Abstract
Background
Multicomponent, interdisciplinary intensive primary care programs target complex patients with the goal of preventing hospitalizations, but programs vary, and their effectiveness is not clear. In this study, we systematically reviewed the impact of intensive primary care programs on all-cause mortality, hospitalization, and emergency department use.
Methods
We searched PubMed, CINAHL, the Cochrane Central Register of Controlled Trials, and the Cochrane Database of Reviews of Effects from inception to March 2017. Additional studies were identified from reference lists, hand searching, and consultation with content experts. We included systematic reviews, randomized controlled trials (RCTs), and observational studies of multicomponent, interdisciplinary intensive primary care programs targeting complex patients at high risk of hospitalization or death, with a comparison to usual primary care. Two investigators identified studies and abstracted data using a predefined protocol. Study quality was assessed using the Cochrane risk of bias tool.
Results
A total of 18 studies (379,745 participants) were included. Three major intensive primary care program types were identified: primary care replacement (home-based; three RCTs, one observational study, N = 367,681), primary care replacement (clinic-based; three RCTs, two observational studies, N = 9561), and primary care augmentation, in which an interdisciplinary team was added to existing primary care (five RCTs, three observational studies, N = 2503). Most studies showed no impact of intensive primary care on mortality or emergency department use, and the effectiveness in reducing hospitalizations varied. There were no adverse effects reported.
Discussion
Intensive primary care interventions demonstrated varying effectiveness in reducing hospitalizations, and there was limited evidence that these interventions were associated with changes in mortality. While interventions could be grouped into categories, there was still substantial overlap between intervention approaches. Further work is needed to identify program features that may be associated with improved outcomes.
KEY WORDS: primary care, primary care redesign, care management, home care, comorbidity
INTRODUCTION
A small proportion of patients are responsible for a large majority of health care costs, often through frequent hospitalization.1 These patients are typically older and suffer from multimorbidity2 – 4 and functional limitations.5 , 6 Such patients may require more frequent primary care visits, care from multiple subspecialists, home care services, or assistance from social workers, pharmacists, or physical therapists to optimize the management of their medical conditions and their overall health. Additionally, as they use more services, the risk of care fragmentation increases, as does the burden of coordinating care. In a traditional primary care setting, the capability to coordinate complex care may be lacking, leading to preventable hospitalizations.
An intuitive approach to caring for these vulnerable patients is to broaden the scope, increase the intensity, and improve the coordination of outpatient care, in the hope that this will lead to reduced hospital use and lower costs.7 , 8 Increased access to primary care is associated with decreased rates of hospitalizations for certain conditions,9 , 10 and discontinuity of primary care is associated with increased risk of hospitalization.11 Outpatient care redesign efforts include patient-centered medical homes (PCMH),12 , 13 hospital-based care transition programs,14 , 15 and external case management.16 , 17 However, the effects of such efforts on utilization and clinical outcomes have been inconsistent.18 – 23
An alternative approach is intensive primary care (IPC), also referred to as ambulatory intensive care.7 , 24 In such programs, patients at high risk for hospitalization or death are enrolled in a separate program (that may be within an existing primary care setting) that addresses a spectrum of medical and social needs, and coordinates care across settings. While PCMH and IPC both use interdisciplinary teams, IPC programs differ in that they specifically target populations at high risk of hospitalization, and provide a higher intensity of care and a broader range of services. IPC programs vary substantially in structure, function, and site of care, but the impact of these different approaches on hospitalization or mortality is unknown.
The objective of this work was to classify interdisciplinary, multicomponent IPC programs according to program characteristics,25 and to evaluate the effectiveness of these programs in reducing hospitalizations, emergency department (ED) visits, and mortality among patients at high risk for hospitalization or death.
METHODS
This review was initially requested for the Department of Veterans Affairs Patient Aligned Care Team (PACT) Health Delivery Committee to inform the creation of an IPC program.26
Data Sources and Searches
We searched for systematic reviews, controlled clinical trials, and observational studies in PubMed (1946 through 3/01/2017), CINAHL (1981 through 3/01/2017), the Cochrane Central Register of Controlled Trials (first quarter 2017), and the Cochrane Database of Reviews of Effects (first quarter 2017) using standard search terms for high utilizing complex patients, IPC, and hospital use. Additional citations were identified from reference lists, hand searching, and consultation with content experts. We limited the search to articles involving human subjects and available in the English language.
Study Selection
A description of study selection criteria is presented in Table 1. Two investigators reviewed titles, abstracts, and full text articles. One reviewer had methodological expertise, and the other had clinical content expertise. There was a high level of agreement, and all disagreements were resolved by consensus.
Table 1.
Study Selection Criteria
| Population: | Patients identified as high risk for hospital admission and/or death |
|---|---|
| Intervention: | Multicomponent, interdisciplinary intensive primary care programs (primary care defined as longitudinal, continuous, and whole person-focused care27) |
| Comparator: | Usual care |
| Outcomes: | All-cause mortality, hospitalization, emergency department use, and hospital days |
| Timing: | Studies that include a follow-up period of more than 30 days |
| Setting: | Ambulatory setting |
| Study design: | Systematic reviews, controlled clinical trials, observational studies |
Data Extraction and Quality Assessment
We abstracted data from all included studies on population, intervention, comparator, and timing characteristics and results for each included outcome. All data abstraction and internal validity ratings were first completed by one reviewer and then checked by another. All disagreements were resolved using consensus.
We used predefined criteria to rate the internal validity of all individual studies. Risk of bias in controlled clinical trials was assessed using the Cochrane Collaboration’s tool and was judged as low, unclear, or high.28 We rated the internal validity (quality) of controlled observational studies as good, fair, or poor, using methods developed by the Drug Effectiveness Review Project (DERP) and based on the adequacy of the patient selection process, completeness of follow-up, adequacy of outcome ascertainment, use of acceptable statistical techniques to minimize potential confounding factors, and whether the duration of follow-up was reasonable to capture investigated events. Ratings of low risk of bias and good quality indicate high confidence in study findings. Ratings of unclear risk of bias and fair quality indicate the presence of plausible bias or some flaws that raise some doubt about study findings. Ratings of high risk of bias and poor quality indicate the presence of plausible bias or serious flaws that seriously weaken our confidence in study findings.29 We graded strength of a body of evidence based on the guidance established for the AHRQ Evidence-based Practice Centers program.30 This approach incorporates four key domains: risk of bias (includes study design and aggregate quality), consistency, directness, and precision of the evidence.
Program Classification
We classified programs as primary care replacement (home-based), primary care replacement (clinic-based), or primary care augmentation, and assessed the impact of outcomes separately for each category. We derived our approach primarily from a classification developed by Yee et al.,25 which grouped programs based on similarities in patient selection methods, scope of provided services, and organization of service delivery. As themes emerged during the review, we considered alternative ways to classify programs, but found that these categories were the most effective and consistent with other literature.7
RESULTS
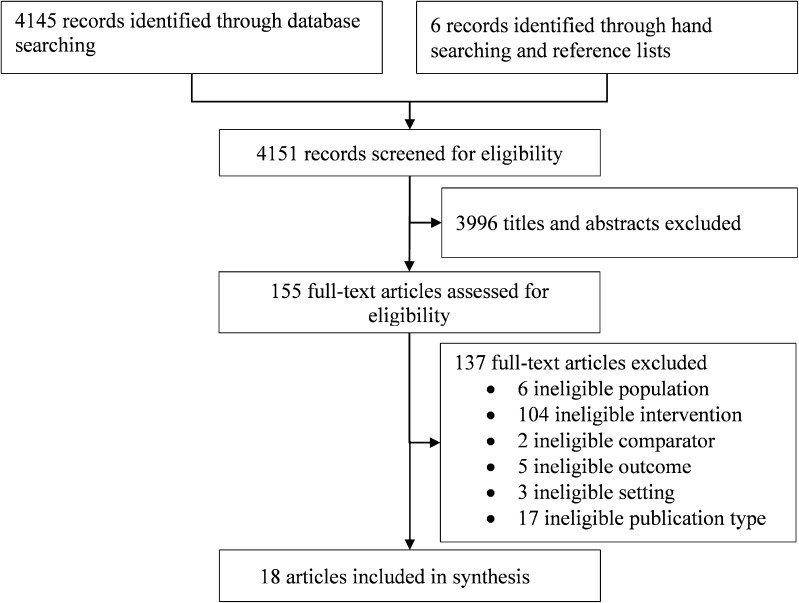
The literature flow diagram (Fig. 1) summarizes the results of the search and study selection processes. Searches resulted in 4151 potentially relevant articles. Of these, we included 11 randomized controlled trials31 – 41 and seven observational studies.42 – 48
Figure 1.
Study identification and selection.
Tables 2 and 3 summarize the study and intervention characteristics. The majority of the studies involved mostly elderly, non-white females, but four studies focused on veterans who were predominantly male.36 , 37 , 45 , 48 Most studies were small (N < 300), and ranged in size from 59 to 3889 participants, with one observational study that included 364,972 participants. Follow-up duration ranged from 6 months to 4 years. Three studies selected patients based on high utilization of inpatient or emergency department services.39 , 44 , 47 The remaining studies selected patients who were at least moderately disabled in activities of daily living 31 , 35 – 38 , 48 , 57; homeless46; aged ≥ 65, 31 , 33 , 34 , 48 , 58 ≥ 67,45 ≥ 70,32 , 35 or ≥ 75 years42; had income below 200% of the federal poverty level34 , 58; and/or who met nursing home eligibility criteria.43 Detailed patient selection criteria are described in Table 2.
Table 2.
Study and Intervention Characteristics (Stratified by Program Type)
| Author, year Study name |
Study design | Setting | Sample size | Follow-up | Patient selection criteria | Usual care comparison | Validity assessment |
|---|---|---|---|---|---|---|---|
| Home-based | |||||||
| Edwards et al., 2017 VA HBPC45 |
OBS | VA, nationwide | 364,972 | 3–4 years | Veterans aged ≥67 with diabetes mellitus, national HBPC enrollment guidelines49 | Not described | Probably valid |
| Hughes et al., 1990 VA HBPC36 |
RCT | Hines VA Hospital, Illinois | 233 | 6 months | Recent hospitalization, severely disabled (≥ 2 ADL impairments) | Customary care, including use of Medicare or other community home care | Not valid |
| Hughes et al., 2000 VA HBPC37 |
RCT | 16 VA medical centers | 1966 | 12 months | Hospitalized within 3 months, 2 or more ADL impairments or a prognosis of terminal illness, or homebound with a primary diagnosis of congestive heart failure (CHF) or chronic obstructive pulmonary disease (COPD) | Access to any VA-sponsored services, except HBPC and non-VA post-acute services | Possibly valid |
| Melin, 199538 | RCT | County general hospital and primary care center, Stockholm, Sweden | 249 | 6 months | Hospitalized, “chronically ill,” dependent in ≥1 Katz ADL50 | Continued treatment in an acute or long-stay hospital, followed by standard district nurse administered care at home | Not valid |
| Vila et al., 201544 | OBS | Hospital de Barcelona, Spain | 261 | 203 days (mean) | Adults with one or more severe, progressive, chronic conditions and limited longevity, admitted to the hospital at least twice in the preceding year for ≥ 25 days | Not described | Not valid |
| Clinic-based | |||||||
| Beland et al., 2006 SIPA31 |
RCT | Public community organizations | 1309 | 19 months | Age ≥ 65 years, “Frail elderly”: moderate disability (< −10 on SMAF score, which includes ADL, IADL, incontinence, mental status, mobility)51 | Usual home care services with limited time/availability and no case management | Possibly valid |
| Boult et al., 2001 GEM32 |
RCT | Ambulatory clinic, community hospital | 568 | 18 months | Medicare beneficiaries, age ≥ 70, with a probability of repeated admission of 0.4 or higher using survey based risk score52 | Usual care with primary care physician | Possibly valid |
| Ekdahl et al., 201541 | RCT | Ambulatory geriatric unit, Sweden | 252 | 24 months | Age ≥ 75, hospitalized ≥3 times in previous year and had ≥3 medical diagnoses | Usual health and social care with primary care centers, outpatient hospital care, and social care | Probably valid |
| Meret-Hanke, 2011 PACE43 |
OBS | Variety of U.S. urban and rural settings | 3889 | 2 years | Age ≥ 55, nursing home-eligible, typically dual Medicare/Medicaid-eligible | Not described | Possibly valid |
| O'Toole et al., 201646
H-PACT |
OBS | National VA H-PACT program | 3543 | 12 months | Homeless veterans | Not described | Not valid |
| Primary care augmentation | |||||||
| Coleman et al., 1999 Chronic Care Clinic33 |
RCT | Ambulatory clinic in a large staff-model HMO | 169 | 24 months | Age ≥ 65, highest risk of hospitalization or functional decline based on risk score53 (included age, gender, recent utilization, chronic disease score based on medication use)54 | Not described | Not valid |
| Counsell et al., 2007 GRACE34 |
RCT | Six community-based health centers, Indianapolis, Indiana | 951 | 24 months | Age ≥ 65, recent primary care visit, income <200% of federal poverty level | Access to all existing primary and specialty care services | Possibly valid |
| Fairhall et al., 2015 FIT35 |
RCT | Community-based intervention, Sydney, Australia | 241 | 12 months | Age ≥ 70, score of ≥3 on Fried frailty criteria,55 life expectancy of >12 months, no severe cognitive impairment | Usual care provided to older residents from community services and their general practitioner | Probably valid |
| Jiwa et al., 200242 | OBS | Single general practitioner practice, UK | 59 | 6 months | Age ≥ 75 years, Clinician judgment of patients at risk of hospital admission based on frailty, multiple diagnoses, polypharmacy, or poor social support | Not described | Not valid |
| Ritchie et al., 2016 GRACE/Care Support47 |
OBS | Academic medical center, UCSF | 148 | ≥ 6 months | ≥ 5 ED visits or ≥2 inpatient hospitalization in past 12 months | Not described | Not valid |
| Schubert et al., 2016 GRACE48 |
OBS | Indianapolis VA Medical Center | 256 | 12 months | Age ≥ 65, life expectancy >6 months, clinician judgment with focus on geriatric syndromes (delirium, cognitive impairment, frailty, multimorbidity, polypharmacy, falls) | Geriatric care clinic | Possibly valid |
| Sledge et al., 2006 PIC39 |
RCT | Primary care center in northeastern U.S. | 96 | 12 months | ≥ 2 Medical or surgical hospital admissions in prior 12 months. Patients with costs >2 SD of log-transformed mean or Charlson Comorbidity Index of ≥5 excluded | Usual care directed by PCP; on-site psychiatric consultation available by PCP referral | Not valid |
| Zulman et al., 2017 ImPACT40 |
RCT | VA Palo Alto | 583 | 16 months | Health care costs or risk for hospitalization in top 5% of facility (calculated using risk score)56 | Not described | Probably valid |
Abbreviations: OBS = observational; SIPA = System of Integrated Care for Older Persons, HBPC = home-based primary care; PACE = Program of All-Inclusive Care for the Elderly; GRACE = Geriatric Resources for Assessment and Care of Elders; PIC = primary intensive care; PCP = primary care physician; FIT = Frailty Intervention Trial; GEM = geriatric evaluation and management; ED = emergency department; H-PACT = Homeless Patient Aligned Care Team; UCSF = University of California San Francisco; ImPACT = Intensive Management Patient Aligned Care Team; SMAF = Systeme de mesure de l'autonomie fonctionelle; RCT = Randomized Controlled Trial; ADL = activities of daily living; IADL = instrumental activities of daily living
*We converted DERP and Cochrane risk of bias (ROB) ratings to validity assessments as follows: ROB “Low” or DERP Good”-➔“Probably valid”; ROB “High” or DERP “Poor”-➔ “Not valid”; ROB “Unclear” or DERP “Fair”➔“Possibly valid”
Table 3.
Intervention Characteristics (Stratified by Program Type)
| Program | Key process elements |
|---|---|
| Home-based | |
| Edwards et al., 201745
Hughes et al., 199036 Hughes et al., 200037 VA HBPC/HBHC |
Hospital-based home care (HBHC) to “heavy care” patients (terminally ill and severely disabled), alternative to nursing home. Management inside and outside of hospital; integrated networks; 24-h contact; salaried physicians who designate a specific percentage of time |
| Melin, 199538 | In-home hospital care as alternative to in-hospital care prompted by bed availability crisis; patients at risk of long-stay care; rehab on a round-the-clock basis |
| Vila et al., 201544 | Home health care; continued proactive monitoring for early detection and treatment of disease exacerbation: direct telephone available 12 h a day; rapid (≤ 6 h) mobilization of intensive hospital home care (IV drug therapy, oxygen therapy, comprehensive assessment within 24 h of discharge) |
| Clinic-based | |
| Beland et al., 2006 SIPA31 |
Full integration: solely responsible for the full range and coordination of community and institutional (acute and long-term) health and social services. Rapid mobilization of resources through 24-h on-call service and intensive home care |
| Boult et al., 2001 GEM32 |
Geriatrician, gerontological nurse practitioner (GNP), nurse, and social worker team-led primary care, including comprehensive assessment, 24-h-a-day on-call services, monthly clinic visits with free transportation, regular telephone calls between visits to monitor and coordinate care |
| Ekdahl et al., 201541
AGe-FIT |
Ambulatory geriatric unit with nurse, geriatrician/resident physician, municipal care manager, occupational therapist, physiotherapist, dietician, and administrative assistant |
| Meret-Hanke, 2011 PACE43 |
Full integration of financing and delivery of acute and long-term care services for nursing home-eligible patients; management of care across all settings; regular clinical monitoring; capitated funding allows more flexibility |
| O'Toole et al., 201646
H-PACT |
Low-threshold access to care with walk-in capacity and clinical outreach to homeless people, integrated primary care and mental health services, intensive health care management, and staff training in homeless care skills |
| Primary care augmentation | |
| Coleman et al., 1999 Chronic Care Clinic33 |
Scheduled half-day multidisciplinary visits every 3–4 months; physician and nurse education |
| Counsell et al., 200734
Ritchie et al., 201647 GRACE |
Integration of the geriatrics team within the primary care environment; in-home assessment and care management provided by a social worker and nurse practitioner team; extensive use of specific care protocols; utilization of an integrated electronic medical record and a Web-based care management tracking tool; and integration with affiliated pharmacy, mental health, home health, community-based, and inpatient geriatric care services; monthly proactive phone follow-ups; dedicated phone line; coordination of care between providers |
| Fairhall et al., 2015 FIT35 |
Case management; targeting domains of frailty—focus on exercise and nutritional supplementation; case conferences; staff experienced in aged care |
| Jiwa et al., 200242 | Proactive outreach (regular visits, phone calls), emphasis on care at home and self-management with support of community teams |
| Sledge et al., 2006 PIC39 |
Comprehensive assessment and follow-up care management with strong mental health component; eligibility based on recent hospitalizations, rather than frailty as in all other studies; minimum follow-up was monthly telephone call and phone/pager availability 5 days a week—more as needed |
| Zulman et al., 2017 ImPACT40 |
Multidisciplinary team (nurse practitioner, physician, social worker, recreational therapist) partnered with patients' medical home, comprehensive patient assessment and tracking, care management of medical and social service needs, frequent contact, and coordination of care with VA and non-VA clinicians |
Among the programs, five were home-based primary care replacement models (referred to as home-based care models), where a dedicated interdisciplinary primary care team delivered all needed services in patients' homes.36 – 38 , 44 Five were clinic-based primary care replacement models (referred to as clinic-based IPC), where patients transferred care from their regular primary care physicians to dedicated clinics that provided only high-intensity care,31 , 32 , 43 , 57 and eight used primary care augmentation, where additional high-intensity services enhanced ongoing care from their regular primary care physician.33 – 35 , 39 , 42 , 58 Because of the clinical heterogeneity in design, patient population, interventions, and outcome assessment methods, we did not pool any data across studies.
Most studies were fair quality/unclear risk of bias or poor quality/high risk of bias. Common methodological limitations among RCTs were lack of blinding, unclear allocation concealment, and unclear methods for addressing incomplete outcome data. Among observational studies, common methodological limitations were lack of unbiased patient selection and lack of control for potential confounding variables. Study results are summarized in Table 4.
Table 4.
Summary of Results (Stratified by Program Type, Intervention vs. Control)
| Author, year Study name |
Mortality rate | Hospital admission/readmission | Hospital days | Emergency department visits |
|---|---|---|---|---|
| Home-based | ||||
| Edwards et al., 2017 VA HBPC45 |
NR | ACSC hospitalization OR = 0.35 (95% CI, 0.30 to 0.42) | NR | NR |
| Hughes et al., 1990 VA HBPC36 |
NR | NR | 13.68 vs. 13.53 (P = NS**) Non-VA hospital days: 2.11 vs. 0.82 (P = 0.10) |
VA ER visits: 0.76 vs. 0.61 (P = NS**) Non-VA ER visits: 0.11 vs. 0.10 (P = NS**) |
| Hughes et al., 2000 VA HBPC37 |
Excluding deaths before discharge: 34.7% vs. 34.1% (P = 0.08) Including deaths before discharge: 38% vs. 37% (P NR) |
Months 1–6: 49% vs. 53% (P = 0.07) Months 1–12: 61% vs. 63% (P = 0.35) |
Months 1–6: 9.3 vs. 9.5 (P = 0.16) Months 1–12: 14.7 vs. 13.3 (P = 0.95) |
NR |
| Melin, 199538 | 26% vs. 27% (P NR) | NR | Survivors (N = 183) Short-term (days): 24 vs. 25 (P = 0.50) Long-term (days): 16 vs. 49 (P < 0.001) Rehab hosp. (days): 2 vs. 3 (P = 0.87) Decedents (N = 66) Short-term (days): 149.9 vs. 179.9 (P = 0.93) Long-term (days): 7.4 vs. 53.6 (P = 0.18) Rehab hosp. (days): 5.4 vs. NR (P NR) |
NR |
| Vila et al., 201544 | 40% vs. 56% (P NR) | 0.19 vs. 0.39 (P = 0.02) | 1 vs. 3.2 (P < 0.001) | 0.3 vs. 0.2 (P = NS**) |
| Clinic-based | ||||
| Beland et al., 2006 SIPA31 |
19 vs. 22% | OR = 0.52 (95% CI, 0.33 to 0.82) | OR = 0.93 (95% CI, 0.71 to 1.18) | OR = 0.92 (95% CI, 0.73 to 1.20) |
| Boult et al., 2001 GEM32 |
9.5% vs. 10.2%, P = 0.88 | NR | NR | NR |
| Ekdahl et al., 201541 | 18.8% vs. 27.0% HR = 1.51 (95% CI, 0.99 to 2.31) |
NR | NR | NR |
| Meret-Hanke, 2011 PACE43 |
Difference (all P < 0.01) 1st 6 months: −0.01 2nd 6 months: −0.02 3rd 6 months: −0.01 4th 6 months: 0.02 |
OR = 0.16 (P = 0.01) | Weighted mean difference: −0.54 (P < 0.01) |
NR |
| O'Toole et al., 2016 H-PACT46 |
NR | Pre-enrollment: 812 hospitalizations Post-enrollment: 503 hospitalizations (34.7% reduction) |
NR | Pre-enrollment: 3022 ED visits Post-enrollment: 2447 ED visits (19.0% reduction) |
| Primary care augmentation | ||||
| Coleman et al., 1999 Chronic Care Clinic33 |
16% vs. 17%; (P = NS)** | 36.5% vs. 34.3% (P = 0.72) | 6.4 vs. 5.4 (P = 0.57) | 0.23 vs. 0.27 (P = 0.73) |
| Counsell et al., 2007 GRACE34 |
7.0% vs. 7.0% (P = 0.64) | Full sample: 700 vs. 740 (P = 0.66) High-risk: Year 1: 705 vs. 798 (P = 0.60) Year 2: 396 vs. 705 (P = 0.03) per 1000 |
Full sample: 700 vs. 740 (P = 0.66) High-risk: Year 1: 705 vs. 798 (P = 0.60) Year 2: 396 vs. 705 (P = 0.03) per 1000 |
Full sample: Year 1: 823 vs. 937 (P = 0.22) Year 2: 643 vs. 841 (P = 0.01) High-risk subgroup Year 1: 1098 vs. 1149 (P = 0.79) Year 2: 848 vs. 1314 (P = 0.03) |
| Fairhall et al., 2015 FIT35 |
NR | Average admissions per participant:1.31 vs. 1.14 (P NR) | 15 vs. 14 days/admission (P NR) | NR |
| Jiwa et al., 200242 | 15% vs. 5% (P = NS**) | 4 vs. 7 (P = NS**) | NR | NR |
| Ritchie et al., 2016 GRACE47 |
NR | Pre-enrollment: median of 5.5 hospitalizations Post-enrollment: median of 0 hospitalizations (P < 0.001) |
NR | Pre-enrollment: median of 5.5 ED visits Post-enrollment: median of 0 ED visits (P = 0.015) |
| Schubert et al., 2016 GRACE48 |
NR | Annual admission: −44.1 (95% CI, −110.7 to 22.5)* 30-day readmissions: −13.1 (95% CI, −36.4 to 10.2)* |
−221.4 (95% CI, −362.7 to −80.1)* | −19.4 (95% CI, −111.2 to 72.4)* |
| Sledge et al., 2006 PIC39 |
6% vs. 10% (P NR) | Mean change: −0.1 vs. −0.3 (P = 0.55) | NR | Mean change: −0.52 vs. −0.6 (P = 0.9) |
| Zulman et al., 2017 ImPACT40 |
12.1% vs. 13.6%, (1.4% difference, 95% CI, −5.7% to 7.3%) | Medicine/surgery ward: 0.06* (P = NS**) | Medicine/surgery ward: −0.73* (P = NS**) | −0.12* (P = NS**) |
*Difference-in-differences; **numerical P-value not reported
Abbreviations: NR = not reported; NS = non-significant; SIPA = System of Integrated Care for Older Persons, HBPC = home-based primary care; PACE = Program of All-Inclusive Care for the Elderly; GRACE = Geriatric Resources for Assessment and Care of Elders; PIC = primary intensive care; FIT = Frailty Intervention Trial; GEM = geriatric evaluation and management; ED = emergency department; H-PACT = Homeless Patient Aligned Care Teams; ImPACT = Intensive Management Patient Aligned Care Team; ACSC = ambulatory care-sensitive conditions
Home-Based Models
There is limited evidence that home-based care models reduce hospital utilization. VA HBPC enrollment was associated with fewer ACSC hospitalizations over 3–4 years in a recent national VA cohort study of 364,972 elderly veterans with diabetes (Table 2).45 VA HBPC enrollment did not reduce hospitalization in two earlier studies, but this may be due to their smaller sample sizes (N = 233 to 1966), shorter follow-up (6–12 months), or changes in HBPC over time.36 , 37 Other home-based programs implemented by single centers in Barcelona and Stockholm had mixed findings regarding hospital utilization, but our confidence in these findings is very low due to their serious limitations.38 , 44 For example, it is possible that the reduced hospital utilization in Hospital de Barcelona’s home care program was exaggerated, because the urban setting characteristic of this home care program is typically associated with higher health care utilization than the rural setting of the control group.59 Similarly, in Stockholm’s in-home hospital care program, the reduction in long-stay hospital utilization at 6 months may have been exaggerated, because participants in the home care group had a higher number of medical diagnoses than the control group (4.5 vs. 3.9; P = 0.003).
Clinic-Based Intensive Primary Care
The SIPA (French acronym for System of Integrated Care for Older Persons)31 , 57 in Montreal and the PACE (Program of All-Inclusive Care for the Elderly) in the U.S.43 were both associated with reduced hospital utilization at 19 to 24 months.31 , 43 , 57 Additionally, PACE demonstrated reduced risk of mortality in the first 18 months compared to usual care, but it increased between months 18 and 24. Both focused on assisting frail elderly individuals with functional limitations. The main methodological limitation of these studies was possible attrition bias. For example, in the PACE study, there were five times as many exclusions from the intervention group due to incomplete data.
Geriatric evaluation and management (GEM), ambulatory geriatric units (AGe-FIT), and Homeless PACT (H-PACT) were generally not associated with significant reductions in mortality or hospital utilization over a period of 12–25 months.32 , 41 , 46 The exception was AGe-FIT, which reduced the mean number of inpatient days over 24 months (11.5 vs. 15.2, p = 0.035) in 382 elderly individuals in Sweden who had been hospitalized at least three times during the past year.41 Among these models, H-PACT is unique in that it focuses on homeless individuals at high risk of hospitalization and offers a separate high-intensity primary care clinic that includes resources such as improved walk-in capacity, co-located mental health, housing, hygiene, clothing, and food vouchers, and intensive case management. While a 6-month pre–post assessment of utilization demonstrated a 19% decrease in ED visits and a 34.7% reduction in hospitalizations, there was no control group, no statistical test of significance, and no attempt to control for pre-existing trend or regression to the mean.43
Primary Care Augmentation
Three studies using the Geriatric Resources for Assessment and Care of Elders (GRACE) model34 , 47 , 48 , 58 demonstrated reductions in hospitalization rates and/or emergency room visits. A GRACE randomized trial was performed among 951 low-income seniors across six community-based health centers in Indianapolis, Indiana.34 , 58 The trial’s main methodological limitation was that it compared groups of physicians who served different patient populations, as evidenced by the significantly lower rate of county medical assistance use in the GRACE intervention group at baseline (83.7% vs. 89.0%; P = 0.02). GRACE’s impact on hospital utilization was limited to a subgroup of 226 patients with the highest risk of baseline hospitalization (Probability of Repeated Admission score of 0.4 or higher), and only in the second year. Also, as baseline characteristics were not provided for the high-risk subgroup, we could not rule out that potential differences in patient characteristics may have mediated the positive effect of the GRACE intervention on outcomes. In a study of GRACE in a VA Medical Center in Marion County, Indiana, the new model was implemented in four clinics, while one clinic that declined to participate served as a control group. Utilization was compared in the 12 months prior to and after implementation, in both GRACE and control groups, using a difference-in-differences approach. There were no significant differences in 30-day readmission or hospital admission, but there was a significant decrease in inpatient days associated with GRACE (−221.4 annual bed days/100 veterans, p = 0.01).47 In a second study, GRACE was adapted to an academic medical center in San Francisco, and named Care Support. Comparison of utilization in the 6 months pre- and post-Care Support enrollment demonstrated a decline in the mean number of ED visits (5.5 to 0, p = 0.015) and hospitalizations (5.5 to 0, p < 0.001). This study included no comparison group, and no method to control for regression to the mean or pre-existing trend.47
None of the other primary care augmentation interventions succeeded in reducing mortality or hospital utilization outcomes. Among the remaining studies, ImPACT is the largest, most recent, and highest-quality randomized controlled trial. In ImPACT, patients were identified as the 5% highest-cost patients or those with the 5% highest risk of hospitalization using a risk prediction algorithm. A multidisciplinary team consisting of a nurse practitioner, physician, social worker, and recreational therapist performed a comprehensive patient assessment, provided care management for medical and social service needs, and coordinated care with VA and non-VA providers. Notably, in ImPACT, both the control group and the intervention group saw reductions in acute and extended care utilization and cost of care, and there was no significant difference between groups.
DISCUSSION
In this review, we identified 18 studies of IPC interventions and categorized them into three major types: home-based programs, clinic-based IPC, and primary care augmentation programs. The programs varied in the way they identified and screened patients for enrollment, though most focused on older adults with functional limitations. Formalized care planning was present in all interventions, many relying upon the comprehensive geriatric assessment. All programs utilized multidisciplinary staff to meet a range of patient needs, and most commonly included physicians, nurses, social workers, physical therapists, mental health providers, and pharmacists. Some clinic-based IPC programs and primary care augmentation programs also included home visits. Overall, we found moderate- to low-strength evidence that some programs (VA-HBPC, PACE, SIPA, and GRACE) were associated with fewer hospital admissions, but no evidence of impact on ED visits or mortality.
While there has been increasing interest in IPC,60 relatively few studies met our inclusion criteria. Notably, many of the studies we did identify were older and were completed before recent changes in health care such as the widespread adoption of electronic health records. Additionally, several studies used weak study designs—for example, pre–post assessment of utilization without a control group. This design is susceptible to both temporal trends, such as decreasing hospitalization rates,61 and regression to the mean. In the ImPACT trial, reductions in utilization and cost were seen in both intervention and control patients, demonstrating the need for study designs that include control groups.40 Additionally, our review lacked some often-cited IPC interventions including the Camden Coalition or the AtlantiCare Special Care Center,62 – 64 as evaluations of these programs do not appear in the peer-reviewed literature. This is likely because programs often do not include evaluations, and among those that do, evaluations are difficult to perform and may take years to complete. Future IPC interventions should include integrated evaluations with rigorous study designs, ideally including randomization.
The current evidence is not adequate to reach a conclusion as to what the right population is for IPC interventions. Patient selection criteria were not well described in many of the studies we reviewed, and no clear patterns of impact on outcomes emerged. Some programs used risk prediction algorithms that included prior diagnoses, laboratory values, and utilization.33 , 40 Others focused on specific subpopulations and selected patients based on older age, an inability to perform activities of daily living, or homelessness. One chose patients based purely on prior health care utilization.47 A potential strategy for new programs would be to identify their specific high-risk patients and assess their needs early in program design, to ensure that provided services fit population needs.
Most of the programs we reviewed used large multidisciplinary teams to deliver a range of specialized services and to coordinate the care of complex patients. However, for complex patients, adding more providers to their care may increase discontinuity with their primary provider and increase the burden of care coordination. Given the negative results of many of these studies, it is possible that attempts to manage complex care using large multidisciplinary teams may be ineffective for some high-needs patients, as the burden of coordination may outweigh the benefits of the specialized skills of each team member. An alternative approach would be to explicitly simplify care to minimize discontinuities and coordination burden by using fewer providers who have a wider scope of practice to care for patients in all settings, such as both in and out of the hospital. In such models, the role of “care coordinator” may be irrelevant.65
Among the studies reviewed, success in reducing hospitalization was achieved in some programs and not others, and success was context-dependent. We had hoped to identify key program features, such as patient selection criteria, that may have contributed to the success or failure of these programs. Unfortunately, reporting of key intervention characteristics was inconsistent, which is a common problem in complex multicomponent intervention studies.66 In addition, the data collected on intervention fidelity, implementation process, and contextual factors at individual intervention sites varied among studies. Future works could use both qualitative and quantitative methods to examine intervention fidelity, implementation, and context.67 – 69 Such data could provide insight into what makes IPC programs work, for whom, and why.70 The VA’s PACT Intensive Management Program has incorporated many of these strategies in their implementation and evaluation of a multisite IPC program within PACT.71 , 72
Our work has several limitations. First, while we attempted to create a precise definition of IPC and differentiate it from other efforts to intensify ambulatory care for high-risk patients (such as care management), we found significant overlap in approaches, both between our identified care models and with other care intensification strategies. Additionally, the lack of a standard taxonomy made this topic difficult to search for. Although we attempted to use an exhaustive list of search terms, our search may have missed some relevant studies. Second, our criteria primarily identified geriatric care models focused on older adults with multiple chronic conditions and functional limitations. Fewer identified programs focused on other at-risk populations, and thus our review may have more limited generalizability. However, this highlights the fact that older adults with multiple chronic conditions and functional limitations are an at-risk population that may be an appropriate target for IPC interventions. Finally, some efforts by accountable care organizations to improve care coordination for complex patients spanned the fields of primary care, specialty care, hospital care, and post-acute care, combining features of the PCMH, transitional care programs, and IPC models.73 The principle underlying these approaches is that in order to achieve the triple aim of improving quality, reducing costs, and improving patient experience, one must go beyond innovations in primary care delivery. However, this review does not address such integrated approaches. IPC programs that had unimpressive results in our review might turn out to be valuable components of a more integrated approach.73
We identified studies representing each of three models of IPC: home-based models, clinic-based IPC, and primary care augmentation. Programs varied in their success in reducing hospitalizations. Future IPC interventions should include rigorous evaluations that include consistent and standardized reporting of intervention characteristics and that collect data on implementation and contextual factors, to further our understanding of what makes such programs successful.
Acknowledgements
We would like to thank the staff of the Evidence-based Synthesis Program Coordinating Center for their assistance.
Funders
Funding was provided by the U.S. Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Quality Enhancement Research Initiative. Dr. Chan is supported in part by grant number K12HS022981 from the Agency for Healthcare Research and Quality.
Conflict of Interest
All authors declare that they have no conflict of interest.
Prior Presentations
None.
References
- 1.Zulman DM, Pal Chee C, Wagner TH, et al. Multimorbidity and healthcare utilisation among high-cost patients in the US Veterans Affairs Health Care System. BMJ Open. 2015;5(4):e007771. doi: 10.1136/bmjopen-2015-007771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolff JL, Starfield B, Anderson G. Prevalence, expenditures, and complications of multiple chronic conditions in the elderly. Arch Intern Med. 2002;162(20):2269–2276. doi: 10.1001/archinte.162.20.2269. [DOI] [PubMed] [Google Scholar]
- 3.Prados-Torres A, Calderón-Larrañaga A, Hancco-Saavedra J, Poblador-Plou B, van den Akker M. Multimorbidity patterns: a systematic review. J Clin Epidemiol. 2014;67(3):254–266. doi: 10.1016/j.jclinepi.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 4.Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380(9836):37–43. doi: 10.1016/S0140-6736(12)60240-2. [DOI] [PubMed] [Google Scholar]
- 5.Greysen SR, Cenzer IS, Auerbach AD, Covinsky KE. Functional impairment and hospital readmission in Medicare seniors. JAMA Intern Med. 2015;175(4):559–565. doi: 10.1001/jamainternmed.2014.7756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koroukian SM, Schiltz N, Warner DF, et al. Combinations of chronic conditions, functional limitations, and geriatric syndromes that predict health outcomes. J Gen Intern Med 2016:1–8. [DOI] [PMC free article] [PubMed]
- 7.Hochman M, Asch SM. Disruptive models in primary care: caring for high-needs, high-cost populations. J Gen Intern Med. 2017;32(4):392–397. doi: 10.1007/s11606-016-3945-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bodenheimer T, Berry-Millett R. Follow the money—controlling expenditures by improving care for patients needing costly services. N Engl J Med. 2009;361(16):1521–1523. doi: 10.1056/NEJMp0907185. [DOI] [PubMed] [Google Scholar]
- 9.Bindman AB, Grumbach K, Osmond D, et al. Preventable hospitalizations and access to health care. JAMA. 1995;274(4):305–311. doi: 10.1001/jama.1995.03530040033037. [DOI] [PubMed] [Google Scholar]
- 10.Agency for Healthcare Research and Quality. Guide to Prevention Quality Indicators: Hospital Admission for Ambulatory Care Sensitive Conditions. Agency for Healthcare Research and Quality, 2006.
- 11.Wasson JH, Sauvigne AE, Mogielnicki RP, et al. Continuity of outpatient medical care in elderly men: a randomized trial. JAMA. 1984;252(17):2413–2417. doi: 10.1001/jama.1984.03350170015011. [DOI] [PubMed] [Google Scholar]
- 12.Stange KC, Nutting PA, Miller WL, et al. Defining and measuring the patient-centered medical home. J Gen Intern Med. 2010;25(6):601–612. doi: 10.1007/s11606-010-1291-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosland A-M, Nelson K, Sun H, et al. The patient-centered medical home in the Veterans Health Administration. Am J Manag Care. 2013;19(7):e263–272. [PubMed] [Google Scholar]
- 14.Coleman EA, Parry C, Chalmers S, Min SJ. The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822–1828. doi: 10.1001/archinte.166.17.1822. [DOI] [PubMed] [Google Scholar]
- 15.Jack BW, Chetty VK, Anthony D, et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178–187. doi: 10.7326/0003-4819-150-3-200902030-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong CS, Siegel AL, Ferris TG. Caring for high-need, high-cost patients: what makes for a successful care management program? Issue Brief (Commonw Fund) 2014;19:1–19. [PubMed] [Google Scholar]
- 17.Hong CS, Abrams MK, Ferris TG. Toward increased adoption of complex care management. N Engl J Med. 2014;371(6):491–493. doi: 10.1056/NEJMp1401755. [DOI] [PubMed] [Google Scholar]
- 18.Bott DM, Kapp MC, Johnson LB, Magno LM. Disease management for chronically ill beneficiaries in traditional medicare. Health Aff (Millwood) 2009;28(1):86–98. doi: 10.1377/hlthaff.28.1.86. [DOI] [PubMed] [Google Scholar]
- 19.Hickam DH, Weiss JW, Guise J-M, et al. Outpatient case management for adults with medical illness and complex care needs. Agency for Healthcare Research and Quality Effective Health Care Program, Comparative Effectiveness Review #99, 2013. [PubMed]
- 20.Rosenthal MB, Friedberg MW, Singer SJ, Eastman D, Li Z, Schneider EC. Effect of a multipayer patient-centered medical home on health care utilization and quality: the Rhode Island chronic care sustainability initiative pilot program. JAMA Intern Med. 2013;173(20):1907–1913. doi: 10.1001/jamainternmed.2013.10063. [DOI] [PubMed] [Google Scholar]
- 21.Fifield J, Forrest DD, Martin-Peele M, et al. A randomized, controlled trial of implementing the patient-centered medical home model in solo and small practices. J Gen Intern Med. 2013;28(6):770–777. doi: 10.1007/s11606-012-2197-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nutting PA, Miller WL, Crabtree BF, Jaen CR, Stewart EE, Stange KC. Initial lessons from the first national demonstration project on practice transformation to a patient-centered medical home. Ann Fam Med. 2009;7(3):254–260. doi: 10.1370/afm.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friedberg MW, Schneider EC, Rosenthal MB, Volpp KG, Werner RM. Association between participation in a multipayer medical home intervention and changes in quality, utilization, and costs of care. JAMA. 2014;311(8):815–825. doi: 10.1001/jama.2014.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milstein A, Gilbertson E. American medical home runs. Health Aff (Millwood) 2009;28(5):1317–1326. doi: 10.1377/hlthaff.28.5.1317. [DOI] [PubMed] [Google Scholar]
- 25.Yee T, Lechner A, Carrier E. High-Intensity Primary Care: Lessons for Physician and Patient Engagement. 2012; October 2012:1–7. http://nihcr.org/analysis/improving-care-delivery/prevention-improving-health/high-intensity-primary-care/. Accessed 21 Jul 2017.
- 26.Peterson K, Heland M, Humphrey L, Christensen V, Carson S. Evidence brief: effectiveness of intensive primary care programs. VA-ESP Project #09–199; 2013. [PubMed]
- 27.Starfield B, Shi L, Macinko J. Contribution of primary care to health systems and health. Milbank Q. 2005;83(3):457–502. doi: 10.1111/j.1468-0009.2005.00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDonagh M, Jonas D, Gartlehner G, et al. Methods for the drug effectiveness review project. BMC Med Res Methodol. 2012;12(1):140. doi: 10.1186/1471-2288-12-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berkman ND, Lohr KN, Ansari M, et al. Grading the strength of a body of evidence when assessing health care interventions for the effective health care program of the agency for healthcare research and quality: an update methods guide for effectiveness and comparative effectiveness reviews. 2013. [PubMed]
- 31.Beland F, Bergman H, Lebel P, et al. A system of integrated care for older persons with disabilities in Canada: results from a randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2006;61(4):367–373. doi: 10.1093/gerona/61.4.367. [DOI] [PubMed] [Google Scholar]
- 32.Boult C, Boult LB, Morishita L, Dowd B, Kane RL, Urdangarin CF. A randomized clinical trial of outpatient geriatric evaluation and management. J Am Geriatr Soc. 2001;49(4):351–359. doi: 10.1046/j.1532-5415.2001.49076.x. [DOI] [PubMed] [Google Scholar]
- 33.Coleman EA, Grothaus LC, Sandhu N, Wagner EH. Chronic care clinics: a randomized controlled trial of a new model of primary care for frail older adults. J Am Geriatr Soc. 1999;47(7):775–783. doi: 10.1111/j.1532-5415.1999.tb03832.x. [DOI] [PubMed] [Google Scholar]
- 34.Counsell SR, Callahan CM, Clark DO, et al. Geriatric care management for low-income seniors: a randomized controlled trial. JAMA. 2007;298(22):2623–2633. doi: 10.1001/jama.298.22.2623. [DOI] [PubMed] [Google Scholar]
- 35.Fairhall N, Sherrington C, Kurrle SE, et al. Economic evaluation of a multifactorial, interdisciplinary intervention versus usual care to reduce frailty in frail older people. J Am Med Dir Assoc. 2015;16(1):41–48. doi: 10.1016/j.jamda.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 36.Hughes SL, Cummings J, Weaver F, Manheim LM, Conrad KJ, Nash K. A randomized trial of Veterans Administration Home Care for Severely Disabled Veterans. Med Care. 1990;28(2):135–145. doi: 10.1097/00005650-199002000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Hughes SL, Weaver FM, Giobbie-Hurder A, et al. Effectiveness of team-managed home-based primary care: a randomized multicenter trial. JAMA. 2000;284(22):2877–2885. doi: 10.1001/jama.284.22.2877. [DOI] [PubMed] [Google Scholar]
- 38.Melin AL. A randomized trial of multidisciplinary in-home care for frail elderly patients awaiting hospital discharge. Aging (Milan, Italy) 1995;7(3):247–250. doi: 10.1007/BF03324326. [DOI] [PubMed] [Google Scholar]
- 39.Sledge WH, Brown KE, Levine JM, et al. A randomized trial of primary intensive care to reduce hospital admissions in patients with high utilization of inpatient services. Dis Manag. 2006;9(6):328–338. doi: 10.1089/dis.2006.9.328. [DOI] [PubMed] [Google Scholar]
- 40.Zulman DM, Pal Chee C, Ezeji-Okoye SC, et al. Effect of an intensive outpatient program to augment primary care for high-need Veterans Affairs Patients: a randomized clinical trial. JAMA Intern Med. 2017;177(2):166–175. doi: 10.1001/jamainternmed.2016.8021. [DOI] [PubMed] [Google Scholar]
- 41.Ekdahl AW, Wirehn AB, Alwin J, et al. Costs and effects of an Ambulatory Geriatric Unit (the AGe-FIT Study): a randomized controlled trial. J Am Med Dir Assoc. 2015;16(6):497–503. doi: 10.1016/j.jamda.2015.01.074. [DOI] [PubMed] [Google Scholar]
- 42.Jiwa M, Gerrish K, Gibson A, Scott H. Preventing avoidable hospital admission of older people. Br J Community Nurs. 2002;7(8):426–431. doi: 10.12968/bjcn.2002.7.8.10650. [DOI] [PubMed] [Google Scholar]
- 43.Meret-Hanke LA. Effects of the program of all-inclusive care for the elderly on hospital use. Gerontologist. 2011;51(6):774–785. doi: 10.1093/geront/gnr040. [DOI] [PubMed] [Google Scholar]
- 44.Vilà A, Villegas E, Cruanyes J, et al. Cost-effectiveness of a Barcelona home care program for individuals with multimorbidity. J Am Geriatr Soc. 2015;63(5):1017–1024. doi: 10.1111/jgs.13396. [DOI] [PubMed] [Google Scholar]
- 45.Edwards ST, Saha S, Prentice JC, Pizer SD. Preventing hospitalization with Veterans affairs home-based primary care: which individuals benefit most? J Am Geriatr Soc. 2017. [DOI] [PMC free article] [PubMed]
- 46.O'Toole TP, Johnson EE, Aiello R, Kane V, Pape L. Tailoring care to vulnerable populations by incorporating social determinants of health: the Veterans Health Administration's "homeless patient aligned care team" program. Prev Chronic Dis. 2016;13:E44. doi: 10.5888/pcd13.150567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ritchie C, Andersen R, Eng J, et al. Implementation of an interdisciplinary, team-based complex care support health care model at an Academic Medical Center: impact on health care utilization and quality of life. PLoS One. 2016;11(2):e0148096. doi: 10.1371/journal.pone.0148096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schubert CC, Myers LJ, Allen K, Counsell SR. Implementing geriatric resources for assessment and care of elders team care in a Veterans Affairs Medical Center: lessons learned and effects observed. J Am Geriatr Soc. 2016;64(7):1503–1509. doi: 10.1111/jgs.14179. [DOI] [PubMed] [Google Scholar]
- 49.Veterans Health Administration, Geriatrics and Extended Care. Home based primary care technical manual. 2009:1–33.
- 50.Katz S, Ford AB, Moskowitz RW. Studies of illness in the Aged. The index of ADL: a standardize measure of biological and psychosocial function. JAMA. 1963;185:914. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 51.Hébert R, Carrier R, Bilodeau A. The functional autonomy measurement system (SMAF [Système de mesure de l’autonomie fonctionnelle]): description and validation of an instrument for the measurements of handicaps. Age Ageing. 1988;17:293–302. doi: 10.1093/ageing/17.5.293. [DOI] [PubMed] [Google Scholar]
- 52.Boult C, Dowd B, McCaffrey D, Boult L, Hernandez R, Krulewitch H. Screening elders for risk of hospital admission. J Am Geriatr Soc. 1993;41(8):811–817. doi: 10.1111/j.1532-5415.1993.tb06175.x. [DOI] [PubMed] [Google Scholar]
- 53.Coleman EA, Wagner EH, Grothaus LC. Predicting hospitalization and functional decline in older health plan enrollees: Are administrative data as accurate as self-report? J Am Geriatr Soc. 1998;46:419–425. doi: 10.1111/j.1532-5415.1998.tb02460.x. [DOI] [PubMed] [Google Scholar]
- 54.VonKorff MV, Wagner EH, Saunders K. A chronic disease from automated pharmacy data. J Clin Epidemiol. 1992;45:197–203. doi: 10.1016/0895-4356(92)90016-G. [DOI] [PubMed] [Google Scholar]
- 55.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol Series A Biol Sci Med Sci. 2001;56A(3):M146–M156. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- 56.Wang L, Porter B, Maynard C, et al. Predicting risk of hospitalization or death among patients receiving primary care in the Veterans Health Administration. Med Care. 2013;51(4):368–373. doi: 10.1097/MLR.0b013e31827da95a. [DOI] [PubMed] [Google Scholar]
- 57.Bergman H, Beland F, Lebel P, et al. Care for Canada's frail elderly population: fragmentation or integration? Can Med Assoc J. 1997;157(8):1116–1121. [PMC free article] [PubMed] [Google Scholar]
- 58.Counsell SR, Callahan CM, Buttar AB, Clark DO, Frank KI. Geriatric Resources for Assessment and Care of Elders (GRACE): a new model of primary care for low-income seniors. J Am Geriatr Soc. 2006;54(7):1136–1141. doi: 10.1111/j.1532-5415.2006.00791.x. [DOI] [PubMed] [Google Scholar]
- 59.Mooney C, Zwanziger J, Phibbs CS, Schmitt S. Is travel distance a barrier to veterans' use of VA hospitals for medical surgical care? Soc Sci Med. 2000;50(12):1743–1755. doi: 10.1016/S0277-9536(99)00414-1. [DOI] [PubMed] [Google Scholar]
- 60.Gawande A. The hot spotters: can we lower medical costs by giving the neediest patients better care? New Yorker (New York, NY : 1925). 2011:40–51. [PubMed]
- 61.MedPAC. March 2012 Report to the Congress: Medicare Payment Policy. 2012:1–443.
- 62.Milstein A, Kothari PP. Are higher value care models replicable? Health Aff Blog 2009.
- 63.Blash L, Chapman S, Dower C. The Special care center - a joint venture to address chronic disease. http://www.iorahealth.com/wp-content/uploads/2014/07/UCSF_The_Special_Care_Center_A_Joint_Venture_to_Address_Chronic_Disease.pdf, 2010. Last accessed 21 Jul 2017.
- 64.Green SR, Singh V, O'Byrne W. Hope for New Jersey's city hospitals: the Camden initiative. Perspect Health Inf Manag. 2010;7(Spring):1d. [PMC free article] [PubMed] [Google Scholar]
- 65.Meltzer DO, Ruhnke GW. Redesigning care for patients at increased hospitalization risk: the comprehensive care physician model. Health Aff (Millwood) 2014;33(5):770–777. doi: 10.1377/hlthaff.2014.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guise J-M, Chang C, Viswanathan M, et al. Agency for Healthcare Research and Quality Evidence-based Practice Center methods for systematically reviewing complex multicomponent health care interventions. J Clin Epidemiol. 2014;67(11):1181–1191. doi: 10.1016/j.jclinepi.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 67.Stange K, Glasgow R. Considering and reporting important contextual factors in research on the patient-centered medical home. Agency Healthc Res Qual. 2013.
- 68.Kahwati L, Jacobs S, Kane H, Lewis M, Viswanathan M, Golin CE. Using qualitative comparative analysis in a systematic review of a complex intervention. Syst Rev. 2016;5(1):1. doi: 10.1186/s13643-016-0256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cohen DJ, Crabtree BF, Etz RS, et al. Fidelity versus flexibility: translating evidence-based research into practice. Am J Prev Med. 2008;35(5):S381–S389. doi: 10.1016/j.amepre.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 70.Pawson R, Tilley N. Realistic Evaluation. London: SAGE; 1997. [Google Scholar]
- 71.Zulman DM, Ezeji-Okoye SC, Shaw JG, et al. Partnered research in healthcare delivery redesign for high-need, high-cost patients: development and feasibility of an Intensive Management Patient-Aligned Care Team (ImPACT) J Gen Intern Med. 2014;29(4):861–869. doi: 10.1007/s11606-014-3022-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Breland JY, Asch SM, Slightam C, Wong A, Zulman DM. Key ingredients for implementing intensive outpatient programs within patient-centered medical homes: a literature review and qualitative analysis. Healthcare. 2016;4(1):22–29. doi: 10.1016/j.hjdsi.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zucco LJ, Urato C, McCall N, et al. Evaluation of the Extended Medicare Care Management for High Cost Beneficiaries (CMHCB) demonstration: Massachusetts General Hospital (MGH). 2013.