Abstract
Extensive preclinical studies have identified mammalian target of rapamycin (mTOR) activation as a frequent molecular signature underlying head and neck squamous cell carcinoma (HNSCC), including the distinct clinical subtype that is human papillomavirus (HPV) related, and have demonstrated the potential therapeutic utility of mTOR inhibitors in the treatment of these cancers. Numerous clinical studies have begun to evaluate this potential, however few have selected for and fewer have focused specifically on HPV-related disease. While HPV-positive (HPV+) HNSCC patients have a generally favorable prognosis, the overall number of patients who suffer failed treatment, recurrent disease, metastasis, and death is increasing due to the rapidly increasing incidence of HPV-related cancers. In this review, we discuss the rationale for proposing the adjuvant use of mTOR inhibition in the treatment of HPV+ HNSCC, highlighting the interplay of virally activated mTOR signaling, cellular metabolism, and the anti-tumor immune response.
Keywords: Head and neck oropharyngeal carcinoma, Human papillomavirus, Metabolism, mTOR, Rapamycin
Introduction
The sixth most common cancer in the world, head and neck squamous cell carcinoma (HNSCC) annually accounts for nearly 600,000 new cases worldwide and approximately 10,000 deaths in the United States alone.1, 2 Human papillomavirus (HPV)-related HNSCC accounts for up to 25% of all HNSCCs,3 and HPV type 16 (HPV-16) is now recognized as the driving etiologic factor for the majority of HNSCCs of the oropharynx in North America and Europe.4 In oropharyngeal squamous cell carcinoma (OPSCC), at least 60%–80% of the arising cancers are HPV-positive (HPV+).5 Despite their more advanced stage at presentation, HPV+ HNSCCs have a clear survival benefit over HPV-negative (HPV−).6, 7 Treatment is successful in approximately 80% of patients8 but often imparts significant comorbidities associated with eating and speaking, and approximately 10% of HPV+ HNSCC patients develop metastasis, typically culminating in incurable disease.9 Considering that the total numbers of HPV+ HNSCC have been increasing at a near epidemic rate,5 improved therapies that address where our standard-of-care treatments are failing are desperately needed.
HPV+ HNSCCs are universally induced by exposure to the same, molecularly defined oncogenic agents.10 Expression of the HPV oncogenes is required for malignant transformation by the virus. The E6 and E7 oncoproteins play a role in both initiation and progression of related cancers. Persistent expression is achieved via integration of the E6 and E7 oncogenes into the host cell genome, though episomal cancers have been described.11 The resulting gene products (oncoproteins) contribute to genomic instability and numerous other oncogenic mechanisms. The most well known oncogenic contributions of these oncoproteins are E6-mediated degradation of the tumor suppressor p53 and E7-mediated inactivation and proteasome-targeted degradation of retinoblastoma protein (Rb). However, E6 and E7 are also oncogenic due to effects on many other signaling pathways.1, 7, 10
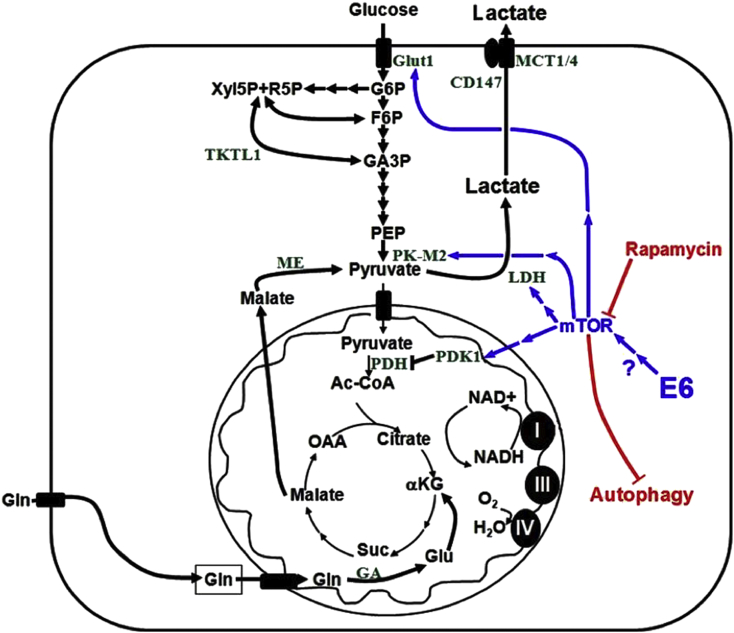
A key difference setting them apart from normal cells, the hypermetabolic nature of cancer cells is of great potential for therapeutic exploitation. HPV oncogenes play a role in conferring the metabolic phenotype of related HNSCC.1 The E6 oncoprotein, in addition to promoting degradation of p53, has been implicated in promoting a highly metabolic phenotype via activation of the mammalian target of rapamycin (mTOR) signaling pathway,12, 13, 14 a key regulator of metabolism (Fig. 1).15 This is consistent with studies showing that the mTOR signaling axis is activated in 80%–90% of HNSCCs, including those HPV-related.16, 17 A vast majority of p16 positive SCCs (a surrogate of HPV-positivity), both oral and cervical, display activated mTOR signaling, suggesting this activation is irrespective of the anatomical site of origin and likely virally-related.17 The mTOR signaling axis is a critical controller of factors that impact local recurrence and survival in HNSCC, including metabolism.16, 18, 19 Signaling through mTOR leads to accumulation of hypoxia-inducible factor 1 alpha (HIF1α) and subsequently pyruvate kinase (PK), lactate dehydrogenase (LDH), pyruvate dehydrogenase kinase 1 (PDK1), glucose transporter 1 (GLUT1), and other hypoxia response element regulated proteins (Fig. 1).20, 21 Upregulation of these proteins promotes a highly glycolytic phenotype: increased levels of glucose uptake, increased rate of glycolysis, and high levels of lactate production even in the presence of sufficient oxygen, known as the Warburg effect. The resultant lactate rich tumor microenvironment has been demonstrated to be inhibitory to immune cell functions,22, 23, 24 while an immune response induced by standard-of-care cisplatin/radiation therapy (CRT) has been demonstrated to be required for HPV+ HNSCC clearance.25, 26 Together with its regulation of numerous other tumor-promoting cellular path ways including growth, proliferation, survival signaling, and angiogenesis, mTOR's potential as a molecular target in HPV+ HNSCC is underscored.
Fig. 1.
HPV+ cancer cell metabolic scheme. Activation of mTOR upregulates numerous proteins involved in cellular metabolism, which together in a cancer cell promote the Warburg effect and excessive lactate production. The HPV-16 E6 oncoprotein has been implicated in activating mTOR, though the specific mechanism remains undefined. Not depicted, the E7 oncoprotein has also been described to contribute to this highly glycolytic phenotype by blocking entrance into the TCA cycle through inhibition of the terminal glycolytic enzyme, pyruvate kinase, specifically the embryonic M2 splice variant (PK-M2) which reemerges as the dominant isoform in many cancers.15
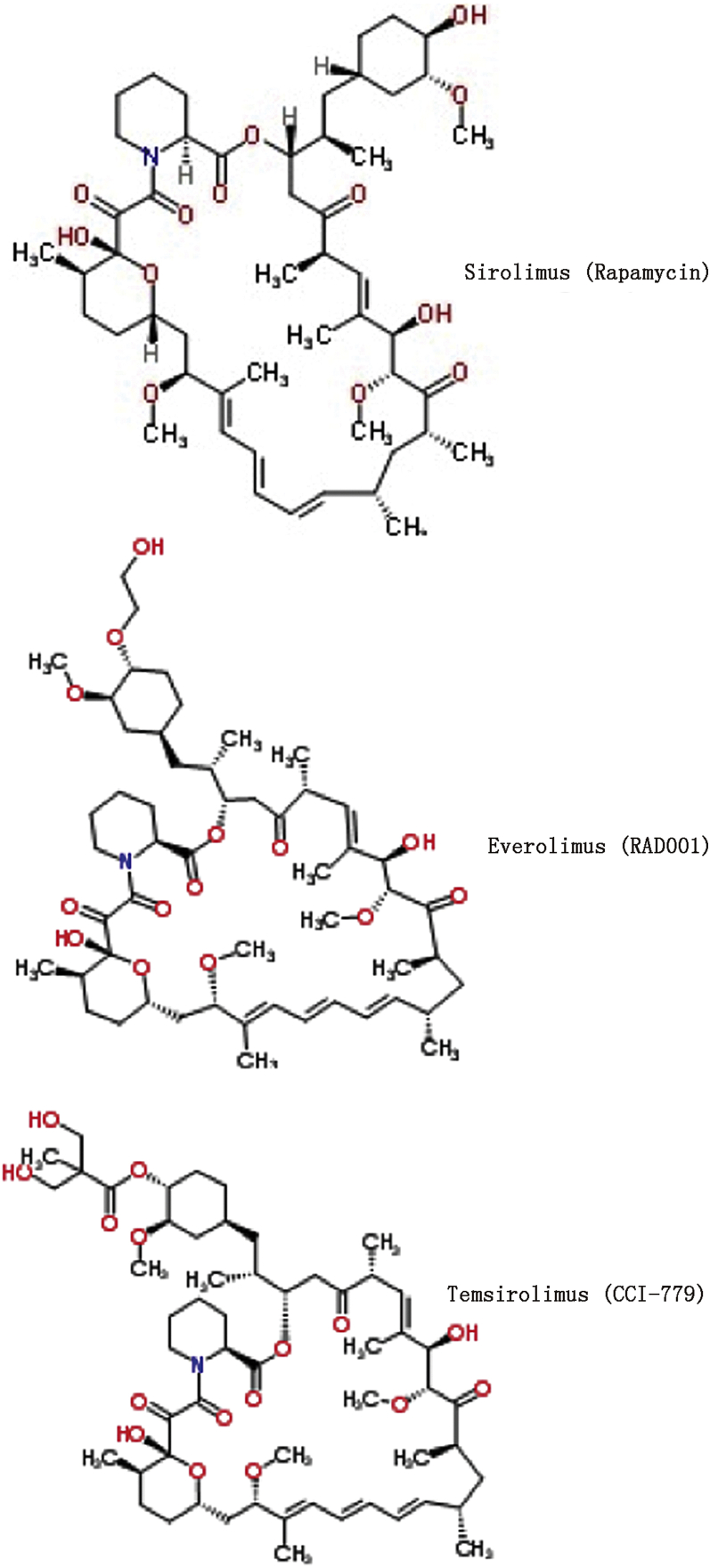
The mTOR inhibitor, rapamycin (sirolimus), is best known for its use in combination with glucocorticoids and cyclosporine to prevent organ rejection. Even at immunosuppressive doses it is a well-tolerated agent,18, 19 making it an attractive adjuvant. Rapamycin does not cause nephrotoxicity, neurotoxicity, or insulin insensitivity, commonly seen with other immune suppressants, and hypertriglyceridemia returns to baseline as early as a month after discontinuation.27 Importantly, rapamycin is already Food and Drug Administration (FDA) approved for particular human indications (i.e. kidney transplant) along with many currently patented analogs, or rapalogs (Fig. 2), making translation to the clinic potentially rapid. While classified as an immune suppressant, rapamycin has also been shown to enhance certain immune cell functions and improve the efficacy of vaccinations.28, 29 Used alone, rapamycin does not cause significant immune suppression but instead has been shown to prevent progression, slow growth, impede angiogenesis and lymphangiogenesis, and enhance the effects of chemotherapy, radiation, and the anti-tumor immune response in various tumor models of HNSCC, as discussed below.
Fig. 2.
Structural comparison of commonly used mTOR inhibitors. Structure of the first discovered, naturally occurring, and prototype drug of the mTOR inhibitors, rapamycin. Shown in comparison are two commonly used rapalogs possessing improved pharmacokinetic profiles and FDA approved indications including refractory renal cell carcinoma and in combination therapies for other solid tumors. CCI-779: cell cycle Inhibitor 779.
mTOR inhibition in preclinical studies of head and neck cancer
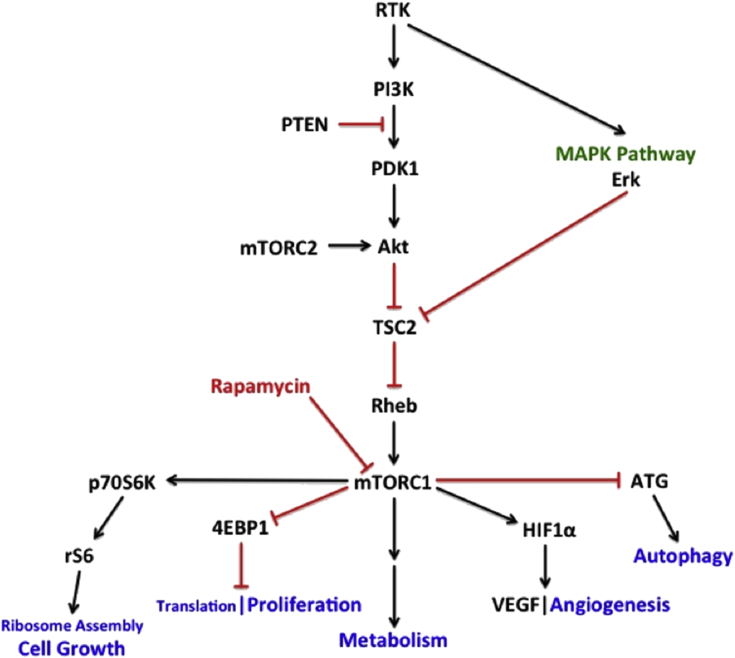
Since the firm establishment of mTOR dysregulation as a frequent signature underlying HNSCC pathogenesis,16, 30 rapamycin has garnered much interest as a potential therapeutic agent representing its class. Rapamycin has been demonstrated to be as effective as the patented temsirolimus in a xenograft established tumor model and thus suggested to be an economical and comparative targeted agent to potentially treat HNSCC.16 Studies have also demonstrated that expression levels of mTOR and downstream targets eukaryotic initiation factor 4E (eIF-4E), eukaryotic initiation factor 4E-binding protein 1 (4EBP1), p70 S6 kinase (S6K), and S6 (Fig. 3) are potential diagnostic and prognostic biomarkers for head and neck cancer,31 contributing to the potential utility of mTOR inhibitors in cancer therapy.
Fig. 3.
mTOR signaling. A simplified schematic of the PI3K/Akt/mTOR signaling pathway, selected integrating pathways, and some of their key effector functions. RTK: receptor tyrosine kinase; PI3K: phosphoinositide-3-kinase; PTEN: phosphatase and tensin homolog; PDK1: phosphoinositide-dependent kinase-1; mTORC2: mammalian target of rapamycin complex 2; MAPK: mitogen-activated protein kinase; TSC2: tuberous sclerosis complex 2; mTORC1: mammalian target of rapamycin complex 1 (mTOR); p70S6K: p70 S6 kinase; rS6: ribosomal S6; 4EBP1: eukaryotic initiation factor 4E-binding protein 1; HIF1α: hypoxia inducible factor 1 alpha; VEGF: vascular endothelial growth factor; ATG: autophagy related protein.
Whether directly by rapamycin or indirectly with a metabolism-inhibiting drug mimicking a low energy state such as metformin, mTOR inhibition has been shown to prevent progression, slow growth, and induce regression in models of carcinogen-induced premalignant SCC lesions, a p53 and K-Ras two-hit carcinogenesis model, and a phosphoinositide-3-kinase catalytic subunit alpha (PIK3CA) mutated OPSCC model.32, 33, 34, 35 The rapalog, everolimus, has shown effective anti-tumor activity in a large panel of head and neck cancer patient-derived xenografts.36 Rapamycin specifically has been shown to reduce the aberrant accumulation of phospho-S6 in HNSCC derived human cell lines, displaying potent anti-tumor activity through DNA synthesis inhibition, induction of apoptosis, and ultimately tumor regression in vivo at clinically relevant doses.37 mTOR inhibition has also been shown to minimize microscopic residual disease within surgical margins of HNSCC, increasing tumor-free survival in a microscopic residual disease mouse model.38 This is supported by a study that found tumor margins of surgical HNSCC patients exhibited over expression of eIF-4E in 90% of patients.27
Using an in vivo retroinhibition approach in which HNSCC cells were genetically engineered to over express a rapamycin-insensitive mTOR protein, mTOR has been demonstrated to control accumulation of HIF1α and subsequent expression of vascular endothelial growth factor (VEGF) in the tumor microenvironment, as well as GLUT1 expression in HNSCC cells.39 Rapamycin and analogs have been shown to accordingly prevent angiogenesis, lymphangiogenesis, and lymph node metastasis of HNSCC.39, 40 mTOR inhibition has also been shown to radio sensitize, enhance the antiangiogenic effects of radiation, and to prevent radiation-induced mTOR upregulation, synergizing with radiation therapy to greatly inhibit HNSCC growth and improve survival in xenograft models.41, 42 This could be explained in part by renormalization of aberrant tumor vasculature by rapamycin, which may improve tumor oxygenation to provide a therapeutic window for improved radiotherapy and possibly delivery of anti-tumor agents.43
Regarding mTOR inhibition in HPV+ HNSCC specifically, tissue microarray was used to confirm mTOR activation in a majority of HPV+ HNSCCs and cervical SCCs (CSCC), and HNSCC and CSCC derived cell lines were then xenografted into mice treated with or without everolimus or rapamycin. Both of these mTOR inhibitors effectively reduced mTOR activity and remarkably decreased tumor burden of HPV+ SCCs of both anatomical origins.17 Metabolic modulation and inhibition of mTOR by metformin has also been demonstrated to inhibit tumor growth in HPV+ HNSCC cells.35
Such work has led our laboratory to explore how inhibition of mTOR, with its master regulation of cellular metabolism as outlined above, might be modulating another, essential facet of therapeutic response in antigenic, HPV+ HNSCCs, the immune system.
Cellular metabolism and the anti-tumor immune response
Our laboratory previously developed a preclinical murine model of HPV+ HNSCC consisting of mouse oropharyngeal epithelial cells stably expressing HPV-16 E6 and E7 together with H-Ras ± Luciferase (mEER(L)cells) as well as an invasively growing HPV− control cell line consisting of small hairpin knockdown of an E6 oncoprotein target phosphatase together with H-Ras.25, 44 Using this model to compare therapeutic responses in immune-competent versus immune-compromised animals, we validated that HPV+ OPSCCs are more curable following standard CRT than HPV− counterparts and demonstrated that an immune response is both responsible and required for these long-term cures.25 HPV+ OPSCCs were not more curable based on increased sensitivity to cisplatin or radiation, but rather CRT induced an immune response to this antigenic cancer subtype leading to immune-mediated clearance. We have demonstrated this process to be cluster of differentiation 8 positive (CD8+) cytotoxic T-lymphocyte (CTL) dependent,26 and CTLs have been demonstrated to be predominant among the intratumoral immune cell infiltrate in HPV+ HNSCC.45, 46 While activated mTOR signaling promotes lactate production as a byproduct of upregulated aerobic glycolysis, lactate in the tumor microenvironment has been shown to inhibit immune cell function,22, 23, 24 including proliferation, cytokine production, and cytotoxic activity of CTLs.47 It has been postulated that this is due to abolishment of the lactate/proton co-transport concentration gradient, leading to intracellular accumulation of lactate and ultimately metabolic blockade, reducing the translation and subsequent availability of intracellular cytotoxic proteins, such as perforin.47 Other details of the tumor microenvironment in immune evasion have been reviewed elsewhere.46, 48 This inhibitory effect on CTLs has been demonstrated to be specific to lactic acid.47 Accordingly, LDH has been shown to be over expressed and an indicator of poor prognosis in HNSCC,49 as well as in other antigenic cancers such as metastatic melanoma.50
We have thus hypothesized that appropriately inhibiting tumor metabolism may be an effective therapeutic strategy for HPV+ HNSCC, with mTOR being a very logical target. While expansion of antigen-stimulated CTLs switches to dependence on glycolytic metabolism like cancer cells, which is inhibited consequently with mTOR, it has been demonstrated that inhibiting glycolytic flux in CTLs leads to the preferential development of antigen-specific precursor memory T-cells and improved anti-tumor function.51 This can be accomplished with transient mTOR inhibition in CTLs via a short course of high-dose rapamycin, which has also been shown to favor CTL persistence and antigen-recall responses.29 Rapamycin has also been shown to inhibit regulatory T-cell expansion and promote their apoptosis.52 In theory, brief, systemic, temporally specific rapamycin could then block mTOR-related, tumor-promoting processes as well as microenvironmental inhibition of the anti-tumor immune response, while simultaneously improving antigen-specific memory T-cell development and thus possibly long-term therapeutic responses after cessation of the drug.
This interplay between metabolism, the immune system, and the role they play in therapeutic response to known antigenic tumors has been largely unexplored in the context of an inhibitor of a key metabolic regulatory protein. Using the murine model of HPV+ OPSCC described above, we have shown that rapamycin inhibits mTOR signaling, cell proliferation, lactate production, and enhances CRT-induced cytotoxicity to HPV+ OPSCC cells in vitro.53 In vivo, rapamycin administered alone inhibited mTOR signaling and tumor growth in both immune-competent and immune-compromised animals, but did not lead to any long-term cures.53 However, in a generally well-tolerated combination with CRT, rapamycin significantly improved tumor clearance and long-term survival in immune-competent animals.53 Immune-compromised animals, alternatively, showed inhibited tumor growth but failed to clear their tumors.53 We correlated these findings with decreased intratumor lactate in rapamycin treated animals ex vivo using an enzymatic, bioluminescent technique.53 Furthermore, we showed that splenocytes from HPV-16 oncogene vaccinated animals had decreased cytotoxic activity against HPV+ OPSCC cells, which we correlated with the splenocytes harboring decreasing levels of intracellular perforin, in increasing, physiologic levels of lactic acid.3, 53 These preclinical data suggest mTOR inhibition to be a strong candidate therapeutic adjuvant that may be a relatively safe way to enhance both direct CRT-induced cytotoxicity and the tumor-clearing immune response to HPV+ OPSCC without further increasing systemic toxicities, a description of the ideal therapeutic strategy for HPV+ HNSCC.
mTOR in therapy resistance and metastasis
We substantiated these findings using a newly characterized metastatic model of HPV+ OPSCC derived from the model described above.54, 55 Several lung metastases were identified in an animal that failed CRT, cultured, and confirmed to have arisen from the primary tumor.54 These metastatic clones were CRT-resistant and more aggressive in vivo, re-metastasizing to the lungs at significantly higher rates than their parental cell line.54 Reverse phase protein arrays and Illumina expression microarrays showed each metastatic clone to be unique to one another and their parental cell line,54 yet notably and consistently activated in mTOR signaling.55 Using this new metastatic model closely recapitulating refractory human disease in heterogeneity, therapy resistance, and regional lymph node and lung metastasis, we showed that rapamycin adjuvant to CRT greatly re-sensitizes multiple, heterogeneous, clonal, therapy-resistant metastatic cell populations to treatment at the primary tumor site and substantially limits lymph node and lung metastasis.55 This phenomenon was observed in both immune-competent and immune-compromised animals,55 further demonstrating the potential of rapamycin in addition to immune-enhancement to be an ideal adjuvant therapy for HPV+ HNSCC.
Considering the preclinical data comprehensively, a multifaceted anti-tumor approach may be provided by the addition of but a single, well-tolerated agent to the standard-of-care treatment for HPV+ HNSCC: inhibited tumor growth, angiogenesis and metastasis, chemo and radio sensitization, and enhancement of the anti-tumor immune response.
mTOR inhibition in clinical studies of head and neck cancer
Beyond the extensive preclinical data supporting therapeutic mTOR inhibition for HNSCC, clinical data is beginning to emerge from many trials, with numerous others currently recruiting, testing, or analyzing data from trials of mTOR inhibitors in HNSCC administered alone or with various other agents and treatment modalities (clinicaltrials.gov).27, 31 A wealth of clinical data regarding mTOR inhibitors is also available from trials of renal cell carcinoma, for which some rapalogs are already FDA approved.31
Importantly, administration regimens that do not lead to clinically significant immune suppression have been observed.27 Perhaps more importantly, plasma levels correlating to those inhibiting tumor growth in animal models are able to be achieved.27 A recently completed phase I trial has determined a dose of everolimus that reaches therapeutic levels and is tolerated concurrent with cisplatin and radiation therapy in head and neck cancer patients.56 Everolimus has been tolerated in other combinations as well, such as with erlotinib or as induction chemotherapy plus cisplatin and docetaxel.57, 58
Yet, multiple clinical trials utilizing mTOR inhibitors for HNSCC have seen high numbers of adverse events, particularly with newer generation agents,59, 60, 61 leading to termination of some trials over the past few years (i.e. NCT01058408). These adverse events may be the result of overzealous targeting of the mTOR signaling pathway with newer or multiple/dual-molecular-targeted agents. While 80%–90% of HNSCCs have clearly been shown to be activated in mTOR signaling,17 this signaling pathway is essential to cellular homeostasis throughout the body almost ubiquitously. The possibility remains that using rapamycin over newer agents of improved pharmacokinetic profiles may yet be effective and limit toxicities. Interestingly, rapamycin, generally considered mTOR Complex 1 (mTORC1) specific, has been seen to inhibit both mTORC1 and mTORC2 in HNSCC cells specifically,17 which may contribute to its potential efficacy. Of course, identifying the appropriate treatment combination, regimen, and patient population most likely to benefit will help to tilt the risk-benefit ratio in the patients' favor. The prospect of combining an mTOR inhibitor with one of the many emerging direct immune modulators in the treatment of HPV+ HNSCC is certainly intriguing.46 Most clinical trials of HNSCC to date have used an mTOR inhibitor as a solo agent, often in patients who have already failed other therapies, have dually-targeted the mTOR pathway often in toxic combinations, or have been general to HNSCC without patient selection. Very few clinical trials have been designed to assess mTOR inhibition adjuvant to standard CRT for HNSCC, let alone for HPV+ disease specifically, though early studies warrant continued evaluation of such an approach.56
In fact, no trials of mTOR inhibitors to date have studied HPV+ HNSCCs specifically. Careful retrospective analysis of current data may thus be necessary as HPV+ HNSCCs are of an improved response to standard-of-care CRT, attributable to their antigenic nature, which may be a confounding factor. This unique treatment response may also be uniquely exploitable for the reasons reviewed above. Therefore, though studies of mTOR inhibitors are warranted in HNSCC broadly, comparisons of treatment response must be made among cancers of known oncogenic drivers in order to identify the appropriate disease stage and patient subpopulations that are likely to benefit. Future trials must directly address HPV status, and though some have begun to do so, none involve the use of mTOR inhibitors.62 Many clinical studies of HPV+ HNSCC have instead focused on de-escalating the toxic components of the standard treatment regimen, replacing cisplatin and/or radiation with a less toxic agent, with the rationale of this being feasible since HPV+ HNSCCs respond better to treatment than HPV− cancers.62, 63 The concept of de-escalation of a standard-of-care treatment with such a favorable prognosis is not without controversy or risk, however.62 First establishing an improved standard-of-care treatment for HPV+ disease, such as-potentially-with the adjuvant use of an mTOR inhibitor, may allow for more safely backing off of the more toxic components of such a treatment regimen.
Conclusion
Whether for lack of sufficient supporting preclinical data, failure to recognize the rapidly increasing incidence and the desperate need to improve quality of life, focus on de-escalation, and/or lack of interest in improving treatment of a cancer subtype already seen as very treatable, studies focusing on improving treatment of HPV+ HNSCC are still needed. Successful treatment of 80% of patients means treatment failure, recurrent disease, metastasis, and/or death in 20%,8 with the total number of these individuals increasing with the rapidly increasing incidence of these cancers. The overwhelming and increasing numbers of HPV+ HNSCC necessitate efforts to better treat this disease, minimizing significant treatment-related comorbidities and addressing the cases where our standard-of-care treatments are failing. In addition to enhanced direct cytotoxicity and inhibition of key growth, survival, and metastatic signals, the ability of mTOR inhibition to also dampen hyperactive tumor cell metabolism may eliminate the metabolic blockade on the standard-of-care-induced, tumor-clearing immune response to improve therapy. As rapamycin and analogs are already FDA approved for human use, and with numerous potential diagnostic and prognostic biomarkers already in place, mTOR inhibition may be an ideal adjuvant to improve treatment of HPV+ HNSCC with translation to the clinic having the potential to be rapid. Clinical studies of mTOR inhibitors targeting HPV+ HNSCC specifically are certainly warranted.
Conflicts of interest
The authors report no financial or other conflict of interest relevant to the subject of this article.
Funding
J.H. Lee acknowledges support of National Institutes of Health 1R01CA193522.
Acknowledgments
We thank W.K. Miskimins for insightful discussions.
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Leemans C.R., Braakhuis B.J., Brakenhoff R.H. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011;11:9–22. doi: 10.1038/nrc2982. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Lee D.W., Anderson M.E., Wu S., Lee J.H. Development of an adenoviral vaccine against E6 and E7 oncoproteins to prevent growth of human papillomavirus-positive cancer. Arch Otolaryngol Head Neck Surg. 2008;134:1316–1323. doi: 10.1001/archoto.2008.507. [DOI] [PubMed] [Google Scholar]
- 4.Gooi Z., Chan J.Y., Fakhry C. The epidemiology of the human papillomavirus related to oropharyngeal head and neck cancer. Laryngoscope. 2016;126:894–900. doi: 10.1002/lary.25767. [DOI] [PubMed] [Google Scholar]
- 5.Marur S., D'Souza G., Westra W.H., Forastiere A. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol. 2010;11:781–789. doi: 10.1016/S1470-2045(10)70017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koch W.M. Clinical features of HPV-related head and neck squamous cell carcinoma: presentation and work-up. Otolaryngol Clin North Am. 2012;45:779–793. doi: 10.1016/j.otc.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Marklund L., Hammarstedt L. Impact of HPV in oropharyngeal cancer. J Oncol. 2011;2011 doi: 10.1155/2011/509036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Rorke M.A., Ellison M.V., Murray L.J., Moran M., James J., Anderson L.A. Human papillomavirus related head and neck cancer survival: a systematic review and meta-analysis. Oral Oncol. 2012;48:1191–1201. doi: 10.1016/j.oraloncology.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 9.Ruzevick J., Olivi A., Westra W.H. Metastatic squamous cell carcinoma to the brain: an unrecognized pattern of distant spread in patients with HPV-related head and neck cancer. J Neurooncol. 2013;112:449–454. doi: 10.1007/s11060-013-1075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McLaughlin-Drubin M.E., Münger K. Oncogenic activities of human papillomaviruses. Virus Res. 2009;143:195–208. doi: 10.1016/j.virusres.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mellin H., Dahlgren L., Munck-Wikland E. Human papillomavirus type 16 is episomal and a high viral load may be correlated to better prognosis in tonsillar cancer. Int J Cancer. 2002;102:152–158. doi: 10.1002/ijc.10669. [DOI] [PubMed] [Google Scholar]
- 12.Spangle J.M., Münger K. The human papillomavirus type 16 E6 oncoprotein activates mTORC1 signaling and increases protein synthesis. J Virol. 2010;84:9398–9407. doi: 10.1128/JVI.00974-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu Z., Hu X., Li Y. Human papillomavirus 16 E6 oncoprotein interferences with insulin signaling pathway by binding to tuberin. J Biol Chem. 2004;279:35664–35670. doi: 10.1074/jbc.M403385200. [DOI] [PubMed] [Google Scholar]
- 14.Zheng L., Ding H., Lu Z. E3 ubiquitin ligase E6AP-mediated TSC2 turnover in the presence and absence of HPV16 E6. Genes Cells. 2008;13:285–294. doi: 10.1111/j.1365-2443.2008.01162.x. [DOI] [PubMed] [Google Scholar]
- 15.Zwerschke W., Mazurek S., Massimi P., Banks L., Eigenbrodt E., Jansen-Dürr P. Modulation of type M2 pyruvate kinase activity by the human papillomavirus type 16 E7 oncoprotein. Proc Natl Acad Sci U S A. 1999;96:1291–1296. doi: 10.1073/pnas.96.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molinolo A.A., Hewitt S.M., Amornphimoltham P. Dissecting the Akt/mammalian target of rapamycin signaling network: emerging results from the head and neck cancer tissue array initiative. Clin Cancer Res. 2007;13:4964–4973. doi: 10.1158/1078-0432.CCR-07-1041. [DOI] [PubMed] [Google Scholar]
- 17.Molinolo A.A., Marsh C., El Dinali M. mTOR as a molecular target in HPV-associated oral and cervical squamous carcinomas. Clin Cancer Res. 2012;18:2558–2568. doi: 10.1158/1078-0432.CCR-11-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liao Y.M., Kim C., Yen Y. Mammalian target of rapamycin and head and neck squamous cell carcinoma. Head Neck Oncol. 2011;3:22. doi: 10.1186/1758-3284-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu M., Ekshyyan O., Herman Ferdinandez L., Rong X., Caldito G., Nathan C.O. Efficacy and comparative effectiveness of sirolimus as an anticancer drug. Laryngoscope. 2011;121:978–982. [Google Scholar]
- 20.Tennant D.A. PK-M2 makes cells sweeter on HIF1. Cell. 2011;145:647–649. doi: 10.1016/j.cell.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 21.Luo W., Semenza G.L. Pyruvate kinase M2 regulates glucose metabolism by functioning as a coactivator for hypoxia-inducible factor 1 in cancer cells. Oncotarget. 2011;2:551–556. doi: 10.18632/oncotarget.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gottfried E., Kunz-Schughart L.A., Ebner S. Tumor-derived lactic acid modulates dendritic cell activation and antigen expression. Blood. 2006;107:2013–2021. doi: 10.1182/blood-2005-05-1795. [DOI] [PubMed] [Google Scholar]
- 23.Shime H., Yabu M., Akazawa T. Tumor-secreted lactic acid promotes IL-23/IL-17 proinflammatory pathway. J Immunol. 2008;180:7175–7183. doi: 10.4049/jimmunol.180.11.7175. [DOI] [PubMed] [Google Scholar]
- 24.Goetze K., Walenta S., Ksiazkiewicz M., Kunz-Schughart L.A., Mueller-Klieser W. Lactate enhances motility of tumor cells and inhibits monocyte migration and cytokine release. Int J Oncol. 2011;39:453–463. doi: 10.3892/ijo.2011.1055. [DOI] [PubMed] [Google Scholar]
- 25.Spanos W.C., Nowicki P., Lee D.W. Immune response during therapy with cisplatin or radiation for human papillomavirus-related head and neck cancer. Arch Otolaryngol Head Neck Surg. 2009;135:1137–1146. doi: 10.1001/archoto.2009.159. [DOI] [PubMed] [Google Scholar]
- 26.Williams R., Lee D.W., Elzey B.D., Anderson M.E., Hostager B.S., Lee J.H. Preclinical models of HPV+ and HPV- HNSCC in mice: an immune clearance of HPV+ HNSCC. Head Neck. 2009;31:911–918. doi: 10.1002/hed.21040. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen S.A., Walker D., Gillespie M.B., Gutkind J.S., Day T.A. mTOR inhibitors and its role in the treatment of head and neck squamous cell carcinoma. Curr Treat Options Oncol. 2012;13:71–81. doi: 10.1007/s11864-011-0180-2. [DOI] [PubMed] [Google Scholar]
- 28.Amiel E., Everts B., Freitas T.C. Inhibition of mechanistic target of rapamycin promotes dendritic cell activation and enhances therapeutic autologous vaccination in mice. J Immunol. 2012;189:2151–2158. doi: 10.4049/jimmunol.1103741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Srivastava R.K., Utley A., Shrikant P.A. Rapamycin: a rheostat for CD8(+) T-cell-mediated tumor therapy. Oncoimmunology. 2012;1:1189–1190. doi: 10.4161/onci.20663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freudlsperger C., Burnett J.R., Friedman J.A., Kannabiran V.R., Chen Z., Van Waes C. EGFR-PI3K-AKT-mTOR signaling in head and neck squamous cell carcinomas: attractive targets for molecular-oriented therapy. Expert Opin Ther Targets. 2011;15:63–74. doi: 10.1517/14728222.2011.541440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao W., Li J.Z., Chan J.Y., Ho W.K., Wong T.S. mTOR pathway and mTOR inhibitors in head and neck cancer. ISRN Otolaryngol. 2012;2012 doi: 10.5402/2012/953089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vitale-Cross L., Molinolo A.A., Martin D. Metformin prevents the development of oral squamous cell carcinomas from carcinogen-induced premalignant lesions. Cancer Prev Res (Phila) 2012;5:562–573. doi: 10.1158/1940-6207.CAPR-11-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amornphimoltham P., Leelahavanichkul K., Molinolo A., Patel V., Gutkind J.S. Inhibition of Mammalian target of rapamycin by rapamycin causes the regression of carcinogen-induced skin tumor lesions. Clin Cancer Res. 2008;14:8094–8101. doi: 10.1158/1078-0432.CCR-08-0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raimondi A.R., Molinolo A., Gutkind J.S. Rapamycin prevents early onset of tumorigenesis in an oral-specific K-ras and p53 two-hit carcinogenesis model. Cancer Res. 2009;69:4159–4166. doi: 10.1158/0008-5472.CAN-08-4645. [DOI] [PubMed] [Google Scholar]
- 35.Madera D., Vitale-Cross L., Martin D. Prevention of tumor growth driven by PIK3CA and HPV oncogenes by targeting mTOR signaling with metformin in oral squamous carcinomas expressing OCT3. Cancer Prev Res (Phila) 2015;8:197–207. doi: 10.1158/1940-6207.CAPR-14-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klinghammer K., Raguse J.D., Plath T. A comprehensively characterized large panel of head and neck cancer patient-derived xenografts identifies the mTOR inhibitor everolimus as potential new treatment option. Int J Cancer. 2015;136:2940–2948. doi: 10.1002/ijc.29344. [DOI] [PubMed] [Google Scholar]
- 37.Amornphimoltham P., Patel V., Sodhi A. Mammalian target of rapamycin, a molecular target in squamous cell carcinomas of the head and neck. Cancer Res. 2005;65:9953–9961. doi: 10.1158/0008-5472.CAN-05-0921. [DOI] [PubMed] [Google Scholar]
- 38.Nathan C.O., Amirghahari N., Rong X. Mammalian target of rapamycin inhibitors as possible adjuvant therapy for microscopic residual disease in head and neck squamous cell cancer. Cancer Res. 2007;67:2160–2168. doi: 10.1158/0008-5472.CAN-06-2449. [DOI] [PubMed] [Google Scholar]
- 39.Amornphimoltham P., Patel V., Leelahavanichkul K., Abraham R.T., Gutkind J.S. A retroinhibition approach reveals a tumor cell-autonomous response to rapamycin in head and neck cancer. Cancer Res. 2008;68:1144–1153. doi: 10.1158/0008-5472.CAN-07-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patel V., Marsh C.A., Dorsam R.T. Decreased lymphangiogenesis and lymph node metastasis by mTOR inhibition in head and neck cancer. Cancer Res. 2011;71:7103–7112. doi: 10.1158/0008-5472.CAN-10-3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leiker A.J., DeGraff W., Choudhuri R. Radiation enhancement of head and neck squamous cell carcinoma by the dual PI3K/mTOR inhibitor PF-05212384. Clin Cancer Res. 2015;21:2792–2801. doi: 10.1158/1078-0432.CCR-14-3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ekshyyan O., Rong Y., Rong X. Comparison of radiosensitizing effects of the mammalian target of rapamycin inhibitor CCI-779 to cisplatin in experimental models of head and neck squamous cell carcinoma. Mol Cancer Ther. 2009;8:2255–2265. doi: 10.1158/1535-7163.MCT-08-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saito K., Matsumoto S., Yasui H. Longitudinal imaging studies of tumor microenvironment in mice treated with the mTOR inhibitor rapamycin. PLoS One. 2012;7:e49456. doi: 10.1371/journal.pone.0049456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoover A.C., Spanos W.C., Harris G.F., Anderson M.E., Klingelhutz A.J., Lee J.H. The role of human papillomavirus 16 E6 in anchorage-independent and invasive growth of mouse tonsil epithelium. Arch Otolaryngol Head Neck Surg. 2007;133:495–502. doi: 10.1001/archotol.133.5.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krupar R., Robold K., Gaag D. Immunologic and metabolic characteristics of HPV-negative and HPV-positive head and neck squamous cell carcinomas are strikingly different. Virchows Arch. 2014;465:299–312. doi: 10.1007/s00428-014-1630-6. [DOI] [PubMed] [Google Scholar]
- 46.Allen C.T., Clavijo P.E., Van Waes C., Chen Z. Anti-tumor immunity in head and neck cancer: understanding the evidence, how tumors escape and immunotherapeutic approaches. Cancers (Basel) 2015;7:2397–2414. doi: 10.3390/cancers7040900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fischer K., Hoffmann P., Voelkl S. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood. 2007;109:3812–3819. doi: 10.1182/blood-2006-07-035972. [DOI] [PubMed] [Google Scholar]
- 48.Curry J.M., Sprandio J., Cognetti D. Tumor microenvironment in head and neck squamous cell carcinoma. Semin Oncol. 2014;41:217–234. doi: 10.1053/j.seminoncol.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 49.Koukourakis M.I., Giatromanolaki A., Winter S., Leek R., Sivridis E., Harris A.L. Lactate dehydrogenase 5 expression in squamous cell head and neck cancer relates to prognosis following radical or postoperative radiotherapy. Oncology. 2009;77:285–292. doi: 10.1159/000259260. [DOI] [PubMed] [Google Scholar]
- 50.Diem S., Kasenda B., Spain L. Serum lactate dehydrogenase as an early marker for outcome in patients treated with anti-PD-1 therapy in metastatic melanoma. Br J Cancer. 2016;114:256–261. doi: 10.1038/bjc.2015.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sukumar M., Liu J., Ji Y. Inhibiting glycolytic metabolism enhances CD8+ T cell memory and antitumor function. J Clin Invest. 2013;123:4479–4488. doi: 10.1172/JCI69589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Y., Camirand G., Lin Y. Regulatory T cells require mammalian target of rapamycin signaling to maintain both homeostasis and alloantigen-driven proliferation in lymphocyte-replete mice. J Immunol. 2011;186:2809–2818. doi: 10.4049/jimmunol.0903805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coppock J.D., Wieking B.G., Molinolo A.A., Gutkind J.S., Miskimins W.K., Lee J.H. Improved clearance during treatment of HPV-positive head and neck cancer through mTOR inhibition. Neoplasia. 2013;15:620–630. doi: 10.1593/neo.13432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vermeer D.W., Coppock J.D., Zeng E. Metastatic model of HPV+ oropharyngeal squamous cell carcinoma demonstrates heterogeneity in tumor metastasis. Oncotarget. 2016 doi: 10.18632/oncotarget.8254. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Coppock J.D., Vermeer P.D., Vermeer D.W. mTOR inhibition as an adjuvant therapy in a metastatic model of HPV+ HNSCC. Oncotarget. 2016 doi: 10.18632/oncotarget.8286. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fury M.G., Lee N.Y., Sherman E. A phase 1 study of everolimus + weekly cisplatin + intensity modulated radiation therapy in head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2013;87:479–486. doi: 10.1016/j.ijrobp.2013.06.2043. [DOI] [PubMed] [Google Scholar]
- 57.Massarelli E., Lin H., Ginsberg L.E. Phase II trial of everolimus and erlotinib in patients with platinum-resistant recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol. 2015;26:1476–1480. doi: 10.1093/annonc/mdv194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fury M.G., Sherman E., Ho A.L. A phase 1 study of everolimus plus docetaxel plus cisplatin as induction chemotherapy for patients with locally and/or regionally advanced head and neck cancer. Cancer. 2013;119:1823–1831. doi: 10.1002/cncr.27986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Piha-Paul S.A., Munster P.N., Hollebecque A. Results of a phase 1 trial combining ridaforolimus and MK-0752 in patients with advanced solid tumours. Eur J Cancer. 2015;51:1865–1873. doi: 10.1016/j.ejca.2015.06.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grünwald V., Keilholz U., Boehm A. TEMHEAD: a single-arm multicentre phase II study of temsirolimus in platin- and cetuximab refractory recurrent and/or metastatic squamous cell carcinoma of the head and neck (SCCHN) of the German SCCHN Group (AIO) Ann Oncol. 2015;26:561–567. doi: 10.1093/annonc/mdu571. [DOI] [PubMed] [Google Scholar]
- 61.Perri F., Pacelli R., Della Vittoria Scarpati G. Radioresistance in head and neck squamous cell carcinoma: biological bases and therapeutic implications. Head Neck. 2015;37:763–770. doi: 10.1002/hed.23837. [DOI] [PubMed] [Google Scholar]
- 62.Chung C.H., Schwartz D.L. Impact of HPV-related head and neck cancer in clinical trials: opportunity to translate scientific insight into personalized care. Otolaryngol Clin North Am. 2012;45:795–806. doi: 10.1016/j.otc.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 63.Wierzbicka M., Szyfter K., Milecki P. The rationale for HPV-related oropharyngeal cancer de-escalation treatment strategies. Contemp Oncol (Pozn) 2015;19:313–322. doi: 10.5114/wo.2015.54389. [DOI] [PMC free article] [PubMed] [Google Scholar]