Abstract
Two embedded sulfur atoms in a novel [2 + 2] Schiff-base macrocyclic dinuclear Zn(II) complex were found to be easily autoxidized to the sulfone units on air exposure, and the resultant sulfone-functionalized macrocyclic complex was obtained by the post-modification strategy exhibiting enhanced antimicrobial activities because of the presence of dual active sites in comparison with the sulfur-containing Schiff-base macrocycle.
Introduction
Macrocyclic molecules have attracted considerable and increasing attention because of the mainstay role they play in supramolecular chemistry1–5, biochemistry6–8, material science9,10, separation11,12, encapsulation13–15 and catalysis16,17. Among them, Schiff-base macrocycles occupy a special status owing to their facile syntheses from the Schiff-base condensation and versatile coordination fashions with metal ions. On the other hand, heteroatom-functionalized macrocycles also represent one challenging research topic due to the difficulties in tuning the ring size, configuration and binding modes18–21. The incorporation of heteroatoms into Schiff-base macrocycles is often achieved by using easily accessible heteroatom-containing diamines and triamines, such as diethylenetriamine, 1,2-bis(2-aminoethoxy)ethane and tris(2-aminoethyl)amine22–25. In contrast, the attempts to design and synthesize extended dialdehydes are believed as another impressive method to yield heteroatom-functionalized Schiff-base macrocycles22,26–30.
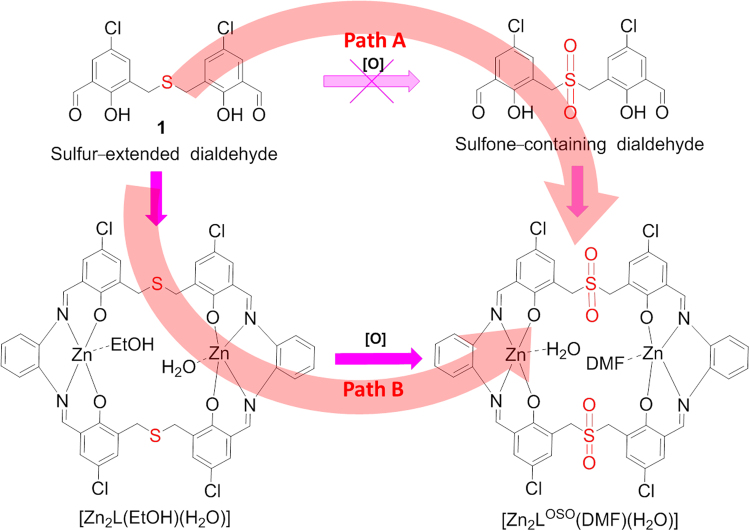
However, sulfur-bridged Schiff-base macrocycles especially the flexible ones are rarely reported up till now31–38. It is known that thiacalixarenes are an important subclass of calixarenes and they can be readily oxidized into the forms of sulfoxides and even sulfones by post-oxidation process displaying improved properties than corresponding thiacalixarenes39–41. This post-modification is regarded as a powerful synthetic strategy for finely tuning functionalities of resultant products. In this work, we intend to introduce the sulfur atoms into the Schiff-base macrocyclic skeletons and implant the post-modification synthetic strategy of thiacalixarenes to generate the sulfur-containing Schiff-base macrocycles and corresponding oxidized products. Thus, a new sulfur-extended dialdehyde 1 (Fig. 1) was prepared via the nucleophilic substitution between 5-chloro-3-(chloromethyl)-2-hydroxybenzaldehyde and sodium sulfide and a [2 + 2] sulfur-bridged Schiff-base dinuclear macrocyclic Zn(II) complex [Zn2L]42 was produced by the template-assisted synthesis. Furthermore, The sulfone-functionalized [2 + 2] Schiff-base macrocyclic Zn(II) complex [Zn2LOSO] was obtained successfully via post-oxidation of [Zn2L].
Figure 1.
Synthetic illustration of macrocyclic Zn(II) complexes.
Results and Discussion
Different from thiacalixarenes having two oxidized forms, only one type of oxidized product [Zn2LOSO] could be detected by ESI-MS (Figure S1) and isolated in a high yield of 67.8% from the H2O2 oxidation of [Zn2L]. The difference may be ascribed to the presence of aliphatic sulfur ether moieties and coordinated metal ions in [Zn2L] compared with the metal free aromatic sulphide in thiacalixarenes. Actually, the air was found to be adequate in this oxidized process because [Zn2LOSO] was first obtained as crystals in the process of growing single crystals under ambient condition. However, H2O2 was used in a control experiment to accelerate the oxidation process and H2O2 was proved to be a more effective reagent to produce [Zn2LOSO]. The good consistency of the sulfone-product obtained from air and H2O2 oxidation was verified by corresponding 1H NMR spectra. (Figure S2). In addition, we intended to prepare [Zn2LOSO] through the direct Schiff-base condensation between sulfone-containing dialdehyde and 1,2-diaminobenzene (Path A in Scheme 1), but sulfur-extended dialdehyde 1 showed no selectivity under H2O2 oxidation and sulfone-containing dialdehyde could not be isolated (Figures S3 and S4). The reason is that aldehyde, phenolic hydroxyl and sulfur units in compound 1 are all sensitive to the oxidant. That is to say, it is invalid to access the targeting [Zn2LOSO] via Path A. In contrast, only path B is available for preparing macrocycle [Zn2LOSO] where the two uncoordinated sulfur atoms are selectively oxidized to sulfone groups, because the easily oxidized aldehyde groups in 1 have been transformed into the imine connected macrocycle and stabilized by the following Zn(II) ion complexation in [Zn2L]. In addition, another oxygen-unstable phenolic hydroxyl groups have been deactivated via electron delocalization originated from the formation of coordinative bonds with Zn(II) ions. So it is concluded that the post-modification synthetic strategy is particularly useful in preparing sulfone-functionalized Schiff-base macrocycle in our case.
FT-IR, ESI-MS and 1H NMR spectra were performed to verify the two types of macrocyclic complexes. A medium FT-IR absorption peak was observed at 1649 cm−1 in the dialdehyde precursor, indicative of the presence of aldehyde groups (Figure S5). However, a new absorption peak was found in the sulfur-bridged macrocyclic complex (1615 cm−1) indicating the transformation from the aldehyde groups to Schiff-base C=N units (Figure S6). ESI-MS of [Zn2L] revealed one positive peak at m/z = 1075.00 corresponding to {[Zn2L] + EtOH + H2O + H}+ (Figure S7). The successful generation of sulfone-based complex was evidenced by the observation of a new positive peak at m/z = 1121.25 in [Zn2LOSO] assigned as {[Zn2LOSO] + EtOH + H}+ specie (Figure S1) by contrast. Furthermore, 1H NMR spectral comparisons were carried out to verify the changes in chemical shifts between the sulfur and sulfone bridged macrocyclic complexes. As depicted in Fig. 2a, the chemical shift of methylene protons (δ = 3.92 ppm) adjacent to the sulfur atoms in [Zn2L] is significantly shifted to the lower field (δ = 4.64 ppm) in [Zn2LOSO], because of the stronger deshielding effects of sulfone groups. In addition, the FT-IR spectrum of sulfone-functionalized macrocyclic complex manifests the disappearance of aldehyde groups and the emergence of imine moieties (Figure S8).
Figure 2.
(a) 1H NMR spectral comparisons of dried macrocyclic Zn(II) complexes [Zn2L] and [Zn2LOSO] in DMSO-d 6; (b) ORTEP drawing of [Zn2LOSO(DMF)(H2O)] at the 50% probability level; (c) View of the dihedral angle between the two N2O2 Salen coordination planes in [Zn2LOSO(DMF)(H2O)], where the apical ligands are omitted for clarity.
X-ray single-crystal diffraction analysis of the sulfur-bridged dialdehyde precursor reveals a folded structure in which the two phenolic rings have a dihedral angle of 15.2(1)°. Intramolecular π-π stacking interactions are present between them with the centroid-to-centroid separation of 3.675(2) Å. Furthermore, intermolecular π-π stacking interactions are found between neighboring molecules with the longer centroid-to-centroid distance of 3.708(2) Å forming an infinite supramolecular chain (Figure S9). In comparison with dialdehyde precursor 1, the two phenolic rings are expanded in macrocycle [Zn2LOSO(DMF)(H2O)] accompanied by the destruction of intramolecular π-π stacking. The tetra Schiff-base macrocyclic complex [Zn2LOSO(DMF)(H2O)] has a [2 + 2] dinuclear structure (Fig. 2b), and the two Zn(II) centers have similar square-pyramidal coordination geometry with slightly different τ values of 0.043 and 0.064, respectively43. The basal plane is composed of two imine nitrogen atoms and two phenolic oxygen atoms, and the apical position for each is occupied by a water molecule and a N,N-dimethylformamide molecule, respectively. The related Zn-O and Zn-N bond lengths fall within the normal range of 1.944(3) ~ 2.092(4) Å. Different from the conventional µ 2 bridging mode for each phenolic oxygen atom, the two N2O2 Salen coordination planes in our 30-membered macrocyclic Zn(II) complex are essentially perpendicular with a bite angle of 89.8(3)° (Fig. 2c), and the distance between two Zn(II) centers is measured as 6.977(3) Å.
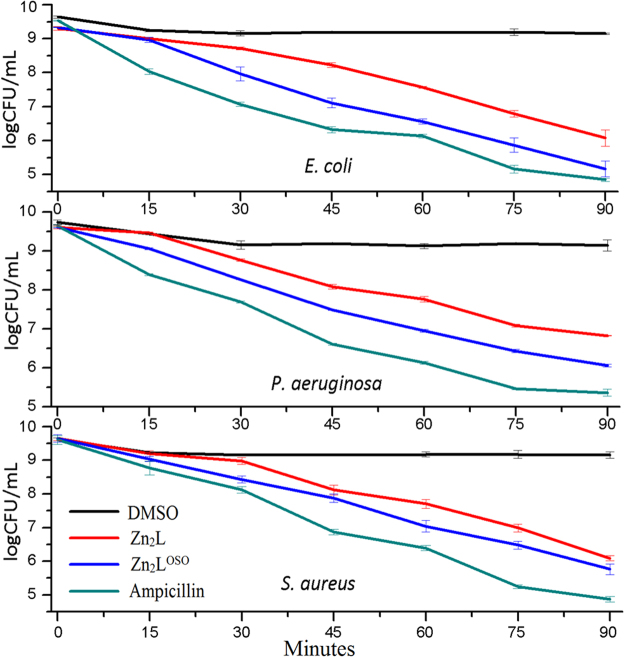
Considering sulfones and Schiff-base macrocycles are both antibacterially active44–48, enhanced antibacterial activities are expected for our dual-functional macrocyclic complex [Zn2LOSO]. In the following work, antimicrobial experiments of [Zn2LOSO] have been carried out for E. coli (ATCC 2567), P. aeruginosa (ATCC 2036) and S. aureus (ATCC 2079) together with [Zn2L] for comparison. The preliminary antimicrobial activities are assessed by determining their MIC (minimum inhibitory concentration) and MBC (minimum bactericidal concentration) values. As can be seen in Table 1, both [Zn2L] and [Zn2LOSO] show significantly lower MIC and MBC values than dialdehyde 1. Compared to the reference compound ampicillin, the results of two macrocyclic complexes are lower, but undoubted improvement of MIC and MBC values can be observed from [Zn2L] to [Zn2LOSO]. Moreover, the enhanced antimicrobial activities could be confirmed through a time-kill assay over 1.5 h, together with ampicillin as a standard and DMSO as the blank. In contrast to ampicillin, both sulfone-functionalized and sulfur-bridged macrocycles exhibit less effective antimicrobial activities against all the three bacterial strains along with time prolonged, but the former displays more obvious decrease of logCFU/mL than the latter (Fig. 3). It is suggested that the enhanced antimicrobial activities from macrocycle [Zn2L] to [Zn2LOSO] could be ascribed to the incorporation of two bioactive sites into one molecule in our case.
Table 1.
Antibacterial activities of the related compounds against three kinds of bacteria. The antibacterial activities are expressed as the MIC and MBC. The data in the first line for every compound (green) represent MIC and those in the second line (purple) stand for MBC. The unit of all value is mg/mL.
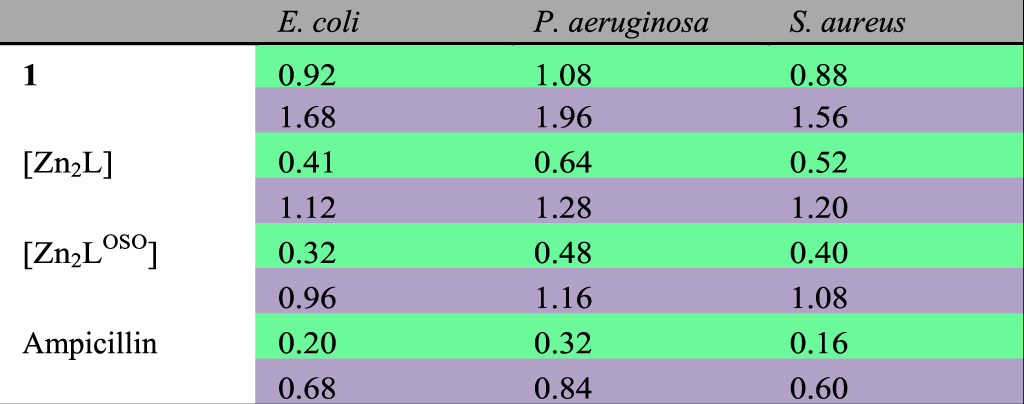
Figure 3.
Time-kill assay of three bacteria after the treatment with two macrocyclic complexes over 1.5 h.
Conclusions
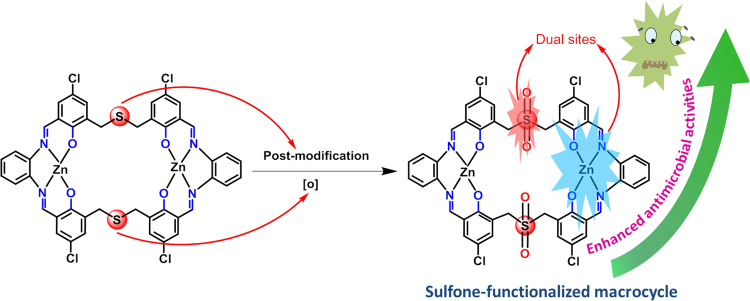
In summary, a novel [2 + 2] sulfur-bridged Schiff-base macrocyclic dinuclear Zn(II) complex was synthesized from a sulfur-extended dialdehyde via the Schiff-base condensation, and the sulfur atoms in the macrocyclic skeleton were found to be facilely in situ oxidized to the sulfone units even in ambient condition. It is noted that this effective post-modification from sulfur to sulfone after forming the macrocyclic complex is necessary because the phenolic hydroxyl and aldehyde units in the dialdehyde precursor are both sensitive to the oxidant. More interestingly, the sulfone-functionalized Schiff-base macrocyclic complex with dual bioactive sites displays enhanced antimicrobial activities in contrast to the undecorated macrocycle (Fig. 4). Considering the scarcity of flexible Schiff-base macrocyclic backbones with sulfur-containing bridges, the current study is believed to provide a practical approach to access multi-functional macrocyclic molecules by means of the post-modification strategy.
Figure 4.
Schematic illustration for the air-oxidation from sulfur to sulfone-bridged Schiff-base macrocyclic complexes with dual bioactive sites displaying enhanced antimicrobial activities.
Electronic supplementary material
Acknowledgements
This work was financially supported by the Major State Basic Research Development Programs (No. 2013CB922101), the Natural Science Foundation of Jiangsu Province (No. BK20171334), the National Natural Science Foundation of China (No. 21171088), and Program B for outstanding PhD candidate of Nanjing University.
Author Contributions
Genfeng Feng synthesized the complexes, performed the characterization and wrote the first draft of the manuscript. Yunshan Shi, Rongguang Shi and Ruiyong Wang contributed to antimicrobial experiments and analyzed the results. Lei Zhang contributed to the single crystal X-ray data collection. Wei Huang designed the experiments and corrected the manuscript. All authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-15898-1.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Wei Huang, Email: whuang@nju.edu.cn.
Ruiyong Wang, Email: wangry@nju.edu.cn.
References
- 1.Lee JW, et al. Cucurbituril Homologues and Derivatives: New Opportunities in Supramolecular Chemistry. Acc. Chem. Res. 2003;36:621–630. doi: 10.1021/ar020254k. [DOI] [PubMed] [Google Scholar]
- 2.Gokel GW, et al. Crown Ethers: Sensors for Ions and Molecular Scaffolds for Materials and Biological Models. Chem. Rev. 2004;104:2723–2750. doi: 10.1021/cr020080k. [DOI] [PubMed] [Google Scholar]
- 3.Xue M, et al. Pillararenes, A New Class of Macrocycles for Supramolecular Chemistry. Acc. Chem. Res. 2012;45:1294–1308. doi: 10.1021/ar2003418. [DOI] [PubMed] [Google Scholar]
- 4.Jie K, et al. Macrocyclic Amphiphiles. Chem. Soc. Rev. 2015;44:3568. doi: 10.1039/C4CS00390J. [DOI] [PubMed] [Google Scholar]
- 5.Chen G, Jiang M. Cyclodextrin-Based Inclusion Complexation Bridging Supramolecular Chemistry and Macromolecular Self Assembly. Chem. Soc. Rev. 2011;40:2254–2266. doi: 10.1039/c0cs00153h. [DOI] [PubMed] [Google Scholar]
- 6.Yang H, et al. Supramolecular Chemistry at Interfaces: Host–Guest Interactions for Fabricating Multifunctional Biointerfaces. Acc. Chem. Res. 2014;47:2106–2115. doi: 10.1021/ar500105t. [DOI] [PubMed] [Google Scholar]
- 7.Ogoshi T, Yamagishi T, Nakamoto Y. Pillar-Shaped Macrocyclic Hosts Pillar[n]arenes: New Key Players for Supramolecular Chemistry. Chem. Rev. 2016;116:7937–8002. doi: 10.1021/acs.chemrev.5b00765. [DOI] [PubMed] [Google Scholar]
- 8.Krężel A, Lisowski J. Enantioselective Cleavage of Supercoiled Plasmid DNA Catalyzed by Chiral Macrocyclic Lanthanide (III) Complexes. J. Inorg. Biochem. 2012;107:1–5. doi: 10.1016/j.jinorgbio.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 9.Descalzo AB, et al. The Supramolecular Chemistry of Organic–Inorganic Hybrid Materials. Angew. Chem., Int. Ed. 2006;45:5924–5948. doi: 10.1002/anie.200600734. [DOI] [PubMed] [Google Scholar]
- 10.Chen Q-Y, et al. Heterodinuclear Cryptates [EuML(dmf)](ClO4)2 (M = Ca, Cd, Ni, Zn): Tuning the Luminescence of Europium(III) through the Selection of the Second Metal Ion. Chem.-Eur. J. 2002;8:3984–3990. doi: 10.1002/1521-3765(20020902)8:17<3984::AID-CHEM3984>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 11.Ilisz I, Berkecz R, Peter A. HPLC separation of amino acid enantiomers and small peptides on macrocyclic antibiotic-based chiral stationary phases: A review. J. Sep. Sci. 2006;29:1305–1321. doi: 10.1002/jssc.200600046. [DOI] [PubMed] [Google Scholar]
- 12.Jyothi RK, Lee J-Y. The role of macrocyclic compounds in the extraction and possible separation of platinum and rhodium from chloride solutions. Sci. Rep. 2016;6:27668. doi: 10.1038/srep27668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frontera A. Encapsulation of anions: macrocyclic receptors based on metal coordination and anion–π interactions. Coord. Chem. Rev. 2013;257:1716–1727. doi: 10.1016/j.ccr.2013.01.032. [DOI] [Google Scholar]
- 14.Leeland JW, White FJ, Love JB. Encapsulation of a magnesium hydroxide cubane by a bowl-shaped polypyrrolic schiff base macrocycle. J. Am. Chem. Soc. 2011;133:7320–7323. doi: 10.1021/ja201630b. [DOI] [PubMed] [Google Scholar]
- 15.Koner AL, et al. Supramolecular encapsulation of benzimidazole-derived drugs by cucurbit[7]uril. Can. J. Chem. 2011;89:139–147. doi: 10.1139/V10-079. [DOI] [Google Scholar]
- 16.Raynal M, et al. Supramolecular Catalysis. Part 1: Noncovalent Interactions as a Tool for Building and Modifying Homogeneous Catalysts. Chem. Soc. Rev. 2014;43:1660–1733. doi: 10.1039/C3CS60027K. [DOI] [PubMed] [Google Scholar]
- 17.Raynal M, Ballester P, Vidal-Ferran A, van Leeuwen PWNM. Supramolecular catalysis. Part 2: artificial enzyme mimics. Chem. Soc. Rev. 2014;43:1734–1787. doi: 10.1039/C3CS60037H. [DOI] [PubMed] [Google Scholar]
- 18.Maes W, Dehaen W. Oxacalix[n](het)arenes. Chem. Soc. Rev. 2008;37:2393–2402. doi: 10.1039/b718356a. [DOI] [PubMed] [Google Scholar]
- 19.Wang, M.-X. Heterocalixaromatics, new generation macrocyclic host molecules in supramolecular chemistry. Chem. Commun. 4541–4551 (2008). [DOI] [PubMed]
- 20.Wang M-X. Nitrogen and oxygen bridged calixaromatics: synthesis, structure, functionalization, and molecular recognition. Acc. Chem. Res. 2012;45:182–195. doi: 10.1021/ar200108c. [DOI] [PubMed] [Google Scholar]
- 21.König, B. & Fonseca, M. H. Heteroatom-Bridged Calixarenes. Eur. J. Inorg. Chem. 2303–2310 (2000).
- 22.Vigato PA, Tamburini S, Bertolo L. The development of compartmental macrocyclic Schiff bases and related polyamine derivatives. Coord. Chem. Rev. 2007;251:1311–1492. doi: 10.1016/j.ccr.2006.11.016. [DOI] [Google Scholar]
- 23.Borisova NE, Reshetova MD, Ustynyuk YA. Metal-free methods in the synthesis of macrocyclic Schiff bases. Chem. Rev. 2007;107:46–79. doi: 10.1021/cr0683616. [DOI] [PubMed] [Google Scholar]
- 24.Radecka-Paryzek W, Patroniak V, Lisowski J. Metal Complexes of Polyaza and Polyoxaaza Schiff Base Macrocycles. Coord. Chem. Rev. 2005;249:2156–2175. doi: 10.1016/j.ccr.2005.02.021. [DOI] [Google Scholar]
- 25.Fontecha JB, Goetz S, McKee V. Di-, Tri-, and Tetracopper(II) Complexes of a Pseudocalixarene Macrocycle. Angew. Chem., Int. Ed. 2002;41:4553–4556. doi: 10.1002/1521-3773(20021202)41:23<4553::AID-ANIE4553>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 26.Maity NC, et al. Organic carbonates as solvents in macrocyclic Mn(III) salen catalyzed asymmetric epoxidation of non-functionalized olefins. J. Mol. Catal. A: Chem. 2013;366:380–389. doi: 10.1016/j.molcata.2012.10.021. [DOI] [Google Scholar]
- 27.Bera PK, et al. Macrocyclic Mn(III) salen complexes as recyclable catalyst for oxidative kinetic resolution of secondary alcohols. Appl. Catal. A: Gen. 2013;467:542–551. doi: 10.1016/j.apcata.2013.07.055. [DOI] [Google Scholar]
- 28.Zhang K, et al. Base-induced self-assembly for one-dimensional coordination polymers via chiral pendant-armed Schiff base mononuclear Pb(II) macrocycles. Inorg. Chem. 2014;53:7803–7805. doi: 10.1021/ic5008846. [DOI] [PubMed] [Google Scholar]
- 29.Zhang K, et al. Distinguishable Zn(II) and Pb(II) template effects on forming pendant-armed Schiff-base macrocyclic complexes including a remarkable Pb(II)–π macrocyclic complex. Dalton Trans. 2014;43:15351–15358. doi: 10.1039/C4DT01927J. [DOI] [PubMed] [Google Scholar]
- 30.Hu Y, et al. Construction of Chiral [4 + 4] and [2 + 2] Schiff-Base Macrocyclic Zinc(II) Complexes Influenced by Counterions and Pendant Arms. Inorg. Chem. 2016;55:8260–8262. doi: 10.1021/acs.inorgchem.6b01493. [DOI] [PubMed] [Google Scholar]
- 31.Lozan V, et al. Coordination chemistry of Robson-type polyamine-dithiophenolate macrocycles: Syntheses, structures and magnetic properties of dinuclear complexes of first-row transition metals. Coord. Chem. Rev. 2009;253:2244–2260. doi: 10.1016/j.ccr.2008.08.016. [DOI] [Google Scholar]
- 32.Vigato PA, Tamburini S. The challenge of cyclic and acyclic Schiff bases and related derivatives. Coord. Chem. Rev. 2004;248:1717–2128. doi: 10.1016/j.cct.2003.09.003. [DOI] [Google Scholar]
- 33.Popa RA, Silvestru A, Pop A. Silver(I) complexes of a new multidentate macrocyclic ligand with N/S/Se donor atoms. Polyhedron. 2016;110:197–202. doi: 10.1016/j.poly.2016.02.045. [DOI] [Google Scholar]
- 34.Shinoura M, et al. A Heterodinuclear CoIICuI Complex with Co(salen) in a Macrocyclic Framework. Oxygenation Studies in Comparison with Analogous CuIICuI and CoIIPbII Complexes. Inorg. Chem. 2000;39:4520–4526. doi: 10.1021/ic000305e. [DOI] [Google Scholar]
- 35.Granzhan A, et al. Macrocyclic DNA-Mismatch-Binding Ligands: Structural Determinants of Selectivity. Chem.-Eur. J. 2010;16:878–889. doi: 10.1002/chem.200901989. [DOI] [PubMed] [Google Scholar]
- 36.Comba, P. et al. Two different types of folding of isomeric macrocycles induced by metal ion co-ordination. J. Chem. Soc., Dalton Trans. 1691–1696 (1997).
- 37.Guerriero P, et al. Lanthanide complexes with compartmental schiff bases. Inorg. Chim. Acta. 1987;129:127–138. doi: 10.1016/S0020-1693(00)85915-0. [DOI] [Google Scholar]
- 38.Casellato U, et al. New acyclic and cyclie Schiff bases derived from 2, 6-diformyl-4-chlorophenol and their interaction with uranyl (Vl), copper (II) and nickel (Il) ions. Inorg. Chim. Acta. 1985;110:181–190. doi: 10.1016/S0020-1693(00)82305-1. [DOI] [Google Scholar]
- 39.Morohashi N, et al. Thiacalixarenes. Chem. Rev. 2006;106:5291–5316. doi: 10.1021/cr050565j. [DOI] [PubMed] [Google Scholar]
- 40.Lhoták, P. Chemistry of thiacalixarenes. Eur. J. Org. Chem. 1675–1692 (2004).
- 41.Kumar R, et al. Recent developments of thiacalixarene based molecular motifs. Chem. Soc. Rev. 2014;43:4824–4870. doi: 10.1039/c4cs00068d. [DOI] [PubMed] [Google Scholar]
- 42.L, Zn2L and Zn2LOSO represent Zn(II)-free Schiff-base macrocycle (hypothetical mode), sulfur-bridged and sulfone-functionalized dinuclear macrocyclic Zn(II) complexes.
- 43.Addison, A. W. et al. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen sulfur donor lignands - The crystal and molecular structure of aqua 1,7-bis(N-methylbenzimidazon-2′-yl)-2,6-dithiaheptane copper(II) perchlorate. J. Chem. Soc., Dalton Trans. 1349–1356 (1984).
- 44.Zhu YI, Stiller MJ. Dapsone and sulfones in dermatology: overview and update. J. Am. Acad. Dermatol. 2001;45:420–434. doi: 10.1067/mjd.2001.114733. [DOI] [PubMed] [Google Scholar]
- 45.Arulmurugan S, Kavitha HP, Venkatraman R. Biological activities of Schiff base and its complexes: a review. Rasayan J. Chem. 2010;3:385–410. [Google Scholar]
- 46.Kumar S, Dhar DN, Saxena PN. Applications of metal complexes of Schiff bases-A review. J. Sci. Ind. Res. 2009;68:181–187. [Google Scholar]
- 47.Sreedaran, S. et al. Synthesis, electrochemical, catalytic and antimicrobial activities of novel unsymmetrical macrocyclic dicompartmental binuclear nickel (II) complexes. Polyhedron27, 1867–1874 (2008).
- 48.Avaji PG, et al. Synthesis, spectral characterization, in-vitro microbiological evaluation and cytotoxic activities of novel macrocyclic bis hydrazone. Eur. J. Med. Chem. 2009;44:3552–3559. doi: 10.1016/j.ejmech.2009.03.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.