Abstract
The genotoxic effects of Vitamin C (ascorbic acid) on human lymphocytes in vitro were estimated by analyzing and identifying various chromosome abnormalities, in relation to the concentration of Vitamin C. Testing concentrations of Vitamin C induced different aberrations including the impairment of spindle function. The spindle disturbances can result in mitotic arrest, multipolar spindles and multipolar segregation, errors in chromosome segregation, formation of chromosome bridges and chromosome laggards. The most frequent irregularities were found in anaphase and telophase. A certain number of lymphocytes were arrested at anaphase or telophase (in colchicine-untreated cultures of human lymphocytes). Testing concentrations of ascorbic acid did not induce a significant increase in the number of aneuploid mitoses and were not clastogenic except at the highest concentration (1,000 μg/ml) in colchicine-treated cultures, and in colchicine-untreated cultures of human lymphocytes the pulverization of chromosome was observed. Vitamin C changed the mitotic index value of lymphocytes notably at the higher concentrations (250, 500 and 1,000 μg/ml).
Keywords: Vitamin C (ascorbic acid), human lymphocytes, chromosome abnormalities
INTRODUCTION
The great number of previous investigations indicate that Vitamin C is an antioxidant and a strong free-radical scavenger (1, 2, 3). This vitamin can protect cellular macromolecules, including DNA, from oxidative damage induced by different agents. Besides the antimutagenic and anticlastogenic properties of Vitamin C, it is also known that this vitamin has mutagenic and clastogenic effects. Shamberger (1) summarized the genotoxic effects of Vitamin C in different test systems. Vitamin C possesses mutagenic activity inducing DNA strand breakage and chromosome aberrations, increasing the frequency of sister-chromatid exchanges (SCEs) in cells and increasing the number of somatic mutations. However, Vitamin C is not an active mutagen itself. Oxy radicals, appearing during the oxidation of the vitamin, are implicated in toxic actions of Vitamin C in vitro. Nowhere in the experiments in vivo did Vitamin C display genotoxicity. All of the mutagenic effects of this vitamin were demonstrated in tests in vitro. This may be due to the presence of an antioxidant system which inactivates oxy radicals before their interaction with DNA, and due to the presence of a spatial separation of the components of oxy-radical generating system in living organisms.
In some cases Vitamin C acts as a comutagen by enhancing the gene mutations, SCEs and chromosome aberrations induced by different chemicals. It enhanced the mutagenicity at high concentrations, whereas at lower concentrations it decreased the frequency of induced aberrations by different chemicals. Vitamin C reduced the clastogenic effects of many agents, decreased the number of SCEs, inhibited the gene mutations and decreased the frequency of micronuclei formation. At lower concentrations, it is probable that the process of O2- elimination by Vitamin C was predominant, but at higher concentrations Vitamin C displayed its own genotoxic properties. As an antioxidant, Vitamin C may also have a potential anticarcinogenic activity, but its possible role and mechanism of action in cancer prevention has not been clearly established. In this study, the genotoxicity of different concentrations of Vitamin C on human peripheral blood lymphocytes in cultures have been estimated by the analysis of chromosome aberrations in standard cytogenetics test in colchicinetreated cultures and by the analysis of genotoxic effects of Vitamin C at all phases of the mitotic process in colchicine-untreated cultures of human lymphocytes.
MATERIALS AND METHODS
Vitamin C (Pliva, Zagreb) or ascorbic acid was used to investigate effects of this vitamin on the genetic material of cultured human lymphocytes. Medium RPMI-1640, Fetal calf serum (FCS), Phytohemagglutinin (PHA) were purchased from Sigma Chemical Co., St. Louis, MO., Colchicine (COL) from Kemika, Zagreb. The test of chromosome aberrations was modified according to Moorhead et al. (4). The study was performed on the peripheral blood lymphocytes obtained from six healthy donors, three females and three males aged 22 to 61 years. The heparinised human blood was cultured in medium RPML1640 and supplemented with 20% fetal calf serum. The lymphocytes were stimulated to divide by adding phytohemagglutinin (10 μg/ml). All cultures were incubated at 37°C for a total 72 hours in the dark. Vitamin C was added to lymphocyte cultures after 48 hours of incubation at following final concentrations: 1, 10, 20, 100, 250, 500 and 1000 μg/ml culture medium and the cultures incubated for a further 24 hours. Vitamin C was dissolved in sterile water just before each experiment. These concentrations of Vitamin C were chosen on the basis of published data (5, 6) and preliminary tests. Two cultures were prepared simultaneously for each concentration of Vitamin C: one culture was without colchicines, enabling the analysis of genotoxic effects of Vitamin C at all phases of the mitotic process, and in the other culture cells were arrested in metaphase by the addition of colchicine at a final concentration of 0,4 μg/ml culture medium two hours before harvesting for cytogenetic analysis of metaphases karyotype. The control, untreated cultures of lymphocytes, were established as well. After hypotonic treatment with 0,075 mol/dm3 KCl for 20 min at 37 °C, cells were gently fixed four times with freshly prepared fixative (3 parts methanol: 1 part glacial acetic acid) at room temperature. The cells were harvested by centrifuging. A small amount of cell suspension was drawn into a Pasteur pipette and, as normally done, three drops were expelled carefully on the slides and air-dried. Five to six slides were made from each culture. The chromosome preparations were stained for 10 min in 10% Giemsa. Data for calculating the value of the mitotic index were obtained by analyzing the number of mitotic cells per 1000 cells of a culture, i.e. per 6000 cells for each concentration of Vitamin C and control. In order to obtain the frequencies of chromosomal aberrations, 100 metaphases from each colchicine-treated culture of one subject were examined for a total of 600 cells for each concentration of Vitamin C and control. But, for Vitamin C concentrations of 250, 500 and 1000 μg/ml were analyzed 1000 cells and 100 metaphases for each concentration of Vitamin C and control because of a small number of cells in mitosis for analysis. Numerical and structural chromosome aberrations were analyzed separately. In colchicine-untreated cultures of human lymphocytes, the genotoxic activity of Vitamin C was studied scoring 100 dividing cells for each concentration of Vitamin C and control for the frequency of anaphase/telophase cells and the frequency of mitotic cells with different aberrations. The significance of the value of the mitotic index, the number of metaphases with numerical and structural chromosome aberrations and the number of cells at anaphase/telophase and the number of cells with different aberrations induced by Vitamin C in comparison with the control cultures were analyzed using the chi-square test (x2-test).
RESULTS
Analysis of chromosome aberrations in standard cytogenetics test in colchicine-treated cultures showed that, in comparison with the control cultures, treatments with Vitamin C at the test concentrations of 1, 10, 20 and 100 μg/ml culture medium, did not change the mitotic index value of lymphocytes notably (Table 1). The mitotic index value of human lymphocytes treated with Vitamin C concentrations of 250, 500 and 1000 μg/ml culture medium was significantly reduced (statistically significant at 250 μg/ml: p < 0,05; at 500 μg/ml: p < 0,01 and at 1000 μg/ml: p < 0,001) (Table 2). The number of abnormal metaphases with numerical and structural chromosome aberrations (chromosome and chromatid breaks, chromosome rearrangements and acentric fragments) was not statistically significant in treated cultures when compared with that of the control except in culture treated with 1000 μg/ml Vitamin C (Table 1. and Table 2). The highest testing concentration of Vitamin C (1000 μg/ml) in the presence of colchicine had clastogenic effect (p < 0,001). This vitamin did not induce aneuploidy and polyploidy remarkably excluding at the highest concentration of Vitamin C (1,000 μg/ml: p < 0,05). Endoreduplicated chromosomes (chromosomes which replicate but cannot divide correctly) have been noted in lymphocyte cultures after treatments with Vitamin C. Analysis of genotoxic effects of Vitamin C at all phases of the mitotic process in colchicine-untreated lymphocyte cultures of human lymphocytes showed that effects of this vitamin already became evident after treatment with the concentration of 1 μg/ml, and reached a maximum at the concentration of 10 μg/ml (Table 3). In lymphocyte cultures treated in vitro with Vitamin C, but not treated with colchicine, a certain number of mitoses were arrested at anaphase or telophase when compared with the control culture (Table 3). The photomicrographs of human lymphocytes arrested at anaphase-telophase are shown in Figure 1. Also evident is the presence of other phases: prophases and metaphases in these cultures (Figure 1a). An increase in the concentration of Vitamin C did not show concentration-dependent increase in the number of aberrant changes. The analysis of human lymphocyte cultures treated with different concentrations of Vitamin C (colchicine-untreated cultures) showed an increase in the number of aberrant phases of mitosis when compared with the lymphocytes of untreated cultures (Table 3). Such an increase was particularly remarkable in lymphocytes treated with 10 μg/ml Vitamin C (p < 0,001). The predominant aberration was irregular kinetics of chromosomes during anaphase and telophase as is shown in Table III (statistically significant at 10 μg/ml: p < 0,001 and at 20 μg/ml: p < 0,05 to the total number of aberrant phases) and Figure 1. The frequency of aberrant anaphases and telophases at all concentrations exceed over 50% of the total number of aberrant cells. The aberrant anaphases contained lagging chromatin, including both whole chromatids and chromosomes lagging behind between the poles (Figure 1c), multipolar anaphases (tripolar and tetrapolar) with disrupted poles and unequal distribution of chromosomes (Figure 1d) and chromosome bridges stretching between the poles (Figure 1e). Some chromosomes or a group of chromosomes may be apart in relation to the main groups of chromosomes (Figure 1c). Similar aberrations were found at telophase cells (Figure 1f). Also, the frequent types of mitosis-disruptive changes were chromosome adhesiveness and stickiness (Figure 1e). The results of the cytogenetic analysis of metaphase cells showed that the aberrant metaphases involved dislocation of whole chromosomes or groups of chromosomes from metaphase plate. A pulverization of chromosomes (the total disintegration of chromosomes) has been reported after treatments with Vitamin C in colchicine-untreated lymphocyte cultures.
TABLE 1.
The mitotic index of human lymphocytes, the number of abnormal metaphases with numerical and structural chromosome aberration: in colchicine-treated cultures of human lymphocytes induced by Vitamin C, in comparison with the control cultures (X±SD: the means ± standar deviation of mitotic index or abnormal metaphases). For each concentration and control 6,000 cells and 600 metaphases were scored.
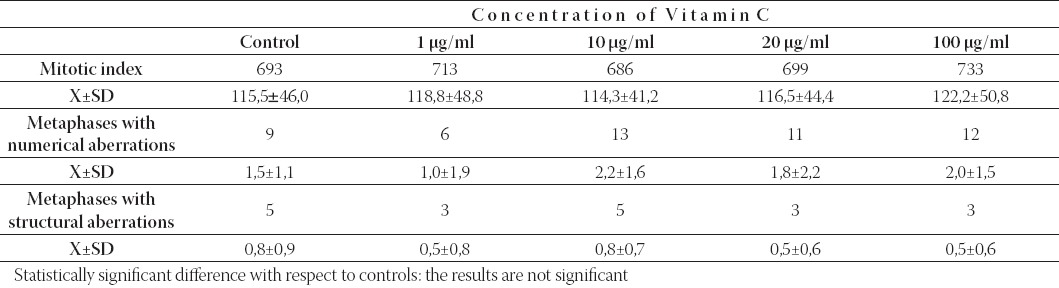
TABLE 2.
The mitotic index of human lymphocytes, the number of abnormal metaphases with numerical and structural chromosome aberrations in colchicine-treated cultures of human lymphocytes treated in vitro with 250, 500 and 1,000 |ig/ml Vitamin C. For each concentration and control 1,000 cells and 100 metaphases were scored.
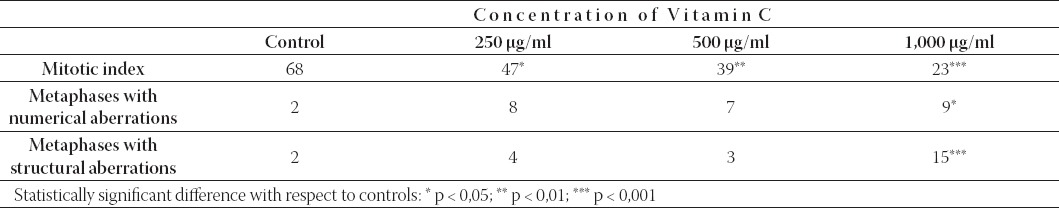
TABLE 3.
The number of anaphases/telophases, the total number of aberrant phases with and the number and percentage of aberrant anaphases and telophases to the total number of aberrant phases of mitosis in colchicine-untreated cultures of human lymphocytes induced by Vitamin C. A hundred mitotic cells were analyzed for each concentration and control.
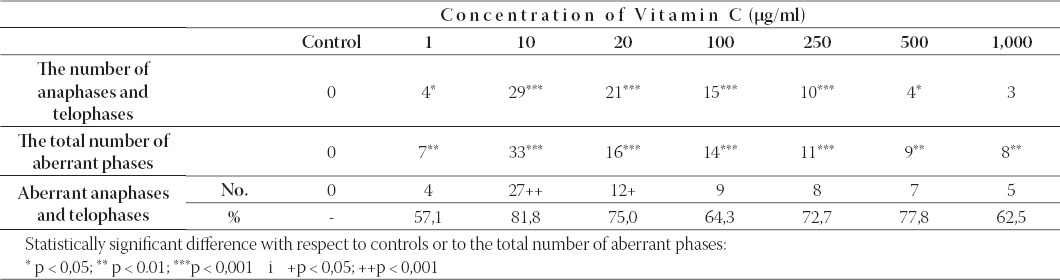
FIGURE 1.
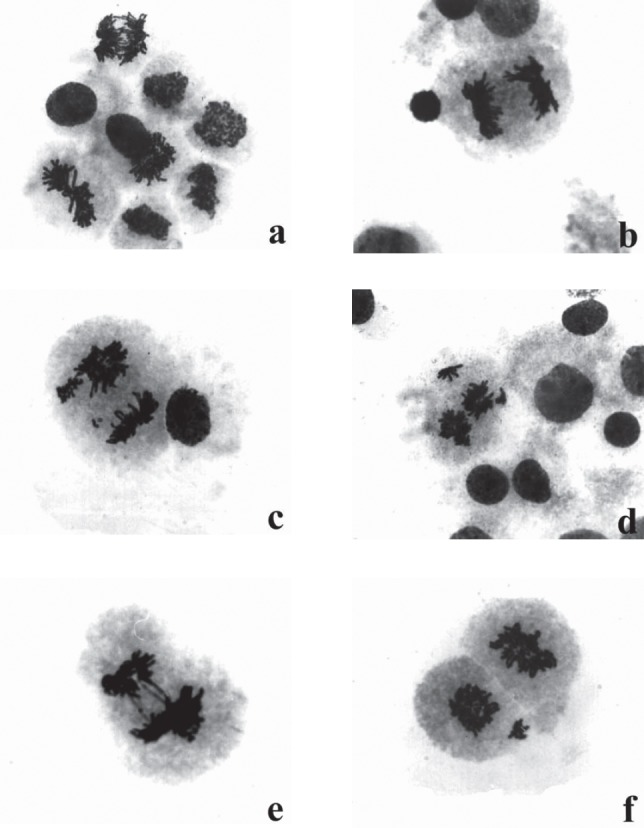
Photomicrographs of human lymphocytes treated in vitro with different concentrations of Vitamin C - Giemsa staining: (a) different phases of mitosis in lymphocyte cultures; (b) a normal anaphase; (c) anaphase with a lagging chromosomes and one pole disrupted; (d) quadripolar anaphase with unequal distribution of chromosomes at poles; (e) disrupted anaphase with chromosome bridges and unequal distribution of chromosomes at poles; (f) lagging chromosomes in telophase (treatment: Vitamin C1, 10 and 20 ng/ml, 24 hours).
DISCUSSION
The results obtained from the chromosome aberrations analysis indicate that the genotoxicity of Vitamin C in human lymphocyte cultures varies depending on concentration. Vitamin C had the most heterogeneous genotoxic effect at the concentration of 10 μg/ml and most efficient genotoxic effects at the concentrations of 250, 500 and 1000 μg/ml. The testing concentrations of Vitamin C induced various aberrations including the impairment of spindle function. The spindle disturbances can result in mitotic arrest, multipolar spindles, errors in chromosome segregation, chromosome bridges and chromosome laggards. The most frequent irregularities were found at anaphase and telophase. The testing concentrations of ascorbic acid were not clastogenic except at the highest concentration (1000 μg/ml) in colchicine-treated cultures, and pulverization of chromosome was observed in colchicine-untreated cultures indicating that certain higher concentrations of ascorbic acid have clastogenic effects. The results obtained by Antunes and Takahashi (5) showing that ascorbic acid at concentration of 1000 μg/ml produced clastogenic effects in human lymphocyte cultures also confirmed this possibility. The treatments with Vitamin C (1, 10, 20 and 100 μg/ ml) did not significantly change the mitotic index value of lymphocytes in the cytogenetics test of chromosome aberrations. This indicates that Vitamin C did not inhibit cell proliferation at these testing concentrations. The results of many in vitro studies have indicated that Vitamin C can be mutagenic (clastogenic) and cytotoxic. Vitamin C at high concentrations possesses genotoxic properties towards Chinese hamster ovary (CHO) cells: induced sister chromatid exchanges (7) and chromosome aberrations (5). Exposure of separated lymphocytes in vitro to Vitamin C, at doses greater than 200 μσ^/ dm3induced strand breakage (8). Vitamin C produced a dose-related increase in DNA damage (9). Ascorbate has caused increase in SCEs in human lymphocytes (10). Contrary to in vitro studies, vitamin C did not induce SCEs in the bone marrow cells of Chinese hamsters (7), was not cytotoxic and did not inhibit proliferation of lymphocytes in cultures (11). Vitamin C, in vitro, successfully reduced the clastogenic effect of many antitumour agents such as cyclophosphamide (12), doxorubicin (5, 13, 14), cisplatin (6) and bleomycin (9, 11). It also has in vivo anticlastogenic effect against chromosomal damage and the number of abnormal metaphases induced by antitumour agents such as cisplatin in rodents (14, 15, 16, 17) and induced by cyclophosphamide (12). Ascorbic acid also reduces the chromosome aberrations induced by ethyl methanesulfonate (18). It decreases the number of SCEs induced by cyclophosphamide (19), mitomycin C (19) and oxygen radicals (9) and inhibits the gene mutations induced by ethyl methane sulfonate (18, 20) and cisplatin (15). Vitamin C decreases the frequency of micronucleus formation induced by cisplatin (16), bleomycin (11) and doxorubicin (21). Sometimes, Vitamin C acted as a comutagen and increased H2O2- and bleomycin-induced chromosomal aberrations (22). In combination with H2O2 there were small protective effects at low doses and exacerbating effects at high doses (9) towards DNA damage in human lymphocytes. According to Park (23) and Osmak et al. (24) Vitamin C inhibited tumour cell growth. It may also have potential anticarcinogenic and chemopreventive activity although the results of these studies are often conflicting (22, 25).
CONCLUSION
Testing concentrations of Vitamin C induced different aberrations including the impairment of spindle function. The spindle disturbances can result in mitotic arrest, multipolar spindles and multipolar segregation, errors in chromosome segregation, formation of chromosome bridges and chromosome laggards. The most frequent irregularities were found in anaphase and telophase. The ascorbic acid was not clastogenic except at the highest concentration (1000 μg/ml) in colchicine-treated cultures, and pulverization of chromosome was observed in colchicine-untreated cultures indicating that certain higher concentrations of ascorbic acid have clastogenic effects.
A certain number of lymphocytes were arrested at anaphase or telophase (in colchicine-untreated cultures of human lymphocytes) indicating that ascorbic acid may also have inhibited tumour cell growth and have potential anticarcinogenic activity.
REFERENCES
- 1.Shamberger R.J. Genetic toxicology of ascorbic acid. Mutat. Res. 1984;133:135–159. doi: 10.1016/0165-1110(84)90005-8. [DOI] [PubMed] [Google Scholar]
- 2.Anderson D. Antioxidant defenses against reactive oxygen species causing genetic and other damage. Mutat. Res. 1996;350:103–108. doi: 10.1016/0027-5107(95)00096-8. [DOI] [PubMed] [Google Scholar]
- 3.Odin A.P. Vitamins as antimutagens: Advantages and some possible mechanisms of antimutagenic action. Mutat. Res. 1997;386:39–67. doi: 10.1016/s1383-5742(96)00044-0. [DOI] [PubMed] [Google Scholar]
- 4.Moorhead P.S, Nowell P.C, Mellman W.J, Bat1pps D.M, Hungerford D.A. Chromosome preparations of leucocytes cultured from human peripheral blood. Exp. Cell Res. 1960;20:613–616. doi: 10.1016/0014-4827(60)90138-5. [DOI] [PubMed] [Google Scholar]
- 5.Antunes L.M.G, Takahashi C.S. Protection and Induction of Chromosomal Damage by Vitamin C in Human Lymphocyte Cultures. Teratog, Carcinog, Mutagen. 1999;19:53–59. [PubMed] [Google Scholar]
- 6.Nefic H. Anticlastogenic effect of vitamin C on cisplatin induced chromosome aberrations in human lymphocyte cultures. Mutat. Res. 2001;498:89–98. doi: 10.1016/s1383-5718(01)00269-8. [DOI] [PubMed] [Google Scholar]
- 7.Speit G, Wolf M, Vogel W. The SCE-inducing capacity of vitamin C: investigations in vitro and in vivo. Mutat. Res. 1980;78:273–278. doi: 10.1016/0165-1218(80)90109-3. [DOI] [PubMed] [Google Scholar]
- 8.Green M.H.L, Lowe J.E, Waugh A.P.W, Aldridge K.E, Cole J, Arlett C.F. Effect of diet and vitamin C on DNA strand breakage in freshly-isolated human white blood cells. Mutat. Res. 1994;316:91–102. doi: 10.1016/0921-8734(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 9.Anderson D, Yu T.-W, Phillips B.J, Schmezer P. The effect of various antioxidants and other modifying agents on oxygen-radical-generated DNA damage in human lymphocytes in the COMET assay. Mutat. Res. 1994;307:261–271. doi: 10.1016/0027-5107(94)90300-x. [DOI] [PubMed] [Google Scholar]
- 10.Galloway S.M, Painter R.B. Vitamin C is positive in the DNA synthesis inhibition and sister-chromatid exchange tests. Mutat. Res. 1979;60:321–327. doi: 10.1016/0027-5107(79)90022-8. [DOI] [PubMed] [Google Scholar]
- 11.Anderson D, Basaran N, Blowers S.D, Edwards A.J. The effect of antioxidants on bleomycin treatment in in vitro and in vivo genotoxicity assays. Mutat. Res. 1995;329:37–47. doi: 10.1016/0027-5107(95)00017-d. [DOI] [PubMed] [Google Scholar]
- 12.Ghaskadbi S, Rajmachikar S, Agate C, Kapadi A.H, Vaidya V.G. Modulation of cyclophosphamide mutagenicity by vitamin C in the in vivo rodent micronucleus assay. Teratog, Carcinog, Mutagen. 1992;12:11–17. doi: 10.1002/tcm.1770120103. [DOI] [PubMed] [Google Scholar]
- 13.Antunes L.M.G, Takahashi C.S. Effects of high doses of vitamins C and E against doxorubicin-induced chromosomal damage in Wistar rat bone marrow cells. Mutat. Res. 1998;419:137–143. doi: 10.1016/s1383-5718(98)00134-x. [DOI] [PubMed] [Google Scholar]
- 14.Antunes L.M.G, Darin J.D.C, Bianchi M.L.P. Anticlastogenic effect of vitamin C on cisplatin in vivo. Genet. Mol. Biol. 1999;22:415–418. [Google Scholar]
- 15.Gentile J.M, Rahimi S, Zwiesler J, Gentile G.J, Ferguson L.R. Effect of selected antimutagens on the genotoxicity of antitumor agents. Mutat. Res. 1998;402:289–298. doi: 10.1016/s0027-5107(97)00308-4. [DOI] [PubMed] [Google Scholar]
- 16.Giri A, Khynriam D, Prasad S.B. Vitamin C medited protection on cisplatin-induced mutagenicity in mice. Mutat. Res. 1998;421:139–148. doi: 10.1016/s0027-5107(98)00158-4. [DOI] [PubMed] [Google Scholar]
- 17.Antunes L.M.G, Araújo M.C.P, Darin J.D.C, Bianchi M.L.P. Effects of the antioxidants curcumin and vitamin C on cisplatin-induced clastogenesis in Wistar rat bone marrow cells. Mutat. Res. 2000;465:131–137. doi: 10.1016/s1383-5718(99)00220-x. [DOI] [PubMed] [Google Scholar]
- 18.Kojima H, Konishi H, Kuroda Y. Effects of L-ascorbic acid on the mutagenicity of ethyl methanesulfonate in cultured mammalian cells. Mutat. Res. 1992;266:85–91. doi: 10.1016/0027-5107(92)90175-2. [DOI] [PubMed] [Google Scholar]
- 19.Krishna G, Nath J, Ong T. Inhibition of cyclophosphamide and mitomycin C-induced sister chromatid exchanges in mice by vitamin C. Cancer Res. 1986;46:2670–2674. [PubMed] [Google Scholar]
- 20.Kuroda Y. Antimutagenic activity of vitamins in cultured mammalian cells. In: Kuroda Y, Shankel D.M, Waters M.D, editors. Antimutagenesis and Anticarcinogenesis Mechanisms II. New York: Plenum Press; 1990. [Google Scholar]
- 21.Amara-Mokrane Y.A, Lebucher-Michel M.P, Balansard G, Duménil G, Botta B. Protective effects of α-hederin, chlorophillin and ascorbic acid towards the induction of micronuclei by doxorubicin in cultured human lymphocytes. Mutagenesis. 1996;11:161–167. doi: 10.1093/mutage/11.2.161. [DOI] [PubMed] [Google Scholar]
- 22.Cozzi R, Ricordy R, Aglitti T, Gatta V, Perticone P, De Salvia R. Ascorbic acid and β-carotene as modulators of oxidative damage. Carcinogenesis. 1997;18:223–228. doi: 10.1093/carcin/18.1.223. [DOI] [PubMed] [Google Scholar]
- 23.Park C.H. Biological nature of the effect of ascorbic acids on the growth of human leukemic cells. Cancer Res. 1985;45:3969–3973. [PubMed] [Google Scholar]
- 24.Osmak M, Kovacek I, Ljubenkov I, Spaventi R, Eckert-Maksic M. Ascorbic acid and 6-deoxy-6-chloro-ascorbic acid: potential anticancer drugs. Neoplasma. 1997;44:101–107. [PubMed] [Google Scholar]
- 25.Block G. Vitamin C status and cancer: epidemiologic evidence of reduced risk. Ann NY Acad Sci. 1992;669:280–290. doi: 10.1111/j.1749-6632.1992.tb17107.x. [DOI] [PubMed] [Google Scholar]


