Abstract
Purpose
To validate the accuracy of previously published equations that estimate pleural effusion volume using ultrasonography.
Methods
Only equations using simple measurements were tested. Three measurements were taken at the posterior axillary line for each case with effusion: lateral height of effusion (H), distance between collapsed lung and chest wall (C) and distance between lung and diaphragm (D). Cases whose effusion was aspirated to dryness were included and drained volume was recorded. Intra-class correlation coefficient (ICC) was used to determine the predictive accuracy of five equations against the actual volume of aspirated effusion.
Results
46 cases with effusion were included. The most accurate equation in predicting effusion volume was (H + D) × 70 (ICC 0.83). The simplest and yet accurate equation was H × 100 (ICC 0.79).
Conclusion
Pleural effusion height measured by ultrasonography gives a reasonable estimate of effusion volume. Incorporating distance between lung base and diaphragm into estimation improves accuracy from 79% with the first method to 83% with the latter.
Keywords: Ultrasound, Pleural effusion, Prediction equation, Real-time, Probes
Sommario
Scopo
Per convalidare l’accuratezza di equazioni che stimano il volume versamento pleurico.
Metodi
Abbiamo testato le equazioni che hanno usato le misurazioni semplici. Sono state prese tre misure: altezza di effusione (H), la distanza tra il polmone collassato e la parete toracica (C) e la distanza tra polmone e diaframma (D). Il versamento è stato aspirato e il volume è stato registrato. Coefficiente di correlazione intra-classe (ICC) è stato utilizzato per determinare l’accuratezza predittiva delle misurazioni.
Risultati
46 pazienti sono stati inclusi. L’equazione più accurata nel predire il volume di effusione era (H + D) × 70 (ICC 0,83). La più semplice equazione accurata era H × 100 (ICC 0,79).
Conclusione
L’altezza del versamento pleurico misurato con ecografia fornisce una stima ragionevole del volume di versamento. La distanza tra la base del polmone e il diaframma migliora la precisione.
Parole chiave: Ultrasuoni, Versamento pleurico, Equazione previsione, Sonde
Introduction
The use of ultrasonography (US) in examining the pleural space has become a standard practice worldwide [1]. Physicians in many different disciplines are acquiring the skills to adequately examine the pleural space, using US. This has positive impact on patient’s overall care as there are many advantages of US over other radiological investigations. US is a relatively cheap test which does not expose the patient to the risk of radiation and the easy mobility of US machines makes the procedure useful as a bedside test. This has revolutionised the practice in different medical disciplines such as the emergency rooms, intensive care units and pulmonology departments [2]. The scope of thoracic US has expanded from evaluating the pleural space to encompass newer domains like evaluating lung parenchymal changes in the acutely breathless patient [3] and assessing diaphragmatic kinetics in patients on mechanical ventilation [4].
The use of US in the identification and management of pleural disease is one of the oldest indications in the field of pulmonology. In addition to its value in diagnosing the presence of pleural effusion, it is possible to evaluate the echogenicity of the fluid and the presence and degree of septations, which are key parameters in stratifying pleural infections and choosing the optimum treatment pathway [5]. Given its real-time potential, US has the advantage of safely guiding pleural procedures, leading to lower complication rates and reduced healthcare costs [6] which has been translated in recent guidelines for pleural procedures [7].
Among the interesting uses of US, is estimating the volume of pleural effusion. Ultrasound is much more sensitive than standard X ray in detecting small volumes of effusion. At least 150 ml of fluid is required to be picked up by a chest X ray even if the procedure is done under favourable conditions [8]. The threshold of US for detecting pleural effusion is lower than 5 ml [9].
Various attempts have been made to derive equations to predict the volume of pleural effusion. There have been many endeavours to develop formulas to estimate effusion volume using computed tomography (CT) [10]. Such formulas have not been validated against the actual volume of fluid after aspiration. In addition, CT is an expensive technique with large radiation dose that makes repeating the procedure for comparison impractical unlike US examination, which can be repeated without any radiation hazard.
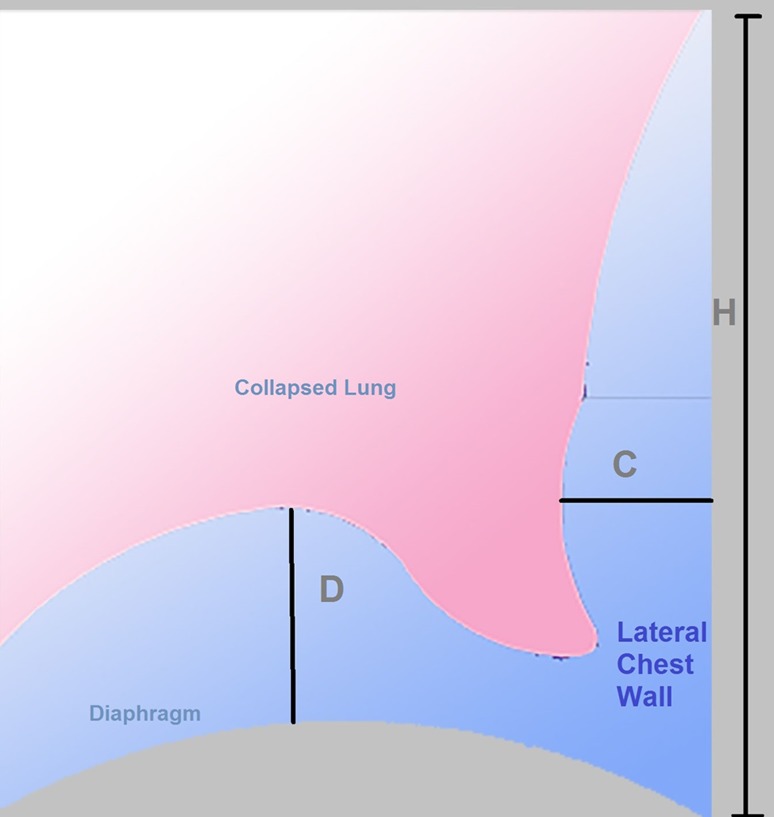
Some practitioners prefer to use qualitative estimations of volume size based on crude measurements such as number of probe ranges [11] or rib spaces [1] where effusion is visible. Others have tested using the depth of effusion from chest wall laterally [12] or posteriorly [13] to evaluate the need of aspiration in ICU patients with pleural effusion. There are several methods in the literature to accurately estimate the volume of effusion. Most methods have evaluated patients in the sitting position, which makes examining effusion easier at the posterior axillary line [11]. Examination at the supine position is more difficult because most of effusion gravitates posteriorly. Table 1 summarises some of the methods reported, their strengths and the setting of the patients. These equations have not been independently validated on different samples which questions their accuracy [1]. This study aimed to examine the validity of equations that rely on simple measurements (Fig. 1) to accurately predict the volume of pleural effusion in seated patients.
Table 1.
Equations for estimation of pleural effusion sonographically
| Authors | Setting | Probe used | Equation | R 2 |
|---|---|---|---|---|
| Goecke and Schwerk [14] | Wards, outpatients | Curvilinear | H × 90 | 0.68 |
| Goecke and Schwerk [14] | Wards, outpatients | Curvilinear | (H + D) × 70 | 0.87 |
| Balik et al. [12] | ICU | Sector | C × 20 | 0.52 |
| Usta at al [13] | Post-cardiac surgery | Sector | D × 16 | 0.79 |
| Remérand et al. [15] | ICU | Curvilinear | Paravertebral effusion area × H | 0.70 |
C chest wall, D diaphragm, H lateral height, R 2 squared regression coefficient
Fig. 1.
Schematic representation for the three parameters measured in study subjects. C chest wall, D diaphragm, H height
Methods
This study was approved by the Medical Ethics Department of Alexandria Faculty of Medicine. Informed consent was obtained from all involved patients.
Patients with pleural effusion, in whom therapeutic aspiration or medical thoracoscopy was clinically indicated, presenting to the Chest Diseases Department at Alexandria University Hospital between January and June 2016 were included in the study. Cases with evidence of encystment/loculation or diaphragmatic pathology were excluded.
Ultrasound examination was performed at the posterior axillary line using either a 3–5 MHz convex-array probe or a 3.5 MHz phased-array probe. An image that captures the effusion, collapsed lung and the hemi-diaphragm at end expiration in B mode was frozen for measurements (Fig. 2). Two measurements were taken:
Distance from visceral pleura to chest wall (C)
Distance from lung base to apex of diaphragm cupola (D)
Fig. 2.
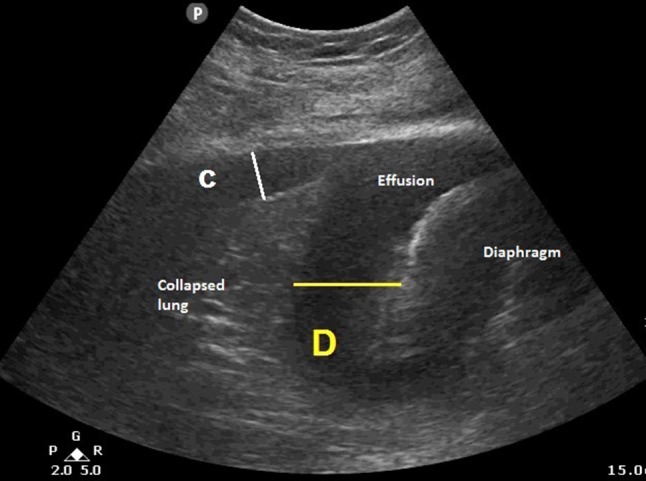
Ultrasound image at the basal part of the effusion showing how C and D are measured. (C chest wall, D diaphragm)
The probe was then moved to more superior and inferior rib spaces marking points where effusion was last detectable on the skin. The lateral height (H) was measured as the distance between the two marks (Fig. 1).
Aspiration was performed at the site determined by US examination. Only cases that were aspirated to dryness or near-dryness (post procedure D ≤ 2 mm) were included. Volume of effusion was then recorded.
The value of C, H and D were used to calculate the predicted volume based on the following equations [14–16]:
| 1 |
| 2 |
| 3 |
| 4 |
In addition, to further simplify Eq. 3 we tested the following equation:
| 5 |
The authors found the method suggested by Remérand et al. [17] (Table 1) impractical due to two reasons. First, the measurements were taken at the paravertebral line which is very challenging in critically unwell and immobile patients. Second, measuring the area of the effusion is not straightforward we felt that it can lead to variability between different operators. It was decided not to include this method in the study.
Statistical analysis
This study used the intraclass correlation coefficient (ICC) to measure the degree of agreement between the volume estimation made by each equation with the actual aspirated volume. ICC is used to assess the reliability of a given instrument to measure the parameter which it is supposed to measure [18] and it is more appropriate than Pearson’s correlation coefficient to achieve this purpose [19].
Estimated effusion volume was calculated for all cases using the aforementioned equations. The results were compared to the actual volume aspirated by means of ICC. Level of agreement was defined as being:
Poor for ICC < 0.5
Moderate for ICC 0.5–0.75
Good for ICC 0.75–0.9
Excellent for ICC > 0.9 [18].
All statistics were performed using PASW software (version 19; SPSS inc, Chicago, IL, USA).
Results
Forty-six cases were available for analysis. 29 (63%) cases had right effusion. 20 cases (43%) were admitted to the ICU. Regarding aetiology, the two commonest causes were heart failure (48%), followed by malignant pleural disease (23%). Other aetiologies included uraemia, liver failure and trauma.
The mean volume aspirated was 1350 + 540 ml (min 330 ml, max 3100 ml). None of the cases developed serious complications (e.g., pneumothorax, re-expansion pulmonary oedema, bleeding). Therapeutic aspiration was performed in 42 cases (91%) and effusion was drained during medical thoracoscopy in the remaining 4 patients.
Table 2 shows the ICC for each of the five equations with 95% confidence intervals. There was poor agreement between actual and estimated volumes using Eqs. 1 and 2. Good agreement was noted with Eqs. 3 and 5 which were very close in accuracy. Equation 4 had excellent agreement with the highest ICC noted among the studied equations.
Table 2.
ICC with 95% confidence intervals for the five tested equations against measured volume
| Equation | ICC | 95% confidence interval | |
|---|---|---|---|
| Upper limit | Lower limit | ||
| C × 20 | 0.342 | − 0.103 | 0.679 |
| D × 16 | 0.382 | − 0.103 | 0.719 |
| H × 90 | 0.773 | 0.452 | 0.894 |
| (H + D) × 70 | 0.835 | 0.687 | 0.913 |
| H × 100 | 0.798 | 0.651 | 0.888 |
C chest wall, D diaphragm, H lateral height, ICC intra-class correlation coefficient
Discussion
Estimating the size of pleural effusion can be useful in mechanically ventilated patients to determine if aspiration is needed and in patients with transudative effusions to monitor their response to therapy.
This study provides independent validation that ultrasound can be used in volume estimation of free-flowing pleural effusion. In previous reports, the study population was either ICU patients on positive pressure ventilation [14, 15] or ward/outpatients [16]. We included patients from the both settings for two reasons; first, we wanted to examine the feasibility of performing these measurements in ICU patients who can, at times, be difficult to mobilise in bed. Second, we wanted to establish the reliability of a given equation to accurately predict volume in the presence of positive pressure ventilation or lack thereof. More than one-third of our patients were receiving positive pressure ventilation, and measurements were feasible in all of them. In addition, the inclusion for their measurements provides evidence that the accurate equations have good predictive capability in this setting.
Different practitioners use different probes. The most commonly used probes to examine the chest are the curvilinear and sector probes [1, 2]. We used both types of probes and we did not find any difficulty to obtain the desired image that captures the collapsed lung just above the diaphragm. The factor that is most important is how comfortable a sonographer is with a certain probe. It is worth noting, however, that if real-time guidance is deemed necessary during pleural aspiration, the curvilinear probe proves superior due to its wider footprint, which allows visualisation of the full course of intervening needles.
The oldest equation studied, proposed by Goerke and Schwerk, was published in 1990 (Table 1) [16]. It is interesting that one of these equations (H + D × 70) showed the highest level of accuracy. The other equation they proposed (H × 90) also showed good accuracy but to a lesser degree (in concordance to their own findings). We thought of trying a modification on the latter equation which makes the process of calculation much easier which is to multiply effusion height by 100. This simplification did not weaken the equation, but rather led to slightly increased accuracy to predict effusion volume (Table 2).
It was noticed that the equations performed differently according to the side of the effusion (data not shown in results). The most accurate equations tended to overestimate the size of the effusion on the left size. This is not surprising, given that left hemithorax is smaller in size in comparison with the right side. In our view, this discrepancy did not lead to large calculation errors. A larger study with more patients would be needed to address whether different equations should be used according to the side of the effusion.
Conclusion
Pleural effusion height measured by US gives a reasonable estimate of effusion volume with 79% accuracy when correlated with the actual aspirated volume. The formula with the highest level of accuracy used both the distance from chest wall to visceral pleura added to the distance from the lung base to the apex of the diaphragm and it could predict the actual volume with 83% accuracy.
Acknowledgements
The authors thank Marwa Hassan for providing the schematic drawings and are very grateful to Dr. Rachel Mercer, who critically revised the final manuscript for language and scientific content.
Compliance with ethical standards
Conflict of interest
None to be declared.
Ethical approval
This study was approved by the Medical Ethics Department of Alexandria Faculty of Medicine.
Informed consent
Informed consent was obtained from all involved patients.
Funding
MH is a recipient of European Respiratory Society long-term research fellowship—ERS 2016-7333. This is not related to and has not affected the substance of this paper.
References
- 1.Mercer RM, Psallidas I, Rahman NM. Ultrasound in the management of pleural disease. Expert Rev Respir Med. 2017;11:323–331. doi: 10.1080/17476348.2017.1300531. [DOI] [PubMed] [Google Scholar]
- 2.Lichtenstein D. Novel approaches to ultrasonography of the lung and pleural space: where are we now? Breathe. 2017;13:100–111. doi: 10.1183/20734735.004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soldati G, Demi M. The use of lung ultrasound images for the differential diagnosis of pulmonary and cardiac interstitial pathology. J Ultrasound. 2017;20:91–96. doi: 10.1007/s40477-017-0244-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zanforlin A, Smargiassi A, Inchingolo R, et al. Ultrasound in obstructive lung diseases: the effect of airway obstruction on diaphragm kinetics. A short pictorial essay. J Ultrasound. 2015;18:379–384. doi: 10.1007/s40477-014-0122-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies HE, Davies RJO, Davies CWH, et al. Management of pleural infection in adults: British Thoracic Society pleural disease guideline. Thorax. 2010;65:ii41–ii53. doi: 10.1136/thx.2010.137000. [DOI] [PubMed] [Google Scholar]
- 6.Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143:532–538. doi: 10.1378/chest.12-0447. [DOI] [PubMed] [Google Scholar]
- 7.Havelock T, Teoh R, Laws D, et al. Pleural procedures and thoracic ultrasound: British Thoracic Society pleural disease guideline 2010. Thorax. 2010;65:i61–i76. doi: 10.1136/thx.2010.137026. [DOI] [PubMed] [Google Scholar]
- 8.Dietrich CF, Mathis G, Cui X-W, et al. Ultrasound of the pleurae and lungs. Ultrasound Med Biol. 2015;41:351–365. doi: 10.1016/j.ultrasmedbio.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Kocijancic I, Kocijancic K, Cufer T. Imaging of pleural fluid in healthy individuals. Clin Radiol. 2004;59:826–829. doi: 10.1016/j.crad.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 10.Moy MP, Levsky JM, Berko NS, et al. A new, simple method for estimating pleural effusion size on CT scans. Chest. 2013;143:1054–1059. doi: 10.1378/chest.12-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prina E, Torres A, Carvalho CRR. Lung ultrasound in the evaluation of pleural effusion. J Bras Pneumol. 2014;40:1–5. doi: 10.1590/S1806-37132014000100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vignon P, Chastagner C, Berkane V, et al. Quantitative assessment of pleural effusion in critically ill patients by means of ultrasonography. Crit Care Med. 2005;33:1757–1763. doi: 10.1097/01.CCM.0000171532.02639.08. [DOI] [PubMed] [Google Scholar]
- 13.Roch A, Bojan M, Michelet P, et al. Usefulness of ultrasonography in predicting pleural effusions > 500 mL in patients receiving mechanical ventilation. Chest. 2005;127:224–232. doi: 10.1378/chest.127.1.224. [DOI] [PubMed] [Google Scholar]
- 14.Balik M, Plasil P, Waldauf P, et al. Ultrasound estimation of volume of pleural fluid in mechanically ventilated patients. Intensive Care Med. 2006;32:318–321. doi: 10.1007/s00134-005-0024-2. [DOI] [PubMed] [Google Scholar]
- 15.Usta E, Mustafi M, Ziemer G. Ultrasound estimation of volume of postoperative pleural effusion in cardiac surgery patients. Interact Cardiovasc Thorac Surg. 2010;10:204–207. doi: 10.1510/icvts.2009.222273. [DOI] [PubMed] [Google Scholar]
- 16.Goecke W, Schwerk W, et al. Die real-time-sonographie in der Diagnostik von Pleuraergüssen. In: Gebhardt J, Hackelöer BJ, Klinggräff VG, et al., editors. Ultraschalldiagnostik. Berlin: Springer; 1990. p. 89. [Google Scholar]
- 17.Remérand F, Dellamonica J, Mao Z, et al. Multiplane ultrasound approach to quantify pleural effusion at the bedside. Intensive Care Med. 2010;36:656–664. doi: 10.1007/s00134-010-1769-9. [DOI] [PubMed] [Google Scholar]
- 18.Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15:155–163. doi: 10.1016/j.jcm.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. doi: 10.1016/S0140-6736(86)90837-8. [DOI] [PubMed] [Google Scholar]