Abstract
This retrospective study investigated whether the volume or density of the thrombus is predictive of recanalization in stent retriever (SR) treatment. Consecutive patients treated with SR thrombectomy as the first endovascular modality were enrolled. The thrombus volume and density were measured on thin-section noncontrast computed tomography using 3-dimensional software. The patients were grouped by recanalization status and the number of SR passes. Among 165 patients, recanalization was achieved with the first pass in 68 (50.0%), 2–3 passes in 43 (31.6%), and ≥4 passes in 25 (18.4%) patients. The thrombus volume was smaller in patients with (107.5 mm3) than without (173.7 mm3, p = 0.025) recanalization, and tended to be larger with increasing number of passes (p for trend = 0.001). The thrombus volume was an independent predictor of first-pass recanalization (odds ratio 0.93 per 10 mm3, 95% confidence interval 0.89–0.97). However, the thrombus density was not associated with recanalization success. Recanalization within 3 passes was associated with a favorable outcome. In conclusion, the thrombus volume was significantly related to recanalization in SR thrombectomy. Measuring the thrombus volume was particularly predictive of first-pass recanalization, which was associated with a higher likelihood of a favorable outcome.
Introduction
Thrombectomy with a stent retriever (SR) is recommended as a first-line endovascular treatment, with the highest level of evidence1,2. Recent meta-analyses have shown that recanalization could be achieved in up to 80% of patients using an SR3,4. However, only 54% of patients experienced favorable outcomes, which suggests that recanalization is futile in many patients.
The chance of futile recanalization increases with the number of passes. However, the optimal number of passes with the SR to avoid futile recanalization is uncertain. Moreover, responses to recanalization treatment may differ according to the characteristics of the thrombus, such as volume and physical properties. A small thrombus may be easily retrieved with an SR, requiring only a few passes and yielding a greater chance of a favorable outcome. However, few studies have investigated the association between thrombus characteristics and recanalization in view of the number of passes5,6.
We have previously shown that the volume and density of the thrombus can be measured quantitatively on thin-section noncontrast computed tomography (NCCT) scans by means of software7,8. This study aimed to investigate whether the volume or density of the thrombus measured on thin-section NCCT is predictive of recanalization after intra-arterial recanalization therapy (IART) with an SR. We also investigated whether there is an association between the volume or density of the thrombus and the number of SR passes to achieve recanalization.
Results
Of 288 patients who received IART during the study period, 211 patients were treated with SR thrombectomy as the first endovascular modality (Fig. 1). After excluding patients without thin-section NCCT (n = 27) or without hyperdense arteries on NCCT (n = 19), 165 patients were finally included.
Figure 1.
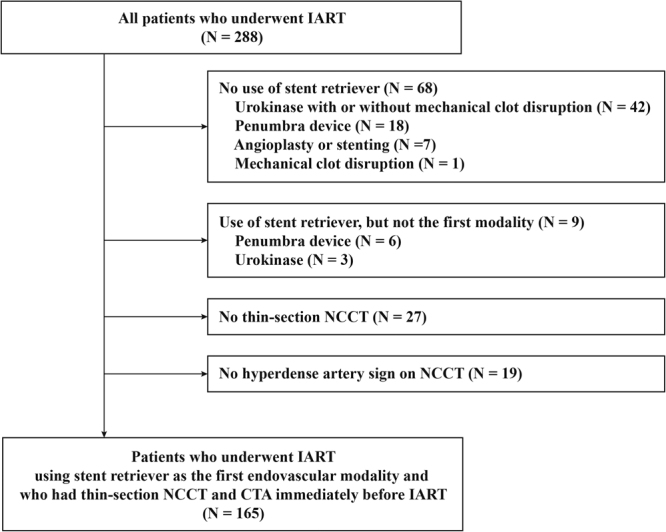
Patient selection flow chart. IART, intra-arterial recanalization therapy; NCCT, non-contrast computed tomography; CTA, computed tomography angiography.
For the 165 patients, the mean age was 70.3 ± 11.2 years and 82 (49.7%) were men. The median thrombus volume was 119.6 mm3 (interquartile range [IQR] 53.9–205.4), and the median corrected Hounsfield unit (HU) of the thrombus was 53.3 (IQR 48.8–58.2). The most common occlusion site was the middle cerebral artery M1 segment, followed by the internal carotid artery (Table 1).
Table 1.
Occlusion site and thrombus characteristics.
| Occlusion site | Number of patients | Volume, mm3 | Density, corrected HU |
|---|---|---|---|
| Anterior circulation | |||
| Internal carotid artery | 46 (27.9) | 211.2 [136.8–287.9] | 56.5 [52.0–60.6] |
| Middle cerebral artery, M1 | 81 (49.1) | 104.8 [59.2–151.1] | 52.6 [48.1–57.0] |
| Middle cerebral artery, M2 | 14 (8.5) | 33.2 [27.6–51.0] | 52.2 [49.1–56.8] |
| Posterior circulation | |||
| Basilar artery | 23 (13.9) | 62.9 [39.2–156.3] | 52.2 [49.4–56.7] |
| Posterior cerebral artery | 1 (0.6) | 9.31 | 65.9 |
| Total | 165 (100) | 119.6 [53.9–205.4] | 53.3 [48.8–58.2] |
Values are number (%) or median [interquartile range]. HU, Hounsfield unit.
Association between thrombus volume and successful recanalization
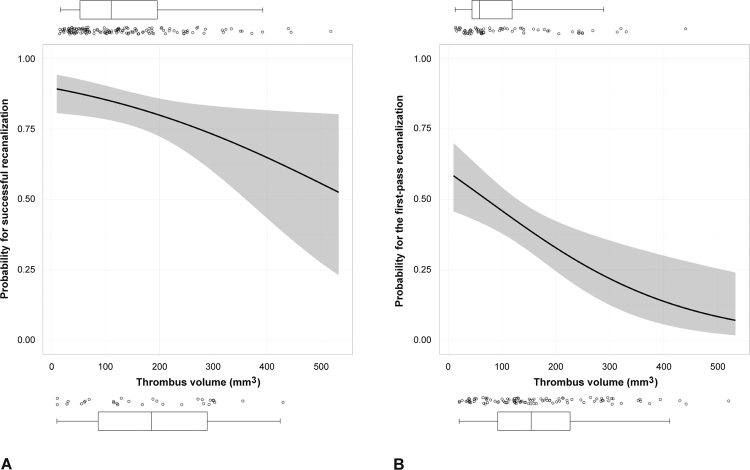
Successful recanalization was achieved in 136 of 165 patients (82.4%). The median thrombus volume was smaller in patients with (107.5 mm3) than in those without successful recanalization (173.7 mm3, p = 0.025) (Table 2). On multivariable analysis, the use of a balloon-guiding catheter (BGC) and the thrombus volume were independent factors for successful recanalization (Table 2 and Fig. 2A). The thrombus density did not differ statistically significantly between patients with and those without successful recanalization.
Table 2.
Factors associated with a successful recanalization.
| Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
| With successful recanalization (n = 136) | Without successful recanalization (n = 29) | P | OR | (95% CI) | P | |
| Age, years | 69.9 ± 10.7 | 72.0 ± 13.3 | 0.357 | 0.99 | (0.95–1.03) | 0.577 |
| Sex, men | 69 (50.7) | 13 (44.8) | 0.563 | 1.27 | (0.54–2.97) | 0.585 |
| Hypertension | 95 (69.9) | 23 (79.3) | 0.306 | |||
| Diabetes | 43 (31.6) | 9 (31.0) | 0.951 | |||
| Smoking | 21 (15.4) | 2 (6.9) | 0.375 | |||
| Hypercholesterolemia | 25 (18.4) | 5 (17.2) | 0.885 | |||
| Initial NIHSS score | 16.0 [13.0–9.0] | 18.0 [15.0–21.0] | 0.082 | 0.97 | (0.90–1.05) | 0.486 |
| Atrial fibrillation | 85 (62.5) | 19 (65.5) | 0.760 | |||
| Occlusion sites | 0.274 | |||||
| Internal carotid artery | 35 (25.7) | 11 (37.9) | ||||
| Middle cerebral artery | 82 (60.3) | 13 (44.8) | ||||
| Posterior circulation | 19 (14.0) | 5 (17.3) | ||||
| Use of intravenous tPA | 55 (40.4) | 9 (31.0) | 0.345 | |||
| Use of BGC | 75 (55.1) | 9 (31.0) | 0.018 | 2.59 | (1.08–6.26) | 0.034 |
| Size of stent retriever | 0.964 | |||||
| 4/15 | 4 (2.9) | 1 (3.4) | ||||
| 4/20 | 126 (92.7) | 27 (93.2) | ||||
| 6/30 | 6 (4.4) | 1 (3.4) | ||||
| Time from onset to puncture, min | 202.5 [150.0–323.2] | 229.0 [160.0–411.0] | 0.292 | |||
| Thrombus density, corrected HU | 52.8 [48.6–57.7] | 54.7 [52.1–60.6] | 0.175 | |||
| Thrombus volume, mm3 | 107.5 [50.8–183.8] | 173.7 [73.2–289.2] | 0.025 | 0.96a | (0.93–0.98) | 0.040 |
| Favorable outcome | 72 (52.9) | 4 (13.8) | <0.001 | |||
Values are mean ± standard deviation, number (%), or median [interquartile range]. aOdds ratio per 10 mm3 of thrombus volume. OR, odds ratio; CI, confidence interval; NIHSS, National Institutes of Health Stroke Scale; tPA, tissue plasminogen activator; BGC, balloon-guiding catheter; HU, Hounsfield unit.
Figure 2.
Logistic regression curves representing the probability of recanalization depending on thrombus volume. Increasing thrombus volume was associated with a significantly decreased probability of (A) successful recanalization and (B) the first-pass recanalization. Dots represent the thrombus volume of each case. The boxplot represents the median and interquartile range.
Association between thrombus volume and the number of stent retriever passes
Of 136 patients with successful recanalization, recanalization was achieved with the first pass of the SR in 68 patients (50.0%), in 2 or 3 passes in 43 patients (31.6%), and in ≥4 passes in 25 patients (18.4%) (Table 3). The thrombus volume tended to be larger as the number of passes for recanalization increased (p for trend = 0.001). The median thrombus volume in the first-pass recanalization group was 63.9 mm3, which was markedly smaller than in the other groups. As the number of passes increased, the time from puncture to recanalization also increased (p = 0.001).
Table 3.
Comparison of variables according to the number of stent retriever passes.
| First-pass recanalization (n = 68) | 2- or 3-Pass recanalization (n = 43) | ≥4-Pass recanalization (n = 25) | No recanalization (n = 29) | Pa | P for trendb | |
|---|---|---|---|---|---|---|
| Age, years | 69.4 ± 11.5 | 71.7 ± 9.4 | 68.2 ± 10.5 | 72.0 ± 13.3 | 0.449 | 0.588 |
| Sex, men | 31 (45.6) | 23 (53.5) | 15 (60.0) | 13 (44.8) | 0.564 | 0.724 |
| Hypertension | 45 (66.2) | 32 (74.4) | 18 (72.0) | 23 (79.3) | 0.573 | 0.203 |
| Diabetes | 22 (32.4) | 11 (25.6) | 10 (40.0) | 9 (31.0) | 0.668 | 0.849 |
| Smoking | 10 (14.7) | 4 (9.3) | 7 (28.0) | 2 (6.9) | 0.135 | 0.828 |
| Hypercholesterolemia | 16 (23.5) | 6 (14.0) | 3 (12.0) | 5 (17.2) | 0.478 | 0.303 |
| Initial NIHSS score | 15.0 [11.8–19.0] | 17.0 [13.0–20.0] | 17.0 [14.0–22.0] | 18.0 [15.0–21.0] | 0.054‡ | 0.012 |
| Atrial fibrillation | 41 (60.3) | 29 (67.4) | 15 (60.0) | 19 (65.5) | 0.861 | 0.714 |
| Occlusion sites | 0.423 | 0.158 | ||||
| Internal carotid artery | 15 (22.1) | 10 (23.3) | 10 (40.0) | 11 (37.9) | ||
| Middle cerebral artery | 42 (61.8) | 28 (65.1) | 12 (48.0) | 13 (44.8) | ||
| Posterior circulation | 11 (16.1) | 5 (11.6) | 3 (12.0) | 5 (17.3) | ||
| Use of intravenous tPA | 29 (42.7) | 19 (44.2) | 7 (28.0) | 9 (31.0) | 0.405 | 0.162 |
| Use of BGC | 40 (58.8%) | 25 (58.1%) | 10 (40.0%) | 9 (31.0) | 0.039‡ | 0.006 |
| Size of stent retriever | 0.232 | 0.114 | ||||
| 4/15 | 1 (1.5) | 1 (2.3) | 2 (8.0) | 1 (3.4) | ||
| 4/20 | 66 (97.0) | 40 (93.0) | 20 (80.0) | 27 (93.2) | ||
| 6/30 | 1 (1.5) | 2 (4.7) | 3 (12.0) | 1 (3.4) | ||
| Time from onset to puncture, min | 190.0 [150.0–313.0] | 210.0 [153.0–311.0] | 205.0 [150.0–372.0] | 229.0 [160.0–411.0] | 0.571 | 0.201 |
| Time from puncture to recanalization, min | 45.0 [34.0–64.5] | 65.0 [55.0–99.0] | 120.0 [82.0–146.0] | NA | 0.001* | 0.001 |
| Thrombus density, corrected HU | 54.0 [50.1–59.4] | 51.0 [46.8–55.9] | 53.9 [47.7–60.1] | 54.7 [52.2–60.6] | 0.050‡ | 0.481 |
| Thrombus volume, mm3 | 63.9 [37.9–146.6] | 122.7 [74.6–210.1] | 157.1 [108.9–187.5] | 173.7 [73.2–289.2] | 0.001† | 0.001 |
Values are mean ± standard deviation, number (%), or median [interquartile range]. aAnalysis of variance, Kruskal-Wallis test, or chi-square or Fisher’s exact test. bAnalysis of variance for trend, Jonckheere-Terpstra test, or Mantel-Haenszel chi-square test. Multiple comparisons were calculated by Bonferroni-corrected Dunn procedure or Bonferroni-corrected chi-square test for initial NIHSS score, use of BGC, time from puncture to recanalization, thrombus density, and thrombus volume. *First-pass recanalization vs. 2- or 3-pass recanalization, P < 0.001; First-pass recanalization vs. ≥4-pass recanalization, P < 0.001; 2- or 3-pass recanalization vs. ≥4-pass recanalization, P < 0.001. †First-pass recanalization vs. ≥4-pass recanalization, P = 0.016; First-pass recanalization vs. no recanalization, P = 0.006. ‡>0.05 on multiple comparisons. NIHSS, National Institutes of Health Stroke Scale; tPA, tissue plasminogen activator; BGC, balloon-guiding catheter; HU, Hounsfield unit; NA, not applicable.
We determined factors that were associated with recanalization according to the number of SR passes. On univariable multinomial logistic regression analysis (see Supplementary Table S1) and multivariable multinomial logistic regression analysis, the thrombus volume was found to be an independent predictor for first-pass recanalization (OR 0.94 per 10 mm3, 95% CI 0.89–0.98; p = 0.005) (Table 4 and Fig. 2B). The use of BGC was an independent predictor for successful recanalization within 3 passes.
Table 4.
Multivariable multinomial logistic regression analysis for the number of stent retriever passes.
| First-pass recanalization | 2- or 3-Pass recanalization | ≥4-Pass recanalization | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Age, per 1 year | 0.99 (0.94–1.04) | 0.646 | 1.00 (0.95–1.05) | 0.907 | 0.97 (0.92–1.02) | 0.236 |
| Sex, men | 1.00 (0.39–2.57) | 0.999 | 1.39 (0.52–3.73) | 0.511 | 1.70 (0.56–5.18) | 0.353 |
| Initial NIHSS score | 0.94 (0.86–1.02) | 0.151 | 0.98 (0.89–1.07) | 0.629 | 1.03 (0.94–1.13) | 0.543 |
| Use of BGC | 3.19 (1.21–8.39) | 0.019 | 2.99 (1.09–8.20) | 0.034 | 1.49 (0.47–4.73) | 0.499 |
| Thrombus volume, per 10 mm3 | 0.94 (0.89–0.98) | 0.005 | 0.97 (0.93–1.02) | 0.241 | 0.99 (0.94–1.03) | 0.619 |
Odds ratio was calculated over no recanalization group. OR, odds ratio; CI, confidence interval; NIHSS, National Institutes of Health Stroke Scale; BGC, balloon-guiding catheter.
As the thrombus volume was the only predictive factor for first-pass recanalization, we determined the cutoff thrombus volume that predicted first-pass recanalization, and found it to be 76.4 mm3 (sensitivity 55.9%, specificity 74.4%, and positive-predictive value 62.3%). The discrimination between the first-pass recanalization and other passes, according to this cutoff thrombus volume, was statistically significant (generalized c-index 0.630 with 95% CI 0.566–0.695; p < 0.001).
Factors associated with functional outcomes
Of the 165 patients, 76 (46.1%) had a favorable outcome. Favorable outcomes were achieved in 64.7% (44 of 68) of the first-pass recanalization group and in 46.5% (20 of 43) of the 2- or 3-pass recanalization group. However, only 32.0% (8 of 25) of the ≥4-pass recanalization group and 13.8% (4 of 29) of the failure group showed favorable outcomes. A younger age, lower initial NIHSS score, and recanalization within 3 passes were independent predictors of a favorable outcome (Table 5). Although patients with unfavorable outcome had higher thrombus density and larger thrombus volume in univariable analysis, no associations were found between them in multivariable analysis, which suggests that they were not independent factors for functional outcome.
Table 5.
Factors associated with a favorable outcome.
| Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
| Unfavorable outcome (n = 89) | Favorable outcome (n = 76) | P | OR | (95% CI) | P | |
| Age, years | 72.9 ± 10.5 | 67.3 ± 11.2 | 0.001 | 0.95a | (0.92–0.99) | 0.010 |
| Sex, men | 41 (46.1) | 41 (53.9) | 0.313 | 1.17 | (0.54–2.52) | 0.692 |
| Hypertension | 65 (73.0) | 53 (69.7) | 0.640 | |||
| Diabetes | 30 (33.7) | 22 (28.9) | 0.512 | |||
| Smoking | 9 (10.1) | 14 (18.4) | 0.125 | |||
| Hypercholesterolemia | 11 (12.4) | 19 (25.0) | 0.036 | 2.55 | (0.90–7.20) | 0.077 |
| Initial NIHSS score | 18.0 [16.0–21.0] | 14.0 [10.0–18.2] | 0.001 | 0.88 | (0.81–0.95) | 0.001 |
| Atrial fibrillation | 61 (68.5) | 43 (56.6) | 0.123 | |||
| Occlusion sites | 0.012 | |||||
| Internal carotid artery | 32 (36.0) | 14 (18.4) | Reference | |||
| Middle cerebral artery | 42 (47.2) | 53 (69.7) | 1.83 | (0.69–4.88) | 0.226 | |
| Posterior circulation | 15 (16.8) | 9 (11.9) | 1.41 | (0.30–6.63) | 0.665 | |
| Use of intravenous tPA | 31 (34.8) | 33 (43.4) | 0.259 | |||
| Use of BGC | 39 (43.8) | 45 (59.2) | 0.049 | 1.53 | (0.65–3.61) | 0.330 |
| Time from onset to puncture, min | 225.0 [154.0–320.0] | 199.0 [149.2–340.0] | 0.412 | |||
| Time from onset to recanalization, minb | 323.5 [239.8–396.2] | 278.0 [202.0–390.8] | 0.155 | |||
| Thrombus density, corrected HU | 55.6 [50.8–60.5] | 52.1 [47.2–56.6] | 0.004 | 0.97 | (0.92–1.01) | 0.169 |
| Thrombus volume, mm3 | 138.5 [65.4–222.1] | 84.8 [46.9–170.7] | 0.014 | 0.99c | (0.96–1.04) | 0.804 |
| Endovascular outcome | 0.001 | |||||
| First-pass recanalization | 24 (27.0) | 44 (57.9) | 9.25 | (2.39–35.8) | 0.001 | |
| 2- or-3-Pass recanalization | 23 (25.8) | 20 (26.3) | 4.94 | (1.18–20.6) | 0.028 | |
| ≥4-Pass recanalization | 17 (19.1) | 8 (10.5) | 3.10 | (0.63–15.2) | 0.162 | |
| No recanalization | 25 (28.1) | 4 (5.3) | Reference | |||
Values are mean ± standard deviation, number (%), or median [interquartile range]. aOdds ratio per 1 year. bValues for 136 patients with successful recanalization. cOdds ratio per 10 mm3. OR, odds ratio; CI, confidence interval; NIHSS, National Institutes of Health Stroke Scale; tPA, tissue plasminogen activator; BGC, balloon-guiding catheter; HU, Hounsfield unit.
Discussion
The thrombus is the ultimate target of recanalization treatment. The volume and composition of the thrombus may affect the response to recanalization treatment. This study showed that the thrombus volume was significantly related to successful recanalization with an SR. Previous studies have shown inconsistent findings in terms of the association between the thrombus burden and recanalization after mechanical thrombectomy by means of an SR6,9–15. The inconsistency between the studies might be partly ascribed to differences in the method used to measure thrombus burden and the number of patients16. Thrombus length and clot burden score could be surrogates for clot burden. It is very difficult to measure thrombus length on NCCT images without the aid of specific software. Therefore, the measurement is usually based on CT angiography (CTA). Determination of thrombus length on CTA is based on the presence of contrast opacification. This method indirectly measures the thrombus length as the portion lacking contrast opacification is assumed to be thrombi, and is influenced by the degree of backflow from the collateral circulation17. Furthermore, for curved or branched arteries and arteries with change in caliber, measurement of thrombus length is not easy on 2-dimensional images, and might not accurately represent the actual thrombus burden. The thrombus volume measured using 3-dimensional software in this study may provide direct, accurate, and reliable information on thrombus burden, irrespective of angioarchitecture and collaterals.
We also compared the thrombus volume and recanalization according to the number of passes for recanalization. Although there was a trend that the number of passes needed for recanalization increased as the volume of the thrombus increased, the association between the volume and recanalization was significant only in the first-pass recanalization group. This first-pass recanalization group had a much smaller thrombus and a markedly higher chance of a favorable outcome than did the other groups. The cutoff volume for predicting first-pass recanalization was calculated as 76.4 mm3 in this study population. However, this cutoff value could differ according to the study population and the use of different mechanical devices. In this study, BGC was also an independent predictor for successful recanalization and recanalization within 3 passes. BGC was known to be effective for reducing distal emboli during mechanical thrombectomy18, and for achieving more recanalization with fewer SR passes19,20.
We also investigated whether thrombus density is predictive of recanalization after SR treatment. This study showed no association between the average thrombus density and recanalization. Previous studies on patients undergoing endovascular treatment showed inconsistent findings in terms of the association between thrombus density and recanalization6,10,21. The thrombus density on CT scan may represent the constituents of the thrombus, in that the HU is high in red blood cell-rich regions and low in fibrin- or platelet-rich regions22. However, the distribution of each constituent within the thrombus is neither even, nor homogeneous, among thrombi23. Thus, defining the region of interest (ROI) might be important for accurate measurement of thrombus density, and, ideally, the entire thrombus should be included for averaging. In previous studies, the ROI was defined manually by outlining the margin of the thrombus or drawing small circles within the thrombus6,10,24–26. The former approach might underestimate the density, because the low-density areas in the periphery or outside of thrombus could be included during manual outlining of the thrombus margin16. On the contrary, the latter approach might overestimate the density by including only high-density areas within the thrombus16. In this study, pixels of almost the entire thrombus could be averaged as the ROI was defined automatically, based on HU, without the risk of a selection bias, using 3-dimensional software8,27,28. Besides the average density, the age and architecture of the thrombus might also affect the physical properties of the thrombus. These factors may also be associated with the retrieval of the thrombus using an SR; however, they cannot be assessed by simple measurement of the average HU of the thrombus.
This study has several limitations. First, this study involved retrospective analysis of data. The use of mechanical devices and the number of passes were not controlled. Thus, this study is not free from selection bias. Second, this study included patients who were treated with an SR. Therefore, the interpretation of our results should be limited to the use of an SR, because the association between the thrombus characteristics and recanalization might differ among the mechanical devices used. Third, patients without hyperdense artery signs were not included, because thrombus volume could not be measured in these cases. The absence of a hyperdense artery sign may be associated with the presence of thrombi with very low HU or non-embolic infarctions, such as intracranial artery stenosis29,30. Fourth, this study included patients who were treated with IV tPA before the endovascular treatment, which is currently the standard treatment. The guideline recommends that patients eligible for IV tPA should receive IV tPA even if endovascular treatments are being considered31. Although the frequency of recanalization was not different between patients with bridging IV thrombolysis and those with direct endovascular treatment in this study, IV tPA treatment might affect on the thrombus composition and structure. This should be considered for the interpretation of findings in this study. Finally, although the benefit of the use of an SR was observed when recanalization could be achieved within 3 passes in this study population, the effective number of passes may be different in other clinical settings, with different onset-to-recanalization time, because the benefit is time-dependent32.
Conclusions
Our findings suggest that thin-section NCCT-based thrombus imaging can be useful for the prediction of successful recanalization, in that the volume of the thrombus was significantly related to recanalization after the use of an SR. Measuring the volume of the thrombus may be particularly useful for predicting first-pass recanalization after SR treatment, which can be achieved in many patients and has higher chances of achieving favorable clinical outcomes. Our findings also suggest that measuring the volume of the thrombus may assist the neurointerventionist to select the appropriate device for thrombectomy, for example, larger thrombi need longer or larger SRs, or aspiration devices.
Methods
Study population
We retrospectively analyzed the data of a prospective cohort that had been studied to investigate the predictive value of thrombus imaging based on thin-section NCCT27,28. The subjects of this study were consecutive patients with acute intracranial artery occlusion who underwent IART from September 2010 to August 2015. We included patients who were treated with SR thrombectomy as the first endovascular modality, and who had undergone thin-section (1-mm or 1.2-mm thickness) NCCT immediately before the IART. A Solitaire (Medtronic, Dublin, Ireland) stent retriever was used exclusively for endovascular treatment. Imaging protocols and IART procedures are described in detail in the Supplementary Methods.
The prospective cohort and the protocol of this study were approved by the Institutional Review Board of Severance Hospital, Yonsei University Health System. All methods were performed in accordance with the relevant guidelines and regulations. We obtained written informed consent from the patients or their qualified next of kin. The need to obtain informed consent for this specific study was waived due to its retrospective nature.
Measurements of thrombus volume and Hounsfield units
The volume and density (HU) of the thrombus were measured on thin-section NCCT in a semi-automatic manner by using 3-dimensional software (Xelis; Infinitt, Seoul, Korea), as published previously27,28. For patients who received IV tPA, the volume and density of thrombus were assessed on follow-up NCCT images, which were taken just before endovascular treatment. Briefly, after the identification of the clot based on the pixel segmentation at a threshold between 50 and 100 HU, an ROI was drawn within any portion of the thrombus by using a brush tool or by a simple click. Then, by clicking the “dilate” button, automatic pixel dilation and region growing were performed to the margin of the thrombus at a threshold between 40 and 100 HU. It was unnecessary to define the margin of the thrombus manually. After this process, the volume and density of thrombus were automatically calculated and presented in table format. The calculated HUs were standardized to the HU value of the contralateral M1 segment of the middle cerebral artery (corrected HU; see Supplementary Methods)27. Although beam-hardening artifacts may occur in the skull base, which may disturb the assessment of thrombi in the internal carotid artery or basilar artery, the artifact was minimized using a reconstruction algorithm and post-imaging processing. In fact, no case in this study population had such an artifact.
Two investigators who were blinded to the clinical details measured the volume and density of the thrombus independently. The inter-rater agreements were excellent for both volume (interclass coefficient 0.989) and density (0.950).
Outcomes
Functional outcome was determined using the modified Rankin Scale (mRS) score at 3 months after stroke onset. A favorable outcome was defined as an mRS 0–2. Successful recanalization was defined as the modified Thrombolysis In Cerebral Infarction (mTICI) 2b or 3 on the final angiogram during IART33. mTICI 2b was defined as antegrade partial perfusion of half or greater of the downstream ischemic territory, and mTICI 3 was defined as antegrade complete perfusion of the downstream ischemic territory. All reperfusion grades were determined with consensus from stroke neurologists, neurointerventionists, and neuroradiologists during the regular stroke conference. The basic principle of mTICI grading was also applied to posterior circulation stroke. Based on the specific target arterial lesion and its relevant target downstream territory, the ratio of final reperfusion area was estimated34. We divided the patients into 4 groups based on the recanalization status and the number of SR passes for achieving recanalization, which included the first-pass recanalization, 2- or 3-pass recanalization, ≥4-pass recanalization, and no recanalization.
Statistical analyses
We compared demographic and clinical variables, and the volume and HU of the thrombus between the groups, using analysis of variance, the Kruskal-Wallis test, chi-square test, or Fisher’s exact test, as appropriate. We also performed analysis of variance for trend, the Jonckheere-Terpstra test, or the Mantel−Haenszel test for trend analysis. To determine independent factors for predicting recanalization, multivariable binary or multinomial logistic regression was performed by entering age, sex, and variables of p < 0.10 on univariable analyses. The diagnostic performances for predicting recanalization were calculated using the cutoff values for each histogram parameter according to the Youden index. The generalized c-index and 95% CI were calculated using the bootstrapping method with resampling of 1,000 times. The factors associated with the functional outcome were compared using Student’s t-test, Wilcoxon’s signed-rank test, chi-square test, or Fisher’s exact test. We also performed multivariable binary logistic regression analysis to determine independent factors for a favorable outcome.
All statistical analyses were performed with R software package version 3.2.2 (http://www.R-project.org) or SAS (version 9.2; SAS Inc., Cary, NC, USA). P < 0.05 was considered statistically significant.
Electronic supplementary material
Acknowledgements
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health and Welfare, Republic of Korea (HI15C2814, HC15C1056).
Author Contributions
J.H.B. and J.H.H. designed the study, made the draft of manuscript, and revised the manuscript. J.H.B. and J.Y. measured thrombus volume and density. J.H.B., D.S., Y.D.K., H.S.N., B.M.K., and D.J.K. collected clinical and angiographic data. H.S.L., J.H.B., and D.S. performed the statistical analysis. J.H.B., J.H.H., Y.D.K., and H.S.N. analyzed the data and discussed the results. All authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-16274-9.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Powers WJ, et al. 2015 American Heart Association/American Stroke Association focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46:3020–3035. doi: 10.1161/STR.0000000000000074. [DOI] [PubMed] [Google Scholar]
- 2.Hong KS, et al. Update of the Korean clinical practice guidelines for endovascular recanalization therapy in patients with acute ischemic stroke. J Stroke. 2016;18:102–1s13. doi: 10.5853/jos.2015.01655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell BC, et al. Safety and efficacy of solitaire stent thrombectomy: individual patient data meta-analysis of randomized trials. Stroke. 2016;47:798–806. doi: 10.1161/STROKEAHA.115.012360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hong KS, Ko SB, Lee JS, Yu KH, Rha JH. Endovascular recanalization therapy in acute ischemic stroke: updated meta-analysis of randomized controlled trials. J Stroke. 2015;17:68–81. doi: 10.5853/jos.2015.17.3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Linfante I, et al. Predictors of poor outcome despite recanalization: a multiple regression analysis of the NASA registry. J Neurointerv Surg. 2016;8:224–229. doi: 10.1136/neurintsurg-2014-011525. [DOI] [PubMed] [Google Scholar]
- 6.Mokin M, et al. Thrombus density predicts successful recanalization with Solitaire stent retriever thrombectomy in acute ischemic stroke. J Neurointerv Surg. 2015;7:104–107. doi: 10.1136/neurintsurg-2013-011017. [DOI] [PubMed] [Google Scholar]
- 7.Kim EY, et al. Detection of thrombus in acute ischemic stroke: value of thin-section noncontrast-computed tomography. Stroke. 2005;36:2745–2747. doi: 10.1161/01.STR.0000185720.03803.41. [DOI] [PubMed] [Google Scholar]
- 8.Kim EY, Yoo E, Choi HY, Lee JW, Heo JH. Thrombus volume comparison between patients with and without hyperattenuated artery sign on CT. AJNR Am J Neuroradiol. 2008;29:359–362. doi: 10.3174/ajnr.A0800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gralla J, et al. Occlusion length is a crucial determinant of efficiency and complication rate in thrombectomy for acute ischemic stroke. AJNR Am J Neuroradiol. 2008;29:247–252. doi: 10.3174/ajnr.A0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spiotta AM, et al. Hounsfield unit value and clot length in the acutely occluded vessel and time required to achieve thrombectomy, complications and outcome. J Neurointerv Surg. 2014;6:423–427. doi: 10.1136/neurintsurg-2013-010765. [DOI] [PubMed] [Google Scholar]
- 11.Jindal G, et al. Relationship of thrombus length to number of stent retrievals, revascularization, and outcomes in acute ischemic stroke. J Vasc Interv Radiol. 2014;25:1549–1557. doi: 10.1016/j.jvir.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 12.Angermaier A, Michel P, Khaw AV, Kirsch M, Kessler C. & Langner, S. Intravenous Thrombolysis and Passes of Thrombectomy as Predictors for Endovascular Revascularization in Ischemic Stroke. J Stroke Cerebrovasc Dis. 2016;25:2488–2495. doi: 10.1016/j.jstrokecerebrovasdis.2016.06.024. [DOI] [PubMed] [Google Scholar]
- 13.Mokin M, et al. Association of clot burden score with radiographic and clinical outcomes following Solitaire stent retriever thrombectomy: analysis of the SWIFT PRIME trial. J Neurointerv Surg. 2017;9:929–932. doi: 10.1136/neurintsurg-2016-012631. [DOI] [PubMed] [Google Scholar]
- 14.Soize S, et al. Susceptibility vessel sign on T2* magnetic resonance imaging and recanalization results of mechanical thrombectomy with stent retrievers: a multicentre cohort study. Eur J Neurol. 2015;22:967–972. doi: 10.1111/ene.12693. [DOI] [PubMed] [Google Scholar]
- 15.Treurniet KM, et al. Clot Burden Score on Baseline Computerized Tomographic Angiography and Intra-Arterial Treatment Effect in Acute Ischemic Stroke. Stroke. 2016;47:2972–2978. doi: 10.1161/STROKEAHA.116.014565. [DOI] [PubMed] [Google Scholar]
- 16.Heo JH, et al. Computed Tomography-Based Thrombus Imaging for the Prediction of Recanalization after Reperfusion Therapy in Stroke. J Stroke. 2017;19:40–49. doi: 10.5853/jos.2016.01522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yan S, et al. Thrombus Length Estimation on Delayed Gadolinium-Enhanced T1. Stroke. 2016;47:756–761. doi: 10.1161/STROKEAHA.116.014686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chueh JY, et al. Reduction in distal emboli with proximal flow control during mechanical thrombectomy: a quantitative in vitro study. Stroke. 2013;44:1396–1401. doi: 10.1161/STROKEAHA.111.670463. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen TN, et al. Balloon guide catheter improves revascularization and clinical outcomes with the Solitaire device: analysis of the North American Solitaire Acute Stroke Registry. Stroke. 2014;45:141–145. doi: 10.1161/STROKEAHA.113.002407. [DOI] [PubMed] [Google Scholar]
- 20.Velasco A, et al. Comparison of a balloon guide catheter and a non-Balloon guide catheter for mechanical thrombectomy. Radiology. 2016;280:169–176. doi: 10.1148/radiol.2015150575. [DOI] [PubMed] [Google Scholar]
- 21.Moftakhar P, et al. Density of thrombus on admission CT predicts revascularization efficacy in large vessel occlusion acute ischemic stroke. Stroke. 2013;44:243–245. doi: 10.1161/STROKEAHA.112.674127. [DOI] [PubMed] [Google Scholar]
- 22.Kirchhof K, Welzel T, Mecke C, Zoubaa S, Sartor K. Differentiation of white, mixed, and red thrombi: value of CT in estimation of the prognosis of thrombolysis phantom study. Radiology. 2003;22:126–130. doi: 10.1148/radiol.2273020530. [DOI] [PubMed] [Google Scholar]
- 23.Ahn SH, et al. Histologic features of acute thrombi retrieved from stroke patients during mechanical reperfusion therapy. Int J Stroke. 2016;11:1036–1044. doi: 10.1177/1747493016641965. [DOI] [PubMed] [Google Scholar]
- 24.Puig J, et al. Quantification of thrombus hounsfield units on noncontrast CT predicts stroke subtype and early recanalization after intravenous recombinant tissue plasminogen activator. AJNR Am J Neuroradiol. 2012;33:90–96. doi: 10.3174/ajnr.A2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shu, L., Riedel, C., Meyne, J., Jansen, O. & Jensen-Kondering, U. Successful recanalization in acute basilar artery occlusion treated with endovascular therapy is independent of thrombus length. J Neurointerv Surg. pii: neurintsurg-2016-012634. 10.1136/neurintsurg-2016-012634 (2016 Oct 27). [DOI] [PubMed]
- 26.Niesten JM, et al. Predictive value of thrombus attenuation on thin-slice non-contrast CT for persistent occlusion after intravenous thrombolysis. Cerebrovasc Dis. 2014;37:116–122. doi: 10.1159/000357420. [DOI] [PubMed] [Google Scholar]
- 27.Nam HS, et al. Prediction of thrombus resolution after intravenous thrombolysis assessed by CT-based thrombus imaging. Thromb Haemost. 2012;107:786–794. doi: 10.1160/TH11-08-0585. [DOI] [PubMed] [Google Scholar]
- 28.Kim YD, et al. Time-dependent thrombus resolution after tissue-type plasminogen activator in patients with stroke and mice. Stroke. 2015;46:1877–1882. doi: 10.1161/STROKEAHA.114.008247. [DOI] [PubMed] [Google Scholar]
- 29.Liebeskind DS, et al. CT and MRI early vessel signs reflect clot composition in acute stroke. Stroke. 2011;42:1237–1243. doi: 10.1161/STROKEAHA.110.605576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim SK, Yoon W, Heo TW, Park MS, Kang HK. Negative susceptibility vessel sign and underlying intracranial atherosclerotic stenosis in acute middle cerebral artery occlusion. AJNR Am J Neuroradiol. 2015;36:1266–1271. doi: 10.3174/ajnr.A4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Powers WJ, et al. 2015 American Heart Association/American Stroke Association Focused Update of the 2013 Guidelines for the Early Management of Patients With Acute Ischemic Stroke Regarding Endovascular Treatment: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2015;46:3020–3035. doi: 10.1161/STR.0000000000000074. [DOI] [PubMed] [Google Scholar]
- 32.Khatri P, et al. Time to angiographic reperfusion and clinical outcome after acute ischaemic stroke: an analysis of data from the Interventional Management of Stroke (IMS III) phase 3 trial. Lancet Neurol. 2014;13:567–574. doi: 10.1016/S1474-4422(14)70066-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoo AJ, et al. Refining angiographic biomarkers of revascularization: improving outcome prediction after intra-arterial therapy. Stroke. 2013;44:2509–2512. doi: 10.1161/STROKEAHA.113.001990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zaidat OO, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke. 2013;44:2650–2563. doi: 10.1161/STROKEAHA.113.001972. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.