Abstract
It has been recognized that some people have a genetic variant which leads to elevated levels of homocysteine and impairs ability to process folate. This condition was recognized as independent risk factor of coronary heart disease. Recently, connection between this termolabile mutation of the methylenetetrahydrofolate reductase and numerous conditions and diseases has been established. Aim of this review is to draw attention to this interesting area in medicine. Additionally, well defined study about presence and frequency of gene polymorphism in our region will provide proper diagnosis and achieve possible delay of development of diseases with vitamin supplementation.
Keywords: MTHFR, Homocysteine, polymorphism, diseases
INTRODUCTION
Methylenetetrahydrofolate reductase (MTHFR) is an enzyme that exists in the cytoplasm of cells. MTHFR contains a bound flavin cofactor and uses NAD(P)H as the reducing agent. Methylenetetrahydrofolate reductase irreversibly reduces 5,10-methylenetetrahydrofolate to 5-methyltet- rahydrofolate (5-MTHF). 5-Methyltetrahydrofolate is used as methyl donor to convert homocysteine to methionine by the enzyme methionine synthase. This process of remethylation has a cobolamin as the cofactor. MTHFR gene is located on chromosome 1 (1p36.3). Two common polymorphisms C677T (change from a C to a T) and A1298C have been identified. These polymorphisms are responsible for the synthesis of a thermolabile form of MTHFR enzyme with decreased enzymatic activity. The 677CT mutation in the MTHFR gene causes an alanine to valine amino acid supstitution. This condition is an important cause of higher level of homocysteine and lower folate level. Low dietary intake of the folic acid can also cause mild hyperhomocysteinemia so poor vitamin status can rise level of total homocysteine. This polymorphism is autosomal recessive and hyperhomocysteinemia is associated with neural tube defects in offspring, arterial and venous thrombosis and cardiovascular disease. Also, we can find a wide range of clinical symptoms, such as developmental delay, severe mental retardation, psychiatric disturbances, and later-onset neurodegenerative disorders. It is interesting to note that 677TT individuals are at a decreased risk for certain leukemias and colon cancer, but only when their dietary intake of folate is high. The second polymorphism at nucleotide position 1298 is not as well characterized. Homocysteine, a sulfur containing amino acid, normally occurs in the plasma at a concentration of about 8-12 μM. The nature of plasma homocysteine is really complex. Only 1-2% is found as free (reduced) homocysteine. Approximately 20% occurs as free, oxidized forms, generally mixed disulfide, mostly as homocysteine-cystein, but also as homocystine. Approximately 80%, is proteinbound; the great bulk of this is linked by a disulfide linkage to cysteine of albumin (1). But, recent data suggest that approximately 10%-30% of protein bound Hcy and cystein are disulfide-linked to globulins (Figure 1.) (2,3).
FIGURE 1.
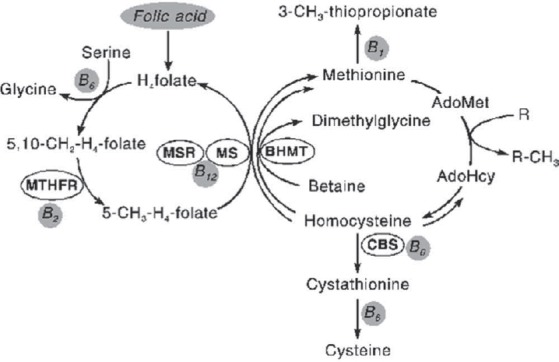
Diagram of Hey metabolism.
Methylenetetrahydrofolate reductase (MTHFR) is a key enzyme in the folate cycle. In the cell, 5-methyltetrahydrofolate (5-CH3-H4-foIate) serves as a methyl donor and as a source of tetrahydrofolate (Htfolate), synthesized by methionine synthase reductase (MSR) and methionine synthase (MS). One of the reactions requiring 5,10-methyIenetetrahydrofolate and 5-methyltetrahydrofolate is the synthesis of methionine from homocysteine, a remethylation pathway of the homocysteine metabolism. The second remethylation pathway involves betaine-homocysteine methyltransferase (BHMT) to form dimethylglycine from betaine. Figure taken from www.multi-media.com/home hemo/hemoint01/sunderl6/ (3).
GENE POLYMORPHISM, HOMOCYSTEINE AND RISK OF DISEASES
Homocysteine has been implicated as a risk factor for a number of important diseases. Hyperhomocysteinemia is a major independent and graded risk factor for atherothrombotic diseases. It has been estimated that up to 10% of all coronary artery disease deaths, i.e. more than 50 000 a year in the U.S.A, can be attributed to mild hyperhomocysteinemia. Homocysteine elevations of only 5 μσ^ can increase the risk of coronary artery disease by 60% in men and by 80% in women (4). This increment was also associated with a 50% and 6.8-fold increase in the risk of cerebrovascular and peripheral arterial diseases. It is registered that extremely high tHcy lead to serious complication and often early death. The most common genetic cause of elevated homocysteine in the general population is the C677T mutation of the methylenetetrahydrofolate reductase (MTHFR) gene (5). There is substantial variability in the prevalence of this mutation among different ethnic groups with a prevalence of 0-2% in Africans, 12% in Whites and 20% in Asians. The 677TT genotype was particularly common in Australia (11,5%), northern China (20%), southern Italy (26%), and Mexico (32%) (the incidence is higher in Mediterranean countries and lower in African-Americans). This regional difference can be attributable to other genetic or environmental factors (dietary habits; low intake of folate, coffee consumption) (6). TT homozygotes have homocysteine levels 25% higher (about 2,5 μmol/l) than those with the wild type CC genotype resulting in reduced enzyme activity and a need for increased dietary folate to maintain adequate enzyme function (7).
NEURODEGENERATIVE DISORDERS
Hyperhomocysteinemia and the 677TT genotype were significantly related to depression. In Hordaland Homocysteine Study II (HHS) on nearly 6000 subject there was found a concentration-dependence relation between tHcy and anxiety and depression. Those with TT allele had a 70% higher risk of depression compared with CC genotype (8). The MTHFR gene could be one of the factors of overall schizophrenia risk. Schizophrenic patients having the risk allele (T\T) show more deficiencies in executive function tasks (9). Sohda et al. (10) found that the MTHFR polymorphism and homozygosity for the 677TT allele was significantly increased in a group of patients with preeclampsia. They concluded that the 677T variant of the MTHFR gene is one of the genetic risk factors for preeclampsia development. Recent findings show a relationship between elevated tHcy and neurodegenerative diseases, in particular with Alzheimer’s disease (11).
High level of homocysteine has been encountered in some Multiple sclerosis (MS) patients (12). An elevated homocysteine concentration in blood may trigger oxidation of low-density lipoproteins (LDL) extending to lipid peroxidation and atherosclerosis. Also, high Hcy level may be responsible for the sensation of neurons suffering oxidative stress. Homocysteine markedly increases the vulnerability of hippocampal neurons to excitotoxic and oxidative injury in cell culture and in vivo, suggesting a mechanism by which homocysteine may contribute to the pathogenesis of neurodegenerative disorders (13).
KIDNEY AND HOMOCYSTEINE
Several prospective studies showed that plasma total homocysteine concentrations are elevated in patients with renal insufficiency and that a lower glomerular filtration rate is associated with a higher plasma homocysteine concentration. Those patients were at particularly high risk of ischemic heart disease and stroke. Individuals who had the TT genotype for MTHFR gene compared with those with CC had 25% higher homocysteine levels and a 16% higher risk of ischemic heart disease (14). Homocysteine causes endothelial cell disfunction and injury via production of potent reactive oxygen species during its outooxidation. This metabolite increases tromboxane formation, enhances platelet aggregation and is potent mitogen for vascular smooth muscle cells. Also, Hcy is antagonist of nitric oxide. Those facts may explain why renal impairment patients have a frequent cardiovascular complication and higher overall mortality rate than general population (15). Also, C677T polymorphism is risk factor for diabetic nephropathy development in patients with diabetes mellitus type 2 (16).
PHARMACOGENETICS IN CANCER DISEASE
MTHFR single nucleotide polymorphisms (SNPs) may be an important pharmacogenetic determinant of predicting cancer response and toxicity of methotrexate and 5-fluorouracil and antinflammatory treatments because of their well-defined and highly relevant biochemical effects on intracellular folate composition and one-carbon transfer reactions. Activity of both drugs is dependent on a competitive interaction with folate metabolism. The C677T polymorphism appears to decrease the risk of colorectal cancer, hepatocellular carcinoma, acute lymphocytic leukemia in adults, certain infant/childhood leukemias, and lymphoma.
In contrast, the C677T polymorphism seems to increase the risk of cancer of the breast, endometrium, cervix, esophagus, stomach, and bladder (17). The MTHFR 677T mutation significantly increased chemosensitivity of colon and breast cancer cells to 5-fluorouracil alone and to 5-fluorouracil plus leucovorin in vitro (18). The MTHFR polymorphisms also appear to modulate chemosensitivity of cancer cells to methotrexate, a classic antifolate that inhibits dihydrofolate reductase, decreases intracellular 5,10- methylenetetrahydrofolate levels for thymidylate biosynthesis, and directly inhibits purine biosynthesis (19). Investigation of the role of MTHFR SNPs in pharmacogenetics has important role in health, since the polymorphism is not so rare and methotrexate and 5-fluorouracil are widely used for the treatment of common cancers and inflammatory conditions (20).
Risk of cardiovascular disease
The 677TT MTHFR allele was correlated with coronary artery disease (CHD). Many study show that MTHFR polymorphism had significantly higher risk of atherosclerosis and coronary artery disease, particularly in the low folate status (21). Recent data show that a prolonged increase in just 1 μσ^ is associated with 5% increase in artery diseases. Persons with MTHFR 677 TT genotype had a 16% (OR, 1.16; 95% confidence interval, 1.05-1.28) higher odds for CHD compared with person with CC genotype. Meta-analyses suggest that MTHFR 677 TT genotype is associated with increase tHcy that is risk factor for venous thrombosis and stroke (22). Elevated blood homocysteine is independent risk factor for ischemic stroke. MTHFR mutation is strongly associated with arterial stroke especially in young adults. Data of Alluri and associates (23) indicated that almost 32% patients with ischemic stroke and hyperhomocystinemia had the mutant allele. Furthermore, 80% patients with TT allele were below age of 50 years. So, clinical evaluation on MTHFR allele status helps in prevention management which would reduce morbidity in young adults with ischemic stroke. Still, the role of this polymorphism in the causation of cardiovascular disease is controversial. It is necessary to do studies with a large number of patients and controls to resolve a mistery is homocysteine causes a disease per se or hyperhomocysteinemia is product of impaired renal function, impaired folate metabolism or even marker of poor lifestyle.
Clinical perspective in cardiovascular disease
The importance of homocysteine-lowering treatment with folic acid remains controversial. Initial reports suggested that lowering tHcy with folic acid may retard progression of atherosclerosis, but recent large-scale clinical trials have not been confirming that fact (24). Understanding the mechanisms by which folic acid affects vascular function is critical to the design of future therapeutic strategies. Antoniades and his coworkers (25) demonstrate that 5-MTHF rapidly improves endothelial function and decreases superoxide production in vessels from patients with coronary artery disease by mechanisms that appear independent of homocysteine lowering. The effects of 5-MTHF are due in part to direct scavenging of the oxidant radical peroxynitrite. By this mechanism, 5-MTHF improves bioavailability of the endothelial nitric oxide synthase (eNOS) cofactor, tetrahydrobiopterin, which leads to an improvement in eNOS “coupling” and a decrease in eNOS-derived superoxide production. Future clinical studies are required to evaluate how these effects of 5-MTHF, may be related to clinical benefits in vascular disease.
REFERENCES
- 1.Jacobsen D.W. Practical chemistry of homocysteine and other thiols. In: Carmel R, Jacobsen D.W, editors. In: Homocysteine in health and disease. Cambridge, New York: Cambridge University Press; 2001. pp. 9–20. [Google Scholar]
- 2.Hortin G.L, Seam N, Hoehn G.T. Bound homocysteine, cysteine, and cysteinylglycine distribution between albumin and globulins. Clin. Chem. 2006;52(12):2258–2264. doi: 10.1373/clinchem.2006.074302. [DOI] [PubMed] [Google Scholar]
- 3.Sunder-Plassmann G, Hörl W.H. Pathophysiology and treatment of hyperhomocysteinemia in end-stage renal disease patients. Hemodial. Int. 2001;5:86–91. doi: 10.1111/hdi.2001.5.1.86. [DOI] [PubMed] [Google Scholar]
- 4.Boushey C.J, Beresford S.A.A, Omenn G.S, Motulsky A.G. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease: probable benefits of increasing folic acid intakes. J. Am. Med. Assoc. 1995;274:1049–1057. doi: 10.1001/jama.1995.03530130055028. [DOI] [PubMed] [Google Scholar]
- 5.Frosst P, Blom H.J, Milos R, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahy-drofolate reductase. Nat. Genet. 1995;10:111–113. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- 6.Cortese C, Motti C. MTHFR gene polymorphism, homocysteine and cardiovascular disease. Public Health Nutr. 2001;4(2B):493–497. doi: 10.1079/phn2001159. [DOI] [PubMed] [Google Scholar]
- 7.Wilcken D.E. Novel risk factors for vascular disease: the homocysteine hypothesis of cardiovascular disease. J. Cardiovasc. Risk. 1998;5:217–221. [PubMed] [Google Scholar]
- 8.Refsum H, Nurk E, Smith A.D, Ueland P.M, Gjesdal C.G, Bjelland I, Tverdal A, Tell G.S, Nygård O, Vollset S.E. The Hordaland Homocysteine Study: a community-based study of homocysteine, its determinants, and associations with disease. J. Nutr. 2006;136(Suppl. 6):1731S–1740S. doi: 10.1093/jn/136.6.1731S. [DOI] [PubMed] [Google Scholar]
- 9.Roffman J.L, Weiss A.P, Deckersbach T, Freudenreich O, Henderson D.C, Purcell S, Wong D.H, Halsted C.H, Goff D.C. Effects of the methylenetetrahydrofolate reductas (MTHFR) C677T polymorphism on executive function in schizophrenia. Schizophr. Res. 2007;92(1-3):181–189. doi: 10.1016/j.schres.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Sohda S, Arinami T, Hamada H, Yamada N, Hamaguchi H, Kubo T. Methylenetetrahydrofolate reductase polymorphism and pre-eclampsia. J Med. Genet. 1997;34:525–526. doi: 10.1136/jmg.34.6.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seshadri S, Beiser A, Selhub J, Jacques P.F, Rosenberg I.H, D’Agostino R.B, Wilson P.W, Wolf P.A. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N. Engl. J. Med. 2002;346:476–483. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- 12.Sciacca F.L, Ciusani E, Silvani A, Corsini E, Frigerio S, Pogliani S, Parati E, Croci D, Boiardi A, Salmaggi A. Genetic and plasma markers of venous thromboembolism in patients with high grade glioma. Clin. Cancer Res. 2004;15(10(4)):1312–1317. doi: 10.1158/1078-0432.ccr-03-0198. [DOI] [PubMed] [Google Scholar]
- 13.Kruman I.I, Culmsee C, Chan S.L, Kruman Y, Guo Z, Penix L, Mattson M.P. Homocysteine elicits a DNA damage response in neurons that promotes apoptosis and hypersensitivity to excitotoxicity. J. Neurosci. 2000;15(20(18)):6920–6926. doi: 10.1523/JNEUROSCI.20-18-06920.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clarke R, Lewington S, Landray M. Homocysteine, renal function, and risk of cardiovascular disease. Kidney Int. 2003;84(Suppl):S131–133. doi: 10.1046/j.1523-1755.63.s84.7.x. [DOI] [PubMed] [Google Scholar]
- 15.Kimura H, Gejyo F, Suzuki S, Takeda T, Miyazaki R, Yoshida H. A C677T mutation in the methylenetetrahydrofolate reductase gene modifies serum cysteine in dialysis patients. Am. J. Kidney Dis. 2000;36(5):925–933. doi: 10.1053/ajkd.2000.19085. [DOI] [PubMed] [Google Scholar]
- 16.Moczulski D, Fojcik H, Zukowska-Szczechowska E, Szydlowska Grzeszczak W. Effects of the C677T and A1298C polymorphisms of the MTHFR gene on the genetic predisposition for diabetic nephropathy. Nephrol. Dial. Transplant. 2003;18(8):1535–1540. doi: 10.1093/ndt/gfg211. [DOI] [PubMed] [Google Scholar]
- 17.Ueland P.M, Hustad S, Schneede J, Refsum H, Vollset SE. Biological and clinical implications of the MTHFR C677T polymorphism. Trends. Pharmacol. Sci. 2001;22:195–201. doi: 10.1016/s0165-6147(00)01675-8. [DOI] [PubMed] [Google Scholar]
- 18.Sohn K.J, Croxford R, Yates Z, Lucock M, Kim Y.I. Effect of the methylenetetrahydrofolate reductase C677T polymorphism on chemosensitivity of colon and breast cancer cells to 5-fluorouracil and methotrexate. J. Natl. Cancer Inst. 2004;96:134–144. doi: 10.1093/jnci/djh015. [DOI] [PubMed] [Google Scholar]
- 19.Calvert H. An overview of folate metabolism: features relevant to the action and toxicities of antifolate anticancer agents. Semin. Oncol. 1999;26:3–10. [PubMed] [Google Scholar]
- 20.Kim Y.I. 5,10-Methylenetetrahydrofolate reductase polymorphisms and pharmacogenetics: a new role of single nucleotide polymorphisms in the folate metabolic pathway in human health and disease. Nutr. Rev. 2005;63(11):398–407. doi: 10.1111/j.1753-4887.2005.tb00377.x. [DOI] [PubMed] [Google Scholar]
- 21.Klerk M, Verhoef P, Clarke R, Blom H.J, Kok F.J, Schouten E.G. MTHFR Studies Collaboration Group. MTHFR 677C-->T polymorphism and risk of coronary heart disease: a meta-analysis. JAMA. 2002;288(16):2023–2031. doi: 10.1001/jama.288.16.2023. [DOI] [PubMed] [Google Scholar]
- 22.Den Heijer M, Lewington S, Clarke R. Homocysteine, MTHFR and risk of venous thrombosis: a meta-analysis of published epidemiological studies. J. Thromb. Haemost. 2005;3:292–299. doi: 10.1111/j.1538-7836.2005.01141.x. [DOI] [PubMed] [Google Scholar]
- 23.Alluri R.V, Mohan V, Komandur S, Chawda K, Chaudhuri J.R, Hasan Q. MTHFR C677T gene mutation as a risk factor for arterial stroke: a hospital based study. Eur. J. Neurol. 2005;12(1):40–44. doi: 10.1111/j.1468-1331.2004.00938.x. [DOI] [PubMed] [Google Scholar]
- 24.Lange H, Suryapranata H, De Luca G, Borner C, Dille J, Kallmayer K, Pasalary M.N, Scherer E, Dambrink J.H. Folate therapy and in-stent restenosis after coronary stenting. N. Engl. J. Med. 2004;350:2673–2681. doi: 10.1056/NEJMoa032845. [DOI] [PubMed] [Google Scholar]
- 25.Antoniades C, Shirodaria C, Warrick N, Cai S, de Bono J, Lee J, Leeson P, Neubauer S, Ratnatunga C, Pillai R, Refsum H, Channon K.M. 5-methyltetrahydrofolate rapidly improves endothelial function and decreases superoxide production in human vessels: effects on vascular tetrahydrobiopterin availability and endothelial nitric oxide synthase coupling. Circulation. 2006;114(11):1193–1201. doi: 10.1161/CIRCULATIONAHA.106.612325. [DOI] [PubMed] [Google Scholar]


