Abstract
Reactive oxygen species have emerged as key participants in a broad range of physiological and pathophysiological processes, not least within the vascular system. Diverse cellular functions which have been attributed to some of these pro-oxidants within the vasculature include the regulation of blood pressure, neovascularisation and vascular inflammation. We here highlight the emerging roles of the enzymatically-generated reaction oxygen species, O2- and H2O2, in the regulation of the functions of the gaseous signalling molecules: nitric oxide (NO), carbon monoxide (CO), and hydrogen sulphide (H2S). These gasotransmitters are produced on demand from distinct enzymatic sources and in recent years it has become apparent that they are capable of mediating a number of homeostatic processes within the cardiovascular system including enhanced vasodilation, angiogenesis, wound healing and improved cardiac function following myocardial infarction. In common with O2- and/or H2O2 they signal by altering the functions of target proteins, either by the covalent modification of thiol groups or by direct binding to metal centres within metalloproteins, most notably haem proteins. The regulation of the enzymes which generate NO, CO and H2S have been shown to be influenced at both the transcriptional and post-translational levels by redox-dependent mechanisms, while the activity and bioavailability of the gasotransmitters themselves are also subject to oxidative modification. Within vascular cells, the family of nicotinamide adenine dinucleotide phosphate oxidases (NAPDH oxidases/Noxs) have emerged as functionally significant sources of regulated O2- and H2O2 production and accordingly, direct associations between Nox-generated oxidants and the functions of specific gasotransmitters are beginning to be identified. This review focuses on the current knowledge of the redox-dependent mechanisms which regulate the generation and activity of these gases, with particular reference to their roles in angiogenesis.
Abbreviations: SDF-1, stromal cell-derived factor 1; PAD, peripheral arterial disease; GPx, glutathione peroxidase; TAK1, transforming growth factor-B-activated-kinase 1; PTP1B, protein tyrosine phosphatase 1B; EDRF, endothelial-derived relaxing factor (EDRF); BH4, tetrahydrobiopterin; L-NMMA, NG-monomethyl-L-arginine; L-NAME, NG-nitro-L-arginine-methyl ester; HRE, hypoxia response element; ADMA, asymmetric dimethylarginine; DDAHI, dimethylarginine dimethylaminohydrolase I; DDAHII, dimethylarginine dimethylaminohydrolase II; NAC, N-acetylcysteine; CORM-1, tricarbonyl-dichlororuthenium (II); CORM-3, tricarbonylchloro(glucinato)ruthenium (II); CORM, carbon monoxide releasing molecule; SnPPIX, tin protoporphyrin IX; ZnPP, zinc protoporphyrin; Bach-1, BTB Domain and CNC Homolog 1 (Bach1); HNO, nitroxyl
Keywords: Reactive oxygen species, Nitric oxide, Carbon monoxide, Hydrogen sulphide, Angiogenesis, NADPH oxidase 4
Graphical abstract
Highlights
-
•
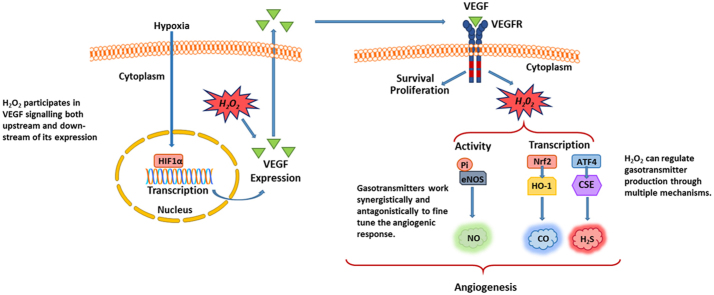
Reactive Oxygen Species and Gasotransmitters are critical regulators of angiogenesis and are involved in processes both upstream and downstream of VEGF expression.
-
•
The enzymes which generate NO, CO and H2S are redox regulated at the levels of their transcription, translation, activity and bioavailability.
-
•
NAPDH oxidases are functionally significant sources of superoxide and hydrogen peroxide that can direct the functions of specific gasotransmitters.
-
•
The products of the chemical interactions of reactive oxygen species and the gasotransmitters are emerging as novel biomolecules that may be exploited therapeutically.
1. Introduction
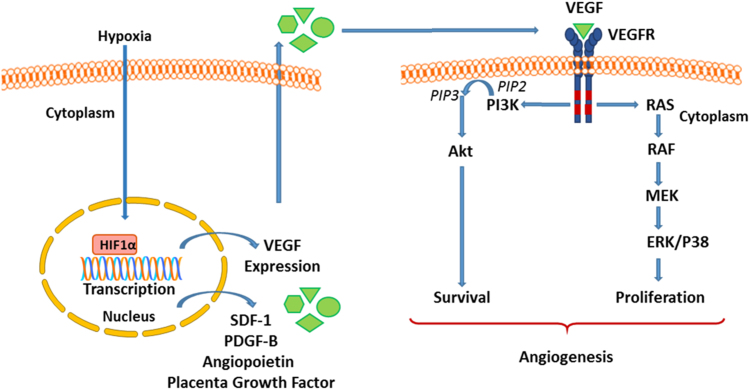
The vascular system represents a closed circuit of vessels that collectively transports oxygen and nutrients to all tissues. This delivery system has evolved multiple mechanisms that allow it to adapt to dynamic changes in tissue physiology in order to ensure that the supply of oxygen and nutrients matches the tissue demand at any given time [1]. One way in which this is achieved is through the growth of new blood vessels from the existing vasculature in a multistep, highly coordinated process known as angiogenesis. In this process, activated (“sprouting”) endothelial cells (from either arterial or venous vessels) release proteases which degrade the underlying basement membrane and subsequently migrate into the extracellular matrix. Adjacent endothelial cells in turn proliferate, differentiate and form luminal tubes which eventually fuse to the pre-established vascular network [2]. These adaptive responses are biochemically coordinated by a number of cell signalling mediators, most notably the vascular endothelial growth factor (VEGF) family of protein growth factors [3]. VEGF targets the endothelium to initiate angiogenesis and comprises multiple isoforms of which VEGF-A is a key regulator of vessel growth which has been shown to induce endothelial cell growth and survival both in vitro and in vivo [4], [5], [6]. The central role of VEGF-A in physiological angiogenesis (and vasculogenesis) has been demonstrated during embryonic and early postnatal development [6] as well as in pathological angiogenesis seen in solid tumour formation [7]. At the biochemical level, the effects of VEGF are mediated via its binding to endothelial-expressed, plasma membrane-bound, tyrosine kinase receptors, Flt-1 (VEGFR-1) and primarily, Flk-1/KDR (VEGFR-2). VEGF binding to VEGFR-2 initiates its autophosphorylation, dimerization and the subsequent activation of its tyrosine kinase domain [8]. This in turn activates downstream signalling cascades, including the MEK-ERK1/2 pathway to support cell growth and proliferation [4] as well as the anti-apoptotic phosphoinositide 3-kinase- (PI3-K-)Akt pathway to promote cell survival [5] (Fig. 1).
Fig. 1.
A schematic illustration of hypoxia- and VEGF-mediated signalling in the endothelium leading to angiogenesis through the promotion of cell survival and proliferation. In response to hypoxia, the upregulation of HIF-1α leads to increased expression of a number of pro-angiogenic factors including SDF-1, PDGF-B, angiopoietin, placenta growth factor and importantly VEGF. VEGF signals have been the best characterised and have been shown to cause the stimulation of VEGFR2 within the endothelium. In turn this activates downstream signalling pathways including P13K/Akt and MEK/MAPK to promote pro-angiogenic cellular responses.
Increased VEGF-dependent signalling triggers the angiogenic response and therefore the control of VEGF expression is critical to the regulation of angiogenesis. In this regard, the transcriptional regulation of VEGF appears to play the pre-eminent role, and multiple transcription factors which are positive mediators of VEGF transcription have been identified, together with cellular agents which stimulate their activity through diverse signalling pathways [9]. An important stimulus for angiogenesis is tissue hypoxia and, accordingly, VEGF is a known direct transcriptional target of hypoxia-inducible factor 1 (HIF-1). Similarly, the expressions of other known pro-angiogenic factors including angiopoietin 1 and 2, stromal cell-derived factor-1 (SDF-1), placenta growth factor and platelet-derived growth factor B are also known to be upregulated by HIF-1 [10], [11].
These regulatory pathways, both upstream and downstream of the action of VEGF, have been extensively studied and emerging data indicate the involvement of redox-dependent molecular signalling mechanisms at multiple stages [12]. Further, angiogenic responses have increasingly been shown to be mediated in part by the biological actions of a small family of gases, termed “gasotransmitters”, which are enzymatically generated within vascular cells [13]. The precise mechanisms of the regulation of action of these short-lived mediators, which comprise nitric oxide (NO), carbon monoxide (CO) and hydrogen sulphide (H2S) are not currently fully understood. However, there is growing evidence that their generation may be regulated in part by redox-dependent mechanisms, while their chemical nature in some cases makes them highly susceptible to oxidation. In this review we summarise the current knowledge of the biochemistry which links reactive oxygen species generation, redox signalling and the action of the gasotransmitters in angiogenesis. A more comprehensive understanding of these mechanisms would be of great potential benefit in identifying new therapeutic targets for both cancer and vascular diseases such as peripheral arterial disease (PAD) [14].
1.1. Reactive oxygen species and redox-signalling
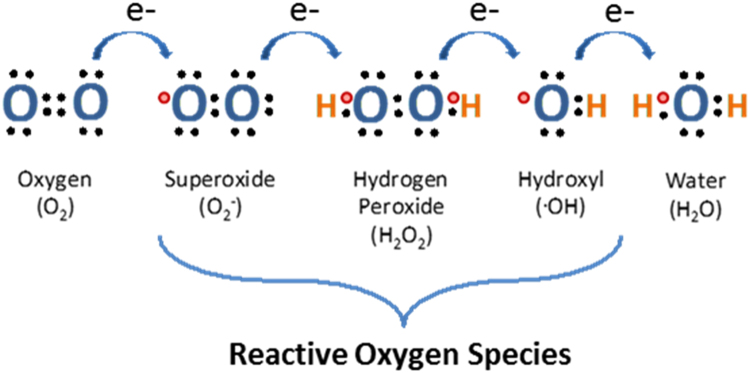
Reactive oxygen species are partial reduction products of molecular oxygen (O2) and include superoxide (O2-), hydrogen peroxide (H2O2) and the hydroxyl radical (•OH) (Fig. 2). Historically, they have been thought of as merely potentially detrimental by-products of aerobic metabolism in the mitochondria or the result of unregulated uncoupling of various O2-dependent enzymatic reactions [15]. The harmful biological effects of these oxidants are countered by the actions of enzymatic and non-enzymatic antioxidants that collectively form the cellular antioxidant system. Enzymatic antioxidants include superoxide dismutase (SOD) that removes O2-, as well as catalase, peroxiredoxin and glutathione peroxidase (GPx) that metabolise H2O2 [16]. Non-enzymatic antioxidants include vitamins C and E as well as the major redox buffer, glutathione (GSH). GSH is present at millimolar concentrations in the cell and scavenges both H2O2 and free radicals through the formation of oxidised GSH (GSSG). The “steady state” cellular redox status is maintained through the balance of constitutively expressed pro-oxidants and these antioxidant systems (reviewed in [17]).
Fig. 2.
Reactive oxygen species. A diagrammatic illustration depicting the gradual reduction of molecular oxygen (O2), through its sequential gain of electrons, to form water. As oxygen is reduced various reactive oxygen species are generated in the process including superoxide (O2-), hydrogen peroxide (H2O2) and hydroxyl radicals (•OH).
However it is now understood that physiological, enzymatic sources of H2O2 and O2- exist which generate these species in a tightly regulated and localised fashion. Moreover, the short-lived, regulated production of these pro-oxidants can mediate various homeostatic aspects of intracellular and extracellular function [18], [19]. Both O2- and H2O2 are capable of orchestrating a variety of cell signalling responses that culminate in a broad range of phenotypic outcomes such as altered proliferation, adhesion and invasion of endothelial cells. Thus they can act as intracellular second messengers in various signalling pathways to control cell function. Due to differences in the chemical properties of each type of oxidant species, such as half-life and lipid solubility, not all reactive oxygen species are able to function as efficient signalling mediators [20]. For example, O2- has a short half-life of only 1 μS and is membrane-impermeable due to its polarity. Therefore its signalling capacity is limited. By contrast, H2O2 is more stable and has a relatively long half-life of 10 μS. It can freely permeate biological membranes and has emerged as the major redox metabolite mediating redox signalling events [21]·H2O2 acts to modulate numerous cellular processes via diverse mechanisms including the regulation of gene transcription, mRNA and/or protein stability, intracellular trafficking and protein activity [22]. The precise mechanisms which underlie the regulation of the generation of H2O2 and the specificity of its action are not fully understood. However it is clear that in complex metazoan systems, the modulation of redox-signalling events must include intracellular compartmentalisation and gradients of the oxidative signal, together with differential reactivity of the target sensors and bioavailability of buffering thiol peroxidase systems [21], [22].
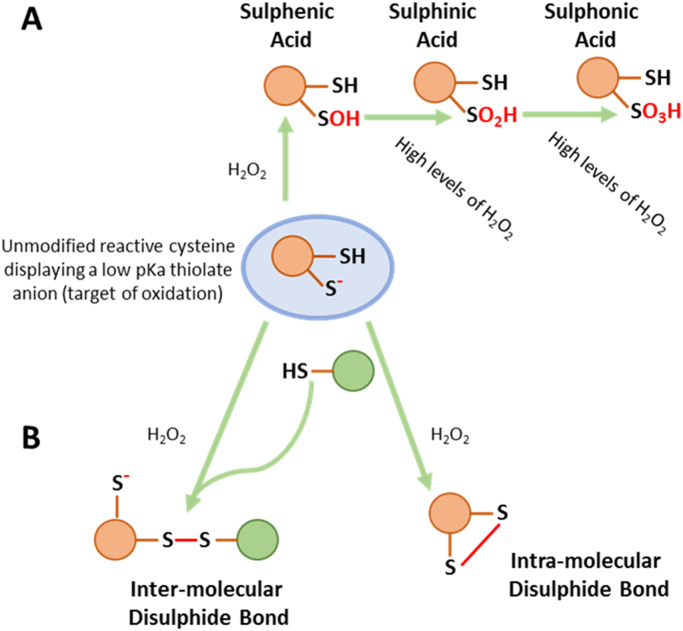
Physiological redox signals are transmitted though the reversible oxidative modification of target proteins to alter conformation and function·H2O2 can act to modify cysteine residues on redox-sensitive proteins [23] that possess reactive thiol groups which exhibit a low pKa [18] (Fig. 3). A number of different types of H2O2-induced, post-translational modifications exist, including those that involve the sequential oxidation of a thiol which proceeds through sulphenic acid (SOH), sulphinic acid (SO2H) and sulphonic acid (SO3H) states [24] (Fig. 3). In addition to these modifications, H2O2 can also induce covalent intra- and inter-disulphide bond formation to elicit a functional change (Fig. 3) [25], [26]. Of these oxidation states, cysteine-sulphenic acids and disulphides are readily reversible via the cell's intrinsic glutathione and thioredoxin antioxidant systems, while cysteine-sulphinic acid derivatives, (once thought to be irreversible), can also be reduced the action of sulfiredoxins [27]. By contrast, cysteine sulphonic acid modifications are not known to be reversible. Both O2- and H2O2 can also react with metal centres, often located within the active sites of target proteins. For example, H2O2 can react with a Mn-Fe centre in the active site of protein phosphatase-1 leading to its inhibition [28].
Fig. 3.
H2O2-induced post-translational modifications. A schematic diagram indicating H2O2-induced post-translational modifications of a cysteine thiol moiety. The pKa, which is a measure of hydrogen dissociation from a given thiol, is determined by the microenvironment in which the thiol is situated, with basic surrounding amino acids tending to deprotonate the thiol more efficiently than acidic amino acids A) The progressive oxidation of a deprotonated, reactive thiol by H2O2 proceeding through sulphenic, sulphinic and sulphonic acid states. B) Inter and Intra-molecular disulphide bond formation. These modifications lead to functional changes within a protein.
1.2. Functional vascular sources of reactive oxygen species: NADPH oxidases
Within vascular cells, O2- and H2O2 is derived from a number of enzymatic sources including the mitochondrial electron transport system, cytochrome p450, xanthine oxidase and uncoupled nitric oxide synthase (NOS). As stated above, the O2- derived from these sources is generally considered to be the result of mis-regulated metabolic functions, although there is some evidence for its potential functional importance in some cellular signalling pathways. By contrast, the family of flavoenzymes known as Nicotinamide Adenine Dinucleotide Phosphate Oxidases (NAPDH oxidases/Noxs) generate O2- or, in some cases, H2O2 in a tightly regulated manner as their sole biological function [29]. The prototype NADPH oxidase, originally identified in phagocytes, comprises the membrane-associated catalytic gp91phox (now termed Nox2) and regulatory p22phox subunits, in addition to the cytosolic subunits; p47phox, p67phox and p40phox together with the small GTPase, Rac1. Nox homologues were subsequently identified in non-phagocytic cells, and in humans, NADPH oxidases now comprise a known family of 7 multi-subunit transmembrane proteins (each containing a distinct catalytic subunit; Nox1-5 or duox 1 and 2) which display distinct cell-type and subcellular expression patterns [29], [30]. Each catalytic Nox subunit contains at least 6 transmembrane alpha helices and, with the exception of Nox5 and duox1/2, all isoforms associate with p22phox. However, different Nox isoforms additionally associate with other varied regulatory proteins which modulate their regulation and function. For example, p22phox can associate with Polymerase Delta Interacting Protein 2 [31] to activate Nox4 in smooth muscle cells (SMCs) whereas Nox1 activation is mediated by association with Nox organiser 1 (NOXO1) and Nox activator 1 (NOXA1) [32]. Mechanistically, all the Nox subunits shuttle electrons from NADPH down an electrochemical gradient across a membrane to molecular oxygen (O2) which is subsequently reduced to superoxide (O2-) [33], [34]. However, in the case of some Noxs (such as Nox4 and duox1/2) the O2- that is produced is very rapidly converted to H2O2. The exact mechanism(s) by which this is achieved is still unclear although some studies suggest that Nox4 may be capable of mediating an intrinsic superoxide dismutase activity [35]. O2- or H2O2 newly-generated by Noxs can be utilised by the cell in order to elicit tightly-controlled, cellular responses [29], [30], [36]. Moreover, the differences in structure, activity, expression, regulation and the type of oxidant species produced enable each specific Nox protein to direct its own distinct and defined functions [37], [38], [39].
Nox proteins are expressed in all of the component cell types of the vascular wall. SMCs express Nox1, Nox4 and Nox5 whereas the endothelium contains 4 Nox isoforms; Nox1, Nox2 (including the associated phagocytic subunits), Nox4 and Nox5 [40]. Their distinct physiological (and pathophysiological) roles in vascular function have, in some cases, been demonstrated in mouse models in vivo. For instance, genetic ablation of Nox1 or Nox2 was shown to elicit beneficial effects on hypertension and vascular dysfunction in certain pathophysiological settings [41], [42], [43], [44], [45]. By contrast, deficiency of Nox4 in mice acted to promote angiotensin II-dependent vascular dysfunction, suggesting that Nox4-generated H2O2 plays a protective role in vascular cells [46]. The different functions of Nox2 and Nox4 within the vasculature have been further illustrated by the contrasting phenotypes of transgenic (TG) mice which overexpress these genes specifically within endothelial cells. Thus we demonstrated that endothelial-specific overexpression of Nox2 (directed by the Tie2 promoter) resulted in increased blood pressure in mice after angiotensin II infusion, and attenuated acetylcholine- (Ach-)induced vasorelaxation in isolated aortas [47]. Meanwhile Tie2-mediated overexpression of Nox4 within endothelial cells acted to reduce basal blood pressure within TG mice, and enhanced endothelial-dependent vasodilation ex-vivo [48]. Therefore Nox4 is increasingly considered to be a positive regulator of vascular homeostasis [48], [46], [49], and in this regard it may be significant that it is abundantly expressed in the endothelium, compared to other Nox isoforms [39], [50], [51].
1.3. Reactive oxygen species and redox signalling in angiogenesis
There is now a large body of evidence which demonstrates that both O2- and H2O2 participate in redox-dependent signalling pathways which can modulate angiogenic responses both in vitro and in vivo. Early in vitro studies demonstrated that topical application of H2O2 to bovine thoracic aortic endothelial cells (BAECs) enhanced proliferation, migration and tube formation compared to controls and that these effects could be ablated by the administration of catalase [52]. Perhaps significantly, it has been reported that these H2O2-induced angiogenic responses occur in a biphasic manner, with low concentrations of H2O2 enhancing angiogenic phenotypes and higher concentrations inhibiting them [53]. In addition, as might be expected from their very diverse properties, there is specificity with regard to the angiogenic effects of individual reactive oxidant species. For example, O2- and H2O2 were shown to be both pro- and anti-angiogenic at varying concentrations, whereas •OH was found only to exert anti-angiogenic effects, or to have no effect at all at low concentrations [53].
Many studies have now shown that some reactive oxygen species can promote the induction of VEGF expression in both endothelial and smooth muscle cells [54], [55]. In addition, VEGF (and other angiogenic growth factors such as angiopoietin-1) have been shown to elicit angiogenic cellular responses via O2-- and/or H2O2-dependent molecular mechanisms [56]. VEGF-induced endothelial cell sprouting, mediated via Transforming growth factor-β-activated-kinase 1 (TAK1), was also shown to involve the increased expression of (mitochondrial-expressed) SOD2. The functional involvement of H2O2 (generated by SOD2) was demonstrated as the impaired angiogenesis, observed in aortic rings in which endothelial TAK1 was ablated, could be rescued by the overexpression of SOD2 [57]. Thus reactive oxygen species are involved in the regulation of cellular angiogenic responses, both upstream and downstream of the induction of VEGF expression. In vivo, a potential role for H2O2 in angiogenesis was first demonstrated in extra-cellular-SOD-transgenic mice, which displayed increased H2O2 production and angiogenesis in a murine model of hind-limb ischemia [58]. Moreover, increased H2O2, VEGF production and Akt phosphorylation, associated with the promotion of physiological pulmonary angiogenesis, was also observed in exercise-trained rats [59].
1.4. Vascular NADPH oxidases and angiogenesis
The physiological sources of reactive oxygen species which regulate angiogenic cellular processes are potentially varied, and there is evidence for the involvement of O2- and H2O2 derived from several different sources in some in vitro settings. For instance, the stimulation of human retinal endothelial cells with high levels of glucose increased VEGF expression and cell proliferation in a manner dependent on mitochondrial O2- production from the electron transport chain (ETC) [60]. Moreover, a recent study demonstrated that treatment of human umbilical vein endothelial cells (HUVECs) with VEGF significantly elevated mitochondrial H2O2 production and subsequent cell migration [61]. In addition, the inhibition of endogenous xanthine oxidase was shown to reduce VEGF-stimulated Akt phosphorylation in HUVECs suggesting that xanthine oxidase may also be important for VEGF- induced endothelial cell survival [62]. However the major sources of O2- and H2O2 which function as signalling molecules in the angiogenic processes in vascular cells are recognised as the NADPH oxidases [56].
The (patho)physiological importance of Nox1, 2 and 4 in angiogenic processes in vivo has, in each case, been demonstrated. Global genetic ablation of Nox1 was shown to impair tumour angiogenesis in mice [63], while (again global) genetic deletion of Nox2 or Nox4 was reported to reduce blood flow recovery in ischemic mouse hindlimb models [46], [64]. Nox4 expression was further shown to be required to support exercise-induced angiogenesis in mice [65]. However, in a recent study in which the consequences of the ablation of Nox1,2 or 4 were assessed in a slow-growing mouse tumour model, only Nox4 was found to contribute positively to angiogenesis. By contrast, ablation of Nox2 had no effect, and deletion of Nox1 actually enhanced angiogenesis [66]. The significance of the apparent discrepancies in these findings is not clear, but likely reflects the different experimental models adopted. The physiological importance specifically of Nox4 in angiogenesis is further evidenced by endothelial-specific TG overexpression of Nox4 in mice which demonstrated increased angiogenesis in an ischemic hind limb model [49]. Accordingly, in vitro, Nox4 overexpression was shown to increase endothelial cell proliferation, migration and tube formation [49], [67]. It is perhaps also significant that Nox4 is an inducible gene, whose transcriptional expression is activated by hypoxia [49], [67]. This clearly may have relevance with regard to its potential functional role in the regulation of angiogenesis.
At the molecular level, the mechanisms which underlie the angiogenic functions of Noxs have been extensively studied in vitro. Nox-dependent upregulation of HIF1α and VEGF expression via Akt and ERK1/2 activation in prostate cancer cells was demonstrated by siRNA-mediated targeting of the common p22 phox subunit [68]. In the specific case of Nox1, overexpression in NIH3T3 fibroblasts promoted VEGF and VEGFR expression in an H2O2-dependent manner [69]. The (mRNA and protein) expression of Nox1 was also shown to be increased by hypoxia in A549 (epithelial) cells, which in turn resulted in increased HIF expression (the transcriptional activator of VEGF), that could be blocked by catalase, or the flavoprotein inhibitor diphenylene iodonium (DPI). Both Nox1 and Nox2 are activated by the small GTPase, Rac1 [29]. AGS cells (gastric cancer cells) in which Rac1 expression had been depleted showed reduced expression of both HIF-1α and VEGF, while media conditioned by these cells inhibited cell proliferation when applied to human endothelial cells ( [70]). A potential function for Nox4 in the upstream activation of VEGF expression has also been demonstrated, as siRNA-mediated inhibition of Nox4 expression resulted in decreased HIF2-α and VEGF promoter activity in HEK-293 fibroblasts [71]. In addition, overexpression of Nox4 in human microvascular endothelial cells (HMVECs) was sufficient to increase VEGF mRNA levels, while downregulation of Nox4 decreased the level of (HIF-1α-dependent) VEGF expression after insulin stimulation [72].
There are also many reports of the involvements of Noxs in the signalling pathways downstream of VEGF and other pro-angiogenic growth factors. VEGF and angiopoietin-1 have been shown to stimulate endothelial cell migration in a Nox2-dependent manner [73], [74]. In addition, Nox4 was shown to mediate VEGF-dependent angiogenic reponses in human microvascular endothelial cells in vitro, by enhancing receptor tyrosine kinase phosphorylation and the activation of ERK1/2 [75]. Moreover in a separate study, VEGF stimulation resulted in a physical interaction between phosphorylated VEGFR2 and Nox4 that promoted STAT3-dependent proliferation of endothelial cells [76]. Finally, a study by Evangelista et al. demonstrated that VEGF induced endothelial migration in a Nox4- and Nox2-dependent manner and that this involved the S-glutathiolation of SERCA2b which subsequently increased Ca2+ influx into endothelial cells [77]. The precise protein targets of Nox-generated O2- and/or H2O2 which are susceptible to oxidation and act to modulate the angiogenic responses in endothelial cells are not known. However it has been demonstrated that H2O2 produced by ectopic expression of extracellular SOD (ecSOD) can increase VEGF-induced phosphorylation of VEGFR2 via the oxidative inactivation of protein tyrosine phosphatase 1B (PTP1B) (a negative regulator of VEGFR2 signalling) [58]. It is of note that in many separate studies PTP1B has also been shown to be a target of oxidative modification by Nox4 [78], [79]. In addition to PTP1B, VEGFR2 has also been shown to have redox-sensitive cysteine residues in its kinase domain [80] and it has been suggested that H2O2 can induce the formation of an inhibitory intramolecular disulphide bond in VEGFR2 [81]. However the functional significance of this with respect to the cellular angiogenic responses to H2O2 remains to be determined.
A central principle in intracellular redox-signalling mechanisms is the localization of the (freely diffusible) signalling molecules close to their targets. The enzyme complexes generating these signals (such as H2O2-generating Nox4), must therefore be localised to specific intracellular compartments in order to effect specific responses. The subcellular location of Nox4 has been the subject of some controversy, and has been reported, in disparate cells types, in the nucleus, endoplasmic reticulum (ER), mitochondria, focal adhesions and plasma membrane (reviewed in [82]). The underlying reasons for these discrepancies may reflect cell-type specific differencies in Nox4 function, or the poor specificity of available antibodies. However, in the case of the Nox4-dependent oxidation of PTP1B, (described above), it has been shown that the co-localization of Nox4 and PTP1B within the ER of endothelial cells was a functional requirement [78].
1.5. Gasotransmitters
It is now known that in addition to the involvement of reactive oxygen species (highlighted above) another group of endogenously-produced small molecules appear to be important for vascular responses both in vitro and in vivo, including angiogenesis, cell survival and the regulation of vascular tone [83], [84], [85]. Collectively termed “gasotransmitters”, NO, CO and H2S comprise a group of small molecule signalling agents which were previously known as toxic gases [86] but are now recognised as physiological regulators and effectors in diverse biological processes. They have become the subject of much research and are now recognised to contribute to signalling pathways in angiogenesis both upstream and downstream of VEGF (and other angiogenic growth factor) expression as described below.
The term gasotransmitters is, however, somewhat misleading as these molecules are completely soluble at physiologically-relevant concentration and pH and so perhaps should not be considered to be gases in these circumstances. None-the-less, their discovery and the characterisation of their mechanisms of action has altered the conventional paradigm of intercellular signalling. Unlike conventional signalling molecules (such as peptides) that are often stored in vesicles, gasotransmitters are generated on demand by distinct enzymes. Furthermore, peptide signals tend to mediate their function by binding to plasma membrane receptors such as receptor tyrosine kinases. By contrast, gasotransmitters can diffuse across membranes both within and between cells and subsequently interact with their protein target(s) directly to modify structure and function [84]. In this regard they share similar properties with some reactive oxygen species, most notably H2O2. Their biochemical targets (in common with both O2- and/or H2O2) are redox metal centres (most notably iron-containing haem proteins) and redox-active amino acids such as cysteine thiol groups. Chemical reactions between the gasotransmitters and some reactive oxygen species (such as the reaction between O2- and NO to form peroxynitrite (NOO-)), together with significant commonality in their targets, results in a significant interdependence between these groups of signalling molecules in their biological functions [87]. Thus the specific chemical properties and reactivity of the signalling oxidant molecules and the gasotransmitters have been utilised together to modulate complex signalling pathways as is beginning to become apparent.
As stated above, gasotransmitters must be synthesised, as required, close to their specific sites of action [84] and therefore both the expression and the activity of their biosynthetic enzymes must be tightly controlled. Within the vascular system, the relevant enzymes involved in the generation of these gases are endothelial nitric oxide synthase (eNOS) for NO [88], haem oxygenase-1 (HO-1) for CO [89] and cystathionine gamma-lyase (CSE) for H2S [90] (Fig. 4). In addition to the modulation of function of the gasotransmitters by their direct reactivity (in some cases) with O2- and/or H2O2, there is also increasing evidence that the expression and/or function of each of these biosynthetic enzymes is regulated by redox-dependent mechanisms. Therefore the gasotransmitters should, as a group, be considered to be important effectors of redox-dependent signalling mechanisms which regulate the angiogenic cellular responses, as detailed below.
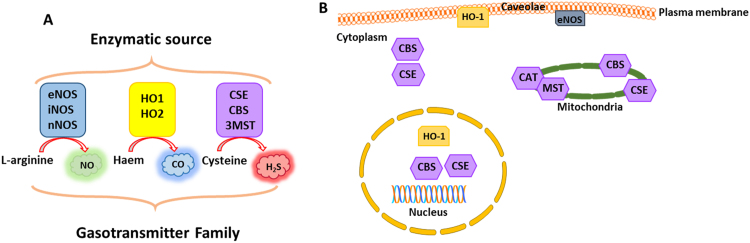
Fig. 4.
Gasotransmitters. A schematic diagram depicting the major gasotransmitters and their respective enzymatic sources and subcellular locations. A) The relevant vascular sources of these gases are eNOS for NO, HO-1 for CO and CSE for H2S. eNOS: Endothelial Nitric Oxide Synthase, nNOS: Neuronal Nitric Oxide Synthase, iNOS: Inducible Nitric Oxide Synthase, HO-1: Haem oxygenase-1, CSE: Cystathionine-γ-lyase, CBS: Cystathionine-β-synthase, and 3MST: 3 mercaptopyruvate sulphur transferase. B) Intracellularly, eNOS is located in caveolae at the plasma membrane, HO-1 can be found in caveolae and the nucleus, CSE and CBS are located in the cytoplasm and may also be found in the nucleus and mitochondria. CAT and MST are located in the mitochondria.
1.6. Nitric oxide (NO)
In 1980, Furchgott and Zawadzki published their seminal research on the function of the endothelium in vasomotion. Here it was demonstrated that an intact endothelium is absolutely required for the mediation of ACh-induced vasorelaxation in rabbit aortic rings [91]. These observations defined a role for the endothelium in the production of a vasoactive substance termed endothelial-derived relaxing factor (EDRF) [91] which was subsequently identified as NO [92]. Since then the study of signalling by NO has expanded exponentially and many important physiological roles within the cardiovascular system have been ascribed to this gaseous mediator [93].
NO is synthesised enzymatically by a family of enzymes collectively termed nitric oxide synthases (NOS). The NOS family encompasses three distinct isoenzymes encoded by separate genes that include neuronal NOS (nNOS, NOS-I), inducible NOS (iNOS, NOS-II) and endothelial NOS (eNOS, NOS-III). eNOS is constitutively-expressed within endothelial cells [94] and utilises the amino acid L-arginine as a substrate for the production of NO and L-citrulline under physiological conditions [95]. Numerous agonists have been shown to induce eNOS activation, including ACh, serotonin, PPARs, bradykinin, histamine, Ca2+ ionophores, adiponectin, VEGF, fluid shear stress and hypoxia [96], [97]. NOS enzymes comprise an N-terminal oxygenase domain with binding sites for haem, L-arginine and tetrahydrobiopterin (BH4), and a reductase domain which binds NADPH, flavin mononucleotide (FMN), FAD, and calmodulin (CaM) (reviewed in [96]). The active eNOS enzyme is a homo-dimer containing a zinc ion, tetrahedrally co-ordinated to cysteine residues at the surface of the dimer [98]. Under quiescent conditions it is anchored to caveolae through its interaction with caveolin-1, and its activation is dependent upon Ca2+/CaM, which binds to and displaces eNOS from caveolin, resulting in a conformational change that promotes the movement of electrons from NADPH to the haem moiety in the reductase domain [99]. Its activity can be modulated by numerous posttranslational modifications including phosphorylation at multiple serine, threonine and tyrosine sites [96], palmitylation, glutathionylation, and S-nitrosylation, and association with other proteins such as heat shock protein 90 (Hsp90) [100]. The bioavailability of its substrate, L-arginine and the BH4 co-factor are additionally crucial determinants of eNOS function. Indeed, loss of BH4 results in eNOS uncoupling and subsequent generation of O2- [101].
1.7. The role of nitric oxide in angiogenesis
Once formed in the endothelium, NO diffuses to neighbouring SMCs where it activates soluble guanylate cyclase (sGC) by binding to its haem group. sGC converts guanosine triphosphate (GTP) into cyclic guanosine monophosphate (cGMP) which then activates Protein Kinase G (PKG). PKG has a number of downstream targets that act to cause SMC relaxation [102]. The importance of eNOS in controlling vasodilation and blood pressure is highlighted by the vessel-constrictive effects of competitive eNOS inhibitors such as NG-monomethyl-L-arginine (L-NMMA) and NG-nitro-L-arginine-methyl ester (L-NAME) on endothelium-induced vasodilation in ex vivo experiments [103], and the clinical use of these inhibitors in the diagnosis of endothelial dysfunction [104]. Direct genetic evidence for eNOS-induced control of blood pressure was confirmed in mice by disruption of the eNOS gene, since these mice displayed enhanced systemic blood pressure compared to wild-type (WT) controls [105]. In addition to its well-established role in regulating vascular tone, NO has also been shown to be a mediator of post-natal angiogenesis (Table 1). Mice in which eNOS has been genetically ablated develop normally, but display a severe form of critical limb ischemia in mouse hindlimb models [106], [107], together with impaired wound healing and defective angiogenesis [108]. Both angiogenic and arteriogenic responses to hindlimb ischemia were severely blunted in eNOS-/- mice, an effect that could be rescued by adenoviral-mediated delivery of a constitutively-active form of eNOS [109].
Table 1.
A summary of the angiogenic effects of each gasotransmitter and its respective synthetic enzyme, both in vitro and in vivo/ex vivo. MI: Myocardial Infarction, CORM: CO releasing molecule, L-NAME: NG-nitro-L-arginine-methyl ester, SDF-1: stromal cell-derived factor-1.
| Gasotransmitter | Role in angiogenesis (In Vitro) | Reference |
|---|---|---|
| eNOS/NO | HUVEC form capillary-like structures when stimulated with VEGF, and this is blocked by L-NAME. | [110] |
| Overexpression of eNOs, or NO-donors promoted VEGF expression in VSMCs which could act in a paracrine manner to promote endothelial cell proliferation | [125] | |
| HO/CO | HO-1 overexpression enhanced endothelial proliferation. | [174] |
| HO-1 gene silencing reduced capillary formation, an effect rescued by CORM. | [89] | |
| CO gas or CORM-2 inhibited VEGF-dependent angiogenic responses in HUVECs | [175] | |
| CORM-2 promoted angiogenic responses in HUVECs, upstream of VEGF | [176] | |
| Chemical inhibition of HO reduced angiogenic activities of endothelial cells both upstream and downstream of VEGF | [177], [180] | |
| HO-1 silencing perturbed VEGF-dependent angiogenic responses in endothelial cells | [182] | |
| CSE/H2S | Exogenous H2S increased HUVEC proliferation. | [90] |
| NaHS administration promoted angiogenic responses in cultured endothelial cells | [232] | |
| Gasotransmitter | Role in angiogenesis (In Vivo/Ex Vivo) | Reference |
| eNOS/NO | eNOS-/- mice show reduced capillary growth in implanted collagen plugs | [111] |
| eNOS-/- mice show impaired angiogenic responses after hindlimb ischemia | [106], [107] | |
| VEGF-induced angiogenesis is blocked by L-NAME in rabbit cornea | [112] | |
| HO/CO | Ablation of HO-1 in mice prevented capillary sprouting in aortic rings in response to SDF-1 | [183] |
| HO1 inhibition blocks angiogenesis in solid tumours in rats. | [185] | |
| HO-1 is necessary for post-injury reparative neovascularization after hind-limb ischemia and cutaneous wounding in mice | [187], [188] | |
| CSE/H2S | NaHS administration increased neovascularisation and haemoglobin content of Matrigel plugs in mice. | [183] |
| NaHS administration promoted angiogenesis after hindlimb ischemia in rats | [233] | |
| Microvessel formation in aortic rings and wound healing was impaired in CSE-/- mice. | [90] | |
| NaHS administration improved wound-healing in type-2 diabetic mice | [234] |
There is much evidence to support NO-mediating signalling events downstream of VEGF. Thus HUVEC form capillary-like structures when stimulated with VEGF, an effect that can be blocked by the NOS antagonist, L-NAME [110]. In vivo, mice with genetic ablation of the eNOS gene (eNOS-/-) demonstrated reduced capillary growth in implanted collagen plugs, and impaired angiogenic responses to hindlimb ischemia compared to control animals, and in both cases the phenotype could not be rescued by administration of VEGF [106], [111]. In addition, VEGF-induced angiogenesis was shown to be blocked by L-NAME in a rabbit cornea model of angiogenesis [112]. Mechanistically, the activation of (human) eNOS is associated with phosphorylation at Ser615, Tyr81, Ser633 and Ser1177 [113], [114], [115], [116], and has been shown to be mediated by the actions of multiple protein kinases, including Akt/PKB, PKA, c-Src and AMPK [117], [118]. Activation of VEGFR2 in endothelial cells (by VEGF binding) results in the Akt-dependent phosphorylation of eNOS at Ser1177 [113], [119], while VEGF treatment of BAECs resulted in the phosphorylation of eNOS at Ser617 and Ser635 (equivalent to human Ser615 and Ser 633) [116]. The kinase responsible for phosphorylation of Ser617 in this study was suggested also to be Akt, while phosphorylation at Ser633 (of human eNOS) has recently been shown to be mediated, in response to VEGF signalling by the serine/threonine-protein kinase, Pim1 [120]. In addition, VEGF-signalling induces the c-Src-dependent phosphorylation and association of Hsp90 with eNOS [121], eNOS is additionally activated by other stimuli, including shear stress [122], which has been shown to be dependent on both Akt- and PKA-dependent mechanisms. NO is believed to promote angiogenesis by a sGC-cGMP-dependent pathway(s), although the precise mechanisms remain to be fully elucidated [123]. There is also evidence that NO can act upstream of VEGF signalling to promote angiogenesis. Thus increased NO, either administered by exogenous NO donors, or due to induction or overexpression of NOS, acted in vascular smooth muscle cells (VSMCs) in vitro to increase VEGF expression [124], [125]. In addition, inhibition of NOS in vivo eliminated the increased expression of VEGF (and VEGFR-2) in electrically stimulated capillary growth in rat muscle for up to 4 days [126]. Mechanistically, NO has been shown to activate the VEGF promoter via the cis-regulatory hypoxia response element (HRE) which mediates the transactivation of VEGF transcription by HIF-1 [127]. It should be noted, however, that in an ex vivo model of balloon angioplasty in rat thoracic aortae, increased VEGF expression was inhibited by NO donors [128] and the reasons for the discrepancies in these observations is currently not clear.
In recent years it has become apparent that asymmetric dimethylarginine (ADMA) and its associated metabolic enzymes dimethylarginine dimethylaminohydrolase I and II (DDAHI and DDAHII) can regulate angiogenesis through their effects on NOS substrate bioavailability [129], [130]. ADMA is an endogenous methylated form of L-arginine that acts as a competitive inhibitor of all NOS enzymes, and is formed from the hydrolysis of proteins that have been methylated on arginine residues. DDAHI and II are enzymes that metabolise ADMA and therefore negate its inhibitory effects on NOS, elevating NO levels [131]. Both in vitro and in vivo studies have demonstrated that DDAH enzymes are involved in angiogenesis. Aortic rings isolated from DDAHI heterozygous mice (DDAH+/-) showed reduced sprouting in Matrigel. This effect could be mimicked through the addition of ADMA to ring explants from WT mice, suggesting that endogenous removal of ADMA by DDAHI is required for vessel growth [132]. Moreover, endothelial-specific ablation of DDAHI blunted angiogenesis both in vitro and in vivo [133], while gene-targeted over-expression of DDAHI enhanced NO production and angiogenesis in a murine model of hindlimb ischemia that was associated with reduced ADMA levels [134].
1.8. Regulation of nitric oxide-dependent signalling by redox mechanisms
The relationship between reactive oxygen species and the signalling functions of NO is complex, and species-type dependent. NO bioavailability is considered to be a fundamental requirement for vascular health [135]. It has long been known that O2- readily reacts with NO to form NOO-, and so acts to reduce NO bioavailability, thereby contributing to vascular disease aetiology [136]. Indeed, Nox1- and Nox2-derived O2- is thought to inactivate NO and enhance hypertension [42], [43], [44], [45] and vascular dysfunction in angiotensin II-induced stress [137]. Nox2 activity has also been shown to directly reduce NO bioavailability leading to enhanced plaque formation in a model of atherosclerosis [138], while detrimental effects of O2- on angiogenesis have also been reported [139]. A more oxidising basal cellular redox state can also act to modulate NOS enzymatic activity by direct oxidation of the enzyme itself, via glutathionylation [140], [141] or by oxidation of its co-factor, BH4 [142]. In both cases this converts the enzyme from a NOS to a superoxide-generating oxidase (or uncoupled NOS), and the NADPH oxidase, Nox2 has been demonstrated to be a potential oxidant source [141], [143]. The responsiveness of the major target of NO, sGC, can also be affected by the cellular redox state. Thus its ferrous haem can become oxidised to an NO-resistant ferric haem which is susceptible to ubiquitylation and degradation [144].
By contrast to O2-, both exogenous and endogenously-generated H2O2 have been shown in endothelial cells in vitro to promote both NO production and NO-dependent signalling [145] and to stimulate angiogenic responses (proliferation and migration) which are blocked by inhibiting eNOS [146]. Moreover, in vivo, the transgenic overexpression of catalase in mice demonstrated that endogenous, endothelial-expressed H2O2 mediates the upregulation of eNOS after exercise [147]. In BAECs H2O2 (but not O2-) was shown to increase both the rate of transcription and the stability of eNOS mRNA in a dose- and time-dependent manner [148]. These increases were shown to be prevented by both the antioxidant N-acetylcysteine (NAC), and the H2O2 scavenger, catalase, although the precise mechanisms of transcriptional regulation remain unknown. H2O2 was also shown to be able to upregulate eNOS activity through the modulation of signalling pathways that contribute to its activation via VEGF-dependent signalling. Thus Thomas et al. demonstrated that H2O2 could induce the phosphorylation of eNOS at Ser1177, which is known to be necessary and sufficient for VEGF-mediated endothelial cell migration, and this was shown to occur via the PI3-K/Akt pathway, shown to be activated by VEGF signalling (see above) [149]. Consistent with this, administration of H2O2 to BAECs was sufficient to promote NO production via Akt and Erk1/2 [150]. Independent of VEGF, H2O2 has also been shown to be a critical intermediate mediating phosphorylation of eNOS via activation of the G-protein-coupled receptor for ADP, P2Y1 [151]. H2O2 administration has also been shown to stimulate phosphorylation of bovine eNOS at Tyr83 (equivalent to Tyr81 in human eNOS) in BAECs via Src kinase, although it is unclear whether this is VEGF-dependent or independent [117].
As stated previously, H2O2 is produced endogenously within vascular cells from a number of enzymatic sources including, (perhaps most notably in endothelial cells) Nox4. Indeed, Nox4 overexpression has been shown to increase eNOS protein expression and activity in cultured endothelial cells and to be sufficient to promote proliferation, migration and tube formation [49]. Moreover, in vivo, endothelial-specific Nox4-overexpressing mice demonstrated accelerated blood-flow recovery in an ischemic hindlimb model as well as enhanced aortic capillary sprouting. Significantly, these Nox4-induced effects were ablated by genetic deletion of eNOS [49]. Perhaps consistent with these observations, global genetic ablation of Nox4 in mice resulted in an approximately 50% decrease in eNOS expression in the carotid artery, and significantly-reduced NO production which potentially contributed to the attenuated angiogenesis in these mice after femoral artery ligation [46]. Taken together, these studies strongly suggest that Nox4 is a physiological source of enzymatic H2O2 which acts to promote post-natal angiogenic responses, at least in part, via modulation of the expression of eNOS and/or modulation of VEGF-mediated activation of eNOS activity (Fig. 5). In this regard, the potential Nox4-generated H2O2-dependent oxidative inactivation of protein tyrosine kinases, including PTP1B and SHP2 (negative regulators of VEGFR2 signalling) may be of functional significance [78], [79], [152].
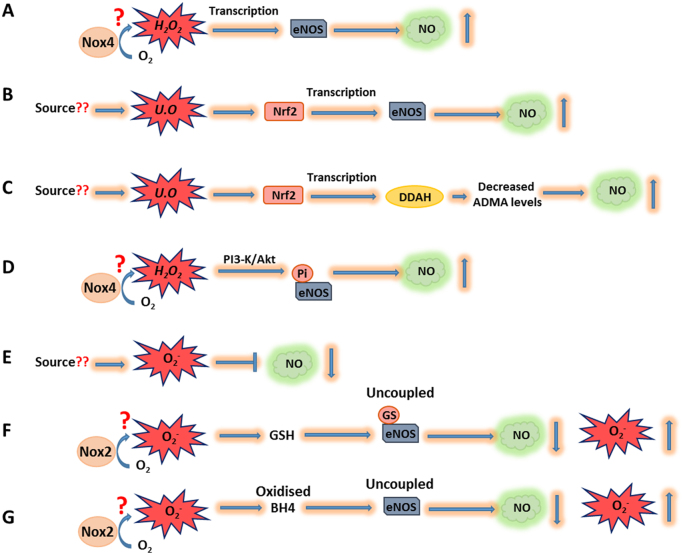
Fig. 5.
Reactive oxygen species-dependent mechanisms regulating NO bioavailability. A) H2O2 (potentially generated by Nox4) can increase eNOS expression via an unknown mechanism leading to increased NO production. B) & C) Oxidant-dependent activation of Nrf2 leads to increased transcription of eNOS (and therefore NO production) and DDAH. Increased DDAH expression in turn acts to reduce ADMA (a competitive inhibitor of NOS) levels resulting in increased NO production. D) H2O2 (potentially generated by Nox4) potentiates the activation of the PI3K/Akt signalling pathway which results in phosphorylation and activation of eNOS and increased NO production. E) O2- is capable of reacting with NO to form peroxynitrite which reduces the bioavailability of NO. F) Glutathionylation of eNOS under oxidative conditions and G) Oxidation of the eNOS co-factor, BH4, leads to eNOS uncoupling, reduced NO production and O2- production. The likely oxidant is O2- generated by Nox2. GSH: glutathione, GS: glutathionylation, Pi: phosphorylation, BH4: tetrahydrobiopterin, DDAH: dimethylarginine dimethylaminohydrolase, U.O: unidentified oxidant.
As mentioned above, the bioavailability of NO is also controlled by DDAH enzymes. A recent study has suggested a role for the redox-sensitive transcription factor, Nuclear factor erythroid 2-related factor (Nrf2) in the coordinated regulation of both DDAH and eNOS in renal glomerular endothelial cells [153]. Nrf2 is a basic leucine-zipper transcription factor that is regulated by the cytoplasmic protein Kelch-like ECH-associated protein 1 (KEAP1). In normal physiological conditions, KEAP1 sequesters Nrf2 in the cytoplasm where it is targeted for ubiquitin-mediated degradation. However, upon activation by pro-oxidants (among other stimuli), critical cysteine residues in KEAP1 are oxidised resulting in the liberation of Nrf2 and its subsequent translocation to the nucleus where it drives the expression of a battery of antioxidant and detoxifying genes [154]. The activation of Nrf2 using tert-butylhydroquinone was demonstrated to enhance Nrf2 translocation to the nucleus and subsequent DDAHI and II expression [131]. This effect was lost upon Nrf2 ablation. In addition, concomitant with the decrease in ADMA levels observed upon Nrf2 activation, both eNOS expression and NO production increased (Fig. 5). It was concluded that Nrf2 upregulates eNOS as well as DDAH enzymes to potentiate NO production and improve endothelial cell function [153]. The physiologically relevant endogenous source(s) of the oxidant(s) which might activate Nrf2 in this process is not known. However, we have shown that increased Nox4 expression can act to promote the expression of several Nrf2-target genes in other in vivo settings (see below) [155], [156], and therefore the increase of Nox4 expression seen in response to hypoxia [49] may be of relevance here.
From these studies it is clear that both O2- and H2O2 play diverse roles in the regulation of NO-dependent signalling, by regulating the expression and activity of eNOS, together with the bioavailability and functional capability of NO itself, and that these regulatory mechanisms have functional consequences in angiogenic phenotypes (Fig. 5).
1.9. Carbon monoxide
CO, first denoted as a ‘silent killer’, has come to be recognised as an important physiological mediator in a broad range of biological processes and systems, perhaps most notably in the neurological and cardiovascular fields [157]. CO has high affinity for haemoglobin, and therefore its subsequent ability to reduce O2 transport in the blood led to its initial characterisation as a toxic pollutant. However, it later became apparent that CO can be generated physiologically by a family of enzymes known as haem oxygenases (HOs) [158]. This family comprises 3 isoenzymes, HO-1 [159], HO-2 [160] and HO-3 [161], of which only HO-1 and −2 are thought to be catalytically-active proteins. HO-1 is an inducible isoform which has been most closely associated with CO-dependent signalling. Its expression and corresponding activity is both positively and negatively regulated by a number of stimuli including hypoxia [162], [163], heat shock [164] and oxidative stress [165]. It is noteworthy that these stimuli have species-specific effects, with hypoxia upregulating HO-1 in rat tissue [163] but decreasing it in human cells [162]. HO-1 has been shown to be localised to caveolae and cytosolic compartments of endothelial cells [166] as well as the nucleus under conditions of “oxidative stress” [167]. By contrast, HO-2 is constitutively expressed and is responsible for basal CO production primarily in the brain and cardiovascular system [168]. Enzymatically, HO catalyses the rate-limiting step in the breakdown of haem into biliverdin, iron and CO in a reaction that requires oxygen and NADPH [169] Once formed, CO can subsequently induce a number of targeted effects as discussed below.
1.10. The role of carbon monoxide in angiogenesis
A number of vascular roles have been ascribed to CO including reduced platelet activation [170] and monocyte-induced inflammation [157]. In addition, CO has been implicated as a vasoactive mediator that can induce vasorelaxation, an effect which has been characterised in a number of vessels including rat aorta [157] as well as mesenteric, renal and pulmonary arteries [171] and has been demonstrated using both CO donors and HO inhibitors [157]. The precise mechanisms by which CO-dependent signalling occurs are not fully understood. However, in common with NO, CO is a non-polar molecule which can freely diffuse across biological membranes and can bind sGC to modulate cGMP production [87]. In addition, some groups have suggested that CO-dependent signalling in the vasculature occurs in an endothelium-dependent manner [172]. Therefore there is likely to be a great deal of interdependence between the vascular functions of CO and NO.
In addition to vascular tone, HO/CO-signalling has also been implicated in angiogenic control (Table 1). HO-1 expression is rapidly induced in bovine aortic endothelial cells in response to hypoxia [173], and early evidence for its functional significance came from studies in which HO-1 overexpression enhanced endothelial cell proliferation [174]. By contrast, targeted gene silencing of HO-1 in endothelial cells reduced capillary formation and cell proliferation. Re-introduction of CO in the form of the CO donors, tricarbonyl-dichlororuthenium (II) dimer (CORM-1) and tricarbonylchloro(glucinato)ruthenium (II) (CORM-3), was sufficient to recover the angiogenic properties of HO-1 deficient endothelial cells [89]. CO-dependent signalling has been reported to act both upstream and downstream of VEGF induction in the angiogenetic response, although it should be noted that there remain inconsistencies within the literature with regard to its pro- or anti-angiogenic effects [175], [176], [177]. This may reflect the differences in cell types and/or differences in levels of CO applied to the cells [178].
Upstream of VEGF-signalling, HO-1 overexpression or activation has been shown to increase VEGF production in VSMCs, macrophages and endothelial cells [177], [179] and accordingly treatment of endothelial cells with CORM was shown to mimic this effect [177]. Consistent with this, inhibition of HO activity by tin protoporphyrin IX (SnPPIX) abrogated the increase in VEGF expression in VSMCs, observed upon stimulation by hypoxia, IL-1β or hemin [180], while ablation of HO-1 was shown to inhibit VEGF expression and the angiogenic responses of endothelial cells [89]. By contrast, in a separate study, the hypoxic induction of VEGF in rat aortic smooth muscle cells was shown to be supressed by both CO and NO [181] in a sGC- and cGMP-mediated pathway that resulted in decreased HIF-1 binding to the VEGF enhancer. Downstream of VEGF signalling, CO has also been reported to promote both pro- and anti-angiogenic effects. Thus VEGF was shown to induce sustained HO-1 expression and activity in cultured endothelial cells, and the angiogenic responses induced by VEGF were perturbed when HO-1 was inhibited [182]. However, in a more recent study using HUVEC, exposure to CO was found to inhibit VEGF-induced angiogenic responses [175]. Mechanistically, CO administration acted to suppress VEGF-stimulated VEGFR-2 phosphorylation and the downstream Akt phosphorylation. The discrepancies in these observations are likely to be due to differences in experimental design and remain to be reconciled. HO-1 has also been shown to mediate the pro-angiogenic effects of SDF-1, through a protein kinase C-ζ-dependent, VEGF-independent mechanism. Thus SDF-1 was shown to induce HO-1 expression in endothelial cells, and ablation of HO-1 in mice, in vivo, prevented aortic rings forming capillary sprouts in response to SDF-1 [183]. Mechanistically, SDF-1 was shown to promote the phosphorylation of vasodilator-stimulated phosphoprotein (VASP) via HO-1 activation to promote angiogenesis. In addition to modulating the expression and associated signalling pathways of pro-angiogenic factors, HO-1 has also been shown to act to deplete the levels of the anti-angiogenic factors, soluble fms-like tyrosine kinase I and soluble Endoglin, thereby potentially further enhancing angiogenesis [184].
In vivo, a potential pro-angiogenic role of HO/CO-dependent signalling has also been demonstrated. Thus the pan HO inhibitor, zinc protoporphyrin (ZnPP) was found to block angiogenesis in solid tumours suggesting a physiological role for HO in neovascularisation [185]. In addition, the induction of HO-1 activity, or the administration of a CO-donor both acted to promote formation of new coronary arteries after myocardial infarction induced by coronary artery ligation in rats [186]. In addition HO-1 was shown to be necessary for efficient post-injury reparative neovascularization after both hind-limb ischemia [187] and cutaneous wounding in mice [188]. A critical role for HO-1 placental vascular formation during embryonic development is also recognised. Thus HO-1 expression and endogenous CO production is highly elevated in the placenta during pregnancy [189] and a partial deficiency of maternal HO-1 results (in mice) in the restriction of the growth of both the placenta and the foetus that is due, at least in part, to impaired angiogenesis [190].
The manipulation of the HO/CO axis using pharmacological and genetic means has therefore implicated this pathway in the regulation of angiogenesis. However little is known about the exact mechanism(s) through which CO acts. As stated above, CO is believed to induce changes in protein activity primarily through its reaction with haem containing proteins [191], and both sGC and NOS have been implicated as potential targets, again suggesting cross-talk between gasotransmitters [192].
1.11. Regulation of carbon monoxide-dependent signalling by redox mechanisms
It is well-established that pro-oxidant-generating stimuli such as UVA radiation and cadmium chloride, in addition to H2O2 per se, are capable of inducing HO gene expression [193]. Indeed, HO-1 expression has been used as a model system for studying redox-regulated gene expression in mammalian cells [194]. Administration of H2O2 has been demonstrated to enhance both HO-1 and VEGF expression in a variety of cell types, including vascular cells, while chemical inhibition of HO-1 activity in part abrogated the increase in VEGF expression, underlying the significance of this redox-regulation to angiogenesis [195]. From several studies it has become apparent that many redox-sensitive cell signalling pathways and transcription factors are involved in regulating HO-1 expression including Mitogen Activated Protein Kinases (MAPKs) such as P38 [196] and JNK [197] as well as the transcription factor Hypoxia Inducible Factor 1α (HIF1α) [163] and, perhaps most notably, Nrf2 [156]. Cigarette smoke contains a number of pro-oxidants and application of aqueous extracts to NIH3T3 cells induced Nrf2 activation leading to HO-1 induction, an effect that was lost when Nrf2 was silenced [198]. Nrf2 has been shown to induce HO-1 transcription through two distal cis-acting enhancer elements in the HO-1 promoter that both contain Nrf2-binding consensus antioxidant response elements (AREs) [198]. The regulation of HO-1 in response to oxidants is further complicated by the involvement of a hypoxia-induced repressor of HO-1 transcription termed BTB Domain and CNC Homolog 1 (Bach1) [199]. By contrast to Nrf2, the activity of Bach1 is inactivated by oxidants and it was shown in human keratinocytes that arsenite-mediated HO-1 induction involves both the removal of Bach1 from the HO-1 promoter and the subsequent Nrf2-directed up-regulation in its expression [200]. Indeed, hypoxia-dependent suppression of HO-1 in human endothelial cells appears to be regulated by a complex interplay between Bach1 and Nrf2 [162], which is in turn potentially mediated by redox-dependent mechanisms.
Although it is well accepted that the Nrf2/HO-1 response is (in part) oxidant driven, the physiological endogenous source(s) of these oxidants remains unknown. However we have shown that Nox4-generated H2O2 can activate Nrf2 and induce HO-1 expression in cardiac-specific Nox4-overexpressing hearts [155]. Moreover, in vitro simulation of hemodynamic stress, using phenylephrine-stimulated neonatal rat ventricular myocytes, resulted in an increase in both Nox4 and Nrf2 protein expression, and a subsequent up-regulation in HO-1 mRNA expression. This effect was ablated when either Nox4 or Nrf2 were silenced [156] (Fig. 6). Consistent with these observations, global Nox4-null mice display reduced HO-1 expression in isolated lung endothelial cells, an effect that resulted in enhanced apoptosis. This has been attributed to perturbed CO production as re-introduction of CO using CORMs enhanced lung endothelial cell survival [46].
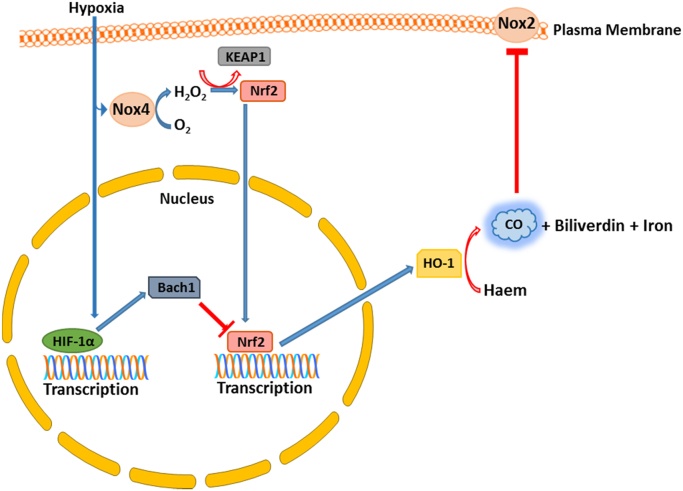
Fig. 6.
The inter-relationships between hypoxia, NADPH oxidases and CO. Hypoxia can act to increase Nox4 expression and H2O2 production, potentially leading to Nrf2 activation and increased HO-1 expression. In opposition to this, hypoxia also increases the expression of Bach1, a negative regulator of Nrf2 function and HO-1 expression. This interplay between transcription factors may therefore modulate CO production by HO-1. CO can itself also inhibit the activity of Nox2.
Intriguingly, there is also potential for the converse cross talk between CO- and Nox-dependent signalling mechanisms. Thus it has been demonstrated that CO administration can act to inhibit the activity of Nox2 within human airway smooth muscle cells and neutrophils [201] due to binding to the haem moiety. It will be of interest to determine whether this is a common regulatory mechanism, potentially modulating the activity of other NADPH oxidases.
1.12. Hydrogen sulphide
H2S was considered a toxic environmental pollutant until Abe and Kimura first proposed it to be an endogenous cellular signalling mediator [202]. Subsequent extensive research efforts have established H2S as a third member of the gasotransmitter family of signalling molecules and have ascribed to it a number of diverse functions within different physiological systems including the cardiovascular, immunological and nervous systems [203]. H2S is synthesised endogenously through the action of 3 pyridoxal 5′ phosphate- (vitamin B6)-dependent enzymes, cystathionine β-synthase (CBS), CSE and the combined action of 3-mercaptopyruvate sulphur transferase (3MST) and cysteine aminotransferase (CAT) [204]. Each of these enzyme systems displays promiscuity with respect to substrate specificity and function, and can additionally catalyse other reactions in the sulphur metabolic network [205]. These enzymes also display tissue-specific patterns of expression. Thus CBS plays a major role in H2S generation in the nervous system [206], while 3MST is widely distributed in multiple cell types including neurons, hepatocytes, cardiac cells and endothelial cells [207], [208]. However, within the vascular system, the major enzymatic source of H2S is CSE, which has been shown to be expressed and be active in a number of vascular cell types including SMCs [209], perivascular adipose cells [210] and endothelial cells [211], [212], [213]. CSE is a tetrameric protein and it generates H2S from a number of substrates including cysteine, cystine and homocysteine [205], [214]. Subcellularly CSE and CBS have been found in the cytoplasm [215], but have also been suggested to be in the nucleus [216] and under hypoxic conditions in the mitochondria [217], [218]. CAT and 3MST are generally considered to be localised to the mitochondria (see Fig. 4) [219], [220].
The chemical properties of H2S are such that under physiological conditions in aqueous solutions at pH 7.4, H2S exists in a dissociated state with approximately two thirds of total H2S existing as hydrosulphide anions (HS-) and protons (H+) with the remaining third being in the form of H2S undissociated, (dissolved) gas. HS- can subsequently form sulphide ions (S2-) through further dissociation but only at high, non-physiological pH. [S2-] is therefore considered negligible in physiological systems [221]. Undissociated H2S is a non-polar, lipophilic molecule which can diffuse through membranes in a similar manner to NO and CO. However, due to its dissociation at physiological pH, H2S should be considered to be less lipid- permeable than the other gasotransmitter molecules [222], and this may impact on its signalling functions. The term H2S is typically used collectively to denote both H2S and HS- anions as it is currently unknown which of these is the most physiologically relevant species. Once generated, H2S is believed to function as a secondary messenger by directly modulating the function of downstream targets. In common with both NO and CO, H2S can form coordination complexes with metal centres (such as haem groups) within proteins [223]. In addition, it acts to covalently modify cysteine residues on target proteins in a process known as S-sulfhydration [224]. Here, sulphur derived from H2S becomes added to the thiol group of a target cysteine residue to render the formation of a hydropersulphide moiety (-SSH). In one study it was shown that as much as 10–25% of some abundant proteins in mouse liver including actin, tubulin and glyceraldehyde-3-phosphate (GAPDH) exist in the S-sulfhydration state under physiological conditions [224]. Further, this was shown to be mediated by H2S generated enzymatically from L-cysteine by CSE. Thus the limited number of specific H2S target proteins currently identified [224] seems likely to grow.
1.13. The role of hydrogen sulphide in angiogenesis
One of the first biological activities attributed to H2S was in the regulation of vascular tone [225]. Subsequent research efforts using a combination of H2S donors [210], [226], [227], [228], [229] and direct genetic manipulation of the CSE gene have confirmed the physiological relevance of the CSE/H2S pathway in vasorelaxation and blood pressure regulation [230]. At the cellular level, studies using patch clamp techniques have demonstrated that both endogenous and exogenous H2S can directly hyperpolarize resistance artery VSMCs via modulation of ATP-gated K+(KATP) channel activity, and that these effects are independent of cGMP-mediated phosphorylation [231]. More recently, H2S has been demonstrated to be an endothelial-derived hyperpolarizing factor (EDHF) which exerts its vasodilatory actions on VSMCs by causing the opening of KATP channels, at least in part, through S-Sulfhydration of the Kir 6.1 subunit at cysteine-43 [212]. In addition to its actions as an EDHF acting on adjacent smooth muscle, H2S signalling is also thought to be important within endothelial cells themselves. Endothelial CSE can be activated by Ca2+/calmodulin [230] in response to cholinergic stimulation or by the calcium ionophore, A23187 leading to enhanced H2S production [230]. Moreover, stimulation of isolated endothelial cells with ACh resulted in hyperpolarisation in cells isolated from WT but not CSE-/- mice. This effect is mediated not by KATP channels but by small and intermediate calcium-gated K+ channels present within the endothelium, as the hyperpolarisation could be blocked by apamin and charybdotoxin [212]. Whether specific cysteine residues within these calcium-gated K+ channels are targets of S-Sulfhydration remains to be demonstrated.
H2S has also been shown to be a key regulator of angiogenesis (Table 1). Thus topical application of H2S to endothelial cells in vitro increased HUVEC proliferation as well as migration and capillary morphogenesis on Matrigel, which are considered essential initiating steps in the angiogenic response [90], [232]. In vivo, administration of NaHS (an H2S donor) increased neovascularisation and haemoglobin content of Matrigel plugs in mice [232] and, perhaps consistent with this, also led to improved capillary sprouting and blood flow recovery in a rat hindlimb ischemia model [233]. The significance of CSE-derived H2S has also been demonstrated in vivo, since both microvessel formation in response to VEGF and wound healing were impaired in CSE-/- mice compared to wild-type littermates [90]. In a separate study H2S was also shown to improve wound healing in type 2 diabetic mice via the activation of angiopoietin-1 [234]. Clinically, low plasma levels of H2S and reduced placental CSE expression are associated with women with pre-eclampsia and it has therefore been suggested that CSE may have important roles in placenta vascularisation [213].
Mechanistically, several studies have demonstrated that the proangiogenic effects of H2S involve the activation of both P13K-Akt and MAPK signalling pathways [90], [211], [232]. In this regard it might be significant that Phosphatase and Tensin Homolog (PTEN), a negative regulator of Akt-signalling has been proposed to be a potential direct target of H2S-mediated modification [235]. There is also increasing evidence to support the involvement of H2S-mediated modification of KATP channels in the stimulation of the MAPK signalling pathway(s). Thus it has been shown that the activation of KATP channels per se is sufficient to promote angiogenesis in endothelial cells in vitro [236] and conversely that KATP inhibitors abrogate VEGF-dependent angiogenic responses. Moreover, in one study the H2S-dependent activation of p38 MAPK was shown to occur via a KATP channel-dependent mechanism [90], that was shown to act downstream of VEGF-signalling. In addition, NaHS administration to tumour-derived human EC cells resulted in a proangiogenic cellular phenotype that was characterised by increased K+ and nonselective cationic currents, and increased cytosolic calcium [237]. These effects were again shown to act downstream of VEGF-signalling. CSE was identified in both these studies as the relevant endogenous source of H2S, as genetic [90] or pharmacological [237] ablation of CSE activity in both cases ablated the pro-angiogenic effects of VEGF. Another potential mechanism of action of H2S in promoting angiogenesis might be via the modulation of cGMP levels. As stated above, the importance of sGC/cGMP-dependent signalling (downstream of VEGF) in angiogenesis has been demonstrated previously. H2S has been shown to reduce the sGC haem-Fe from a ferric to a ferrous state, thereby promoting cGMP production [238]. In addition, H2S is an inhibitor of phosphodiesterase (PDE) activity (which breaks down cGMP), and therefore can further promote cGMP levels and signalling functions [239].
Recently the potential roles of miRNAs in the mediation of the proangiogenic effects of H2S were investigated. miR-640 was identified as an miRNA which was significantly downregulated in vascular ECs by H2S treatment and conversely overexpression of miR-640 reduced the proangiogenic effects of H2S [240]. The altered expression of miR-640 in response to H2S was blocked by inhibition of either VEGFR2 or mTOR, again suggesting the involvement of VEGF- and Akt-dependent signalling in the angiogenic response. However, mechanistically, miR-640 was shown to be a negative regulator of HIF1α, via direct binding to 3′UTR sequences within HIF1α mRNA. Perhaps consistent with these observations, the loss of CSE-derived H2S has been shown to be associated with decreased HIF-1α [241]. Thus H2S may potentially act upstream as well as downstream of VEGF-signalling to promote angiogenic responses and a feed-forward cycle between H2S production and HIF-signalling has been proposed [242]. CSE-derived H2S production has also been implicated in the negative regulation of expression of anti-angiogenic factors. For instance, CSE silencing in HUVEC resulted in the increased release of soluble fms-like tyrosine kinase I as well as soluble endoglin [213], while over-expression of CSE conversely dampened their release. Moreover, administration of the slow-releasing H2S donor, GYY4137, caused a reduction in circulating levels of these anti-angiogenic factors. Therefore H2S may potentially act upon multiple (potentially inter-related) signalling pathways to modulate the cellular angiogenic response.
1.14. Regulation of hydrogen sulphide-signalling by redox-dependent mechanisms
As is the case for NO and CO, the signalling functions of H2S that involve binding to metal centres can be modulated by the redox state of the coordinated metal ion, which in turn may be modulated by both the “steady state” redox potential of the cellular environment and/or the localised production of reactive oxygen species such as O2- or H2O2. In addition, the signalling mechanisms of H2S which involve thiol modification may also be subject to redox-dependent regulation. The precise mechanism through which H2S induces its modifications remains contentious. It was thought initially that H2S attacks previously oxidised cysteine residues such as those in the sulphenic acid (SOH) or disulphide bonded (S-S) state in a reduction reaction [224]. However recent studies have suggested that in order to mediate its function, H2S acts as an oxidant molecule through an initial reaction with H2O2 to form polysulphides (H2Sn). These polysulphides can then oxidise cysteine residues on target proteins yielding the persulphide moiety [226], [235]. Such a mechanism of H2S-mediated oxidative activation, ultimately resulting in the formation of an intermolecular disulphide bond, has been demonstrated in the case of PKG [226]. Moreover, the H2O2-dependent conversion of H2S to polysulphides results in the loss of polarity, and therefore greater lipid solubility of the signalling moiety. This therefore potentially increases the ability of H2S to signal to adjacent cells, and may be an important contributory factor in regulating the signalling functions of H2S.
Another potential level at which reactive oxygen species may act to mediate H2S-dependent signalling is by regulation of CSE-dependent H2S production. At the level of gene expression, Wang et al. showed that exogenous H2O2 increased the activity of the CSE promoter [243] while in another study CSE transcription was upregulated in rat mesangial cells by treatment with platelet-derived growth factor, and this upregulation was abolished by antioxidants including NAC and the Nox inhibitor, DPI [244]. However in the interpretation of these results it should be noted that DPI is an inhibitor of all flavoproteins, including NOS [245]. A potential role for Nox4 in the transcriptional regulation of CSE is also suggested by studies conducted in our group. Thus we have demonstrated that in endothelial cells in vitro, Nox4-generated H2O2 acts as a positive regulator of CSE transcription, via activation of a haem-regulated inhibitor kinase/ eIF2α/activating transcription factor 4 (ATF4) signalling module and CSE was found to be a direct transcriptional target of ATF4 [246]. In vivo, endothelial-specific Nox4 transgenic mice exhibited lowered blood pressure [48] and a hypo-contractile phenotype in response to phenylephrine that was abolished when vessels were incubated with the CSE inhibitor, propargylglycine [246]. We therefore conclude that Nox4 may be a physiologically relevant source of H2O2 that positively regulates CSE expression and H2S production [246] in endothelial cells (Fig. 7).
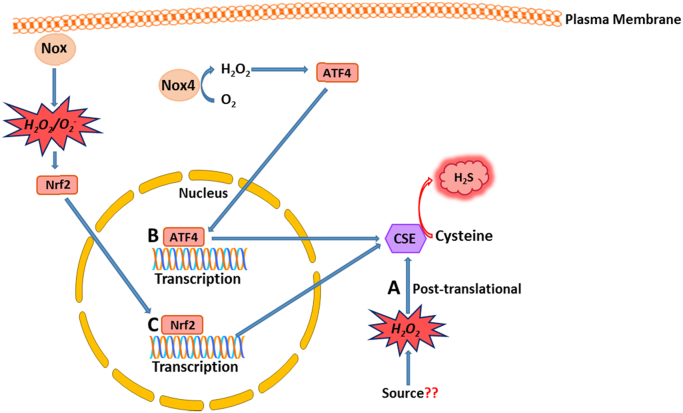
Fig. 7.
Redox-dependent mechanisms regulating H2S production by CSE. A) H2O2 (generated from an unknown source) can enhance CSE activity. B) Nox4 represents a potential source of H2O2 that can upregulate the transcriptional expression of CSE through an ATF4-dependent mechanism. C) NADPH-derived O2-/H2O2 can activate Nrf2 leading to increased CSE transcription. These redox-dependent transcriptional and post-translational changes in CSE expression may lead to altered H2S production. CSE: Cystathionine-γ-lyase.
In addition to playing a role in the transcriptional regulation of CSE, H2O2-dependent signalling may also modulate CSE activity. Thus in HUVECs it was shown, using a cell-trappable fluorescent H2S probe, that VEGF application acted to increase CSE-derived H2S production in a H2O2- dependent manner. The relevant source of H2O2 in these experiments was again suggested to be a Nox, as the increase in H2S production could be inhibited by DPI (although, as stated above, DPI is not a specific Nox inhibitor). Clearly, however, crosstalk between H2S and H2O2, in endothelial cells after VEGF-activation, may be important in the regulation of angiogenic cellular responses [247].
1.15. Interactions between gasotransmitters
The sequential discovery and characterisation of each of the gasotransmitters had initially led them to be considered as biologically distinct entities that mediate their functions independently of each other. While this is no doubt the case in certain settings, it has become apparent that considerable synergistic and antagonistic interplay occurs between this family of gases at multiple levels creating a multifaceted signalling platform though which key cellular and physiological processes, such as angiogenesis, are orchestrated [87]. Coletta et al. studied the synergistic effects of H2S and NO in the angiogenic response. Here, the pro-angiogenic effects of H2S were ablated in endothelial cells isolated from eNOS gene-deficient mice. Conversely the silencing of CSE and concomitant reduction in H2S production prevented NO-stimulated angiogenesis. Mechanistically, the mutual dependency between H2S and NO in the endothelium occurs on multiple levels and appears to be centred on the production of cGMP. NO is known to bind to and activate sGC leading to elevated cGMP production. By contrast H2S inhibits phosphodiesterase-5 (PDE5) preventing cGMP degradation. Furthermore H2S was also shown to enhance NO production in endothelial cells. Thus these gasotransmitters act in synergy to potentiate the effect of cGMP leading to protein kinase G (PKG) activation and subsequent modulation of key aspects of angiogenesis [123]. Interestingly, the haem group of sGC can be bound and activated by CO and NO but with varying affinities. CO, for example, binds at lower affinity than NO, an observation that may relate to compensatory actions of the gases [248]. By contrast H2S can reduce the haem iron of sGC from its ferric form to its ferrous form, an effect that promotes the binding of NO [238]. This ability for a single protein to become the target of multiple gasotransmitters has been shown to extend to a number of other proteins such as GAPDH which can be S-sulfhydrated by H2S and nitrosolated by NO [224].
The interrelationship between NO and CO extends further as it has been shown that NO can stimulate HO in the endothelium [249] but inhibit it in the brain [250]. To add a further layer of complexity to the interplay between the gases, it is becoming apparent that they may be able to react together to form novel signalling intermediates. Indeed the NO donor sodium nitroprusside (SNP) and H2S can react to form nitroxyl (HNO) [251] which has been shown to elicit anti-angiogenic effects in tumours [252]. Taken together it is clear that the effects of these gases cannot be viewed simply in terms of their individual functions and must be considered as part of a more complex system, in which many aspects of their biology are crucial, including, for example, their subcellular location. It is conceivable that compartmentalisation of the gasotransmitter producing enzymes into the same subcellular location may facilitate the interaction of their respective gases thereby helping to fine-tune the varied signalling responses associated with them. Perhaps consistent with this notion, O2- and H2O2 (enzymatically produced in a highly-regulated, compartmentalised manner), can potentially act in a similar way through varied and complex interactions with the gasotransmitters to yield diverse signalling molecules such as polysulphide [226] and peroxynitrite [253]. A greater understanding of the chemistries and interrelated actions of these gases is now very much needed and will no doubt lead to a fuller understanding of their roles in many biological processes, including angiogenesis.
2. Conclusion and future perspectives
In the last decade the conventional paradigm of reactive oxygen species as deleterious by-products of aerobic metabolism has shifted, and now it is largely accepted that they represent a group of highly regulated and coordinated secondary messengers capable of mediating a number of physiological processes [254] including angiogenesis. In particular, H2O2 has been shown to induce endothelial cell phenotypes such as increased proliferation, tube formation and migration that are all fundamental to the angiogenic response [52]. Nox enzyme complexes have emerged as the significant endogenous sources of O2- and H2O2 within the vasculature and Nox4, in particular, seems a likely generator of the H2O2 shown to be important in the angiogenic cellular response. Thus Nox4 is highly expressed in vascular cells compared to other Nox isoforms, and its expression increases in response to tissue hypoxia [67], a condition in which many pro-angiogenic factors such as VEGF are released. Secondly, by contrast to Nox1 and Nox2 which generate O2-, Nox4 is considered to be a generator of H2O2, the oxidant species shown to be most pro-angiogenic [53]. Lastly, genetic gain- and loss-of-function models, both in vitro and in vivo, have consistently highlighted a positive role for Nox4 in ischemia-induced angiogenesis [46], [49].
The roles of reactive oxygen species, notably Nox-generated O2- and H2O2, in orchestrating the angiogenic cellular responses are being demonstrated to be increasingly complex and diverse. Moreover it is clear that their functions are intimately connected to those of the gastransmitters, NO, CO and H2S. The production of all these reactive signalling molecules occurs in a highly coordinated manner, close to their biological sites of action. They can also act independently, synergistically or antagonistically to elicit their downstream effects often culminating in a highly fine-tuned response. The transient chemical nature of the gasotransmitters, as well as their inherent need to be produced at their sites of action, and their toxicity in non-physiological concentrations, precludes their direct use in modulating angiogenesis therapeutically. However, the potential use of (sometimes slow-releasing) donor compounds of NO, CO and H2S as clinical therapeutics is being vigourously explored [255]. In this regard a critical issue is the adverse, off target, potential pro-oncogenic effects which might be induced by promoting angiogenesis. Thus it will be necessary to specifically target the activity of such drugs, and a better understanding of the complexity of the interplay within and between different reactive oxygen species and the gasotransmitters is crucial. This represents a major challenge to the scientific community, and better methodologies to measure the physiological (and pathophysiological) levels of these mediators accurately and efficiently both in vitro and in vivo are in high demand. Interestingly, donor molecules have begun to be designed which exploit the beneficial synergistic actions of the gasotransmitters. One such molecule; ZYZ-803, is a novel H2S- NO hybrid molecule which has been shown to exhibit greater proangiogenic potency than either H2S or NO alone [256]. It can be hoped that in the future a more integrative approach to studying the roles of the gasotransmitters in angiogenesis, and their modes of regulation by diverse redox-dependent mechanisms may lead to the development of more efficacious therapeutic strategies.
Conflict of interest statement
None.
Acknowlegement
This work was supported by BHF project grant; PG/15/119/31970, awarded to Dr Alison Brewer.
References
- 1.Vito R.P., Dixon S.A. Blood vessel constitutive models-1995–2002. Annu. Rev. Biomed. Eng. 2003;5:413–439. doi: 10.1146/annurev.bioeng.5.011303.120719. [DOI] [PubMed] [Google Scholar]
- 2.Otrock Z.K., Mahfouz R.A., Makarem J.A., Shamseddine A.I. Understanding the biology of angiogenesis: review of the most important molecular mechanisms. Blood Cells Mol. Dis. 2007;39:212–220. doi: 10.1016/j.bcmd.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Ferrara N., Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr. Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 4.Gerber H.P., McMurtrey A., Kowalski J., Yan M., Keyt B.A., Dixit V., Ferrara N. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3'-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J. Biol. Chem. 1998;273:30336–30343. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- 5.Gerber H.P., Dixit V., Ferrara N. Vascular endothelial growth factor induces expression of the antiapoptotic proteins Bcl-2 and A1 in vascular endothelial cells. J. Biol. Chem. 1998;273:13313–13316. doi: 10.1074/jbc.273.21.13313. [DOI] [PubMed] [Google Scholar]
- 6.Gerber H.P., Hillan K.J., Ryan A.M., Kowalski J., Keller G.A., Rangell L., Wright B.D., Radtke F., Aguet M., Ferrara N. VEGF is required for growth and survival in neonatal mice. Development. 1999;126:1149–1159. doi: 10.1242/dev.126.6.1149. [DOI] [PubMed] [Google Scholar]
- 7.Kim K.J., Li B., Winer J., Armanini M., Gillett N., Phillips H.S., Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 8.Ferrara N., Gerber H.P., LeCouter J. The biology of VEGF and its receptors. Nat. Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 9.Josko J., Mazurek M. Transcription factors having impact on vascular endothelial growth factor (VEGF) gene expression in angiogenesis. Med. Sci. Monit. 2004;10 (RA89-98) [PubMed] [Google Scholar]
- 10.Ceradini D.J., Kulkarni A.R., Callaghan M.J., Tepper O.M., Bastidas N., Kleinman M.E., Capla J.M., Galiano R.D., Levine J.P., Gurtner G.C. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat. Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 11.Manalo D.J., Rowan A., Lavoie T., Natarajan L., Kelly B.D., Ye S.Q., Garcia J.G., Semenza G.L. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood. 2005;105:659–669. doi: 10.1182/blood-2004-07-2958. [DOI] [PubMed] [Google Scholar]
- 12.Ushio-Fukai M., Alexander R.W. Reactive oxygen species as mediators of angiogenesis signaling: role of NAD(P)H oxidase. Mol. Cell. Biochem. 2004;264:85–97. doi: 10.1023/b:mcbi.0000044378.09409.b5. [DOI] [PubMed] [Google Scholar]
- 13.Wang R. Shared signaling pathways among gasotransmitters. Proc. Natl. Acad. Sci. USA. 2012;109:8801–8802. doi: 10.1073/pnas.1206646109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collinson D.J., Donnelly R. Therapeutic angiogenesis in peripheral arterial disease: can biotechnology produce an effective collateral circulation? Eur. J. Vasc. Endovasc. Surg. 2004;28:9–23. doi: 10.1016/j.ejvs.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 15.Valko M., Leibfritz D., Moncol J., Cronin M.T., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Pham-Huy L.A., He H., Pham-Huy C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008;4:89–96. [PMC free article] [PubMed] [Google Scholar]
- 17.Blokhina O., Virolainen E., Fagerstedt K.V. Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann. Bot. 2003;91:179–194. doi: 10.1093/aob/mcf118. (Spec No) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winterbourn C.C. Reconciling the chemistry and biology of reactive oxygen species. Nat. Chem. Biol. 2008;4:278–286. doi: 10.1038/nchembio.85. [DOI] [PubMed] [Google Scholar]
- 19.Galli S., Antico Arciuch V.G., Poderoso C., Converso D.P., Zhou Q., Bal de Kier Joffe E., Cadenas E., Boczkowski J., Carreras M.C., Poderoso J.J. Tumor cell phenotype is sustained by selective MAPK oxidation in mitochondria. PloS One. 2008;3:e2379. doi: 10.1371/journal.pone.0002379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halliwell B., Gutteridge J.M.C. Oxford University Press; Oxford; New York: 2007. Free Radicals in Biology and Medicine. [Google Scholar]
- 21.Sies H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: oxidative eustress. Redox Biol. 2017;11:613–619. doi: 10.1016/j.redox.2016.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marinho H.S., Real C., Cyrne L., Soares H., Antunes F. Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox Biol. 2014;2:535–562. doi: 10.1016/j.redox.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stadtman E.R., Levine R.L. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids. 2003;25:207–218. doi: 10.1007/s00726-003-0011-2. [DOI] [PubMed] [Google Scholar]
- 24.Chung H.S., Wang S.B., Venkatraman V., Murray C.I., Van Eyk J.E. Cysteine oxidative posttranslational modifications: emerging regulation in the cardiovascular system. Circ. Res. 2013;112:382–392. doi: 10.1161/CIRCRESAHA.112.268680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burgoyne J.R., Madhani M., Cuello F., Charles R.L., Brennan J.P., Schroder E., Browning D.D., Eaton P. Cysteine redox sensor in PKGIa enables oxidant-induced activation. Science. 2007;317:1393–1397. doi: 10.1126/science.1144318. [DOI] [PubMed] [Google Scholar]
- 26.Burgoyne J.R., Eaton P. Oxidant sensing by protein kinases a and g enables integration of cell redox state with phosphoregulation. Sensors. 2010;10:2731–2751. doi: 10.3390/s100402731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biteau B., Labarre J., Toledano M.B. ATP-dependent reduction of cysteine-sulphinic acid by S. cerevisiae sulphiredoxin. Nature. 2003;425:980–984. doi: 10.1038/nature02075. [DOI] [PubMed] [Google Scholar]
- 28.Santos C.X., Hafstad A.D., Beretta M., Zhang M., Molenaar C., Kopec J., Fotinou D., Murray T.V., Cobb A.M., Martin D., Zeh Silva M., Anilkumar N., Schroder K., Shanahan C.M., Brewer A.C., Brandes R.P., Blanc E., Parsons M., Belousov V., Cammack R., Hider R.C., Steiner R.A., Shah A.M. Targeted redox inhibition of protein phosphatase 1 by Nox4 regulates eIF2alpha-mediated stress signaling. EMBO J. 2016;35:319–334. doi: 10.15252/embj.201592394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bedard K., Krause K.H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 30.Brown D.I., Griendling K.K. Nox proteins in signal transduction. Free Radic. Biol. Med. 2009;47:1239–1253. doi: 10.1016/j.freeradbiomed.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lyle A.N., Deshpande N.N., Taniyama Y., Seidel-Rogol B., Pounkova L., Du P., Papaharalambus C., Lassegue B., Griendling K.K. Poldip2, a novel regulator of Nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circ. Res. 2009;105:249–259. doi: 10.1161/CIRCRESAHA.109.193722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Banfi B., Clark R.A., Steger K., Krause K.H. Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J. Biol. Chem. 2003;278:3510–3513. doi: 10.1074/jbc.C200613200. [DOI] [PubMed] [Google Scholar]
- 33.Sheppard F.R., Kelher M.R., Moore E.E., McLaughlin N.J., Banerjee A., Silliman C.C. Structural organization of the neutrophil NADPH oxidase: phosphorylation and translocation during priming and activation. J. Leukoc. Biol. 2005;78:1025–1042. doi: 10.1189/jlb.0804442. [DOI] [PubMed] [Google Scholar]
- 34.Griendling K.K., Sorescu D., Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ. Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 35.Takac I., Schroder K., Zhang L., Lardy B., Anilkumar N., Lambeth J.D., Shah A.M., Morel F., Brandes R.P. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J. Biol. Chem. 2011;286:13304–13313. doi: 10.1074/jbc.M110.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lambeth J.D. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 37.Anilkumar N., Weber R., Zhang M., Brewer A., Shah A.M. Nox4 and nox2 NADPH oxidases mediate distinct cellular redox signaling responses to agonist stimulation. Arterioscler., Thromb., Vasc. Biol. 2008;28:1347–1354. doi: 10.1161/ATVBAHA.108.164277. [DOI] [PubMed] [Google Scholar]
- 38.Hilenski L.L., Clempus R.E., Quinn M.T., Lambeth J.D., Griendling K.K. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler., Thromb., Vasc. Biol. 2004;24:677–683. doi: 10.1161/01.ATV.0000112024.13727.2c. [DOI] [PubMed] [Google Scholar]
- 39.Van Buul J.D., Fernandez-Borja M., Anthony E.C., Hordijk P.L. Expression and localization of NOX2 and NOX4 in primary human endothelial cells. Antioxid. Redox Signal. 2005;7:308–317. doi: 10.1089/ars.2005.7.308. [DOI] [PubMed] [Google Scholar]
- 40.Drummond G.R., Sobey C.G. Endothelial NADPH oxidases: which NOX to target in vascular disease? Trends Endocrinol. Metab. 2014;25:452–463. doi: 10.1016/j.tem.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 41.Drummond G.R., Selemidis S., Griendling K.K., Sobey C.G. Combating oxidative stress in vascular disease: nadph oxidases as therapeutic targets. Nat. Rev. Drug Discov. 2011;10:453–471. doi: 10.1038/nrd3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jung O., Schreiber J.G., Geiger H., Pedrazzini T., Busse R., Brandes R.P. gp91phox-containing NADPH oxidase mediates endothelial dysfunction in renovascular hypertension. Circulation. 2004;109:1795–1801. doi: 10.1161/01.CIR.0000124223.00113.A4. [DOI] [PubMed] [Google Scholar]
- 43.Landmesser U., Cai H., Dikalov S., McCann L., Hwang J., Jo H., Holland S.M., Harrison D.G. Role of p47(phox) in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension. 2002;40:511–515. doi: 10.1161/01.hyp.0000032100.23772.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matsuno K., Yamada H., Iwata K., Jin D., Katsuyama M., Matsuki M., Takai S., Yamanishi K., Miyazaki M., Matsubara H., Yabe-Nishimura C. Nox1 is involved in angiotensin II-mediated hypertension: a study in Nox1-deficient mice. Circulation. 2005;112:2677–2685. doi: 10.1161/CIRCULATIONAHA.105.573709. [DOI] [PubMed] [Google Scholar]
- 45.Dikalova A., Clempus R., Lassegue B., Cheng G., McCoy J., Dikalov S., San Martin A., Lyle A., Weber D.S., Weiss D., Taylor W.R., Schmidt H.H., Owens G.K., Lambeth J.D., Griendling K.K. Nox1 overexpression potentiates angiotensin II-induced hypertension and vascular smooth muscle hypertrophy in transgenic mice. Circulation. 2005;112:2668–2676. doi: 10.1161/CIRCULATIONAHA.105.538934. [DOI] [PubMed] [Google Scholar]
- 46.Schroder K., Zhang M., Benkhoff S., Mieth A., Pliquett R., Kosowski J., Kruse C., Luedike P., Michaelis U.R., Weissmann N., Dimmeler S., Shah A.M., Brandes R.P. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ. Res. 2012;110:1217–1225. doi: 10.1161/CIRCRESAHA.112.267054. [DOI] [PubMed] [Google Scholar]
- 47.Murdoch C.E., Alom-Ruiz S.P., Wang M.S., Zhang M., Walker S., Yu B., Brewer A., Shah A.M. Role of endothelial Nox2 NADPH oxidase in angiotensin II-induced hypertension and vasomotor dysfunction. Basic Res. Cardiol. 2011;106:527–538. doi: 10.1007/s00395-011-0179-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ray R., Murdoch C.E., Wang M., Santos C.X., Zhang M., Alom-Ruiz S., Anilkumar N., Ouattara A., Cave A.C., Walker S.J., Grieve D.J., Charles R.L., Eaton P., Brewer A.C., Shah A.M. Endothelial Nox4 NADPH oxidase enhances vasodilatation and reduces blood pressure in vivo. Arterioscler., Thromb., Vasc. Biol. 2011;31:1368–1376. doi: 10.1161/ATVBAHA.110.219238. [DOI] [PubMed] [Google Scholar]
- 49.Craige S.M., Chen K., Pei Y., Li C., Huang X., Chen C., Shibata R., Sato K., Walsh K., Keaney J.F., Jr. NADPH oxidase 4 promotes endothelial angiogenesis through endothelial nitric oxide synthase activation. Circulation. 2011;124:731–740. doi: 10.1161/CIRCULATIONAHA.111.030775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Touyz R.M., Montezano A.C. Vascular Nox4: a multifarious NADPH oxidase. Circ. Res. 2012;110:1159–1161. doi: 10.1161/CIRCRESAHA.112.269068. [DOI] [PubMed] [Google Scholar]
- 51.Frey R.S., Ushio-Fukai M., Malik A.B. NADPH oxidase-dependent signaling in endothelial cells: role in physiology and pathophysiology. Antioxid. Redox Signal. 2009;11:791–810. doi: 10.1089/ars.2008.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yasuda M., Ohzeki Y., Shimizu S., Naito S., Ohtsuru A., Yamamoto T., Kuroiwa Y. Stimulation of in vitro angiogenesis by hydrogen peroxide and the relation with ETS-1 in endothelial cells. Life Sci. 1999;64:249–258. doi: 10.1016/s0024-3205(98)00560-8. [DOI] [PubMed] [Google Scholar]
- 53.Huang S.S., Zheng R.L. Biphasic regulation of angiogenesis by reactive oxygen species. Pharmazie. 2006;61:223–229. [PubMed] [Google Scholar]
- 54.Gonzalez-Pacheco F.R., Deudero J.J., Castellanos M.C., Castilla M.A., Alvarez-Arroyo M.V., Yague S., Caramelo C. Mechanisms of endothelial response to oxidative aggression: protective role of autologous VEGF and induction of VEGFR2 by H2O2. Am. J. Physiol. Heart Circ. Physiol. 2006;291:H1395–H1401. doi: 10.1152/ajpheart.01277.2005. [DOI] [PubMed] [Google Scholar]
- 55.Bassus S., Herkert O., Kronemann N., Gorlach A., Bremerich D., Kirchmaier C.M., Busse R., Schini-Kerth V.B. Thrombin causes vascular endothelial growth factor expression in vascular smooth muscle cells: role of reactive oxygen species. Arterioscler., Thromb., Vasc. Biol. 2001;21:1550–1555. doi: 10.1161/hq0901.095148. [DOI] [PubMed] [Google Scholar]
- 56.Ushio-Fukai M. VEGF signaling through NADPH oxidase-derived ROS. Antioxid. Redox Signal. 2007;9:731–739. doi: 10.1089/ars.2007.1556. [DOI] [PubMed] [Google Scholar]
- 57.Zippel N., Malik R.A., Fromel T., Popp R., Bess E., Strilic B., Wettschureck N., Fleming I., Fisslthaler B. Transforming growth factor-beta-activated kinase 1 regulates angiogenesis via AMP-activated protein kinase-alpha1 and redox balance in endothelial cells. Arterioscler., Thromb., Vasc. Biol. 2013;33:2792–2799. doi: 10.1161/ATVBAHA.113.301848. [DOI] [PubMed] [Google Scholar]
- 58.Oshikawa J., Urao N., Kim H.W., Kaplan N., Razvi M., McKinney R., Poole L.B., Fukai T., Ushio-Fukai M. Extracellular SOD-derived H2O2 promotes VEGF signaling in caveolae/lipid rafts and post-ischemic angiogenesis in mice. PloS One. 2010;5:e10189. doi: 10.1371/journal.pone.0010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Colombo R., Siqueira R., Conzatti A., de Lima Seolin B.G., Fernandes T.R., Godoy A.E., Litvin I.E., Silva J.M., Tucci P.J., da Rosa Araujo A.S., Bello-Klein A. Exercise training contributes to H2O2/VEGF signaling in the lung of rats with monocrotaline-induced pulmonary hypertension. Vasc. Pharmacol. 2016 doi: 10.1016/j.vph.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 60.Sun J., Xu Y., Sun S., Sun Y., Wang X. Intermittent high glucose enhances cell proliferation and VEGF expression in retinal endothelial cells: the role of mitochondrial reactive oxygen species. Mol. Cell. Biochem. 2010;343:27–35. doi: 10.1007/s11010-010-0495-5. [DOI] [PubMed] [Google Scholar]
- 61.Wang Y., Zang Q.S., Liu Z., Wu Q., Maass D., Dulan G., Shaul P.W., Melito L., Frantz D.E., Kilgore J.A., Williams N.S., Terada L.S., Nwariaku F.E. Regulation of VEGF-induced endothelial cell migration by mitochondrial reactive oxygen species. Am. J. Physiol. Cell Physiol. 2011;301:C695–C704. doi: 10.1152/ajpcell.00322.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kou B., Ni J., Vatish M., Singer D.R. Xanthine oxidase interaction with vascular endothelial growth factor in human endothelial cell angiogenesis. Microcirculation. 2008;15:251–267. doi: 10.1080/10739680701651495. [DOI] [PubMed] [Google Scholar]
- 63.Garrido-Urbani S., Jemelin S., Deffert C., Carnesecchi S., Basset O., Szyndralewiez C., Heitz F., Page P., Montet X., Michalik L., Arbiser J., Ruegg C., Krause K.H., Imhof B.A. Targeting vascular NADPH oxidase 1 blocks tumor angiogenesis through a PPARalpha mediated mechanism. PloS One. 2011;6:e14665. doi: 10.1371/journal.pone.0014665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tojo T., Ushio-Fukai M., Yamaoka-Tojo M., Ikeda S., Patrushev N., Alexander R.W. Role of gp91phox (Nox2)-containing NAD(P)H oxidase in angiogenesis in response to hindlimb ischemia. Circulation. 2005;111:2347–2355. doi: 10.1161/01.CIR.0000164261.62586.14. [DOI] [PubMed] [Google Scholar]
- 65.Vogel J., Kruse C., Zhang M., Schroder K. Nox4 supports proper capillary growth in exercise and retina neo-vascularization. J. Physiol. 2015;593:2145–2154. doi: 10.1113/jphysiol.2014.284901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Helfinger V., Henke N., Harenkamp S., Walter M., Epah J., Penski C., Mittelbronn M., Schroder K. The NADPH Oxidase Nox4 mediates tumour angiogenesis. Acta Physiol. 2016;216:435–446. doi: 10.1111/apha.12625. [DOI] [PubMed] [Google Scholar]
- 67.Diebold I., Petry A., Hess J., Gorlach A. The NADPH oxidase subunit NOX4 is a new target gene of the hypoxia-inducible factor-1. Mol. Biol. Cell. 2010;21:2087–2096. doi: 10.1091/mbc.E09-12-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li Q., Fu G.B., Zheng J.T., He J., Niu X.B., Chen Q.D., Yin Y., Qian X., Xu Q., Wang M., Sun A.F., Shu Y., Rui H., Liu L.Z., Jiang B.H. NADPH oxidase subunit p22(phox)-mediated reactive oxygen species contribute to angiogenesis and tumor growth through AKT and ERK1/2 signaling pathways in prostate cancer. Biochim Biophys. Acta. 2013;1833:3375–3385. doi: 10.1016/j.bbamcr.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 69.Arbiser J.L., Petros J., Klafter R., Govindajaran B., McLaughlin E.R., Brown L.F., Cohen C., Moses M., Kilroy S., Arnold R.S., Lambeth J.D. Reactive oxygen generated by Nox1 triggers the angiogenic switch. P Natl. Acad. Sci. USA. 2002;99:715–720. doi: 10.1073/pnas.022630199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xue Y., Bi F., Zhang X., Pan Y., Liu N., Zheng Y., Fan D. Inhibition of endothelial cell proliferation by targeting Rac1 GTPase with small interference RNA in tumor cells. Biochem. Biophys. Res. Commun. 2004;320:1309–1315. doi: 10.1016/j.bbrc.2004.06.088. [DOI] [PubMed] [Google Scholar]
- 71.Maranchie J.K., Zhan Y. Nox4 is critical for hypoxia-inducible factor 2-alpha transcriptional activity in von Hippel-Lindau-deficient renal cell carcinoma. Cancer Res. 2005;65:9190–9193. doi: 10.1158/0008-5472.CAN-05-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Meng D., Mei A., Liu J., Kang X., Shi X., Qian R., Chen S. NADPH oxidase 4 mediates insulin-stimulated HIF-1alpha and VEGF expression, and angiogenesis in vitro. PloS One. 2012;7:e48393. doi: 10.1371/journal.pone.0048393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ushio-Fukai M., Tang Y., Fukai T., Dikalov S.I., Ma Y., Fujimoto M., Quinn M.T., Pagano P.J., Johnson C., Alexander R.W. Novel role of gp91(phox)-containing NAD(P)H oxidase in vascular endothelial growth factor-induced signaling and angiogenesis. Circ. Res. 2002;91:1160–1167. doi: 10.1161/01.res.0000046227.65158.f8. [DOI] [PubMed] [Google Scholar]
- 74.Harfouche R., Malak N.A., Brandes R.P., Karsan A., Irani K., Hussain S.N. Roles of reactive oxygen species in angiopoietin-1/tie-2 receptor signaling. FASEB J. 2005;19:1728–1730. doi: 10.1096/fj.04-3621fje. [DOI] [PubMed] [Google Scholar]
- 75.Datla S.R., Peshavariya H., Dusting G.J., Mahadev K., Goldstein B.J., Jiang F. Important role of Nox4 type NADPH oxidase in angiogenic responses in human microvascular endothelial cells in vitro. Arterioscler., Thromb., Vasc. Biol. 2007;27:2319–2324. doi: 10.1161/ATVBAHA.107.149450. [DOI] [PubMed] [Google Scholar]
- 76.Wang H., Yang Z., Jiang Y., Hartnett M.E. Endothelial NADPH oxidase 4 mediates vascular endothelial growth factor receptor 2-induced intravitreal neovascularization in a rat model of retinopathy of prematurity. Mol. Vis. 2014;20:231–241. [PMC free article] [PubMed] [Google Scholar]
- 77.Evangelista A.M., Thompson M.D., Bolotina V.M., Tong X., Cohen R.A. Nox4- and Nox2-dependent oxidant production is required for VEGF-induced SERCA cysteine-674 S-glutathiolation and endothelial cell migration. Free Radic. Biol. Med. 2012;53:2327–2334. doi: 10.1016/j.freeradbiomed.2012.10.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen K., Kirber M.T., Xiao H., Yang Y., Keaney J.F., Jr. Regulation of ROS signal transduction by NADPH oxidase 4 localization. J. Cell Biol. 2008;181:1129–1139. doi: 10.1083/jcb.200709049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mahadev K., Motoshima H., Wu X., Ruddy J.M., Arnold R.S., Cheng G., Lambeth J.D., Goldstein B.J. The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Mol. Cell Biol. 2004;24:1844–1854. doi: 10.1128/MCB.24.5.1844-1854.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wissner A., Fraser H.L., Ingalls C.L., Dushin R.G., Floyd M.B., Cheung K., Nittoli T., Ravi M.R., Tan X., Loganzo F. Dual irreversible kinase inhibitors: quinazoline-based inhibitors incorporating two independent reactive centers with each targeting different cysteine residues in the kinase domains of EGFR and VEGFR-2. Bioorg. Med. Chem. 2007;15:3635–3648. doi: 10.1016/j.bmc.2007.03.055. [DOI] [PubMed] [Google Scholar]
- 81.Kang D.H., Lee D.J., Lee K.W., Park Y.S., Lee J.Y., Lee S.H., Koh Y.J., Koh G.Y., Choi C., Yu D.Y., Kim J., Kang S.W. Peroxiredoxin II is an essential antioxidant enzyme that prevents the oxidative inactivation of VEGF receptor-2 in vascular endothelial cells. Mol. Cell. 2011;44:545–558. doi: 10.1016/j.molcel.2011.08.040. [DOI] [PubMed] [Google Scholar]
- 82.Chen F., Haigh S., Barman S., Fulton D.J. From form to function: the role of Nox4 in the cardiovascular system. Front. Physiol. 2012;3:412. doi: 10.3389/fphys.2012.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gasotransmitters – Physiology and Pathophysiology, New York, Springer, 2012.
- 84.Mustafa A.K., Gadalla M.M., Snyder S.H. Signaling by gasotransmitters. Sci. Signal. 2009;2 doi: 10.1126/scisignal.268re2. (re2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stein A., Bailey S.M. Redox biology of hydrogen sulfide: implications for physiology, pathophysiology, and pharmacology. Redox Biol. 2013;1:32–39. doi: 10.1016/j.redox.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang R. Gasotransmitters: growing pains and joys. Trends Biochem. Sci. 2014;39:227–232. doi: 10.1016/j.tibs.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 87.Kajimura M., Fukuda R., Bateman R.M., Yamamoto T., Suematsu M. Interactions of multiple gas-transducing systems: hallmarks and uncertainties of CO, NO, and H2S gas biology. Antioxid. Redox Signal. 2010;13:157–192. doi: 10.1089/ars.2009.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cooke J.P. NO and angiogenesis. Atheroscler. Suppl. 2003;4:53–60. doi: 10.1016/s1567-5688(03)00034-5. [DOI] [PubMed] [Google Scholar]
- 89.Li Volti G., Sacerdoti D., Sangras B., Vanella A., Mezentsev A., Scapagnini G., Falck J.R., Abraham N.G. Carbon monoxide signaling in promoting angiogenesis in human microvessel endothelial cells. Antioxid. Redox Signal. 2005;7:704–710. doi: 10.1089/ars.2005.7.704. [DOI] [PubMed] [Google Scholar]
- 90.Papapetropoulos A., Pyriochou A., Altaany Z., Yang G., Marazioti A., Zhou Z., Jeschke M.G., Branski L.K., Herndon D.N., Wang R., Szabo C. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc. Natl. Acad. Sci. USA. 2009;106:21972–21977. doi: 10.1073/pnas.0908047106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Furchgott R.F., Zawadzki J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 92.Palmer R.M., Ferrige A.G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 93.Liu V.W., Huang P.L. Cardiovascular roles of nitric oxide: a review of insights from nitric oxide synthase gene disrupted mice. Cardiovasc. Res. 2008;77:19–29. doi: 10.1016/j.cardiores.2007.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Alderton W.K., Cooper C.E., Knowles R.G. Nitric oxide synthases: structure, function and inhibition. Biochem. J. 2001;357:593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Palmer R.M., Ashton D.S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333:664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- 96.Fleming I. Molecular mechanisms underlying the activation of eNOS. Pflug. Arch. 2010;459:793–806. doi: 10.1007/s00424-009-0767-7. [DOI] [PubMed] [Google Scholar]
- 97.Maccallini C., Mollica A., Amoroso R. The positive regulation of eNOS signaling by PPAR agonists in cardiovascular diseases. Am. J. Cardiovasc. Drugs. 2017 doi: 10.1007/s40256-017-0220-9. [DOI] [PubMed] [Google Scholar]
- 98.Chreifi G., Li H., McInnes C.R., Gibson C.L., Suckling C.J., Poulos T.L. Communication between the zinc and tetrahydrobiopterin binding sites in nitric oxide synthase. Biochemistry. 2014;53:4216–4223. doi: 10.1021/bi5003986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fulton D., Gratton J.P., Sessa W.C. Post-translational control of endothelial nitric oxide synthase: why isn't calcium/calmodulin enough? J. Pharmacol. Exp. Ther. 2001;299:818–824. [PubMed] [Google Scholar]
- 100.Siragusa M., Frohlich F., Park E.J., Schleicher M., Walther T.C., Sessa W.C. Stromal cell-derived factor 2 is critical for Hsp90-dependent eNOS activation. Sci. Signal. 2015;8 doi: 10.1126/scisignal.aaa2819. (ra81) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bendall J.K., Alp N.J., Warrick N., Cai S., Adlam D., Rockett K., Yokoyama M., Kawashima S., Channon K.M. Stoichiometric relationships between endothelial tetrahydrobiopterin, endothelial NO synthase (eNOS) activity, and eNOS coupling in vivo: insights from transgenic mice with endothelial-targeted GTP cyclohydrolase 1 and eNOS overexpression. Circ. Res. 2005;97:864–871. doi: 10.1161/01.RES.0000187447.03525.72. [DOI] [PubMed] [Google Scholar]
- 102.Francis S.H., Busch J.L., Corbin J.D., Sibley D. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol. Rev. 2010;62:525–563. doi: 10.1124/pr.110.002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chang G.J., Lin T.P., Ko Y.S., Lin M.S. Endothelium-dependent and -independent vasorelaxation induced by CIJ-3–2F, a novel benzyl-furoquinoline with antiarrhythmic action, in rat aorta. Life Sci. 2010;86:869–879. doi: 10.1016/j.lfs.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 104.Karlsson W.K., Sorensen C.G., Kruuse C. l-arginine and l-NMMA for assessing cerebral endothelial dysfunction in ischaemic cerebrovascular disease: a systematic review. Clin. Exp. Pharmacol. Physiol. 2017;44:13–20. doi: 10.1111/1440-1681.12679. [DOI] [PubMed] [Google Scholar]
- 105.Huang P.L., Huang Z., Mashimo H., Bloch K.D., Moskowitz M.A., Bevan J.A., Fishman M.C. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- 106.Murohara T., Asahara T., Silver M., Bauters C., Masuda H., Kalka C., Kearney M., Chen D., Symes J.F., Fishman M.C., Huang P.L., Isner J.M. Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J. Clin. Investig. 1998;101:2567–2578. doi: 10.1172/JCI1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Aicher A., Heeschen C., Mildner-Rihm C., Urbich C., Ihling C., Technau-Ihling K., Zeiher A.M., Dimmeler S. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat. Med. 2003;9:1370–1376. doi: 10.1038/nm948. [DOI] [PubMed] [Google Scholar]
- 108.Lee P.C., Salyapongse A.N., Bragdon G.A., Shears L.L., 2nd, Watkins S.C., Edington H.D., Billiar T.R. Impaired wound healing and angiogenesis in eNOS-deficient mice. Am. J. Physiol. 1999;277:H1600–H1608. doi: 10.1152/ajpheart.1999.277.4.H1600. [DOI] [PubMed] [Google Scholar]
- 109.Yu J., deMuinck E.D., Zhuang Z., Drinane M., Kauser K., Rubanyi G.M., Qian H.S., Murata T., Escalante B., Sessa W.C. Endothelial nitric oxide synthase is critical for ischemic remodeling, mural cell recruitment, and blood flow reserve. Proc. Natl. Acad. Sci. USA. 2005;102:10999–11004. doi: 10.1073/pnas.0501444102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Papapetropoulos A., Garcia-Cardena G., Madri J.A., Sessa W.C. Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J. Clin. Investig. 1997;100:3131–3139. doi: 10.1172/JCI119868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fukumura D., Gohongi T., Kadambi A., Izumi Y., Ang J., Yun C.O., Buerk D.G., Huang P.L., Jain R.K. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc. Natl. Acad. Sci. USA. 2001;98:2604–2609. doi: 10.1073/pnas.041359198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ziche M., Morbidelli L., Choudhuri R., Zhang H.T., Donnini S., Granger H.J., Bicknell R. Nitric oxide synthase lies downstream from vascular endothelial growth factor-induced but not basic fibroblast growth factor-induced angiogenesis. J. Clin. Investig. 1997;99:2625–2634. doi: 10.1172/JCI119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fulton D., Gratton J.P., McCabe T.J., Fontana J., Fujio Y., Walsh K., Franke T.F., Papapetropoulos A., Sessa W.C. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fulton D., Ruan L., Sood S.G., Li C., Zhang Q., Venema R.C. Agonist-stimulated endothelial nitric oxide synthase activation and vascular relaxation. Role of eNOS phosphorylation at Tyr83. Circ. Res. 2008;102:497–504. doi: 10.1161/CIRCRESAHA.107.162933. [DOI] [PubMed] [Google Scholar]
- 115.Luo Z., Fujio Y., Kureishi Y., Rudic R.D., Daumerie G., Fulton D., Sessa W.C., Walsh K. Acute modulation of endothelial Akt/PKB activity alters nitric oxide-dependent vasomotor activity in vivo. J. Clin. Investig. 2000;106:493–499. doi: 10.1172/JCI9419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Michell B.J., Harris M.B., Chen Z.P., Ju H., Venema V.J., Blackstone M.A., Huang W., Venema R.C., Kemp B.E. Identification of regulatory sites of phosphorylation of the bovine endothelial nitric-oxide synthase at serine 617 and serine 635. J. Biol. Chem. 2002;277:42344–42351. doi: 10.1074/jbc.M205144200. [DOI] [PubMed] [Google Scholar]
- 117.Fulton D., Church J.E., Ruan L., Li C., Sood S.G., Kemp B.E., Jennings I.G., Venema R.C. Src kinase activates endothelial nitric-oxide synthase by phosphorylating Tyr-83. J. Biol. Chem. 2005;280:35943–35952. doi: 10.1074/jbc.M504606200. [DOI] [PubMed] [Google Scholar]
- 118.Michell B.J., Chen Z., Tiganis T., Stapleton D., Katsis F., Power D.A., Sim A.T., Kemp B.E. Coordinated control of endothelial nitric-oxide synthase phosphorylation by protein kinase C and the cAMP-dependent protein kinase. J. Biol. Chem. 2001;276:17625–17628. doi: 10.1074/jbc.C100122200. [DOI] [PubMed] [Google Scholar]
- 119.Dimmeler S., Fleming I., Fisslthaler B., Hermann C., Busse R., Zeiher A.M. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 120.Chen M., Yi B., Zhu N., Wei X., Zhang G.X., Huang S., Sun J. Pim1 kinase promotes angiogenesis through phosphorylation of endothelial nitric oxide synthase at Ser-633. Cardiovasc. Res. 2016;109:141–150. doi: 10.1093/cvr/cvv250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Duval M., Le Boeuf F., Huot J., Gratton J.P. Src-mediated phosphorylation of Hsp90 in response to vascular endothelial growth factor (VEGF) is required for VEGF receptor-2 signaling to endothelial NO synthase. Mol. Biol. Cell. 2007;18:4659–4668. doi: 10.1091/mbc.E07-05-0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Boo Y.C., Hwang J., Sykes M., Michell B.J., Kemp B.E., Lum H., Jo H. Shear stress stimulates phosphorylation of eNOS at Ser(635) by a protein kinase A-dependent mechanism. Am. J. Physiol. Heart Circ. Physiol. 2002;283:H1819–H1828. doi: 10.1152/ajpheart.00214.2002. [DOI] [PubMed] [Google Scholar]
- 123.Coletta C., Papapetropoulos A., Erdelyi K., Olah G., Modis K., Panopoulos P., Asimakopoulou A., Gero D., Sharina I., Martin E., Szabo C. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc. Natl. Acad. Sci. USA. 2012;109:9161–9166. doi: 10.1073/pnas.1202916109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dulak J., Jozkowicz A., Dembinska-Kiec A., Guevara I., Zdzienicka A., Zmudzinska-Grochot D., Florek I., Wojtowicz A., Szuba A., Cooke J.P. Nitric oxide induces the synthesis of vascular endothelial growth factor by rat vascular smooth muscle cells. Arterioscler., Thromb., Vasc. Biol. 2000;20:659–666. doi: 10.1161/01.atv.20.3.659. [DOI] [PubMed] [Google Scholar]
- 125.Jozkowicz A., Cooke J.P., Guevara I., Huk I., Funovics P., Pachinger O., Weidinger F., Dulak J. Genetic augmentation of nitric oxide synthase increases the vascular generation of VEGF. Cardiovasc. Res. 2001;51:773–783. doi: 10.1016/s0008-6363(01)00344-3. [DOI] [PubMed] [Google Scholar]
- 126.Milkiewicz M., Hudlicka O., Brown M.D., Silgram H. Nitric oxide, VEGF, and VEGFR-2: interactions in activity-induced angiogenesis in rat skeletal muscle. Am. J. Physiol. Heart Circ. Physiol. 2005;289:H336–H343. doi: 10.1152/ajpheart.01105.2004. [DOI] [PubMed] [Google Scholar]
- 127.Kimura H., Weisz A., Ogura T., Hitomi Y., Kurashima Y., Hashimoto K., D'Acquisto F., Makuuchi M., Esumi H. Identification of hypoxia-inducible factor 1 ancillary sequence and its function in vascular endothelial growth factor gene induction by hypoxia and nitric oxide. J. Biol. Chem. 2001;276:2292–2298. doi: 10.1074/jbc.M008398200. [DOI] [PubMed] [Google Scholar]
- 128.Tsurumi Y., Murohara T., Krasinski K., Chen D., Witzenbichler B., Kearney M., Couffinhal T., Isner J.M. Reciprocal relation between VEGF and NO in the regulation of endothelial integrity. Nat. Med. 1997;3:879–886. doi: 10.1038/nm0897-879. [DOI] [PubMed] [Google Scholar]
- 129.Fiedler L.R., Wojciak-Stothard B. The DDAH/ADMA pathway in the control of endothelial cell migration and angiogenesis. Biochem. Soc. Trans. 2009;37:1243–1247. doi: 10.1042/BST0371243. [DOI] [PubMed] [Google Scholar]
- 130.Fiedler L.R., Bachetti T., Leiper J., Zachary I., Chen L., Renne T., Wojciak-Stothard B. The ADMA/DDAH pathway regulates VEGF-mediated angiogenesis. Arterioscler., Thromb., Vasc. Biol. 2009;29:2117–2124. doi: 10.1161/ATVBAHA.109.194035. [DOI] [PubMed] [Google Scholar]
- 131.Palm F., Onozato M.L., Luo Z., Wilcox C.S. Dimethylarginine dimethylaminohydrolase (DDAH): expression, regulation, and function in the cardiovascular and renal systems. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H3227–H3245. doi: 10.1152/ajpheart.00998.2007. [DOI] [PubMed] [Google Scholar]
- 132.Wojciak-Stothard B., Torondel B., Tsang L.Y., Fleming I., Fisslthaler B., Leiper J.M., Vallance P. The ADMA/DDAH pathway is a critical regulator of endothelial cell motility. J. Cell Sci. 2007;120:929–942. doi: 10.1242/jcs.002212. [DOI] [PubMed] [Google Scholar]
- 133.Dowsett L., Piper S., Slaviero A., Dufton N., Wang Z., Boruc O., Delahaye M., Colman L., Kalk E., Tomlinson J., Birdsey G., Randi A.M., Leiper J. Endothelial dimethylarginine dimethylaminohydrolase 1 is an important regulator of angiogenesis but does not regulate vascular reactivity or hemodynamic homeostasis. Circulation. 2015;131:2217–2225. doi: 10.1161/CIRCULATIONAHA.114.015064. [DOI] [PubMed] [Google Scholar]
- 134.Jacobi J., Sydow K., von Degenfeld G., Zhang Y., Dayoub H., Wang B., Patterson A.J., Kimoto M., Blau H.M., Cooke J.P. Overexpression of dimethylarginine dimethylaminohydrolase reduces tissue asymmetric dimethylarginine levels and enhances angiogenesis. Circulation. 2005;111:1431–1438. doi: 10.1161/01.CIR.0000158487.80483.09. [DOI] [PubMed] [Google Scholar]
- 135.Cooke J.P. The pivotal role of nitric oxide for vascular health. Can. J. Cardiol. 2004;20(Suppl B):S7B–S15B. [PubMed] [Google Scholar]
- 136.Cai H., Harrison D.G. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ. Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 137.Brandes R.P., Weissmann N., Schroder K. NADPH oxidases in cardiovascular disease. Free Radic. Biol. Med. 2010;49:687–706. doi: 10.1016/j.freeradbiomed.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 138.Judkins C.P., Diep H., Broughton B.R., Mast A.E., Hooker E.U., Miller A.A., Selemidis S., Dusting G.J., Sobey C.G., Drummond G.R. Direct evidence of a role for Nox2 in superoxide production, reduced nitric oxide bioavailability, and early atherosclerotic plaque formation in ApoE-/- mice. Am. J. Physiol. Heart Circ. Physiol. 2010;298:H24–H32. doi: 10.1152/ajpheart.00799.2009. [DOI] [PubMed] [Google Scholar]
- 139.Liu Y., Fang S., Sun Q., Liu B. Anthelmintic drug ivermectin inhibits angiogenesis, growth and survival of glioblastoma through inducing mitochondrial dysfunction and oxidative stress. Biochem. Biophys. Res. Commun. 2016;480:415–421. doi: 10.1016/j.bbrc.2016.10.064. [DOI] [PubMed] [Google Scholar]
- 140.Chen C.A., Wang T.Y., Varadharaj S., Reyes L.A., Hemann C., Talukder M.A., Chen Y.R., Druhan L.J., Zweier J.L. S-glutathionylation uncouples eNOS and regulates its cellular and vascular function. Nature. 2010;468:1115–1118. doi: 10.1038/nature09599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wu F., Szczepaniak W.S., Shiva S., Liu H., Wang Y., Wang L., Wang Y., Kelley E.E., Chen A.F., Gladwin M.T., McVerry B.J. Nox2-dependent glutathionylation of endothelial NOS leads to uncoupled superoxide production and endothelial barrier dysfunction in acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014;307:L987–L997. doi: 10.1152/ajplung.00063.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wever R.M., van Dam T., van Rijn H.J., de Groot F., Rabelink T.J. Tetrahydrobiopterin regulates superoxide and nitric oxide generation by recombinant endothelial nitric oxide synthase. Biochem. Biophys. Res. Commun. 1997;237:340–344. doi: 10.1006/bbrc.1997.7069. [DOI] [PubMed] [Google Scholar]
- 143.An H., Wei R., Ke J., Yang J., Liu Y., Wang X., Wang G., Hong T. Metformin attenuates fluctuating glucose-induced endothelial dysfunction through enhancing GTPCH1-mediated eNOS recoupling and inhibiting NADPH oxidase. J. Diabetes Complicat. 2016;30:1017–1024. doi: 10.1016/j.jdiacomp.2016.04.018. [DOI] [PubMed] [Google Scholar]
- 144.Stasch J.P., Pacher P., Evgenov O.V. Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation. 2011;123:2263–2273. doi: 10.1161/CIRCULATIONAHA.110.981738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cai H., Griendling K.K., Harrison D.G. The vascular NAD(P)H oxidases as therapeutic targets in cardiovascular diseases. Trends Pharmacol. Sci. 2003;24:471–478. doi: 10.1016/S0165-6147(03)00233-5. [DOI] [PubMed] [Google Scholar]
- 146.Polytarchou C., Papadimitriou E. Antioxidants inhibit human endothelial cell functions through down-regulation of endothelial nitric oxide synthase activity. Eur. J. Pharmacol. 2005;510:31–38. doi: 10.1016/j.ejphar.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 147.Lauer N., Suvorava T., Ruther U., Jacob R., Meyer W., Harrison D.G., Kojda G. Critical involvement of hydrogen peroxide in exercise-induced up-regulation of endothelial NO synthase. Cardiovasc. Res. 2005;65:254–262. doi: 10.1016/j.cardiores.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 148.Drummond G.R., Cai H., Davis M.E., Ramasamy S., Harrison D.G. Transcriptional and posttranscriptional regulation of endothelial nitric oxide synthase expression by hydrogen peroxide. Circ. Res. 2000;86:347–354. doi: 10.1161/01.res.86.3.347. [DOI] [PubMed] [Google Scholar]
- 149.Thomas S.R., Chen K., Keaney J.F., Jr. Hydrogen peroxide activates endothelial nitric-oxide synthase through coordinated phosphorylation and dephosphorylation via a phosphoinositide 3-kinase-dependent signaling pathway. J. Biol. Chem. 2002;277:6017–6024. doi: 10.1074/jbc.M109107200. [DOI] [PubMed] [Google Scholar]
- 150.Cai H., Li Z., Davis M.E., Kanner W., Harrison D.G., Dudley S.C., Jr. Akt-dependent phosphorylation of serine 1179 and mitogen-activated protein kinase kinase/extracellular signal-regulated kinase 1/2 cooperatively mediate activation of the endothelial nitric-oxide synthase by hydrogen peroxide. Mol. Pharmacol. 2003;63:325–331. doi: 10.1124/mol.63.2.325. [DOI] [PubMed] [Google Scholar]
- 151.Kalwa H., Sartoretto J.L., Martinelli R., Romero N., Steinhorn B.S., Tao M., Ozaki C.K., Carman C.V., Michel T. Central role for hydrogen peroxide in P2Y1 ADP receptor-mediated cellular responses in vascular endothelium. Proc. Natl. Acad. Sci. USA. 2014;111:3383–3388. doi: 10.1073/pnas.1320854111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sanchez-Gomez F.J., Calvo E., Breton-Romero R., Fierro-Fernandez M., Anilkumar N., Shah A.M., Schroder K., Brandes R.P., Vazquez J., Lamas S. NOX4-dependent hydrogen peroxide promotes shear stress-induced SHP2 sulfenylation and eNOS activation. Free Radic. Biol. Med. 2015;89:419–430. doi: 10.1016/j.freeradbiomed.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 153.Luo Z., Aslam S., Welch W.J., Wilcox C.S. Activation of nuclear factor erythroid 2-related factor 2 coordinates dimethylarginine dimethylaminohydrolase/PPAR-gamma/endothelial nitric oxide synthase pathways that enhance nitric oxide generation in human glomerular endothelial cells. Hypertension. 2015;65:896–902. doi: 10.1161/HYPERTENSIONAHA.114.04760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kaspar J.W., Niture S.K., Jaiswal A.K. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic. Biol. Med. 2009;47:1304–1309. doi: 10.1016/j.freeradbiomed.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Brewer A.C., Murray T.V., Arno M., Zhang M., Anilkumar N.P., Mann G.E., Shah A.M. Nox4 regulates Nrf2 and glutathione redox in cardiomyocytes in vivo. Free Radic. Biol. Med. 2011;51:205–215. doi: 10.1016/j.freeradbiomed.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Smyrnias I., Zhang X., Zhang M., Murray T.V., Brandes R.P., Schroder K., Brewer A.C., Shah A.M. Nicotinamide adenine dinucleotide phosphate oxidase-4-dependent upregulation of nuclear factor erythroid-derived 2-like 2 protects the heart during chronic pressure overload. Hypertension. 2015;65:547–553. doi: 10.1161/HYPERTENSIONAHA.114.04208. [DOI] [PubMed] [Google Scholar]
- 157.Wu L., Wang R. Carbon monoxide: endogenous production, physiological functions, and pharmacological applications. Pharmacol. Rev. 2005;57:585–630. doi: 10.1124/pr.57.4.3. [DOI] [PubMed] [Google Scholar]
- 158.Tenhunen R., Marver H.S., Schmid R. Microsomal heme oxygenase. characterization of the enzyme. J. Biol. Chem. 1969;244:6388–6394. [PubMed] [Google Scholar]
- 159.Maines M.D., Kappas A. Cobalt induction of hepatic heme oxygenase; with evidence that cytochrome P-450 is not essential for this enzyme activity. Proc. Natl. Acad. Sci. USA. 1974;71:4293–4297. doi: 10.1073/pnas.71.11.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Maines M.D., Trakshel G.M., Kutty R.K. Characterization of two constitutive forms of rat liver microsomal heme oxygenase. only one molecular species of the enzyme is inducible. J. Biol. Chem. 1986;261:411–419. [PubMed] [Google Scholar]
- 161.McCoubrey W.K., Jr, Huang T.J., Maines M.D. Isolation and characterization of a cDNA from the rat brain that encodes hemoprotein heme oxygenase-3. Eur. J. Biochem. 1997;247:725–732. doi: 10.1111/j.1432-1033.1997.00725.x. [DOI] [PubMed] [Google Scholar]
- 162.Kitamuro T., Takahashi K., Ogawa K., Udono-Fujimori R., Takeda K., Furuyama K., Nakayama M., Sun J., Fujita H., Hida W., Hattori T., Shirato K., Igarashi K., Shibahara S. Bach1 functions as a hypoxia-inducible repressor for the heme oxygenase-1 gene in human cells. J. Biol. Chem. 2003;278:9125–9133. doi: 10.1074/jbc.M209939200. [DOI] [PubMed] [Google Scholar]
- 163.Lee P.J., Jiang B.H., Chin B.Y., Iyer N.V., Alam J., Semenza G.L., Choi A.M. Hypoxia-inducible factor-1 mediates transcriptional activation of the heme oxygenase-1 gene in response to hypoxia. J. Biol. Chem. 1997;272:5375–5381. [PubMed] [Google Scholar]
- 164.Shibahara S., Muller R.M., Taguchi H. Transcriptional control of rat heme oxygenase by heat shock. J. Biol. Chem. 1987;262:12889–12892. [PubMed] [Google Scholar]
- 165.Nath K.A., Grande J.P., Haggard J.J., Croatt A.J., Katusic Z.S., Solovey A., Hebbel R.P. Oxidative stress and induction of heme oxygenase-1 in the kidney in sickle cell disease. Am. J. Pathol. 2001;158:893–903. doi: 10.1016/S0002-9440(10)64037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kim H.P., Wang X., Galbiati F., Ryter S.W., Choi A.M. Caveolae compartmentalization of heme oxygenase-1 in endothelial cells. FASEB J. 2004;18:1080–1089. doi: 10.1096/fj.03-1391com. [DOI] [PubMed] [Google Scholar]
- 167.Biswas C., Shah N., Muthu M., La P., Fernando A.P., Sengupta S., Yang G., Dennery P.A. Nuclear heme oxygenase-1 (HO-1) modulates subcellular distribution and activation of Nrf2, impacting metabolic and anti-oxidant defenses. J. Biol. Chem. 2014;289:26882–26894. doi: 10.1074/jbc.M114.567685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Maines M.D. The heme oxygenase system: a regulator of second messenger gases. Annu. Rev. Pharmacol. Toxicol. 1997;37:517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- 169.Kikuchi G., Yoshida T., Noguchi M. Heme oxygenase and heme degradation. Biochem. Biophys. Res. Commun. 2005;338:558–567. doi: 10.1016/j.bbrc.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 170.Mansouri A., Perry C.A. Inhibition of platelet ADP and serotonin release by carbon monoxide and in cigarette smokers. Experientia. 1984;40:515–517. doi: 10.1007/BF01952415. [DOI] [PubMed] [Google Scholar]
- 171.Wang R., Wang Z., Wu L. Carbon monoxide-induced vasorelaxation and the underlying mechanisms. Br. J. Pharmacol. 1997;121:927–934. doi: 10.1038/sj.bjp.0701222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zakhary R., Gaine S.P., Dinerman J.L., Ruat M., Flavahan N.A., Snyder S.H. Heme oxygenase 2: endothelial and neuronal localization and role in endothelium-dependent relaxation. Proc. Natl. Acad. Sci. USA. 1996;93:795–798. doi: 10.1073/pnas.93.2.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Motterlini R., Foresti R., Bassi R., Calabrese V., Clark J.E., Green C.J. Endothelial heme oxygenase-1 induction by hypoxia. modulation by inducible nitric-oxide synthase and S-nitrosothiols. J. Biol. Chem. 2000;275:13613–13620. doi: 10.1074/jbc.275.18.13613. [DOI] [PubMed] [Google Scholar]
- 174.Deramaudt B.M., Braunstein S., Remy P., Abraham N.G. Gene transfer of human heme oxygenase into coronary endothelial cells potentially promotes angiogenesis. J. Cell. Biochem. 1998;68:121–127. doi: 10.1002/(sici)1097-4644(19980101)68:1<121::aid-jcb12>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 175.Ahmad S., Hewett P.W., Fujisawa T., Sissaoui S., Cai M., Gueron G., Al-Ani B., Cudmore M., Ahmed S.F., Wong M.K., Wegiel B., Otterbein L.E., Vitek L., Ramma W., Wang K., Ahmed A. Carbon monoxide inhibits sprouting angiogenesis and vascular endothelial growth factor receptor-2 phosphorylation. Thromb. Haemost. 2015;113:329–337. doi: 10.1160/TH14-01-0002. [DOI] [PubMed] [Google Scholar]
- 176.Choi Y.K., Kim C.K., Lee H., Jeoung D., Ha K.S., Kwon Y.G., Kim K.W., Kim Y.M. Carbon monoxide promotes VEGF expression by increasing HIF-1alpha protein level via two distinct mechanisms, translational activation and stabilization of HIF-1alpha protein. J. Biol. Chem. 2010;285:32116–32125. doi: 10.1074/jbc.M110.131284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Jozkowicz A., Huk I., Nigisch A., Weigel G., Dietrich W., Motterlini R., Dulak J. Heme oxygenase and angiogenic activity of endothelial cells: stimulation by carbon monoxide and inhibition by tin protoporphyrin-IX. Antioxid. Redox Signal. 2003;5:155–162. doi: 10.1089/152308603764816514. [DOI] [PubMed] [Google Scholar]
- 178.Loboda A., Jozkowicz A., Dulak J. Carbon monoxide: pro- or anti-angiogenic agent? Comment on Ahmad et al. (Thromb Haemost 2015; 113: 329-337) Thromb. Haemost. 2015;114:432–433. doi: 10.1160/TH15-01-0082. [DOI] [PubMed] [Google Scholar]
- 179.Jozkowicz A., Huk I., Nigisch A., Weigel G., Weidinger F., Dulak J. Effect of prostaglandin-J(2) on VEGF synthesis depends on the induction of heme oxygenase-1. Antioxid. Redox Signal. 2002;4:577–585. doi: 10.1089/15230860260220076. [DOI] [PubMed] [Google Scholar]
- 180.Dulak J., Jozkowicz A., Foresti R., Kasza A., Frick M., Huk I., Green C.J., Pachinger O., Weidinger F., Motterlini R. Heme oxygenase activity modulates vascular endothelial growth factor synthesis in vascular smooth muscle cells. Antioxid. Redox Signal. 2002;4:229–240. doi: 10.1089/152308602753666280. [DOI] [PubMed] [Google Scholar]
- 181.Liu Y., Christou H., Morita T., Laughner E., Semenza G.L., Kourembanas S. Carbon monoxide and nitric oxide suppress the hypoxic induction of vascular endothelial growth factor gene via the 5' enhancer. J. Biol. Chem. 1998;273:15257–15262. doi: 10.1074/jbc.273.24.15257. [DOI] [PubMed] [Google Scholar]
- 182.Bussolati B., Ahmed A., Pemberton H., Landis R.C., Di Carlo F., Haskard D.O., Mason J.C. Bifunctional role for VEGF-induced heme oxygenase-1 in vivo: induction of angiogenesis and inhibition of leukocytic infiltration. Blood. 2004;103:761–766. doi: 10.1182/blood-2003-06-1974. [DOI] [PubMed] [Google Scholar]
- 183.Deshane J., Chen S., Caballero S., Grochot-Przeczek A., Was H., Li Calzi S., Lach R., Hock T.D., Chen B., Hill-Kapturczak N., Siegal G.P., Dulak J., Jozkowicz A., Grant M.B., Agarwal A. Stromal cell-derived factor 1 promotes angiogenesis via a heme oxygenase 1-dependent mechanism. J. Exp. Med. 2007;204:605–618. doi: 10.1084/jem.20061609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Cudmore M., Ahmad S., Al-Ani B., Fujisawa T., Coxall H., Chudasama K., Devey L.R., Wigmore S.J., Abbas A., Hewett P.W., Ahmed A. Negative regulation of soluble Flt-1 and soluble endoglin release by heme oxygenase-1. Circulation. 2007;115:1789–1797. doi: 10.1161/CIRCULATIONAHA.106.660134. [DOI] [PubMed] [Google Scholar]
- 185.Fang J., Sawa T., Akaike T., Akuta T., Sahoo S.K., Khaled G., Hamada A., Maeda H. In vivo antitumor activity of pegylated zinc protoporphyrin: targeted inhibition of heme oxygenase in solid tumor. Cancer Res. 2003;63:3567–3574. [PubMed] [Google Scholar]
- 186.Lakkisto P., Kyto V., Forsten H., Siren J.M., Segersvard H., Voipio-Pulkki L.M., Laine M., Pulkki K., Tikkanen I. Heme oxygenase-1 and carbon monoxide promote neovascularization after myocardial infarction by modulating the expression of HIF-1alpha, SDF-1alpha and VEGF-B. Eur. J. Pharmacol. 2010;635:156–164. doi: 10.1016/j.ejphar.2010.02.050. [DOI] [PubMed] [Google Scholar]
- 187.Jazwa A., Stepniewski J., Zamykal M., Jagodzinska J., Meloni M., Emanueli C., Jozkowicz A., Dulak J. Pre-emptive hypoxia-regulated HO-1 gene therapy improves post-ischaemic limb perfusion and tissue regeneration in mice. Cardiovasc. Res. 2013;97:115–124. doi: 10.1093/cvr/cvs284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Grochot-Przeczek A., Lach R., Mis J., Skrzypek K., Gozdecka M., Sroczynska P., Dubiel M., Rutkowski A., Kozakowska M., Zagorska A., Walczynski J., Was H., Kotlinowski J., Drukala J., Kurowski K., Kieda C., Herault Y., Dulak J., Jozkowicz A. Heme oxygenase-1 accelerates cutaneous wound healing in mice. PloS One. 2009;4:e5803. doi: 10.1371/journal.pone.0005803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Zhao H., Wong R.J., Doyle T.C., Nayak N., Vreman H.J., Contag C.H., Stevenson D.K. Regulation of maternal and fetal hemodynamics by heme oxygenase in mice. Biol. Reprod. 2008;78:744–751. doi: 10.1095/biolreprod.107.064899. [DOI] [PubMed] [Google Scholar]
- 190.Zhao H., Azuma J., Kalish F., Wong R.J., Stevenson D.K. Maternal heme oxygenase 1 regulates placental vasculature development via angiogenic factors in mice. Biol. Reprod. 2011;85:1005–1012. doi: 10.1095/biolreprod.111.093039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Boczkowski J., Poderoso J.J., Motterlini R. CO-metal interaction: vital signaling from a lethal gas. Trends Biochem. Sci. 2006;31:614–621. doi: 10.1016/j.tibs.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 192.Motterlini R., Otterbein L.E. The therapeutic potential of carbon monoxide. Nat. Rev. Drug Discov. 2010;9:728–743. doi: 10.1038/nrd3228. [DOI] [PubMed] [Google Scholar]
- 193.Keyse S.M., Tyrrell R.M. Heme oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide, and sodium arsenite. Proc. Natl. Acad. Sci. USA. 1989;86:99–103. doi: 10.1073/pnas.86.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Tyrrell R.M. Approaches to define pathways of redox regulation of a eukaryotic gene: the heme oxygenase 1 example. Methods. 1997;11:313–318. doi: 10.1006/meth.1996.0425. [DOI] [PubMed] [Google Scholar]
- 195.Cisowski J., Loboda A., Jozkowicz A., Chen S., Agarwal A., Dulak J. Role of heme oxygenase-1 in hydrogen peroxide-induced VEGF synthesis: effect of HO-1 knockout. Biochem. Biophys. Res. Commun. 2005;326:670–676. doi: 10.1016/j.bbrc.2004.11.083. [DOI] [PubMed] [Google Scholar]
- 196.Alam J., Wicks C., Stewart D., Gong P., Touchard C., Otterbein S., Choi A.M., Burow M.E., Tou J. Mechanism of heme oxygenase-1 gene activation by cadmium in MCF-7 mammary epithelial cells. Role of p38 kinase and Nrf2 transcription factor. J. Biol. Chem. 2000;275:27694–27702. doi: 10.1074/jbc.M004729200. [DOI] [PubMed] [Google Scholar]
- 197.Kietzmann T., Samoylenko A., Immenschuh S. Transcriptional regulation of heme oxygenase-1 gene expression by MAP kinases of the JNK and p38 pathways in primary cultures of rat hepatocytes. J. Biol. Chem. 2003;278:17927–17936. doi: 10.1074/jbc.M203929200. [DOI] [PubMed] [Google Scholar]
- 198.Knorr-Wittmann C., Hengstermann A., Gebel S., Alam J., Muller T. Characterization of Nrf2 activation and heme oxygenase-1 expression in NIH3T3 cells exposed to aqueous extracts of cigarette smoke. Free Radic. Biol. Med. 2005;39:1438–1448. doi: 10.1016/j.freeradbiomed.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 199.Ishikawa M., Numazawa S., Yoshida T. Redox regulation of the transcriptional repressor Bach1. Free Radic. Biol. Med. 2005;38:1344–1352. doi: 10.1016/j.freeradbiomed.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 200.Reichard J.F., Motz G.T., Puga A. Heme oxygenase-1 induction by NRF2 requires inactivation of the transcriptional repressor BACH1. Nucleic Acids Res. 2007;35:7074–7086. doi: 10.1093/nar/gkm638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Taille C., El-Benna J., Lanone S., Boczkowski J., Motterlini R. Mitochondrial respiratory chain and NAD(P)H oxidase are targets for the antiproliferative effect of carbon monoxide in human airway smooth muscle. J. Biol. Chem. 2005;280:25350–25360. doi: 10.1074/jbc.M503512200. [DOI] [PubMed] [Google Scholar]
- 202.Abe K., Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci.: Off. J. Soc. Neurosci. 1996;16:1066–1071. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kimura H. Hydrogen sulfide and polysulfides as biological mediators. Molecules. 2014;19:16146–16157. doi: 10.3390/molecules191016146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kolluru G.K., Shen X., Bir S.C., Kevil C.G. Hydrogen sulfide chemical biology: pathophysiological roles and detection. Nitric Oxide: Biol. Chem./Off. J. Nitric Oxide Soc. 2013;35:5–20. doi: 10.1016/j.niox.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Chiku T., Padovani D., Zhu W., Singh S., Vitvitsky V., Banerjee R. H2S biogenesis by human cystathionine gamma-lyase leads to the novel sulfur metabolites lanthionine and homolanthionine and is responsive to the grade of hyperhomocysteinemia. J. Biol. Chem. 2009;284:11601–11612. doi: 10.1074/jbc.M808026200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kimura H. Hydrogen sulfide as a neuromodulator. Mol. Neurobiol. 2002;26:13–19. doi: 10.1385/MN:26:1:013. [DOI] [PubMed] [Google Scholar]
- 207.Nagahara N., Ito T., Kitamura H., Nishino T. Tissue and subcellular distribution of mercaptopyruvate sulfurtransferase in the rat: confocal laser fluorescence and immunoelectron microscopic studies combined with biochemical analysis. Histochem. Cell Biol. 1998;110:243–250. doi: 10.1007/s004180050286. [DOI] [PubMed] [Google Scholar]
- 208.Shibuya N., Mikami Y., Kimura Y., Nagahara N., Kimura H. Vascular endothelium expresses 3-mercaptopyruvate sulfurtransferase and produces hydrogen sulfide. J. Biochem. 2009;146:623–626. doi: 10.1093/jb/mvp111. [DOI] [PubMed] [Google Scholar]
- 209.Zhao W., Zhang J., Lu Y., Wang R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J. 2001;20:6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kohn C., Schleifenbaum J., Szijarto I.A., Marko L., Dubrovska G., Huang Y., Gollasch M. Differential effects of cystathionine-gamma-lyase-dependent vasodilatory H2S in periadventitial vasoregulation of rat and mouse aortas. PloS One. 2012;7:e41951. doi: 10.1371/journal.pone.0041951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Altaany Z., Yang G., Wang R. Crosstalk between hydrogen sulfide and nitric oxide in endothelial cells. J. Cell. Mol. Med. 2013;17:879–888. doi: 10.1111/jcmm.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Mustafa A.K., Sikka G., Gazi S.K., Steppan J., Jung S.M., Bhunia A.K., Barodka V.M., Gazi F.K., Barrow R.K., Wang R., Amzel L.M., Berkowitz D.E., Snyder S.H. Hydrogen sulfide as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels. Circ. Res. 2011;109:1259–1268. doi: 10.1161/CIRCRESAHA.111.240242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Wang K., Ahmad S., Cai M., Rennie J., Fujisawa T., Crispi F., Baily J., Miller M.R., Cudmore M., Hadoke P.W., Wang R., Gratacos E., Buhimschi I.A., Buhimschi C.S., Ahmed A. Dysregulation of hydrogen sulfide producing enzyme cystathionine gamma-lyase contributes to maternal hypertension and placental abnormalities in preeclampsia. Circulation. 2013;127:2514–2522. doi: 10.1161/CIRCULATIONAHA.113.001631. [DOI] [PubMed] [Google Scholar]
- 214.Paul B.D., Snyder S.H. H(2)S signalling through protein sulfhydration and beyond. Nat. Rev. Mol. Cell Biol. 2012;13:499–507. doi: 10.1038/nrm3391. [DOI] [PubMed] [Google Scholar]
- 215.Allsop J., Watts R.W. Methionine adenosyltransferase, cystathionine beta-synthase and cystathionine gamma-lyase activity of rat liver subcellular particles, human blood cells and mixed white cells from rat bone marrow. Clin. Sci. Mol. Med. Suppl. 1975;48:509–513. doi: 10.1042/cs0480509. [DOI] [PubMed] [Google Scholar]
- 216.Agrawal N., Banerjee R. Human polycomb 2 protein is a SUMO E3 ligase and alleviates substrate-induced inhibition of cystathionine beta-synthase sumoylation. PloS One. 2008;3:e4032. doi: 10.1371/journal.pone.0004032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Fu M., Zhang W., Wu L., Yang G., Li H., Wang R. Hydrogen sulfide (H2S) metabolism in mitochondria and its regulatory role in energy production. Proc. Natl. Acad. Sci. USA. 2012;109:2943–2948. doi: 10.1073/pnas.1115634109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Teng H., Wu B., Zhao K., Yang G., Wu L., Wang R. Oxygen-sensitive mitochondrial accumulation of cystathionine beta-synthase mediated by Lon protease. Proc. Natl. Acad. Sci. USA. 2013;110:12679–12684. doi: 10.1073/pnas.1308487110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Kuo S.M., Lea T.C., Stipanuk M.H. Developmental pattern, tissue distribution, and subcellular distribution of cysteine: alpha-ketoglutarate aminotransferase and 3-mercaptopyruvate sulfurtransferase activities in the rat. Biol. Neonat. 1983;43:23–32. doi: 10.1159/000241634. [DOI] [PubMed] [Google Scholar]
- 220.Shibuya N., Tanaka M., Yoshida M., Ogasawara Y., Togawa T., Ishii K., Kimura H. 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid. Redox Signal. 2009;11:703–714. doi: 10.1089/ars.2008.2253. [DOI] [PubMed] [Google Scholar]
- 221.Kabil O., Banerjee R. Redox biochemistry of hydrogen sulfide. J. Biol. Chem. 2010;285:21903–21907. doi: 10.1074/jbc.R110.128363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Lowicka E., Beltowski J. Hydrogen sulfide (H2S) – the third gas of interest for pharmacologists. Pharmacol. Rep. 2007;59:4–24. [PubMed] [Google Scholar]
- 223.Pietri R., Roman-Morales E., Lopez-Garriga J. Hydrogen sulfide and hemeproteins: knowledge and mysteries. Antioxid. Redox Signal. 2011;15:393–404. doi: 10.1089/ars.2010.3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Mustafa A.K., Gadalla M.M., Sen N., Kim S., Mu W., Gazi S.K., Barrow R.K., Yang G., Wang R., Snyder S.H. H2S signals through protein S-sulfhydration. Sci. Signal. 2009;2 doi: 10.1126/scisignal.2000464. (ra72) (ra72) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Hosoki R., Matsuki N., Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem. Biophys. Res. Commun. 1997;237:527–531. doi: 10.1006/bbrc.1997.6878. [DOI] [PubMed] [Google Scholar]
- 226.Stubbert D., Prysyazhna O., Rudyk O., Scotcher J., Burgoyne J.R., Eaton P. Protein kinase G Ialpha oxidation paradoxically underlies blood pressure lowering by the reductant hydrogen sulfide. Hypertension. 2014;64:1344–1351. doi: 10.1161/HYPERTENSIONAHA.114.04281. [DOI] [PubMed] [Google Scholar]
- 227.Al-Magableh M.R., Hart J.L. Mechanism of vasorelaxation and role of endogenous hydrogen sulfide production in mouse aorta. Naunyn-Schmiedeberg's Arch. Pharmacol. 2011;383:403–413. doi: 10.1007/s00210-011-0608-z. [DOI] [PubMed] [Google Scholar]
- 228.Al-Magableh M.R., Kemp-Harper B.K., Hart J.L. Hydrogen sulfide treatment reduces blood pressure and oxidative stress in angiotensin II-induced hypertensive mice. Hypertens. Res.: Off. J. Jpn. Soc. Hypertens. 2015;38:13–20. doi: 10.1038/hr.2014.125. [DOI] [PubMed] [Google Scholar]
- 229.Chitnis M.K., Njie-Mbye Y.F., Opere C.A., Wood M.E., Whiteman M., Ohia S.E. Pharmacological actions of the slow release hydrogen sulfide donor GYY4137 on phenylephrine-induced tone in isolated bovine ciliary artery. Exp. Eye Res. 2013;116:350–354. doi: 10.1016/j.exer.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 230.Yang G., Wu L., Jiang B., Yang W., Qi J., Cao K., Meng Q., Mustafa A.K., Mu W., Zhang S., Snyder S.H., Wang R. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Tang G., Wu L., Liang W., Wang R. Direct stimulation of K(ATP) channels by exogenous and endogenous hydrogen sulfide in vascular smooth muscle cells. Mol. Pharmacol. 2005;68:1757–1764. doi: 10.1124/mol.105.017467. [DOI] [PubMed] [Google Scholar]
- 232.Cai W.J., Wang M.J., Moore P.K., Jin H.M., Yao T., Zhu Y.C. The novel proangiogenic effect of hydrogen sulfide is dependent on Akt phosphorylation. Cardiovasc. Res. 2007;76:29–40. doi: 10.1016/j.cardiores.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 233.Wang M.J., Cai W.J., Li N., Ding Y.J., Chen Y., Zhu Y.C. The hydrogen sulfide donor NaHS promotes angiogenesis in a rat model of hind limb ischemia. Antioxid. Redox Signal. 2010;12:1065–1077. doi: 10.1089/ars.2009.2945. [DOI] [PubMed] [Google Scholar]
- 234.Liu F., Chen D.D., Sun X., Xie H.H., Yuan H., Jia W., Chen A.F. Hydrogen sulfide improves wound healing via restoration of endothelial progenitor cell functions and activation of angiopoietin-1 in type 2 diabetes. Diabetes. 2014;63:1763–1778. doi: 10.2337/db13-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Greiner R., Palinkas Z., Basell K., Becher D., Antelmann H., Nagy P., Dick T.P. Polysulfides link H2S to protein thiol oxidation. Antioxid. Redox Signal. 2013;19:1749–1765. doi: 10.1089/ars.2012.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Umaru B., Pyriochou A., Kotsikoris V., Papapetropoulos A., Topouzis S. ATP-sensitive potassium channel activation induces angiogenesis in vitro and in vivo. J. Pharmacol. Exp. Ther. 2015;354:79–87. doi: 10.1124/jpet.114.222000. [DOI] [PubMed] [Google Scholar]
- 237.Pupo E., Pla A.F., Avanzato D., Moccia F., Cruz J.E., Tanzi F., Merlino A., Mancardi D., Munaron L. Hydrogen sulfide promotes calcium signals and migration in tumor-derived endothelial cells. Free Radic. Biol. Med. 2011;51:1765–1773. doi: 10.1016/j.freeradbiomed.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 238.Zhou Z., Martin E., Sharina I., Esposito I., Szabo C., Bucci M., Cirino G., Papapetropoulos A. Regulation of soluble guanylyl cyclase redox state by hydrogen sulfide. Pharmacol. Res. 2016;111:556–562. doi: 10.1016/j.phrs.2016.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Bucci M., Papapetropoulos A., Vellecco V., Zhou Z., Pyriochou A., Roussos C., Roviezzo F., Brancaleone V., Cirino G. Hydrogen sulfide is an endogenous inhibitor of phosphodiesterase activity. Arterioscler., Thromb., Vasc. Biol. 2010;30:1998–2004. doi: 10.1161/ATVBAHA.110.209783. [DOI] [PubMed] [Google Scholar]
- 240.Zhou Y., Li X.H., Zhang C.C., Wang M.J., Xue W.L., Wu D.D., Ma F.F., Li W.W., Tao B.B., Zhu Y.C. Hydrogen sulfide promotes angiogenesis by downregulating miR-640 via the VEGFR2/mTOR pathway. Am. J. Physiol. Cell Physiol. 2016;310:C305–C317. doi: 10.1152/ajpcell.00230.2015. [DOI] [PubMed] [Google Scholar]
- 241.Flannigan K.L., Agbor T.A., Motta J.P., Ferraz J.G., Wang R., Buret A.G., Wallace J.L. Proresolution effects of hydrogen sulfide during colitis are mediated through hypoxia-inducible factor-1alpha. FASEB J. 2015;29:1591–1602. doi: 10.1096/fj.14-266015. [DOI] [PubMed] [Google Scholar]
- 242.Katsouda A., Bibli S.I., Pyriochou A., Szabo C., Papapetropoulos A. Regulation and role of endogenously produced hydrogen sulfide in angiogenesis. Pharmacol. Res. 2016;113:175–185. doi: 10.1016/j.phrs.2016.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Wang M., Guo Z., Wang S. Cystathionine gamma-lyase expression is regulated by exogenous hydrogen peroxide in the mammalian cells. Gene Expr. 2012;15:235–241. doi: 10.3727/105221613x13571653093286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Hassan M.I., Boosen M., Schaefer L., Kozlowska J., Eisel F., von Knethen A., Beck M., Hemeida R.A., El-Moselhy M.A., Hamada F.M., Beck K.F., Pfeilschifter J. Platelet-derived growth factor-BB induces cystathionine gamma-lyase expression in rat mesangial cells via a redox-dependent mechanism. Br. J. Pharmacol. 2012;166:2231–2242. doi: 10.1111/j.1476-5381.2012.01949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Stuehr D.J., Fasehun O.A., Kwon N.S., Gross S.S., Gonzalez J.A., Levi R., Nathan C.F. Inhibition of macrophage and endothelial cell nitric oxide synthase by diphenyleneiodonium and its analogs. FASEB J. 1991;5:98–103. doi: 10.1096/fasebj.5.1.1703974. [DOI] [PubMed] [Google Scholar]
- 246.Mistry R.K., Murray T.V., Prysyazhna O., Martin D., Burgoyne J.R., Santos C., Eaton P., Shah A.M., Brewer A.C. Transcriptional regulation of cystathionine-gamma-lyase in endothelial cells by NADPH oxidase 4-dependent signaling. J. Biol. Chem. 2016;291:1774–1788. doi: 10.1074/jbc.M115.685578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Lin V.S., Lippert A.R., Chang C.J. Cell-trappable fluorescent probes for endogenous hydrogen sulfide signaling and imaging H2O2-dependent H2S production. Proc. Natl. Acad. Sci. USA. 2013;110:7131–7135. doi: 10.1073/pnas.1302193110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Ma X., Sayed N., Beuve A., van den Akker F. NO and CO differentially activate soluble guanylyl cyclase via a heme pivot-bend mechanism. EMBO J. 2007;26:578–588. doi: 10.1038/sj.emboj.7601521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Motterlini R., Foresti R., Intaglietta M., Winslow R.M. NO-mediated activation of heme oxygenase: endogenous cytoprotection against oxidative stress to endothelium. Am. J. Physiol. 1996;270:H107–H114. doi: 10.1152/ajpheart.1996.270.1.H107. [DOI] [PubMed] [Google Scholar]
- 250.Willis D., Tomlinson A., Frederick R., Paul-Clark M.J., Willoughby D.A. Modulation of heme oxygenase activity in rat brain and spleen by inhibitors and donors of nitric oxide. Biochem. Biophys. Res. Commun. 1995;214:1152–1156. doi: 10.1006/bbrc.1995.2406. [DOI] [PubMed] [Google Scholar]
- 251.Filipovic M.R., Eberhardt M., Prokopovic V., Mijuskovic A., Orescanin-Dusic Z., Reeh P., Ivanovic-Burmazovic I. Beyond H2S and NO interplay: hydrogen sulfide and nitroprusside react directly to give nitroxyl (HNO). A new pharmacological source of HNO. J. Med. Chem. 2013;56:1499–1508. doi: 10.1021/jm3012036. [DOI] [PubMed] [Google Scholar]
- 252.Norris A.J., Sartippour M.R., Lu M., Park T., Rao J.Y., Jackson M.I., Fukuto J.M., Brooks M.N. Nitroxyl inhibits breast tumor growth and angiogenesis. Int. J. Cancer. 2008;122:1905–1910. doi: 10.1002/ijc.23305. [DOI] [PubMed] [Google Scholar]
- 253.Pacher P., Beckman J.S., Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.D'Autreaux B., Toledano M.B. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 2007;8:813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 255.Andreadou I., Iliodromitis E.K., Rassaf T., Schulz R., Papapetropoulos A., Ferdinandy P. The role of gasotransmitters NO, H2S and CO in myocardial ischaemia/reperfusion injury and cardioprotection by preconditioning, postconditioning and remote conditioning. Br. J. Pharmacol. 2015;172:1587–1606. doi: 10.1111/bph.12811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Hu Q., Wu D., Ma F., Yang S., Tan B., Xin H., Gu X., Chen X., Chen S., Mao Y., Zhu Y.Z. Novel angiogenic activity and molecular mechanisms of ZYZ-803, a slow-releasing hydrogen sulfide-nitric oxide hybrid molecule. Antioxid. Redox Signal. 2016;25:498–514. doi: 10.1089/ars.2015.6607. [DOI] [PubMed] [Google Scholar]