Abstract
Nitroxyl anion (NO−) is the one-electron reduction product of nitric oxide (NO⋅) and is enzymatically generated by NO synthase in vitro. The physiologic activity and mechanism of action of NO− in vivo remains unknown. The NO− generator Angeli's salt (AS, Na2N2O3) was administered to conscious chronically instrumented dogs, and pressure–dimension analysis was used to discriminate contractile from peripheral vascular responses. AS rapidly enhanced left ventricular contractility and concomitantly lowered cardiac preload volume and diastolic pressure (venodilation) without a change in arterial resistance. There were no associated changes in arterial or venous plasma cGMP. The inotropic response was similar despite reflex blockade with hexamethonium or volume reexpansion, indicating its independence from baroreflex stimulation. However, reflex activation did play a major role in the selective venodilation observed under basal conditions. These data contrasted with the pure NO donor diethylamine/NO, which induced a negligible inotropic response and a more balanced veno/arterial dilation. AS-induced positive inotropy, but not systemic vasodilatation, was highly redox-sensitive, being virtually inhibited by coinfusion of N-acetyl-l-cysteine. Cardiac inotropic signaling by NO− was mediated by calcitonin gene-related peptide (CGRP), as treatment with the selective CGRP-receptor antagonist CGRP(8–37) prevented this effect but not systemic vasodilation. Thus, NO− is a redox-sensitive positive inotrope with selective venodilator action, whose cardiac effects are mediated by CGRP-receptor stimulation. This fact is evidence linking NO− to redox-sensitive cardiac contractile modulation by nonadrenergic/noncholinergic peptide signaling. Given its cardiac and vascular properties, NO− may prove useful for the treatment of cardiovascular diseases characterized by cardiac depression and elevated venous filling pressures.
Nitric oxide (NO⋅)-related species play a crucial role in diverse physiological processes, including blood pressure regulation, neurotransmission, and cytostatic/cytotoxic signaling (1). Whereas some NO⋅-mediated cardiovascular effects are firmly established, its control over myocardial contractility remains controversial in that positive, negative, or neutral effects can be observed. The net result varies with the tissue preparation, NO⋅ concentration and donor, and myocardial redox state (2, 3). These factors can critically influence the particular NO⋅ species generated and, thereby, the net contractile response.
Among the NO⋅-related species is nitroxyl anion (NO−), the one-electron reduction product of NO⋅ that is formed by NO⋅ synthase in vitro by direct enzyme action or metabolism of the decoupled NO⋅ synthase product NG-hydroxy-l-arginine (4–8). At high concentrations of 0.1–5 mM, NO− seems more cytotoxic than NO⋅ in vitro, causing DNA strand breaks and base oxidation (9, 10). Like NO⋅, NO− induces vasodilation in vivo and in vitro in association with the formation of iron–nitrosyl complexes and the conversion of NO− to NO⋅ (11–13). These effects are highly sensitive to redox state, being effectively eliminated by the addition of reduced thiols (13, 14) or copper-containing enzymes (15). Whether and how NO− affects the intact heart itself remains unknown, but this too might be anticipated to display marked redox sensitivity.
A signaling pathway that might link NO− with inotropic modulation is the stimulation of nonadrenergic/noncholinergic (NANC) neuromodulators such as calcitonin gene-related peptide (CGRP). Recent studies have proposed that certain NO⋅ donors may trigger this pathway as an alternative redox-sensitive mechanism for vasodilation (16). CGRP has positive inotropic effects in isolated myocytes (17) and is thought to act primarily by cAMP/protein kinase A-dependent signaling (18). Accordingly, the present study determined the effects of NO− on cardiac contractile and diastolic function and systemic vascular tone in conscious dogs. Load-independent pressure–dimension analysis was used to delineate cardiac from vascular loading alterations. We determined the involvement of cGMP signaling, redox modulation, and CGRP signaling in NO− cardiovascular responses. The results provide evidence that NO− is a potent, highly redox-sensitive inotropic agent in vivo, acting independently from cGMP and through the release of CGRP from NANC fibers. In contrast to NO⋅, NO− favors venous over arterial vasodilation in the basal state by mechanisms that differ from those altering contractility. These data highlight a signaling pathway that has therapeutic potential in treating depressed hearts operating under high resistive load.
Materials and Methods
Chemicals.
The NO− donor Angeli's salt (AS, sodium trioxodinitrate, Na2N2O3) and NO⋅ donor diethylamine (DEA)/NO (more completely, Na[Et2NN(N⩵O)O]) were synthesized as described (9, 19). Dilutions of both compounds were prepared in 0.9% NaCl just before use from a 100 mM stock solution made up in 10 mM NaOH. Dextran was obtained from Abbott, whereas all other drugs were purchased from Sigma. The CGRP fragment CGRP-(8–37) was dissolved in saline just before use.
Experimental Preparation.
The protocol was performed with adult male mongrel dogs (22–25 kg) and was approved by the Animal Care and Use Committee of the Johns Hopkins University. Details of the surgical preparation have been reported (20). The instrumentation included a micromanometer placed within the distal cavity to record left ventricular (LV) pressure, sonomicrometers to measure anteroposterior LV dimension, an inferior vena caval perivascular occluder to manipulate cardiac load, a central arterial catheter, an ultrasound coronary-flow probe placed around the proximal circumflex artery, and epicardial-pacing electrodes for atrial pacing.
Experimental Protocols.
The effect on basal cardiovascular function of NO− donated by AS (10 μg/kg of body weight per min for 5–20 min) was tested in 14 animals. The dose used was comparable to the low-dose group in a prior in vivo rabbit study of ischemia–reperfusion (21). Studies were performed at a constant heart rate during atrial pacing (130–160 beats per min). To identify the role of baroreflex activation on NO− effects, studies were performed in the presence of either ganglionic blockade (hexamethonium chloride, 10 mg/kg of body weight, i.v., every 15 min) or volume infusion to restore chamber loading to baseline [10% (wt/vol) dextran, 60 ml every 5 min; n = 6]. To test the influence of the redox state, NO− was infused after pretreatment with N-acetyl-l-cysteine (NAC; 6.7 μmol/kg of body weight per min for 30 min; n = 5). NAC was infused by means of a venous port separate from that used for AS; pilot studies revealed that coinfusion led to a sudden dramatic decline in systemic blood pressure, complicating the interpretation of other hemodynamic changes. Last, to test the relation between the inotropic action of NO− and CGRP signaling, CGRP receptors were blocked with the selective antagonist CGRP-(8–37) (400 μg in 30 ml of saline bolus, then 2.6 μg/kg of body weight per min for 15 min).
Hemodynamic Analysis.
Hemodynamic data were sampled at 250 Hz, and steady-state and pressure–dimension parameters were derived as described (20). Because in vivo cardiac contractility assessment requires separation of the effects of chamber loading, we used pressure–volume relation indexes; specifically, we used the end-systolic elastance (Ees) and the slope of dP/dtmax–end-diastolic dimension (DEDV) relations (22). Isovolumic relaxation was derived from pressure decay waveforms by assuming a nonzero decay asymptote.
cGMP Measurements.
Arterial, venous (both n = 6), and coronary sinus (n = 3) plasma were analyzed for cGMP levels with an enzyme immunoassay (Biotrak, Amersham Pharmacia) based on lyophilized samples. The assay has a detection sensitivity of 2 fM.
Nitrate and Nitrite Analysis.
Serum concentrations of nitrite and nitrate were determined by a modified Griess assay (23) with and without prior chemical reduction of nitrate to nitrite with VCl3. Serum stored at −70°C was deproteinized by ultrafiltration (30-kDa cut-off; Centricon, Sartorius) at 4°C and absorbance at 540 nm was read with a plate reader (Perkin–Elmer HTS 7000 BioAssay Reader controlled by Tecan WINSELECT software) after a 37°C incubation with Griess reagents for 30–45 min.
Statistical Analysis.
Data are presented as means ± SEM. Nonparametric analyses (Wilcoxon or Kruskal–Wallis) were used for within- or between-group comparisons.
Results
Nitroxyl Augments Myocardial Contractility While Reducing Vascular Tone.
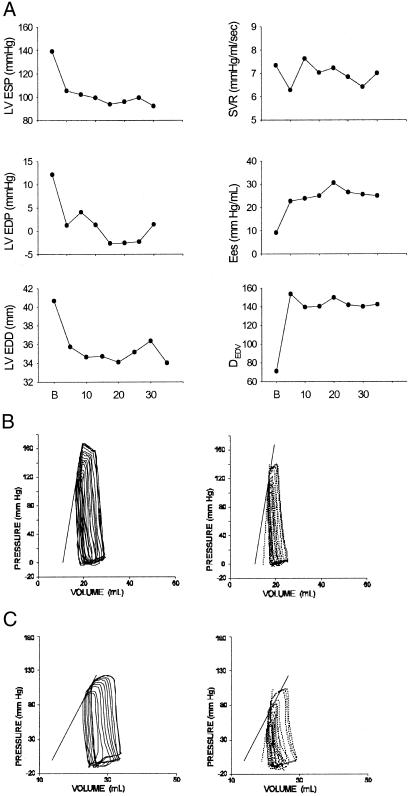
Fig. 1A shows an example of the time course of cardiovascular responses to AS. Systolic pressure, chamber end-diastolic volume and pressure all declined rapidly (within 5 min), and these changes were maintained during 30 min of AS infusion. Arterial resistance remained unchanged. Ventricular contractility indexed by two cardiac-specific indexes rose with a time course similar to that for venous unloading. Fig. 1B displays representative pressure–dimension loops before and after 10 min of NO− infusion (steady state), and Table 1 provides summary data. LV contractility indexed by Ees and DEDV rose by 88.5 ± 27.8% and 99 ± 29.1%, respectively (both P < 0.01), and this change was accompanied by shortening of the systolic period from 42 ± 2.7% to 33 ± 2.1% (P = 0.01) and reduction of the time constant of pressure relaxation (−8.4 ± 1.9%, P < 0.005). LV preload (end-diastolic pressure and volume) also declined, lowering systolic pressure despite enhanced contractility. However, systemic arterial resistance was unchanged.
Figure 1.
(A) Hemodynamic response to AS in a conscious dog. Abscissa is in min. There was a rapid decline in left ventricular systolic pressure (LV ESP) and left ventricular preload (LV EDP, left ventricular end-diastolic pressure; LV EDD, left ventricular end-diastolic dimension) that occurred within 5–10 min of bolus infusion H(B), and persisted for at least 35 min of sustained AS infusion. Systemic vascular resistance (SVR) was unchanged. Ventricular contractility (Ees, slope of end-systolic pressure–dimension relation; DEDV relation slope) also rose rapidly and markedly and remained elevated throughout drug infusion. (B) Representative pressure–dimension loops before (Left) and after (Right) AS infusion. There was an increase in Ees (dotted line at upper left) and decline in chamber volume. (C) Similar results for heart with hexamethonium-induced autonomic blockade (Right).
Table 1.
Hemodynamic effects of AS infusion
| Hemodynamics | Baseline (n = 9) | NO− (n = 9) | NAC (n = 6) | NAC + NO− (n = 6) |
|---|---|---|---|---|
| Heart rate, bpm | 134 ± 4 | 141 ± 5.5 | 150 ± 7 | 155 ± 7 |
| Ees, mmHg/s | 10.46 ± 1.5 | 19.5 ± 3.8* | 10.7 ± 0.6* | 12.1 ± 1.3† |
| DEDV | 100.2 ± 16 | 177.3 ± 19‡ | 125.4 ± 29* | 141.6 ± 26† |
| SVR, mmHg/mm | 7.28 ± 0.3 | 7.33 ± 0.5 | 5.9 ± 0.21 | 5.22 ± 0.25§¶ |
| Tau (dP/dt), ms | 31.1 ± 1.3 | 28.4 ± 1‡ | 29.6 ± 1 | 31.1 ± 2.2¶ |
| LVPes, mmHg | 132.4 ± 6 | 109.5 ± 5‡ | 119.4 ± 5.5 | 108 ± 5.6§ |
| LVEDV, ml | 37.3 ± 2 | 34.8 ± 2‡ | 36.5 ± 1.35 | 33.8 ± 1.2§ |
| LVEDP, mmHg | 10.6 ± 3 | 3.6 ± 2* | 9.8 ± 3.7 | 2.2 ± 2.7§ |
| CorF, ml/min | 52.5 ± 6.1 | 45.4 ± 5.8 | 64.4 ± 7.7 | 66.7 ± 10.3 |
Data are means ± SEM. NO−, nitroxyl anion; NAC, N-acetylcysteine; Ees, slope of the end-systolic pressure–dimension relation; DEDV, preload-normalized maximal dP/dt; SVR, systemic vascular resistance; Tau, time constant of relaxation; LVPes, LV end-systolic pressure; LVEDV, LV end-diastolic volume; LVEDP, LV end-diastolic pressure; CorF, coronary blood flow (circumflex territory).
, P < 0.01 vs. baseline. †, P ≤ 0.01. ‡, P < 0.005 vs. baseline. §, P < 0.05 vs. NAC only. ¶, P = 0.06 for comparison of NO− effect with or without NAC.
The hemodynamic response to AS was reproducible with repeated exposure. A rise in DEDV to 201 ± 33.5 from a baseline of 111 ± 27 mmHg/ml per s on first infusion was followed by a similar rise (218 ± 24) upon second exposure (n = 3). The response was also not specific to venous administration, as similar changes were observed with intraarterial injection (data not shown).
Nitroxyl-Induced Effects Are cGMP-Independent.
The hemodynamic effects of AS at the dose used were not associated with changes in plasma cGMP. For example, cGMP was 22.5 ± 9 pmol/ml before and 20.3 ± 8.8 pmol/ml after NO− infusion in arterial plasma (P = NS). Virtually identical results were obtained in venous plasma. cGMP also did not vary in coronary sinus plasma (19.5 ± 9 vs. 17.3 ± 4.5 pmol/ml). In contrast, infusion of sodium nitroprusside (40 μg/kg of body weight per min for 5 min, titrated to match the systolic-pressure decline with AS) increased cGMP to 42 pmol/ml.
Contractility Change Evoked by Nitroxyl Does Not Depend on Reflex Activation.
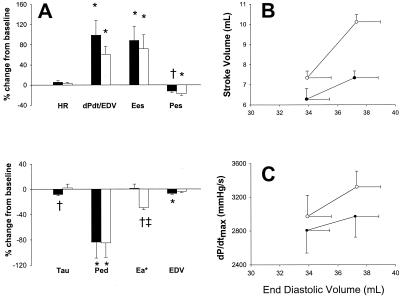
The decline in arterial pressure with AS raised the possibility that the positive inotropic response and lack of systemic arterial vasodilation was related to baroreflex activation. This possibility was tested by determining AS responses in the presence of reflex blockade with hexamethonium or with LV preload restored to minimize reflex activation (Fig. 2A). With either intervention, contractility was enhanced by NO− to levels similar to those in the basal state. However, arterial resistance declined 30% (P < 0.001) in the absence of baroreflex activation, suggesting its role in mediating apparent selective venodilation under basal conditions. Further confirmation of enhanced systolic pump function from NO− is shown in Fig. 2 B and C, which reveal a significant upward shift of two pump-function curves (e.g., Frank–Starling relation). These data were also used to determine cardiac efficiency (external work/myocardial oxygen consumption) at matched preload, and this relationship was not significantly altered by NO−.
Figure 2.
(A) Mean ± SEM for % change in hemodynamics for AS (black bars, n = 9) vs. AS in the presence of preload-volume restoration (white bars, n = 4). HR, heart rate; dPdt/EDV, slope of dP/dtmax-end-diastolic dimension; Pes, end-systolic pressure; Tau, time constant of pressure decay; Ped, end-diastolic pressure; Ea, arterial elastance; EDV, enol-diastolic volume. *, P < 0.05 vs. baseline; †, P < 0.01 vs. baseline; ‡, P < 0.01 vs. nitroxyl alone. (B and C) Cardiac function curves showing stroke volume (B) and maximal rate of pressure rise (C) at two different end diastolic volumes. Control curves (●) were significantly shifted upward after AS infusion (○).
Positive Inotropic Effect of Nitroxyl Is Not Mimicked by Nitric Oxide.
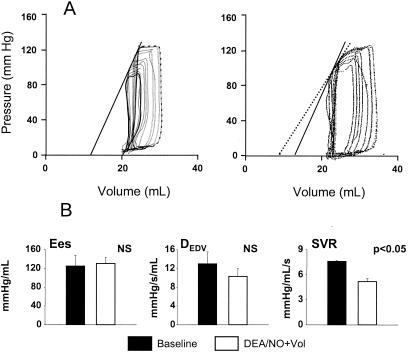
The cardiovascular response to NO− differed from that of an NO⋅ donor (DEA/NO titrated to achieve the same 18% decline in systolic pressure as AS). DEA/NO induced mixed venous and arterial vasodilation. After restoration of preload volume, however, it had no effect on myocardial contractility (unlike NO−; Fig. 3), whereas the arterial dilation was similar to AS under this condition.
Figure 3.
(A) Pressure–dimension loops before (Left) and after (Right) infusion of DEA/NO, a NO donor. Unlike AS, the NO donor did not significantly alter contractility. However, there was a decline in systemic resistance reflected by widening of the loop with less preload change. (B) Summary results (n = 3) show the lack of contractile increase (Ees or DEDV) with DEA/NO, but arterial resistance (SVR) decreased by 25% .
NAC Blocks NO−-Mediated Contractile Augmentation.
Table 1 (two right columns) shows the effects of NAC pretreatment on the cardiovascular response to AS. NAC alone had minimal hemodynamic effects, only slightly increasing cardiac preload and systolic pressure (4–6%). Coinfusion of NAC with AS, however, effectively prevented the contractility change observed with AS alone. In contrast, both the decline in systolic pressure and venodilation were similar to that of combined NAC + AS to AS alone.
Nitrite and Nitrate Plasma Levels During AS Infusion.
The preceding observations suggested that the mechanisms by which AS altered systemic vascular loading differed from the mechanisms related to positive inotropy. One possible explanation was that the former related to the effects of nitrite (converted to nitrate in red blood cells), a decomposition product of AS (in addition to NO−). Direct measurements confirmed a rise in nitrite with AS infusion that was similar in arterial and venous plasma (from 3.2 ± 0.9 μM to 7.4 ± 1.8 μM; P = 0.007). Nitrate levels increased in venous plasma (from 22.4 ± 10 μM to 30.74 ± 13.8 μM; P = 0.02) but not arterial plasma (26.7 ± 11.3 μM vs. 26.2 ± 10.6 μM; NS); potentially, this difference is related to the venous route of administration. However, with coadministration of NAC and AS, nitrite and nitrate levels were virtually unchanged from control in arterial and venous blood.
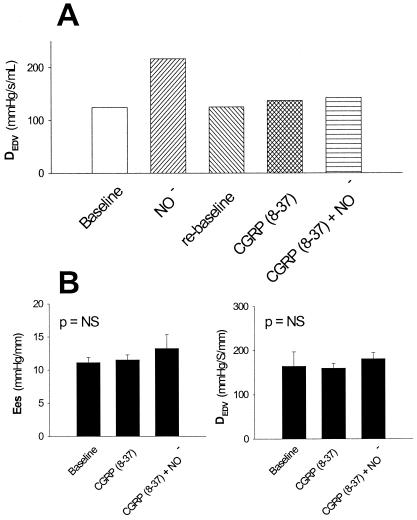
CGRP Receptor Blockade Prevents Nitroxyl-Induced Inotropic Action.
Fig. 4 displays an example and summary data for the contractile response to AS before and after administration of CGRP-(8–37) blocking peptide (n = 5). CGRP-(8–37) alone did not have significant effects on systemic blood pressure, coronary flow, or cardiac contractility. However, NO−-mediated inotropy was effectively prevented by CGRP-(8–37). Both cardiac preload and systemic arterial pressure still declined significantly (−1.8 ± 0.6% and −6.6 ± 1.1%, respectively; P < 0.05 for both) despite the presence of CGRP-(8–37), although the magnitude of both changes was less than those with AS alone (P < 0.05). We also confirmed that CGRP (0.05 μg/kg of body weight per min) itself enhanced contractility in our preparation, and this, too, was fully blocked by CGRP-(8–37) at the same dose used for the NO− studies (data not shown).
Figure 4.
(A) Example of inotropic response to AS (NO−) before and after administration of the CGRP receptor-blocking peptide CGRP-(8–37). The blocking peptide effectively eliminated the inotropic response to AS. (B) Summary data for two measures of LV contractility showing inhibition of AS-induced positive inotropy by CGRP-(8–37) (n = 5). The blocking peptide did not produce significant inotropic effects per se.
Discussion
This study shows that NO− derived from AS has a unique cardiovascular profile that differs strikingly from NO⋅; it increases myocardial contractility, and, in control (reflex intact) conditions, preferentially dilates the venous circulation. These effects are not reflected in altered plasma cGMP levels, but they are strongly redox-dependent and seem to be linked to CGRP release from NANC nerve fibers. The AS-mediated inotropic response occurs without a disproportionate rise in myocardial oxygen consumption, thereby maintaining chamber efficiency, and is not dependent on concomitant changes in vascular or venous load or reflex activation.
The biological effects of NO⋅ and its redox-related species are primarily determined by their individual chemistries. NO⋅ itself is a relatively inert molecule that reacts with transition metals, oxygen, or superoxide to form oxidizing or nitrosating species. In contrast, NO− and its conjugate acid, HNO, are far more reactive than and rapidly interact with amines, thiols, and ferric heme proteins. A direct comparison of the biological effects of these two redox-related species often reveals them to have opposite biochemical and physiological properties. For instance, NO⋅ reacts with O2 to form a nitrosating species, whereas NO− reacts with O2 to form an oxidizing species (24). At a physiological level, NO⋅ is protective in myocardial ischemia, whereas high levels of NO− can aggravate ischemia/reperfusion injury (21). Thus, it is not surprising that these two compounds might differ substantially in their basal effects on the peripheral circulation and the heart, as demonstrated in the present study. Furthermore, such effects are dose dependent. In this regard, the AS dose used in the present study was <10−4-fold lower than that used in vitro (9, 10), in which cytotoxic effects were observed, and was comparable to the lowest dose used in the only prior in vivo study done in an ischemia/reperfusion model (21).
In the conscious dog, the inotropic response to AS was considerably greater than that of a pure NO⋅-donor, DEA/NO. Several studies have shown modest effects with low-dose nitrovasodilators and other NO⋅ donors on myocardial contractility, both in vivo (25–28) and in vitro (29). In particular, we reported that both DEA/NO and SIN-1 (the latter, 3-morpholinosynodiomine, being a coreleaser of NO⋅ and peroxynitrite) have small positive inotropic effects in isolated rat hearts (29), whereas Preckel et al. (28) reported enhanced regional function without loading changes by intracoronary administration of nitrosodilators in anesthetized dogs. Consistent with this report, small but significant reductions in contractility have been observed in dogs administered the nitric oxide synthase inhibitor N-monomethyl l-arginine (30). However, these positive inotropic effects have been uniformly modest changes (+10–15%), whereas the present study revealed far greater effects mediated by NO−. It remains unknown whether analogous inotropic responses would be observed with the infusion of an alternative oxidant such as peroxynitrite in a similar conscious animal preparation. SIN-1 has positive inotropic effects in the anesthetized mouse heart in vivo (29). Although this effect was not assessed independently of reflex activation, rat studies have revealed cardiodepression from peroxynitrite (31). There are no data regarding links between oxidants such as peroxynitrite and CGRP-signaling in vivo. Furthermore, given the known interactions between anesthesia and NO⋅ signaling and the potential differences in oxidative stress between preparations, an appropriate test would be conducted best in a conscious animal like one used in the present study.
The nearly complete inhibition of an AS-mediated positive inotropic effect (but not vasodilation) by NAC suggests that NO− or a related metabolite is the active mediator of the cardiac response. Because at physiological pH, NAC and other thiols do not directly react with AS, nitrite, or NO⋅, these potential mechanistic explanations for an inhibitory effect of NAC can be discounted. Thiols such as NAC do have a higher affinity for HNO than do oxidants such as ROS or peroxynitrite (ONOO−; ref. 24). Quenching of dihydrorhodamine oxidation required less than 10 μM GSH, whereas oxidation by equimolar N2O3 or ONOO− required concentrations of thiol 100× higher. In the present in vivo experiments, the effective plasma concentration of NAC was estimated to be less than 100 μM, arguing in favor of HNO as the most likely effector molecule of the positive inotropic action of AS. The lack of effect of NAC on basal hemodynamics suggests that HNO is not involved in normal cardiovascular regulation.
The striking evidence that CGRP-receptor blockade abolishes nitroxyl-induced inotropy reveals a mechanism for NO⋅-related species modulation of cardiac contractility via a noncGMP dependent pathway. Besides its localization in the central nervous system, CGRP is found in the heart and periadventitial nerve fibers throughout the coronary and peripheral vascular system (32, 33). CGRP has prominent cardiovascular effects including vasodilatation (34) and positive inotropy (32, 35, 36). One role of basal-CGRP signaling in vivo was suggested by Grewal et al. (37), who observed increased arterial pressure in the presence of CGRP-(8–37) in anesthetized rats. This result was not observed in the present study, which may be related to differences in the preparation (i.e., conscious condition) and CGRP-(8–37) dose. Importantly, NO⋅ has been shown to evoke the release of CGRP from NANC nerve terminals in rat heart and isolated aorta (16, 38), which has been hypothesized to occur by means of conversion to NO− (16). Through its receptors, described recently in both atrial and ventricular human trabeculae (17), CGRP activates protein kinase A to increase calcium-mediated calcium release in myocytes (18). The observed shortening of systolic ejection period, improved relaxation rate, and increased dP/dtmx and Ees are all consistent with this signaling pathway.
Whereas the dominant mechanism for selective venodilation with AS was largely caused by concomitant reflex activation, several features deserve comment. First, administration of DEA/NO (which also lowered systemic pressures) similarly did not mimic this result but rather generated more balanced arterial–venous dilation. Similar results were achieved by combining AS with NAC to yield SNAC (a NO⋅ donor). These findings suggest that HNO may have enhanced arterial baroreflex responsiveness. In addition, the data with CGRP-(8–37) suggest that some, but not all, of the veno- and arterial vasorelaxation from AS were related to NANC nerve CGRP release.
It remains unknown whether the NO− signaling observed in the present study can occur endogenously, or indeed, whether NO− is generated in vivo. This deficit is caused largely by the current lack of an assay for detecting NO− in vivo directly or even indirectly, although ongoing efforts may provide methods to obtain such data in the future. Studies (4–8) have shown that NO− can be synthesized by nitric oxide synthase isoforms in vitro, so there is reason to suspect that in vivo synthesis may indeed occur. Regardless of whether endogenous synthesis is ultimately confirmed, the present results suggest that NO− donors may be useful in disease situations such as cardiac failure where combined venodilation and inotropy are desired.
In conclusion, we have shown that NO− is a potent, positive inotropic agent endowed with preferential venous dilatative properties in the intact circulation. The inotropic effect of NO− is redox-sensitive, not related to cGMP-synthesis, and fully antagonized by a CGRP-receptor blocker. The latter finding demonstrates that a NO-related species can modulate cardiac contractility by this pathway. Differences between the pharmacologic profile of NO− and traditional NO⋅ donors (e.g., nitrates) suggest the former may provide an attractive alternative in conditions with increased cardiac loading and depressed function.
Acknowledgments
We thank Richard S. Tunin for his excellent surgical preparations and Koenraad M. Vandegaer for measuring the cGMP plasma levels. This research was supported by National Institute of Health Grants HL-47511 and P50-HL52307 (to D.A.K.) and a doctoral fellowship grant (to C.M.) from the University of Bologna, Italy.
Abbreviations
- AS
Angeli's salt
- CGRP
calcitonin gene-related peptide
- LV
left ventricular
- NAC
N-acetyl-l-cysteine
- NANC
nonadrenergic/noncholinergic
- Ees
end-systolic elastance
- DEDV
end-diastolic dimension
- DEA
diethylamine
- NS
not significant
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Moncada S, Palmer R M J, Higgs E A. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 2.Balligand J L, Feron O, Kelly R A. In: Nitric Oxide. Biology and Pathobiology. Ignarro L J, editor. San Diego: Academic; 2000. pp. 585–632. [Google Scholar]
- 3.Campbell D L, Stamler J S, Strauss H C. J Gen Physiol. 1996;108:277–293. doi: 10.1085/jgp.108.4.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hobbs A J, Fukuto J M, Ignarro L J. Proc Natl Acad Sci USA. 1994;91:10992–10996. doi: 10.1073/pnas.91.23.10992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pufahl R A, Wishnok J S, Marletta M A. Biochemistry. 1995;34:1930–1941. doi: 10.1021/bi00006a014. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt H H H W, Hofmann H, Schindler U, Shutenko Z S, Cunningham D D, Feelisch M. Proc Natl Acad Sci USA. 1996;93:14492–14497. doi: 10.1073/pnas.93.25.14492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rusche K M, Spiering M M, Marletta M A. Biochemistry. 1998;37:15503–15512. doi: 10.1021/bi9813936. [DOI] [PubMed] [Google Scholar]
- 8.Adak S, Wang Q, Struehr D J. J Biol Chem. 2000;275:33554–33561. doi: 10.1074/jbc.M004337200. [DOI] [PubMed] [Google Scholar]
- 9.Wink D A, Feelisch M, Fukuto J, Chistodoulou D, Jourd'heuil D, Grisham M B, Vodovotz Y, Cook J A, Krishna M, DeGraff W G, et al. Arch Biochem Biophys. 1998;351:66–74. doi: 10.1006/abbi.1997.0565. [DOI] [PubMed] [Google Scholar]
- 10.Ohshima H, Gilibert I, Bianchini F. Free Radical Biol Med. 1999;26:1305–1313. doi: 10.1016/s0891-5849(98)00327-x. [DOI] [PubMed] [Google Scholar]
- 11.Vanin A F, Vedernikov Y I, Galagan M E, Kubrina L N, Kuzmanis I A, Kalvin'sh I, Mordvintsev P I. Biokhimiiia (Moskva) 1990;55:1408–1413. [PubMed] [Google Scholar]
- 12.Fukuto J M, Chiang K, Hszieh R, Wong P, Chaudhuri G. J Pharmacol Exp Ther. 1992;263:546–551. [PubMed] [Google Scholar]
- 13.Zamora R, Feelisch M. Biochem Biophys Res Commun. 1994;210:54–62. doi: 10.1006/bbrc.1994.1668. [DOI] [PubMed] [Google Scholar]
- 14.Ellis A, Li C G, Rand M J. Br J Pharmacol. 2000;129:315–322. doi: 10.1038/sj.bjp.0703058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nelli S, Hillen M, Buyukafsar K, Martin W. Br J Pharmacol. 2000;131:356–362. doi: 10.1038/sj.bjp.0703550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Booth B P, Tabrizi-Fard M A, Fung H-L. Biochem Pharmacol. 2000;59:1603–1609. doi: 10.1016/s0006-2952(00)00290-2. [DOI] [PubMed] [Google Scholar]
- 17.Opgaard O S, Hasbak P, de Vries R, Saxena P R, Edvinsson L. Eur J Pharmacol. 2000;397:373–382. doi: 10.1016/s0014-2999(00)00233-8. [DOI] [PubMed] [Google Scholar]
- 18.Huang M-H, Knight P R, III, Izzo J L., Jr Am J Physiol. 1999;276:R259–R264. doi: 10.1152/ajpregu.1999.276.1.R259. [DOI] [PubMed] [Google Scholar]
- 19.Maragos C M, Morley D, Wink D A, Dunams T M, Saavedra J E, Hoffman A, Bove A A, Isaac L, Hrabie J A, Keefer L K. J Med Chem. 1991;34:3242–3247. doi: 10.1021/jm00115a013. [DOI] [PubMed] [Google Scholar]
- 20.Senzaki H, Isoda T, Paolocci N, Ekelund U, Hare J M, Kass D A. Circulation. 2000;101:1040–1048. doi: 10.1161/01.cir.101.9.1040. [DOI] [PubMed] [Google Scholar]
- 21.Ma X L, Gao F, Liu G-L, Lopez B L, Christopher T A, Fukuto J M, Wink D A, Feelisch M. Proc Natl Acad Sci USA. 1999;96:14617–14622. doi: 10.1073/pnas.96.25.14617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Little W C. Circ Res. 1985;57:706–717. doi: 10.1161/01.res.57.5.706. [DOI] [PubMed] [Google Scholar]
- 23.Miranda K M, Espey M G, Wink D A. Nitric Oxide. 2001;5:62–71. doi: 10.1006/niox.2000.0319. [DOI] [PubMed] [Google Scholar]
- 24.Miranda K M, Espey M G, Yamada K, Krishna M, Ludwick N, Kim S, Jourd'heuil D, Grisham M B, Feelisch M, Fukuto J M, Wink D A. J Biol Chem. 2001;276:1720–1727. doi: 10.1074/jbc.M006174200. [DOI] [PubMed] [Google Scholar]
- 25.Raff W K, Drechsel U, Scholtholt J, Lochner W. Pflügers Arch. 1970;317:336–343. doi: 10.1007/BF00586582. [DOI] [PubMed] [Google Scholar]
- 26.Brodie B R, Grossman W, Mann T, McLaurin L P. J Clin Invest. 1977;59:59–68. doi: 10.1172/JCI108622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paulus W J, Vantrimpont P J, Shah A M. Circulation. 1994;89:2070–2078. doi: 10.1161/01.cir.89.5.2070. [DOI] [PubMed] [Google Scholar]
- 28.Preckel B, Kojda G, Schlack W, Ebel D, Kottenberg K, Noack E, Thamer V. Circulation. 1997;96:2675–2682. doi: 10.1161/01.cir.96.8.2675. [DOI] [PubMed] [Google Scholar]
- 29.Paolocci N, Ekelund U E, Isoda T, Ozaki M, Vandegaear K, Georgakopoulos D, Harrison R W, Kass D A, Hare J M. Am J Physiol. 2000;279:H1982–H1988. doi: 10.1152/ajpheart.2000.279.4.H1982. [DOI] [PubMed] [Google Scholar]
- 30.Harrison R W, Thakkar R N, Senzaki H, Ekelund U E, Cho E, Kass D A, Hare J M. Crit Care Med. 2000;28:1263–1268. doi: 10.1097/00003246-200005000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Lopez B L, Liu G-L, Christopher T A, Ma X L. Coron Artery Dis. 1997;8:149–153. doi: 10.1097/00019501-199703000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Bell D, McDermott B J. Pharmacol Rev. 1996;48:253–288. [PubMed] [Google Scholar]
- 33.Chang Y, Stover S R, Hoover D B. J Mol Cell Cardiol. 2001;33:745–754. doi: 10.1006/jmcc.2000.1342. [DOI] [PubMed] [Google Scholar]
- 34.Brain S D, Williams T J, Tippins J R, Morris H R, MacIntyre I. Nature (London) 1985;313:54–56. doi: 10.1038/313054a0. [DOI] [PubMed] [Google Scholar]
- 35.Franco-Cereceda A, Gennari C, Nami R, Agnusdei D, Pernow J, Lund J M, Fischer J A. Circ Res. 1987;60:393–397. doi: 10.1161/01.res.60.3.393. [DOI] [PubMed] [Google Scholar]
- 36.Wimalawansa S J. Endocr Rev. 1996;17:533–585. doi: 10.1210/edrv-17-5-533. [DOI] [PubMed] [Google Scholar]
- 37.Grewal M, Cuevas J, Chaudhuri G, Nathan L. Am J Physiol. 1999;276:H2063–H2068. doi: 10.1152/ajpheart.1999.276.6.H2063. [DOI] [PubMed] [Google Scholar]
- 38.Hu C P, Li Y J, Deng H W. Eur J Pharmacol. 1999;369:189–194. doi: 10.1016/s0014-2999(99)00050-3. [DOI] [PubMed] [Google Scholar]