All patients were admitted to the hospital for observation after the procedure.
Technical Success, Technique Efficacy, and Local Tumor Progression
Patients were imaged at 24 hours with MRI with and without IV contrast material (or CT with IV contrast material if MRI was contraindicated) and then with MRI, CT, or PET/CT at 3-month intervals for the first 6 9 months and then every 3 6 months thereafter. After 2 years, patients were imaged yearly. A procedure was considered technically successful for the 295 tumors treated with curative intent if the tumor was completely included in the hypoenhancing ablation zone at 24 hours [28]. Four tumors that were treated for debulking or pain palliation were excluded from the technical success, technique efficacy, and local tumor progression calculations.
Technique efficacy that is, the percentage of tumors without local progression (defined as the absence of nodular or masslike enhancing components at MRI or CT or the lack of focal 18F-FDG avidity at PET/CT located in or contiguous with the ablation zone) at 3 months after an ablation procedure was assessed for 258 tumors (209 metastases and 49 primary tumors) [26] (Fig. 1). Thirty-seven tumors (12.5% [28 < 4 cm and 9 ≥ 4 cm]) lacked sufficient follow-up imaging (less than 3 months without documented recurrence) and were therefore excluded in the determination of technique efficacy. A single tumor with less than 3 months of imaging follow-up recurred at 2 months and was included. Local tumor progression rates for the same 258 tumors, including all subsequent imaging follow-up, were calculated; 31.4% (81/258) of tumors had less than 12 months of follow-up, 25.6% (66/258) had 12–23 months of follow-up, 6.7% (43/258) had 24–35 months of follow-up, 7.4% (19/258) had 36–47 months of follow-up, and 19.0% (49/258) had at least 4 years of follow-up (range, 4.2–14.6 years). The mean follow-up period was 2.5 years (range, 2 months to 14.6 years).
Fig. 1.
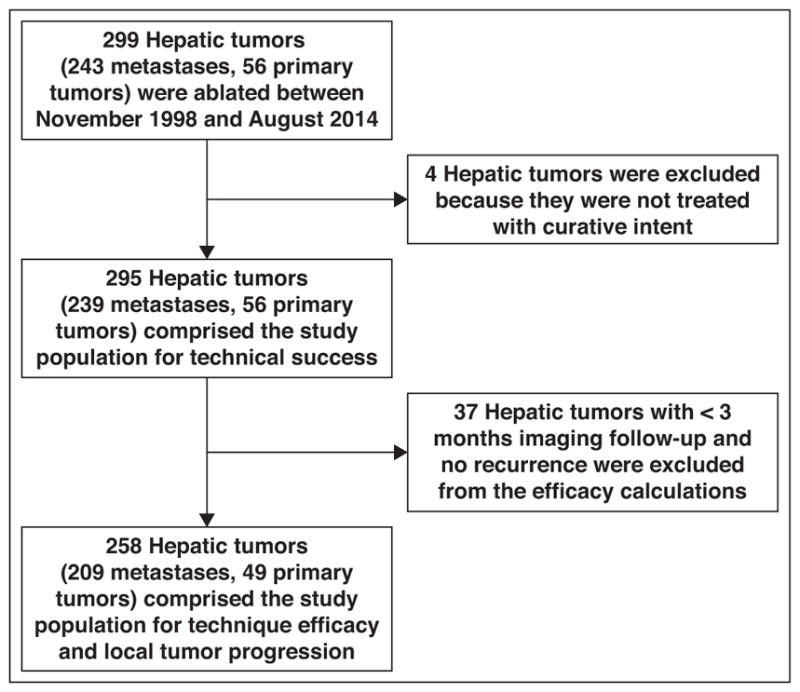
Flowchart of inclusion and exclusion criteria for determining technical success, technique efficacy, and local tumor progression of patients undergoing percutaneous image-guided cryoablation of hepatic tumors.
Adverse Event Rate
Adverse events were graded using the National Cancer Institute’s Common Terminology Criteria for Adverse Events [29] and were identified according to a retrospective review of the electronic medical record for each patient. Acute kidney injury was defined as an increase in serum creatinine level of 0.3 mg/dL or greater above baseline [29]. Pre- and postablation platelet count, serum creatinine level, and serum myoglobin level were recorded for each procedure. All adverse events occurring within 30 days after each procedure were tabulated. The overall adverse event rate and adverse event rates by grade, using the most severe adverse event for each procedure, were calculated.
Statistical Analysis
The primary study endpoints were technical success, 3-month technique efficacy, local tumor progression, and adverse event rates. Secondary endpoints were postprocedural platelet count, serum myoglobin level, and serum creatinine level. Further analysis compared groups according to tumor size, guidance modality, applicator density, and histologic profile. The threshold diameter used to determine the effect of tumor size on results was selected using recursive partitioning, as implemented in the rpart (Recursive Partitioning and Regression Trees) package of R (version 4.1-8, R Foundation for Statistical Computing). Pearson chi-square tests were used to compare technical success, technique efficacy, local tumor progression, and adverse event rates between tumors smaller than 4 cm and larger tumors, between primary tumors and metastases, and between guidance modalities. The t test was used to compare mean laboratory test values between groups, mean applicator density, and mean time to local tumor recurrence. A one-tailed z-test was used to compare rates of technical success and technique efficacy between tumor types to a threshold value (90%). A two-tailed z-test was used to compare local tumor progression to a threshold value (25%). A Kaplan-Meier curve was used to compare the time to progression between small and large tumors. The statistical significance level was set at p < 0.05. Data analyses were performed using R (version 3.1.0, R Foundation for Statistical Computing) and Stata (version 13, StataCorp).
Results
Technical Success
The overall technical success rate at 24 hours was 94.6% (279/295) in tumors treated with curative intent (Table 1 and Figs. 2 and 3). Of the 16 tumors that were not successfully treated at 24 hours, two were ablated a second time. The remaining 14 tumors were not ablated a second time; these patients were treated with systemic chemotherapy. The technical success rate was 96.1% (246/256) in tumors smaller than 4 cm and 84.6% (33/39) in tumors 4 cm or larger (p = 0.003) (Table 1). Technical success rates for metastases and primary hepatic tumors were 94.1% (225/239) and 96.4% (54/56), respectively (p = 0.50) (Table 1). Technical success varied among tumor types; ovarian cancer had the highest rate (100%; 30/30) and lung cancer had the lowest (72.7%; 8/11) (p = 0.02) (Table 2). Technical success rates were 92.5% (62/67) for colorectal carcinoma metastases and 95.5% (42/44) for HCC (p = 0.54) (Table 2). There was no statistically significant difference in technical success rates between procedures performed with CT, MRI, or PET/CT guidance (Table 1). The mean applicator density was higher when technical success was achieved (1.7 applicators/cm tumor) compared with tumors in which technical success was not achieved (1.2 applicators/cm tumor) (p = 0.02).
TABLE 1.
Technical Success, Technique Efficacy, and Local Tumor Progression of Percutaneous Image-Guided Cryoablation of Hepatic Tumors, by Tumor Size and Type and Guidance Modality
| Variable | Technical Success (n = 295) | Technique Efficacy at 3 Months (n = 258) | Local Tumor Progression (n = 258) |
|---|---|---|---|
|
| |||
| Tumor size (cm) | |||
| < 4 | 96.1 (246/256) | 93.4 (213/228) | 18 (41/228) |
| ≥ 4 | 84.6 (33/39) | 60.0 (18/30) | 63.3 (19/30) |
| p | 0.003 | < 0.0001 | < 0.0001 |
| Tumor type | |||
| Metastases | 94.1 (225/239) | 88.5 (185/209) | 23.0 (48/209) |
| Primary liver tumors | 96.4 (54/56) | 93.9 (46/49) | 24.5 (12/49) |
| p | 0.50 | 0.27 | 0.82 |
| Guidance modality | |||
| CT | 96.1 (146/152) | 91.0 (122/134) | 23.1 (31/134) |
| MRI | 92.3 (120/130) | 86.5 (96/111) | 25.2 (28/111) |
| PET/CT | 100 (13/13) | 100 (13/13) | 7.7 (1/13) |
| p | 0.26 | 0.23 | 0.37 |
Note—Except for p values, data are percentage (number/total) of hepatic tumors.
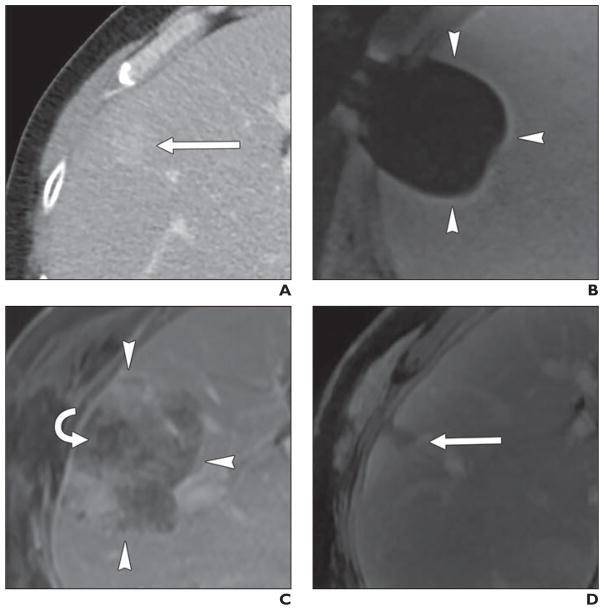
Fig. 2. 55-year-old man with renal cell carcinoma metastatic to liver treated with percutaneous MRI-guided cryoablation.
A, Preablation contrast-enhanced CT shows 2.1-cm peripheral metastasis (arrow) in segment VIII.
B, Intraprocedural T1-weighted unenhanced MR image shows ice ball as signal void (arrowheads).
C, Twenty-four-hour contrast-enhanced MR image shows ablation zone (arrowheads) surrounding nonenhancing tumor (curved arrow) with minimal enhancement of adjacent hepatic parenchyma.
D, Four-year follow-up subtraction image from contrast-enhanced MR image shows involution of ablation zone (arrow) and absence of residual or recurrent tumor.
Fig. 3. 75-year-old man with colon cancer metastatic to liver treated with percutaneous CT-guided cryoablation.
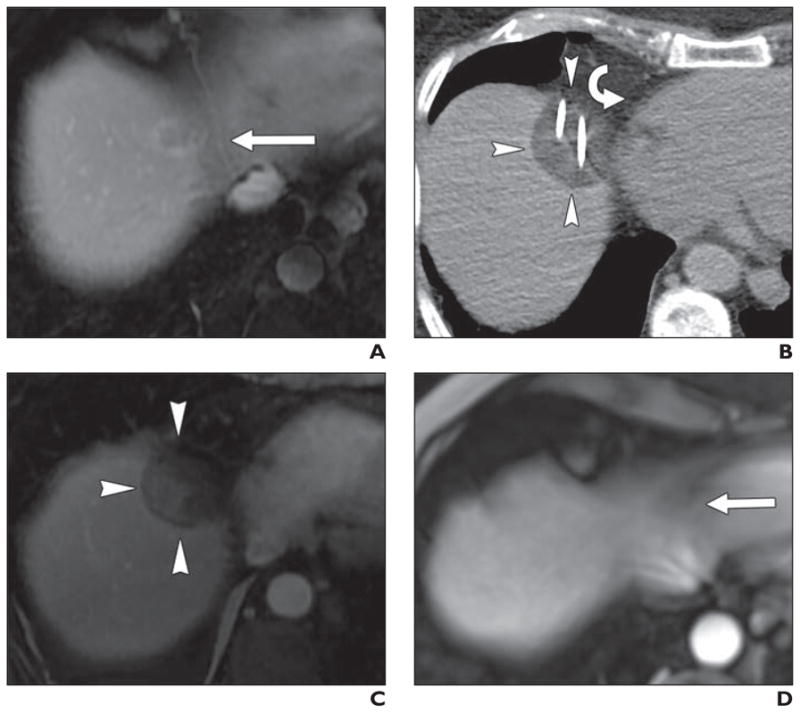
A, Preablation contrast-enhanced MR image shows 1.2-cm metastasis (arrow) at liver dome medially, area often difficult to separate from diaphragm and heart using artificial ascites.
B, Intraprocedural unenhanced CT image shows hypoattenuating ice ball (arrowheads) directly adjacent to right atrium (curved arrow).
C, Twenty-four-hour contrast-enhanced MR image shows nonenhancing ablation zone (arrowheads).
D, Four-year follow-up contrast-enhanced MR image shows involution of ablation zone (arrow) and absence of residual or recurrent tumor.
TABLE 2.
Technical Success, Technique Efficacy, and Local Tumor Progression of Percutaneous Image-Guided Cryoablation of Hepatic Tumors, by Type of Tumor
| Type of Tumor | Technical Success | Technique Efficacy | Local Tumor Progression |
|---|---|---|---|
|
| |||
| Colorectal cancer | 92.5 (62/67) | 88.5 (54/61) | 24.6 (15/61) |
| Hepatocellular carcinoma | 95.5 (42/44)a | 93.2 (41/44) | 25.0 (11/44) |
| Ovarian cancer | 100 (30/30)a | 96.6 (28/29)a | 17.2 (5/29) |
| Gastrointestinal stromal tumor | 95.2 (20/21) | 85.7 (18/21) | 23.8 (5/21) |
| Breast cancer | 93.3 (14/15) | 92.9 (13/14) | 21.4 (3/14) |
| Renal cell carcinoma | 100 (13/13) | 100 (11/11) | 15.4 (2/13) |
| Lung cancer | 72.7 (8/11) | 62.5 (5/8) | 50.0 (4/8) |
| Esophageal cancer | 100 (11/11) | 85.7 (6/7) | 28.6 (2/7) |
| Pancreatic neuroendocrine tumor | 100 (9/9) | 100 (9/9) | 0 (0/9)b |
Note—Data are percentage (number/total) of tumors.
Significantly different from 90% (p < 0.05).
Significantly different from 25% (p < 0.05).
Technique Efficacy
Overall, the technique efficacy rate at 3 months was 89.5% (231/258). The technique efficacy rate was 93.4% (213/228) for tumors smaller than 4 cm and 60.0% (18/30) for tumors 4 cm and larger (p < 0.0001) (Table 1). Technique efficacy rates were 88.5% (185/209) for metastases and 93.9% (46/49) for primary hepatic tumors (p = 0.27) (Table 1). When stratified by tumor type, the ablation of renal cell carcinoma metastases had the highest technique efficacy rate (100%; 11/11) and lung cancer metastases had the lowest (62.5%; 5/8) (p = 0.03) (Table 2). Technique efficacy rates were 88.5% (54/61) for colorectal carcinoma metastases and 93.2% (41/44) for HCC (p = 0.42) (Table 2). There was no statistically significant difference in technique efficacy between the procedures performed with CT, MRI, or PET/CT guidance (Table 1). The mean applicator density was higher in tumors that were effectively treated at 3 months (1.7 applicators/cm tumor) compared with tumors that were not effectively treated at 3 months (1.2 applicators/cm tumor) (p = 0.007).
Local Tumor Progression
Local tumor progression based on all available imaging follow-up occurred in 27.1% (70/258) of tumors. Ten of the recurrences were treated successfully with repeat cryoablation procedures. Sixty of the recurrences were either not retreated (n = 53) or were retreated unsuccessfully (n = 7). Therefore, the local tumor progression rate adjusted for repeat treatments was 23.3% (60/258) (Fig. 4). Allowing for repeat treatments, local tumor progression occurred in 18.0% (41/228) of tumors smaller than 4 cm and in 63.3% (19/30) of tumors 4 cm or larger (p < 0.0001) (Table 1 and Fig. 4). Local tumor progression occurred in 23.0% (48/209) of metastases and 24.5% (12/49) of primary tumors (p = 0.82) (Table 1). Local tumor progression occurred in 24.6% (15/61) of colorectal carcinoma metastases and in 25% (11/44) of HCCs (p = 0.96) (Table 2).
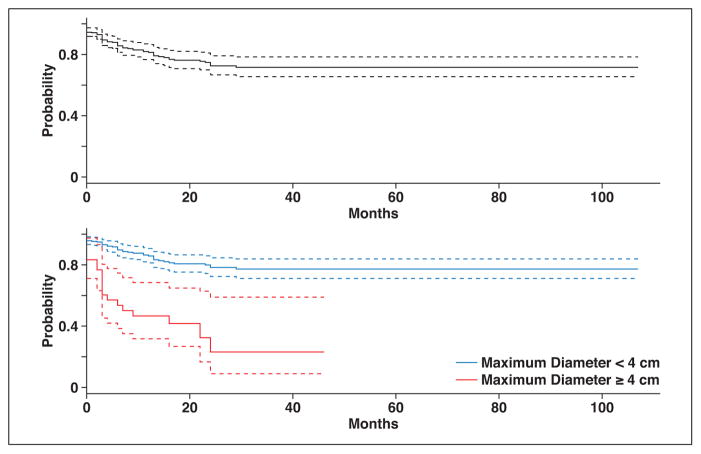
Fig. 4.
Graphs of progression-free survival for all tumors (top) and according to tumor size (bottom). Dashed lines represent 95% CIs.
The time to local tumor progression ranged from 1 to 29 months (mean, 9.4 months; median, 7 months). Local tumor progression rates were higher for tumors 4 cm or larger compared with tumors smaller than 4 cm (Fig. 4). There was no significant difference in the mean time to local tumor progression between tumors smaller than 4 cm and those 4 cm or larger (10.3 vs 7.6 months; p = 0.25). There also was no difference in mean time to local tumor progression between metastases and primary tumors (9.5 vs 9.5 months; p = 0.99). The mean applicator density was higher in tumors without local tumor progression (1.7 applicators/cm tumor) compared with tumors with local progression (1.4 applicators/ cm tumor) (p = 0.005).
Adverse Events
One hundred fifteen adverse events were recorded in 80 procedures for an overall procedural adverse event rate of 33.8% (80/236) (Table 3). The adverse events were mild or moderate (grade 1 or 2) in 23.3% (55/236) of procedures and severe or greater (grade 3–5) in 10.6% (25/236) of procedures (Table 3). The overall adverse event rate was 31.3% (61/195) for tumors smaller than 4 cm and 42.9% (19/41) for tumors 4 cm or larger (p = 0.06). The grade 3–5 adverse event rate was lower after the treatment of tumors smaller than 4 cm (8.7%; 17/195) compared with tumors 4 cm or larger (19.5%; 8/41) (p = 0.04). There was no difference in the adverse event rate between procedures for metastases (33.7%; 64/190) and primary hepatic tumors (34.8%; 16/46) (p = 0.88). The adverse event rate was 42.9% (54/126) after procedures performed with CT guidance, 22.0% (22/100) after procedures performed with MRI guidance, and 40.0% (4/10) after PET/CT guidance (p = 0.004).
TABLE 3.
Classification and Frequency of 115 Adverse Events After 80 of 236 Percutaneous Image-Guided Cryoablations of Hepatic Tumor, by Tumor Size
| Variable | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|---|---|
|
| |||||
| Description of adverse event | Segmental portal vein thrombus (n = 14) | Thrombocytopenia (platelet count 50–75 × 103/μL) (n = 15) | Symptomatic hemorrhage requiring nonemergent intervention or blood transfusion (n = 10) | Abdominal hemorrhage requiring emergent intervention (n = 3) | Death (n = 1) |
| Acute kidney injury (creatinine level increase of > 0.3 mg/dL) (n = 13) | Pneumothorax requiring catheter placement (n = 9) | Thrombocytopenia (platelet count 25–50 × 103/μL) (n = 8) | Disseminated intravascular coagulation (life threatening) (n = 2) | ||
| Asymptomatic pneumothorax (n = 12) | Asymptomatic hemorrhage requiring observation or blood transfusion (n = 5) | Abscess (n = 3) | Thrombocytopenia (platelet count < 25 × 103/μL) (n = 2) | ||
| Hepatic vein thrombus (n = 5) | Acute kidney injury (creatinine level 2–3 times above baseline) (n = 2) | Cellulitis treated with IV antibiotics (n = 1) | Hemothorax (n = 1) | ||
| Frostbite (n = 2) | Cellulitis treated with oral antibiotics (n = 1) | Disseminated intravascular coagulation (laboratory findings and bleeding) (n = 1) | Pulmonary embolism (n = 1) | ||
| Pleural effusion requiring catheter drainage (n = 1) | Pneumonia (n = 1) | Acute kidney injury requiring dialysis (n = 1) | |||
| Acute kidney injury (creatinine level > 3 times baseline or > 4.0 mg/dL) (n = 1) | |||||
| Tumors < 4 cm (n = 195 procedures) | 38 | 21 | 18 | 4 | 0 |
| Tumors ≥ 4 cm (n = 41 procedures) | 8 | 12 | 7 | 6 | 1 |
| Total (n = 236 procedures) | 46 | 33 | 25 | 10 | 1 |
Note—Adverse events were graded using the Common Terminology Criteria for Adverse Events [29]. Some patients had multiple adverse events or more than one grade. Laboratory abnormalities were considered adverse events only if they developed after the procedure.
The 10 grade 4 adverse events (each in a different patient) included intraabdominal hemorrhage requiring emergent angiography and embolization (n = 3), disseminated intravascular coagulation managed with supportive care and blood products (n = 2), severe thrombocytopenia (platelet count < 25 × 103/μL) (n= 2), hemothorax requiring emergent thoracotomy (n = 1), pulmonary embolism (n= 1), and acute kidney injury requiring dialysis (n = 1) (Table 3). All patients with grades 1–4 adverse events recovered fully. The single grade 5 adverse event involved an 88-year-old man who died 3 days after cryoablation of a 7.8-cm colorectal cancer metastasis. Acute oliguric renal failure developed, thought to be secondary to myoglobinuria, and the proximate cause of death was aspiration with associated pulmonary edema. The mortality rate was 0.4% (1/236).
After cryoablation, a mean decrease in platelet count of 95.6 × 103/μL below baseline was observed, and in 36.4% (86/236) of procedures, the platelet count decreased below 100 × 103/μL. Platelet transfusion was administered after 11.4% (27/236) of procedures, and more commonly after the treatment of tumors 4 cm or larger (22.0%; 9/41) compared with tumors smaller than 4 cm (9.2%; 18/195) (p = 0.02). The platelet transfusion rate was 18.1% (8/44) in patients who had thrombocytopenia (platelet count < 150 × 103/μL) before the procedure compared with 9.9% (19/192) in patients with a normal platelet count before the procedure (p = 0.12).
Acute kidney injury occurred after 7.2% (17/236) of procedures. The mean (± SD) maximum serum myoglobin level after cryoablation was 397.5 ± 511 ng/mL. Acute kidney injury was more common with a maximum serum myoglobin level greater than 1000 mg/dL (26.3%; 5/19) compared with serum myoglobin levels below 1000 ng/mL (5.5%; 12/217) (p = 0.001). All patients’ renal function returned to baseline within 3 weeks except for the single grade 5 adverse event described already. Patients were discharged the next day after 67.8% (160/236) of procedures (mean hospital stay, 1.9 days; range, 1–31 days).
Discussion
Relative to heat-based ablation techniques, the principal advantage of cryoablation is that the effects of freezing (visualized as an ice ball) are well depicted intraprocedurally with CT and MRI and, to a lesser extent, with ultrasound; thus, the ablation can be monitored intraprocedurally [30, 31]. Intraprocedural monitoring of ice ball formation maximizes the chance of treating a tumor completely and minimizes the chance of harming adjacent critical structures. In addition, limited ice ball extension beyond the liver into the diaphragm or body wall has been shown to be associated with less pain and injury than RFA [32]. For these reasons, cryoablation has been favored by some to treat hepatic masses, particularly those that are close to critical structures, such as the diaphragm, chest wall, heart, lung, gallbladder, and biliary tree [24, 33]. The majority of experience with percutaneous image-guided cryoablation has been in the treatment of HCC [16, 19, 22, 34] and, to a lesser extent, metastases [12, 19, 35]. However, to our knowledge, the overall safety and efficacy of percutaneous image-guided percutaneous cryoablation has not been defined in patients with a variety of primary and secondary hepatic tumors in a series using both CT and MRI guidance.
Our results show that a technically successful percutaneous cryoablation was achieved in 94.6% of tumors. Of the 16 tumors that were not successfully treated at 24 hours, only two were ablated a second time. The remaining 14 patients all received systemic chemotherapy after the unsuccessful ablation but were not treated with another interventional procedure. The reasons for not repeating cryoablation are difficult to determine given the retrospective nature of the study, although half of these cases were performed very early in our institutional experience with cryoablation (in the first year) and therefore referring physicians or the radiologist performing the ablation may not have been comfortable repeating treatment.
Our technical success rate of 94.6%, technique efficacy rate of 89.5% at 3 months, and local tumor progression rate of 23.3% compare favorably to the limited previously published data for percutaneous cryoablation of liver metastases, with technical success rates of 83 92% and technique efficacy rates of 47–85.4% [12, 18, 35, 36]. Previous studies of percutaneous cryoablation of HCC have reported technical success rates of 51–98.5% and technique efficacy rates of 69 75.8% [16, 22, 34]. A more recently published large single-center study of percutaneous CT-guided ablation of both primary and metastatic liver tumors found a local tumor progression rate of 5.5% for HCC, 11.1% for colorectal metastases, and 9.4% for noncolorectal metastases with a mean follow-up of 1.8 years [19]. The reason for our overall higher local tumor progression rate is uncertain but could relate to the type of metastases treated, overall length of follow-up imaging, or perhaps to technical factors such as applicator density. Indeed, in our study, mean applicator density was higher in tumors in which technical success and technique efficacy were achieved, and was also higher in patients without local tumor progression. The discrepancy between the technical success rate and local tumor progression rate shows that the 24-hour assessment of the ablation zone is not fully predictive of success. The fact that the tumor was fully encompassed at 24 hours but not fully treated suggests that viable neoplastic cells likely remained at the periphery of the ablation zone and, hence, a sufficient ablation margin was not achieved. We did not evaluate margins in this cohort, but theoretically, if the lethal isotherm is 5–10 mm within an ice ball’s edge, the ice ball needs to extend at least 1.0 cm beyond the tumor’s edge [37]. This likely was not achieved in many patients in our cohort and could explain local tumor progression in some patients.
The mean time to local tumor progression in our study was 9.5 months for primary hepatic tumors and metastases, similar to what has previously been reported for cryoablation (11.9 months for HCC, 9.5 months for colorectal metastases, and 7.9 months for noncolorectal metastases) and RFA (13.7 months for HCC and 9.1 months for metastases) [19, 38]. Of those patients who did experience local progression, more than half did so within 6 months of their ablation and only one after 2 years.
Our results are also comparable to those reported with RFA in the treatment of hepatic metastases and HCC [9, 10, 30]. Reported technical success rates of RFA for metastases have varied from 93% to 100%, and technique efficacy rates have varied from 60.9% to 88.1% [9, 39]. A recent study reported a local tumor progression rate of 22% at 12 months after RFA of colorectal cancer metastases [40]. However, technical success and technique efficacy rates for microwave ablation may be superior to those for cryoablation, with reported technique efficacy rates of 95.7% for primary hepatic tumors and 95.9% for metastases [41]. Our less efficacious results may be because we treated larger tumors, many of which may have been in locations adjacent to critical structures. However, the degree to which ours and others’ results were affected by proximity to critical structures is unknown.
Percutaneous cryoablation was more effective in treating tumors smaller than 4 cm. The effect of size on ablation efficacy is common to virtually all ablation technologies [33, 42]. In our series, technical success, technique efficacy, and local tumor progression rates were all favorable for tumors smaller than 4 cm.
The effect of margin size on local tumor progression has been validated for heat-based ablations as well [43–45]. Indeed, a recent study of RFA revealed that an ablation margin of less than 5 mm was an independent predictor of local tumor progression [40]. However, complex tumor geometry and adjacent critical structures made achieving a sufficient margin difficult at times, particularly in large tumors.
In addition to tumor size, tumor biology affected technique efficacy. Cryoablation was more effective in treating less biologically aggressive tumors, such as pancreatic neuroendocrine tumor, renal cell carcinoma, and ovarian cancer, relative to more aggressive tumors, such as esophageal and lung cancer.
Regarding guidance modality, some have considered MRI the preferred imaging technique to guide cryoablation because of its ability to depict the ice ball signal void apart from the often T2-hyperintense tumor and its ability to more easily image in multiple planes [46] (Fig. 2). We were not able to show improved technical success, technique efficacy, or local tumor progression with MRI guidance in this study. However, adverse events were less frequent when MRI guidance was used compared with CT and PET/CT. The reason for this is uncertain, but could have been secondary to the improved visualization of the tumor and ice ball with MRI when compared with CT, allowing less freezing of adjacent normal hepatic parenchyma and perhaps less freezing of nearby critical structures.
The overall adverse event rate in the current study is concordant with previously published smaller single-center reports for percutaneous cryoablation of both metastases and HCC [22, 36]. A recently published larger single-center study had an overall adverse event rate of 5.8%, lower than ours, but with a similar mortality rate of 1.2% [19]. Although the exact explanation for our higher adverse event rate is uncertain, patients in this prior series received preprocedural corticosteroids and were aggressively hydrated with alkalinized fluids [19]. Our study had a higher adverse event rate (overall, 33.8%; severe or greater, 10.6%) than microwave ablation (overall, 10.2%; major, 2.9%) or RFA (overall, 6.9%; major, 2.2%) [47, 48]. The reason for this discrepancy in adverse events between heat-based techniques and cryoablation is unknown. Comparing our data with other studies is confounded by the fact that the patient populations and other factors known to affect the likelihood of an adverse event, such as tumor size, tumor location, and applicator diameter, may differ. Although this notion is speculative, the systemic inflammatory or hematologic effects caused by liver tumor cryoablation may contribute to the overall increased adverse event rate [49].
Ablations of tumors 4 cm or larger were associated with an increased number of severe adverse events relative to smaller tumors, including the need for platelet transfusion. Thrombocytopenia after cryoablation has been described elsewhere [49]. A decrease in platelet count after hepatic tumor ablations can also be seen after heat-based ablations [50]. We encountered disseminated intravascular coagulation after three procedures, all of which occurred after the ablation of tumors larger than 3 cm (two larger than 4 cm) that necessitated including a large volume of adjacent normal hepatic parenchyma into the ablation zone. The ablation of a large volume of liver parenchyma during cryoablation has been shown to be associated with subsequent alterations in liver enzymes and platelet count [49]. To minimize the risk of bleeding due to thrombocytopenia, we monitor the platelet count immediately after the procedure and then every 12–24 hours until nadir is reached. Platelet transfusion is typically administered if the platelet count falls below 50 × 103/μL or if there is bleeding. To minimize the risk of hemorrhage, we typically perform hepatic cryoablation only in patients with an international normalized ratio of less than or equal to 1.5 and a platelet count of greater than or equal to 75 × 103/μL.
Acute kidney injury occurred after 7.2% (17/236) of procedures. Its cause is likely multifactorial but myoglobinuria may have played a role [49]. Because acute kidney injury was more common with a maximum serum myoglobin level of over 1000 mg/dL, we measure serum creatinine and myoglobin levels immediately after the procedure, particularly in patients with preexisting renal insufficiency. For all patients with preprocedural renal insufficiency, or if the postprocedural serum myoglobin level exceeds 1000 ng/mL, we administer alkalinized fluids (5% dextrose solution with 150 mEq/L sodium bicarbonate, 100–150 mL/hr for 24 hours) to decrease the risk of acute kidney injury. As with thrombocytopenia, myoglobinuria may also occur after heat-based ablations [50].
Our study had limitations. The retrospective nature of the study prompted excluding 12.5% of tumors from the assessment of technique efficacy because of insufficient imaging follow-up. Most of these patients were lost to clinical follow-up but none had documented recurrences. There may have been a selection bias in selecting patients for cryoablation rather than heat-based techniques. Although selection bias would typically skew the data in a positive (favorable outcomes) fashion, it may have skewed the efficacy and adverse event rates in our series negatively because cryoablation was likely selected to treat tumors in challenging locations and adjacent to critical structures. In addition, determination of technical success and local tumor progression was based on imaging of the liver rather than pathologic assessment. The comparative analyses that evaluated the effect of tumor type and size, imaging guidance modality, and applicator density may have been affected by confounding variables. A prospective controlled study would be needed to fully evaluate the effect of these parameters on the efficacy and safety of hepatic tumor cryoablation. Finally, an analysis of the cost of the cryoablation procedures was not performed in this study but could be considered in a future study comparing cryoablation with heat-based ablation technologies.
In conclusion, the ability to monitor ice ball formation during CT- and MRI-guided liver tumor cryoablation procedures has been the principal reason for selecting cryoablation instead of heat-based techniques. In patients who undergo hepatic cryoablation, we recommend postprocedure assessment of platelet count and myoglobin as a way to monitor for frequently encountered hematologic alterations. Our 15-year experience shows that percutaneous cryoablation of hepatic tumors is a reasonably effective and safe alternative, particularly for the treatment of hepatic tumors smaller than 4 cm.
Acknowledgments
Supported by grant R25CA89017 to D. I. Glazer and grant P41EB015898 to M. G. Vangel from the National Institutes of Health.
References
- 1.American Cancer Society. Cancer facts & figures 2015. Atlanta, GA: American Cancer Society; 2015. [Google Scholar]
- 2.Danet IM, Semelka RC, Leonardou P, et al. Spectrum of MRI appearances of untreated metastases of the liver. AJR. 2003;181:809–817. doi: 10.2214/ajr.181.3.1810809. [DOI] [PubMed] [Google Scholar]
- 3.Hwang M, Jayakrishnan TT, Green DE, et al. Systematic review of outcomes of patients undergoing resection for colorectal liver metastases in the setting of extra hepatic disease. Eur J Cancer. 2014;50:1747–1757. doi: 10.1016/j.ejca.2014.03.277. [DOI] [PubMed] [Google Scholar]
- 4.Bala MM, Riemsma RP, Wolff R, Kleijnen J. Cryotherapy for liver metastases. Cochrane Database Syst Rev. 2013;6:CD009058. doi: 10.1002/14651858.CD009058.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Shin DS, Ingraham CR, Dighe MK, et al. Surgical resection of a malignant liver lesion: what the surgeon wants the radiologist to know. AJR. 2014;203:W21–W33. doi: 10.2214/AJR.13.11701. [web] [DOI] [PubMed] [Google Scholar]
- 6.Garden OJ, Rees M, Poston GJ, et al. Guidelines for resection of colorectal cancer liver metastases. Gut. 2006;55(suppl 3):iii1–iii8. doi: 10.1136/gut.2006.098053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hinshaw JL, Lee FT. Cryoablation for liver cancer. Tech Vasc Interv Radiol. 2007;10:47–57. doi: 10.1053/j.tvir.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Gillams A, Goldberg N, Ahmed M, et al. Thermal ablation of colorectal liver metastases: a position paper by an international panel of ablation experts, The Interventional Oncology Sans Frontières meeting 2013. Eur Radiol. 2015;25:3438–3454. doi: 10.1007/s00330-015-3779-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solbiati L, Ahmed M, Cova L, Ierace T, Brioschi M, Goldberg SN. Small liver colorectal metastases treated with percutaneous radiofrequency ablation: local response rate and long-term survival with up to 10-year follow-up. Radiology. 2012;265:958–968. doi: 10.1148/radiol.12111851. [DOI] [PubMed] [Google Scholar]
- 10.Lee DH, Lee JM, Lee JY, et al. Radiofrequency ablation of hepatocellular carcinoma as first-line treatment: long-term results and prognostic factors in 162 patients with cirrhosis. Radiology. 2014;270:900–909. doi: 10.1148/radiol.13130940. [DOI] [PubMed] [Google Scholar]
- 11.Shady W, Petre EN, Gonen M, et al. Percutaneous radiofrequency ablation of colorectal cancer liver metastases: factors affecting outcomes a 10-year experience at a single center. Radiology. 2016;278:601–611. doi: 10.1148/radiol.2015142489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang W, Yu H, Guo Z, et al. Percutaneous cryoablation of liver metastases from breast cancer: initial experience in 17 patients. Clin Radiol. 2014;69:231–238. doi: 10.1016/j.crad.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 13.Xin’an L, Jianying Z, Lizhi N, et al. Alleviating the pain of unresectable hepatic tumors by percutaneous cryoablation: experience in 73 patients. Cryobiology. 2013;67:369–373. [PubMed] [Google Scholar]
- 14.Mu F, Niu L, Li H, et al. Percutaneous comprehensive cryoablation for metastatic hepatocellular cancer. Cryobiology. 2013;66:76–80. doi: 10.1016/j.cryobiol.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Bang HJ, Littrup PJ, Currier BP, et al. Percutaneous cryoablation of metastatic lesions from colorectal cancer: efficacy and feasibility with survival and cost-effectiveness observations. ISRN Minim Invasive Surg. 2012;2012:942364. doi: 10.5402/2012/942364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Y, Wang C, Lu Y, et al. Outcomes of ultrasound-guided percutaneous argonhelium cryoablation of hepatocellular carcinoma. J Hepatobiliary Pancreat Sci. 2012;19:674–684. doi: 10.1007/s00534-011-0490-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shimizu T, Sakuhara Y, Abo D, et al. Outcome of MR-guided percutaneous cryoablation for hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2009;16:816–823. doi: 10.1007/s00534-009-0124-4. [DOI] [PubMed] [Google Scholar]
- 18.Xu KC, Niu LZ, He WB, Hu YZ, Zuo JS. Percutaneous cryosurgery for the treatment of hepatic colorectal metastases. World J Gastroenterol. 2008;14:1430–1436. doi: 10.3748/wjg.14.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Littrup PJ, Aoun HD, Adam B, Krycia M, Prus M, Shields A. Percutaneous cryoablation of hepatic tumors: long-term experience of a large U.S. series. Abdom Radiol (NY) 2016;41:767–780. doi: 10.1007/s00261-016-0687-x. [DOI] [PubMed] [Google Scholar]
- 20.Sarantou T, Bilchik A, Ramming KP. Complications of hepatic cryosurgery. Semin Surg Oncol. 1998;14:156–162. doi: 10.1002/(sici)1098-2388(199803)14:2<156::aid-ssu7>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 21.Ravikumar TS, Kane R, Cady B, et al. Hepatic cryosurgery with intraoperative ultrasound monitoring for metastatic colon carcinoma. Arch Surg. 1987;122:403–409. doi: 10.1001/archsurg.1987.01400160029002. [DOI] [PubMed] [Google Scholar]
- 22.Wang C, Wang H, Yang W, et al. Multicenter randomized controlled trial of percutaneous cryoablation versus radiofrequency ablation in hepatocellular carcinoma. Hepatology. 2015;61:1579–1590. doi: 10.1002/hep.27548. [DOI] [PubMed] [Google Scholar]
- 23.Dunne RM, Shyn PB, Sung JC, et al. Percutaneous treatment of hepatocellular carcinoma in patients with cirrhosis: a comparison of the safety of cryoablation and radiofrequency ablation. EurJ Radiol. 2014;83:632–638. doi: 10.1016/j.ejrad.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Fairchild AH, Tatli S, Dunne RM, Shyn PB, Tuncali K, Silverman SG. Percutaneous cryoablation of hepatic tumors adjacent to the gallbladder: assessment of safety and effectiveness. J Vasc Interv Radiol. 2014;25:1449–1455. doi: 10.1016/j.jvir.2014.04.023. [DOI] [PubMed] [Google Scholar]
- 25.Silverman SG, Tuncali K, Adams DF, et al. MR imaging-guided percutaneous cryotherapy of liver tumors: initial experience. Radiology. 2000;217:657–664. doi: 10.1148/radiology.217.3.r00dc40657. [DOI] [PubMed] [Google Scholar]
- 26.Shyn PB, Mauri G, Alencar RO, et al. Percutaneous imaging-guided cryoablation of liver tumors: predicting local progression on 24-hour MRI. AJR. 2014;203:W181–W191. doi: 10.2214/AJR.13.10747. [web] [DOI] [PubMed] [Google Scholar]
- 27.Littrup PJ, Jallad B, Vorugu V, et al. Lethal isotherms of cryoablation in a phantom study: effects of heat load, probe size, and number. JVasc Interv Radiol. 2009;20:1343–1351. doi: 10.1016/j.jvir.2009.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria a 10-year update. Radiology. 2014;273:241–260. doi: 10.1148/radiol.14132958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.U.S. Department of Health and Human Services. Common terminology criteria for adverse events (CTCAE), version 4.0. Department of Health and Human Services website; [Accessed July 25, 2017]. evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. Published May 28, 2009. Updated June 14, 2010. [Google Scholar]
- 30.Tokuda J, Plishker W, Torabi M, et al. Graphics processing unit-accelerated nonrigid registration of MR images to CT images during CT-guided percutaneous liver tumor ablations. Acad Radiol. 2015;22:722–733. doi: 10.1016/j.acra.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu X, Tuncali K, Wells WM, Zientara GP. Automatic iceball segmentation with adapted shape priors for MRI-guided cryoablation. J Magn Reson Imaging. 2015;41:517–524. doi: 10.1002/jmri.24531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thacker PG, Callstrom MR, Curry TB, et al. Palliation of painful metastatic disease involving bone with imaging-guided treatment: comparison of patients’ immediate response to radiofrequency ablation and cryoablation. AJR. 2011;197:510–515. doi: 10.2214/AJR.10.6029. [DOI] [PubMed] [Google Scholar]
- 33.Hinshaw JL, Lubner MG, Ziemlewicz TJ, Lee FT, Jr, Brace CL. Percutaneous tumor ablation tools: microwave, radiofrequency, or cryoablation what should you use and why? RadioGraphiccs. 2014;34:1344–1362. doi: 10.1148/rg.345140054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rong G, Bai W, Dong Z, et al. Long-term outcomes of percutaneous cryoablation for patients with hepatocellular carcinoma within Milan criteria. PLoS One. 2015;10:e0123065. doi: 10.1371/journal.pone.0123065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao W, Guo Z, Zhang X, et al. Percutaneous cryoablation of ovarian cancer metastasis to the liver: initial experience in 13 patients. Int J Gynecol Cancer. 2015;25:802–808. doi: 10.1097/IGC.0000000000000420. [DOI] [PubMed] [Google Scholar]
- 36.Adam R, Hagopian EJ, Linhares M, et al. A comparison of percutaneous cryosurgery and percutaneous radiofrequency for unresectable hepatic malignancies. Arch Surg. 2002;137:1332–1339. doi: 10.1001/archsurg.137.12.1332. discussion, 1340. [DOI] [PubMed] [Google Scholar]
- 37.Gage AA, Baust J. Mechanisms of tissue injury in cryosurgery. Cryobiology. 1998;37:171–186. doi: 10.1006/cryo.1998.2115. [DOI] [PubMed] [Google Scholar]
- 38.Kei SK, Rhim H, Choi D, Lee WJ, Lim HK, Kim YS. Local tumor progression after radiofrequency ablation of liver tumors: analysis of morphologic pattern and site of recurrence. AJR. 2008;190:1544–1551. doi: 10.2214/AJR.07.2798. [DOI] [PubMed] [Google Scholar]
- 39.Solbiati L, Livraghi T, Goldberg SN, et al. Percutaneous radio-frequency ablation of hepatic metastases from colorectal cancer: long-term results in 117 patients. Radiology. 2001;221:159–166. doi: 10.1148/radiol.2211001624. [DOI] [PubMed] [Google Scholar]
- 40.Sotirchos VS, Petrovic LM, Gönen M, et al. Colorectal cancer liver metastases: biopsy of the ablation zone and margins can be used to predict oncologic outcome. Radiology. 2016;280:949–959. doi: 10.1148/radiol.2016151005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu J, Liang P, Yu XL, et al. Local tumour progression after ultrasound-guided microwave ablation of liver malignancies: risk factors analysis of 2529 tumours. Eur Radiol. 2015;25:1119–1126. doi: 10.1007/s00330-014-3483-4. [DOI] [PubMed] [Google Scholar]
- 42.Ryan MJ, Willatt J, Majdalany BS, et al. Ablation techniques for primary and metastatic liver tumors. World J Hepatol. 2016;8:191–199. doi: 10.4254/wjh.v8.i3.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim YS, Lee WJ, Rhim H, Lim HK, Choi D, Lee JY. The minimal ablative margin of radiofrequency ablation of hepatocellular carcinoma (> 2 and < 5 cm) needed to prevent local tumor progression: 3D quantitative assessment using CT image fusion. AJR. 2010;195:758–765. doi: 10.2214/AJR.09.2954. [DOI] [PubMed] [Google Scholar]
- 44.Kim KW, Lee JM, Klotz E, et al. Safety margin assessment after radiofrequency ablation of the liver using registration of preprocedure and post-procedure CT images. AJR. 2011;196:W565–W572. doi: 10.2214/AJR.10.5122. [web] [DOI] [PubMed] [Google Scholar]
- 45.Wang X, Sofocleous CT, Erinjeri JP, et al. Margin size is an independent predictor of local tumor progression after ablation of colon cancer liver metastases. Cardiovasc Intervent Radiol. 2013;36:166–175. doi: 10.1007/s00270-012-0377-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Silverman SG, Sun MR, Tuncali K, et al. Three-dimensional assessment of MRI-guided percutaneous cryotherapy of liver metastases. AJR. 2004;183:707–712. doi: 10.2214/ajr.183.3.1830707. [DOI] [PubMed] [Google Scholar]
- 47.Livraghi T, Meloni F, Solbiati L, Zanus G. Complications of microwave ablation for liver tumors: results of a multicenter study. Cardiovasc Intervent Radiol. 2012;35:868–874. doi: 10.1007/s00270-011-0241-8. [DOI] [PubMed] [Google Scholar]
- 48.Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radiofrequency ablation: complications encountered in a multicenter study. Radiology. 2003;226:441–451. doi: 10.1148/radiol.2262012198. [DOI] [PubMed] [Google Scholar]
- 49.Nair RT, Silverman SG, Tuncali K, Obuchowski NA, van Sonnenberg E, Shankar S. Biochemical and hematologic alterations following percutaneous cryoablation of liver tumors: experience in 48 procedures. Radiology. 2008;248:303–311. doi: 10.1148/radiol.2481061874. [DOI] [PubMed] [Google Scholar]
- 50.Cizginer S, Tatli S, Hurwitz S, Tuncali K, vanSonnenberg E, Silverman SG. Biochemical and hematologic changes after percutaneous radiofrequency ablation of liver tumors: experience in 83 procedures. J Vasc Interv Radiol. 2011;22:471–478. doi: 10.1016/j.jvir.2010.12.033. [DOI] [PubMed] [Google Scholar]