Abstract
AIM
To establish a simplified, reproducible D-galactosamine-induced cynomolgus monkey model of acute liver failure having an appropriate treatment window.
METHODS
Sixteen cynomolgus monkeys were randomly divided into four groups (A, B, C and D) after intracranial pressure (ICP) sensor implantation. D-galactosamine at 0.3, 0.25, 0.20 + 0.05 (24 h interval), and 0.20 g/kg body weight, respectively, was injected via the small saphenous vein. Vital signs, ICP, biochemical indices, and inflammatory factors were recorded at 0, 12, 24, 36, 48, 72, 96, and 120 h after D-galactosamine administration. Progression of clinical manifestations, survival times, and results of H&E staining, TUNEL, and Masson staining were recorded.
RESULTS
Cynomolgus monkeys developed different degrees of debilitation, loss of appetite, and jaundice after D-galactosamine administration. Survival times of groups A, B, and C were 56 ± 8.7 h, 95 ± 5.5 h, and 99 ± 2.2 h, respectively, and in group D all monkeys survived the 144-h observation period except for one, which died at 136 h. Blood levels of ALT, AST, CK, LDH, TBiL, Cr, BUN, and ammonia, prothrombin time, ICP, endotoxin, and inflammatory markers [(tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6)] significantly increased compared with baseline values in different groups (P < 0.05). Pathological results showed obvious liver cell necrosis that was positively correlated with the dose of D-galactosamine.
CONCLUSION
We successfully established a simplified, reproducible D-galactosamine-induced cynomolgus monkey model of acute liver failure, and the single or divided dosage of 0.25 g/kg is optimal for creating this model.
Keywords: Cynomolgus monkey, D-galactosamine, Acute liver failure, Artificial liver support systems, Intracranial pressure
Core tip: This is an article about a novel D-galactosamine-induced cynomolgus monkey model of acute liver failure (ALF). In this study, we used small saphenous vein puncture instead of jugular vein intubation for different doses of D-gal administration, which not only effectively avoided the trauma caused by intubation, but also significantly reduced the anesthesia time and greatly improved the convenience of operation. This study concluded that a simplified, reproducible D-gal-induced large-animal ALF model with an appropriate treatment window had been established successfully, which is suitable for assessing the safety and efficacy of artificial liver support systems, studying the pathogenesis of ALF, and developing new drugs.
INTRODUCTION
Acute liver failure (ALF) results from various causes and is a serious threat to human health[1-3]. Therefore, the establishment of an ALF animal model is of great significance for studying its pathogenesis, developing new drugs, and determining the comprehensive treatment of ALF[4]. In recent years, artificial liver technology has become a topic of great interest to researchers in the ALF field[5-8]. Artificial liver support systems (ALSS) can significantly improve the clinical manifestations and prolong the survival time of patients with liver failure or those awaiting liver transplantation[9]. The safety and efficacy of ALSS must be verified before clinical application because they contain biological substances, such as liver cells; at this point, an ideal animal model of ALF would be an indispensable verification platform[10,11]. Therefore, it is necessary to establish a simplified and reproducible animal model of ALF with an appropriate treatment window.
There are current literature reports of many drugs that have been used to induce animal models of ALF[5,12-14]. D-galactosamine (D-gal) is a disruptor of uridine triphosphate of hepatocytes, causing diffuse hepatic necrosis and an inflammatory response, similar to the pathological changes of clinical viral hepatitis[15,16]. Compared with other drugs, D-gal has many advantages, including better reproducibility and easier dosage control; it is generally accepted as the ideal drug to induce ALF.
At present, large animals used to establish liver failure models are mainly pigs and dogs[15,17,18], but their physiological and biochemical characteristics are dissimilar to those of humans, and results are relatively poor for guiding clinical treatment. As for the methods of drug administration, the main method used is intubation through the jugular vein[8], which is complex and increases the trauma to experimental animals.
In this study, we used different doses of D-gal administered through the small saphenous vein of cynomolgus monkeys, and then observed the clinical manifestations, survival times, changes in biochemical indices, intracranial pressure (ICP) changes, and resulting pathological and histological characteristics, in order to establish a simplified, reproducible D-gal-induced large-animal ALF model with an appropriate treatment window, suitable for assessing the safety and efficacy of ALSS, studying the pathogenesis of ALF, and developing new drugs.
MATERIALS AND METHODS
Animals
Sixteen 6-9-year-old male cynomolgus monkeys, weighing 9.4-11 kg, were purchased from Guangdong Landao Biological Technology Co. Ltd. (Guangzhou, Guangdong Province, China; Certificate of Conformity SCXK [Guangdong] 2014-0010) (Table 1). The experimental protocol was reviewed and approved by the Institutional Review Board of Zhujiang Hospital, Southern Medical University, China (No. ZJYY-2014-GDEK-003).
Table 1.
The general condition of cynomolgus monkeys before drug administration
| No. | Age (yr) | Weight (kg) | Sexual (F/M) | Dose (g/kg) | BP (mmHg) | T (°C) | Amm (µmol/L) | PT (s) |
| 1 | 6 | 9.5 | M | 0.20 | 110/68 | 37.3 | 37 | 10.3 |
| 2 | 9 | 10.2 | M | 0.25 | 101/76 | 37.0 | 43 | 10.7 |
| 3 | 7.5 | 9.4 | M | 0.20 | 108/79 | 36.3 | 41 | 11.3 |
| 4 | 8 | 9.8 | M | 0.30 | 100/58 | 36.4 | 37 | 10.5 |
| 5 | 6.5 | 9.4 | M | 0.30 | 121/54 | 36.5 | 41 | 10.3 |
| 6 | 8.5 | 11 | M | 0.250 | 104/70 | 37.9 | 41 | 11.7 |
| 7 | 6.5 | 9.7 | M | 0.20 + 0.05 | 123/67 | 36.6 | 49 | 10.2 |
| 8 | 7 | 9.6 | M | 0.25 | 123/74 | 36.9 | 46 | 10.6 |
| 9 | 8.5 | 10.4 | M | 0.30 | 103/56 | 36.6 | 45 | 9.5 |
| 10 | 9 | 10.6 | M | 0.20 | 102/79 | 37.4 | 39 | 9.6 |
| 11 | 8.5 | 10.3 | M | 0.25 | 116/64 | 36.6 | 34 | 10 |
| 12 | 9 | 10.7 | M | 0.20 + 0.05 | 113/77 | 36.7 | 29 | 9.9 |
| 13 | 7.8 | 10.3 | M | 0.30 | 105/73 | 37.4 | 58 | 10.2 |
| 14 | 6.5 | 11 | M | 0.20 + 0.05 | 118/67 | 36.6 | 32 | 9.8 |
| 15 | 7.5 | 9.5 | M | 0.20 | 112/69 | 37.3 | 45 | 9.8 |
| 16 | 8.5 | 10.1 | M | 0.20 + 0.05 | 113/63 | 36.8 | 31 | 9.9 |
F: Female; M: Male; BP: Blood pressure; T: Body temperature; Amm: Ammonia; PT: Prothrombin time.
Experimental drugs and preparation
D-gal, purchased from Sigma-Aldrich (United States), was dissolved in 5% glucose solution to a concentration of 1.0 g/10 mL, with pH adjusted to 6.8 using 1.0 mol/L NaOH solution. Then, the solution was sterilized by filtration through a membrane with a pore diameter of 0.22 µm and administrated within 2 h after preparation.
Experimental groups
The study design is presented in Figure 1. The 16 monkeys were randomly divided into four groups after an ICP sensor was implanted and then were given different doses of D-gal according to the results of our previous study[19]. The study groups and dosages given were as follows: group A (n = 4), 0.30 g/kg D-gal; group B (n = 4), 0.25 g/kg D-gal; group C (n = 4), 0.20 g/kg D-gal plus 0.05 g/kg D-gal after 24 h; group D (n = 4), 0.20 g/kg D-gal.
Figure 1.
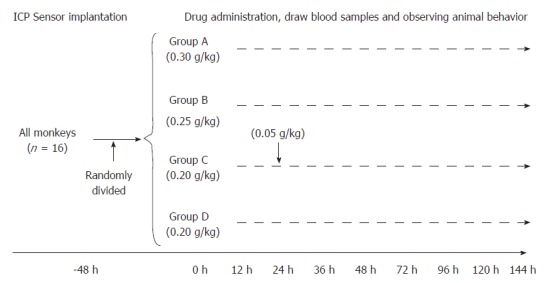
Study design. All monkeys were randomly divided into four groups after ICP sensor implantation; the interval of D-gal administration to group C was 24 h. ICP: Intracranial pressure.
Anesthesia and preparation
Basic anesthesia was induced by intramuscular injection of Zoletil (Virbac Laboratory, Carros, France) (15 mg/kg) and atropine (0.5 mg/kg). The experimental monkey was placed on an operating table with a hot blanket after basic anesthesia. After peroral endotracheal intubation, spontaneous breathing was maintained by continuous inhalation of isoflurane (1%-2%) and O2 (2 L/min) during implantation of the ICP sensor. Animals were placed on the operating table in the prone position; the limbs and head were fixed in place after anesthesia. Skin preparation of the head (for ICP sensor implantation), arms (for collecting blood samples), and hind legs (for drug administration) was performed by using an electric shaver and cleansing with soap and water.
ICP sensor implantation
The detail surgical procedure to implant an ICP sensor in cynomolgus monkeys is shown in Supplementary Meterial 1 and Supplementary Figure 1 .
Establishing the ALF model
Study monkeys were fasted (free access to water) for 12 h before drug administration. Anesthesia was induced by intramuscular injection of Zoletil (15 mg/kg) and atropine (0.5 mg/kg). Blood samples were collected from the forearm and vital signs and ICP were measured as baseline values (0 h). Finally, the prepared D-gal solution was drawn into a 50 mL syringe connected to a disposable needle, air was expelled from the syringe, and then the D-gal solution was administered slowly through the small saphenous vein (Supplementary Figure 2). After D-gal administration, animals were given regular feed and free access to water and fresh fruit.
Parameters
The general condition of study animals was monitored during the experiment and the subsequent observation period, as follows: the ability to stand, to walk, and to eat; the response to sight, sound, and stimulation; and presence of cramps or convulsions. When the animal was conscious, these were recorded every 12 h, whereas the animals were observed every 2 h after unconsciousness occurred. The recorded survival time was defined as the time interval from completion of injection of D-gal to death, and surviving animals were observed for 144 h in total.
ICP, ammonia level, and levels of inflammatory markers [tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6] and endotoxin were recorded at 0, 12, 24, 36, 48, 72, 96, and 120 h after D-gal administration. An ammonia determination kit (end-point method) purchased from Sysmex Corporation (Japan) was used to measure whole blood ammonia levels. TNF-α, IL-1β, and IL-6 levels were determined with ELISA kits purchased from Sigma-Aldrich. Endotoxin levels were determined with a Tachypleus Amebocyte Lysate kit purchased from Sigma-Aldrich.
Vital signs were monitored and blood samples to measure liver function indices (AST, ALT, ALB, TBiL, CK, and LDH), renal function indices (BUN and Cr), blood glucose, prothrombin time (PT), and routine blood chemistry tests were collected at 0, 12, 24, 36, 48, 72, 96, and 120 h after drug administration. All tests of blood samples were conducted in the clinical laboratory of Zhujiang Hospital, Southern Medical University, China.
Histopathological examination
From each of the four study groups, one monkey was randomly selected for liver biopsy, which was conducted under ultrasound guidance before D-gal administration (Supplementary Figure 3). Histopathological examination was then performed.
Animals surviving at 144 h were sacrificed with a lethal intravenous injection of pentobarbital and KCl, and a detailed autopsy was performed immediately after animal death. Each animal’s liver, heart, kidneys, spleen, lungs, large intestine, small intestine, brain, and pancreas were collected, and all tissue specimens were fixed in 10% formalin solution and cut into 5 mm3 blocks which were paraffin-embedded and thin-sectioned. Subsequently, slides underwent stepwise alcohol dehydration before hematoxylin-eosin (H&E) staining for observation under a light microscope. In addition, liver specimens were collected from all four groups and TUNEL assays were performed to assess cell apoptosis and necrosis. Finally, liver specimens from all four groups also underwent Masson staining to assess the extent of ALF fibrosis.
Animal care and use statement
The monkeys were cared for in strict accordance with the institution’s guidelines for experimental animals. Each animal was kept individually in a special iron cage under standard conditions and fed three times a day with free access to water. Animals surviving at 144 h were sacrificed with a lethal intravenous injection of pentobarbital and KCl for tissue collection.
Statistical analysis
Data are expressed as mean ± SD and were analyzed using the SPSS 21.0 statistical package. Differences between baseline values and values at different study time points were analyzed using Student’s t-test and ANOVA for multiple comparisons. Animal survival was analyzed using the Kaplan-Meier log rank method. P-values < 0.05 were considered significant.
RESULTS
General condition
The general condition of the experimental monkeys before D-gal administration is shown in Table 1. After D-gal injection, the experimental monkeys in group A began to eat less at 12 h, responded slowly to the sound stimulus, and two were apparently vomiting at 16 h and 21 h. All animals in group A had jaundice and very yellow urine after 24 h; their general condition subsequently declined rapidly into a persistent coma, and all animals died within 68 h. In group B, one monkey appeared nauseated and was vomiting at 48 h, while another was discovered to have convulsions and liver coma at 96 h and died a short time later. In group C, two monkeys had nausea, vomiting, and very yellow urine at 72 h after D-gal administration. Group D monkeys eat less and had slower responses at 48 h after D-gal administration, but they were recovering slowly after 96 h.
Survival
All experimental animals in groups A, B, and C died within 5 days. Compared with group D, the survival times of groups A, B, and C animals were significantly shortened (56 ± 8.7 h, 95 ± 5.5 h, and 99 ± 2.2 h, respectively; P < 0.01 for all), whereas three monkeys in group D survived until the end of the 144-h observation period, and one died at 136 h. Kaplan-Meier survival analysis suggested that the survival time of each group of monkeys was significantly different (χ2 = 22.42, P < 0.001) (Figure 2).
Figure 2.
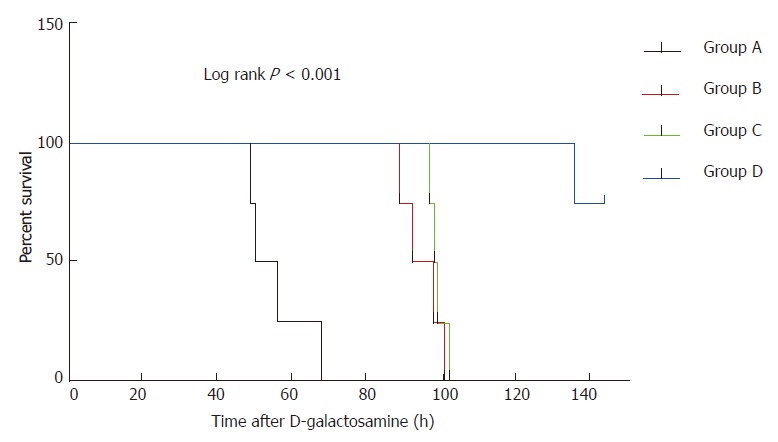
Survival times of monkeys in different study groups. Group A vs group B: P = 0.007; group A vs group C: P = 0.007; Group A vs group D: P < 0.001; Group B vs group C: P = 0.375.
Changes in ICP and ammonia
Significantly increased levels of ICP and ammonia were observed after D-gal administration in all study groups, compared with baseline values (P < 0.05 for all). The ICP and ammonia levels in group A increased to their peaks at 48 h to about 3-fold and 4-fold of baseline, respectively, whereas those in groups B and C had no significant increase (P > 0.05) except at 72 h and 96 h, when they all increased to a peak. In group D, ICP and ammonia levels increased slowly and declined after peaking at 96 h (Figure 3 and B).
Figure 3.
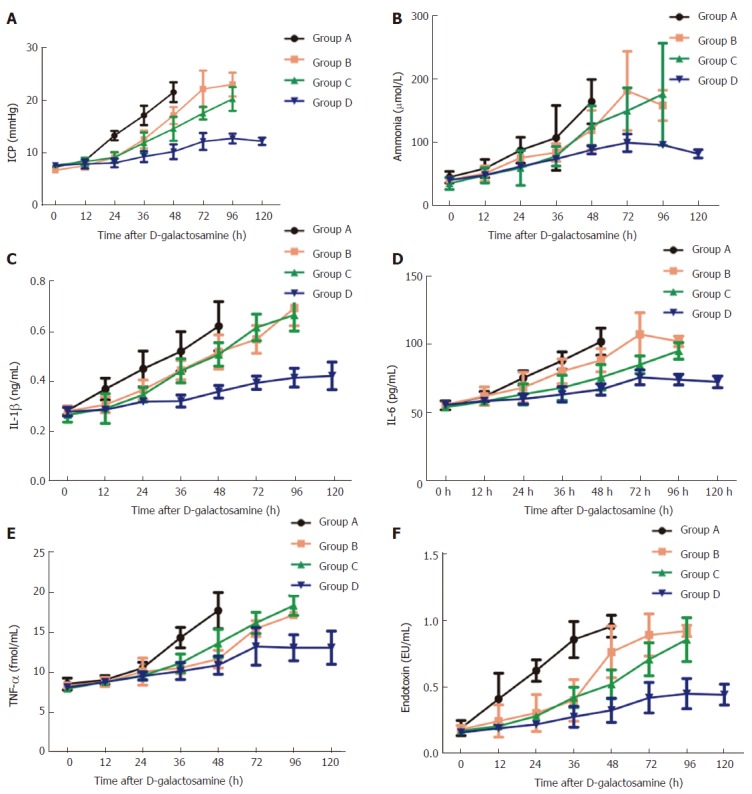
Changes of intracranial pressure, ammonia, inflammation markers and endotoxin at different time points in each group. All data points are mean ± SD, n = 4. ICP: Intracranial pressure; Amm: Ammonia; IL-1β: Interleukin-1β; IL-6: Interleukin-6; TNF-α: Tumor necrosis factor-α.
Changes in inflammatory markers and endotoxin levels
As shown in Figure 3C-F, compared with baseline values, IL-1β, IL-6, TNF-α, and endotoxin all significantly increased in group A at all time points except at 12 h (P < 0.05 for all). IL-1β and TNF-α levels were not significantly different between groups B and C at any time point; IL-6 and endotoxin were not significantly different between groups B and C except at 48 h and 72 h. In group D, these values were all lower than those in the other groups.
Biochemical parameters
The progressive increase in the levels of liver enzymes (ALT, AST, LDH, and CK), and TBiL indicated serious liver damage after D-gal administration. The liver enzymes and TBiL in group A significantly increased compared with baseline levels and those of the other groups (P < 0.05 for all). However, the liver enzymes in groups B and C were not significantly different (P > 0.05), and neither were levels of TBiL except at 72 h and 96 h (Figure 4A-C, F and G).
Figure 4.
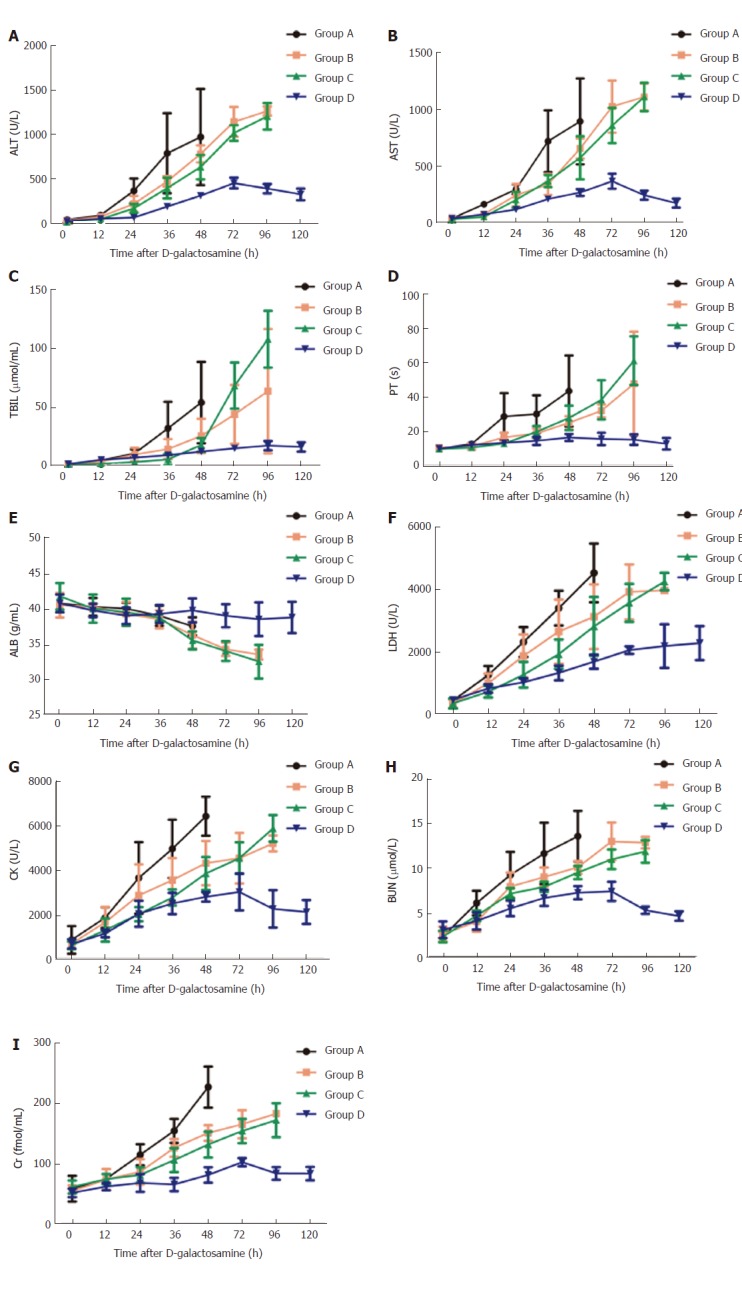
Changes of biochemical indices at different time points in each group. All data points are mean ± SD, n = 4. ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; TBIL: Total bilirubin; PT: Prothrombin time; ALB: Albumin; LDH: Lactic dehydrogenase; CK: Creatine kinase; BUN: Blood urea nitrogen; Cr: Creaninine.
The PT in all monkeys was prolonged significantly and there were significant differences between different time points after D-gal administration (P < 0.05 for all). The PT in group A increased to a peak at 48 h, about 4-fold of baseline; that of groups B and C significantly increased to a peak at 96 h, about 5-fold and 6-fold of baseline, respectively; whereas in group D, the PT increased slowly (Figure 4D).
Significant reductions in the plasma levels of ALB were also observed after D-gal administration in groups A, B, and C, to 37.50 ± 1.29 g/L at 48 h, and 33.50 ± 0.71 g/L and 32.50 ± 2.38 g/L at 96 h, respectively. The plasma level of ALB in group D did not change significantly (Figure 4E).
The BUN and Cr levels of all experimental monkeys significantly increased after D-gal administration. The BUN and Cr in group A increased to a peak at 48 h to about 6-fold and 4-fold compared with baseline levels. In groups B and C, the increase was progressive. In group D, the increase was slow and declined after peaking at 72 h to the baseline level at 120 h (Figure 4H, I).
Histopathology
The histopathology of normal liver clearly showed the expected findings of the central vein, portal area, liver cords, and liver lobules. No swelling, vacuoles, or necrosis of liver cells was observed. In group A after D-gal administration, liver cells presented with extensive necrosis, had visible nuclear fragments, and a large number of vacuolar structures. The situation in groups B and C was similar, with areas of necrotic lesions with diffuse swelling of liver cells having cytoplasmic and vacuolar degeneration. In group D, liver cells had mainly degenerative edema and the liver sinus structure was visible. The TUNEL assay demonstrated obvious positive cells in group A, while in groups B and C positive cells were present in comparatively lower quantities. No positive cells were detected in group D samples. Masson staining revealed mild fibrosis in groups A, B, and C, and no obvious abnormality in group D animals (Figure 5).
Figure 5.
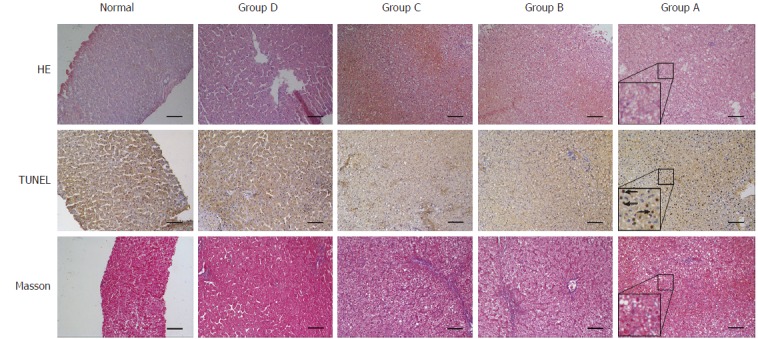
H&E staining, Tunel and Masson assays of post-mortem liver specimens from different groups. H&E: Hematoxylin-eosin staining; TUNEL: Terminal -deoxynucleotidyl transferase mediated nick end labeling; Arrows: Apoptotic bodies. Lower left corner detail: enlarged scale for group A (× 100 magnification, 200 μm scale bars).
Results of examination of gross specimens of the other organs are shown in Figure 6. The results of HE staining of other organs are shown in Figure 7.
Figure 6.
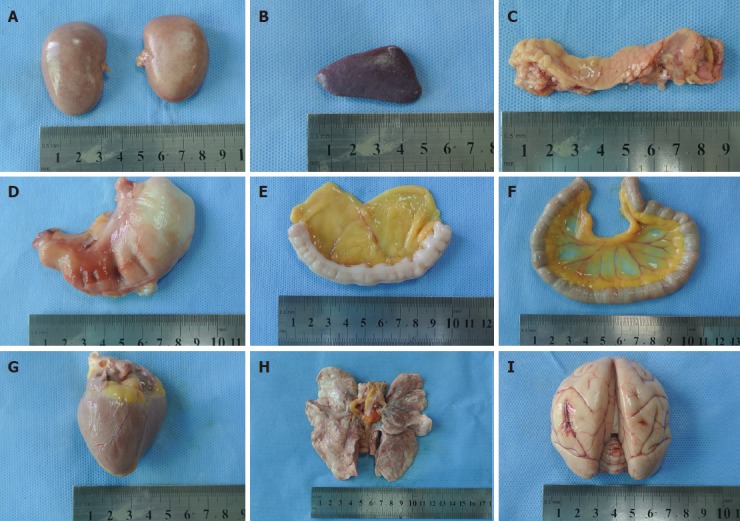
Gross specimens of other organs post-mortem (in group C). A: Renal; B: Spleen; C: Pancreas; D: Stomach; E: Large intestine; F: Small intestine;G: Heart; H: Lung; I: Brain.
Figure 7.
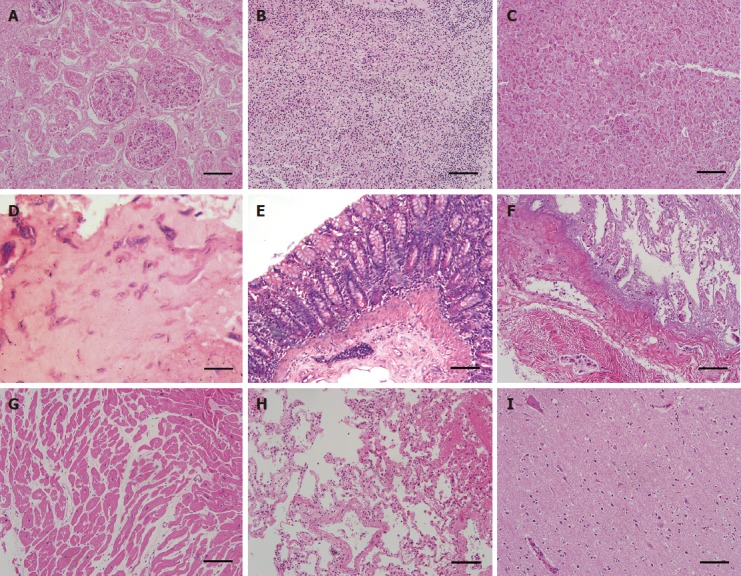
HE staining of other organs post-mortem (in group C). A: The renal tissue profile was clear, and the glomerular capillaries and renal interstitial blood vessels were slightly dilated and congested; B: The splenic sinusoids were mildly to moderately expanded with a large number of red blood cells; C: Pancreas, no abnormalities; D: Stomach, no abnormalities; E: Large intestine, no abnormalities; F: Small intestine, no abnormalities; G: Heart, no abnormalities; H: Lung, the bronchial and alveolar structures of pulmonary tissues were complete, and the interstitial capillaries were diffusely expanded and congested with few red blood cells; I: Brain, the nerve cells were diffusely enlarged with mild degenerative changes (× 100 magnification, 200 μm scale bars).
DISCUSSION
A simplified, reproducible D-gal-induced cynomolgus monkey model of ALF that is suitable for use to assess the safety and efficacy of ALSS has been successfully established. The ideal criteria for animal models were first proposed by Terblanche et al[20], which were promoted and supplemented as a result of subsequent studies[14]. They mainly comprise the following points: reversibility; reproducibility; death from liver failure; suitable treatment window; large animals; causing minimal harm to environment and researchers; consciousness level making hepatic encephalopathy easy to evaluate; similarity to human beings; and ethically acceptable.
At present, large animal models of ALF meeting the above criteria mainly include drug-induced models[5] and surgery-induced models[21]. Drug-induced models are easy to create, having short anesthesia times, and can be accomplished without use of the highly skilled technical work required to establish surgery-induced models. Although sometimes drug-induced models are unstable because of great individual differences in drug tolerance and metabolic function, these models are of great interest to research scholars because the most common reason for ALF in clinical setting is drug toxicity[4].
Current literature reports of drugs that can induce ALF include those on D-gal, acetaminophen (APAP), and carbon tetrachloride, to name a few[12-14]. Yu et al[22] reported on the pharmacokinetics, drug metabolism, and hepatic toxicity of APAP in cynomolgus monkeys and found significant tolerance to APAP; therefore, APAP is not suitable to create a cynomolgus monkey model to study related hepatic injury. Compared with other drugs, D-gal has many advantages for this purpose, including better reproducibility and easier control of the dosage; it is generally accepted as the ideal drug to induce ALF.
For this study, we chose cynomolgus monkeys because their anatomy, physiology, biochemical metabolism, and immune system characteristics are very similar to those of human beings, making them the ideal animal to establish an ALF model. Given the rarity of primate species and the instability of other models, there are few relevant published reports of primate models of ALF. Zhou et al[23] induced fulminant hepatic failure (FHF) in the Macaca mulatta by intraperitoneal injection of amatoxin and endotoxin, and evaluated the animal model by progressive analysis of clinical features, biochemical indices, and histopathology. However, their study included only two monkeys, so the stability and reproducibility need further verification, and the effective treatment window of this model would make the study of use of ALSS difficult.
Drug dosages and administration methods are important for establishing drug-induced models. The method of drug administration affects the convenience of using a model. Various ALF studies have different requirements for the survival time, which usually means exploring the optimal dosage and induction methods for different purposes. Glorioso et al[8] successfully established a pig model of ALF by injecting 0.75 g/kg D-gal through the external jugular vein, which was successfully used in the study of artificial livers. Li et al[7,24] established a pig model of FHF by intravenous injection of 1.3 g/kg and 1.5 g/kg[25] D-gal, which was used in studies to verify the safety and efficacy of ALSS. Ding et al[26] established a pig model of ALF by injecting 0.45 g/kg D-gal intravenously to study treatment with a novel bio-artificial liver.
Currently, D-gal is usually administered through the external jugular vein or the abdominal cavity[23-25,27]. The abdominal cavity injection is simple and convenient, but resulting models are unstable, while administration through the external jugular vein and portal vein usually requires a long anesthesia time and surgical venous intubation, so the method is more complicated.
In our early study, we administered 0.45, 0.3, and 0.15 g/kg of D-gal through the external jugular vein to establish an ALF model to explore the optimal basic dosage to establish the primate model of ALF[19]. However, venous intubation is not only inconvenient, but also brings certain trauma to the experimental animal. In this study, we used small saphenous vein puncture instead of jugular vein intubation for D-gal administration, which not only effectively avoided the trauma caused by intubation, but also significantly reduced the anesthesia time and greatly improved the convenience of operation. Moreover, we further adjusted and optimized the dosage of D-gal using the previous dose of 0.3 g/kg, as well as a 0.25 g/kg single dose, 0.25 g/kg as a divided dose (0.20 + 0.05 g/kg), and a single 0.20 g/kg dose, and then compared in the different groups for changes of clinical manifestation, survival time, liver function, inflammatory factors, PT, ICP, and histopathology.
The results showed that the experimental monkeys developed different levels of anorexia, anemia, jaundice, and coagulopathy after intravenous injection of different doses of D-gal that were similar to the various degrees of clinical ALF. The animals administered 0.30 g/kg of D-gal had the shortest survival time (56 ± 8.7 h), and there was no significant difference in survival time after 0.25 g/kg given as a single or divided dose (95 ± 5.5 h and 99 ± 2.2 h, respectively). In our study, 81.3% (13/16) of experimental monkeys died, and the survival time of experimental animals was positively correlated with the dose of D-gal.
D-gal can cause liver cell necrosis and lead to ALF, as well as abnormally elevated serum TNF-α, which then triggers the cascade of inflammatory mediators and is closely related to the pathophysiology of ALF[28,29]. In our study, a strong inflammatory response was observed, as evidenced by markedly increased levels of TNF-α, IL-1β, IL-6, and endotoxin, all of which were positively correlated with the dose of D-gal.
Liver enzymes are important indices to assess clinical liver injury. When liver cells are necrotic, inflammation and toxicity can cause damage to the liver cell membrane, leading to serum transaminase elevations; transaminase levels 10-fold higher than the baseline indicate acute liver damage[30]. In this study, ALT, AST, CK, and LDH increased rapidly in a short time after injection of D-gal, with results demonstrating that acute liver injury and the degree of damage were positively correlated with the dose of D-gal.
ALB and PT are important indicators of liver synthesis and reserve function. In our study, serum ALB levels showed a progressive decline after D-gal administration except in group D, and this may explain the anomalous finding of abdominal and pleural effusions on autopsy of the study animals. The PT in the four groups was significantly prolonged, with the peak times 4-, 5-, 6-, and 1.5-fold of the baseline time in groups A, B, C and D, respectively. At autopsy, the livers in groups A and B had obvious ecchymosis, and four lung specimens had obvious bleeding; these findings are likely associated with the coagulation dysfunction caused by liver failure.
Hepatic encephalopathy is a serious complication of ALF and is closely related to the blood ammonia level, elevations of which cause brain edema, oxidative stress, and inflammation[31,32]. In our study, we measured the progression of ammonia levels and ICP to monitor for hepatic encephalopathy. Ammonia and ICP were significantly increased in groups A and B, and were associated with the clinical manifestations of consciousness changes and hepatic coma before death, as well as histopathological changes, all indicating that the experimental animals developed hepatic encephalopathy before death.
The model established in our study has some limitations. First, we used Zoletil to induce anesthesia before administering D-gal, and although Zoletil has many advantages, including short induction time, minimal side effects, and maximum security compared with ketamine, whether it can affect the effect of D-gal is unknown. In addition, the number of animals used was small, and further studies with larger experimental groups are warranted to verify our results.
In conclusion, we have successfully established a simplified, reproducible D-gal-induced cynomolgus monkey model of ALF that is suitable for assessing the safety and efficacy of ALSS, studying the pathogenesis of ALF, and developing new drugs, and the dosage of 0.25 g/kg as either a single or divided dose is optimal.
ARTICLE HIGHLIGHTS
Research Background
Acute liver failure (ALF) is a serious threat to human health. Artificial liver support system (ALSS) is a novel method to deal with ALF. However, the safety and efficacy of ALSS must be verified before clinical application. Therefore, the establishment of an ALF animal model is of great significance for testing ALSS, studying the pathogenesis of ALF, and determining the comprehensive treatment of ALF. Nowadays, there have been many studies about the acute liver failure in large animals, such as pigs and dogs. However, there have been few previously reported studies of ALF models in cynomolgus monkey. Furthermore, the methods of drug administration are complex and increase the trauma to experimental animals.
Research motivation
In this study, our motivation was to establish an ideal animal model of ALF with an appropriate treatment window which is suitable for assessing the safety and efficacy of ALSS, studying the pathogenesis of ALF, developing new drugs, and determining the comprehensive treatment of ALF.
Research objectives
The primary objective of this study was to establish a simplified, reproducible D-gal-induced large-animal ALF model with an appropriate treatment window. In addition, we wanted to explore the optimal dosage of D-gal to induce ALF in cynomolgus monkey.
Research methods
In this study, we used small saphenous vein puncture instead of jugular vein intubation for different doses of D-gal administration, and then observed the clinical manifestations, survival times, changes in biochemical indices, intracranial pressure changes, and resulting pathological and histological characteristics. This method not only effectively avoided the trauma caused by intubation, but also significantly reduced the anesthesia time and greatly improved the convenience of operation. All experimental data were analyzed using SPSS 21.0 statistical package.
Research results
The results showed that the experimental monkeys developed different levels of anorexia, anemia, jaundice, and coagulopathy after intravenous injection of different doses of D-gal that were similar to the various degrees of clinical ALF. The animals administered 0.30 g/kg of D-gal had the shortest survival time, and there was no significant difference in survival time after 0.25 g/kg was given as a single or divided dose. The degree of acute liver damage and the survival time of experimental animals were positively correlated with the dose of D-gal. The experimental animals given 0.25 g/kg as a single or divided dose had an appropriate treatment window. However, the number of animals used was limited, and further studies with larger experimental groups are warranted to verify our results.
Research conclusions
The authors have successfully established a simplified, reproducible D-gal-induced cynomolgus monkey model of ALF and found that the optimal dosage to induce ALF in cynomolgus monkey is 0.25 g/kg as either a single or divided dose.
Research perspectives
From this study, we found that drug dosages and the administration methods are important for establishing drug-induced models. The method of drug administration affects the convenience of using a model. In addition, we think small saphenous vein puncture for D-gal administration is the best method to induce ALF in cynomolgus monkey and the dosage of 0.25 g/kg as either a single or divided dose is optimal. Furthermore, we can use this method and dosage to induce ALF in cynomolgus monkey to test ALSS or study the pathogenesis of ALF in the future.
ACKNOWLEDGMENTS
The authors would like to thank Mr. Yue-Feng Li and Mr. Xing-Wen Qin at the Department of Experimental Animals, Guangdong Landao Biological Technology Co., who kindly provided anesthesia assistance, blood sample collection, and animal care services.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study was reviewed and approved by the Zhujiang Hospital Institutional Review Board.
Institutional animal care and use committee statement: All procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of Guangdong Province (IACUC protocol number: SCXK (Guangdong) 2014-0010).
Conflict-of-interest statement: No conflict of interest exists.
Peer-review started: August 24, 2017
First decision: September 13, 2017
Article in press: October 17, 2017
P- Reviewer: Isik A S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Ma YJ
Contributor Information
Lei Feng, Department of Hepatobiliary Surgery II, Guangdong Provincial Research Center for Artificial Organ and Tissue Engineering, Guangzhou Clinical Research and Transformation Center for Artificial Liver, Institute of Regenerative Medicine, Zhujiang Hospital, Southern Medical University, Guangzhou 510282, Guangdong Province, China.
Lei Cai, Department of Hepatobiliary Surgery II, Guangdong Provincial Research Center for Artificial Organ and Tissue Engineering, Guangzhou Clinical Research and Transformation Center for Artificial Liver, Institute of Regenerative Medicine, Zhujiang Hospital, Southern Medical University, Guangzhou 510282, Guangdong Province, China.
Guo-Lin He, Department of Hepatobiliary Surgery II, Guangdong Provincial Research Center for Artificial Organ and Tissue Engineering, Guangzhou Clinical Research and Transformation Center for Artificial Liver, Institute of Regenerative Medicine, Zhujiang Hospital, Southern Medical University, Guangzhou 510282, Guangdong Province, China.
Jun Weng, Department of Hepatobiliary Surgery II, Guangdong Provincial Research Center for Artificial Organ and Tissue Engineering, Guangzhou Clinical Research and Transformation Center for Artificial Liver, Institute of Regenerative Medicine, Zhujiang Hospital, Southern Medical University, Guangzhou 510282, Guangdong Province, China.
Yang Li, Department of Hepatobiliary Surgery II, Guangdong Provincial Research Center for Artificial Organ and Tissue Engineering, Guangzhou Clinical Research and Transformation Center for Artificial Liver, Institute of Regenerative Medicine, Zhujiang Hospital, Southern Medical University, Guangzhou 510282, Guangdong Province, China.
Ming-Xin Pan, Department of Hepatobiliary Surgery II, Guangdong Provincial Research Center for Artificial Organ and Tissue Engineering, Guangzhou Clinical Research and Transformation Center for Artificial Liver, Institute of Regenerative Medicine, Zhujiang Hospital, Southern Medical University, Guangzhou 510282, Guangdong Province, China.
Ze-Sheng Jiang, Department of Hepatobiliary Surgery II, Guangdong Provincial Research Center for Artificial Organ and Tissue Engineering, Guangzhou Clinical Research and Transformation Center for Artificial Liver, Institute of Regenerative Medicine, Zhujiang Hospital, Southern Medical University, Guangzhou 510282, Guangdong Province, China.
Qing Peng, Department of Hepatobiliary Surgery II, Guangdong Provincial Research Center for Artificial Organ and Tissue Engineering, Guangzhou Clinical Research and Transformation Center for Artificial Liver, Institute of Regenerative Medicine, Zhujiang Hospital, Southern Medical University, Guangzhou 510282, Guangdong Province, China.
Yi Gao, Department of Hepatobiliary Surgery II, Guangdong Provincial Research Center for Artificial Organ and Tissue Engineering, Guangzhou Clinical Research and Transformation Center for Artificial Liver, Institute of Regenerative Medicine, Zhujiang Hospital, Southern Medical University, Guangzhou 510282, Guangdong Province, China; State Key Laboratory of Organ Failure Research, Southern Medical University, Guangzhou 510282, Guangdong Province, China. drgaoy@126.com.
References
- 1.Cardoso FS, Marcelino P, Bagulho L, Karvellas CJ. Acute liver failure: An up-to-date approach. J Crit Care. 2017;39:25–30. doi: 10.1016/j.jcrc.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Isik A, Firat D. Bilateral intra-areolar polythelia. Breast J. 2017 doi: 10.1111/tbj.12838. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 3.Isik A, Idiz O, Firat D. Novel Approaches in Pilonidal Sinus Treatment. Prague Med Rep. 2016;117:145–152. doi: 10.14712/23362936.2016.15. [DOI] [PubMed] [Google Scholar]
- 4.Tuñón MJ, Alvarez M, Culebras JM, González-Gallego J. An overview of animal models for investigating the pathogenesis and therapeutic strategies in acute hepatic failure. World J Gastroenterol. 2009;15:3086–3098. doi: 10.3748/wjg.15.3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee KC, Palacios Jimenez C, Alibhai H, Chang YM, Leckie PJ, Baker LA, Stanzani G, L Priestnall S, Mookerjee RP, Jalan R, et al. A reproducible, clinically relevant, intensively managed, pig model of acute liver failure for testing of therapies aimed to prolong survival. Liver Int. 2013;33:544–551. doi: 10.1111/liv.12042. [DOI] [PubMed] [Google Scholar]
- 6.Saliba F, Samuel D. Artificial liver support: a real step forward. Minerva Med. 2015;106:35–43. [PubMed] [Google Scholar]
- 7.Zhou N, Li J, Zhang Y, Lu J, Chen E, Du W, Wang J, Pan X, Zhu D, Yang Y, et al. Efficacy of coupled low-volume plasma exchange with plasma filtration adsorption in treating pigs with acute liver failure: A randomised study. J Hepatol. 2015;63:378–387. doi: 10.1016/j.jhep.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 8.Glorioso JM, Mao SA, Rodysill B, Mounajjed T, Kremers WK, Elgilani F, Hickey RD, Haugaa H, Rose CF, Amiot B, et al. Pivotal preclinical trial of the spheroid reservoir bioartificial liver. J Hepatol. 2015;63:388–398. doi: 10.1016/j.jhep.2015.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicolas CT, Hickey RD, Chen HS, Mao SA, Lopera Higuita M, Wang Y, Nyberg SL. Concise Review: Liver Regenerative Medicine: From Hepatocyte Transplantation to Bioartificial Livers and Bioengineered Grafts. Stem Cells. 2017;35:42–50. doi: 10.1002/stem.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Chalabi A, Matevossian E, V Thaden AK, Luppa P, Neiss A, Schuster T, Yang Z, Schreiber C, Schimmel P, Nairz E, et al. Evaluation of the Hepa Wash® treatment in pigs with acute liver failure. BMC Gastroenterol. 2013;13:83. doi: 10.1186/1471-230X-13-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Z, Ding YT. Functional evaluation of a new bioartificial liver system in vitro and in vitro. World J Gastroenterol. 2006;12:1312–1316. doi: 10.3748/wjg.v12.i8.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He GL, Feng L, Cai L, Zhou CJ, Cheng Y, Jiang ZS, Pan MX, Gao Y. Artificial liver support in pigs with acetaminophen-induced acute liver failure. World J Gastroenterol. 2017;23:3262–3268. doi: 10.3748/wjg.v23.i18.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ning Y, Kim JK, Min HK, Ren S. Cholesterol metabolites alleviate injured liver function and decrease mortality in an LPS-induced mouse model. Metabolism. 2017;71:83–93. doi: 10.1016/j.metabol.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Maes M, Vinken M, Jaeschke H. Experimental models of hepatotoxicity related to acute liver failure. Toxicol Appl Pharmacol. 2016;290:86–97. doi: 10.1016/j.taap.2015.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Awad SS, Hemmila MR, Soldes OS, Sawada S, Rich PB, Mahler S, Gargulinski M, Hirschl RB, Bartlett RH. A novel stable reproducible model of hepatic failure in canines. J Surg Res. 2000;94:167–171. doi: 10.1006/jsre.2000.5997. [DOI] [PubMed] [Google Scholar]
- 16.Xiong Q, Hase K, Tezuka Y, Namba T, Kadota S. Acteoside inhibits apoptosis in D-galactosamine and lipopolysaccharide-induced liver injury. Life Sci. 1999;65:421–430. doi: 10.1016/s0024-3205(99)00263-5. [DOI] [PubMed] [Google Scholar]
- 17.de Groot GH, Reuvers CB, Schalm SW, Boks AL, Terpstra OT, Jeekel H, ten Kate FW, Bruinvels J. A reproducible model of acute hepatic failure by transient ischemia in the pig. J Surg Res. 1987;42:92–100. doi: 10.1016/0022-4804(87)90070-9. [DOI] [PubMed] [Google Scholar]
- 18.Benoist S, Sarkis R, Baudrimont M, Delelo R, Robert A, Vaubourdolle M, Balladur P, Calmus Y, Capeau J, Nordlinger B. A reversible model of acute hepatic failure by temporary hepatic ischemia in the pig. J Surg Res. 2000;88:63–69. doi: 10.1006/jsre.1999.5778. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Z, Zhao YC, Cheng Y, Jian GD, Pan MX, Gao Y. Hybrid bioartificial liver support in cynomolgus monkeys with D-galactosamine-induced acute liver failure. World J Gastroenterol. 2014;20:17399–17406. doi: 10.3748/wjg.v20.i46.17399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Terblanche J, Hickman R. Animal models of fulminant hepatic failure. Dig Dis Sci. 1991;36:770–774. doi: 10.1007/BF01311235. [DOI] [PubMed] [Google Scholar]
- 21.Cai L, Weng J, Feng L, He G, Qin J, Zhang Z, Li Y, Peng Q, Jiang Z, Pan M, et al. Establishment of a Novel Simplified Surgical Model of Acute Liver Failure in the Cynomolgus Monkey. Biomed Res Int. 2016;2016:3518989. doi: 10.1155/2016/3518989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu H, Barrass N, Gales S, Lenz E, Parry T, Powell H, Thurman D, Hutchison M, Wilson ID, Bi L, et al. Metabolism by conjugation appears to confer resistance to paracetamol (acetaminophen) hepatotoxicity in the cynomolgus monkey. Xenobiotica. 2015;45:270–277. doi: 10.3109/00498254.2014.973000. [DOI] [PubMed] [Google Scholar]
- 23.Zhou P, Xia J, Guo G, Huang ZX, Lu Q, Li L, Li HX, Shi YJ, Bu H. A Macaca mulatta model of fulminant hepatic failure. World J Gastroenterol. 2012;18:435–444. doi: 10.3748/wjg.v18.i5.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li LJ, Du WB, Zhang YM, Li J, Pan XP, Chen JJ, Cao HC, Chen Y, Chen YM. Evaluation of a bioartificial liver based on a nonwoven fabric bioreactor with porcine hepatocytes in pigs. J Hepatol. 2006;44:317–324. doi: 10.1016/j.jhep.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Lv G, Zhao L, Zhang A, Du W, Chen Y, Yu C, Pan X, Zhang Y, Song T, Xu J, et al. Bioartificial liver system based on choanoid fluidized bed bioreactor improve the survival time of fulminant hepatic failure pigs. Biotechnol Bioeng. 2011;108:2229–2236. doi: 10.1002/bit.23150. [DOI] [PubMed] [Google Scholar]
- 26.Shi XL, Gao Y, Yan Y, Ma H, Sun L, Huang P, Ni X, Zhang L, Zhao X, Ren H, et al. Improved survival of porcine acute liver failure by a bioartificial liver device implanted with induced human functional hepatocytes. Cell Res. 2016;26:206–216. doi: 10.1038/cr.2016.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou P, Shao L, Zhao L, Lv G, Pan X, Zhang A, Li J, Zhou N, Chen D, Li L. Efficacy of Fluidized Bed Bioartificial Liver in Treating Fulminant Hepatic Failure in Pigs: A Metabolomics Study. Sci Rep. 2016;6:26070. doi: 10.1038/srep26070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Puengel T, Tacke F. Repair macrophages in acute liver failure. Gut. 2017 doi: 10.1136/gutjnl-2017-314245. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 29.Li J, Zhong L, Zhu H, Wang F. The Protective Effect of Cordycepin on D-Galactosamine/Lipopolysaccharide-Induced Acute Liver Injury. Mediators Inflamm. 2017;2017:3946706. doi: 10.1155/2017/3946706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shehu AI, Ma X, Venkataramanan R. Mechanisms of Drug-Induced Hepatotoxicity. Clin Liver Dis. 2017;21:35–54. doi: 10.1016/j.cld.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 31.Weiss N, Jalan R, Thabut D. Understanding hepatic encephalopathy. Intensive Care Med. 2017 doi: 10.1007/s00134-017-4845-6. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 32.Jamshidzadeh A, Heidari R, Abasvali M, Zarei M, Ommati MM, Abdoli N, Khodaei F, Yeganeh Y, Jafari F, Zarei A, et al. Taurine treatment preserves brain and liver mitochondrial function in a rat model of fulminant hepatic failure and hyperammonemia. Biomed Pharmacother. 2017;86:514–520. doi: 10.1016/j.biopha.2016.11.095. [DOI] [PubMed] [Google Scholar]


