Abstract
Nasopharyngeal carcinoma (NPC) is a special type of head and neck cancer with a widely variable geographical variation in incidence. The central location of the tumor inside the head coupled with the radiosensitivity of the tumor to radiation made radiation therapy the first choice in primary treatment of NPC. Advances in radiotherapy and chemotherapy have markedly improved the local control of NPC. Unfortunately, a small but significant number of patients still suffered from loco-regional failures that would be amenable to re-treatment. Traditional form of retreatment was to employ a second course of radiation. The efficacy of re-irradiation to treat local of regional recurrent NPC has been suboptimal. Moreover, the local tissue had already received a high dose of radiation and the second radiation could result in radiation toxicities to the local tissue, leading to significant complications.
Surgical salvage, on the other hand, could spare the patients from complications of re-treatment. Due to the difficult access of the nasopharynx, various surgical approaches had been devised for nasopharyngectomy. The maxillary swing approach had the largest published experience with over 300 cases from various centers. In the recent decade, the endoscopic approach with or without robotic assistance had gained popularity for resecting small, centrally located recurrences. This minimally invasive approach further reduced the morbidity for treating locally recurrent NPC.
Nodal recurrences had been a rare entity after the introduction of modern radiotherapy technique and concurrent chemotherapy. Treatment of nodal failure with second radiation has dismal results. Surgical removal of the lymph node harboring the recurrence should be in the form of a formal radical neck dissection. In cases of extensive nodal recurrence where microscopic disease may be present after a formal neck dissection, additional radiotherapy can be delivered with after-loading brachytherapy.
Surgical treatment played a definitive role in salvage of loco-regional failures of nasopharyngeal carcinoma.
Keywords: Nasopharyngeal carcinoma, Nasopharyngectomy, Salvage surgery, Recurrent cancer
Introduction
Nasopharyngeal carcinoma (NPC) is a distinct type of head and neck cancer that distinguishes itself from other head and neck cancers. The incidence of NPC has a wide geographical difference with Southern China being of highest incidences followed by Southeast Asia, North Africa and Arabia. Incidence in rest of northern Asia and in Caucasians is more ten times less than the high incidence area.1 Endemic form of nasopharyngeal carcinoma, being of the undifferentiated or poorly differentiated histological type, is highly radiosensitive. Access to the nasopharynx is also difficult as the nasopharynx is situated in the central part of the human head and surrounded by bone in all sides except the exit to the oropharynx inferiorly. Radiotherapy thus becomes the mainstay of treatment for primary NPC. Advances in radiotherapy treatments included the advent of three-dimensional and intensity modulated radiotherapy (IMRT) together with the addition of concurrent chemotherapy has markedly improved the cure rate of NPC. Reviews of contemporary treatment strategies showed the local recurrence rate is around 11%and nodal failure rate is around 5%.2, 3 The predominant failure pattern is distant failure, accounting to 30% of all treated patients.3
While surgery has a limited diagnostic role in treatment of primary NPC, surgical treatment played an increasing important role in salvage of recurrent or persistent disease after primary radiotherapy. Traditionally, recurrent local and nodal diseases were treated with additional course of radiotherapy. Unfortunately, the local tissue usually had received the maximum dosage of radiation during the primary treatment and additional radiotherapy will increase the local tissue toxicity. Moreover, the recurrent disease may harbor clones of tumor cells that were resistant to radiation, contribution to low success rate of salvage radiation. A large series of over 100 patients employing conventional 2D radiotherapy techniques to re-irradiate local recurrences had shown poor survival results of 9.4% 5-year survival with significant complications.4 Newer radiotherapy techniques like three-dimensional conformal radiotherapy or IMRT marginally improved the outcome of re-irradiation but patients still suffered from significant late toxicities from radiation.5, 6, 7 For nodal diseases, the result of re-irradiation was also dismal with 5-year survival of less than 20% after re-treatment with radiation.8
The alternative strategy for salvage is surgical treatment. Surgical salvage can avoid the toxicity of radiation, which most local tissue has already reached the limits of tolerance to radiation. Surgical salvage can be divided into salvage for locally recurrent cancer and salvage for nodal recurrence.
Salvage surgery for locally recurrent nasopharyngeal cancer
Nasopharyngectomy is the surgical resection of the nasopharynx and its adjacent structure. It is primarily employed for surgical salvage of recurrent nasopharyngeal carcinoma but occasional used for resection of radiation resistant cancers in the nasopharynx like salivary gland cancers of the nasopharynx. As the nasopharynx is situated in the central part of the head, access to the nasopharynx has been difficult and nasopharyngectomy traditionally has been considered a difficult operation. Multiple route of access has been devised for nasopharyngectomy and the commonest approaches will be discussed.
Before embarking on nasopharyngectomy as salvage, patients suffering from recurrent nasopharyngeal cancers should be fully investigated to ensure the disease is salvageable. A nasopharyngoscopy with biopsy should be performed to determine the location of the recurrent tumor and to obtain histological proof of recurrence. Radiation necrosis can mimic recurrence on cross-sectional imaging and radionecrosis is often metabolically active on positron emission tomography (PET) scan.9, 10 Occasionally, deep-seated recurrence may not be amenable to endoscopic biopsy and the surgeon may need to operate without histological proof. The surgeon then should discuss with the patient the implication of risk of a histologically negative resection. Cross-sectional imaging should always be obtained before embarking on nasopharyngectomy. Contrasted enhanced MRI is the preferred imaging modality due to its superior soft tissue delineation.11 Computer tomography (CT) scan alternatively is more superior in delineating the extent of bony involvement. The two imaging modalities are complimentary. Cross-sectional imaging can determine the extent of the recurrent cancer and its relationship to adjacent vital structures including the internal carotid artery, bones of the skull base and cranial nerves. Extensive recurrence with encasement of internal carotid artery or intracranial involvement may not be completely resected without significant morbidities or mortality. If available, PET scan before the operation can detect synchronous distant failure that prevents long-term cure and avoid futile operation.
Different approaches for nasopharyngectomy will be discussed in the following review.
Infratemporal fossa approach – lateral approach
This is the first approach described by Ugo Fisch in 1979 for resection of malignant lesions in temporal bone and base of skull.12 It is a neuro-otological approach. The procedure started with a total parotidectomy and mastoidectomy, aiming to reroute the facial nerve for access to the lateral skull base. The zygomatic arch and master muscle will then be detached and the temporalis muscle insertion to the mandible will then be divided. The base of the middle cranial fossa can then be followed from lateral to medial to approach the parapharyngeal space. The mandibular branch of the trigeminal nerve had to be divided and the pterygoid muscles removed from the attachment to the lateral pterygoid plate. At this juncture, the internal carotid artery (ICA) can then be traced from the neck to the carotid canal. The ICA can then be retracted away and the whole ipsilateralparapharygneal space and lateral nasopharynx together with the Eustachian tube can then be resected enbloc. As the internal carotid artery is identified and isolated before resection of the pathology, it can be fully protected during the resection. This is the main advantage of this approach. Unfortunately, the operation can result in multiple cranial nerve palsies including mandibular branch of trigeminal nerve, facial nerve, glossopharyngeal nerve and vagus nerve, either temporarily or permanently. The masticatory function is also damaged by the operation. The other disadvantage is the poor exposure of the contralateral nasopharynx.
Fisch et al. in 1983 reported their experience of treating 13 patients with local failure of nasopharyngeal carcinoma.13 Six patients with small diseases (T1–T2) had no recurrence two to five years after surgery but all patients with advance local disease (T4) developed local recurrence and died of disease within three years. Subsequently there were few reports of the use of this approach for resecting recurrent nasopharyngeal carcinoma.
Transpalatal approach – inferior approach
Willard Fee of Stanford University and Tu et al. from Beijing, China separately described similar approach to the nasopharynx for resection of recurrent NPC in 1988.14, 15 The approach was modified from the palatal fenestration approach for treatment of pediatric choanal atresia and juvenile angiofibroma first described by Wilson in 1957.16 A posteriorly based U-shaped mucosa flap was raised from the hard palate. The soft palate was then detached from the hard palate and retracted posteriorly. The nasopharynx would then be exposed and resection of the nasopharynx pathologies could then be performed under direct vision. To improve the exposure, the posterior of the hard palate (palatine bone) and posterior part of the inferior turbinate could be removed. After completion of the resection, the palatal flap would then be resutured back to the anterior hard palate mucosal incision to close the wound.
Fee reported the long-term result of this approach for 37 patients in 2002.17 The 5-year overall survival was 52% and 5-year local control was 67%. Twenty percent of the patients in the cohort developed surgical complication but serious complications only occurred in two patients (5%) with one death and one permanent dysphagia. When compared with the treatment results of re-irradiation of that era, the Fee's cohort was comparable or even better than re-irradiation in terms of local control.
Transcervico-mandibulo-palatal approach – inferolateral approach
This approach was similar to the mandibular split approach to the parapharyngeal space. The procedure started with a lip-splitting paramedian mandibulotomy and extending the incision to the ipsilateral upper neck. The floor of mouth was then incised and the incision carried superiorly and medially to the anterior pillar of the ipsilateral tonsils. The soft palate was then divided and detached to from the hard palate to expose the nasopharynx. The approach had 2 distinct advantages. The exposure was excellent the internal carotid artery could be traced form the neck all the way to the skull base and protected during the procedure. Morton et al. in 1996 reported his experience of this approach in managing seven cases of recurrent nasopharyngeal carcinoma with one patient developed early recurrence within one year after operation and one late recurrence more than ten years after the nasopharyngectomy.18 The major disadvantage of the approach was the large amount of normal tissue that needed to be transgressed and the associated morbidities. King et al. reported in his series of eleven patients who had underwent nasopharyngectomy via this approach with significant proportion of patients suffering from post-operative complications.19 Eight patients had a palatal defect, 6 patients suffered from trismus, 6 patients had dysphagia and three patients had malunion of the mandible. The approach was less employed nowadays as other approaches offered similar exposure with less morbidity.
Maxillary swing approach and facial translocation approach – anterolateral approach
Both approaches were similar in that the nasopharynx was exposed after removing the ipsilateral maxillary antrum. In the maxillary swing approach, the procedure was similar to a maxillectomy but the maxillary bone was left attached to the cheek flap where the flap continued to provide blood supply to the maxilla. The procedure started with a Weber-Ferguson incision similar performing a maxillectomy. The subciliary incision was extended laterally to the anterior zygoma. Intraorally, the lip-split incision was extended posteriorly in the midline of the hard palate until it reached the hard palate-soft palate junction. The lip split incision was also extended laterally in the ginviobuccal sulcus and turning medially at the maxillary tuberosity to meet in the hard palate incision. Osteotomies were made at the anterior maxilla inferior to the orbital rim; midline at the hard plate just lateral to the nasal septum; at the anterior zygoma separating the maxilla from the zygoma and finally between the posterior maxillary wall and the pterygoid plates at the maxillary tuberosity. The maxilla was left attached to the anterior cheek skin and the whole maxilla with the cheek skin would be retracted laterally to expose the parapharyngeal space and nasopharynx. To improve the exposure of the parapharyngeal space, the medial pterygoid plate and part of the medial pterygoid muscles could be removed. A posterior septectomy can be performed to improve the exposure of the contralateral nasopharynx. This approach provided excellent exposure of the whole nasopharynx and ipsilateral parapharyngeal space. While the internal carotid artery was not readily exposed during the procedure, the surgeon could palpate for arterial pulsations during the dissection in the parapharyngeal space. At the conclusion of the resection, the maxilla would be repositioned back to the anatomical position and the osteotomies would be secured with titanium plates. Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5 illustrates a case of the maxillary swing approach to the nasopharynx.
Fig. 1.
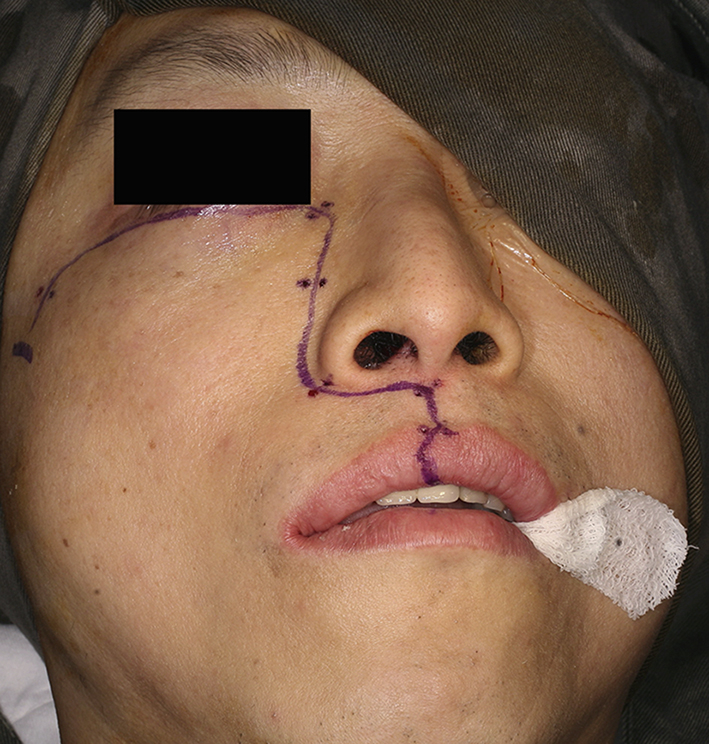
Clinical photo showing the incision for right maxillary swing operation.
Fig. 2.
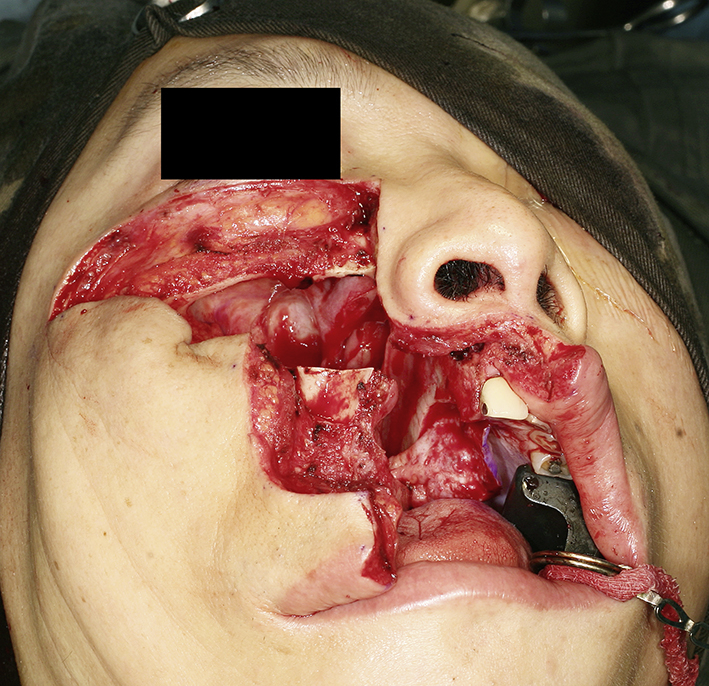
Clinical photo showing the right maxilla mobilized after osteotomies. The maxillary bone was left attached to the cheek flap.
Fig. 3.
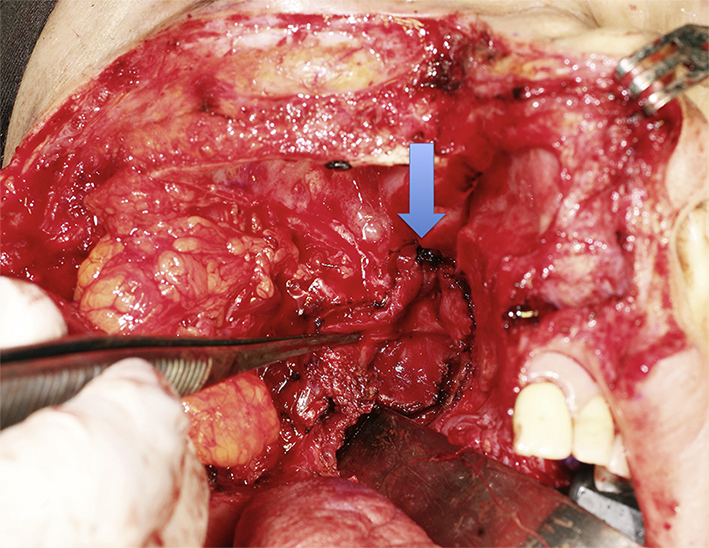
Clinical photo showing the right nasopharynx after swinging the right maxilla laterally. Arrow pointed the planned resection margin, which was marked with ink.
Fig. 4.
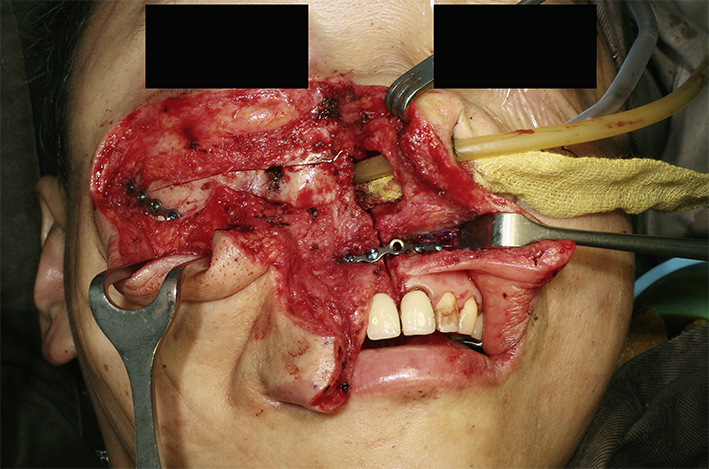
Clinical photo showing the right maxilla repositioned back to the facial skeleton and fixed with titanium plates.
Fig. 5.
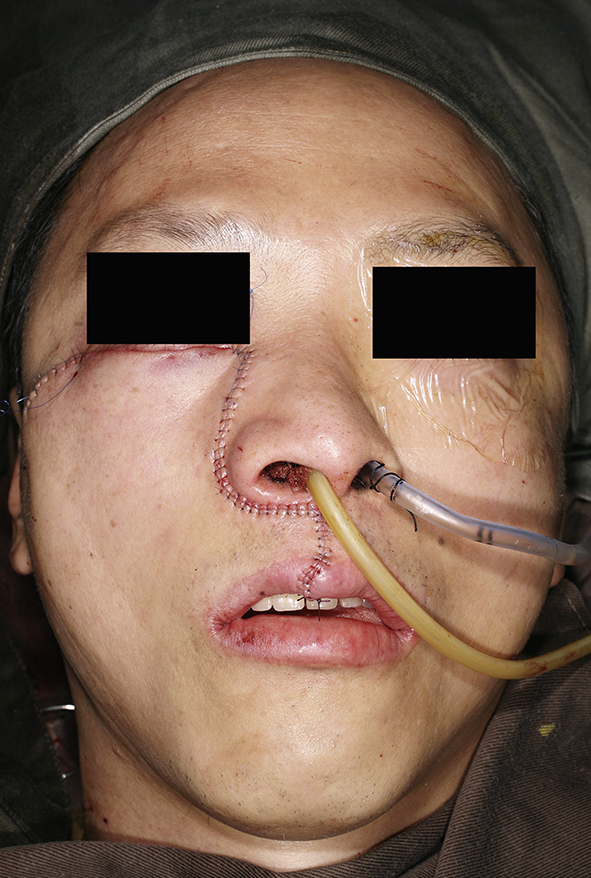
Clinical photo showing the completion of the maxillary swing operation. The incision was carefully sutured for better cosmetic outcome.
For the facial translocation approach, the whole maxilla including the floor of the orbit, lateral orbital rim, malar prominence and zygomatic arch were all removed enbloc. The maxilla was detached from the cheek skin as a free bone graft and reattached to the facial skeleton with plates at the conclusion of the operation. To improve exposure to the infratemporal fossa the coronoid process of the mandible was out-fractured. The approach provided better exposure of the infratemporal fossa than the maxillary swing approach though this was usually not necessary for resection of tumors confined in the nasopharynx and parapharyngeal space.20
Wei et al. first reported the technique of maxillary swing approach for resecting recurrent nasopharyngeal carcinoma in 1991.21 In 1995, he reported the results of 18 patients with recurrent NPC treated with maxillary swing approach with a 3.5 years actuarial survival of 42%.22 In 2011, he updated his cohort of 246 patients with an encouraging 5-year local control of 74% and 5-year disease free survival of 56%.23
For facial translocation approach, the surgical results were similar to the maxillary swing approach. Hao et al. reported their cohort of 53 patients treated with this technique with a 5-year local control of 54% and 5-year overall survival of 49%.24 Hao also reported that 21% of the patients treated with this technique developed avascular necrosis of part of the maxilla that required surgical debridement.25
Mid-facial degloving approach – anterior approach
The approach was first described by Casson et al. in 1974 for treatment of fibrous dysplasia of the mid-face.26 Howard and Lund later popularized the approach for resection of sinonasal neoplasms and juvenile nasopharyngeal angiofibromas.27, 28 The major advantage of the approach was lack of a facial incision and no disruption of palatal function. The approach started with an incision on the whole upper gingivabuccal sulcus. Two separate intercartilgenous incisions in both nasal alar were then extended to a transfixation incision of the nasal columellar would allow the soft tissue of the nasal alar and nasal tip to be separated from the nasal bone. The sublabial incision would then be dissected down towards the premaxilla and connected with the nasal incisions. The soft tissue of the whole midface could then be elevated superiorly up to the infraorbital foramen and the bony anterior midface could be fully exposed. In order to improve the expose of the nasopharynx and increase space for instrument manipulation, bilateral medial maxillectomies (Denker's procedure) could be performed.
To et al. reported a case series of 15 patients with recurrent NPC treated with the mid-facial degloving approach.29 They were able to achieve clear surgical margins in 12 (80%) cases. The major disadvantage of the approach is the relative narrow view of the nasopharynx with minimal lateral reach. The surgeon would not be able to put the finger into the parapharyngeal space to localize the ICA. To et al. circumvented this limitation with the use of stereotactic navigation system to localize the internal carotid artery.30
Endoscopic approach
As the techniques of endoscopic sinus surgeries matured in later 1990's, surgeons started to perform endoscopic resection of sinonasal malignancies and endoscopic nasopharyngectomy was a natural extension of application of endoscopic sinus surgery. The first reported case of endoscopic nasopharyngectomy was reported by Yoshizaki et al.31 In order to increase the space for instrument manipulation and improve visualization, the authors performed a posterior septectomy and introduced the endoscope and dissection instruments on separate nostrils. They also used an endoscope holder to allow the surgeon to use two hands for surgical dissection. Since 2004, there were several case series in the literature on endoscopic nasopharyngectomy.
The largest series of endoscopic nasopharyngectomy to date is from MY Chen and his colleagues from People's Republic of China. The cohort consisted of 72 patients; 32 rT1, 27 rT2 and 13 limited rT3 disease. The survival results were a respectable 5-year overall survival of 77.1% and 5-year loco-regional relapse free survival was 67.4%. The authors also did a case controlled comparison with IMRT and found endoscopic nasopharyngectomy superior to IMRT in terms of overall survival, disease specific survival and quality of life.32
The use of the endoscope in nasopharyngectomy improved the visualization of the surgical field during resection but instrumentation can be difficult in the narrow spaces of the nasal cavities and nasopharynx. In order to increase space for instrumentation, Al-Sheibani et al. described the endoscopic endonasal transpterygoid approach for nasopharyngectomy.33 The approach increased the working space and lateral extension of dissection by first removing the medial maxillary and posterior maxillary wall. The pterygopalatine fossa was entered with the internal maxillary artery and its branches clipped. The Vidian canal was then identified and drilled posteriorly. The Vidian canal was used as a landmark leading to the paraclival internal carotid artery. Resection of the nasopharynx was started by removal of the medial pterygoid plates and then division of the cartilaginous Eustachian tube. The dissection was then carried out from the lateral to medial fashion. This approach allowed a much wider lateral extent of resection the pure endonasal nasopharyngectomy.
Table 1 is a summary of the case series on endoscopic endonasalnasopahryngectomy. The oncological results of endoscopic nasopharyngectomy were respectable, with most series having a 2-year local control of over 80%.
Table 1.
Table summarizing the case series of endoscopic nasopharyngectomy in the literature to date.
| Authors | Location | Number of cases/follow up period (months) | T stage of recurrent disease (UICC 2002 staging) | Resection margin status | Local recurrence | Survival results | Comments |
|---|---|---|---|---|---|---|---|
| Yoshizaki et al. 200531 | Japan | 4/Not mentioned | rT2 – 4 | Not mentioned | 1 | Not mentioned | |
| Chen et al., 200734 | Taiwan | 6/16–59, median 29 | rT1 – 3 rT2a – 3 |
Not mentioned | 1 | Local control rate 83.3% at 29 months | |
| Rohaizam et al., 200935 | Malaysia | 6/3–14, median 5 | rT1 – 6 | Negative – 6 | 0 | All patients alive with no local recurrence | |
| Ko et al., 200936 | Taiwan | 28/6–32, median 13 | rT1 – 12 rT2 – 16 |
Negative – 25 Positive – 3 |
7 | 2-year OS – 57.6% 2-year DFS – 59.4% |
3 patients died of ORN 2 patients had synchronous nodal recurrence |
| Chen et al., 200937 | Guangzhou, China | 37/6–45, median 24 | rT1 – 17 rT2a – 4 rT2b – 14 rT3 – 2 |
Negative – 36 Positive – 1 |
8 | 2-year OS – 84.2% 2-year PFS – 82.6% |
1 patient died of intracranial infection 6 months after operation |
| Tay et al., 200938 | Singapore | 4/66–120 | rT1 – 1 3 unknown |
Negative – 1 Positive – 1 Unknown – 2 |
2 | DFS 66–120 months | 2 cases were adenocarcinoma |
| Ho et al., 201239 | Stanford, USA | 13 (19 surgeries)/3–48.5 mean 24.2 | rT1 – 6 rT2a – 3 rT2b – 2 rT3 – 2 |
Negative – 15 Positive – 4 |
4 | 2-year OS 100% 2-year local control – 69.2% |
|
| Cuastelnuovo et al., 201340 | Italy | 27/3–137 | rT1 – 12 rT2a – 1 rT3 – 13 rT4 – 1 |
Not mentioned | ? | 5-year OS – 72.5% 5-year DFS – 55.6% |
Mixed histology including salivary gland cancers and adenocarcinomas |
| You et al. 201532 | Guangzhou, China | 72/49.3 | rT1 – 32 rT2 – 27 rT3 – 13 |
Not mentioned | ? | 5-year OS – 77.1% 5-year LRRFS 67.4% |
OS, overall survival; DFS, disease free survival; PFS, progression free survival, LRRFS, loco-regional relapse free survival.
Robotic-assisted nasopharyngectomy
The surgical robot was designed to improve instrumentation and surgical dexterity during endoscopic and laparoscopic surgery. In order to circumvent the limitations of poor instrumentation in endoscopic nasopharyngectomy, the da Vinci surgical robot (Intuitive Surgical Inc, Sunnyvale CA) has been employed to perform endoscopic nasopharyngectomy. Unfortunately, the current generation of da Vinci surgical robot has not been designed for use in the head and neck region and needed to be adapted for use in the nasopharynx. In order for the robotic arms to reach the nasopharynx via a transoral route, the soft palate needed to be split for access. The first cadaveric experiment on robotic nasopharyngectomy was described by Ozer and Waltonen in 2008,34 and Wei and Ho published the first clinical case of salvaging recurrent nasopharyngeal cancer with the da Vinci surgical robot.35 Tsang et al. later published the first case series of robotic-assisted nasopharyngectomy of 12 cases with a 2-year local control rate of 86%.36 The case selection for robotic nasopharyngectomy was similar to endoscopic nasopharyngectomy, limited to small centrally located tumors.
Factors affecting survival and local control for salvage nasopharyngectomy
Recurrent nasopharyngeal cancer outside endemic area is still a rare entity, therefore analysis of survivals in the literature are limited to single case series only. Still these case series can offer insights on factors affecting the success of nasopharyngectomy.
Yu et al. in 2005 analyzed the results of salvaging of 275 patients with locally recurrent NPC with only 22 patients received radiotherapy. They found only survival benefits in patients with rT1 and rT2 disease, whether they were treated with re-irradiation or surgery.2
Hsu et al. reported in 2001 their cohort of 60 patients treated with salvage nasopharyngectomy via a variety of approaches.37 The 2-year overall survival was 56% and 2-year local control rate was only 50%. The suboptimal results can be explained by the fact that 32 out of 60 patients had rT3 or rT4 diseases. Univariate analysis of survival also showed that the transmandibular approach was associated with poor survival. The authors noted that the transmandibular approach was used in advanced stage disease; therefore it may well be the stage of the disease and not the approach that governed the poor outcome.
Wei et al. described their centers 20 years of experience in nasopharyngectomy via the maxillary swing approach.23 Their cohort consisted of 246 patients and they were able to achieve a negative resection margin in 78% of the patients. The 5-year actuarial local control 78% and 5-year actuarial overall survival was 56%. Negative surgical margin and a tumor size smaller than 1.5 cm in diameter were independent prognostic factors for local control and disease free survival. Chan et al. updated the cohort in 2014 and found tumor size, resection margin status, and gross tumor in the sphenoid sinus were independent prognostic factors for local tumor control. Resection margin status, synchronous cervical nodal recurrence and cavernous sinus invasion were poor prognostic factors for overall survival.38
Vlantis et al. independently in their cohort also found positive or close margin was associated with poor outcome.39 Patients with a clear surgical margin had significantly better 5-year overall survival (77%) and local progression free survival (85%) than patients with close margin (overall survival 46%, local progression free survival 72%) and patients with positive margin (overall survival 23%, local progression free survival 31%). For patients with positive resection margins, the use of post-operative radiotherapy did not improve the survival. In 2011, the authors updated their cohort and confirmed that positive margin was an associated with poor local relapse free survival.
In a separate analysis, Vlantis et al. also analyzed the difference in survival outcome of different surgical approaches.40 For patients with rT1 and rT2 diseases, the maxillary swing approach was significantly better than mid-facial degloving approach (88% vs 50%, P = 0.021) in terms of local progression free survival but there was no significant difference in the 5-year overall survival. The authors commented that the maxillary swing approach allowed a better exposure of the tissue lateral to the nasopharynx and was ideal for unilateral tumors with lateral extension to the parapharyngeal space. For the mid-facial degloving approach, the lateral access would be limited by the medial maxillary sinus walls and pterygoid plates while the inferior access would be limited by the floor of the nose. As nasopharyngeal carcinoma frequently had lateral extension, the inability of the mid-facial degloving approach might explain the worse surgical outcome compared to the maxillary swing approach.
Overall, it can be concluded that positive resection margin is associated with poor local control and overall survival. The surgeon should always attempt to achieve negative resection margins when embarking on nasopharyngectomy for salvaging locally recurrent nasopharyngeal cancer. The effect of surgical approach is less clear and probably dictated by the site and stage of the recurrent tumor.
Salvage surgery for nodal recurrences in nasopharyngeal cancer
With improvement in primary treatment, isolated nodal recurrence in NPC is now uncommon. A recent review of the treatment result in a large cohort in Hong Kong showed that only 5% of patients had isolated nodal failures. Patients presented with nodal failures should always be investigated for concurrent local failure. Results of treatment of nodal failures with irradiation had been poor with 5-year survival of only 19.7%. Re-irradiation of the neck for nodal failures was also associated with significant radiation toxicities. Therefore re-irradiation should not be the first choice in treating nodal failures. Surgical resection of the lymph nodes with recurrent disease on the other hand can avoid the toxicities of re-irradiating an area previously treated with high dose radiotherapy. A pathological study on 43 neck dissection specimens by Wei et al. has shown that 70% of the nodal recurrences had extra-capsular spread.41 The lymph nodes also had propensity to spread along the spinal accessory chain the posterior triangle. Tumor tissue in close proximity of the spinal accessory nerve was found in 27.5% of the specimens and 35% of the specimens had isolated clusters of tumor cells in the soft not inside the lymph nodes. Wei performed a follow up study in 2001, showed that metastatic cancers can spread to all five levels of lymph nodes in the neck in patients with extensive nodal metastasis.42 With these findings, Wei proposed to adopt classical radical neck dissection for salvaging nodal failures in NPC in order to eradicate the tumors in the soft tissue and extended beyond the capsule of the lymph nodes. More recently, advances in imaging especially functional imaging modality like PET scan increased the diagnostic accuracy of the extent of lymph node involvement in head and neck cancer. This leads to an recent editorial commenting that a modified or selective neck dissection, sparing some levels not involved by metastatic disease, may be adequate for salvaging nodal failures in NPC, especially for persistent disease after radiotherapy.43
The surgeon should make special consideration of the surgical techniques when performing radical neck dissection for salvaging nodal recurrence in NPC. The neck skin has been heavily radiated so when designing the neck incisions, it is advisable to avoid a 3-point junction to prevent non-healing ad break down of the wound. Instead, two parallel incisions as described by MacFee should be employed.44 If there is any break down of the wound with exposure of the underlying great vessels, a strategy of early repair with flaps like pectoralis major myocutaneous flap should be employ instead of adopting a conservative approach to avoid a vascular catastrophe.
Cross-sectional imaging ideally should be performed in all cases planned for salvage neck dissection. Special attention should be paid note the presence of carotid artery encasement by tumor, tumor invasion to the brachial plexus or deep muscle of the neck. When the tumor had invaded or encases these vital structures, it may not be feasible to completely remove all microscopic tumor surgically but may have to leave microscopic disease in the neck. The area with microscopic residual disease can then be irradiated with brachytherapy technique, delivering a high dose of radiation to a localized area. Nylon tubes are place 1 cm apart over the area with microscopic residual tumor for placement of radioactive source after the operation. The overlying skin should be resected and replaced with a loco-regional flap like a pectoralis major flap or a deltopectorial flap to avoid radiation necrosis of the skin. Once the patient is stable after the operation, radioactive sources like iridium-111 can be after-loaded into the nylon tubes to deliver high dose concentrated radiation to the concerned area.42 The reported 5-year local control of radical neck dissection of nodal recurrence in NPC was 66% while the 5-year overall survival rate was 38%.45 With the combined modality treatment of surgery and brachytherapy for extensive nodal recurrent in NPC, the local control was reported to be 60% in 3 years.42 Fig. 6, Fig. 7, Fig. 8, Fig. 9 shows a case of extended neck dissection with placement of nylon tubes for brachytherapy.
Fig. 6.
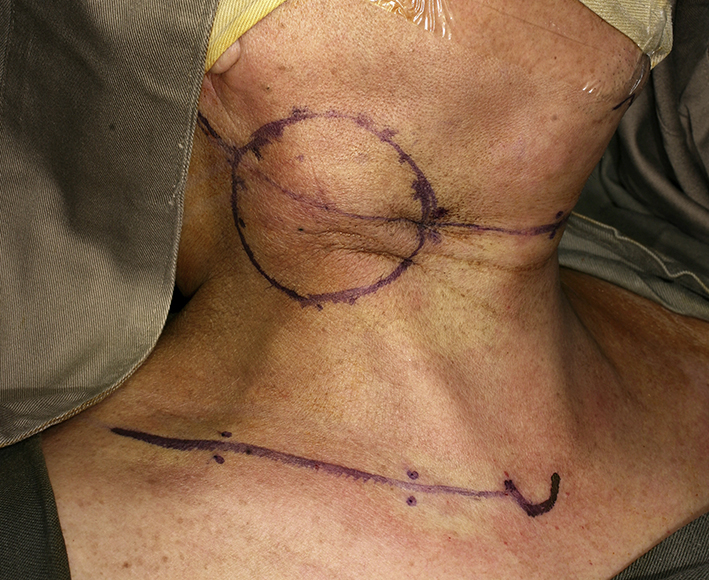
Clinical photo of patient with nodal recurrence of nasopharyngeal carcinoma. The incision was a Macfee's incision with upper and lower incision 7 cm apart. The metastatic lymph node had invaded the overlying skin and the involved skin was marked for excision.
Fig. 7.
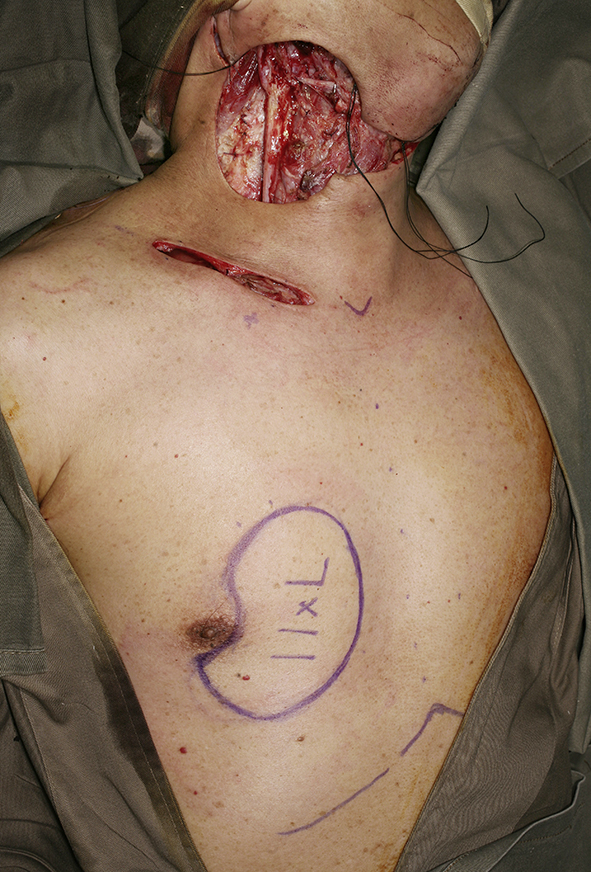
Clinical photo showing the completion of the extended neck dissection. A pectoralis myocutaneous flap was planned for coverage of the neck skin defect.
Fig. 8.
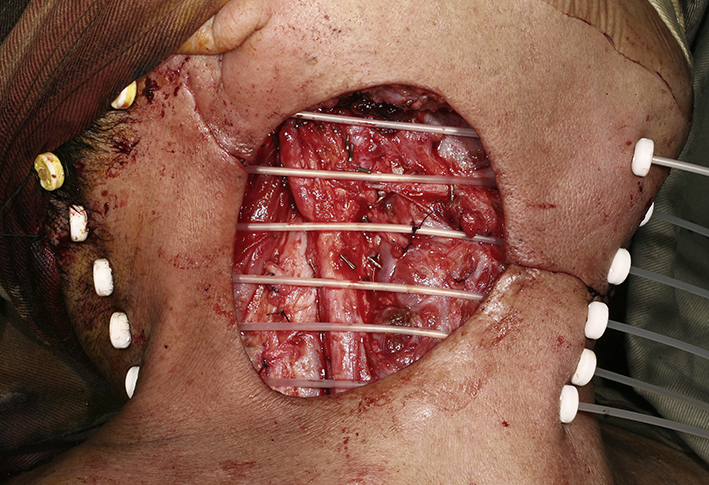
Close up view of the bed of neck dissection. Nylon tubes were inserted 1 cm apart and fixed. Radioactive iridium-111 wires would be inserted to the nylon tubes with the after-loading technique after the operation for brachytherapy.
Fig. 9.
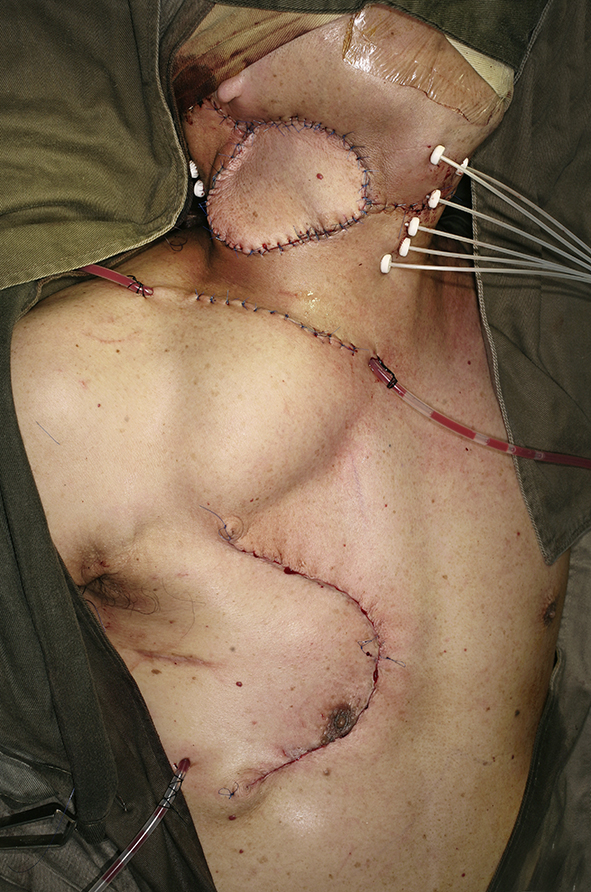
Completion of the extended right neck dissection with insertion of brachytherapy tubes and pectoralis myocutaneous flap reconstruction.
Conclusion
Although surgical treatment is not the prime modality of treatment for NPC, it played a significant role in managing loco-regional recurrence. Compared with re-irradiation, surgical treatment for recurrent NPC can avoid the toxicities associated with second radiation on a previously highly radiated field. Theoretically it can also be more effective in controlling the resistant clones of tumor cells present in the recurrent disease. Therefore when managing recurrent NPC, the patient should always be investigated for the possibility of surgical treatment.
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Chang E.T., Adami H.O. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15:1765–1777. doi: 10.1158/1055-9965.EPI-06-0353. [DOI] [PubMed] [Google Scholar]
- 2.Yu K.H., Leung S.F., Tung S.Y. Survival outcome of patients with nasopharyngeal carcinoma with first local failure: a study by the Hong Kong Nasopharyngeal Carcinoma Study Group. Head Neck. 2005;27:397–405. doi: 10.1002/hed.20161. [DOI] [PubMed] [Google Scholar]
- 3.Lee A.W., Sze W.M., Au J.S. Treatment results for nasopharyngeal carcinoma in the modern era: the Hong Kong experience. Int J Radiat Oncol Biol Phys. 2005;61:1107–1116. doi: 10.1016/j.ijrobp.2004.07.702. [DOI] [PubMed] [Google Scholar]
- 4.Teo P.M., Kwan W.H., Chan A.T. How successful is high-dose (> or = 60 Gy) reirradiation using mainly external beams in salvaging local failures of nasopharyngeal carcinoma? Int J Radiat Oncol Biol Phys. 1998;40:897–913. doi: 10.1016/s0360-3016(97)00854-7. [DOI] [PubMed] [Google Scholar]
- 5.Chua D.T., Sham J.S., Hung K.N. Stereotactic radiosurgery as a salvage treatment for locally persistent and recurrent nasopharyngeal carcinoma. Head Neck. 1999;21:620–626. doi: 10.1002/(sici)1097-0347(199910)21:7<620::aid-hed6>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 6.Chua D.T., Sham J.S., Leung L.H. Re-irradiation of nasopharyngeal carcinoma with intensity-modulated radiotherapy. Radiother Oncol. 2005;77:290–294. doi: 10.1016/j.radonc.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 7.Qiu S., Lin S., Tham I.W., Pan J., Lu J., Lu J.J. Intensity-modulated radiation therapy in the salvage of locally recurrent nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2012;83:676–683. doi: 10.1016/j.ijrobp.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Sham J.S., Choy D. Nasopharyngeal carcinoma: treatment of neck node recurrence by radiotherapy. Australas Radiol. 1991;35:370–373. doi: 10.1111/j.1440-1673.1991.tb03050.x. [DOI] [PubMed] [Google Scholar]
- 9.Hung G.U., Tsai S.C., Lin W.Y. Extraordinarily high F-18 FDG uptake caused by radiation necrosis in a patient with nasopharyngeal carcinoma. Clin Nucl Med. 2005;30:558–559. doi: 10.1097/01.rlu.0000170039.14351.0b. [DOI] [PubMed] [Google Scholar]
- 10.Liu S.H., Chang J.T., Ng S.H. False positive fluorine-18 fluorodeoxy-D-glucose positron emission tomography finding caused by osteoradionecrosis in a nasopharyngeal carcinoma patient. Br J Radiol. 2004;77:257–260. doi: 10.1259/bjr/69516821. [DOI] [PubMed] [Google Scholar]
- 11.Ng S.H., Chan S.C., Yen T.C. Comprehensive imaging of residual/recurrent nasopharyngeal carcinoma using whole-body MRI at 3 T compared with FDG-PET-CT. Eur Radiol. 2010;20:2229–2240. doi: 10.1007/s00330-010-1784-9. [DOI] [PubMed] [Google Scholar]
- 12.Fisch U., Pillsbury H.C. Infratemporal fossa approach to lesions in the temporal bone and base of the skull. Arch Otolaryngol. 1979;105:99–107. doi: 10.1001/archotol.1979.00790140045008. [DOI] [PubMed] [Google Scholar]
- 13.Fisch U. The infratemporal fossa approach for nasopharyngeal tumors. Laryngoscope. 1983;93:36–44. doi: 10.1288/00005537-198301000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Fee W.E., Gilmer P.A., Goffinet D.R. Surgical management of recurrent nasopharyngeal carcinoma after radiation failure at the primary site. Laryngoscope. 1988;98:1220–1226. doi: 10.1288/00005537-198811000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Tu G.Y., Hu Y.H., Xu G.Z., Ye M. Salvage surgery for nasopharyngeal carcinoma. Arch Otolaryngol Head Neck Surg. 1988;114:328–329. doi: 10.1001/archotol.1988.01860150110026. [DOI] [PubMed] [Google Scholar]
- 16.Wilson C.P. Observations on the surgery of the nasopharynx. Ann Otol Rhinol Laryngol. 1957;66:5–40. doi: 10.1177/000348945706600101. [DOI] [PubMed] [Google Scholar]
- 17.Fee W.E., Jr., Moir M.S., Choi E.C. Nasopharyngectomy for recurrent nasopharyngeal cancer: a 2- to 17-year follow-up. Arch Otolaryngol Head Neck Surg. 2002;128:280–284. doi: 10.1001/archotol.128.3.280. [DOI] [PubMed] [Google Scholar]
- 18.Morton R.P., Liavaag P.G., McLean M. Transcervico-mandibulo-palatal approach for surgical salvage of recurrent nasopharyngeal cancer. Head Neck. 1996;18:352–358. doi: 10.1002/(SICI)1097-0347(199607/08)18:4<352::AID-HED7>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 19.King W.W., Ku P.K., Mok C.O. Nasopharyngectomy in the treatment of recurrent nasopharyngeal carcinoma: a twelve-year experience. Head Neck. 2000;22:215–222. doi: 10.1002/(sici)1097-0347(200005)22:3<215::aid-hed2>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 20.Arriaga M.A., Janecka I.P. Facial translocation approach to the cranial base: the anatomic basis. Skull Base Surg. 1991;1:26–33. doi: 10.1055/s-2008-1056975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei W.I., Lam K.H., Sham J.S. New approach to the nasopharynx: the maxillary swing approach. Head Neck. 1991;13:200–207. doi: 10.1002/hed.2880130306. [DOI] [PubMed] [Google Scholar]
- 22.Wei W.I., Ho C.M., Yuen P.W. Maxillary swing approach for resection of tumors in and around the nasopharynx. Arch Otolaryngol Head Neck Surg. 1995;121:638–642. doi: 10.1001/archotol.1995.01890060036007. [DOI] [PubMed] [Google Scholar]
- 23.Wei W.I., Chan J.Y., Ng R.W. Surgical salvage of persistent or recurrent nasopharyngeal carcinoma with maxillary swing approach – Critical appraisal after 2 decades. Head Neck. 2011;33:969–975. doi: 10.1002/hed.21558. [DOI] [PubMed] [Google Scholar]
- 24.Hao S.P., Tsang N.M., Chang K.P. Nasopharyngectomy for recurrent nasopharyngeal carcinoma: a review of 53 patients and prognostic factors. Acta Otolaryngol. 2008;128:473–481. doi: 10.1080/00016480701813806. [DOI] [PubMed] [Google Scholar]
- 25.Hao S.P. Facial translocation approach to the skull base: the viability of translocated facial bone graft. Otolaryngol Head Neck Surg. 2001;124:292–296. doi: 10.1067/mhn.2001.112308. [DOI] [PubMed] [Google Scholar]
- 26.Casson P.R., Bonanno P.C., Converse J.M. The midface degloving procedure. Plast Reconstr Surg. 1974;53:102–103. doi: 10.1097/00006534-197401000-00026. [DOI] [PubMed] [Google Scholar]
- 27.Howard D.J., Lund V.J. The midfacial degloving approach to sinonasal disease. J Laryngol Otol. 1992;106:1059–1062. doi: 10.1017/s0022215100121759. [DOI] [PubMed] [Google Scholar]
- 28.Howard D.J., Lund V.J. The role of midfacial degloving in modern rhinological practice. J Laryngol Otol. 1999;113:885–887. doi: 10.1017/s0022215100145505. [DOI] [PubMed] [Google Scholar]
- 29.To E.W., Teo P.M., Ku P.K. Nasopharyngectomy for recurrent nasopharyngeal carcinoma: an innovative transnasal approach through a mid-face deglove incision with stereotactic navigation guidance. Br J Oral Maxillofac Surg. 2001;39:55–62. doi: 10.1054/bjom.2000.0479. [DOI] [PubMed] [Google Scholar]
- 30.To E.W., Yuen E.H., Tsang W.M. The use of stereotactic navigation guidance in minimally invasive transnasal nasopharyngectomy: a comparison with the conventional open transfacial approach. Br J Radiol. 2002;75:345–350. doi: 10.1259/bjr.75.892.750345. [DOI] [PubMed] [Google Scholar]
- 31.Yoshizaki T., Wakisaka N., Murono S. Endoscopic nasopharyngectomy for patients with recurrent nasopharyngeal carcinoma at the primary site. Laryngoscope. 2005;115:1517–1519. doi: 10.1097/01.MLG.0000165383.35100.17. [DOI] [PubMed] [Google Scholar]
- 32.You R., Zou X., Hua Y.J. Salvage endoscopic nasopharyngectomy is superior to intensity-modulated radiation therapy for local recurrence of selected T1-T3 nasopharyngeal carcinoma – A case-matched comparison. Radiother Oncol. 2015;115:399–406. doi: 10.1016/j.radonc.2015.04.024. [DOI] [PubMed] [Google Scholar]
- 33.Al-Sheibani S., Zanation A.M., Carrau R.L. Endoscopic endonasal transpterygoid nasopharyngectomy. Laryngoscope. 2011;121:2081–2089. doi: 10.1002/lary.22165. [DOI] [PubMed] [Google Scholar]
- 34.Ozer E., Waltonen J. Transoral robotic nasopharyngectomy: a novel approach for nasopharyngeal lesions. Laryngoscope. 2008;118:1613–1616. doi: 10.1097/MLG.0b013e3181792490. [DOI] [PubMed] [Google Scholar]
- 35.Wei W.I., Ho W.K. Transoral robotic resection of recurrent nasopharyngeal carcinoma. Laryngoscope. 2010;120:2011–2014. doi: 10.1002/lary.21059. [DOI] [PubMed] [Google Scholar]
- 36.Tsang R.K., To V.S., Ho A.C. Early results of robotic assisted nasopharyngectomy for recurrent nasopharyngeal carcinoma. Head Neck. 2015;37:788–793. doi: 10.1002/hed.23672. [DOI] [PubMed] [Google Scholar]
- 37.Hsu M.M., Hong R.L., Ting L.L. Factors affecting the overall survival after salvage surgery in patients with recurrent nasopharyngeal carcinoma at the primary site: experience with 60 cases. Arch Otolaryngol Head Neck Surg. 2001;127:798–802. [PubMed] [Google Scholar]
- 38.Chan J.Y., To V.S., Chow V.L. Multivariate analysis of prognostic factors for salvage nasopharyngectomy via the maxillary swing approach. Head Neck. 2014;36:1013–1017. doi: 10.1002/hed.23403. [DOI] [PubMed] [Google Scholar]
- 39.Vlantis A.C., Tsang R.K., Yu B.K. Nasopharyngectomy and surgical margin status: a survival analysis. Arch Otolaryngol Head Neck Surg. 2007;133:1296–1301. doi: 10.1001/archotol.133.12.1296. [DOI] [PubMed] [Google Scholar]
- 40.Vlantis A.C., Yu B.K., Kam M.K. Nasopharyngectomy: does the approach to the nasopharynx influence survival? Otolaryngol Head Neck Surg. 2008;139:40–46. doi: 10.1016/j.otohns.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 41.Wei W.I., Ho C.M., Wong M.P. Pathological basis of surgery in the management of postradiotherapy cervical metastasis in nasopharyngeal carcinoma. Arch Otolaryngol Head Neck Surg. 1992;118:923–929. doi: 10.1001/archotol.1992.01880090039012. discussion 930. [DOI] [PubMed] [Google Scholar]
- 42.Wei W.I., Ho W.K., Cheng A.C. Management of extensive cervical nodal metastasis in nasopharyngeal carcinoma after radiotherapy: a clinicopathological study. Arch Otolaryngol Head Neck Surg. 2001;127:1457–1462. doi: 10.1001/archotol.127.12.1457. [DOI] [PubMed] [Google Scholar]
- 43.Khafif A., Ferlito A., Takes R.P. Is it necessary to perform radical neck dissection as a salvage procedure for persistent or recurrent neck disease after chemoradiotherapy in patients with nasopharyngeal cancer? Eur Arch Otorhinolaryngol. 2010;267:997–999. doi: 10.1007/s00405-010-1235-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.MacFee W.F. Transverse incisions for neck dissection. Ann Surg. 1960;151:279–284. doi: 10.1097/00000658-196002000-00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wei W.I., Lam K.H., Ho C.M. Efficacy of radical neck dissection for the control of cervical metastasis after radiotherapy for nasopharyngeal carcinoma. Am J Surg. 1990;160:439–442. doi: 10.1016/s0002-9610(05)80561-6. [DOI] [PubMed] [Google Scholar]


