Abstract
Cotton leaf curl disease (CLCuD), caused by cotton leaf curl viruses (CLCuVs), is among the most devastating diseases in cotton. While the widely cultivated cotton species Gossypium hirsutum is generally susceptible, the diploid species G. arboreum is a natural source for resistance against CLCuD. However, the influence of CLCuD on the G. arboreum transcriptome and the interaction of CLCuD with G. arboreum remains to be elucidated. Here we have used an RNA-Seq based study to analyze differential gene expression in G. arboreum under CLCuD infestation. G. arboreum plants were infested by graft inoculation using a CLCuD infected scion of G. hirsutum. CLCuD infested asymptomatic and symptomatic plants were analyzed with RNA-seq using an Illumina HiSeq. 2500. Data analysis revealed 1062 differentially expressed genes (DEGs) in G. arboreum. We selected 17 genes for qPCR to validate RNA-Seq data. We identified several genes involved in disease resistance and pathogen defense. Furthermore, a weighted gene co-expression network was constructed from the RNA-Seq dataset that indicated 50 hub genes, most of which are involved in transport processes and might have a role in the defense response of G. arboreum against CLCuD. This fundamental study will improve the understanding of virus-host interaction and identification of important genes involved in G. arboreum tolerance against CLCuD.
Introduction
Cotton (Gossypium spp.) is a leading source of natural textile fiber, oil and protein meal, making it one of the most important cash crops in many countries1. The major cotton producing countries include China, India, United States, and Pakistan which contribute more than 70% of the total cotton production around the globe (https://apps.fas.usda.gov/). Among the 50 Gossypium species, two diploid species (G. herbaceum and G. arboreum) and two tetraploid species (G. hirsutum and G. barbadense) are cultivated worldwide, but G. hirsutum (upland cotton) accounts for more than 90% of overall cotton production2.
Tetraploid cotton, G. hirsutum, evolved about 1–1.5 million years ago (Mya) as a result of a polyploidization event with an A-genome species G. arboreum and a D-genome species G. raimondii the putative progenitors3. G. hirsutum cultivars have superior lint yield and produce very high-quality fiber, however, these tetraploid cultivars are often more susceptible to several abiotic and biotic stresses4,5. In contrast, diploid cotton species, particularly G. arboreum, are more tolerant to many biotic and abiotic stresses including CLCuD. G. arboreum is a valuable source for novel genes to enhance genetic diversity as well as for specific traits6,7.
Bemisia tabaci, commonly known as silverleaf whitefly, is one of the sap sucking pests that causes damage to more than 500 plant species including cotton. Among the list of sap-sucking insects that cause damage to a cotton crop, whitefly is responsible for more than 50% crop losses8. Whitefly serves as a vector for viruses causing cotton leaf curl disease (CLCuD), which is a major threat to cotton crops in several countries including Pakistan and India9. Viruses causing CLCuD, collectively referred to as cotton leaf curl viruses (CLCuVs), belong to the family Geminiviridae and the genus Begomovirus, which is the most important genera of this family9. Other members of this genus also infect important crops including tomato, beans, chillies, cucurbits, mungbean and other vegetables10–12. Plants infected with CLCuD show typical begomovirus infection symptoms such as leaf curling, vein yellowing, leaf enations and stunted growth9,13. CLCuD is among the most devastating of viral diseases, responsible for serious crop losses annually that negatively impact a country’s economy14.
As their defense system, plants mobilize a variety of intrinsic mechanisms to deal with pathogens and it is necessary to understand the mechanisms that plants use to cope with different stresses at the genetic level15. Abiotic and biotic stresses activate a relay network of plant gene expression mechanisms that lead to the reprogramming of a variety of physiological and metabolic processes in accordance with the stress response. Early studies mainly used model plant species to identify a wide spectrum of genes that are involved in different levels of metabolism, signal transduction, osmotic regulation and stress response. Microarray gene expression profiling has been widely used to study differential gene expression in plants under different stresses16,17. Advances in the field of transcriptomics has made RNA-Sequencing (RNA-Seq) a major “-omics” technology for studying global gene expression of plants under different stress conditions18,19. Several RNA-Seq studies have been done to reveal mechanisms in cotton under different biotic stresses. Transcriptome analysis of cotton flower buds infested with the cotton boll weevil highlighted the diversity of genes and pathways regulated by pest infestation, such as kinase cascades, transcription factors (TFs), and phytohormone-signaling pathways20. Significant changes in the expression of transcripts were found to be associated with sugar and amino acid metabolism in cotton following aphid and whitefly infestation21. Another recent transcriptomic study reported several genes for control of phloem-feeding pests22.
Viral infection initiates a complex interaction between the virus and the host. Understanding host responses during viral infection can help in the development of effective strategies for virus control. In recent years, RNA-Seq studies have been applied extensively to uncover the responses of plant hosts to viral infection23–29. However, the comprehensive molecular mechanisms underlying CLCuD-cotton interactions remain poorly defined.
Therefore, to elucidate the mechanisms involved in cotton defense against CLCuD, we aimed to identify important differentially expressed genes in cotton under CLCuD infestation and to elucidate the resistance mechanisms of the naturally immune cotton species G. arboreum at a molecular level. We infested G. arboreum with CLCuD by grafting, then performed RNA-Seq followed by data analysis, weighted gene co-expression network analysis (WGCNA), gene ontology enrichment and functional annotation.
Results and Discussion
Cotton is an economically important crop and CLCuD causes drastic losses in crop production. Compared to normally susceptible tetraploid G. hirsutum, the diploid species G. arboreum is a natural source for resistance against CLCuD. Typically, whitefly is the vector that transmits CLCuD in tetraploid cotton, but G. arboreum remains unaffected when infected with viruliferous whiteflies. Recent studies have revealed evidence that G. arboreum plants support CLCuD replication and its long-distance spread only if CLCuD is transmitted by graft inoculation. In the presence of CLCuV/CLCuMBBur, mild symptoms of CLCuD have been shown to be induced in G. arboreum by its graft inoculation with scions from CLCuD infested G. hirsutum plants30,31. Interestingly, only a few leaves of G. arboreum have been observed to develop symptoms of the disease following grafting. The nature of this resistance pattern remains unknown and might be correlated with gene expression levels or other indigenous immune mechanisms of G. arboreum 30,32. The present study aims to detect changes G. arboreum gene expression in response to CLCuD infestation by graft inoculation, even in the absence of severe symptoms. This study will help in understanding the mechanisms involved in resistance of G. arboreum to the begomoviruses causing CLCuD.
Grafting and transcriptome sequencing
The standard method for CLCuD, delivery into G. hirsutum is by whitefly-mediated virus transmission. However, in most studies, G. arboreum remained asymptomatic and thought to be a non-host against CLCuD33. As an alternative, a graft infestation method was developed for systemic delivery of the CLCuD complex (cotton leaf curl geminivirus with its associated betasatellite) into G. arboreum 30. In this study, we used the graft-infestation method for delivery of CLCuD complex to G. arboreum. Characteristic viral infection symptoms were observed on G. arboreum grafted with CLCuD infected G. hirsutum scions (Fig. 1). Out of 30 plants grafted with scions, 27 plants established a successful graft. Among these 27 G. arboreum plants, 20 plants showed characteristic symptoms of CLCuD on the leaves near the grafted scion, while 7 plants remained asymptomatic for CLCuD (Fig. 1). These results are comparable with previous observations where most of the graft-infested G. arboreum developed mild symptoms with a few plants remaining asymptomatic32. RNA was extracted from three biological replicates of graft-infested, symptomatic and asymptomatic G. arboreum plants. This RNA was processed for library preparation followed by RNA sequencing.
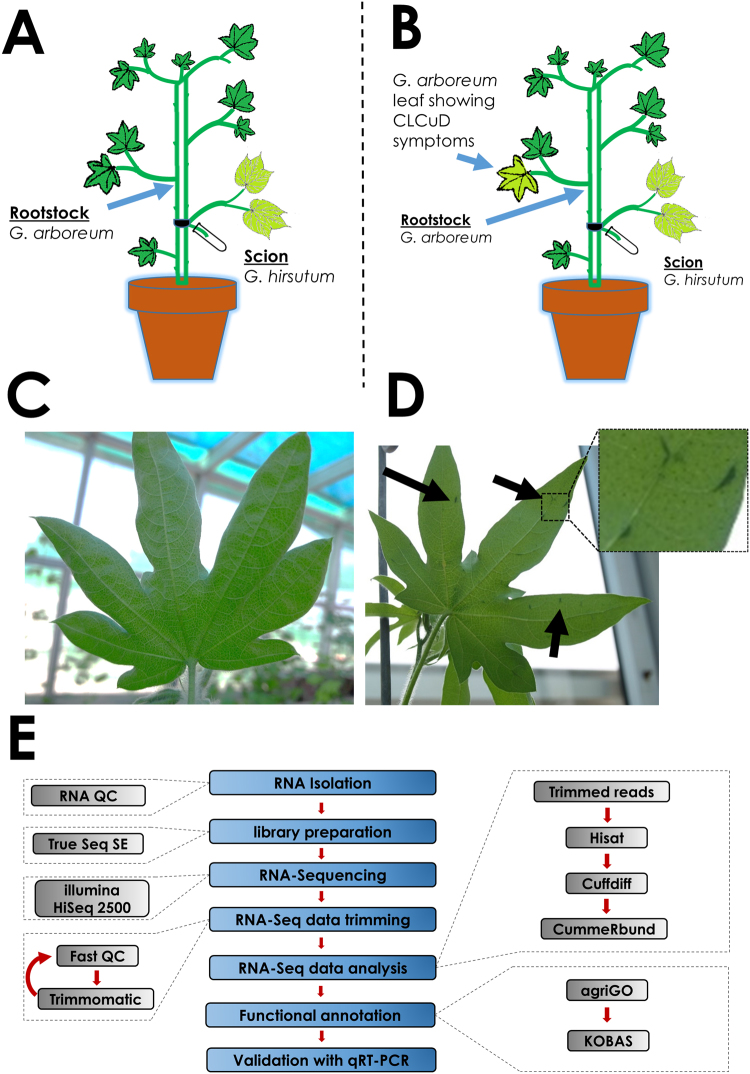
Figure 1.
Experimental design, methodology and RNA-Seq pipeline used in study. Panel A and B show the graphical representation of grafting experiments (A) scion of cotton leaf curl disease (CLCuD) infected Gossypium hirsutum was used on a rootstock of G arboreum; asymptomatic leaves of CLCuD were collected for RNA extraction and sequencing. (B) Leaves showing symptoms of CLCuD were collected for RNA extraction and sequencing. (C) Shows an asymptomatic CLCuD free leaf of G. arboreum. (D) Shows a leaf with very mild symptoms of CLCuD including vein swelling and darkening, highlighted with black arrows. (E) Shows the workflow of the RNA-Seq experiment and the tools used at each step.
RNA-Seq data analysis
All six libraries were sequenced using a HiSeq™ 2500 platform. On average, 10 million total reads were obtained from each replicate (Table 1). Quality of individual sequences was evaluated using FastQC analysis, including per base sequence quality analysis which plots the Phred quality score distribution on the y axis for each read generated per sample and for each nucleotide base call plotted on the x axis (Figure S1). All FASTQ sequencing files obtained in this study have an average per base Phred score of 64, a conventional threshold denoting high quality base calls. High quality sequence reads were aligned to the G. arboreum reference genome with 13 chromosomes and 41,330 gene models (Table S1). The percentage of mapped reads obtained from the alignment summary of HISAT2 is provided in Table 1 (Fig. 2A).
Table 1.
Summary of RNA-Seq runs used in this study.
| Sample | Condition | No. of Reads | % Mapping |
|---|---|---|---|
| RZN7 | Grafted - CLCuD symptomatic | 11304210 | 76.25 |
| RZN8 | Grafted - CLCuD symptomatic | 9833891 | 66.97 |
| RZN9 | Grafted - CLCuD symptomatic | 10201621 | 62.17 |
| RZN10 | Grafted - asymptomatic | 10049099 | 69.77 |
| RZN11 | Grafted - asymptomatic | 10941016 | 68.2 |
| RZN12 | Grafted - asymptomatic | 9667217 | 77.69 |
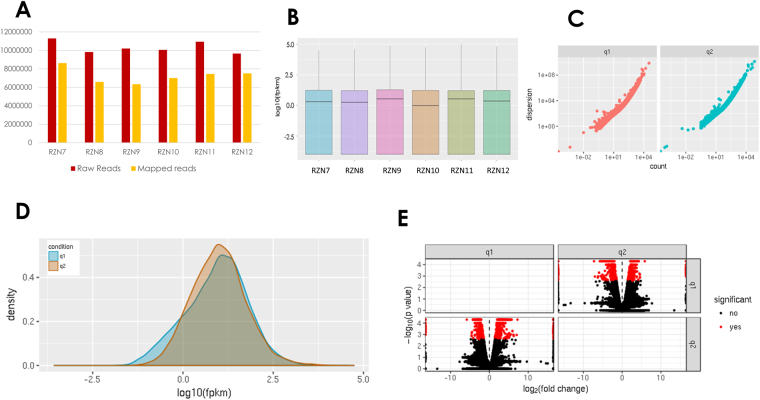
Figure 2.
RNA-Seq data quality check and efficiency of mapping with G. arboreum genome. Panel A shows the raw sequencing reads compared the mapped reads where the y axis indicates the number of reads and x axis indicates the samples used in study. Panel B, C, D and E show the amount of data among replicates in terms of log10_(FPKM), gene dispersion, density and differentially expressed transcripts in the dataset. q1 and q2 represent the two conditions of symptomatic and asymptomatic G. arboreum respectively.
Differential gene expression was quantified and grafted CLCuD symptomatic G. arboreum (RZN7-RZN9) were compared to the grafted asymptomatic G. arboreum (RZN10-RZN12). To assess the biological reproducibility of the experiment, each sample was treated as an individual biological replicate using the cuffdiff package of Cufflinks34. We compared individual runs from each condition and calculated the transcript abundances of each independent run (Fig. 2B). A quality check of compared transcripts provided standard gene density and dispersion (Fig. 2C,D). The expression levels of mapped genes were normalized with a FPKM (fragments per kilobase of exon per million fragments mapped) value. To confirm the quality of RNA-Seq, the eight highest-ranking housekeeping control genes in G. arboreum were selected to evaluate gene expression35. Based on comparisons of our samples, none of these reference genes were significantly differentially expressed (Table 2), suggesting that the sequences obtained and the transcript expression levels qualified for further transcriptome analysis. In the comparison of gene expression levels of symptomatic and asymptomatic G. arboreum plants, an absolute value of log2 fold change >1 and the False Discovery Rate (FDR) <0.05 was set to declare differentially expressed genes (DEGs) involved in the response of CLCuD infestation. Overall, 1062 genes were differentially expressed in this comparison, out of which 563 and 499 were up and downregulated, respectively (Fig. 2E; Fig. 3).
Table 2.
Expression levels of housekeeping control genes in G. arboretum.
| No | Function | Gene_Id | log2FC | significant |
|---|---|---|---|---|
| 1 | Clathrin adaptor complexes medium subunit family protein | Cotton_A_09164_BGI-A2_v1.0 | −0.363142 | no |
| 2 | Catalytic subunit of protein phosphatase 2 A | Cotton_A_09192_BGI-A2_v1.0 | 0.619919 | no |
| 3 | F-box family | Cotton_A_12507_BGI-A2_v1.0 | 0.510816 | no |
| 4 | Betatubulin | Cotton_A_14308_BGI-A2_v1.0 | −0.7817 | no |
| 5 | Elongation factor | Cotton_A_23419_BGI-A2_v1.0 | −0.452078 | no |
| 6 | Glyceraldehyde-3-phosphate dehydrogenase C-2 | Cotton_A_31637_BGI-A2_v1.0 | −0.539749 | no |
| 7 | Ubiquitin | Cotton_A_32873_BGI-A2_v1.0 | 0.0623818 | no |
| 8 | Actin | Cotton_A_38366_BGI-A2_v1.0 | −0.395614 | no |
Figure 3.
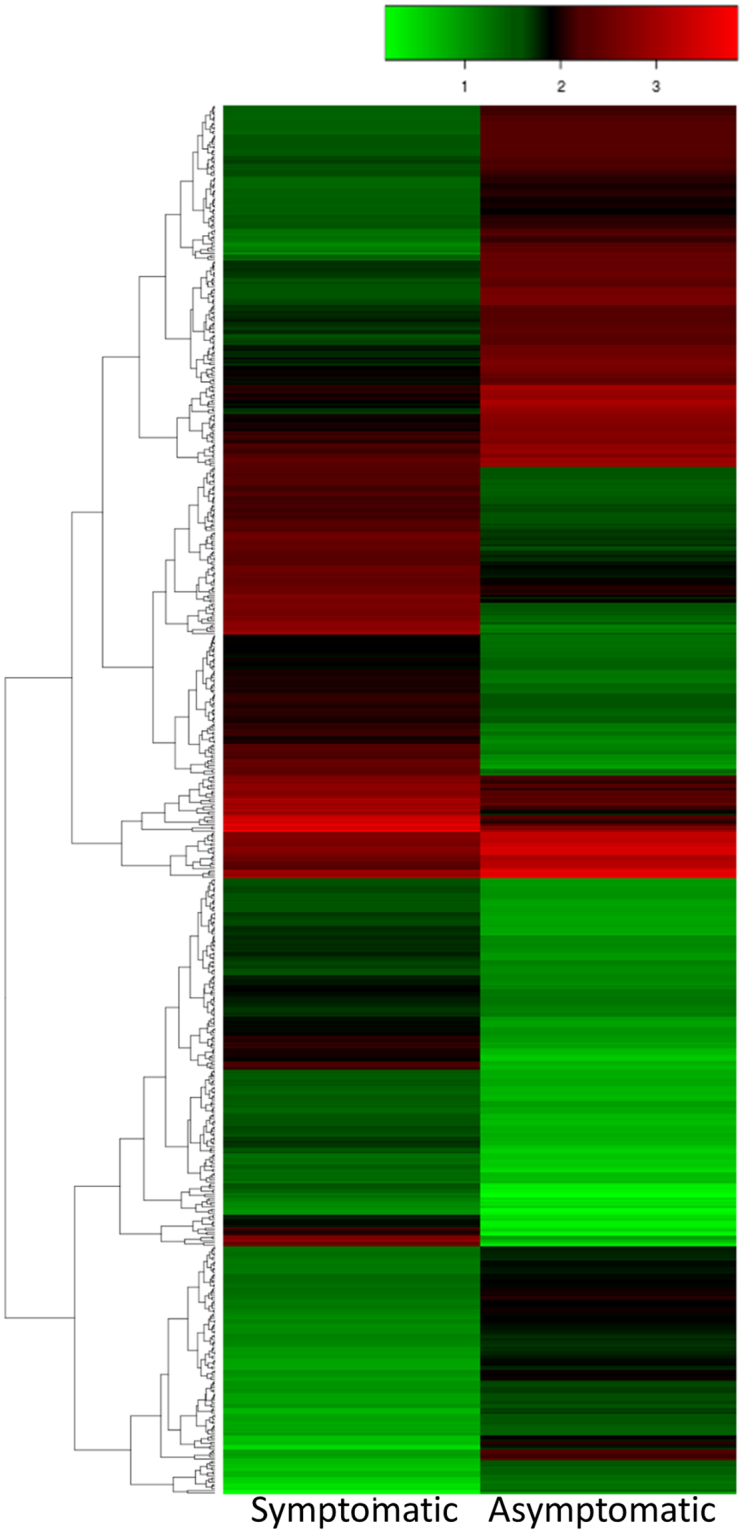
Hierarchical clustering of differentially expressed genes. A heat map of G. arboreum differentially expressed genes in response to cotton leaf curl disease with respect to hierarchical clustering. Log10 expression values were used for the analysis and negative values were set to zero. Clustering and the heat map were performed using heatmap 2.0 package in R.
The transcriptome data of all replicates from this experiment is publicly available under BioProject accession number PRJNA380937 and a comprehensive list of up- and downregulated DEGs is provided in Table S2. This data is a valuable resource for understanding CLCuD tolerance in G. arboreum. Furthermore, the DEGs identified were narrowed down by log2 fold-change, p values and q values for further experimentation. Many functional classes of genes related to pathogen defense were found to be significantly affected in CLCuD-infected symptomatic plants compared to that of the asymptomatic plants.
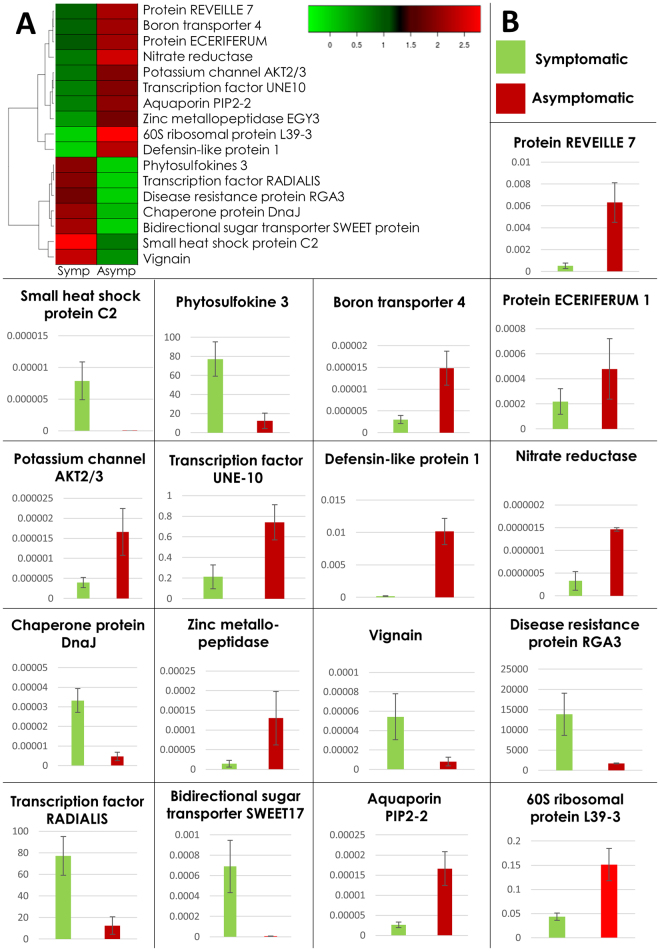
For the validation of our transcriptomic data we selected 17 significant DEGs with a probable role in disease resistance. We designed primers and performed qPCR on cDNA of independent biological replicates of G. arboreum. Our qPCR data strongly correlated with the RNA-Seq expression data (Fig. 4). The implication of these genes has been discussed with reference to published data (Supplementary Discussion; Table 3).
Figure 4.
Validation of gene expression with quantitative RT-PCR. (A) Heat map of 17 selected DEGs for qRT-PCR in G. arboreum in response to cotton leaf curl disease with respect to hierarchical clustering. Log10 expression values were used for the analysis and negative values were set to zero. Clustering and the heat map were performed using heatmap 2.0 package in R. (B) Quantitative RT-PCR was used to measure the relative expression levels of seventeen pathogen resistance related genes with 18 S as an internal reference. Values were expressed as fold changes of transcript levels in the CLCuD infested symptomatic leaf samples with respect to the transcript levels in CLCuD infested asymptomatic leaf samples. Error bars represented standard error (SE) of three biological replicates.
Table 3.
Selected differentially expressed genes for qRT-PCR and their probable functions in plant pathogen defense.
| Gene | Probable role in defense | References |
|---|---|---|
| Probable zinc metallopeptidase EGY3 | Development and stress response | 94 |
| Defensin-like protein 1 | Bacterial and fungal pathogens as well as herbivorous insects | 95–97 |
| Phytosulfokines 3 | Pattern-triggered immunity against pathogens, Leucine Rich Repeat family (LRR) | 98–100 |
| Chaperone protein DnaJ | Pathogen defense, antiviral defense | 101–103 |
| Transcription factor UNE10 | Antiviral defense | 46 |
| Protein REVEILLE 7 | Plant growth, stress, pathogen response | 104,105 |
| Small heat shock protein C2 | Antiviral and antibacterial stress response | 59,106 |
| Transcription factor RADIALIS | Myb encoding genes, plant defense response | 45,107 |
| Putative disease resistance protein RGA3 | R-gene mediated pathogen and disease response | 49,108,109 |
| Bidirectional sugar transporter SWEET17 | Abiotic stress tolerance, pathogenesis related protein | 39 |
| Protein ECERIFERUM 1 | Biotic and abiotic stresses | 110 |
| Probable aquaporin PIP2-2 | Biotic and abiotic stresses, plant immunity | 111–113 |
| Potassium channel AKT2/3 | Plant development, stress responses, antiviral defense | 41,42 |
| 60 S ribosomal protein L39-3 | Pathogen and disease resistance, antiviral defense | 7,114,115 |
| Boron transporter 4 | R-gene mediated viral defense | 38 |
| Vignain | Plant immunity, pathogenesis and plant defense | 116,117 |
| Nitrate reductase [NADH] | Pathogen signal-induced NO production | 118–120 |
Putative defense related genes involved in resistance of G. arboreum to CLCuD infection
Plants have evolved with sophisticated and well-established defense mechanisms to cope with pathogens, such as insects, fungi, bacteria and viruses36. Besides physical barriers (like trichomes), toxic compounds or secondary metabolites, plants have numerous defense pathways to defend themselves against diverse pathogens37. An initial gene ontology (GO) term analysis of the top one hundred most significantly expressed genes present in the RNA-Seq dataset indicated that several GO terms were involved in regulation of responses related to stress, external stimulus, biotic stress and plant defense (Figure S2).
Further dissection of expression levels of individual genes in symptomatic and asymptomatic G. arboreum plants revealed that many groups of genes were differentially expressed in response to CLCuD infection.
Differential expression of membrane transporters and channel proteins
Among the DEGs, some transporter genes were observed such as a boron transporter gene that was upregulated while a SWEET 17 gene was downregulated in G. arboreum asymptomatic plants. An interaction of host boron transporter with viral coat protein can regulate boron transport to induce necrosis, or the interaction can modulate an R gene mediated defense response38. Based on these studies, the upregulation of the boron transporter gene in G. arboreum depicts the protection of the plant from boron toxicity. In G. arboreum, the boron transporter may also be involved in host-viral interaction which modulates the R-gene mediated response in the host to combat virus infection. SWEETs are bidirectional vacuolar fructose transporters in plants predicted to be involved in maintaining sugar homeostasis in plant organs during favorable as well as abiotic and biotic stress conditions. SWEET transporters have been found to be associated with plant pathogen interactions during a pathogen attack in plants39. A reduced level of SWEET gene expression in tobacco and rice was associated with enhanced resistance to pathogens, suggesting a role in pathogen growth and plant disease resistance39,40. The downregulation of G. arboreum SWEET transporter suggests its role in sugar homeostasis under unfavorable conditions and possible involvement in reduced pathogen growth and disease resistance.
Alteration in expression levels of channel proteins in plants has been reported as a response to environmental stimuli41. Significantly enhanced soybean mosaic virus resistance was observed in soybean by the overexpression of GmAKT242. The induction of aquaporin channel proteins encoding genes has been reported with expression profiling of soybean under Pseudomonas syringae infection43. Studies have also shown the involvement of aquaporins in plant-viral interactions. For example, aquaporin genes interact with a cucumber mosaic virus (CMV) replication protein that potentially affects CMV replication in the host plant44. Thus, the upregulation of G. arboreum AKT2 and aquaporin genes might have a role in CLCuD.
Transcription factors and R-gene mediated response
During pathogen attack, transcription factors play an important role in plant innate immunity. MYB transcription factors act as master regulators of cellular responses and are involved in plant development, secondary metabolism, hormone signal transduction, abiotic stress tolerance and disease resistance. These transcription factors have been found to be involved in mechanisms of disease resistance in several plants through regulation of defense genes45. In the present data set, differential gene expression of different MYB transcription factors, including RADIALIS and REVEILLE, indicate their role in plant defense against CLCuD. A recent study on genome-wide analysis of bHLH transcription factors in Solanum lycopersicum shows that bHLH transcription factors are involved in the plant’s defense under infection by Tomato yellow leaf curl virus and upregulation of bHLH is associated with disease resistance46. G. arboreum bHLH transcription factor UNE10 was upregulated during CLCuD stress and suggests a role in disease resistance. Transcription factors, particularly ethylene response factor (ERF), lead to the transcriptional regulation of several jasmonate and ethylene responsive defense genes under pathogen attack47. In our experiment, seven DEGS were found to be ethylene responsive genes (Table S5) that might have a role in G. arboreum defense against CLCuD.
R gene-triggered resistance in plants is associated with a rapid defense response known as hypersensitive response (HR) that can bring localized cell death at the site of infection and trigger a series of downstream defense pathways48. R-genes have been found to be induced in response to several pathogens and diseases in plants, including tobacco mosaic virus, rice blast, Arabidopsis downy mildew, tomato leaf mold and Verticillium wilt-resistance in cotton49. In our experiment, eleven putative R genes were differentially expressed indicating their role in plant defense against CLCuD (Table S6). Protein kinases along with R genes are well known in disease resistance both in a positive and a negative manner50,51. Forty-six kinase genes were identified as DEGs and most of them were downregulated in asymptomatic G. arboreum (Table S7).
Phytohormone signaling
CLCuD infestation in G. arboreum also revealed the induced expression of genes associated with phytohormone signaling pathways. Phytohormones are the key players in signaling cascades induced by pathogens20. In this study auxin, cytokinin, abscisic acid and brassinosteroid related genes were upregulated while genes involved in ethylene and salicylic acid pathways were downregulated, highlighting their role in plant defense. Taken together the complex cascade in phytohormone related gene expression demonstrates the interconnection of the signaling pathways regulated by phytohormones in response to CLCuD infection.
Protein processing in endoplasmic reticulum and ubiquitin proteasome systems are involved in plant-viral interaction
Under virus infection, the plant’s cellular machinery is hijacked by viruses for DNA replication of viral genomes and translation of viral proteins. Like other pathogens including bacteria, phytopathogenic viruses activate endoplasmic reticulum (ER) stress signaling machinery. Viruses act as “pirates” and use host machinery for their spread across the plasmodesmata to neighboring cells using viral movement proteins (MP). The ER signaling mechanism has been found to be involved in cell to cell movement and spread of viruses through plasmodesmata52. In Arabidopsis, a broad range of viruses induce gene expression of ER stress marker genes such as Calreticulins (CRTs). Calreticulin has been shown to interact with movement of viral proteins including MP of tobacco mosaic and turnip crinkle virus (TCV). CRT helps in redirecting virus movement through plasmodesmata which leads to symptom development, however, its overexpression leads to delayed cell to cell movement of virus53. Many other well studied host proteins interact with viral MPs for regulation of their cell to cell movement including β−1,3-glucanase-interacting proteins54. DnaJ and heat shock chaperones act as potential translocation factors and are involved in regulation of viral cell-to-cell movement55. Deposition of β−1,3-glucan or callose in plasmodesmata initiates a hypersensitivity response (HR) in hosts, consequently leading to the blocking of plasmodesmata to hinder cell to cell viral movement. Viral proteins like the TGB2 protein of Potato Virus X (PVX) interacts with the host ß−1,3-glucanase to degrade the callose, followed by PVX movement through the plasmodesmata54. DnaJ (HSP 40) is an important co-chaperon of HSP70 and is important for its function56. HSP70 is proposed to be associated with coat protein interaction, viral transportation and replication of many geminiviruses. Downregulation of HSP70 showed decreased viral load and reduced viral movement in plants infected with tomato yellow leaf curl virus (TYLCV)57.
In G. arboreum symptomatic plants, the induction of many genes involved in protein processing in ER including CRT, lectin like gene OS9, glucanase interacting gene OST and heat shock proteins HSP70 and HSP 40 indicate the possible role of this pathway interacting with CLCuV proteins and helping viral cell to cell movement in the plant, making it susceptible to establishment of mild symptoms from graft inoculation with CLCuD. The downregulation of these genes in asymptomatic plants might have role in impairment of viral movement proteins, restricting viral movement in plasmodesmata (Figure S3).
The ubiquitin proteasome system (UPS) has a very important role in virus host interactions and plant defense against viruses58,59. There are several reports showing the complex interaction of host plant UPS components with viral proteins, suggesting that the ubiquitin pathway is probably a conserved pathway involved in plant virus interaction. UPS in plants is comprised of ubiquitin-activating enzyme (E1), ubiquitin-conjugating enzyme (E2), and ubiquitin-ligase (E3). These three enzymes make an E3 ubiquitin ligase complex which is required for polyubiquitination of cellular proteins, followed by 26 S proteasome targeted degradation60. During viral infection, the virus takes over the plant host’s UPS machinery regulating its gene expression to enhance pathogenesis, while in parallel the plant uses UPS as a strong layer of defense, mainly by targeting of viral proteins. Different components of UPS, from ubiquitin to 26 S proteasome have been found to be involved in plant defense mechanisms. Host UPS machinery is involved in targeted degradation of TMV and Turnip yellow mosaic virus movement proteins and decreased viral pathogenesis suggest a role for UPS in plant antiviral defense59.
Like other plant viruses, geminiviruses also interact with the different components of host UPS. Rep, a replication protein of geminiviruses, interacts with host plant SUMO-conjugating enzyme 1 (SCE1), which is an essential host factor for viral infection in the plant61. Altered expression of SCE1 leads to unsuccessful geminiviral replication and suppression of infection62. SKP1 is another potent component of the CUL1-based SCF ubiquitin E3 ligases that recruits proteins for polyubiquitination. Geminiviral C2 protein interferes with the different components of host UPS including SKP159,63. Suppression of SKP1 in plants abolishes the downstream HR and resistance response in plants60. βC1 from Cotton leaf curl Multan virus (CLCuMV) interacts with the host ubiquitin-conjugating (UBC) enzyme, SlUBC3, leading to symptom induction that is possibly due to downregulation of the UPS64. The upregulation of UPS pathway components including UBC3 (COP1), SCE1(UBE21) and SKP1 in the G. arboreum asymptomatic plants indicates that ubiquitin mediated proteolysis could be a defense response against symptom development of CLCuD (Figure S4).
Weighted co-expression gene network analysis (WGCNA) identifies two novel modules within the network
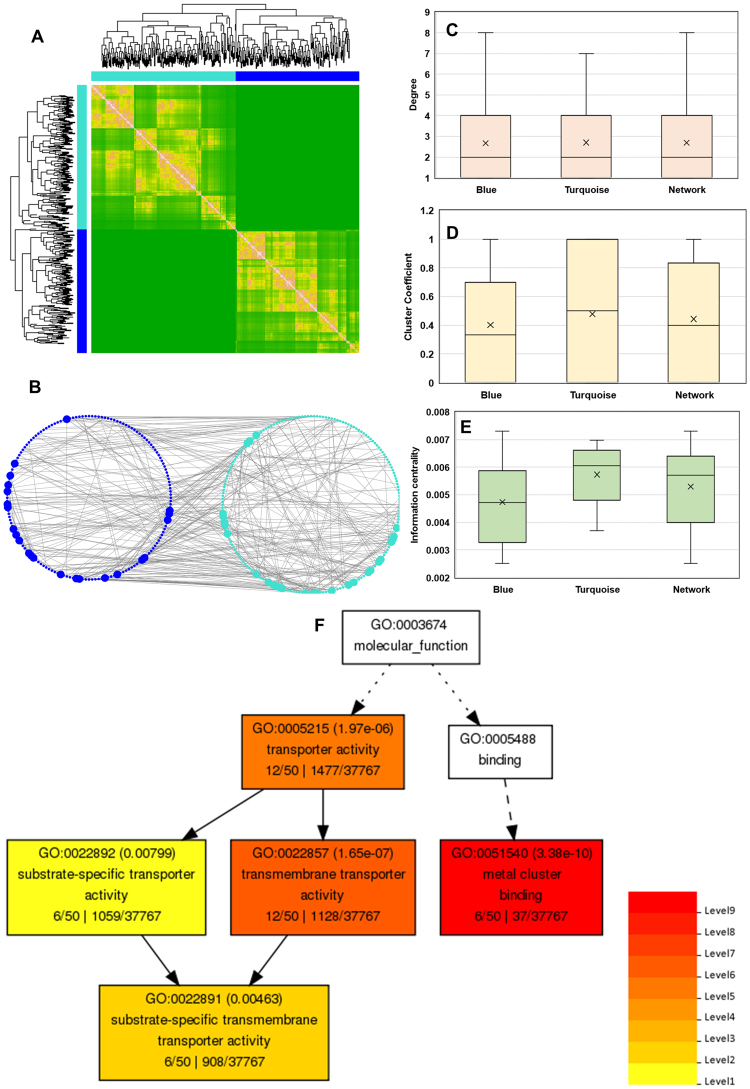
With the availability of large-scale transcriptome datasets, co-expression network analysis allows identification of a cohort of genes with similar expression patterns in response to a given stimulus or physiological condition within a cell65,66. Thus, co-expression networks can identify a set of genes, which might participate in a common biological process. To determine CLCuD-responsive common gene signatures, we performed a weighted gene co-expression network analysis67,68 on 468 selected DEGs. Using the WGCNA platform, we created a topological overlap mapping metric (TOM) plot, a measure of neighborhood proximity that calculates the similarity matrix of gene expression between two nodes69. TOM also features hierarchical clustering dendrograms possessing a range of weighted correlations67. These analyses led us to generate an undirected weighted network with scale-free topology, a network with power-law degree distribution. This weighted co-expression network encompasses two different modules (Fig. 5A,B; Table S8) that are denoted with two different colors. The turquoise and blue modules comprise of 252 and 216 genes, respectively.
Figure 5.
Weighted gene co-expression network construction and analysis for CLCuD-responsive cotton genes in CLCuD symptomatic and asymptomatic leaves. (A) Transcriptomic data from symptomatic and asymptomatic leaves was analyzed by WGCNA using 468 DEGs. Genes were clustered as per expression arrangements characterized by the dendrogram and topological overlap mapping metric (TOM) heat map. Each line of the dendrogram corresponds to a gene. Clusters of similarly-regulated genes are grouped as modules by corresponding color (blue and turquoise) with a threshold minimum module size of 70 genes. The intensity of pink coloring in heatmap specifies high strength and green as no strength of correlation between pairs of genes on a direct scale and features hierarchical clustering dendrograms possessing a range of weighted correlations. (B) Weighted network illustrates correlations (edges) among the nodes (genes) with a weighted correlation threshold of ≥0.85. The network is composed of 468 connections and 348 genes organized in two different modules. The node color corresponds to modules identified via WGCNA. Nodes with high connectivity ( ≥5 connections, hubs) among different modules are indicated with increased node size. (C) Highly connected nodes (hubs) within the co-expression network are shown. Average degree of turquoise and blue modules is not changed in the entire co-expression network (2.689655172). (D) Clustering coefficient (degree to which a node is connected in a neighborhood) for genes within each node is illustrated in a box plot. Turquoise module displays significantly a higher average clustering coefficient than whole co-expression network (0.443746579). (E) The information centrality (the flow of information between any two nodes in a connected network) for the largest component of co-expression network (92 nodes) is presented. Turquoise module displays significantly increased information centrality compared to the entire network (0.005299235). (F) Interactive graph of GO terms associated with cotton leaf curl disease responsive G. arboreum hub genes identified by co-expression network analysis. Analysis performed with online tool agriGO (bioinfo.cau.edu.cn/agriGO/) where a key indicates significance levels of GO terms.
Centrality measures can reveal the most influential vertices in a network. To decipher the most important nodes within this co-expression network, we calculated, degree (number of connections of a node) and clustering coefficients (degree to which a node is connected in a neighborhood) as well as information centrality (the flow of information between any two nodes in a connected network)66,70,71. Degree distribution revealed a total of 50 hubs, nodes with ≥ 5 connections within the co-expression network (Fig. 5B; Table S8).
Out of these identified hub genes, co-expression network upregulated hub genes included Aquaporin TIP4-1, Nitrate/peptide (NRT1/ PTR) transporter, Ammonium transporter 1 member, vacuolar calcium ion transporter, CDGSH iron-sulfur cluster binding protein, 3-isopropylmalate dehydratase and nitrite reductase, while downregulated genes included sugar transport protein 13, NAT3 (Nucleobase-ascorbate transporter 3 ion symporter) and zinc transporter genes. GO term analysis of these hub genes revealed their involvement in transport processes. Plants use a wide array of chemicals as weapons for their defense against pathogens. These chemicals or compounds can accumulate at high levels in the infected or attacked tissue72. Proteins involved in transport processes are vital for long distance transport of these defense compounds to the other tissues and for plant sustainability under viral attack73. The differentially expressed transporters identified here might have a role in transport of secondary metabolites and defense related compounds that further activate other mechanisms involved in plant defense responses under viral attack.
Moreover, we determined that the average degree of turquoise and blue modules was constant. We did not observe any change in the degree for blue and turquoise modules compared to the entire co-expression network or blue module. Intriguingly, we also found that the turquoise module exhibited a significantly heightened average clustering coefficient (Fig. 5C,D). Finally, our information centrality measure in the largest component with 92 nodes determined that the turquoise module displayed significantly increased information centrality compared to the entire network (Fig. 5E; Table S9). These data suggest that nodes in the network may transmit the information via DEGs faster.
Oxidative stress related genes under CLCuD infection
Light has a major role in photosynthesis and meeting energy demands of the plant under pathogen attack. It provides energy as well as elicits protection to plants against invading microbes74. We found GO enrichment of light and radiation based gene expression under a module after co-expression network analysis (Figure S5). Under plant microbe attack, one protective strategy in plants is to produce reactive oxygen species (ROS)75. Nicotinamide adenine dinucleotide (reduced form NADH) and cytochrome oxidase (COX) enzymes are involved in synthesis of glutathione S-transferase a major scavenger of ROS. However, another mechanism involved in generation of ROS under biotic stress includes light dependent ROS production from chloroplasts74. Therefore, upregulation of COX1, COX3, NADH and genes related to light response in asymptomatic G. arboreum plants suggests modulation of gene regulation to combat CLCuD by ROS scavenging.
Role of primary metabolic pathways in plant immunity
Cellular energy demands have been found to be increased under pathogen attacks in plants. Primary metabolic pathways along with secondary metabolic processes support these increased requirements of the plants. Secondary metabolites including terpenes and phenolics are required by plants for protection against microbial pathogens. However, plant secondary metabolic pathways create a large carbon flux by influencing and reconfiguring the primary metabolism for resistance responses76,77. We identified upregulation of genes related to the metabolic pathway specifically involved in steroid and lipid metabolism in asymptomatic plants (Figure S6). These genes include flavonoids, brassinosteroid, sphingolipids and terpenoids, suggesting a role in the defense response to CLCuD. Carbohydrates or sugars act as a fuel to boost energy levels in plants during plant microbe interaction. These are studied as potent players in coordinated plant metabolism with development as well as dealing with different stress responses78. The concept of “sweet immunity” has been established based on their importance in plant immunity. In the present dataset, several genes involved in carbohydrate or saccharide metabolism were found to be highly expressed including phosphoenolpyruvate carboxykinase, fructose-1 6-bisphosphatase and phosphoglucomutase (Figure S6). We detected several genes related to cation or meta ion channel activity by WGCNA (Figure S6). Ca2 + signaling related genes, including calmodulins (CaCML) and cyclic nucleotide-gated ion channels (CNGs), were elevated with significantly higher expression levels during CLCuV infection. The upregulation of these ion channels is correlated with the previous studies where cytosolic Ca2 + levels have been observed to be triggered under pathogen infection. Furthermore, Ca2 + acts as second messenger that induces defense related signaling pathways79,80. In the blue module, we identified upregulated genes in asymptomatic plants related to metabolic pathways specifically involved in steroid and lipid metabolism (Figure S5). These genes encode flavonoids, brassinosteroids, sphingolipids and terpenoids, known to play a role in defense response.
In this study, we extended the fundamental understanding of G. arboreum’s response to CLCuD infection. G. arboreum’s natural resistance to CLCuD may involve a complicated gene network, which starts with a basal response and production of general pathogen-associated molecular pattern molecules, followed by activation of CLCuD defense response signaling cascades leading to the transport of defense compounds to other plant tissues and all these interconnected layers of responses ultimately cause immunity to CLCuD in G. arboreum. The transcriptomic data provided here is a valuable resource to further characterize candidate genes that are responsive to CLCuD infection and involved in the resistance gene network; these genes can be used in future breeding programs using new breeding techniques like CRISPR to engineer resistance against geminiviruses81–83. Consequently, this study will help the scientific community to better understand the mechanisms of G. arboreum resistance against CLCuD.
Materials and Methods
Plant growth and virus inoculation by grafting
Plants of G. arboreum, variety Ravi, were grown in an insect-free glasshouse. G. hirsutum, variety CIM 496, plants were maintained in a separate glasshouse and were inoculated with CLCuD by allowing viruliferous whiteflies to feed on these plants. Severely CLCuD infected CIM496 branches were then used as scions to graft on Ravi plants. G. arboreum plants were grafted using a “bottle shoot” grafting method30,32. In this technique, a graft-scion is placed in a tube filled with distilled water to support plant growth at the higher temperatures maintained for this experiment (Fig. 1). The water in the tube was refreshed daily till the graft union between root stock and scion was established. The tube of water was removed at day 9 after grafting. The temperature of the glasshouse was maintained between 38–45 °C for day time and 25–30 °C for night time. The leaf tissue was collected from CLCuD symptomatic and asymptomatic plants at day 25 post graft infestation. Three biological replicates were processed independently.
RNA extraction
Total RNA was extracted from CLCuD infested symptomatic and asymptomatic leaves using TRIzol reagent according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA, USA). Three biological replicates from each sample were used for this experiment. The quality and quantity of RNA were assessed by electrophoresis on 1% agarose gels and by a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific, USA). The integrity of RNA samples was examined using Bio-analyzer 2100 equipment (Agilent Technologies, Germany).
Library construction and RNA sequencing
The extracted total RNA samples with a concentration of 10 µg were used for cDNA synthesis and strand specific RNA-Seq libraries were constructed as described earlier84. Poly (A) mRNA was isolated using oligo-dT beads (Qiagen, Hilden, Germany). The mRNA was broken into short fragments (~300 nt). First-strand cDNA was synthesized using random hexamer-primed reverse transcription. Second-strand cDNA was generated using RNase H and DNA polymerase I. The cDNA fragments were purified and washed for end repair and ligated to sequencing adapters. The cDNA fragments of suitable size were purified and enriched by PCR to obtain the final cDNA library. The integrity of each cDNA library was examined using Bio-analyzer 2100 equipment (Agilent Technologies, Germany). The cDNA libraries were then sequenced using single-end mode of HiSeq™ 2500 equipment (Illumina, San Diego, CA, USA).
RNA-Seq data analysis
Clean reads were selected after preprocessing with Trimmomatic85 to remove low-quality sequences (i.e. reads containing adaptor sequences, and reads with more than 5% unknown bases). After preprocessing the RNA-Seq data, the quality of reads was checked by FastQC86. The cleaned reads were then mapped to the reference G. arboreum genome87 using HISAT2-build and HISAT2 aligner88. Default HISAT2 parameters, which allow up to two mismatches and report up to 20 alignments for reads mapping at multiple positions, were used. The sequence alignment/map files generated by HISAT2 were used as the input to the software Cufflinks34 which assembles the alignments in the sequence alignment/map file into transfrags. Cufflinks does this assembly independently of the existing gene annotations and constructs a minimum set of transcripts that best describes the RNA-Seq reads. The unit of measurement used by Cufflinks to estimate transcript abundance is FPKM (Fragments Per Kilobase of transcript per Million mapped reads). The Cufflinks statistical model probabilistically assigns reads to the assembled isoforms. Cuffdiff was used to find differentially expressed genes (DEGs). The read coverage of one gene was used to calculate the gene expression level, which was measured with the fragment per kilobase of exon model per million mapped reads (FPKM) method. A q value cutoff of 0.05 was used to determine whether a gene had differential expression between samples.
Quantitative real time PCR
To verify the differential expression detected by the Illumina RNA-Seq data, quantitative real-time RT-PCR (qRT-PCR) was performed on a new set of symptomatic and asymptomatic samples. A set of 17 genes was chosen, including 10 upregulated and 7 downregulated genes (Table S4). Primers for qPCR were designed with the program Primer3 (http://bioinfo.ut.ee/primer3-0.4.0/primer3) with default settings. All primer sequences are provided in Table S3. qRT-PCR was performed using a QUANTSTUDIO 6 flex qRT-PCR instrument and the light cycler fast start DNA Master SYBR Green I kit (Roche, Basel, Switzerland). Reactions were performed in triplicate, and contained 100 ng of cDNA, 0.5 μ L of each primer (10 μ M/μ L), and 10 μ L SYBR Green Master Mix in a final volume of 20 μ L. The amplification reactions were performed under the following conditions: 95 °C for 5 min, followed by 40 cycles of 95 °C for 15 s, 55 °C for 20 s, and 72 °C for 30 s. Melting curve analysis, performed by increasing the temperature from 55 to 95 °C (0.5 °C per 10 s), and gel electrophoresis of the final product confirmed the presence of single amplicons. Relative fold differences for each sample in each experiment were calculated using the ΔΔ Ct method and the amplification of 18 S rRNA was used as an internal control to normalize all data89. To corroborate the expression levels measured by RNA-Seq, the ratio of expression as measured by qRT-PCR was compared to the ratio of expression levels between samples using RNA-Seq.
Construction of co-expression network and network analyses
To construct a co-expression network, we processed transcripts with FPKM count ≥10 and removed all the outliers, which yielded a total number of 468 DEGs. We implemented the R-based Weighted Gene Co-expression Network Analysis (WGCNA)67,68 package to construct a co-expression network. The dendrogram was constructed using the cutreeDynamicTree algorithm68, with a threshold of minimum module size of 70 genes. A weighted correlation threshold of ≥0.85 was set that resulted in the identification of two modules representing the entire network. Python-based NetworkX90 and Cytoscape v. 3.5.1 Plugins91 were utilized for network analyses including information centrality, degree and cluster coefficients. The weighted co-expression network was visualized using “Group Attributes Layout”, a feature of Cytoscape v. 3.5.192. Box plot was used to display the distribution of nodes within each module for information centrality, degree and cluster coefficients. Students t-test describes statistical significance.
Gene ontology and functional annotation of DEGs
To determine the main biological functions and pathways of the DEGs, all DEGs were mapped to terms in the Kyoto Encyclopedia of Genes and Genomes (KEGG: www.kegg.jp/kegg/kegg1.html) databases93 using AgriGO and Kobas 3.0 tools respectively. Functional analysis of DEGs was done by searching the gene IDs of DEGs in the Swissprot and TrEMBL blast alignment hits available on Cottongen database (https://www.cottongen.org/). Furthermore, the GO term analysis of the identified nodes and hub genes in WGCNA was performed to determine their possible roles in plant defense to CLCuD.
Data availability
RNA-Seq data from this study have been deposited at the NCBI under the BioProject accession No. PRJNA380937 and BioSample Nos. SAMN06628122, SAMN06628123, SAMN06628124, SAMN06628125, SAMN06628126 and SAMN06628127.
Electronic supplementary material
Acknowledgements
The authors highly acknowledge USDA, ICARDA and HEC for funding this research project. Any opinions, findings, conclusions or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the views of the USDA or ICARDA. This research was supported by grants from the USDA-ICARDA Cotton Productivity Enhancement Project and International Research Support Initiative Program of Higher Education Commission (HEC) of Pakistan. This work was also supported by the National Science Foundation (IOS-1557796) to MSM.
Author Contributions
R.Z.N. and S.S.Z. contributed equally to this work. M.A., S.M., B.E.S., J.A.S., L.A.M., conceived and designed the current project. R.Z.N. and K.P.A. were involved in grafting experiments. R.Z.N., S.S.Z. and M.A. performed the experiments. G.J. and M.W. contributed in real time experiments and data analysis. R.Z.N., S.S.Z., S.S., B.M., M.S.M. analyzed the data. R.Z.N. wrote the first draft of the paper. M.A., M.S.M., S.M. and J.A.S. critically revised the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Rubab Zahra Naqvi and Syed Shan-e-Ali Zaidi contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-15963-9.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lee JJ, Woodward AW, Chen ZJ. Gene expression changes and early events in cotton fibre development. Annals of botany. 2007;100:1391–1401. doi: 10.1093/aob/mcm232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen ZJ, et al. Toward sequencing cotton (Gossypium) genomes. Plant physiology. 2007;145:1303–1310. doi: 10.1104/pp.107.107672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paterson AH, et al. Repeated polyploidization of Gossypium genomes and the evolution of spinnable cotton fibres. Nature. 2012;492:423–427. doi: 10.1038/nature11798. [DOI] [PubMed] [Google Scholar]
- 4.Maqbool A, et al. Gossypium arboreum GHSP26 enhances drought tolerance in Gossypium hirsutum. Biotechnology progress. 2010;26:21–25. doi: 10.1002/btpr.306. [DOI] [PubMed] [Google Scholar]
- 5.Robinson AF. Reniform in US Cotton: When, Where, Why, and Some Remedies 1. Annual review of phytopathology. 2007;45:263–288. doi: 10.1146/annurev.phyto.45.011107.143949. [DOI] [PubMed] [Google Scholar]
- 6.Li R, Erpelding JE. Genetic diversity analysis of Gossypium arboreum germplasm accessions using genotyping-by-sequencing. Genetica. 2016;144:535–545. doi: 10.1007/s10709-016-9921-2. [DOI] [PubMed] [Google Scholar]
- 7.Gong Q, et al. Salicylic acid-related cotton (Gossypium arboreum) ribosomal protein GaRPL18 contributes to resistance to Verticillium dahliae. BMC plant biology. 2017;17:59. doi: 10.1186/s12870-017-1007-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Razaq M, Abbas G, Farooq M, Aslam M, Athar H-U-R. Effect of Insecticidal Application on Aphid Population, Photosynthetic Parameters and Yield Components of Late Sown Varieties of Canola, Brassica napus L. Pakistan journal of zoology. 2014;46:661–668. doi: 10.3923/pjn.2014.661.665. [DOI] [Google Scholar]
- 9.Zubair M, Zaidi SS, Shakir S, Amin I, Mansoor S. An insight into Cotton leaf curl Multan betasatellite, the most important component of cotton leaf curl disease complex. Viruses. 2017;9:280. doi: 10.3390/v9100280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zaidi SS, Briddon RW, Mansoor S. Engineering dual Begomovirus-Bemisia tabaci resistance in plants. Trends in Plant Science. 2017;22:6–8. doi: 10.1016/j.tplants.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Zaidi SS, Martin DP, Amin I, Farooq M, Mansoor S. Tomato leaf curl New Delhi virus: a widespread bipartite begomovirus in the territory of monopartite begomoviruses. Molecular Plant Pathology. 2017;18:901–911. doi: 10.1111/mpp.12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zaidi, S. S.-e.-A., Tashkandi, M., Mansoor, S. & Mahfouz, M. M. Engineering Plant Immunity: Using CRISPR/Cas9 to Generate Virus Resistance. Frontiers in plant science7 (2016). [DOI] [PMC free article] [PubMed]
- 13.Zaidi SS, et al. Frequent occurrence of Tomato leaf curl New Delhi virus in cotton leaf curl disease affected cotton in Pakistan. Plos one. 2016;11:e0155520. doi: 10.1371/journal.pone.0155520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zubair M, et al. Multiple begomoviruses found associated with cotton leaf curl disease in Pakistan in early 1990 are back in cultivated cotton. Scientific reports. 2017;7:680. doi: 10.1038/s41598-017-00727-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gatehouse JA. Plant resistance towards insect herbivores: a dynamic interaction. New phytologist. 2002;156:145–169. doi: 10.1046/j.1469-8137.2002.00519.x. [DOI] [PubMed] [Google Scholar]
- 16.Park W, Scheffler BE, Bauer PJ, Campbell BT. Genome-wide identification of differentially expressed genes under water deficit stress in upland cotton (Gossypium hirsutum L.) BMC plant biology. 2012;12:90. doi: 10.1186/1471-2229-12-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L, et al. RNA-seq analyses of multiple meristems of soybean: novel and alternative transcripts, evolutionary and functional implications. BMC Plant Biology. 2014;14:169. doi: 10.1186/1471-2229-14-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heidel-Fischer HM, Musser RO, Vogel H. Plant transcriptomic responses to herbivory. Annual plant reviews. 2014;47:155–196. doi: 10.1002/9781118829783.ch5. [DOI] [Google Scholar]
- 19.Thompson GA, Goggin FL. Transcriptomics and functional genomics of plant defence induction by phloem-feeding insects. Journal of experimental botany. 2006;57:755–766. doi: 10.1093/jxb/erj135. [DOI] [PubMed] [Google Scholar]
- 20.Artico S, et al. Transcriptome analysis of Gossypium hirsutum flower buds infested by cotton boll weevil (Anthonomus grandis) larvae. BMC genomics. 2014;15:854. doi: 10.1186/1471-2164-15-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dubey NK, et al. Comparative transcriptome analysis of Gossypium hirsutum L. in response to sap sucking insects: aphid and whitefly. BMC genomics. 2013;14:241. doi: 10.1186/1471-2164-14-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li, J. et al. Transcriptome analysis reveals a comprehensive insect resistance response mechanism in cotton to infestation by the phloem feeding insect Bemisia tabaci (whitefly). Plant biotechnology journal (2016). [DOI] [PMC free article] [PubMed]
- 23.Babu M, Gagarinova AG, Brandle JE, Wang A. Association of the transcriptional response of soybean plants with soybean mosaic virus systemic infection. Journal of general virology. 2008;89:1069–1080. doi: 10.1099/vir.0.83531-0. [DOI] [PubMed] [Google Scholar]
- 24.Zhang F, et al. Genetic regulation of salt stress tolerance revealed by RNA-Seq in cotton diploid wild species, Gossypium davidsonii. Scientific reports. 2016;6:20582. doi: 10.1038/srep20582. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Du P, et al. Viral infection induces expression of novel phased microRNAs from conserved cellular microRNA precursors. PLoS pathogens. 2011;7:e1002176. doi: 10.1371/journal.ppat.1002176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu J, et al. Transcriptome analysis of Nicotiana tabacum infected by Cucumber mosaic virus during systemic symptom development. PLoS one. 2012;7:e43447. doi: 10.1371/journal.pone.0043447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang C, et al. Spatial analysis of Arabidopsis thaliana gene expression in response to Turnip mosaic virus infection. Molecular plant-microbe interactions. 2007;20:358–370. doi: 10.1094/MPMI-20-4-0358. [DOI] [PubMed] [Google Scholar]
- 28.Yang J, Zhang F, Li J, Chen J-P, Zhang H-M. Integrative analysis of the microRNAome and transcriptome illuminates the response of susceptible rice plants to rice stripe virus. PLoS one. 2016;11:e0146946. doi: 10.1371/journal.pone.0146946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Z, et al. De novo transcriptome sequencing in Frankliniella occidentalis to identify genes involved in plant virus transmission and insecticide resistance. Genomics. 2013;101:296–305. doi: 10.1016/j.ygeno.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 30.Akhtar KP, et al. First symptomatic evidence of infection of Gossypium arboreum with Cotton leaf curl Burewala virus through grafting. Internationa Journal of Agricultural Biology. 2013;15:157–160. [Google Scholar]
- 31.Akhtar K, et al. Evaluation of Gossypium species for resistance to cotton leaf curl Burewala virus. Annals of applied biology. 2010;157:135–147. doi: 10.1111/j.1744-7348.2010.00416.x. [DOI] [Google Scholar]
- 32.Ullah R, et al. An analysis of the resistance of Gossypium arboreum to cotton leaf curl disease by grafting. European journal of plant pathology. 2014;139:837–847. doi: 10.1007/s10658-014-0437-2. [DOI] [Google Scholar]
- 33.Azhar MT, Amin I, Bashir A, Mansoor S. Characterization of resistance gene analogs from Gossypium arboreum and their evolutionary relationships with homologs from tetraploid cottons. Euphytica. 2011;178:351–362. doi: 10.1007/s10681-010-0310-0. [DOI] [Google Scholar]
- 34.Trapnell C, et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nature protocols. 2012;7:562–578. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Artico S, Nardeli SM, Brilhante O, Grossi-de-Sa MF, Alves-Ferreira M. Identification and evaluation of new reference genes in Gossypium hirsutum for accurate normalization of real-time quantitative RT-PCR data. BMC plant biology. 2010;10:49. doi: 10.1186/1471-2229-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Henry E, Yadeta KA, Coaker G. Recognition of bacterial plant pathogens: local, systemic and transgenerational immunity. New phytologist. 2013;199:908–915. doi: 10.1111/nph.12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang, C. et al. Overexpression of a novel peanut NBS‐LRR gene AhRRS5 enhances disease resistance to Ralstonia solanacearum in tobacco. Plant biotechnology journal (2016). [DOI] [PMC free article] [PubMed]
- 38.Lim H-S, et al. The coat protein of Alternanthera mosaic virus is the elicitor of a temperature-sensitive systemic necrosis in Nicotiana benthamiana, and interacts with a host boron transporter protein. Virology. 2014;452:264–278. doi: 10.1016/j.virol.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 39.Chandran D. Co‐option of developmentally regulated plant SWEET transporters for pathogen nutrition and abiotic stress tolerance. IUBMB life. 2015;67:461–471. doi: 10.1002/iub.1394. [DOI] [PubMed] [Google Scholar]
- 40.Antony G, et al. Rice xa13 recessive resistance to bacterial blight is defeated by induction of the disease susceptibility gene Os-11N3. The plant cell. 2010;22:3864–3876. doi: 10.1105/tpc.110.078964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y, Wu W-H. Plant sensing and signaling in response to K + -deficiency. Molecular plant. 2010;3:280–287. doi: 10.1093/mp/ssq006. [DOI] [PubMed] [Google Scholar]
- 42.Zhou L, et al. Overexpression of GmAKT2 potassium channel enhances resistance to soybean mosaic virus. BMC plant biology. 2014;14:154. doi: 10.1186/1471-2229-14-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zou J, et al. Expression profiling soybean response to Pseudomonas syringae reveals new defense-related genes and rapid HR-specific downregulation of photosynthesis. Molecular plant-microbe interactions. 2005;18:1161–1174. doi: 10.1094/MPMI-18-1161. [DOI] [PubMed] [Google Scholar]
- 44.Kim MJ, Kim HR, Paek K-H. Arabidopsis tonoplast proteins TIP1 and TIP2 interact with the cucumber mosaic virus 1a replication protein. Journal of general virology. 2006;87:3425–3431. doi: 10.1099/vir.0.82252-0. [DOI] [PubMed] [Google Scholar]
- 45.Katiyar A, et al. Genome-wide classification and expression analysis of MYB transcription factor families in rice and Arabidopsis. BMC genomics. 2012;13:544. doi: 10.1186/1471-2164-13-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang J, et al. Genome-wide analysis of bHLH transcription factor and involvement in the infection by yellow leaf curl virus in tomato (Solanum lycopersicum) BMC genomics. 2015;16:39. doi: 10.1186/s12864-015-1249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang, P.-Y., Catinot, J. & Zimmerli, L. Ethylene response factors in Arabidopsis immunity. Journal of experimental botany, erv518 (2015). [DOI] [PubMed]
- 48.Thomma BP, Nürnberger T, Joosten MH. Of PAMPs and effectors: the blurred PTI-ETI dichotomy. The plant cell. 2011;23:4–15. doi: 10.1105/tpc.110.082602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen J-Y, et al. Genome-wide analysis of the gene families of resistance gene analogues in cotton and their response to Verticillium wilt. BMC plant biology. 2015;15:148. doi: 10.1186/s12870-015-0508-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rowland O, et al. Functional analysis of Avr9/Cf-9 rapidly elicited genes identifies a protein kinase, ACIK1, that is essential for full Cf-9–dependent disease resistance in tomato. The Plant cell. 2005;17:295–310. doi: 10.1105/tpc.104.026013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Song W-Y, Wang G-L, Chen L-L, Kim H-S. A receptor kinase-like protein encoded by the rice disease resistance gene, Xa21. Science. 1995;270:1804. doi: 10.1126/science.270.5243.1804. [DOI] [PubMed] [Google Scholar]
- 52.Kørner CJ, Du X, Vollmer ME, Pajerowska-Mukhtar KM. Endoplasmic reticulum stress signaling in plant immunity—at the crossroad of life and death. International journal of molecular sciences. 2015;16:26582–26598. doi: 10.3390/ijms161125964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen M-H, Tian G-W, Gafni Y, Citovsky V. Effects of calreticulin on viral cell-to-cell movement. Plant physiology. 2005;138:1866–1876. doi: 10.1104/pp.105.064386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stange C. Plant-virus interactions during the infective process. Cien. Inv. Agr. (in English) 33 (1): 1–18. Ciencia e investigacion agraria. 2006;33:1–18. [Google Scholar]
- 55.Liu JZ, Whitham SA. Overexpression of a soybean nuclear localized type–III DnaJ domain‐containing HSP40 reveals its roles in cell death and disease resistance. The plant journal. 2013;74:110–121. doi: 10.1111/tpj.12108. [DOI] [PubMed] [Google Scholar]
- 56.Taguwa S, et al. Defining Hsp70 subnetworks in dengue virus replication reveals key vulnerability in flavivirus infection. Cell. 2015;163:1108–1123. doi: 10.1016/j.cell.2015.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gorovits, R. & Czosnek, H. The involvement of heat shock proteins in the establishment of Tomato yellow leaf curl virus infection. Frontiers in plant science8 (2017). [DOI] [PMC free article] [PubMed]
- 58.Citovsky, V., Zaltsman, A., Kozlovsky, S. V., Gafni, Y. & Krichevsky, A. In Seminars in cell & developmental biology. 1048–1054 (Elsevier). [DOI] [PubMed]
- 59.Mandadi KK, Scholthof K-BG. Plant immune responses against viruses: how does a virus cause disease? The plant cell. 2013;25:1489–1505. doi: 10.1105/tpc.113.111658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hua Z, Vierstra RD. The cullin-RING ubiquitin-protein ligases. Annual review of plant biology. 2011;62:299–334. doi: 10.1146/annurev-arplant-042809-112256. [DOI] [PubMed] [Google Scholar]
- 61.Sánchez-Durán MA, et al. Interaction between geminivirus replication protein and the SUMO-conjugating enzyme is required for viral infection. Journal of virology. 2011;85:9789–9800. doi: 10.1128/JVI.02566-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Castillo, A. et al. Identification of Plant Genes Involved in TYLCV Replication. Tomato Yellow Leaf Curl Virus Disease, 207–221 (2007).
- 63.Lozano-Durán R, et al. Geminiviruses subvert ubiquitination by altering CSN-mediated derubylation of SCF E3 ligase complexes and inhibit jasmonate signaling in Arabidopsis thaliana. The Plant Cell Online. 2011;23:1014–1032. doi: 10.1105/tpc.110.080267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eini O, et al. Interaction with a host ubiquitin-conjugating enzyme is required for the pathogenicity of a geminiviral DNA β satellite. Molecular plant-microbe interactions. 2009;22:737–746. doi: 10.1094/MPMI-22-6-0737. [DOI] [PubMed] [Google Scholar]
- 65.Garbutt, C. C., Bangalore, P. V., Kannar, P. & Mukhtar, M. Getting to the edge: protein dynamical networks as a new frontier in plant–microbe interactions. Frontiers in plant science5 (2014). [DOI] [PMC free article] [PubMed]
- 66.Tully JP, et al. Expression-based network biology identifies immune-related functional modules involved in plant defense. BMC genomics. 2014;15:421. doi: 10.1186/1471-2164-15-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC bioinformatics. 2008;9:559. doi: 10.1186/1471-2105-9-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang, B. & Horvath, S. A general framework for weighted gene co-expression network analysis. Statistical applications in genetics and molecular biology4 (2005). [DOI] [PubMed]
- 69.Langfelder P, Horvath S. Eigengene networks for studying the relationships between co-expression modules. BMC systems biology. 2007;1:54. doi: 10.1186/1752-0509-1-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Seebacher, J. & Gavin, A.-C. SnapShot: Protein-protein interaction networks. Cell144, 1000–1000. e1001 (2011). [DOI] [PubMed]
- 71.Wang J, Chen G, Li M, Pan Y. Integration of breast cancer gene signatures based on graph centrality. BMC systems biology. 2011;5:S10. doi: 10.1186/1752-0509-5-S3-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nour-Eldin HH, et al. NRT/PTR transporters are essential for translocation of glucosinolate defence compounds to seeds. Nature. 2012;488:531–534. doi: 10.1038/nature11285. [DOI] [PubMed] [Google Scholar]
- 73.Sade D, et al. Involvement of the hexose transporter gene LeHT1 and of sugars in resistance of tomato to tomato yellow leaf curl virus. Molecular plant. 2013;6:1707–1710. doi: 10.1093/mp/sst036. [DOI] [PubMed] [Google Scholar]
- 74.Delprato ML, Krapp AR, Carrillo N. Green Light to Plant Responses to Pathogens: The Role of Chloroplast Light‐Dependent Signaling in Biotic Stress. Photochemistry and photobiology. 2015;91:1004–1011. doi: 10.1111/php.12466. [DOI] [PubMed] [Google Scholar]
- 75.Liu X, et al. Reactive oxygen species are involved in plant defense against a gall midge. Plant physiology. 2010;152:985–999. doi: 10.1104/pp.109.150656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bolton MD. Primary metabolism and plant defense—fuel for the fire. Molecular plant-microbe Interactions. 2009;22:487–497. doi: 10.1094/MPMI-22-5-0487. [DOI] [PubMed] [Google Scholar]
- 77.Rojas CM, Senthil-Kumar M, Tzin V, Mysore K. Regulation of primary plant metabolism during plant-pathogen interactions and its contribution to plant defense. Frontiers in plant science. 2014;5:17. doi: 10.3389/fpls.2014.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Trouvelot, S. et al. Carbohydrates in plant immunity and plant protection: roles and potential application as foliar sprays. Frontiers in plant science5 (2014). [DOI] [PMC free article] [PubMed]
- 79.Wu J, Baldwin IT. New insights into plant responses to the attack from insect herbivores. Annual review of genetics. 2010;44:1–24. doi: 10.1146/annurev-genet-102209-163500. [DOI] [PubMed] [Google Scholar]
- 80.Howe GA, Jander G. Plant immunity to insect herbivores. Annu. Rev. Plant Biol. 2008;59:41–66. doi: 10.1146/annurev.arplant.59.032607.092825. [DOI] [PubMed] [Google Scholar]
- 81.Zaidi SS, Tashkandi M, Mahfouz MM. Engineering molecular immunity against plant viruses. Progress in Molecular Biology and Translation Science. 2017;149:167–186. doi: 10.1016/bs.pmbts.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 82.Zaidi SS, Mansoor S, Ali Z, Tashkandi M, Mahfouz MM. Engineering plants for geminivirus resistance with CRISPR/Cas9 system. Trends in Plant Science. 2016;21:279–281. doi: 10.1016/j.tplants.2016.01.023. [DOI] [PubMed] [Google Scholar]
- 83.Ali Z, Ali S, Tashkandi M, Zaidi SS, Mahfouz MM. CRISPR/Cas9-mediated immunity to geminiviruses: Differential interference and evasion. Scientific reports. 2016;6:26912. doi: 10.1038/srep26912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhong, S. et al. High-throughput illumina strand-specific RNA sequencing library preparation. Cold spring harbor protocols 2011, pdb. prot5652 (2011). [DOI] [PubMed]
- 85.Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics, btu170 (2014). [DOI] [PMC free article] [PubMed]
- 86.Andrews, S. FastQC: a quality control tool for high throughput sequence data. Available online at: http://www.bioinformatics.babraham.ac.uk/projects/fastqc (2010).
- 87.Li F, et al. Genome sequence of the cultivated cotton Gossypium arboreum. Nature genetics. 2014;46:567–572. doi: 10.1038/ng.2987. [DOI] [PubMed] [Google Scholar]
- 88.Pertea M, Kim D, Pertea GM, Leek JT, Salzberg SL. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nature protocols. 2016;11:1650–1667. doi: 10.1038/nprot.2016.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang X, et al. mRNA-seq analysis of the Gossypium arboreum transcriptome reveals tissue selective signaling in response to water stress during seedling stage. PLoS one. 2013;8:e54762. doi: 10.1371/journal.pone.0054762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hagberg, A., Swart, P. J. & Schult, D. A. Exploring network structure, dynamics, and function using NetworkX. (Los Alamos National Laboratory (LANL), 2008).
- 91.Saito R, et al. A travel guide to Cytoscape plugins. Nature methods. 2012;9:1069–1076. doi: 10.1038/nmeth.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Demchak, B. et al. Cytoscape: the network visualization tool for GenomeSpace workflows. F1000Research3 (2014). [DOI] [PMC free article] [PubMed]
- 93.Kanehisa M, Furumichi M, Tanabe M, Sato Y, Morishima K. KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic acids research. 2017;45:D353–D361. doi: 10.1093/nar/gkw1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Marino G, Funk C. Matrix metalloproteinases in plants: a brief overview. Physiologia plantarum. 2012;145:196–202. doi: 10.1111/j.1399-3054.2011.01544.x. [DOI] [PubMed] [Google Scholar]
- 95.Lacerda A, Vasconcelos ÉAR, Pelegrini PB, Grossi-de-Sa MF. Antifungal defensins and their role in plant defense. Frontiers in microbiology. 2014;5:116. doi: 10.3389/fmicb.2014.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Thomma BP, Cammue BP, Thevissen K. Plant defensins. Planta. 2002;216:193–202. doi: 10.1007/s00425-002-0902-6. [DOI] [PubMed] [Google Scholar]
- 97.Spoel SH, Dong X. How do plants achieve immunity? Defence without specialized immune cells. Nature reviews immunology. 2012;12:89–100. doi: 10.1038/nri3141. [DOI] [PubMed] [Google Scholar]
- 98.Igarashi D, Tsuda K, Katagiri F. The peptide growth factor, phytosulfokine, attenuates pattern‐triggered immunity. The plant journal. 2012;71:194–204. doi: 10.1111/j.1365-313X.2012.04950.x. [DOI] [PubMed] [Google Scholar]
- 99.Loivamäki M, et al. A role for PSK signaling in wounding and microbial interactions in Arabidopsis. Physiologia plantarum. 2010;139:348–357. doi: 10.1111/j.1399-3054.2010.01371.x. [DOI] [PubMed] [Google Scholar]
- 100.Wang J, et al. Allosteric receptor activation by the plant peptide hormone phytosulfokine. Nature. 2015;525:265–268. doi: 10.1038/nature14858. [DOI] [PubMed] [Google Scholar]
- 101.Park C-J, Seo Y-S. Heat shock proteins: a review of the molecular chaperones for plant immunity. The plant pathology journal. 2015;31:323. doi: 10.5423/PPJ.RW.08.2015.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Verchot J. Cellular chaperones and folding enzymes are vital contributors to membrane bound replication and movement complexes during plant RNA virus infection. Frontiers in plant science. 2012;3:275. doi: 10.3389/fpls.2012.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hofius D, et al. Capsid protein-mediated recruitment of host DnaJ-like proteins is required for Potato virus Y infection in tobacco plants. Journal of virology. 2007;81:11870–11880. doi: 10.1128/JVI.01525-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rawat R, et al. REVEILLE1, a Myb-like transcription factor, integrates the circadian clock and auxin pathways. Proceedings of the national academy of sciences. 2009;106:16883–16888. doi: 10.1073/pnas.0813035106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhong Y, et al. Digital gene expression analysis of Ponkan Mandarin (Citrus reticulata Blanco) in response to Asia citrus psyllid-vectored Huanglongbing infection. International journal of molecular sciences. 2016;17:1063. doi: 10.3390/ijms17071063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Maimbo M, Ohnishi K, Hikichi Y, Yoshioka H, Kiba A. Induction of a small heat shock protein and its functional roles in Nicotiana plants in the defense response against Ralstonia solanacearum. Plant physiology. 2007;145:1588–1599. doi: 10.1104/pp.107.105353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stevenson CE, et al. Crystal structure of the MYB domain of the RAD transcription factor from Antirrhinum majus. Proteins: structure, function, and bioinformatics. 2006;65:1041–1045. doi: 10.1002/prot.21136. [DOI] [PubMed] [Google Scholar]
- 108.Van Der Vossen E, et al. An ancient R gene from the wild potato species Solanum bulbocastanum confers broad-spectrum resistance to Phytophthora infestans in cultivated potato and tomato. The plant journal. 2003;36:867–882. doi: 10.1046/j.1365-313X.2003.01934.x. [DOI] [PubMed] [Google Scholar]
- 109.Song J, et al. Gene RB cloned from Solanum bulbocastanum confers broad spectrum resistance to potato late blight. Proceedings of the national academy of sciences. 2003;100:9128–9133. doi: 10.1073/pnas.1533501100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bourdenx B, et al. Overexpression of Arabidopsis ECERIFERUM1 promotes wax very-long-chain alkane biosynthesis and influences plant response to biotic and abiotic stresses. Plant physiology. 2011;156:29–45. doi: 10.1104/pp.111.172320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Meng D, Walsh M, Fricke W. Rapid changes in root hydraulic conductivity and aquaporin expression in rice (Oryza sativa L.) in response to shoot removal-xylem tension as a possible signal. Annals of botany. 2016;118:809–819. doi: 10.1093/aob/mcw150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Matsumoto T, et al. Role of the aquaporin PIP1 subfamily in the chilling tolerance of rice. Plant and cell physiology. 2009;50:216–229. doi: 10.1093/pcp/pcn190. [DOI] [PubMed] [Google Scholar]
- 113.Tian, S. et al. Plant aquaporin AtPIP1; 4 links apoplastic H2O2 induction to disease immunity pathways. Plant physiology, pp. 01237.02015 (2016). [DOI] [PMC free article] [PubMed]
- 114.Nagaraj S, Senthil-Kumar M, Ramu VS, Wang K, Mysore KS. Plant ribosomal proteins, RPL12 and RPL19, play a role in nonhost disease resistance against bacterial pathogens. Frontiers in plant science. 2016;6:1192. doi: 10.3389/fpls.2015.01192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yang C, Zhang C, Dittman JD, Whitham SA. Differential requirement of ribosomal protein S6 by plant RNA viruses with different translation initiation strategies. Virology. 2009;390:163–173. doi: 10.1016/j.virol.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 116.Figueiredo A, Monteiro F, Sebastiana M. Subtilisin-like proteases in plant–pathogen recognition and immune priming: a perspective. Frontiers in plant science. 2014;5:739. doi: 10.3389/fpls.2014.00739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Xia Y. Proteases in pathogenesis and plant defence. Cellular microbiology. 2004;6:905–913. doi: 10.1111/j.1462-5822.2004.00438.x. [DOI] [PubMed] [Google Scholar]
- 118.Fagard M, et al. Nitrogen metabolism meets phytopathology. Journal of experimental botany. 2014;65:5643–5656. doi: 10.1093/jxb/eru323. [DOI] [PubMed] [Google Scholar]
- 119.Yamamoto-Katou A, Katou S, Yoshioka H, Doke N, Kawakita K. Nitrate reductase is responsible for elicitin-induced nitric oxide production in Nicotiana benthamiana. Plant and cell physiology. 2006;47:726–735. doi: 10.1093/pcp/pcj044. [DOI] [PubMed] [Google Scholar]
- 120.Modolo LV, et al. Decreased arginine and nitrite levels in nitrate reductase-deficient Arabidopsis thaliana plants impair nitric oxide synthesis and the hypersensitive response to Pseudomonas syringae. Plant science. 2006;171:34–40. doi: 10.1016/j.plantsci.2006.02.010. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNA-Seq data from this study have been deposited at the NCBI under the BioProject accession No. PRJNA380937 and BioSample Nos. SAMN06628122, SAMN06628123, SAMN06628124, SAMN06628125, SAMN06628126 and SAMN06628127.