Abstract
Purpose
Peripheral nerves frequently travel close to the bone surface and are, therefore, prone to elastosonographic “bone-proximity” hardening artifacts. The impact of these artifacts on quantitative measurements of median nerve stiffness performed by shear wave elastosonography has not been explored. Our aim was to assess normal median nerve stiffness values at various locations.
Materials and methods
Thirty-six healthy volunteers (24 women and 12 men) aged between 25 and 40 years were evaluated. Two operators performed the evaluation: one expert (6 years of ultrasound experience) and one inexperienced operator (6 months’ experience). The nerve was sampled in cross-section at three different locations: mid-forearm, immediately before the carpal tunnel and within the tunnel. The ultrasound scanner was equipped with a 14-MHz linear probe. The Shear Wave module was activated in one-shot mode. Measurements were performed using a ROI corresponding to the diameter of the nerve.
Results
The mean values of stiffness of the medial nerve were 32.26 kPa ± 18.60 within the carpal tunnel, 22.20 kPa ± 9.84 at the carpal tunnel inlet and 7.62 kPa ± 7.38 in the forearm. Inter-observer agreement assessed using the intraclass correlation coefficient (ICC) was “moderate” within the carpal tunnel (ICC = 0.44), “moderate” at the carpal tunnel inlet (ICC = 0.41) and “fair” in the forearm (ICC = 0.38).
Conclusions
The stiffness of the median nerve progressively increases in its distal portions, where the nerve approaches the bone surface. Inter-observer agreement was generally good (from fair to moderate).
Keywords: Median nerve, Carpal tunnel, Elasticity imaging techniques, Shear wave elastosonography, Artifacts
Sommario
Scopo del lavoro
i nervi periferici frequentemente decorrono in prossimità di superfici ossee e sono perciò soggetti agli artefatti elasosonografici di “prossimità ossea”. L’impatto di questi artefatti sulle misure quantitative di stiffness del nervo mediano realizzate con elastosonografia a onde trasversali non è stata esplorata. Il nostro intento è quello di valutare la stiffness del nervo mediano in varie sedi.
Materiali e metodi
Sono stati valutati trentasei volontari sani (24 donne e 12 uomini) di età compresa tra 25 e 40 anni. Le valutazioni sono state eseguite da 2 operatori: un operatore esperto (6 anni di esperienza in ecografia muscoloscheletrica) e uno non esperto (6 mesi di esperienza). Il nervo è stato campionato in sezione assiale a tre livelli differenti: a livello dell’avambraccio, immediatamente prima del tunnel carpale e entro il tunnel. L’ecografo utilizzato era equipaggiato con una sonda lineare da 14 MHz. Il modulo shear-wave è stato attivato nella modalità “one-shot”. Le misurazioni sono state realizzate usando una ROI della stessa dimensione del nervo.
Risultati
Il valore medio di stiffness del nervo mediano è risultato essere di 32.26 kPa ± 18.60 entro il tunnel carpale, 22.20 kPa ± 9.84 all’inlet del tunnel carpale e 7.62 kPa ± 7.38 a livello dell’avambraccio. L’agreement inter osservatore è stato valutato usando l’Intraclass Correlation Coefficient (ICC) ed è risultato “moderato” entro il tunnel carpale (ICC = 0.44), “moderato” all’inlet del tunnel carpale (ICC = 0.41) e “soddisfacente” nell’avambraccio (ICC = 0.38).
Conclusioni
La stiffness del nervo mediano aumenta progressivamente nelle sue porzioni distali ove il nervo si avvicina alle superfici ossee. L’agreement inter osservatore di queste misurazioni è stato generalmente buono (da soddisfacente a moderato).
Introduction
Elastosonography (ESG) is an ultrasound technique that allows the evaluation of biological tissue stiffness. This technique was introduced in 1991 and was first applied in a clinical setting in 1997. The first studies focused on the assessment of liver stiffness, which remains one of the main fields of application for ESG. Nonetheless, during recent years the use of ESG has expanded into other areas [1–5]. Among these applications, peripheral nerve stiffness evaluation is one of the main areas of growing interest [6].
Carpal tunnel syndrome (CTS) is a common entrapment peripheral neuropathy that occurs inside the carpal tunnel of the wrist. It affects mainly female subjects (3:1) between the 4th and 6th decade. Generally CTS is idiopathic and bilateral (50% of cases). Several factors must be taken into account to confirm clinical suspicion: clinical tests, electroneurography and imaging (e.g. X-rays, ultrasound and magnetic resonance). Ultrasound is generally used to identify causes of secondary CTS (e.g. synovial cysts). In idiopathic CTS the cross-sectional area (CSA) of the median nerve is evaluated (cut off 10 mm2) [7].
Being both a challenging diagnosis and a very diffuse disease, CTS has recently attracted the attention of the scientific community in terms of the possible benefits deriving from the application of ESG in this setting. Currently, there are two different approaches to ESG: strain elastosonography (SE) and shear wave elastosonography (SWE). The first and oldest is SE, which is based on operator-induced compression to evaluate tissue displacement and, therefore, stiffness. SE is not capable of delivering quantitative data but only ratios among structures (for example the nerve and an acoustic coupler), thus reducing the general significance of the results. SWE is a newer technique that is independent from operator compression and is capable of delivering quantitative data (either in kPa or in m/s).
SWE is based on the application of particular types of sound waves obtained through a focused beam. Shear (transversal) waves propagate slower than longitudinal ones but their speed is directly related to the medium shear modulus that is dependent on the density of the examined area. To generate shear waves “acoustic radiation force” or “push pulse” can be used: tiny displacements in soft tissue set up by shear waves that travel sideways away from the “pushing” ultrasound beam; few microns displacement can be detected by conventional ultrasound using tracking algorithms. Another way to image shear waves relies on coupling two concepts: Mach cone (ultrasound beams are successively focused at different depths) and ultrafast imaging (scanning of the entire imaging plane with very good temporal resolution in one single acquisition, typically with a frame rate of around 5000 images per second).
While much enthusiasm has emerged around the possibility of SWE for overcoming the limitations of SE we must bear in mind that SWE also presents some limitations such as a certain degree of variability in results [8]. Peripheral nerves frequently travel close to bone surfaces and are, therefore, prone to elastosonographic “bone-proximity” hardening artifacts. These artifacts occur when the studied structure is near a hard plane (such as bone) that prevents the shear wave homogeneously propagating at depth, causing local stress inhomogeneity [9]. Since the carpal tunnel floor is composed by the carpal bones the median nerve is undoubtedly prone to this artifact. But the impact of these artifacts on quantitative measurements of median nerve stiffness performed by SWE has not been explored. Since these artifacts are relatively different from those of the SE and their impact on measurement is unknown, we decided to focus on their assessment. Our aim was to understand their impact when assessing normal median nerve stiffness values at various locations and, as a secondary endpoint, to evaluate inter-observer agreement.
Materials and methods
Thirty-six healthy volunteers (24 women and 12 men) aged between 25 and 40 years were evaluated; forty volunteers were initially screened.
Exclusion criteria were: a previous diagnosis of CTS, any surgery or fracture involving forearm, hand and wrist and any medication (e.g. Tamoxifen) or medical condition (e.g. hypothyroidism, diabetes) possibly favoring CTS development. In addition, an increase in the median nerve CSA (over 10 mm2) or the presence of anatomical variants was considered as exclusion criteria (e.g. bifid median nerve or persistence of the median artery). Two volunteers were excluded because of the presence of a bifid median nerve, one because of a type I diabetes and one because of a known subclinical hypothyroidism.
Height and weight data were collected.
We analyzed the left side (corresponding to the non-dominant side in all of the subjects examined) and each subject was evaluated by two operators: one expert (6 years of ultrasound experience) and one inexperienced (6 months’ experience). IRB approval was obtained.
All subjects were evaluated seated in front of the operator, with the elbow flexed to 90°, the forearm resting on a flat surface, the hand supinated and fingers in neutral position; the volunteers were at rest from at least 30 min. Preliminarily the median nerve CSA and the presence of anatomical variants were assessed.
The nerve was sampled at three different points three times for each point, in cross-section:
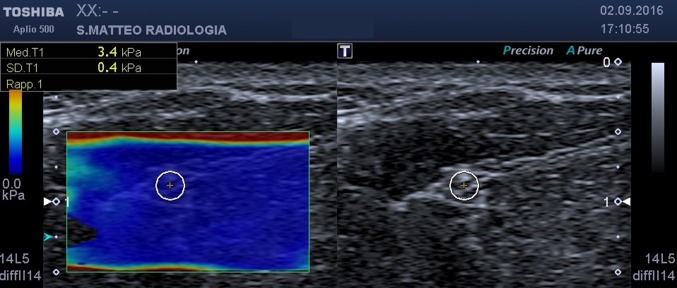
mid-forearm, between flexor digitorum superficialis and flexor digitorum profundus (Fig. 1);
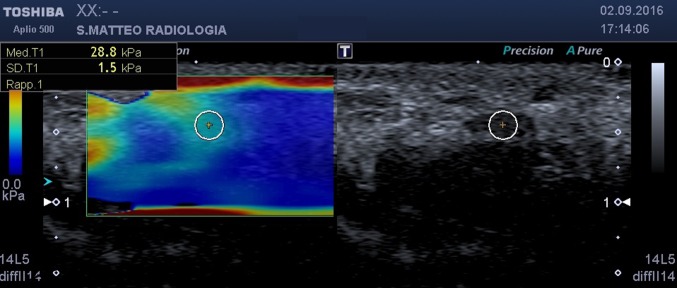
at the carpal tunnel inlet (scaphoid–pisiform level) (Fig. 2);
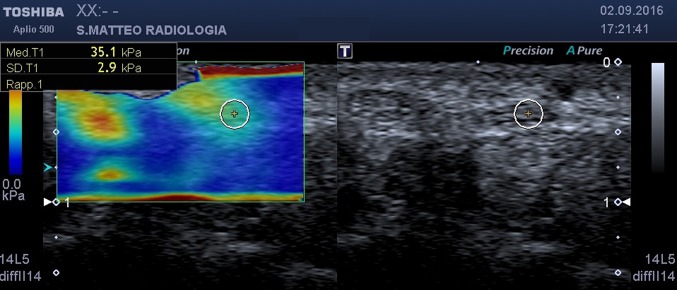
at the carpal tunnel outlet (hamate–trapezium level) (Fig. 3).
Fig. 1.
SWE, axial scan at mid-forearm. On the left the b-mode image, on the right the elastogram. The circular ROI encloses the median nerve
Fig. 2.
SWE, axial scan at carpal tunnel inlet. On the left the b-mode image, on the right the elastogram. The circular ROI encloses the median nerve
Fig. 3.
SWE, axial scan at carpal tunnel outlet. On the left the b-mode image, on the right the elastogram. The circular ROI encloses the median nerve
An ultrasound scanner (Toshiba Aplio 500, Toshiba, Japan) equipped with a 14-MHz linear probe was used.
The Shear Wave module was activated in one-shot mode, which, when holding the probe in the same position, performs a 5-second assessment after which it provides a colorimetric map of tissue stiffness (elastogram).
Finally, a ROI, manually set and corresponding to the diameter of the nerve, was positioned on the elastogram. The diameter of the ROI, however, always ranged between 2 and 3 mm. The ROI was positioned exactly within the borders of the median nerve. No arterial vessels normally travel along with the median nerve; it was surrounded only by muscles (at the mid forearm) and tendons (at the carpal tunnel). The persistence of a median artery, being an anatomic variant, was an exclusion criteria.
To ensure the best quality of the image the ROI was positioned avoiding the periphery of the elastogram. The probe was held exactly perpendicular to the body surface with a generous amount of gel at the probe-skin interface.
Using this approach, the values of the stiffness of the nerve at the various sampling points were obtained (Figs. 1, 2, 3). Statistical analysis was performed using MedCalc (MedCalc Software, Ostend, Belgium).
Results
The mean values of stiffness of the medial nerve were 32.26 kPa ± 18.60 within the carpal tunnel, 22.20 kPa ± 9.84 at the carpal tunnel inlet and 7.62 kPa ± 7.38 in the forearm (see Table 1). The paired Student’s T test demonstrated significantly different values between the three anatomical levels (see Table 2). There was moderate correlation between stiffness of the median nerve and the patients’ weight and height, but only at the levels before the CT and in the forearm. There was no correlation with age. There was a significant difference in mean stiffness between men and women in the carpal tunnel (women 36.54 ± 24.9; men 28.25 ± 8.72; p < 0.001), before the carpal tunnel (women 19.06 ± 7.20; men 26.12 ± 9.98; p 0.05) and in the forearm (women 8.63 ± 10.63; men 6.80 ± 1.55; p < 0.001). Inter-reader agreement assessed using the Intraclass Correlation Coefficient (ICC) was “moderate” in the carpal tunnel (ICC = 0.44), “moderate” proximal to the carpal tunnel (ICC = 0.41) and “fair” in the forearm (ICC = 0.38).
Table 1.
Median nerve stiffness measurements
| Nerve level | Examiner 1 (KPa ± SD) | Examiner 2 (KPa ± SD) |
|---|---|---|
| Carpal tunnel | 32.26 ± 18.60 | 39.81 ± 17.09 |
| Proximal carpal tunnel | 22.20 ± 9.84 | 20.97 ± 9.43 |
| Forearm | 7.62 ± 7.38 | 6.01 ± 2.62 |
Table 2.
Mean differences in median nerve stiffness measurements between anatomic levels
| Anatomic levels | Mean differences | P |
|---|---|---|
| 1–2 | − 10.06 | 0.007 |
| 2–3 | − 9.86 | 0.005 |
| 1–3 | − 24.57 | < 0.001 |
Discussion
The carpal tunnel can be divided into two sections: the proximal carpal tunnel (or carpal tunnel inlet) and the distal carpal tunnel (or carpal tunnel outlet). The proximal section’s walls are formed by the scaphoid (on the radial side) and the pisiform (on the ulnar side). The distal portion’s walls are formed by the hook of the hamate (ulnar side) and the tubercle of the trapezium (radial side). Generally, the proximal section of the CTS is the expected location for maximum swelling of the median nerve [10].
Literature on the use of ESG to diagnose CTS is mainly based on SE. As a recent review underlined [6], only nine articles (the first one published in 2013) have addressed the issue of measuring median nerve stiffness (Table 3) [11–19]. Seven of these articles used SE. Almost all authors agree that the pathological median nerve is stiffer than the unaffected median nerve, but the exact statistics are much more complex to deal with. For example, in Miyamoto and colleagues [16], patients had a 6.9% strain and normal controls 4.1% strain, in Yoshii and colleagues [18] patients had a 3.7% strain and normal controls 1.8% strain and in Orman and colleagues [17] patients had a 0.094 mean tissue strain while in normal controls it was 0.0145. Yoshii and Miyamoto both used an acoustic coupler to create the ratio while Orman used the subcutaneous fat.
Table 3.
Literature on ESG applied to CTS
| Year | Author | Technique | Number of patients | Plane | Position measurement | Elasticity measurement (description) |
|---|---|---|---|---|---|---|
| 2014 | Kantarci | SWE | 37 | Long axis | Proximal carpal row | Mean elasticity |
| 2017 | Fu | SWE | 49 | Short axial | Carpal tunnel inlet | Shear Wave velocity |
| 2015 | Ghajarzadeh | SE | 31 | Short axis | Carpal tunnel inlet | Blue Index and Red Index |
| 2015 | Liao | SE | 12 | Short axis | Carpal tunnel inlet (proximal margin of the flexor retinaculum between the scaphoid and pisiform bones) | Standard deviations of the cumulative strain [SDCS] |
| 2014 | Miyamoto | SE | 31 | Short axis | Carpal tunnel inlet (scaphoidpisiform level) | AC/MN (Acoustic Coupler/Median Nerve) strain ratio |
| 2015 | Ogur | SE | 20 | Long axis | 4 different places: proximal carpal tunnel at the level of the pisiform, distal carpal tunnel at the level of the hamate, middle of the carpal tunnel, forearm area one centimeter the proximal carpal tunnel | |
| 2013 | Orman | SE | 41 | Short axis | Scafoid-pisiform level (no measure were taken at the hamate-trapezium level) | Ratio between median nerve and subcutaneous fat |
| 2014 | Yoshii | SE | 15 healthy volunteers | Short axis | Parallel to the wrist crease (proximal carpal tunnel) | Ratio between median nerve and coupler |
| 2015 | Yoshii | SE | 28 | Short axis | Parallel to the wrist crease (proximal carpal tunnel) | Median nerve strain + ratio between median nerve and coupler |
Some studies have reported that the freehand strain ratio measure shows a low diagnostic power [18, 19]. To overcome this limitation complex cyclic compression apparatus have been proposed [18, 19].
Only two articles on median nerve stiffness evaluation involved SWE [11, 12]. Kantarci and colleague measurements were performed at the proximal carpal row level on sagittal ultrasound images. Thus the evaluation was not performed on short axis images but on long axis ones [11]. The results confirmed the increase in stiffness of the median nerve already seen with strain and strain ratios; nerve stiffness was significantly higher in the CTS group (66.7 kPa) when compared to controls (32.0 kPa) (P < 0.001). Since these results are free from operator-dependent compression artifacts and are quantitative, they are more generalizable and can easily be transposed among different patients and subsequent controls.
Also Zhang and colleague used SWE, but they measured the velocity (SWV; m/s) at the carpal tunnel inlet. The median nerve SWV was significantly higher in the carpal tunnel syndrome group (3.857 m/s) than the control group (2.542 m/s; P < 0.05) [12]. A 3.0-m/s SWV cutoff value revealed sensitivity, specificity, positive predictive value, negative predictive value and accuracy of 83.3, 91.3, 93.8, 77.8 and 86.4%, respectively.
This positive aspect of SWE is counterbalanced by the presence of new kinds of artifacts such as the “bone-proximity” artifacts. These artifacts occur when the nerve travels near a bone plane that prevents the shear wave homogeneously propagating at depth, thus causing local stress inhomogeneity [9]. The importance of these artifacts has already been underlined for other small part settings such as for salivary glands, but never for peripheral nerves.
Our data seem to confirm the presence of these artifacts in the carpal tunnel by showing that the healthy median nerve stiffness progressively increases from proximal to distal portions, where the nerve approaches the bone surface. This increase is substantial between the forearm and the wrist (immediately outside the carpal tunnel). In the forearm the nerve is surrounded by muscle bellies while in the wrist it becomes superficial and approaches the bone surfaces of the ulna and radius. The stiffness increases around three-fold (from around 8 to 22 kPa) without any change in cross-sectional area or b-mode appearance. While travelling through the carpal tunnel another 33% (from around 22 to 32 kPa) increase is seen. These results suggest that even a slight change in the bony landscape through which the nerve travels seems to considerably alter the measured stiffness. Since our patients were healthy volunteers and the nerve showed no difference on B-mode or changes in diameter (such as those related to branching), the only factor that could have changed was the “bone-environment” around the nerve.
Measurements of inter-observer agreement were generally good (from fair to moderate). This seems to confirm that these artifacts are not related to the improper use of the technique by an inexperienced operator but are intrinsic to the measurement itself.
Our study suggests two levels of consideration: local and global. The “local consideration” is that when evaluating CTS with SWE the site of measurement must be as standardized as possible. Historically the short-axis approach has been used and for this reason other approaches, such as the long-axis approach, risk altering the geometry of the measure in ways that are difficult to foresee. Therefore, a short-axis approach seems to be the most reasonable one. The measurement needs to be taken in the proximal carpal tunnel, trying to properly image the scaphoid and pisiform to confirm and ensure the correct site of measurement. A shift from the inlet to outlet of the carpal tunnel can induce a 33% difference in the stiffness value (from around 22 to 32 kPa). Incredible care must be taken to place the probe and properly scan the proximal carpal tunnel.
The “global consideration” is that results on peripheral nerves are not generalizable and every nerve, since it has a particular path and relation to the bone surface, needs in depth study. Also for the median nerve, when the CTS is not bilateral, the contralateral nerve gives us important information on the normal stiffness of the median nerve in a specific subject with a specific osseous conformation. Therefore, the importance of measuring the contralateral nerve, in the same position within the carpal tunnel, must not be diminished in favour of universal cut-offs. Since these cut-offs do not take into account the individual peculiarities of anatomy of the patients they can, in some cases, give wrongful advice instead of helping in the diagnosis. Needless to say, when evaluating nerve pathologies, stiffness must not be compared to its proximal or distal portions to avoid “bone-proximity” hardening artifacts.
The presence of a statistically significant difference between men and women nerve stiffness is unexpected and its interpretation is difficult: it may be related to the difference in global dimensions of the carpal tunnel (wider in men) or may be related to an ability of the SWE to catch minimal changes in elasticity (related to hormonal factors?). Since CTS affects mainly female subjects this difference needs further evaluations with dedicated, wider and more balanced, cohort of healthy man and female.
Our study has some limitations. First, the size of the sample should be increased to strengthen the statistical significance of the results. Future research will need to focus on the difference in median nerve values among CTS patients both on the affected and non-affected side. This may help to understand the real impact of artifacts on the measurements.
In conclusion, SWE is a powerful new tool in the evaluation of the median nerve but it is prone to new types of artifacts such as “bone-proximity” artifacts. Since these artifacts seem to have a major impact on the measurements, future research should consider them in order to control and neutralize their influence.
Compliance with ethical standards
Conflict of interest
Fabrizio Calliada is consultant for Hitachi Medical System Europe, Shenzhen Mindray Bio-Medical Electronics Co., Toshiba Medical System Europe. Vito Cantisani is lecturer for Bracco, Samsung and Toshiba Medical System. No conflict of interest to disclose from the other authors.
Ethical standard statements
All human and animal studies have been approved by the appropriate ethics committee and have, therefore, been performed in accordance with the ethical standards laid down in the Helsinki Declaration of 1975 and its late amendments.
Informed consent
Written informed consent was obtained from every subject.
Bibliography
- 1.Cosgrove D, Piscaglia F, Bamber J, Bojunga J, Correas JM, Gilja OH, Klauser AS, Sporea I, Calliada F, Cantisani V, D’Onofrio M, Drakonaki EE, Fink M, Friedrich-Rust M, Fromageau J, Havre RF, Jenssen C, Ohlinger R, Săftoiu A, Schaefer F, Dietrich CF, EFSUMB EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 2: Clinical applications. Ultraschall Med. 2013;34(3):238–253. doi: 10.1055/s-0033-1335375. [DOI] [PubMed] [Google Scholar]
- 2.Cantisani V, Grazhdani H, Drakonaki E, et al. Strain US elastography for the characterization of thyroid nodules: advantages and limitation. Int J Endocrinol. 2015;2015:908575. doi: 10.1155/2015/908575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cantisani V, Lodise P, Di Rocco G, et al. Diagnostic accuracy and interobserver agreement of quasistatic ultrasound elastography in the diagnosis of thyroid nodules. Ultraschall Med. 2015;36(2):162–167. doi: 10.1055/s-0034-1366467. [DOI] [PubMed] [Google Scholar]
- 4.Cantisani V, Grazhdani H, Ricci P, et al. Q-elastosonography of solid thyroid nodules: assessment of diagnostic efficacy and interobserver variability in a large patient cohort. Eur Radiol. 2014;24(1):143–150. doi: 10.1007/s00330-013-2991-y. [DOI] [PubMed] [Google Scholar]
- 5.Botticelli A, Mazzotti E, Di Stefano D, et al. Positive impact of elastography in breast cancer diagnosis: an institutional experience. J Ultrasound. 2015;18(4):321–327. doi: 10.1007/s40477-015-0177-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miyamoto H, Morizaki Y, Kashiyama T, Tanaka S. Grey-scale sonography and sonoelastography for diagnosing carpal tunnel syndrome. World J Radiol. 2016;8(3):281–287. doi: 10.4329/wjr.v8.i3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chammas M, Boretto J, Burmann LM, Ramos RM, Dos Santos Neto FC, Silva JB. Carpal tunnel syndrome—Part I (anatomy, physiology, etiology and diagnosis) Rev Bras Ortop. 2014;49(5):429–436. doi: 10.1016/j.rbo.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brezak R, Hippe D, Thiel J, Dighe MK. Variability in stiffness assessment in a thyroid nodule using shear wave imaging. Ultrasound Q. 2015;31(4):243–249. doi: 10.1097/RUQ.0000000000000205. [DOI] [PubMed] [Google Scholar]
- 9.Bhatia KS, Cho CC, Tong CS, Lee YY, Yuen EH, Ahuja AT. Shear wave elastography of focal salivary gland lesions: preliminary experience in a routine head and neck US clinic. Eur Radiol. 2012;22(5):957–965. doi: 10.1007/s00330-011-2364-3. [DOI] [PubMed] [Google Scholar]
- 10.Fu T, Cao M, Liu F, Zhu J, Ye D, Feng X, Xu Y, Wang G, Bai Y. Carpal tunnel syndrome assessment with ultrasonography: value of inlet-to-outlet median nerve area ratio in patients versus healthy volunteers. PLoS One. 2015;10(1):e0116777. doi: 10.1371/journal.pone.0116777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kantarci F, Ustabasioglu FE, Delil S, Olgun DC, Korkmazer B, Dikici AS, Tutar O, Nalbantoglu M, Uzun N, Mihmanli I. Median nerve stiffness measurement by shear wave elastography: a potential sonographic method in the diagnosis of carpal tunnel syndrome. Eur Radiol. 2014;24(2):434–440. doi: 10.1007/s00330-013-3023-7. [DOI] [PubMed] [Google Scholar]
- 12.Zhang C, Li M, Jiang J, Zhou Q, Xiang L, Huang Y, Ban W, Peng W. Diagnostic value of virtual touch tissue imaging quantification for evaluating median nerve stiffness in carpal tunnel syndrome. J Ultrasound Med. 2017;36(9):1783–1791. doi: 10.1002/jum.14213. [DOI] [PubMed] [Google Scholar]
- 13.Ghajarzadeh M, Dadgostar M, Sarraf P, Emami-Razavi SZ, Miri S, Malek M. Application of ultrasound elastography for determining carpal tunnel syndrome severity. Jpn J Radiol. 2015;33(5):273–278. doi: 10.1007/s11604-015-0416-3. [DOI] [PubMed] [Google Scholar]
- 14.Liao YY, Lee WN, Lee MR, Chen WS, Chiou HJ, Kuo TT, Yeh CK. Carpal tunnel syndrome: US strain imaging for diagnosis. Radiology. 2015;275(1):205–214. doi: 10.1148/radiol.14140017. [DOI] [PubMed] [Google Scholar]
- 15.Ogur T, Yakut ZI, Teber MA, Alp F, Turan A, Tural A, Gelisen O. Ultrasound elastographic evaluation of the median nerve in pregnant women with carpal tunnel syndrome. Eur Rev Med Pharmacol Sci. 2015;19(1):23–30. [PubMed] [Google Scholar]
- 16.Miyamoto H, Halpern EJ, Kastlunger M, Gabl M, Arora R, Bellmann-Weiler R, Feuchtner GM, Jaschke WR, Klauser AS. Carpal tunnel syndrome: diagnosis by means of median nerve elasticity—improved diagnostic accuracy of US with sonoelastography. Radiology. 2014;270(2):481–486. doi: 10.1148/radiol.13122901. [DOI] [PubMed] [Google Scholar]
- 17.Orman G, Ozben S, Huseyinoglu N, Duymus M, Orman KG. Ultrasound elastographic evaluation in the diagnosis of carpal tunnel syndrome: initial findings. Ultrasound Med Biol. 2013;39(7):1184–1189. doi: 10.1016/j.ultrasmedbio.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 18.Yoshii Y, Ishii T, Etou F, Sakai S, Tanaka T, Ochiai N. Reliability of automatic vibratory equipment for ultrasonic strain measurement of the median nerve. Ultrasound Med Biol. 2014;40(10):2352–2357. doi: 10.1016/j.ultrasmedbio.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 19.Yoshii Y, Ishii T, Tanaka T, Tung WL, Sakai S. Detecting median nerve strain changes with cyclic compression apparatus: a comparison of carpal tunnel syndrome patients and healthy controls. Ultrasound Med Biol. 2015;41(3):669–674. doi: 10.1016/j.ultrasmedbio.2014.09.020. [DOI] [PubMed] [Google Scholar]