Abstract
Whey protein concentrate (WPC) is an amino acid-rich supplement that has been shown to increase cellular antioxidant capacity. Mammalian target of rapamycin (mTOR) is a crucial regulator of signaling in mammalian cells, and serves as a therapeutic target for triple-negative breast cancer (TNBC). This study was designed to investigate the effect of combining WPC with rapamycin on MDA-MB-231 human breast cancer cells. These cells were found to be insensitive to rapamycin and exhibited higher glutathione (GSH) and reactive oxygen species levels than non-tumorigenic MCF-10A cells. However, for MDA-MB-231 cells, the half maximal inhibitory concentration of rapamycin was lower when this drug was administered in combination with WPC than when used alone. Furthermore, combining WPC with rapamycin depleted GSH levels and reduced Nrf2 nuclear accumulation. In addition, WPC activated GSK3β/mTOR signaling, and GSK3β appeared to be involved in the WPC-mediated Nrf2 reduction and mTOR activation. In conclusion, WPC induced rapamycin sensitivity in MDA-MB-231 cells by altering their redox state and activating GSK3β/mTOR signaling. These results not only suggest a novel therapeutic approach for breast cancer treatment, but also provide insight into the critical pathways affecting the resistance to mTOR inhibition observed in a subgroup of TNBC patients.
Introduction
Breast cancer is the most common malignancy affecting women worldwide and its incidence has increased globally over recent decades. Triple-negative breast cancer (TNBC), defined by the absence of estrogen receptor, progesterone receptor, and human epidermal growth factor receptor expression, remains a therapeutically challenging disease with the worst prognosis of any breast cancer subtype1.
Mammalian target of rapamycin (mTOR), a downstream protein of the PI3K/Akt pathway, is activated by growth, changes in nutrient and energy levels, and hypoxia, and has also been implicated in cancer cell proliferation and survival2. mTOR complex 1 (mTORC1) responds to intracellular energy levels and nutrient availability, and once activated, phosphorylates and activates ribosomal p70 S6 kinase (p70S6K) and hyperphosphorylates eukaryotic translation initiation factor 4E (eIF4E)-binding proteins 1 (4E-BP1), influencing cell growth and survival. Recent evidence has indicated that glycogen synthase kinase 3 beta (GSK3β) positively regulates mTORC1 activity in MCF-7 breast cancer cells3. Moreover, GSK3β has been shown to play a permissive role in the amino acid-induced activation of mTORC14.
Reactive oxygen species (ROS) and cellular oxidative stress are associated with cancer5,6, and compared to normal cells, antioxidant capacity is upregulated in malignant cells to adapt to higher ROS levels5. Previous studies have reported that nuclear factor (erythroid-derived 2)-like 2 (Nrf2) responds to increased ROS levels by enhancing the expression of genes involved in maintaining the cellular redox balance, including those encoding glutamate-cysteine ligase (GCL) and glutathione reductase (GR), which are associated with glutathione (GSH) production and regeneration7. Furthermore, deregulation of Nrf2, such as that leading to its increased nuclear accumulation, reduces apoptosis and promotes drug resistance8.
Since mTOR and Nrf2 signaling are known drivers of human oncogenesis9,10, agents targeting these pathways have shown promise as treatments for breast cancer11,12. However, despite the establishment of this treatment strategy and its encouraging results, a proportion of patients with TNBC harbor tumors that are resistant to mTOR inhibitors13. Thus, identifying markers of mTOR inhibitor sensitivity or the development of combination therapy is urgently needed to improve TNBC response to mTOR inhibition.
Whey protein concentrate (WPC) is prepared in a special manner to preserve native forms of the cysteine-rich proteins in whey (serum albumin, lactoferrin, and α-lactalbumin) and functions as a GSH precursor in cells. We have shown previously that WPC supplementation increases antioxidant activity in human peripheral blood mononuclear cells and rats treated with high doses of alcohol14,15. Besides, we have demonstrated that WPC supplementation selectively depletes tumor GSH levels in 7,12-dimethylbenz[a]anthracene (DMBA)-induced mammary tumors in rats16. In addition, prior studies have reported that WPC ingestion promotes activation of the mTOR pathway17, and it was demonstrated that this supplement exerts its antioxidant effects through an Nrf2-dependent mechanism in endothelial cells18. Therefore, in the present work, we aimed to determine whether WPC can influence the susceptibility to mTOR inhibitors using MDA-MB-231 TNBC cells, a cell line that has been reported to be resistant19,20. These results might provide insight into the critical pathways that are involved in resistance to mTOR inhibition in TNBC, and could identify biomarkers of for the responsiveness to such inhibitors.
Results
Determination of cellular redox status
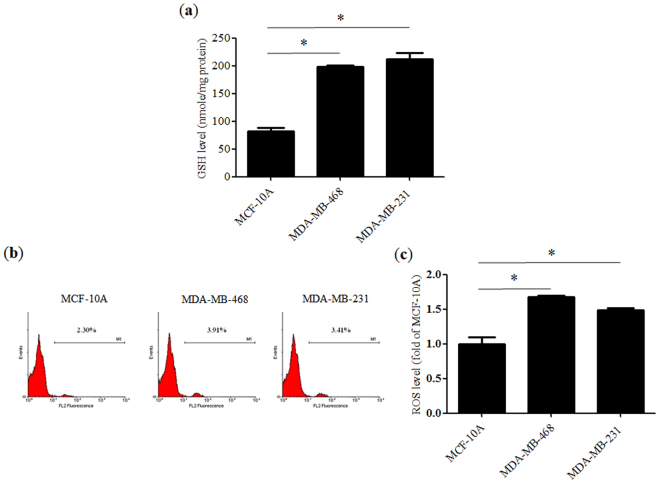
Increased GSH (Fig. 1a) and ROS (Fig. 1b,c) levels were noted in advanced breast cancer cell lines, including MDA-MB-231 and MDA-MB-468, compared to those in MCF-10A non-tumorigenic breast epithelial cells. Since GSH is a key cellular antioxidant, these results indicated that cancer cells have higher tolerance to ROS.
Figure 1.
Redox status of various cell lines. (a) Glutathione (GSH) levels in non-tumorigenic MCF-10A cells and advanced breast cancer cell lines including MDA-MB-463 and MDA-MB-231 cells. (b) Representative flow cytometry histograms showing cellular reactive oxygen species (ROS) content and (c) a bar graph indicating fold-change relative to MCF-10A. Data represent the means ± SD from three experiments. *P < 0.05.
WPC sensitizes MDA-MB-231 cells to rapamycin
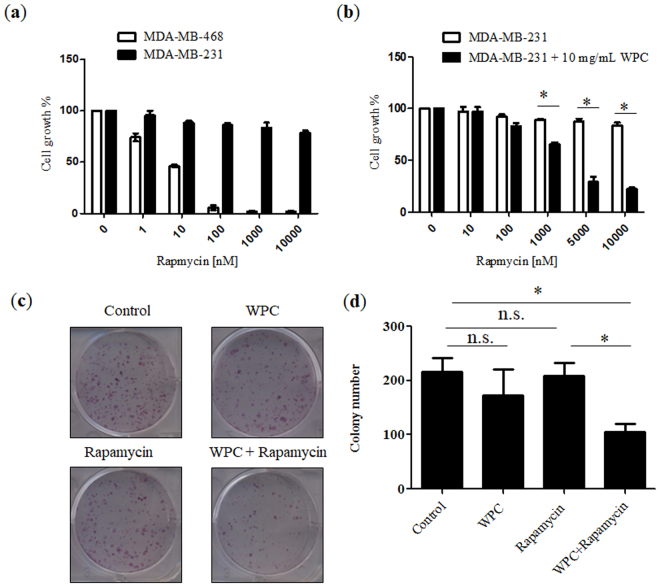
To examine the sensitivity of each cell line to rapamycin, cells were treated with this drug at different concentrations for 72 hours, and cell viability was measured by standard MTT assays. As shown in Fig. 2a, growth of MDA-MB-468 cells was significantly inhibited by rapamycin, whereas MDA-MB-231 cells were resistant to the growth inhibitory effects of this drug, with observed half maximal inhibitory concentration (IC50) values of 8.91 nM and >10 μM, respectively. Given these different responses to rapamycin, we chose the MDA-MB-231 line for further evaluation. In addition, cellular viability of MCF-10A, MDA-MB-468, and MDA-MB-231 cells was measured with WPC at concentrations of 1, 10, and 20 mg/mL. There were no significant differences (P > 0.05) in cell viability among the WPC-treated groups, despite increasing concentrations of WPC (Fig. S1a–c). Regarding viability of MDA-MB-231 cells, Fig. 2b shows the effect of 48-hour exposure to WPC (10 mg/mL) combined with different concentrations of rapamycin. Compared to treatment with rapamycin alone, the growth inhibitory effect of combined treatment resulted in a significant decrease in the IC50 of rapamycin (from >10 μM to 2.54 μM, respectively). In vitro colony formation assays were then performed to further test the effect of WPC combined with rapamycin on cell growth. Figure 2c and d demonstrate that combined treatment led to a significant decrease in the number of colonies compared to that in the control and rapamycin-only groups. However, cells treated with WPC or rapamycin alone did not significantly differ from the control in this respect.
Figure 2.
Whey protein concentrate (WPC) renders MDA-MB-231 cells sensitive to rapamycin and inhibits the colony formation of MDA-MB-231 cells. (a) MDA-MB-468 and MDA-MB-231 cell viability was determined by MTT assay after incubation with rapamycin at the indicated concentration for 72 hours. (b) Inhibition of MDA-MB-231 cell growth following 48 hours of exposure to 10 mg/mL WPC combined with 10, 100, 1,000, 5000, and 10,000 nM rapamycin. (c) Representative images demonstrating the effect of WPC, rapamycin, and their combination on colony formation in MDA-MB-231 cells. (d) Bar graph indicating the number of colonies formed. Data represent the means ± SD from three experiments. *P < 0.05; n.s.: not significant.
Effect of WPC, rapamycin, and their combination on cellular redox status
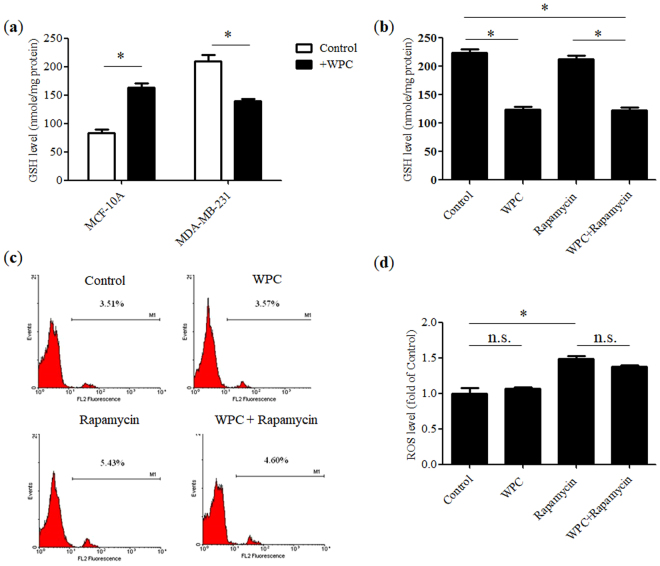
As shown in Fig. 3a, WPC (10 mg/mL) treatment selectively depleted GSH levels in MDA-MB-231 cells, but increased GSH levels in non-tumorigenic MCF-10A cells. Moreover, in MDA-MB-231 cells, rapamycin (1 μM) treatment did not alter GSH levels (Fig. 3b), but did significantly increase ROS concentrations (Fig. 3c,d) compared to those in the control. Furthermore, in comparison to those in the control, GSH levels were significantly lower following administration of WPC alone or in combination with rapamycin. However, WPC alone had no effect on ROS levels in MDA-MB-231 cells and did not affect the rapamycin-mediated elevation in ROS concentrations when administered as part of the combination treatment.
Figure 3.
Effect of whey protein concentrate (WPC), rapamycin, and their combination on the redox status of breast cancer cells. (a) Glutathione (GSH) levels in non-tumorigenic MCF-10A cells and MDA-MB-231 cells after incubation with 10 mg/mL WPC for 48 hours. Effect of 48-hour treatment with 10 mg/mL WPC, 1 μM rapamycin, and their combination on (b) GSH levels and (c) reactive oxygen species (ROS) content in MDA-MB-231 cells, displayed as representative flow cytometry histograms and (d) a bar graph indicating fold-change relative to control levels. Data represent the means ± SD from three experiments. *P < 0.05; n.s.: not significant.
Effect of WPC, rapamycin, and their combination on the expression of Nrf2, GCL, and GR in MDA-MB-231 cells
Since the intracellular GSH redox state is regulated by the Nrf2-GCL-GR-GSH signaling pathway, whole cell lysates were subjected to immunoblotting for Nrf2, the GCL catalytic subunit (GCLC), and GR to clarify the effects of WPC. As shown in Fig. 4, there were no significant differences (P > 0.05) in whole-cell Nrf2 protein expression between groups with WPC treatment alone or combined treatment, when compared to that in the control group. However, treatment with rapamycin alone increased whole-cell Nrf2 protein expression. Compared to the that in the control group, WPC alone and combined treatment resulted in reduced expression of GCLC and GR proteins. However, treatment with rapamycin alone had no effect on the expression of these proteins.
Figure 4.
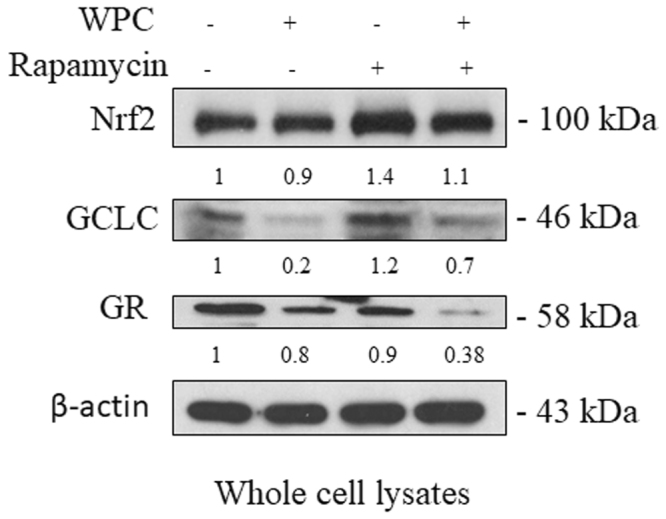
Effect of whey protein concentrate (WPC), rapamycin, and their combination on the expression of Nrf2, GCLC, and glutathione reductase (GR) proteins in MDA-MB-231 cells. Representative western blots showing the effect of 48 hours of exposure to 10 mg/mL WPC, 1 μM rapamycin, and their combination on whole cell Nrf2, GCLC, and GR protein levels. β-actin was used as a loading control. Data represent the means ± SD from three experiments.
Rapamycin-induced nuclear accumulation of Nrf2 in MDA-MB-231 cells is reduced by WPC treatment
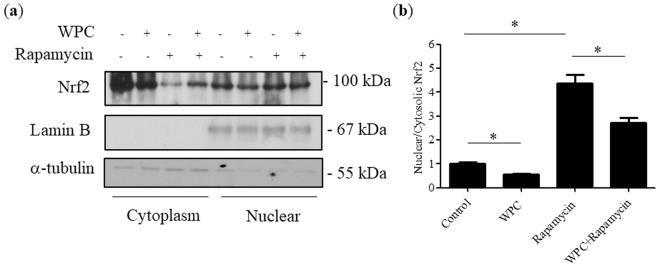
Since there were no significant differences in whole-cell Nrf2 protein expression among groups, we next evaluated the cellular translocation of Nrf2. As indicated in Fig. 5a and b, rapamycin treatment induced Nrf2 nuclear accumulation; however, WPC administration decreased the presence of Nrf2 in the nucleus, and inhibited the effect of rapamycin on Nrf2 translocation.
Figure 5.
Effect of when protein concentrate (WPC), rapamycin, and their combination on Nrf2 translocation in MDA-MB-231 cells. (a) Representative western blots showing the effect of 48 hours of exposure to 10 mg/mL WPC, 1 μM rapamycin, and their combination on cytosolic and nuclear Nrf2 protein levels. α-tubulin and lamin B were used as loading controls for cytosolic and nuclear fractions, respectively. (b) Bar graphs showing relative nuclear/cytosolic Nrf2 ratios. Data represent the means ± SD from three experiments. *P < 0.05.
WPC activates the GSK3/mTOR pathway
We next assessed the combined effects of WPC and rapamycin on mTORC1 in MDA-MB-231 cells, since this complex is the direct target of rapamycin and is activated by nutritional factors. The level of phosphorylated (p)-mTOR (Ser2448) is a marker of mTORC1 activation. As shown in Fig. 6, the p-mTOR/mTOR ratio was approximately 2.2-fold higher in the WPC-only group compared to that in the control group. In contrast, compared to that in the control, the p-mTOR/mTOR ratio was found to be reduced with combined treatment. In addition, the p-p70S6K/p70S6K and the p-4E-BP1/4E-BP1 ratios were 1.4-fold and 1.5-fold higher, respectively, in the WPC-only group than the control group, whereas this effect was negated by the administration of rapamycin together with WPC. Strikingly, relative to that in the control, WPC upregulated p-GSK3β/GSK3β, both alone (1.32-fold) and with combined treatment (2.2-fold).
Figure 6.
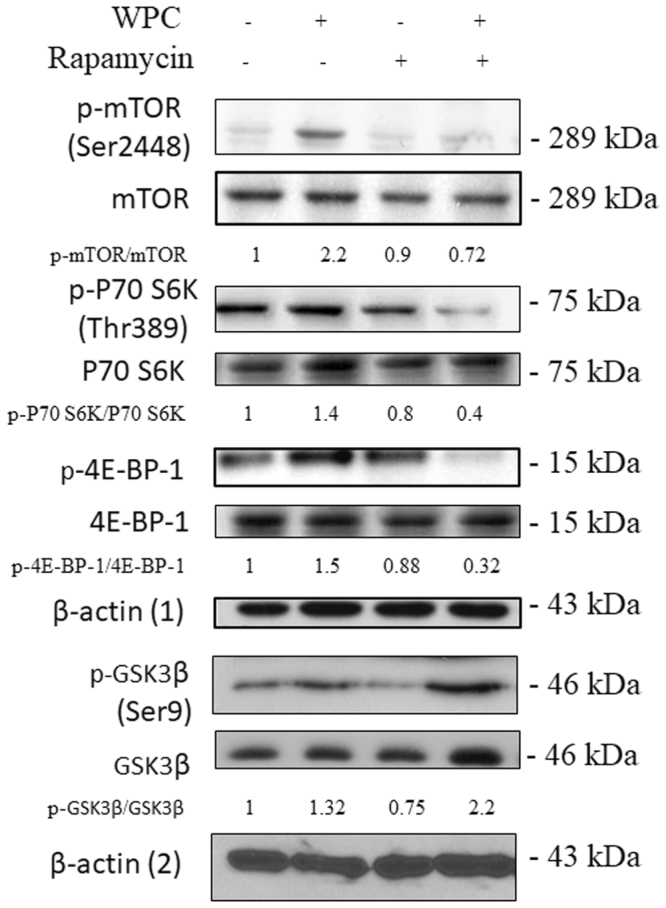
Effect of whey protein concentrate (WPC), rapamycin, and their combination on the mTOR/GSK3β signaling pathway in MDA-MB-231 cells. Representative western blots showing levels of p-mTOR (Ser 2448), mTOR, p-p70S6K (Thr 389), p70S6K, p-4E-BP-1, 4E-BP-1, p-GSK3β, and GSK3β in cells treated with 10 mg/mL WPC, 1 μM rapamycin, or both for 48 hours. β-actin (1) was used as a loading control of p-mTOR (Ser 2448), mTOR, p-p70S6K (Thr 389), p70S6K, p-4E-BP-1, and 4E-BP-1. β-actin (2) was used as a loading control of p-GSK3β, and GSK3β Data represent the means ± SD from three experiments.
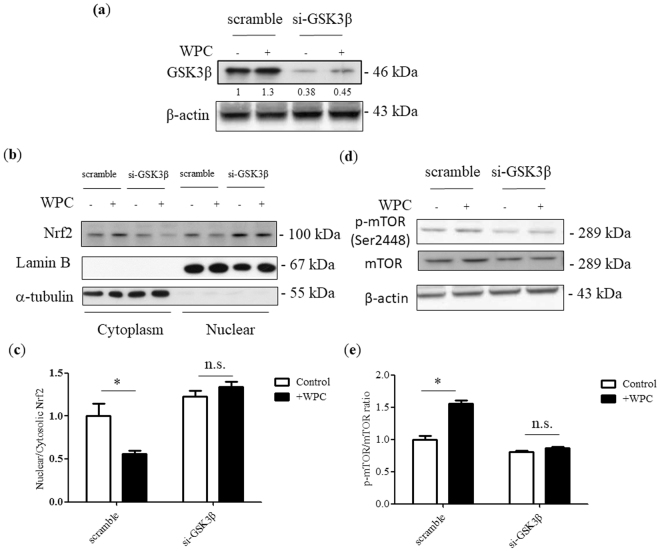
WPC reduces Nrf2 nuclear accumulation and induces mTORC1 activation via GSK3β activity
To determine the mechanism underlying the WPC-mediated reduction in Nrf2 nuclear accumulation, we downregulated GSK3β protein expression using siRNA specific to the corresponding mRNA. Cells were transfected with non-specific (scrambled) siRNA or GSK3β siRNA, and were then treated with WPC. Protein expression was then detected by immunoblotting (Fig. 7a). GSK3β was inhibited, as expected, and this was found to result in increased nuclear Nrf2, compared to cytosolic levels (Fig. 7b,c). Moreover, cells treated with GSK3β siRNA exhibited a reduced p-mTOR/mTOR ratio in response to WPC treatment (Fig. 7d,e).
Figure 7.
Whey protein concentrate (WPC)-induced reduction of Nrf2 nuclear accumulation and mTORC1 activation are disrupted by GSK3β siRNA. MDA-MB-231 cells were transfected with non-specific siRNA or GSK3β-targeting siRNA and treated for 48 hours with 10 mg/mL WPC. (a) Representative western blots showing GSK3β protein levels. β-actin was used as a loading control. (b) Representative western blots showing cytosolic and nuclear levels of Nrf2. Lamin B and α-tubulin were used as loading controls for nuclear and cytosolic fractions, respectively. (c) Bar graphs showing relative nuclear/cytosolic Nrf2 ratios. (d) Representative western blots demonstrating levels of p-mTOR (Ser2448) and mTOR. β-actin was used as a loading control. (e) Bar graphs displaying relative p-mTOR/mTOR ratios. Data represent the means ± SD from three experiments. *P < 0.05; n.s.: not significant.
Discussion
Although many studies have investigated molecular markers such as VEGF21, EGFR22, and mTOR23 as drug targets for TNBC, a greater understanding of the molecular basis of this disease is required for the development of effective treatments. In the present study, we demonstrated that WPC treatment renders MDA-MB-231 cells susceptible to rapamycin by (i) altering the cellular redox state and (ii) activating GSK3β/mTORC1 signaling.
Basal-like TNBC cells were reported to be highly sensitive to the mTOR inhibitor everolimus, whereas a subgroup of TNBC cells, comprising lines characterized as stem-like cancer cells, are less sensitive to this drug24. Given that the cellular redox state affects the regulation of signal transduction pathways and the development of drug resistance, this could be used to dictate therapeutic strategies for TNBC25. Many studies have shown that redox mechanisms regulate mTORC1 activity26. For example, Sarbassov et al. demonstrated that cysteine oxidants such as phenylarsine oxide and diamide activate mTORC1 in vivo and in vitro 27.
In the current work, we first observed that GSH and ROS levels were elevated in MDA-MB-231 cells compared to those in non-tumorigenic MCF-10A cells. Our previous results indicated an imbalance in GSH redox status in breast cancer patients. The high GSH levels observed in breast cancer tissues might be associated with enhanced cell proliferation and resistance to oxidative stress, representing a selective growth advantage for tumor cells over their normal counterparts28. In addition, Panis et al. reported that compared to adjacent normal breast tissue, tumors are sites of elevated oxidative and nitrosative stress, with concomitant augmented antioxidant capacity29.
WPC constitutes a rich source of bioavailable cysteine that can be used for GSH synthesis, and contains all nine essential amino acids. It is used to promote muscle protein synthesis and as a nutritional supplement for chemotherapy patients30. The PTEN-positive cell line MDA-MB-231 has been reported to be resistant to mTOR inhibition; however, we observed here that WPC treatment rendered these cells sensitive to rapamycin. Furthermore, WPC selectively altered GSH levels, affecting those in MDA-MB-231 cells, but not those in non-tumorigenic MCF-10A cells. This might be explained by the fact that GSH synthesis is regulated by negative feedback. Since GSH levels are much higher in tumor cells than in normal cells, it is easier to reach the threshold at which GSH synthesis is inhibited by negative feedback in the former than in the latter31,32. In addition, we found that treatment with 1 μM rapamycin, with or without WPC, induces ROS production in MDA-MB-231 cells. The relationship between rapamycin and ROS remains disputed, with some studies suggesting that rapamycin reduces cellular ROS levels33 and others claiming the opposite34. Such discrepancies might be explained by the important effect of rapamycin concentration35,36. In addition, there were no obvious changes in the viability of cells with WPC treatment. It is notable that in the present work, WPC selectively affected GSH levels but did not change cellular ROS content and viability, implying that a synergistic antitumor effect might be achieved by decreasing GSH levels in tandem with WPC treatment37.
Nrf2 regulates the inducible antioxidant program in response to cellular stress38. Here, we revealed that rapamycin treatment induces the nuclear accumulation of Nrf2, consistent with the observations of other researchers. It has also been reported that TORC1 inhibition by rapamycin increases transcription of the Nrf2 gene39,40. Notably, combining rapamycin with WPC negated the promotion of Nrf2 nuclear accumulation by the rapamycin treatment in the present study. Moreover, we observed reduced expression of GCLC and GR proteins when cells were treated with WPC. Consistent with the observations of our previous in vivo study, we found that WPC supplementation reduced the nuclear accumulation of Nrf2 in tumor tissue16. Kerasioti et al. indicated that whey protein exerts its antioxidant activity through an Nrf2-dependent mechanism in endothelial cells; however, it was cell type dependent18.
It has been suggested that p-p70S6Klow/p-4E-BP1high cancer cells might be intrinsically resistant to mTORC1 inhibitors41. Of note, we found here that WPC treatment reversed this phenotype by enhancing phosphorylation of mTOR (Ser2448), p70S6K (Thr389), and 4E-BP1. Furthermore, we revealed that WPC upregulated GSK3β expression, consistent with a study by Stretton et al., in which GSK3β activity was shown to be required for the support of mTORC1 signaling in response to nutrient availability4. It has also been recognized that amino acid treatment promotes mTORC1 activation42,43. Our investigation suggests a combined therapy that might be beneficial for the subgroup of TNBC patients with tumors resistant to mTOR inhibitors.
Finally, in the present study, we inhibited GSK3β activity using siRNA, the results of which suggested that GSK3β is required for the reduction in Nrf2 nuclear accumulation mediated by WPC treatment. This is consistent with a report that activation and direct inhibition of GSK3β leads to nuclear exclusion and accumulation of Nrf2, respectively44. In addition, GSK3β was found to be associated with WPC-induced mTORC1 activation. Taken together, our results indicate that WPC leads to the depletion of GSH by reducing Nrf2 nuclear accumulation and activating GSK3β/mTOR signaling, rendering MDA-MB-231 cells susceptible to rapamycin.
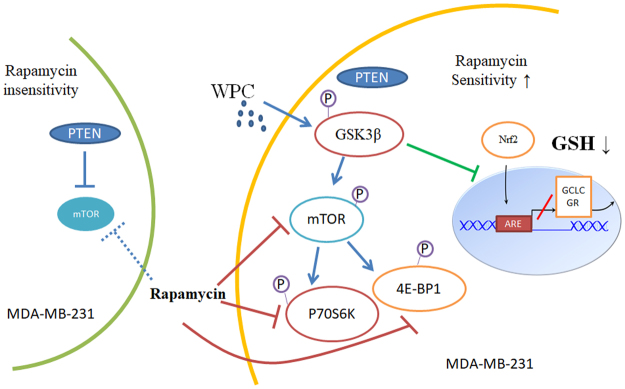
In conclusion, we explored the result of combining antioxidant and mTOR inhibitory effects, and clarified the underlying mechanism for the first time (Fig. 8). Our findings might serve as a reference for the development of such combination therapies for the treatment of breast cancer, and particularly TNBC.
Figure 8.
A working model of the mechanism through which whey protein concentrate (WPC) renders MDA-MB-231 cells sensitive to rapamycin. MDA-MB-231 cells express wild-type PTEN and are insensitive to rapamycin. WPC treatment activates mTOR/GSK3β signaling, involving increased p-p70S6K and p-4E-BP1 levels. In addition, activation of GSK3β inhibits translocation of Nrf2 from the cytoplasm to the nucleus, altering the cellular redox state by downregulating the glutamate-cysteine ligase catalytic subunit (GCLC) and glutathione reductase (GR), and finally reducing glutathione (GSH) levels. Taking together, WPC treatment thus induces rapamycin sensitivity in MDA-MB-231 cells.
Materials and Methods
Reagents and antibodies
The WPC (Immunocal, Immunotec Research Ltd., Quebec, Canada) contained about 90% whey protein isolate, < 1.5% lactose, < 0.5% fat, and <5.0% moisture, with a solubility index of 99% at pH 4.6. WPC was freshly prepared before each experiment. The rapamycin and dimethylsulfoxide (DMSO) were purchased from Sigma-Aldrich (St. Louis, MO, USA). DMSO used as a solvent for rapamycin and control cells, which was not beyond 0.1%. The antibodies used in this experiment included: GCLC (abcam, ab53179), GR (abcam, ab16801), Nrf2 (Cell Signaling Technology (CST), #12721), mTOR (CST, #2972), p-mTOR (Ser 2448) (CST, #2971), p70S6K (CST, #9202), p-p70S6K (Thr389) (CST, #9205), 4E-BP1 (CST, #9644), p-4E-BP1 (Thr 37/46) (CST, #2855), GSK3β (CST, #9332), p-GSK3β (Ser9) (CST, #9336), β-actin (Sigma, A5441), α-tubulin (Santa Cruz Biotechnology, sc-8035), and Lamin B (sc-6216).
Cell culture
The TNBC cell lines, MDA-MB-231 and MDA-MB-468 were obtained from American Type Culture Collection (Manassas, VA, USA). The nonmalignant MCF-10A breast epithelial cells were a kind gift from Taipei Medical University Hospital. MDA-MB-231 and MDA-MB-468 cells were cultured in DMEM supplemented with 10% fetal bovine serum (FBS), and MCF-10A cells were maintained in DMEM/F-12 medium supplemented with 5% horse serum, 0.5 μg/mL hydrocortisone, 100 ng/ml cholera toxin, 10 μg/mL insulin, and 20 ng/ml recombinant human EGF. All cells were cultured in a humidified incubator containing 5% CO2 at 37 °C.
Preparation of WPC
Preparation of WPC was the same as in our previous study15. We followed the instructions of Immunocal to design the concentrations of WPC and WPC was freshly prepared before each experiment. Briefly, 50 mg of WPC was dissolved in a fresh 5 mL serum-free medium and centrifuged at 12,000 × g for 10 minutes at room temperature. The supernatant was filtered with a 0.2 μm filter. Therefore, concentrations of 10 mg/mL of WPC were used in this experiment.
Determination of GSH
The 100 μL cell suspension, as indicated above, in an amber microcentrifuge tube was mixed with 0.1% MPA in a 1:2 volumetric ratio, and then it was centrifuged at 12,000 × g for 10 minutes after being adequately mixed by a vortex mixer. The supernatant was assayed on a capillary electrophoresis analyzer (P/ACE MDQ, Beckman Coulter) after being filtered with a 0.2 μm syringe set. The analysis was performed at a constant temperature (28 °C) with 300 mM borate running buffer (pH 7.8) and a UV absorbance detector set to 200 nm.
Flow cytometric analysis of ROS levels
The intracellular ROS levels were assessed by flow cytometry using DCFH-DA. To assess the ROS levels, the cells were resuspended in PBS at 1,000,000 cells per mL and incubated in the presence of DCFH-DA (10 μM) in the dark at 37 °C for 30 minutes. Then, the cells were washed and resuspended in PBS. Finally, submitted the sample into flow cytometric analysis using a FACScan flow cytometer (Coulter EPICS XL Flow Cytometer, Beckman Coulter). Analyses were performed on 10,000 cells per sample and fluorescence intensities were measured on a logarithmic scale of four decades of log of fluorescence. Each experiment was repeated at least three times.
Cell viability assays
Cell viability was assessed by 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) dye conversion at 570 nm following the manufacturer’s instructions. Briefly, cells were seeded 5,000 per well in a 96-well flat bottom plate and grown for 24 hours. Cells were then treated for 48 hours or 72 hours with indicated concentrations of rapamycin and WPC in the presence of 10% FBS. 20 μl of MTT (5 mg/mL in PBS) was then added to each well and incubated 2 hours at 37 °C. The formazan in viable cells were dissolved with 100 μl of dimethyl sulfoxide and determined by reading optical densities in a microplate reader (Dynatech Laboratories, Chantilly, VA, USA).
Colonies formation assay
MDA-MB-231 cells were seeded in 6-well plate at a density of 1,500 cells per well duplicate. These cells were cultured with respective treatment including WPC (2 mg/mL), rapamycin (200 nM), and their combination. The medium was replaced every 2–3 days until day 14. Colonies were stained with 0.2% (w/v) crystal violet in buffered formalin for 10 minutes. The number of colonies was counted.
Preparation of cytosolic and nuclear extracts
Cytosolic and nuclear extracts were isolated according to Schreiber et al.45 with some modify cations. After rinsing with cold PBS, cells were resuspended in cold lysis buffer A (20 mM HEPES pH 8.0, 1 mM EDTA, 1.5 mM MgCl2, 10 mM KCl, 1 mM DTT, 1 mM sodium orthovanadate, 1 mM NaF, 1 mM PMSF, 0.5 mg/mL benzamidine, 0.1 mg/ml leupeptin, and 1.2 mg /mL aprotinin). They were incubated on ice for 15 min, after which 7.5 μL of 10% NP-40 detergent was added. Afterwards the cells were intensively stirred in a vortex mixer for 10 s. The homogenate was centrifuged at 15,000 × g for 50 seconds, and the supernatant was used as the cytosolic extract. The nuclear pellet was resuspended in cold extraction buffer B (20 mM HEPES pH 8.0), 1 mM EDTA, 1.5 mM MgCl2, 10 mM KCl, 1 mM DTT, 1 mM sodium orthovanadate, 1 mM NaF, 1 mM PMSF, 0.5 mg/mL benzamidine, 0.1 mg/mL leupeptin, 1.2 mg/mL aprotinin, and 20% glycerol). All protein fractions were stored at −70 °C until used.
Western blotting
Cells were seeded, cultured, and treated with WPC (10 mg/mL), rapamycin (1 μM), and their combination for 48 hours. The proteins were separated by SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membrane. The membranes were blocked with 5% milk for 1 hour and incubated with targeted primary antibodies overnight at 4 °C, and then incubated with HRP-conjugated secondary antibodies at room temperature for 1 hour. Finally, the signals were enhanced by ECL (Millipore, Temecula, CA, USA) and exposed to X-ray film for autoradiogram. The intensity of the bands was quantified by densitometry and the results were evaluated relative to the expression of the constitutively expressed protein β-actin.
Small interfering RNA (siRNA)
GSK-3β was silenced using siRNA (Santa Cruz Biotechnology, sc-35527) and a nonspecific scrambled siRNA (sc-37007) used as the negative control. Cells at 80% confluency were transfected with 2 μg of siRNA/well in 6-well plates using lipofectamine 2000 (Invitrogen). After they had been transfected, the cells were incubated for 18 hours in DMEM supplemented with 10% FBS at 37 °C, then treated with WPC or rapamycin for additional 48 hours.
Statistical analysis
Data were expressed as mean ± standard deviation (SD) from three experiments. P-values were determined by Student’s t-test or one-way analysis of variance (ANOVA), followed by the Bonferroni post-test. Statistics were calculated using GraphPad Prism 5.0 software. Values of P < 0.05 was considered statistically significant.
Electronic supplementary material
Acknowledgements
This work was supported by grant from the Ministry of Science and Technology, Taiwan (NSC 102-2320-B037-018), and Kaohsiung Veterans General Hospital Research Program (VGHKS104-55).
Author Contributions
Shih-Hsuan Cheng wrote the manuscript, researched the data and contributed to the design of the study. Yang-Ming Tseng and Shih-Meng Tsai researched the data and contributed to the discussion. Szu-Hsien Wu provided technical support. Li-Yu Tsai is the guarantor of this work. All the authors read and approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-14159-5.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shih-Meng Tsai, Email: tsaism@kmu.edu.tw.
Li-Yu Tsai, Email: tsliyu@kmu.edu.tw.
References
- 1.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363:1938–1948. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 2.Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell. 2010;40:310–322. doi: 10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azoulay-Alfaguter I, Elya R, Avrahami L, Katz A, Eldar-Finkelman H. Combined regulation of mTORC1 and lysosomal acidification by GSK-3 suppresses autophagy and contributes to cancer cell growth. Oncogene. 2015;34:4613–4623. doi: 10.1038/onc.2014.390. [DOI] [PubMed] [Google Scholar]
- 4.Stretton C, et al. GSK3-mediated raptor phosphorylation supports amino-acid-dependent mTORC1-directed signalling. Biochem J. 2015;470:207–221. doi: 10.1042/BJ20150404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liou GY, Storz P. Reactive oxygen species in cancer. Free Radic Res. 2010;44:479–496. doi: 10.3109/10715761003667554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Current biology: CB. 2014;24:R453–462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Q, Pi J, Woods CG, Andersen ME. A systems biology perspective on Nrf2-mediated antioxidant response. Toxicol Appl Pharmacol. 2010;244:84–97. doi: 10.1016/j.taap.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niture SK, Khatri R, Jaiswal AK. Regulation of Nrf2-an update. Free Radic Biol Med. 2014;66:36–44. doi: 10.1016/j.freeradbiomed.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Owonikoko, T. K. & Khuri, F. R. Targeting the PI3K/AKT/mTOR pathway: biomarkers of success and tribulation. American Society of Clinical Oncology educational book. American Society of Clinical Oncology. Meeting, 10.1200/EdBook_AM.2013.33.e395 (2013). [DOI] [PMC free article] [PubMed]
- 10.Jaramillo MC, Zhang DD. The emerging role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev. 2013;27:2179–2191. doi: 10.1101/gad.225680.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paplomata E, O’Regan R. The PI3K/AKT/mTOR pathway in breast cancer: targets, trials and biomarkers. Therapeutic advances in medical oncology. 2014;6:154–166. doi: 10.1177/1758834014530023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis NM, et al. Deregulation of the EGFR/PI3K/PTEN/Akt/mTORC1 pathway in breast cancer: possibilities for therapeutic intervention. Oncotarget. 2014;5:4603–4650. doi: 10.18632/oncotarget.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang WL, et al. [Molecular mechanisms of resistance to phosphatidyl inositol 3-kinase inhibitors in triple-negative breast cancer cells] Zhonghua zhong liu za zhi [Chinese journal of oncology] 2016;38:578–588. doi: 10.3760/cma.j.issn.0253-3766.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Tseng YM, et al. Effects of whey protein concentrate (WPC) on the distributions of lymphocyte subpopulations in rats with excessive alcohol intake. J Agric Food Chem. 2010;58:12729–12734. doi: 10.1021/jf103518u. [DOI] [PubMed] [Google Scholar]
- 15.Tseng, Y. M. et al. Effects of alcohol-induced human peripheral blood mononuclear cell (PBMC) pretreated whey protein concentrate (WPC) on oxidative damage. J Agric Food Chem56, 8141–8147, 10.1021/jf801034k (2008). [DOI] [PubMed]
- 16.Cheng SH, Tseng YM, Wu SH, Tsai SM, Tsai LY. Selective effects of whey protein concentrate on glutathione levels and apoptosis in rats with mammary tumors. Food Chem Toxicol. 2017;107:440–448. doi: 10.1016/j.fct.2017.07.024. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell CJ, et al. Consumption of Milk Protein or Whey Protein Results in a Similar Increase in Muscle Protein Synthesis in Middle Aged Men. Nutrients. 2015;7:8685–8699. doi: 10.3390/nu7105420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kerasioti E, Stagos D, Tzimi A, Kouretas D. Increase in antioxidant activity by sheep/goat whey protein through nuclear factor-like 2 (Nrf2) is cell type dependent. Food Chem Toxicol. 2016;97:47–56. doi: 10.1016/j.fct.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 19.Lehmann BD, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noh WC, et al. Determinants of rapamycin sensitivity in breast cancer cells. Clin Cancer Res. 2004;10:1013–1023. doi: 10.1158/1078-0432.CCR-03-0043. [DOI] [PubMed] [Google Scholar]
- 21.Burstein HJ, et al. Phase II study of sunitinib malate, an oral multitargeted tyrosine kinase inhibitor, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2008;26:1810–1816. doi: 10.1200/JCO.2007.14.5375. [DOI] [PubMed] [Google Scholar]
- 22.Manupati K, et al. Inhibiting Epidermal Growth Factor Receptor Signalling Potentiates Mesenchymal-Epithelial Transition of Breast Cancer Stem Cells and their Responsiveness to Anticancer Drugs. Febs j. 2017 doi: 10.1111/febs.14084. [DOI] [PubMed] [Google Scholar]
- 23.Dey, N., De, P. & Leyland-Jones, B. PI3K-AKT-mTOR inhibitors in breast cancers: From tumor cell signaling to clinical trials. Pharmacology & therapeutics, 10.1016/j.pharmthera.2017.02.037 (2017). [DOI] [PubMed]
- 24.Miller TW, Balko JM, Arteaga CL. Phosphatidylinositol 3-kinase and antiestrogen resistance in breast cancer. J Clin Oncol. 2011;29:4452–4461. doi: 10.1200/JCO.2010.34.4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim D, Koo JS, Lee S. Overexpression of reactive oxygen species scavenger enzymes is associated with a good prognosis in triple-negative breast cancer. Oncology. 2015;88:9–17. doi: 10.1159/000358365. [DOI] [PubMed] [Google Scholar]
- 26.Yoshida S, et al. Redox regulates mammalian target of rapamycin complex 1 (mTORC1) activity by modulating the TSC1/TSC2-Rheb GTPase pathway. J Biol Chem. 2011;286:32651–32660. doi: 10.1074/jbc.M111.238014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sarbassov DD, Sabatini DM. Redox regulation of the nutrient-sensitive raptor-mTOR pathway and complex. J Biol Chem. 2005;280:39505–39509. doi: 10.1074/jbc.M506096200. [DOI] [PubMed] [Google Scholar]
- 28.Yeh CC, et al. A study of glutathione status in the blood and tissues of patients with breast cancer. Cell Biochem Funct. 2006;24:555–559. doi: 10.1002/cbf.1275. [DOI] [PubMed] [Google Scholar]
- 29.Panis C, et al. Can Breast Tumors Affect the Oxidative Status of the Surrounding Environment? A Comparative Analysis among Cancerous Breast, Mammary Adjacent Tissue, andPlasma. Oxidative medicine and cellular longevity. 2015;2015:6429812. doi: 10.1155/2016/6429812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marshall K. Therapeutic applications of whey protein. Alternative medicine review: a journal of clinical therapeutic. 2004;9:136–156. [PubMed] [Google Scholar]
- 31.Bounous G. Whey protein concentrate (WPC) and glutathione modulation in cancer treatment. Anticancer Res. 2000;20:4785–4792. [PubMed] [Google Scholar]
- 32.Huang CS, Chang LS, Anderson ME, Meister A. Catalytic and regulatory properties of the heavy subunit of rat kidney gamma-glutamylcysteine synthetase. J Biol Chem. 1993;268:19675–19680. [PubMed] [Google Scholar]
- 33.Suzuki M, Endo M, Shinohara F, Echigo S, Rikiishi H. Rapamycin suppresses ROS-dependent apoptosis caused by selenomethionine in A549 lung carcinoma cells. Cancer Chemother Pharmacol. 2011;67:1129–1136. doi: 10.1007/s00280-010-1417-7. [DOI] [PubMed] [Google Scholar]
- 34.Chen W, et al. Synergistic antitumor activity of rapamycin and EF24 via increasing ROS for the treatment of gastric cancer. Redox biology. 2016;10:78–89. doi: 10.1016/j.redox.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yellen P, et al. High-dose rapamycin induces apoptosis in human cancer cells by dissociating mTOR complex 1 and suppressing phosphorylation of 4E-BP1. Cell Cycle. 2011;10:3948–3956. doi: 10.4161/cc.10.22.18124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miwa S, et al. Low abundance of the matrix arm of complex I in mitochondria predicts longevity in mice. Nature communications. 2014;5:3837. doi: 10.1038/ncomms4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Glasauer A, Chandel NS. Targeting antioxidants for cancer therapy. Biochem Pharmacol. 2014;92:90–101. doi: 10.1016/j.bcp.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 38.DeNicola GM, et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475:106–109. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robida-Stubbs S, et al. TOR signaling and rapamycin influence longevity by regulating SKN-1/Nrf and DAF-16/FoxO. Cell metabolism. 2012;15:713–724. doi: 10.1016/j.cmet.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Calap-Quintana P, et al. TORC1 Inhibition by Rapamycin Promotes Antioxidant Defences in a Drosophila Model of Friedreich’s Ataxia. PLoS One. 2015;10:e0132376. doi: 10.1371/journal.pone.0132376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Faes, S., Demartines, N. & Dormond, O. Resistance to mTORC1 Inhibitors in Cancer Therapy: From Kinase Mutations to Intratumoral Heterogeneity of Kinase Activity. 2017, 1726078, 10.1155/2017/1726078 (2017). [DOI] [PMC free article] [PubMed]
- 42.Sancak Y, et al. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141:290–303. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sancak Y, et al. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320:1496–1501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rojo AI, Sagarra MR, Cuadrado A. GSK-3beta down-regulates the transcription factor Nrf2 after oxidant damage: relevance to exposure of neuronal cells to oxidative stress. J Neurochem. 2008;105:192–202. doi: 10.1111/j.1471-4159.2007.05124.x. [DOI] [PubMed] [Google Scholar]
- 45.Schreiber E, Matthias P, Muller MM, Schaffner W. Rapid detection of octamer binding proteins with ‘mini-extracts’, prepared from a small number of cells. Nucleic acids research. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.