Abstract
AIM
To investigate the effect of Hemp seed soft capsule (HSCC) on colonic ion transport and its related mechanisms in constipation rats.
METHODS
Sprague-Dawley male rats were randomly divided into three groups: normal group, constipation group and HSSC group. Rats in the constipation and HSSC groups were administrated loperamide 3 mg/kg per day orally for 12 d to induce the constipation model. Then, the HSSC group was given HSSC 0.126 g/kg per day by gavage for 7 d. The normal and constipation groups were treated with distilled water. After the treatment, the fecal wet weight and water content were measured. The basal short-circuit current (Isc) and resistance were measured by an Ussing Chamber. Besides the in vivo drug delivery experiment above, an in vitro drug application experiment was also conducted. The accumulative concentrations of HSSC (0.1 mg/mL, 0.5 mg/mL, 1.0 mg/mL, 2.5 mg/mL, 5.0 mg/mL, 10.0 mg/mL and 25.0 mg/mL) were added to the normal isolated colonic mucosa and the Isc was recorded. Further, after the application of either ion (Cl- or HCO3-) substitution, ion channel-related inhibitor (N-phenylanthranilic acid, glybenclamide, 4,4-diisothiocyano-2,2-stilbenedisulfonic acid or bumetanide) or neural pathway inhibitor [tetrodotoxin (TTX), atropine, or hexamethonium], the Isc induced by HSSC was also measured.
RESULTS
In the constipation group, the fecal wet weight and the water content were decreased in comparison with the normal group (P < 0.01). After the treatment with HSSC, the fecal wet weight and the water content in the HSSC group were increased, compared with the constipation group (P < 0.01). In the constipation group, the basal Isc was decreased and resistance was increased, in comparison with the normal group (P < 0.01). After the treatment with HSSC, the basal Isc was increased (P < 0.05) and resistance was decreased (P < 0.01) in the HSSC group compared with the constipation group. In the in vitro experiment, beginning with the concentration of 1.0 mg/mL, differences in Isc were found between the experimental mucosa (with HSSC added) and control mucosa. The Isc of experimental mucosa was higher than that of control mucosa under the same concentration (1.0 mg/mL, P < 0.05; 2.5-25 mg/mL, P < 0.01). After the Cl- or HCO3- removal and pretreated with different inhibitors (cAMP-dependent and Ca2+-dependent Cl- channels, Na+-K+-2Cl- cotransporter (NKCC), Na+-HCO3- cotransporter or Cl-/HCO3- exchanger inhibitor), there were differences between experimental mucosa and control mucosa; the Isc of experimental mucosa was lower than that of control mucosa under the same concentration (P < 0.05). Meanwhile, after pretreatment with neural pathway inhibitor (TTX, atropine, or hexamethonium), there were no differences between experimental mucosa and control mucosa under the same concentration (P > 0.05).
CONCLUSION
HSSC ameliorates constipation by increasing colonic secretion, which is mediated via the coaction of cAMP-dependent and Ca2+-dependent Cl- channels, NKCC, Na+-HCO3- cotransporter or Cl-/HCO3- exchanger.
Keywords: Hemp seed soft capsule, Constipation, Ion transport, Cl-, HCO3-
Core tip: In this study, we established a constipation model using the application of loperamide and found that Hemp seed soft capsule could improve the symptom of constipation. Further, it was found that the effect of Hemp seed soft capsule might be achieved by increasing colonic secretion, which is mediated via the combined action of cAMP-dependent and Ca2+-dependent Cl- channels, Na+-K+-2Cl- cotransporter, Na+-HCO3- cotransporter or Cl-/HCO3- exchanger. However, the submucosal neurons seemed to not play a key role in the process.
INTRODUCTION
Constipation is one of the most common gastrointestinal complaints in clinical practice. With the modern changes in living and working environments, constipation maintains a high incidence and greatly influences the life quality of patients. According to an epidemiological survey, the average incidence of constipation in the worldwide general population was 16%[1]. In China, the prevalence rate of constipation was 8.2% in the general population, being higher in the elderly population (18.1%) and pediatric population (18.8%)[2].
As reported, multiple factors (diet, lifestyle, certain medication, organic or functional diseases, etc) and various potential pathogeneses (disturbed intestinal motility, sensory and secretion, microbiota, etc) contribute to the occurrence and development of constipation. A variety of treatment methods have been adopted for constipation, such as lifestyle or dietary modification, secretagogues and prokinetics[3]. However, the current treatment options are limited due to various side effects, such as diarrhea, melanosis coli and so on[4,5].
In recent years, Traditional Chinese Medicine (TCM) has been reported to be effective in the treatment of constipation[6,7], and Hemp seed soft capsule (HSSC) is one of the safe and effective Chinese herbal medicines[8]. However, the treatment mechanism of HSSC is unclear, which restricts its widespread application. Intestinal secretion is known to play an important role in constipation[9]. Besides, Semen Cannabis as the monarch drug of HSSC was reported to possess rich fatty oil and stimulate intestinal mucosa secretion[10]. Hence, in the present study, we aimed to explore whether the regulation of intestinal secretion was a possible mechanism of HSSC in the improvement of constipation.
MATERIALS AND METHODS
Animals
Specific pathogen-free male Sprague-Dawley rats (250 g ± 10 g) were purchased from the Chinese People’s Liberation Army Military Medical Academy Experimental Animal Center. Animals were housed in the Institute of Basic Theory, China Academy of Chinese Medical Sciences. The level II-feeding condition maintains the room temperature at 23 °C-26 °C, the light /dark cycle of 12h/12h and relative humidity from 45% to 60%. All the animals were given free access to food and water.
Reagents and apparatus
Preparation of the Krebs solution involved NaCl 117 mmol/L, KCl 4.7 mmol/L, MgCl2 1.2 mmol/L, NaHCO3 24.8 mmol/L, KH2PO4 1.2 mmol/L, CaCl2 2.56 mmol/L, and glucose 11.1 mmol/L. For preparation of the Krebs solution without Cl-, the NaCl, KCl, MgCl2 and CaCl2 were replaced by sodium gluconate, potassium gluconate, magnesium gluconate and calcium gluconate, respectively. For preparation of the Krebs solution without HCO3-, the NaHCO3 was replaced by NaCl and the solution was buffered with 10 mmol/L HEPES-free acid.
HSSC was acquired from Tianjin Central Pharmaceutical Co., LTD (Tianjin, China). N-Phenylanthranilic acid (DPC; 144509), glybenclamide (G0639), 4,4-diisothiocyano-2,2-stilbenedisulfonic acid (DIDS; D3514), bumetanide (B3023), and tetrodotoxin (TTX; T8024) were obtained from Sigma-Aldrich Co., Ltd (St. Louis, MO, United States). Atropine sulfate monohydrate (A800762) was purchased from Macklin Biochemical Co., Ltd (Shanghai, China). Hexamethonium bromide (H0481) was obtained from TCI Chemicals Co., Ltd (Tokyo, Japan).
Multichannel voltage-current clamp (VCC MC6) was purchased from Physiologic Instruments Corporation (San Diego, CA, United States). The bridge amplifier (ML228) and the recording and analysis system (Power Lab) were purchased from AD Instruments Corporation (New South Wales, Australia).
Animal groups and interventions
Twenty-four rats were randomly divided into three groups: normal group, constipation group and HSSC group. The constipation and HSSC groups were given oral administration of loperamide 3 mg/kg daily for 12 d[11,12]. The control rats were administered with the same volume of distilled water.
After the 12-d loperamide treatment, the rats in the HSSC group were given HSSC by gavage (0.126 g/kg per day). HSSC was water soluble and diluted into aqueous solution. The concentration of the stock solution was 1 g/mL. Whereas, the normal and constipation groups were treated with distilled water (2 mL/100 g per day). The interventions for all the groups were given for 7 consecutive days.
Fecal water content
After the treatment, the rats were placed in metabolic cages, individually. Fecal samples in 24 h were collected, weighed and dried. The weight of the samples before and after drying were made for measuring the fecal water content (%) using the following formula[13,14]: (wet weight - dry weight)/wet weight × 100%.
Tissue preparation
The distal colon (6-7 cm from the anus) was obtained and cut longitudinally along the mesenteric border. Then, the serosal and muscular layers were carefully separated from the mucosal and submucous layers with fine tweezers. The tissue preparations were cut into small sheets, with an area of more than 0.5 cm2.
Isc measurement after application of HSSC
Isc was the main evaluation index for colonic mucosa secretion. Ussing Chamber was the indispensable instrument for measuring Isc, which was conducted after first mounting the tissue preparations in the Ussing Chamber. In the experiment, the Krebs solution was circulated with 95% oxygen and 5% carbon dioxide, the pH was maintained at 7.35-7.45 and temperature was at 37 °C[15,16].
Two experiments were designed to detect the effect of HSSC on colonic mucosa secretion: in vivo drug delivery experiment and in vitro drug application experiment.
The in vivo drug delivery experiment involved intragastric administration of HSSC to constipation rats. Specifically, the tissues were left to incubate for 60 min and the voltage across the tissues was clamped to 0, then the basal Isc was measured (μA/cm2). To measure the transmembrane resistance, 1 mV electrical stimulation was applied and the resistance was calculated according to Ohm’s Law (R = V/I).
The in vitro drug application experiment involved application of HSSC on normal isolated colonic mucosa. The final concentration of HSSC (0.1 mg/mL, 0.5 mg/mL, 1.0 mg/mL, 2.5 mg/mL, 5.0 mg /mL, 10.0 mg/mL and 25 mg/mL) was added into the serosal side for 10 min and the Isc curve was recorded. The Isc at each concentration (%) equaled (Isc maximum peak)/(basal Isc). In the control group, the same volume of normal saline was added as blank control.
Effect of HSSC on Isc after removal of Cl- or HCO3- and pretreated with different inhibitors
When Cl- or HCO3- was removed from the Krebs solution, or the colonic mucosa was pretreated with a nonselective Cl- channel blocker (DPC at 1 mmol/L), a cAMP-dependent Cl- channel blocker (glibenclamide at 1 mmol/L) or a Ca2+-dependent Cl- channel blocker (DIDS at 500 μmol/L) apically, a Na+-K+-2Cl- cotransporter inhibitor (bumetanide at 100 mol/L), a Na+-HCO3- cotransporter or Cl-/HCO3- exchanger inhibitor (DIDS at 200 μmol/L), a neural inhibitor (TTX at 1 μmol/L), a muscarinic receptor inhibitor (atropine at 1 μmol/L ) or a nicotinic receptor antagonist (hexamethonium at 100 μmol/L) basolaterally, then the HSSC (1.0 mg/mL, 2.5 mg/mL, 5.0 mg/mL and 10.0 mg/mL) was added consecutively. The Isc curve was recorded and its concentration (%) was calculated using the method mentioned above. In the Cl- or HCO3- removal experiment, the normal Krebs solution was used as blank control, and in the inhibitor treatment experiment, the same volume of normal saline was used to replace the inhibitors as blank control. The above experiment was repeated 6 times, each using 6 different rats.
Statistical analysis
The statistical analyses were performed by using SPSS 17.0 software (SPSS, Chicago, IL, United States); data are presented as mean ± SEM. All the original data in the study were distributed normally and conformed to homogeneity of variance. Differences among the three groups were analyzed using one-way analysis of variance (commonly known as ANOVA) followed by the least-significant difference test to compare the differences between two groups. Differences between two groups were analyzed using the t-test. P < 0.05 was considered statistically significant.
RESULTS
Effects of HSSC on fecal index in constipation rats
In the constipation group, the fecal wet weight and water content were decreased compared with those in the normal group (P < 0.01). After the treatment with HSSC, the fecal wet weight and the water content were increased in the HSSC group, in comparison with the constipation group (P < 0.01) (Figure 1).
Figure 1.
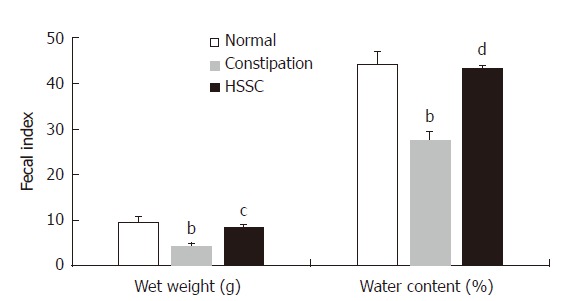
Effect of Hemp seed soft capsule on fecal index in constipation rats. Data are presented as mean ± SEM (n = 8). aP < 0.01 vs normal group; bP < 0.01 vs constipation group.
Effects of HSSC on colonic mucosa secretion
In the constipation group, the basal Isc was decreased and the resistance was increased compared to the normal group (P < 0.01). After treatment with HSSC, the basal Isc was increased (P < 0.05) and the resistance was decreased (P < 0.01) in the HSSC group, in comparison with the constipation group (Figure 2A).
Figure 2.
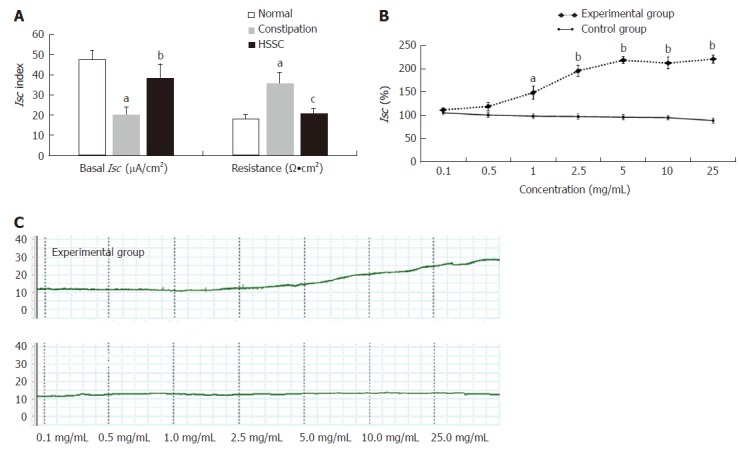
Effect of Hemp seed soft capsule on colonic mucosa secretion. A: In constipation rats (mean ± SEM, n = 8). bP < 0.01 vs normal group; cP < 0.05, dP < 0.01 vs constipation group; B: In normal isolated colonic mucosa (mean ± SEM, n = 6); C: Representative Isc trace in experimental group and control group. aP < 0.05, bP < 0.01 vs control group.
HSSC at cumulative concentrations from 0.1 mg/mL to 25 mg/mL (experimental group), increased Isc dose-dependently, which was not noted in the control group. As shown in Figure 2B, starting from the concentration of 1.0 mg/mL, the difference in Isc was found to be significant between the experimental group and the control group. The Isc in the experimental group was higher than that in the control group at the same concentration (1.0 mg/mL, P < 0.05; 2.5-25 mg/mL, P < 0.01).
Effects of Cl- and HCO3- on the secretagogue role of HSSC
When Cl- or HCO3- was removed from the Krebs solution (-Cl- or -HCO3- group), the Isc induced by the effective concentration of HSSC (1.0 mg/mL, 2.5 mg/mL, 5.0 mg/mL or 10.0 mg/mL) in the -Cl- or -HCO3- group was lower than that in the +Cl- or +HCO3- group (1.0 mg/mL-2.5 mg/mL, P < 0.05; 5.0 mg/mL-10.0 mg/mL, P < 0.01) (Figure 3).
Figure 3.
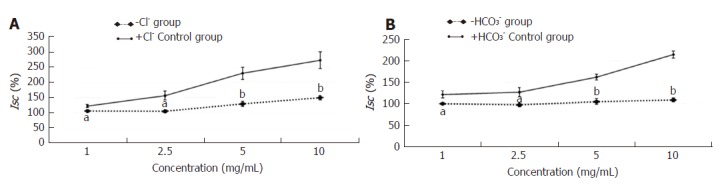
Effect of Cl- and HCO3- on the secretagogue role of Hemp seed soft capsule. Data are presented as mean ± SEM (n = 6). A: When Cl- was removed from the Krebs solution. The effect of Hemp seed soft capsule on colonic mucosa secretion was determined; B: When HCO3- was removed from the Krebs solution. The effect of Hemp seed soft capsule on colonic mucosa secretion was determined. aP < 0.05, bP < 0.01 vs +Cl- group or +HCO3- group.
Effects of Cl- channel on the secretagogue role of HSSC
The experimental mucosa was pretreated apically with the nonselective Cl- channel blocker DPC (+DPC group), cAMP-dependent Cl- channel blockers glibenclamide (+glibenclamide group) or Ca2+-dependent Cl- channel blocker DIDS (+apical DIDS group) for 30 min. The control mucosa was pretreated with the same volume of normal saline (-DPC group, -glibenclamide group, -apical DIDS group, respectively). After that, the effective concentration of HSSC (1.0 mg/mL, 2.5 mg/mL, 5.0 mg/mL or 10.0 mg/mL) was added to solicit the Isc response. As shown in Figure 4, at the concentrations of 1.0 mg/mL, 2.5 mg/mL, 5.0 mg/mL and 10.0 mg/mL, the Isc in the +DPC group was lower than that in the -DPC group (1.0 mg/mL-2.5 mg/mL, P < 0.05; 5.0 mg/mL-10.0 mg/mL, P < 0.01). At the concentration of 2.5 mg/mL, 5.0 mg/mL and 10.0 mg/mL, the Isc in the +glibenclamide group was lower than that in the -glibenclamide group (2.5 mg/mL, P < 0.05; 5.0 mg/mL-10.0 mg/mL, P < 0.01). At the concentration of 5.0 mg/mL and 10.0 mg/mL, the Isc in the +apical DIDS group was lower than that in the -apical DIDS group (5.0 mg/mL, P < 0.05; 10.0 mg/mL, P < 0.01).
Figure 4.
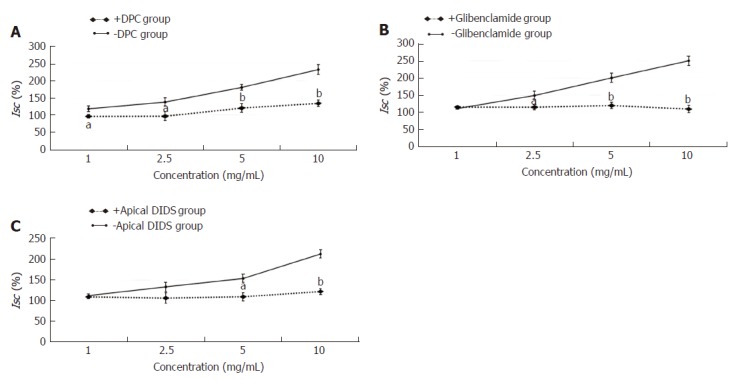
Effect of Cl- channel on the secretagogue role of Hemp seed soft capsule. Data are presented as mean ± SEM (n = 6). A: Pretreated with nonselective Cl- channel blocker DPC; B: Pretreated with cAMP-dependent Cl- channel blocker glibenclamide; C: Pretreated with Ca2+-dependent Cl- channel blocker DIDS. aP < 0.05, bP < 0.01 vs -DPC group, -glibenclamide group or -apical DIDS group.
Effects of Na+-K+-2Cl- cotransporter on the secretagogue role of HSSC
The experimental mucosa was pretreated with the Na+-K+-2Cl- cotransporter inhibitor bumetanide (+bumetanide group); the control mucosa was pretreated with the same volume of normal saline (-bumetanide group). After that, the effective concentration of HSSC (1.0 mg/mL, 2.5 mg/mL, 5.0 mg/mL or 10.0 mg/mL) was added to solicit the Isc response. As shown in Figure 5, at the concentrations of 2.5 mg/mL, 5.0 mg/mL and 10.0 mg/mL, the Isc in the +bumetanide group was lower than that in the -bumetanide group (2.5 mg/mL, P < 0.05; 5.0 mg/mL-10.0 mg/mL, P < 0.01).
Figure 5.
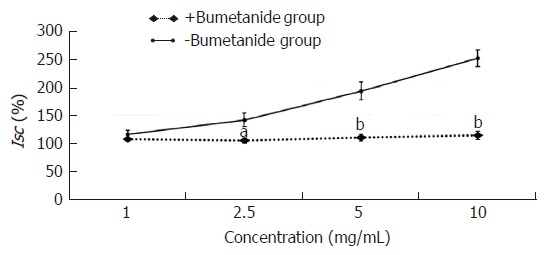
Effect of Na+-K+-2Cl- cotransporter on the secretagogue role of Hemp seed soft capsule. Data are presented as mean ± SEM (n = 6). aP < 0.05, bP < 0.01 vs -bumetanide group.
Effects of Na+-HCO3- cotransporter or Cl-/HCO3- exchanger on the secretagogue role of HSSC
The experimental mucosa was pretreated with the Na+-HCO3- cotransporter or Cl-/HCO3- exchanger inhibitor (+basolateral DIDS group); the control mucosa was pretreated with the same volume of normal saline (-basolateral DIDS group). After that, the effective concentration of HSSC (1.0 mg/mL, 2.5 mg/mL, 5.0 mg/mL or 10.0 mg/mL) was added to solicit the Isc response. As shown in Figure 6, at the concentrations of 5.0 mg/mL and 10.0 mg/mL, the Isc in +bumetanide group was lower than that in the -bumetanide group (5.0 mg/mL, P < 0.05; 10.0 mg/mL, P < 0.01).
Figure 6.

Effect of Na+-HCO3- cotransporter or Cl-/HCO3- exchanger on the secretagogue role of Hemp seed soft capsule. Data are presented as mean ± SEM (n = 6). aP < 0.05, bP < 0.01 vs -basolateral DIDS group.
Effects of neural pathway on the secretagogue role of HSSC
The experimental mucosa was pretreated with the neural inhibitor TTX (+TTX group), muscarinic receptor inhibitor atropine (+atropine group) or nicotinic receptor antagonist hexamethonium (+hexamethonium group); the control mucosa was pretreated with the same volume of normal saline (-TTX group, -atropine group, -hexamethonium group, respectively). After that, the effective concentration of HSSC (1.0 mg/mL, 2.5 mg/mL, 5.0 mg/mL or 10.0 mg/mL) was added to solicit the Isc response. As shown in Figure 7, at each concentration, there was no difference between the experimental group (+TTX group, +atropine group, +hexamethonium group) and the control group (-TTX group, -atropine group, -hexamethonium group) (P > 0.05).
Figure 7.
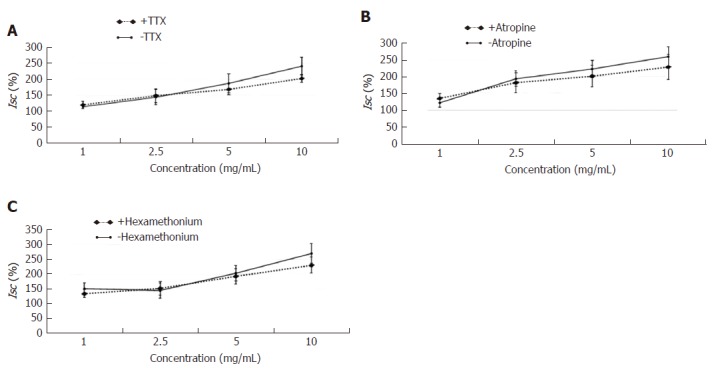
Effects of neural pathway on the secretagogue role of Hemp seed soft capsule. Data are presented as mean ± SEM (n = 6). A: Pretreated with neural inhibitor tetrodotoxin; B: Pretreated with muscarinic receptor inhibitor atropine; C: Pretreated with nicotinic receptor antagonist hexamethonium.
DISCUSSION
In our study, HSSC was found to increase the fecal wet weight and water content in constipation rats; meanwhile, colonic secretion was increased, possibly attributable to the combined action of cAMP-dependent and Ca2+-dependent Cl- channels, NKCC, Na+-HCO3- cotransporter or Cl-/HCO3- exchanger. However, the submucosal neurons, especially the cholinergic neurons, might play little or no role in the secretagogue role of HSSC.
HSSC is developed from the ancient traditional prescription “Hemp seed pills”, which consists of Semen Cannabis, Magnolia officinalis, Fructus Aurantii Immaturus, Radix Paeoniae Alba, Almond and Rheum rhabarbarum. The Hemp seed pill is a representative prescription of TCM in the treatment of constipation, in which Semen Cannabis as the monarch drug plays the role of Runchang Tongbian[17]. A series of clinical trials have confirmed the efficiency and safety of HSSC in the treatment of constipation[18,19]. In our study, we established a rodent model of constipation in which the fecal wet weight and water content were decreased and found that HSSC increased the fecal wet weight and water content; these findings confirmed that the HSSC could improve the stool properties in constipation rats.
As research progresses, the pharmacological properties of HSSC have been gradually revealed and the therapeutic mechanisms of HSSC for constipation have been partly clarified. Animal experimental studies have confirmed that HSSC could obviously increase the intestinal motility, fecal pellets and weight in constipation rats[20,21]. Further, in rabbits, the intestinal contractile frequency and amplitude were found to be increased after intragastric administration of Hemp seed pills. These previous findings suggested that HSSC might play a laxative effect by regulating the intestinal motility and gastrointestinal hormonal secretions, findings which were consistent with the pathophysiologies of constipation.
Constipation is a common clinical complaint caused by multiple physiological changes. In addition to the above-mentioned intestinal motility and gastrointestinal hormones, the imbalance of intestinal water and electrolytes has also been recognized as one of the most important pathophysiologies[22]. However, it hasn’t been reported whether HSSC could regulate the intestinal epithelium electrolyte secretion. In our study, HSSC was found to cause an upward Isc response and to have a role of secretagogue.
It is well known that under normal conditions, the Cl- and HCO3- transport in the colon can generate an osmotic driving force for water. The components of Cl- secretion have been elaborated by Frizzell et al[23]: the apical cAMP-dependent and Ca2+-dependent Cl- channels (CaCC), basolateral NKCC are the main participants for the Cl- secretion; the HCO3- transport is regulated by the Na+-HCO3- cotransporter or Cl-/HCO3- exchanger. In our experiment, it was found that when the Cl- and HCO3- was removed from the normal Krebs solution, the Isc response induced by HSSC was reduced, suggesting that Cl- and HCO3- participated in the ionic regulation of HSSC. Further, the pretreatment of colonic epithelium with the inhibitors of Cl- and HCO3- channels inhibited the Isc response induced by HSSC, suggesting that HSSC regulated the anionic transport through the cAMP-dependent and Ca2+-dependent Cl- channels, NKCC, Na+-HCO3- cotransporter or Cl-/HCO3- exchanger.
As reported, anion secretion can be regulated by neural and non-neural pathways[24]. In the neural pathway, acetylcholine is one of the most important neurotransmitters that mediates intestinal secretion[25]. To determine the role of submucosal neurons, especially the cholinergic neurons, on secretagogue effect of HSSC, the neural inhibitor TTX, muscarinic receptor inhibitor atropine or nicotinic receptor antagonist hexamethonium was used to pretreat the mucosa/submucosa preparation; we found that there was no difference between experimental mucosa and control mucosa, which implied that the secretagogue effect of HSSC seemed not to be dependent on the submucosal nervous system.
In conclusion, the effect of HSSC on constipation was achieved by regulating the colonic secretion, which was mediated via cAMP-dependent and Ca2+-dependent Cl- channels, NKCC, Na+-HCO3- cotransporter or Cl-/HCO3- exchanger.
ARTICLE HIGHLIGHTS
Research background
Constipation is one of the most common gastrointestinal disorders, which maintains a high incidence rate and greatly influences the life quality of patients. As reported, multiple factors and potential pathogenesis contribute to the occurrence and development of constipation. And, a variety of treatment methods have been adopted for constipation, including Traditional Chinese Medicine, in which Hemp seed soft capsule is one of the safe and effective Chinese patent medicines. However, the treatment mechanisms of Hemp seed soft capsule have been unclear, which restricts its popularization and application. Hence, the exploration of possible mechanisms about Hemp seed soft capsule for treating constipation is necessary.
Research motivation
This study aimed to investigate the effect of Hemp seed soft capsule on colonic ion transport and its related mechanisms in constipation rats, the findings of which will have an important significance for clarifying the pharmacological effect of Traditional Chinese Medicine.
Research objectives
The main objective of the study was to explore whether the regulation of the intestinal secretion was the possible mechanism of Hemp seed soft capsule for treating constipation. This research provided a new thought for us to study Hemp seed soft capsule, and more molecules related to the colonic secretion could be discussed in the future.
Research methods
The constipation rats were induced by oral administration of loperamide (3 mg/kg per day for 12 d). The Hemp seed soft capsule group was given Hemp seed soft capsule by gavage (0.126 g/kg per day for 7 d). The normal and constipation groups were treated with the same volume of distilled water. After treatment, the fecal wet weight and water content were measured. The basal short-circuit current (Isc) and resistance were acquired by an Ussing Chamber. Further, after the ion substitution or inhibitor application, the Isc induced by Hemp seed soft capsule was also measured.
The statistical analyses were performed by using SPSS 17.0 software and the differences among the three groups were analyzed using one-way analysis of variance followed by least-significant difference test to compare the differences between two groups. The differences among the two groups were analyzed using t-test. P < 0.05 was considered statistically significant.
Research results
In this study, it was found that Hemp seed soft capsule could increase the fecal wet weight and water content in constipation rats. Meanwhile, Hemp seed soft capsule could increase colonic secretion, and after the application of cAMP- dependent or Ca2+- dependent Cl- channels inhibitor, NKCC inhibitor, Na+-HCO3- cotransporter inhibitor or Cl-/HCO3- exchanger inhibitor, the colonic secretion induced by HSSC were decreased in experimental group than that in the control group, which demonstrated that cAMP- dependent and Ca2+- dependent Cl- channels, Na+-K+-2Cl- cotransporter (NKCC), Na+-HCO3- cotransporter or Cl-/HCO3- exchanger may participate in the HSSC treatment. Meanwhile, after pretreatment with a neural pathway inhibitor (tetrodotoxin, atropine or hexamethonium), there were no differences between experimental mucosa and control mucosa, which implied that the secretagogue effect of HSSC was not dependent on the submucosal nervous system.
Though some results were obtained, the effect of Hemp seed soft capsule on real-time ion current and expression of ion channel protein should be explored in further studies, which will also be important for exploring the specific targets.
Research conclusions
The secretagogue effect of Hemp seed soft capsule for constipation may be achieved via the combined action of cAMP-dependent and Ca2+-dependent Cl- channels, NKCC, Na+-HCO3- cotransporter or Cl-/HCO3- exchanger. However, the submucosal neurons seem to not play a key role in the process. Decreased colonic secretion is found in constipation rats and Hemp seed soft capsule can reverse it. Besides, Cl- and HCO3- participate in the regulative process. The effect of Hemp seed soft capsule for constipation can be achieved by increasing colonic secretion, which is related with the coaction of cAMP-dependent and Ca2+-dependent Cl- channels, NKCC, Na+-HCO3- cotransporter or Cl-/HCO3- exchanger. The discoveries in this study imply that Cl-, HCO3- and related channels/cotransporters/exchangers participate in the effect of HSSC on constipation.
The new hypotheses proposed in this study is that regulation of the intestinal secretion was the possible mechanism of Hemp seed soft capsule for treating constipation. Ussing Chamber is the acknowledged method, although it is new, to be used in the discovery of the Hemp seed soft capsule therapeutic mechanism. Hemp seed soft capsule can increase the fecal wet weight and water content in constipation rats, while the colonic secretion is increased. Besides, cAMP-dependent and Ca2+-dependent Cl- channels, NKCC, Na+-HCO3- cotransporter and Cl-/HCO3- exchanger are involved in the effect of Hemp seed soft capsule on colonic secretion. The effect of Hemp seed soft capsule on constipation can be achieved by regulating the colonic secretion, which is related with cAMP-dependent and Ca2+-dependent Cl- channels, NKCC, Na+-HCO3- cotransporter or Cl-/HCO3- exchanger. Hemp seed soft capsule may increase the fecal water content in constipation patients and can be used as an effective laxative in clinical practice.
Research perspectives
In the study, we found Hemp seed soft capsule could relieve the symptom of constipation and increase the colonic secretion, which implied that the effect of Hemp seed soft capsule on constipation may be achieved by regulating colonic secretion. Further, it was found that Cl- and HCO3- participated in the process, mediated via the related channels/cotransporters/exchangers. But, several questions remain unanswered, all of which can be discussed in the future (e.g., what specific pathways, receptors or molecules may be involved in the effect of Hemp seed soft capsule on colonic secretion, such as the 5-HT/5-HTR pathway or the dopamine pathway, or what structural and functional changes can be found in the ion transport protein on the effect of Hemp seed soft capsule, as the expression of cystic fibrosis transmembrane conductance regulator (CFTR, a cAMP- dependent Cl- channel) protein).
In further studies, we will focus on the effect of Hemp seed soft capsule on Cl- current and CFTR protein in constipation rats. The methods of patch-clamp and short-circuit current will be used to measure the Cl- current. Immunofluorescence will be used to investigate the location and expression of the CFTR protein. Finally, western blot and PCR will be conducted to evaluate the expression of CFTR protein and phosphorylated CFTR protein.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study was reviewed and approved by the Beijing Hospital of Traditional Chinese Medicine Affiliated to Capital Medical University Review Board.
Institutional animal care and use committee statement: All procedures involving animals were reviewed and approved by the Institute of Basic Theory, China Academy of Chinese Medical Sciences Ethics Committee (No. 20160905).
Conflict-of-interest statement: None of the authors declared any conflicts of interest related to this study.
Data sharing statement: No additional data were available.
Peer-review started: July 20, 217
First decision: August 21, 2017
Article in press: September 19, 2017
P- Reviewer: Akiba Y, Liu S S- Editor: Qi Y L- Editor: Filipodia E- Editor: Huang Y
Contributor Information
Xiao-Fang Lu, Digestive Disease Center, Beijing Hospital of Traditional Chinese Medicine Affiliated to Capital Medical University, Beijing 100010, China. zhss2000@163.com.
Meng-Di Jia, Digestive Disease Center, Beijing Hospital of Traditional Chinese Medicine Affiliated to Capital Medical University, Beijing 100010, China.
Sheng-Sheng Zhang, Digestive Disease Center, Beijing Hospital of Traditional Chinese Medicine Affiliated to Capital Medical University, Beijing 100010, China.
Lu-Qing Zhao, Digestive Disease Center, Beijing Hospital of Traditional Chinese Medicine Affiliated to Capital Medical University, Beijing 100010, China.
References
- 1.Mugie SM, Benninga MA, Di Lorenzo C. Epidemiology of constipation in children and adults: a systematic review. Best Pract Res Clin Gastroenterol. 2011;25:3–18. doi: 10.1016/j.bpg.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 2.Chu H, Zhong L, Li H, Zhang X, Zhang J, Hou X. Epidemiology characteristics of constipation for general population, pediatric population, and elderly population in china. Gastroenterol Res Pract. 2014;2014:532734. doi: 10.1155/2014/532734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bardsley A. Assessment and treatment options for patients with constipation. Br J Nurs. 2017;26:312–318. doi: 10.12968/bjon.2017.26.6.312. [DOI] [PubMed] [Google Scholar]
- 4.Kakino M, Tazawa S, Maruyama H, Tsuruma K, Araki Y, Shimazawa M, Hara H. Laxative effects of agarwood on low-fiber diet-induced constipation in rats. BMC Complement Altern Med. 2010;10:68. doi: 10.1186/1472-6882-10-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siegers CP, von Hertzberg-Lottin E, Otte M, Schneider B. Anthranoid laxative abuse--a risk for colorectal cancer? Gut. 1993;34:1099–1101. doi: 10.1136/gut.34.8.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng CW, Bian ZX, Wu TX. Systematic review of Chinese herbal medicine for functional constipation. World J Gastroenterol. 2009;15:4886–4895. doi: 10.3748/wjg.15.4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng CW, Bian ZX, Zhu LX, Wu JC, Sung JJ. Efficacy of a Chinese herbal proprietary medicine (Hemp Seed Pill) for functional constipation. Am J Gastroenterol. 2011;106:120–129. doi: 10.1038/ajg.2010.305. [DOI] [PubMed] [Google Scholar]
- 8.Li DD, Xie ZN, Li YF, Cai T, Wang SY. Effect and safety of Hemp seed soft capsule on constipation. Zhongcaoyao. 2013;44:1645–1647. [Google Scholar]
- 9.Harada Y, Iizuka S, Saegusa Y, Mogami S, Fujitsuka N, Hattori T. Mashiningan Improves Opioid-Induced Constipation in Rats by Activating Cystic Fibrosis Transmembrane Conductance Regulator Chloride Channel. J Pharmacol Exp Ther. 2017;362:78–84. doi: 10.1124/jpet.117.240630. [DOI] [PubMed] [Google Scholar]
- 10.He HB, Shi MJ. Research progress of Hemp seed soft capsule about Chemical composition and pharmacological activity. Zhongcaoyao. 2010;9:1575–1577. [Google Scholar]
- 11.Choi JS, Kim JW, Cho HR, Kim KY, Lee JK, Sohn JH, Ku SK. Laxative effects of fermented rice extract in rats with loperamide-induced constipation. Exp Ther Med. 2014;8:1847–1854. doi: 10.3892/etm.2014.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim JE, Lee YJ, Kwak MH, Jun G, Koh EK, Song SH, Seong JE, Kim JW, Kim KB, Kim S, et al. Metabolomics approach to serum biomarker for loperamide-induced constipation in SD rats. Lab Anim Res. 2014;30:35–43. doi: 10.5625/lar.2014.30.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang S, Jiao T, Chen Y, Gao N, Zhang L, Jiang M. Methylglyoxal induces systemic symptoms of irritable bowel syndrome. PLoS One. 2014;9:e105307. doi: 10.1371/journal.pone.0105307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han SH, Park K, Kim EY, Ahn SH, Lee HS, Suh HJ. Cactus (Opuntia humifusa) water extract ameliorates loperamide-induced constipation in rats. BMC Complement Altern Med. 2017;17:49. doi: 10.1186/s12906-016-1552-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ning Y, Zhu JX, Chan HC. Regulation of ion transport by 5-hydroxytryptamine in rat colon. Clin Exp Pharmacol Physiol. 2004;31:424–428. doi: 10.1111/j.1440-1681.2004.04015.x. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Li LS, Zhang XL, Zhang Y, Xu JD, Zhu JX. An enhanced cAMP pathway is responsible for the colonic hyper-secretory response to 5-HT in acute stress rats. Physiol Res. 2015;64:387–396. doi: 10.33549/physiolres.932863. [DOI] [PubMed] [Google Scholar]
- 17.Deng ZJ. Formulas of Chinese Medicine, Chinese Press of Traditional Chinese Medicine, Beijing, China, 2003 [Google Scholar]
- 18.Li DB, Xie ZN, LI YF, Cai T, Wang SY. Effect and safety of Hemp seed soft capsule on constipation. Zhongcaoyao. 2013;44:1645–1647. [Google Scholar]
- 19.Tu JM, Ding J, Luo SW, Deng MY, Luo SY. Clinical observation of Hemp seed soft capsule on functional constipation in the elderly. Zhongguo Putong Waike Zazhi. 2011;9:1251–1260. [Google Scholar]
- 20.Zeng Q, Song L, Yang R. Effect of Shutong Capsule and Maren Soft Gelatin Capsule in Promoting Enterokinesia on Large Intestine of Costive Rats. Zhonghua Zhongyiyao Zazhi 2102; 53: 510-512 [Google Scholar]
- 21.Peng ZH, Chen LF, Jian LH, Xiao MY, Yang Y. Effect of Maren Capsule on Defecating Function of Small Rat With Coprostasis Type of Dry Stagnation in Stomach and Intestines. Zhongyiyao Xuebao. 2005;11:73–74. [Google Scholar]
- 22.Sharma A, Rao S. Constipation: Pathophysiology and Current Therapeutic Approaches. Handb Exp Pharmacol. 2017;239:59–74. doi: 10.1007/164_2016_111. [DOI] [PubMed] [Google Scholar]
- 23.Frizzell RA, Hanrahan JW. Physiology of epithelial chloride and fluid secretion. Cold Spring Harb Perspect Med. 2012;2:a009563. doi: 10.1101/cshperspect.a009563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cooke HJ. “Enteric Tears”: Chloride Secretion and Its Neural Regulation. News Physiol Sci. 1998;13:269–274. [PubMed] [Google Scholar]
- 25.Bader S, Diener M. Novel aspects of cholinergic regulation of colonic ion transport. Pharmacol Res Perspect. 2015;3:e00139. doi: 10.1002/prp2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]


