Abstract
Purpose
The distinction between active inflammation and fibrosis of the bowel wall is essential for therapeutic decisions in stricturing Crohn’s disease. We aimed to assess whether real-time elastography (RTE) with strain ratio measurement could be useful in differentiating fibrotic from inflamed bowel strictures and to evaluate the possible relationship between US techniques and the histology of the stenotic bowel wall.
Materials and methods
Bowel ultrasonography (including RTE, color-Doppler and CEUS examination) was prospectively evaluated in 26 patients with symptomatic stricturing Crohn’s disease, before surgery. RTE was adopted to evaluate bowel stiffness: five loops of 20 RTE frames were recorded for each stenotic segment and the mean strain ratio (MSR) was obtained. Histology scoring systems both for inflammation and fibrosis were established for surgical specimens.
Results
No significant correlation was found between MSR and fibrosis score (P = 0.877). Color-Doppler score was significantly related to gut wall and submucosal thicknesses (P = 0.006 and P = 0.032, respectively). There was no significant correlation between the number of vessels counted at histology and color-Doppler and CEUS examinations (P = 0.170 and P = 0.302, respectively).
Conclusion
MSR detection was not able to distinguish fibrotic from inflammatory tissue in our selected population. This result could be influenced by the presence of the superimposed inflammation. Larger cohort of patients, further analysis with shear wave elastography, and validated histopathology classification systems for fibrosis and inflammation are necessary to assess if intestinal fibrosis could be reliably detected on the basis of bowel elastic properties.
Keywords: Real-time strain elastography, Color-Doppler, CEUS, Fibrosis, Inflammation, Crohn’s disease
Sommario
Obiettivo
la distinzione tra infiammazione attiva e fibrosi nella parete intestinale è essenziale nel proceso decisionale della terapia nella malattia di Crohn stenosante. Lo scopo del nostro studio era di stabilire se l’elastografia real-time (RTE) con la misurazione dello strain ratio potesse essere utile nel differenziare il tessuto fibrotico da quello infiammatorio nella parete intestinale stenotica, e di valutare la presenza di correlazioni tra le tecniche ecografiche di studio delle anse intestinali e le caratteristiche istologiche dei segmenti analizzati.
Materiali e metodi
Lo studio ecografico delle anse intestinali che comprendeva anche RTE, valutazione color-Doppler e CEUS, è stato eseguito in maniera prospettica in 26 pazienti con malattia di Crohn stenosante sintomatica, prima dell’ intervento chirurgico resettivo. La RTE è stata utilizzata per valutare la rigidità della parete intestinale: 5 filmati di 20 frames di elastografia sono stati registrati per ogni segmento stenotico, per ogni frame è stato calcolato lo strain ratio e quindi ne è stata ottenuta la media (MSR). E’ stato poi stabilito uno score istologico per l’ infiammazione e la fibrosi per i pezzi operatori analizzati.
Risultati
non è stata rilevata alcuna correlazione significativa tra MSR e score istologico della fibrosi (P = 0877). Il Color-doppler correlava significativamente con lo spessore di parete e l’ispessimento della sottomucosa (P = 0006 e P = 0032, rispettivamente). Non è stata trovata una correlazione significativa tra il numero di vasi rilevato sul pezzo istologico e gli score color-Doppler e CEUS (P = 0170 e P = 0302, rispettivamente).
Conclusioni
il calcolo del MSR non si è rivelato un parametro efficace nel distinguere tra tessuto fibrotico ed infiammatorio nella nostra popolazione. Questo risultato è influenzato da vari fattori, tra cui probabilmente la compresenza di infiammazione. Coorti di pazienti più ampie, ulteriori analisi con l’ausilio eventualmente dell’ elastografia shear-wave, e sistemi di classificazione istopatologici validati sia per la fibrosi che per l’infiammazione, risultano necessari per stabilire se la fibrosi intestinale possa essere rilevata in maniera affidabile sulla base delle proprietà elastiche della parete intestinale.
Introduction
Crohn’s disease is a chronic, transmural bowel wall inflammation that can lead to complications such as strictures or fistulas [1, 2]. Fibrosis, as a result of the recurrent inflammatory episodes, occurs unpredictably and contributes to bowel obstruction [3, 4]. The distinction between active inflammation and fibrosis of the bowel wall is essential for therapeutic decisions. Patients with ‘inflammatory’ strictures can potentially be managed with medical treatment, whereas patients with narrowed fibrotic bowel segments, in particular, if associated with obstructive symptoms, frequently require endoscopic dilatation or surgery [5]. The assessment of the amount of bowel wall fibrosis could allow clinicians to identify patients who could take advantage of early surgical treatment, avoiding potentially harmful and costly immunosuppressant anti-inflammatory medical therapies.
While the anatomic location and length of the inflamed intestinal segments and their possible complications, such as abscess or stenosis, can be accurately assessed both with ultrasonography (US), computed tomography (CT) and magnetic resonance imaging (MRI), to date any imaging method showed enough accuracy to characterize the presence of or assess the degree of fibrosis in a bowel stricture [6, 7].
Ultrasonography, being noninvasive, free of ionizing radiation, easily repeatable and well tolerated, has increasingly emerged as an alternative imaging technique for diagnosis and follow-up of Crohn’s disease patients in clinical practice. The introduction of color-Doppler and contrast-enhanced ultrasound (CEUS) increased US accuracy in the detection of bowel vascularization, with a good correlation with clinical and endoscopic activity findings [8–14].
Recent preliminary studies introduced ultrasound elasticity imaging as a noninvasive technique to assess bowel tissue mechanical properties, as already successfully applied for liver or kidney disease [15–18]. Both strain elastography and shear wave elastography demonstrated good accuracy to differentiate inflammatory from fibrotic intestine in rat models of inflammatory bowel disease [19, 20]. To date, only one small proof-of-concept study by Baumgart et al. investigated in vivo real-time strain elastography (RTE) in Crohn’s disease patients as a useful method to detect fibrotic tissue in affected vs. unaffected segments [21]. RTE provides a colored-scale elastogram on B-mode images where the distribution of the strain (stiffness) of the studied tissue can be obtained within a region of interest.
The primary aim of our study was to measure bowel wall stiffness in stricturing Crohn patients using in vivo RTE and to evaluate its role in differentiating the extent of fibrosis and inflammation assessed by histology. In addition, we also evaluated the relationship between US, color-Doppler and CEUS and the histological features of the stenotic bowel wall.
Materials and methods
Population
Between January 2011 and December 2013, 32 consecutive patients who were followed-up at the IBD unit of our Department required ileocolonic resection for stricturing Crohn’s disease. The primary indication for surgery was a symptomatic bowel obstruction due to stricturing ileocolonic Crohn’s disease. The IBD medical team, made up of gastroenterologists, surgeons and radiologists, indicated elective surgery on the basis of severity of symptoms, instrumental diagnostic findings (endoscopy and CT enterography or MR enterography) and clinical history. All these patients were invited to participate in a prospective study by undergoing ultrasound examination, including color-Doppler, CEUS and strain elastography, within a seven-day period before surgery. The exams were performed by two physicians with strong experience in intestinal bowel disease US (C.S. and E.F.), which were aware of the patient’s diagnosis of Crohn’s disease, but unaware of the patient’s clinical and biochemical data. US data were used for comparison with the histological findings obtained from the surgical specimens. Prior to surgery a careful clinical history was collected, including age, sex, site and duration of disease, type of previous medical treatment and number of previous operations. The Harvey Bradshaw Index (HBI) and C-reactive protein value (CRP) were as well recorded [22].
Patients with pure colonic disease and patients with complicated disease and urgent surgical indication were excluded. Patients with extreme technical US disadvantages (very deep bowel segments, too thin bowel walls, very meteoric abdomen), despite the operators experience, were also excluded.
Ultrasonography examination
Real-time ultrasound was performed using an iU22 Philips (Philips, Bothell, WA, USA), initially employing a 1–5 MHz convex-array transducer and then a 3–9 MHz linear transducer. The entire gastrointestinal tract was submitted to a gray-scale ultrasound examination. Each stenosis was first identified in B mode, as a pathological wall thickness with a narrowed lumen below a significant bowel dilation [23]. The distance from the ileocecal valve, or from the previous anastomosis in already operated patients, was measured. This helped to match between US and histology of the examined gut segments.
US disease activity scores were assigned to the stricture, considering the wall thickness (thickness score 1, 3–5 mm; 2, 6–8 mm; 3, 9–11 mm; 4, > 11 mm) and the wall stratification (stratification score 0 normal; 1 normal but with a thickened submucosal layer; 2 lost, with hypoechoic echo pattern. The intensity of the Doppler signal was subjectively graded as absent (grade 0), with mild vascularity (grade 1), and with marked vascularity (grade 2), according to a modified and simplified Limberg score, [24].
Real-time strain elastography examination
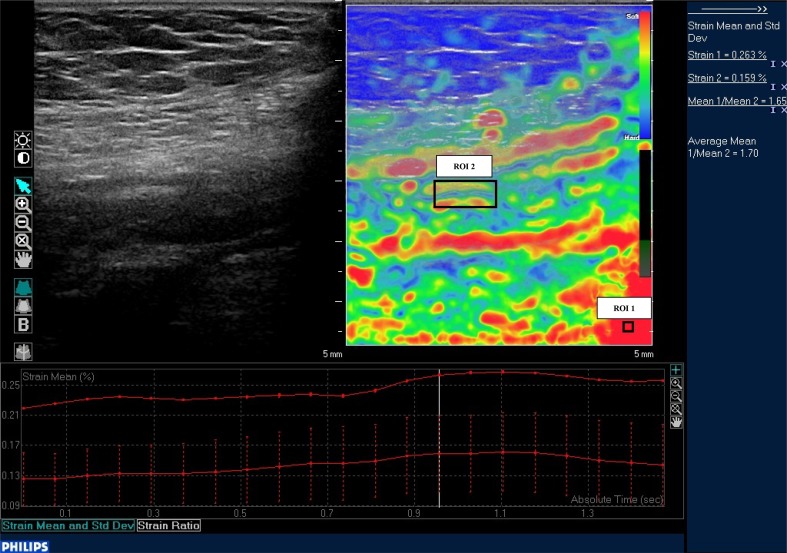
We adopted real-time strain elastography to estimate the gut wall strain-stiffness induced by vascular pulsations. All the patients were thin so that in no case a free-hand compression was necessary and we could obtain good quality elastograms by vascular pulsations. The examination was conducted using a Philips 5–12 MHz linear transducer. The patient was required breath-holding and the probe was held over the stenotic segment, already studied on B-mode. When a good static ultrasound image was obtained, a rectangular elastogram was dimensioned over the stenotic segment. The strain of the tissue included in the elastogram was displayed on B mode images in real-time, with an overlapping color map. Areas with lower strain (relative hard tissue) were depicted in blue and those with higher strain (relative soft tissue) in red, by a color gradation scale. For each segment, five loops of elastography were recorded, each loop made by 20 consecutive elastograms. Each one of the five loops included the same stenotic tract of the studied bowel segment. On each loop two different rectangular ROI (region of interest) were manually drawn and placed, one on a reference tissue (ROI1) and one on the upper part of the cross-sectioned gut wall (ROI2), making sure that luminal content and surrounding tissues were excluded (Fig. 1). We chose as reference tissue the area that was most uniformly red (representing the bottom of the color scale), since a dark-red area could always be detected in the deepest portion of the elastogram [25].
Fig. 1.
B-mode image of a stenotic bowel segment, with a superimposed rectangular colored elastogram. The two black boxes on the elastogram represent the ROI1, on the reference tissue, and the ROI2, on the upper part of the cross-sectioned gut wall. On the column on the right it is displayed the Philips QLAB software numerical examination of the ratio between ROI1 and ROI2 strains (strain ratio, SR)
For each elastogram, the Philips QLAB software calculated the strain ratio (SR), a numerical value that represented the ratio between ROI1 and ROI2 strains and measured the stiffness of the examined tissue. The software calculated the mean SR of the 20 elastograms of each loop (mSR) and then the mean mSR of the 5 loops (MSR). This final MSR value was used to compare RTE stiffness with histological data of the examined bowel segment.
CEUS examination
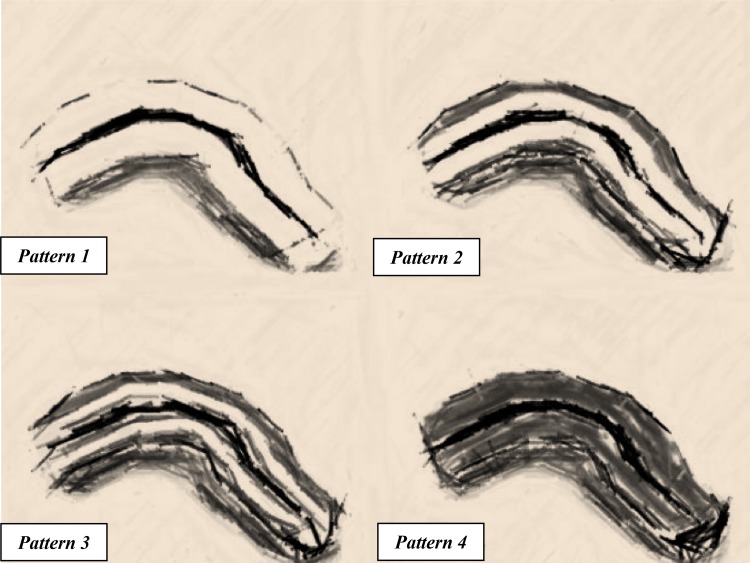
CEUS was performed with the Philips 3–9 MHz linear transducer, using the second generation intravenous echo-contrast agent SonoVue (Bracco, Milan, Italy). SonoVue was prepared just before administration by adding 4 and 8 mL of sterile saline solution (0.9% NaCl) to the vial and then shaking for at least 20 s. It was injected through a three-way 20-gauge catheter into an antecubital vein, followed immediately by an injection of 10 mL of normal saline solution (0.9% NaCl). With the probe held over the stenotic segment previously identified with B mode, the contrast uptake was recorded on a disk, starting a few seconds before the intravenous administration of the contrast until the venous phase of enhancement, at least for 2 min. The enhanced layer arrangement of the bowel wall was then evaluated according the four patterns of enhancement previously proposed by our group: pattern 1, complete enhancement of the entire wall section; pattern 2, absence of enhancement only in the outer border of the muscularis propria; patter 3, absence of enhancement both in the outer and in the inner border of the bowel wall; pattern 4, complete absence of enhancement in the entire wall section [10]. One point was assigned to patterns 1 and 2 (abundant bowel wall enhancement), 0 point to patterns 3 and 4 (poor bowel wall enhancement) (Fig. 2).
Fig. 2.
Representation of bowel wall enhancement with CEUS. The white areas correspond to the distribution of the bowel wall enhancement. Pattern 1 enhancement of the whole bowel section. Pattern 2 absence of enhancement only in the external border of the muscularis propria. Pattern 3 enhancement only in the intermediate layer. Pattern 4 complete absence of enhancement in the whole bowel section. (Figure adapted by author from “Figure. 1”: Serra et al. [10])
Histopathology evaluation
After surgical resection, full gut wall segments were standardly processed (hematoxylin–eosin-stained and Masson trichrome-stained slides). An experienced gastrointestinal pathology specialist evaluated histologic specimens, blinded to the clinical details and ultrasound findings of the patient. A close conversation between the physicians who performed US and the pathologist was established to match the area on which the US probe had been placed during pre-surgery examinations and the studied specimens. The distance between the studied segment and the ileocecal valve or the previous anastomosis measured with US, was used to identify the site of histological evaluation. At least three different histological sections were taken and examined for each segment. The whole gut wall thickness and the different gut wall layers (mucosa, submucosa, serosa and muscularis mucosae) were measured in millimeters on hematoxylin–eosin-stained slides. Histologic evaluation was conducted according to the general recommendations of the histopathology consensus in inflammatory bowel disease published by ECCO [26]. Since there is currently no agreement on any numerical histopathology classification system, we adopted the scoring system for inflammatory and fibrostenotic features of Crohn’s disease proposed by Chiorean et al. [27], with some modifications for the fibrosis score, as shown in Table 1. For the purpose of the study, the highest grading scores for each sectioned specimen were taken into consideration. To assess differences in mean strain ratio, bowel segments were differentiated in low and high fibrosis score (0–2 vs. 3–4) and low and high inflammation scores (0–1 vs. 2–3). The number of vessels in each slide was also measured using CD31 stain for endothelial cells.
Table 1.
Histological scoring system for inflammatory features of Crohn’s disease stenosis
| Inflammation score | ||
|---|---|---|
| Score 0 | No polymorphonuclear or mononuclear leukocytes infiltrates | Low inflammation |
| Score 1 | Mild: cryptitis, leukocytes infiltrates limited to mucosa | |
| Score 2 | Moderate: cryptitis, crypt abscess, and leukocytes infiltrates until the submucosa | High inflammation |
| Score 3 | Severe: transmural inflammation with leukocytes infiltrates in all the layers | |
| Fibrosis score | ||
|---|---|---|
| Score 0 | None or normal fibrosis | Low fibrosis |
| Score 1 | Minimal fibrosis limited to submucosa | |
| Score 2 | Submucosal and muscular layer fibrosis < 30% | |
| Score 3 | Submucosal and muscular layer fibrosis between 30 and 60%, with preserved layers | High fibrosis |
| Score 4 | Massive transmural fibrosis > 60%, effacement of normal layers | |
Clinical evaluation
Clinical activity at the time of US examination was assessed using the HBI (clinical remission, HBI ≤ 4; mild disease activity, 5 ≤ HBI ≤ 7; moderate–severe disease activity, HBI ≥ 8) while CRP was used as the biochemical activity marker (positive if > 0.8 mg/dl). For statistical analysis, patients were dichotomized in mild (HBI ≤ 7) and moderate–severe activity (HBI ≥ 8).
Statistical analysis
Mean, median, standard deviation (SD), interquartile range (IQR) and range were used as descriptive statistics for scalar variables while absolute and relative frequencies were reported for discrete data. The variability among the five RTE measurements made for each bowel segment was determined by computing the coefficient of variation as percentage (CV %). The Spearman’s rank correlation and Mann–Whitney U test were used. The IBM SPSS Statistics package (IBM Co., Armonk, NY, USA; version 23) was used to manage and analyze the data and two-tailed P values less than 0.05 were considered statistically significant.
Ethical Considerations
The local Ethic Committee of the S. Orsola-Malpighi Hospital approved the study protocol (Identification Number 067/2011/O/Sper). All patients gave informed written consent, and the study was performed in conformity with the ethical guidelines of the Declaration of Helsinki [28].
Results
Twenty-six of 32 patients were recruited. Six patients were excluded because the stenotic segment could not be clearly localized at the US examination. Among the 26 enrolled patients, ten (38.5%) were females and 16 (61.5%) were males. Three patients (13.6%) were active smokers. The mean age at disease diagnosis was 35.5 ± 11.0 years (20–59 years), with mean disease duration of 11.7 ± 7.5 years (0–25 years). Ten patients (38.5%) had previous ileo or ileocolonic resections due to Crohn’s disease and ten patients (38.5%) were previously treated with anti-TNF alpha. Twelve patients (46.2%) presented moderate–severe activity (HBI ≥ 8), 12 (46.2%) mild disease activity (5 ≤ HBI ≤ 7) and 2 (7.6%) were in clinical remission (HBI ≤ 4) according to the HBI. CRP was positive in 12 patients (46.2%) (Table 2).
Table 2.
Characteristics of the 26 patients
| Sex: male Female |
16 (61.5%) 10 (38.5%) |
| Age (mean ± SD; years) | 35.5 ± 11.0 |
| Smokers | 3/22 (13.6%) |
| Disease duration (mean ± SD; years) | 11.7 ± 7.5 |
| Previous surgery | 10 (38.5%) |
| Previous anti-TNF alpha therapy | 10 (38.5%) |
| CRP: < 0.8 mg/dL ≥ 0.8 mg/dL |
14 (53.8%) 12 (46.2%) |
| HBI: ≤ 4 5–7 ≥ 8 |
2 (7.6%) 12 (46.2%) 12 (46.2%) |
CRP C-reactive protein, HBI Harvey Bradshaw index
Three patients underwent double stenosis resection, so that a total of 29 bowel segments were examined. The histopathology evaluation of the 29 specimens reported mean gut wall thickness of 5.98 ± 2.82 mm (2.39–16.0 mm) and mean submucosal thickness of 2.99 ± 1.75 mm (0.28–8.00 mm), while the mean number of vessels obtained from the analysis of at least three different histological sections for each segment was 11.9 ± 3.8 (6.9–22.5).
Histology inflammation score ranged from grade 1 to 3 (0% of grade 0): 31.0% of segments presented low grade of inflammation (grade 1), 58.6% a moderate grade (grade 2) and 10.4% a high grade (grade 3) of inflammation. At the same time, the fibrosis score ranged from grade 1 to 4, with 6.9% of low grade of fibrosis (grade 1), 51.7% of mild grade (grade 2), 24.1% of moderate grade (grade 3) and 17.3% of high grade (grade 4) of fibrosis (Table 3).
Table 3.
Distribution of the 29 bowel segments according to the inflammatory and fibrotic histological scores
| Score value | Inflammatory score | Fibrosis score |
|---|---|---|
| 0 | 0 | 0 |
| 1 | 9 (31.0%) | 2 (6.9%) |
| 2 | 17 (58.6%) | 15 (51.7%) |
| 3 | 3 (10.4%) | 7 (24.1%) |
| 4 | – | 5 (17.3%) |
Twenty-five patients concluded the whole ultrasound examination while one patient could not complete CEUS due to a cutaneous allergic reaction.
Eleastographic examination
In the 29 bowel segments examined, the mean MSR was 3.41 ± 0.64 (median value 3.15; IQR 2.96-3.88; range 2.53–5.02) with a mean CV % among the 5 measurements of 12.3 ± 4.7% (median value 11.0%; IQR 8.8–15.8%; range 5.7–23.2%). The relationships between MSR and histological, clinical and biochemical parameters are shown in Table 4. There was no significant correlation between MSR and fibrosis and inflammatory scores (P = 0.877 and P = 0.531, respectively) even when they were dichotomized in high- and low-scores (fibrosis score P = 0.894; inflammatory score P = 0.572). In addition, MSR showed no significant correlation also with HBI, CRP and previous anti-TNF alpha therapy.
Table 4.
Relationships between mean strain ratio (MSR) and histological, clinical and biochemical parameters
| Statistics | P value | |
|---|---|---|
| Fibrosis score (0–4) High score (3–4) vs. low score (0–2) |
r
s = 0.030a
z = − 0.133b |
0.877 0.894 |
| Inflammatory score (0–3) High score (2–3) vs. low score (0–1) |
r
s = − 0.121a
z = − 0.566b |
0.531 0.572 |
| HBI Mild (0–7) vs. moderate/severe (≥ 8) |
r
s = − 0.030a
z = − 0.263b |
0.879 0.792 |
| CRP (mg/dL) | r s = − 0.150a | 0.485 |
| Previous anti-TNF alpha therapy (yes vs. no) | z = − 0.045b | 0.964 |
CRP C-reactive protein, HBI Harvey Bradshaw index
aSpearman rank correlation
bMann–Whitney test
US, color-Doppler and CEUS examination
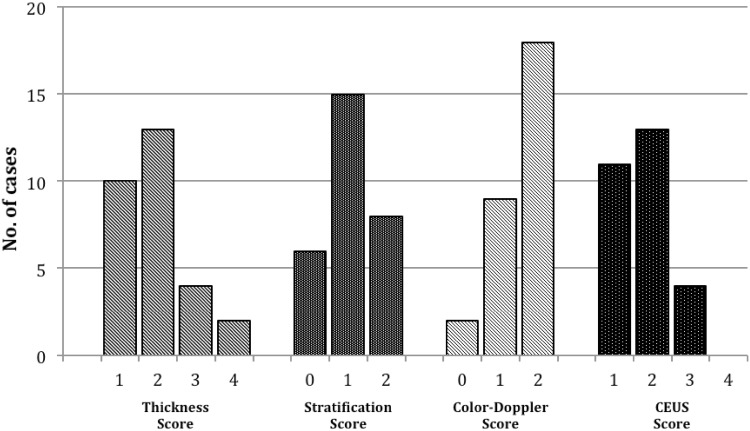
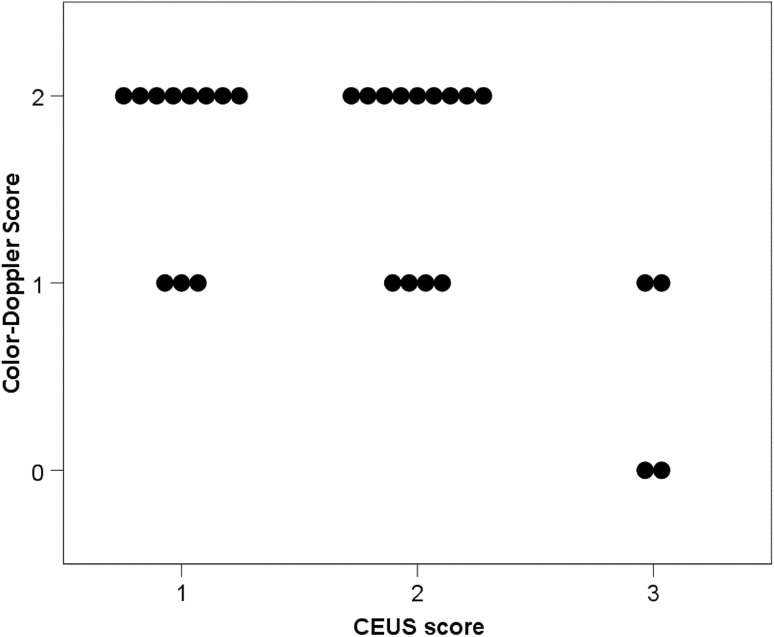
The results of the whole ultrasonographic examination are shown in Fig. 3. The majority of bowel segments presented wall thickness between 3 and 8 mm at ultrasound examination (34.5% between 3 and 5 mm and 44.8% between 6 and 8 mm). The bowel stratification was preserved but with a thickened submucosal layer in 51.7% of cases, while it was lost with hypoechoic echo pattern in 27.6% of cases. Two (6.9%) bowel segments had no vascular signals at color-Doppler evaluation, 9 (31%) presented rare signals and 18 (62.1%) intense signals. The majority of bowel segments presented high contrast uptake at CEUS evaluation, with enhancement of the whole bowel wall (pattern 1) in the 39.3% of cases, and absence of enhancement only in the external border of the muscularis propria (pattern 2) in the 46.4% of cases.
Fig. 3.
Distribution of ultrasound scores (thickness and stratification scores), color-Doppler score and CEUS score
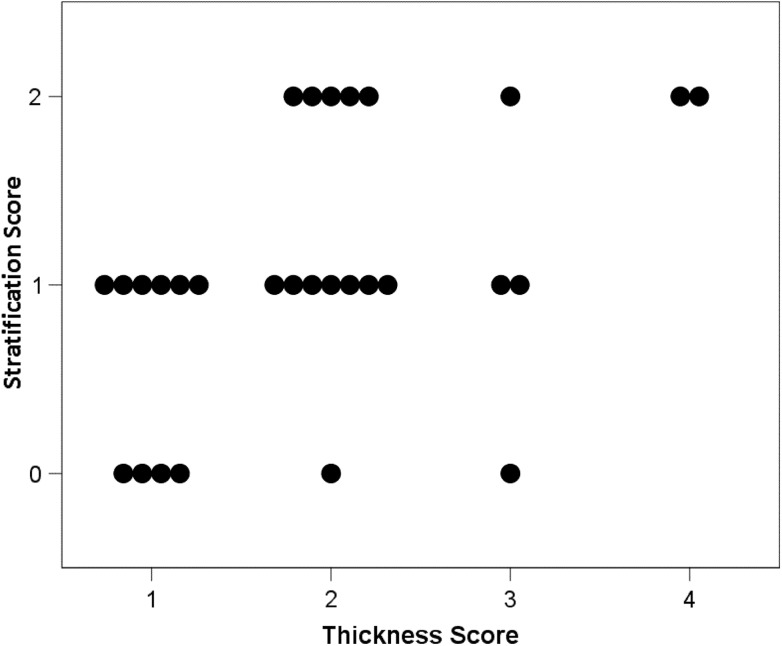
As far as the relationships among US techniques are concerned, a significant positive correlation was observed between B-mode thickness and B-mode stratification score (P = 0.010; Fig. 4) and a significant negative correlation was observed between color-Doppler score and CEUS score (P = 0.026; Fig. 5). B-mode scores were not significantly related with color-Doppler score and CEUS score (P > 0.350).
Fig. 4.
Relationship between gut wall thickness (thickness score) and gut wall layering (stratification score) assessed by B-mode US (P = 0.010; Spearman rank correlation). (Thickness score 1, 3–5 mm; 2, 6–8 mm; 3, 9–11 mm; 4, > 11 mm. stratification score: 0 normal; 1 normal but with a thickened submucosal layer; 2 lost, with hypoechoic echo pattern.)
Fig. 5.
Relationship between color-Doppler signal intensity (color-Doppler score) and CEUS patterns (CEUS score) (P = 0.026; Spearman rank correlation). (Color-Doppler score: grade 0 absent vascularity; grade 1 mild vascularity; grade 2 marked vascularity. CEUS score: pattern 1 enhancement of the whole bowel section; pattern 2 absence of enhancement only in the external border of the muscularis propria; pattern 3 enhancement only in the intermediate layer; pattern 4 complete absence of enhancement in the whole bowel section)
Table 5 shows the relationships between US techniques and histological, clinical and biochemical parameters. A significant correlation was found between color-Doppler score and gut wall thickness and submucosal thickness assessed by histology (P = 0.006 and P = 0.032, respectively), while color-Doppler score did not significantly correlate with the fibrosis and inflammatory scores (P = 0.288 and P = 0.764, respectively). No other significant correlations were found between histology and US or CEUS evaluations of the gut wall (P > 0.063). The number of vessels counted at histology was not significantly related to US techniques (P > 0.170); in particular, we found no significant correlation between the number of vessels and color-Doppler or CEUS scores (P = 0.170 and P = 0.302, respectively). Finally, no significant correlations were observed between the US techniques and clinical and biochemical parameters (P > 0.138).
Table 5.
Correlation between ultrasound scores, Color-Doppler score and CEUS score and histological, clinical and biochemical parameters in the 29 bowel specimens of the 26 patients
| Thickness score | Stratification score | Color-Doppler score | CEUS score | |
|---|---|---|---|---|
| Histological wall thickness |
r
s = − 0.035 P = 0.856 |
r
s = − 0.185 P = 0.337 |
r
s = 0.497 P = 0.006 |
r
s = − 0.246 P = 0.207 |
| Histological submucosal thickness |
r
s = 0.167 P = 0.395 |
r
s = − 0.191 P = 0.330 |
r
s = 0.407 P = 0.032 |
r
s = − 0.123 P = 0.540 |
| Fibrosis score |
r
s = − 0.246 P = 0.198 |
r
s = − 0.093 P = 0.632 |
r
s = 0.204 P = 0.288 |
r
s = − 0.247 P = 0.205 |
| Inflammatory score |
r
s = 0.259 P = 0.176 |
r
s = 0.350 P = 0.063 |
r
s = 0.058 P = 0.764 |
r
s = − 0.097 P = 0.622 |
| No of vessels |
r
s = 0.053 P = 0.783 |
r
s = 0.010 P = 0.958 |
r
s = 0.262 P = 0.170 |
r
s = − 0.202 P = 0.302 |
| HBI |
r
s = − 0.027 P = 0.889 |
r
s = − 0.050 P = 0.796 |
r
s = 0.196 P = 0.309 |
r
s = 0.092 P = 0.641 |
| CRP |
r
s = 0.312 P = 0.138 |
r
s = − 0.123 P = 0.568 |
r
s = 0.106 P = 0.623 |
r
s = 0.089 P = 0.686 |
Spearman rank correlation
CRP: C-reactive protein; HBI: Harvey Bradshaw index
Discussion
Due to the importance of strictures composition in the medical management of Crohn’s disease, several studies investigated for an accurate noninvasive diagnostic tool to differentiate fibrosis from inflammation, but none have fully succeeded. Some of the studies lack an appropriate reference standard: to assess the transmural extent of Crohn’s disease, imaging findings should be compared to full-thickness bowel wall tissue specimens, which is possible only if the subjects undergo surgery immediately after imaging. Two recent studies focused on CT and MRI enterography in detecting fibrosis in stricturing Crohn’s disease before surgery. Adler et al. showed that CT enterography could not reliably establish the presence of fibrosis in small bowel Crohn’s strictures. Moreover, the lack of enterography inflammation signs was not able to predict the presence of fibrotic tissue, as previously assumed [29]. At the same time, Barkmeier et al. failed to significantly correlate the majority of MRI findings with bowel wall histological fibrosis, except for pre-stenotic upstream dilatation [30].
Only recently, a small number of studies have investigated the role of ultrasound elasticity imaging in differentiating inflammatory from fibrotic bowel tissue in both animal models of colitis and in ex vivo Crohn’s disease bowel specimens. First, Kim et al. demonstrated stiffness changes in fibrotic vs unaffected bowel segments of rat models of colitis [19]. Then Stidham et al. found that strain elastography could differentiate between inflammatory and fibrotic intestine in rat models of colitis and could distinguish fibrotic from unaffected bowel segments in subjects with Crohn’s [20]. At the same time, Dillman group used shear wave elastography to discriminate between acutely inflamed and fibrotic intestine in Crohn’s rat models before, and in ex vivo human intestinal specimens after. Bowel wall shear wave speed measurements increased when transmural intestinal fibrosis was present. The non-linear relationship between shear wave speed and fibrosis was related to the coexistence of inflammation in the examined tissues [31, 32].
Finally, Havre et al. showed that RTE with SR could detect fibrotic harder tissue in resected lesions from both colorectal carcinomas and Crohn’s disease but could not detect any strain differences between benign transmural lesions and malignant tumors [33].
In light of these preliminary positive results, we tested RTE to quantify fibrosis in vivo Crohn’s disease bowel strictures. RTE is a noninvasive technique that evaluates tissue’s strain induced by a quasi-static mechanical force (manual compression, cardiovascular pulsation, respiratory motion). Strain imaging is essentially qualitative, because its quantification would require knowledge about the stress distribution within the body [34]. Therefore, some pseudo-quantitative methods have been proposed, like the strain ratio (SR) measurement. SR have been successfully applied to compare the stiffness of breast lesions and to quantify liver fibrosis although the inter-operator reproducibility of this techniques has not been assessed [23, 35–37].
In our study, RTE with SR detection was not able to distinguish fibrotic from inflammatory tissue in Crohn’s stenosis. It also did not correlate with clinical and biochemical markers. Even if elastography seemed to be a good method to differentiate fibrotic and inflammatory tissue in animal bowels, it failed to quantify fibrosis in in vivo human bowel segment. There could be several possible explanations.
First of all, unlike rat models of colitis where inflamed and fibrotic strictures are reproduced separately, our analyzed fibrotic strictures presented a high grade of inflammation (69% of strictures with moderate or severe grade of inflammation). What is recently emerging for Crohn’s strictures is that not only inflammation and fibrosis coexist, but also the majority of highly fibrotic strictures frequently contain large amounts of superimposed active inflammation [29–32, 38, 39]. We do not know how inflammation changes could modify the elasticity of the bowel wall and, since strain elastography furnishes a number that evaluates the elastic properties of the whole bowel wall, it could be influenced by the presence of both fibrosis and inflammation. Thus, with RTE and SR we probably cannot differentiate fibrosis from inflammation at all. Furthermore there is currently no agreement on any numerical histopathology classification system to score inflammatory and fibrotic features of Crohn’s disease. Following the abovementioned considerations, we think that RTE evaluation might take advantage of a unified histologic score for fibrosis and inflammation.
Another matter of issue was the setting of the reference tissue for the SR calculation. We positioned the reference ROI1 on an area of the image represented by the colour at the bottom of the scale, located in the deepest part of the elastographic box. This technique, successfully applied for the static and more homogeneous liver parenchyma, might be less accurate when the complex structures below the bowel wall, its physiological movements and the imposed luminal content pressure are involved [25]. The mesenteric hypertrophy beneath Crohn’s strictures could influence the segment elasticity and create variability in the ROI1 values, and additionally, whereas the affected bowel segments appear more rigid than the near unaffected ones, there are still some remaining peristaltic movements that could affect RTE measurements. Therefore, the lack of a reference tissue might not appear to be adequately compensated by technical standardization and placement of a reference ROI in the deepest part of the elastographic box. Fraquelli et al. [39] showed that the SR had excellent ability to discriminate severe bowel fibrosis with evidence of no influence of the histologic degree of inflammation on strain ratio at multivariate analysis. This conclusion is quite different from that of the present study. One possible explanation could be the different choice of the reference tissue, as in the paper by Fraquelli et al. the mesenchymal tissue surrounding the affected bowel was selected. As the Authors comment in the discussion section, the mesenteric inflammation could be a possible bias. Since there are many factors that could influence its severity and histology composition (disease duration, on-going therapy, etc.), it could eventually differ from patient to patient, with unknown consequences on the elastographic measurements.
In this study we found no correlation between B-mode bowel wall layering and histology, as already known and reported in literature. Ultrasound imaging is generated by the different interfaces between the bowel layers, while the US detection of the wall thickness is influenced by the pulse length and by the axial resolution of the transducer. When the ultrasound wave reaches an interface, it increases the thickness of the superficial layer and generates a subtraction of thickness from the deep echo-poor layer. The influence of this phenomenon is so relevant in the complex and multi-layered structure as the bowel wall, that in clinical practice, it is considered the presence or the loss of stratification rather than the single layer thickness [40].
As expected, a thickened bowel wall was found to be related with loss of normal stratification at B-mode evaluation. We also found a significant positive correlation between color-Doppler signal intensity and gut wall thickness, in particular submucosal thickness, assessed by histology. This could be attributable to the several vascular and microvascular changes, such as vasculitis, neovascularization, and dilation of feeding arteries and draining veins that characterize Crohn’s affected bowel segments, especially the submucosal layer, which could simultaneously enhance Doppler signals and increase bowel wall thickness [23, 41]. Interestingly, color-Doppler and CEUS assessment of bowel wall vascularization showed no correlation with the effective number of vessels at histology, unlike what could be presumably expected. The histologic vascular assessment was made on non-perfused intestinal specimens, while CEUS and color-Doppler were conducted on in vivo bowel segments, where the inflammatory related microvascular changes and the vascular dilatation are marked and variable. The amount of flow rather than the number of vessels could be probably the leading cause of both color-Doppler and CEUS signals increase [42, 43]. We indeed confirmed the presence of hypervascularization in chronically inflamed bowel segments [13]. Although practical and accurate for clinical activity assessment, the CEUS parameters we used to assess inflammation have some limitations because they provide a qualitative and not a quantitative assessment of vascularization. Finally, we found a significant positive correlation between color-Doppler and CEUS vascular evaluation, which could suggest in the pre-surgical setting to use the faster and cheapest color-Doppler analysis, and limit CEUS examination to the dubious or negative color-Doppler evaluations.
In conclusion, despite the abovementioned limitations, we think that this exploratory study could be a starting point to discuss the role and the best method to apply elastography for the study of the gastrointestinal tract. One of the major criticisms made against ultrasonography is the limited reproducibility of ultrasound findings; to address this criticism, it would be necessary to standardize technical settings and find numerical values to describe ultrasound data. We aimed with strain elastography and SR assessment to overcome this problem, and despite our attempt to standardize its evaluation, our method have not proven satisfactory. Further investigations with larger cohort of patients are needed to assess if intestinal fibrosis could be reliably detected on the basis of bowel elastic properties, and to establish which could be the best approach to standardly quantify it, including the shear wave technique. Shear wave elastography is a technique which directly evaluates the elastic properties of a tissue, using an acoustic radiation force impulse technology, that measures the velocity of the propagation of the generated shear wave. Conversely strain elastography gives an undirect measurement of tissue stiffness, because it furnishes a numeric value based on a colored graduated scale. Based on this difference, shear wave might appear a more reliable tool for detecting bowel stiffness. On the other hand, in recent study Lu et al. [44] failed to correlate shear wave elastography with histological fibrosis in Crohn’s affected bowel segments, but demonstrated a moderate correlation between shear wave and muscular hypertrophy. This aspect should be investigated in larger studies and at the same time, because histology is the reference method to assess transmural disease activity, a consensus on a numerical histopathology classification system for fibrosis and inflammation is warranted.
Since there is currently no accurate diagnostic tool to completely detect the amount of intestinal fibrosis in a bowel stricture, the decision to operate or not is still based on the overall patient evaluation made by expert clinicians.
Compliance with ethical standards
Conflict of interest
The authors have no conflict of interest.
Ethical approval
The local Ethic Committee of the S. Orsola-Malpighi Hospital approved the study protocol (Identification 319 Number 067/2011/O/Sper). The study was performed in conformity with the ethical guidelines of the Declaration of Helsinki.
Informed consent
All patients gave informed written consent.
References
- 1.Crohn BB, Ginzburg L, Oppenheimer GD. Regional ileitis. A pathological and clinical entity. JAMA. 1932;99:1323–1329. doi: 10.1001/jama.251.1.73. [DOI] [PubMed] [Google Scholar]
- 2.Mekhjian HS, Switz DM, Melnyk CS, Rankin GB, Brooks RK. Clinical features and natural history of Crohn’s disease. Gastroenterology. 1979;77:898–906. [PubMed] [Google Scholar]
- 3.Cosnes J, Cattan S, Blain A, Beaugerie L, Carbonnel F, Parc R, Gendre JP. Long-term evolution of disease behavior of Crohn’s disease. Inflamm Bowel Dis. 2002;8:244–250. doi: 10.1097/00054725-200207000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785–1794. doi: 10.1053/j.gastro.2011.01.055. [DOI] [PubMed] [Google Scholar]
- 5.Dignass A, Van Assche G, Lindsay JO, Lémann M, Söderholm J, Colombel JF, Danese S, D’Hoore A, Gassull M, Gomollón F, Hommes DW, Michetti P, O’Morain C, Oresland T, Windsor A, Stange EF, Travis SP, European Crohn’s and Colitis Organisation (ECCO) The second European evidence-based consensus on the diagnosis and management of Crohn’s disease: current management. J Crohns Colitis. 2010;4(1):28–62. doi: 10.1016/j.crohns.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Panés J, Bouzas R, Chaparro M, Garcia-Sànchez V, Gisbert JP, Martinez de Guerenu B, Mendoza JL, Paredes JM, Quiroga S, Ripollés T, Rimola J. Systematic review: the use of ultrasonography, computed tomography and magnetic resonance imaging for the diagnosis, assessment of activity and abdominal complications of Crohn’s disease. Aliment Pharmacol Ther. 2011;34:125–145. doi: 10.1111/j.1365-2036.2011.04710.x. [DOI] [PubMed] [Google Scholar]
- 7.Adler J, Stidham RJ, Higgins PD. Bringing the inflamed and fibrotic bowel into focus: imaging in inflammatory bowel disease. Gastroenterol Hepatol (N Y) 2009;5(10):705–715. [PMC free article] [PubMed] [Google Scholar]
- 8.Migaleddu V, Quaia E, Scano D, Virgilio G. Inflammatory activity in Crohn disease: ultrasound findings. Abdom Imaging. 2008;33:589–597. doi: 10.1007/s00261-007-9340-z. [DOI] [PubMed] [Google Scholar]
- 9.Esteban JM, Aleixandre A, Hurtado MJ, Maldonado L, Mora FJ, Nogués E. Contrast-enhanced power Doppler ultrasound in the diagnosis and follow-up of inflammatory abdominal masses in Crohn’s disease. J Gastroenterol Hepatol. 2003;15:253–259. doi: 10.1097/00042737-200303000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Serra C, Menozzi G, Labate A, Giangregorio F, Gionchetti P, Beltrami M, et al. Ultrasound assessment of vascularization of the thickened terminal ileum wall in Crohn’s disease patients using a low-mechanical index real-time scanning technique with a second generation ultrasound contrast agent. Eur J Radiol. 2007;62:114–121. doi: 10.1016/j.ejrad.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 11.Robotti D, Cammarota T, Debani P, Sarno A, Astegiano M. Activity of Crohn disease: value of color-power-Doppler and contrast- enhanced ultrasonography. Abdom Imaging. 2004;29:648–652. doi: 10.1007/s00261-003-0157-0. [DOI] [PubMed] [Google Scholar]
- 12.Rapaccini GL, Pompili M, Orefice R, Covino M, Riccardi L, Cedrone A, et al. Contraste-enhanced power Doppler of the intestinal wall in the evaluation of patients with Crohn disease. Scand J Gastroenterol. 2004;2:188–194. doi: 10.1080/00365520310008223. [DOI] [PubMed] [Google Scholar]
- 13.Kratzer W, von Tirpitz C, Mason R, Reinshagen M, Adler G, Möller P, et al. Contrast-enhanced power Doppler sonography of the intestinal wall in the differentiation of hipervascularized and hipovascularized intestinal obstructions in patients with Crohn’s disease. J Ultrasound Med. 2002;21:149–157. doi: 10.7863/jum.2002.21.2.149. [DOI] [PubMed] [Google Scholar]
- 14.Migaleddu V, Scanu AM, Quaia E, Rocca PC, Dore MP, Scanu D, et al. Contrast-enhanced ultrasonographic evaluation of inflammatory activity in Crohn’s disease. Gastroenterology. 2009;137:43–61. doi: 10.1053/j.gastro.2009.03.062. [DOI] [PubMed] [Google Scholar]
- 15.Weitzel WF, Kim K, Rubin JM, Wiggins RC, Xie H, Chen X, Emelianov SY, O’Donnell M. Feasibility of applying ultrasound strain imaging to detect renal transplant chronic allograft nephropathy. Kidney Int. 2004;65(2):733–736. doi: 10.1111/j.1523-1755.2004.00435.x. [DOI] [PubMed] [Google Scholar]
- 16.Castera L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128(2):343–350. doi: 10.1053/j.gastro.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 17.Arena U, Vizzutti F, Abraldes JG, Corti G, Stasi C, Moscarella S, et al. Reliability of transient elastography for the diagnosis of advanced fibrosis in chronic hepatitis C. Gut. 2008;57(9):1288–1293. doi: 10.1136/gut.2008.149708. [DOI] [PubMed] [Google Scholar]
- 18.Havre R, Gilja OH. Elastography and strain rate imaging of the gastrointestinal tract. Eur J Radiol. 2014;83(3):438–441. doi: 10.1016/j.ejrad.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 19.Kim K, Johnson LA, Jia C, et al. Noninvasive ultrasound elasticity imaging (UEI) of Crohn’s disease: animal model. Ultrasound Med Biol. 2008;34:902–912. doi: 10.1016/j.ultrasmedbio.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stidham RW, Xu J, Johnson LA, et al. Ultrasound elasticity imaging for detecting intestinal fibrosis and inflammation in rats and humans with Crohn’s disease. Gastroenterology. 2011;141:819–826. doi: 10.1053/j.gastro.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baumgart DC, Müller HP, Grittner U, Metzke D, Fischer A, Guckelberger O, Pascher A, Sack I, Vieth M, Rudolph B. US-based Real-time Elastography for the Detection of Fibrotic Gut Tissue in Patients with Stricturing Crohn Disease. Radiology. 2015;275(3):889–899. doi: 10.1148/radiol.14141929. [DOI] [PubMed] [Google Scholar]
- 22.Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet. 1980;315(8167):514. doi: 10.1016/S0140-6736(80)92767-1. [DOI] [PubMed] [Google Scholar]
- 23.Maconi G, Parente F, Bollani S, Cesana B, Bianchi Porro G. Abdominal ultrasound in the assessment of extent and activity of Crohn’s disease: clinical significance and implication of bowel wall thickening. Am J Gastroenterol. 1996;91:1604–1609. [PubMed] [Google Scholar]
- 24.Limberg B. Diagnosis of chronic inflammatory bowel disease by ultrasonography. Z Gastroenterol. 1999;37:495–508. [PubMed] [Google Scholar]
- 25.Fiorini E, Cipriano V, De Molo C, Righi S, Ainora ME, Arcelli A, Bertusi C, Montanari M, Bianchi G, Serra C. Real-time elastography as a noninvasive technique for quantification of fibrosis in patients with chronic viral liver disease: preliminary findings. J Ultrasound. 2012;15(4):220–225. doi: 10.1016/j.jus.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Magro F, Langner C, Driessen A, Ensari A, Geboes K, Mantzaris GJ, Villanacci V, Becheanu G, et al. European Society of Pathology (ESP); European Crohn’s and Colitis Organisation (ECCO). European consensus on the histopathology of inflammatory bowel disease. J Crohns Colitis. 2013;7(10):827–851. doi: 10.1016/j.crohns.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 27.Chiorean MV, Sandrasegaran K, Saxena R, Maglinte DD, Nakeeb A, Johnson CS. Correlation of CT enteroclysis with surgical pathology in Crohn’s disease. Am J Gastroenterol. 2007;102(11):2541–2550. doi: 10.1111/j.1572-0241.2007.01537.x. [DOI] [PubMed] [Google Scholar]
- 28.41st World Medical Assembly Declaration of Helsinki: recommendations guiding physicians in biomedical research involving human subjects. Bull Pan Am Health Organ. 1990;24:606–609. [Google Scholar]
- 29.Adler J, Punglia DR, Dillman JR, et al. Computed tomography enterography findings correlate with tissue inflammation, not fibrosis in resected small bowel Crohn’s disease. Inflamm Bowel Dis. 2012;18:849–856. doi: 10.1002/ibd.21801. [DOI] [PubMed] [Google Scholar]
- 30.Barkmeier DT, Dillman JR, Al-Hawary M, Heider A, Davenport MS, Smith EA, Adler J. MR enterography-histology comparison in resected pediatric small bowel Crohn disease strictures: can imaging predict fibrosis? Pediatr Radiol. 2015;46(4):498–507. doi: 10.1007/s00247-015-3506-6. [DOI] [PubMed] [Google Scholar]
- 31.Dillman JR, Stidham RW, Higgins PD, Moons DS, Johnson LA, Rubin JM. US elastography-derived shear wave velocity helps distinguish acutely inflamed from fibrotic bowel in a Crohn disease animal model. Radiology. 2013;267:757–766. doi: 10.1148/radiol.13121775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dillman JR, Stidham RW, Higgins PD, Moons DS, Johnson LA, Keshavarzi NR, Rubin JM. Ultrasound shear wave elastography helps discriminate low-grade from high-grade bowel wall fibrosis in ex vivo human intestinal specimens. J Ultrasound Med. 2014;33(12):2115–2123. doi: 10.7863/ultra.33.12.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Havre RF, Leh S, Gilja OH, Odegaard S, Waage JE, Baatrup G, Nesje LB. Strain assessment in surgically resected inflammatory and neoplastic bowel lesions. Ultrashall in Med. 2014;35:149–158. doi: 10.1055/s-0032-1325535. [DOI] [PubMed] [Google Scholar]
- 34.Shiina T, Nightingale KR, Palmeri ML, Hall TJ, Bamber JC, Barr RG, Castera L, Choi BI, Chou YH, Cosgrove D, Dietrich CF, Ding H, et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: part 1: basic principles and terminology. Ultrasound Med Biol. 2015;41(5):1126–1147. doi: 10.1016/j.ultrasmedbio.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 35.Farrokh A, Wojcinski S, Degenhardt F. Diagnostic value of strain ratio measurement in the differentiation of malignant and benign breast lesions. Ultraschall Med. 2011;32:400–405. doi: 10.1055/s-0029-1245335. [DOI] [PubMed] [Google Scholar]
- 36.Ueno E, Umemoto T, Bando H, Tohno E, Waki K, Matsumura T (2007) New quantitative method in breast elastography: fat lesion ratio (FLR). In: Proceedings of the Radiological Society of North America Scientific Assembly and Annual Meeting, Radiological Society of North America, Oak Brook, 2007, pp 697
- 37.Itoh A, Ueno E, Tohno E, Kamma H, Takahashi H, Shiina T, Yamakawa M, Matsumura T. Breast disease: clinical application of US elastography for diagnosis. Radiology. 2006;239:341–350. doi: 10.1148/radiol.2391041676. [DOI] [PubMed] [Google Scholar]
- 38.Zappa M, Stefanescu C, Cazals-Hatem D, et al. Which magnetic resonance imaging findings accurately evaluate inflammation in small bowel Crohn’s disease? A retrospective comparison with surgical pathologic analysis. Inflamm Bowel Dis. 2011;17(4):984–993. doi: 10.1002/ibd.21414. [DOI] [PubMed] [Google Scholar]
- 39.Fraquelli M, Branchi F, Cribiu’ FM, et al. The role of ultrasound elasticity imaging in predicting ileal fibrosis in Crohn’s disease patients. Inflamm Bowel Dis. 2015;21:2605–2612. doi: 10.1097/MIB.0000000000000536. [DOI] [PubMed] [Google Scholar]
- 40.Kimmey MB, Martin RW, Haggitt RC, et al. Histological correlates of gastrointestinal endoscopic ultrasound images. Gastroenterology. 1989;96:433–441. doi: 10.1016/0016-5085(89)91568-0. [DOI] [PubMed] [Google Scholar]
- 41.Wakefield AJ, Sawyerr AM, Dhillon AP, et al. Pathogenesis of Crohn’s disease: multifocal gastrointestinal infarction. Lancet. 1989;ii:1057–1062. doi: 10.1016/S0140-6736(89)91078-7. [DOI] [PubMed] [Google Scholar]
- 42.Brahme F, Lindstrom C. A comparative radiographic and pathological study of intestinal vasoarchitecture in Crohn’s disease and ulcerative colitis. Gut. 1970;11:928–940. doi: 10.1136/gut.11.11.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boijsen E, Reuter SR. Mesenteric angiography in the evaluation of inflammatory and neoplastic disease of intestine. Radiology. 1966;87:1028–1036. doi: 10.1148/87.6.1028. [DOI] [PubMed] [Google Scholar]
- 44.Lu C, Gui X, Chen W, et al. Ultrasound shear wave elastography and contrast enhancement: effective biomarkers in Crohn’s disease strictures. Inflamm Bowel Dis. 2017;23:421–430. doi: 10.1097/MIB.0000000000001020. [DOI] [PubMed] [Google Scholar]