Abstract
Interleukin-6 (IL-6) is a pleiotropic cytokine which plays an important role in a number of cellular processes including proliferation, survival, differentiation, migration and invasion. IL-6 mediates its downstream effects by activating a number of signaling cascades including JAK/STAT, PI3K/AKT and MAPK pathways. In addition to its effects on tumor cells, IL-6 also regulates tumor progression and tumor metastasis by modulating tumor angiogenesis and tumor lymphangiogenesis. A number of studies have shown that IL-6 levels are markedly upregulated in cancer patients. We and others have shown that high IL-6 expression independently predicts tumor recurrence, tumor metastasis and poor survival in head and neck cancer patients. Therefore targeting IL-6 signaling is a potential therapeutic strategy for the treatment of head and neck squamous cell carcinoma (HNSCC). In this review, we discuss the current understanding of the role of IL-6 in HNSCC progression and potential therapeutic strategies to target IL-6 signaling for the treatment of head and neck cancer patients.
Keywords: Head and neck squamous cell carcinoma, Interleukin-6, JAK, STAT3, MAPK, PI3K/Akt, Nanog
Introduction
Head and neck squamous cell carcinoma (HNSCC) remains a major health care problem worldwide, comprising almost 50% of all malignancies in some developing nations.1, 2, 3 Although advancements in the anti-cancer treatments including surgery, radiation and chemotherapy have increased the local control of HNSCC, the overall survival rates have not improved significantly over the last three decades.3, 4 Five year survival rates for patients with early stage localized head and neck cancers are more than 80% but drops to 40% when the disease has spread to the neck nodes, and to below 20% for patients with distant metastatic disease.4 Acquisition of chemoresistance and metastatic phenotype are the major causes of treatment failure and mortality in these patients.1, 5 It is therefore imperative that we gain a better understanding of the molecular mechanisms that contribute to the aggressive tumor phenotype in order to develop novel and effective strategies for the treatment of head and neck cancer patients.
Interleukin-6 (IL-6) is one of the key molecules that has been widely studied and implicated in poor clinical outcomes in HNSCC patients.6, 7, 8, 9, 10 IL-6 was initially identified and cloned as B-cell stimulatory factor-2.11, 12, 13 At the same time a number of other molecules (IFN-β2, plasmacytoma growth factor and hepatocyte-stimulating factor) were independently cloned and found to be identical to IL-6.14, 15, 16 Accumulating evidence has shown that IL-6 plays an important role in a number of biological process including immune regulation, hematopoiesis, inflammation and oncogenesis.17, 18, 19 IL-6 is produced by a wide variety of cell types including immune cells (macrophages, dendritic cells and B-cells), endothelial cells and tumor cells.20, 21, 22, 23 We and others have shown that IL-6 levels are markedly elevated in the blood samples from cancer patients including HNSCC and independently predict tumor recurrence, poor survival and tumor metastasis.6, 7, 8 A number of mechanistic studies have corroborated these clinical observations. Using our in vitro and in vivo head and neck cancer models, we were able to demonstrate that IL-6 is a potent inducer of epithelial to mesenchymal transition (EMT) in head and neck cancer cell lines thereby promoting regional (lymph node) and distant (lung) metastasis.20 Similarly, Lederle et al24 demonstrated that IL-6 promotes malignant growth of squamous cell carcinoma by regulating a complex cytokine and protease network. Recent studies have also highlighted the role of IL-6 in the acquisition of chemoresistance and stem cell phenotype in cancer cells.25, 26, 27, 28 We hereby present a review of recent studies that demonstrate the role of IL-6 in head and neck cancer progression.
Clinical significance of IL-6 in HNSCC
The association of IL-6 with clinical parameters (clinicopathological factors) and oncological outcomes in HNSCC has been largely studied over the past two decades. Several studies have shown elevated levels of IL-6 in HNSCC.6, 8, 26, 29 In a study of 65 untreated HNSCC patients and 20 healthy volunteers, Mojtahedi et al29 found that serum levels of IL-6 and IL-18 were significantly increased in HNSCC patients compared to healthy individuals, however only the difference of IL-6 levels was found to be statistically significant. In addition, they showed that IL-6 concentration increased as tumor stage progressed and a significant difference was observed between stage IV vs stage I/II/III disease. These results suggest the activation of the Th2 arm of the immune response in HNSCC patients. However, Lathers et al30 found elevated levels of IL-2 and GM-CSF in addition to IL-4, IL-6 and IL-10, thereby suggesting that HNSCC patients might have incomplete Th2 skewing. This theory of incomplete Th2 immune switch was further supported by the work of Sparano et al31 where they examined blood samples from 58 patients of histologically proven HNSCC and showed that there were significantly higher levels of IL-6 and IL-10 as compared to IL-12. In addition, they showed that T3 and T4 patients had a positive relationship between tumor size and serum IL-6 levels. Similarly, in a case–control study of 90 HNSCC patients and 39 controls, Riedel et al8 showed higher levels of IL-6 in serum of HNSCC compared to healthy controls. They also showed a statistically significant correlation between serum IL-6 concentrations and with higher tumor stage and positive lymph nodes. In a prospective study of 85 patients with primary HNSCC, Tartour et al32 showed a significant association between higher lymph node (N) classification and elevated serum IL-6 levels.
In addition to serum levels of IL-6, tumor IL-6 expression both at mRNA and protein levels are also directly correlated with higher tumor stage and positive lymph nodes.9, 33 Wang et al33 examined IL-6R and IL-6 mRNA expression in 86 oral squamous cell carcinoma tumor specimens and showed significantly higher IL-6R and IL-6 mRNA expression in tumor samples as compared to normal mucosa. IL-6 and IL-6R mRNA levels were also associated with larger tumors and more advanced histological grade. We have recently examined IL-6 expression in HNSCC by immunohistochemistry and our results show a direct correlation between IL-6 expression and tumor stage, tumor recurrence, perineural invasion, extracapsular spread and inversely associated with HPV status.9
Considering the direct correlation between elevated IL-6 levels and high risk clinicopathological features in HNSCC, it does not come as a surprise that increased IL-6 levels are also associated with poor oncological outcomes in HNSCC and are reflective of a high burden of disease. This concept is further strengthened by the reduction of IL-6 levels after treatment in HNSCC patients. Several reports have studied IL-6's association with oncological outcomes in HNSCC. Allen et al34 studied numerous cytokines (IL-6, IL-8, growth-related oncogene-1 [GRO-1], VEGF and hepatocyte growth factors) longitudinally in a small prospective study of 30 patients with advanced oropharyngeal HNSCC receiving chemoradiation treatment by measuring serum levels at baseline and every 3 months. They showed a significant decrease in disease specific survival with longitudinal increase in levels of IL-6, suggesting that IL-6 could be a biomarker of treatment response and survival. Similarly, De Schutter et al35 did a retrospective study of 34 patient samples showing that pre-treatment IL-6 serum level is an independent predictor of local control, disease free survival and overall survival. These concepts where further strengthened by our large prospective, longitudinal cohort study of 444 patients, where we have shown that serum IL-6 level was an independent predictor of recurrence and poor prognosis.
Elevated IL-6 levels are not only linked to tumor progression but have also shown to mediate chemo and radio-resistance in HNSCC. Jinno et al26 have shown that the high IL-6 expressing group showed significantly poor tumor response to the preoperative chemoradiotherapy as compared to the negative or low IL-6 expressing group. Similarly, Argiris et al36 showed that IL-6 levels were inversely associated with tumor response to induction chemotherapy in HNSCC patients with stages III–IVB receiving cisplatin doclataxel and Cetuximab. Recently, Stanam et al37 showed that upregulated IL-6 expression contributes to erlotinib resistance in HNSCC. Additionally, De Schutter et al35 report a potential link between IL-6 and radio-resistance in HNSCC. High IL-6 levels in HNSCC patients have also been found to be associated with health behaviors. We have recently shown that smoking (current and former) and decreased sleep was directly correlated with higher IL-6 levels.38 We also showed that smoking was negatively associated with response to treatment and was an independent predictor of recurrence and poor prognosis. In addition, higher IL-6 levels were associated with mucositis and weight loss in HNSCC patients.
IL-6 effects on head and neck cancer progression and metastasis
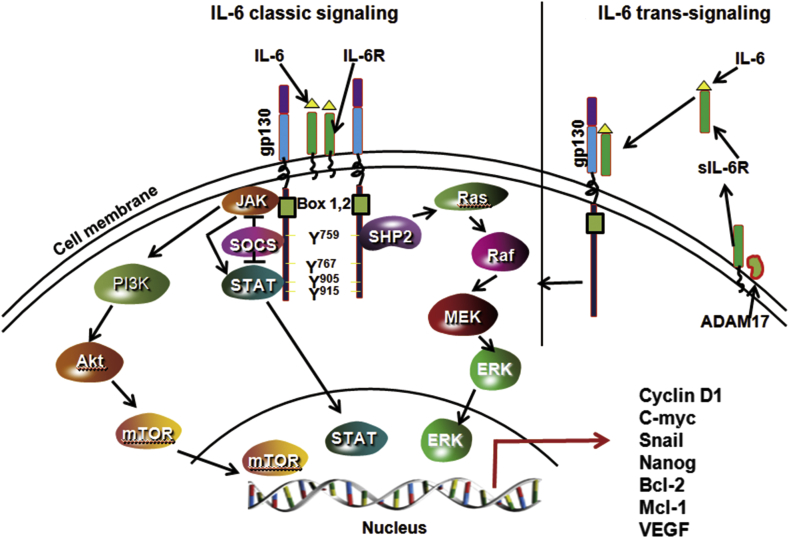
IL-6 is one of the cytokines that is commonly overexpressed in most cancer types including HNSCC.6, 7, 39, 40, 41, 42, 43 IL-6 is a part of the cytokine family that shares a common glycoprotein 130 receptor (gp130; also known as CD130).44, 45 Other members of IL-6 family include IL-11, leukemia inhibitory factor (LIF), ciliar neurotrophic factor (CTNF), oncostatin M (OSM), cardiotrophin-1 (CT-1) and cardiotrophin like cytokine (CLC).46, 47 IL-6 mediates its signaling by binding to an 80 kDa type-1 cell surface cytokine α-receptor subunit (IL-6R; also known as CD126)48 and a universally expressed 130 kDa gp130 to form an IL-6/IL-6R/gp130 complex that is clustered into a dimer structure (Fig. 1).49 Once IL-6/IL-6R/gp130 complex is formed, it recruits multiple signaling partners to mediate the IL-6's wide range of cellular functions. One of the widely studied IL-6-mediated signaling pathway is the Jak family and STAT family of transcription factors.46 Dimerization of IL-6/IL-6R/gp130 leads to the activation of Jak family of kinases (Jak1, Jak2 and Tyk2) and recruitment of STAT proteins (STAT1, STAT3 and STAT5).50 STAT3 is one main STAT family member that is extensively studied in cancer.51 Once STAT3 is activated, it forms a dimer in which the SH2 domain of one phospho-STAT3 binds to the phosphorylated Tyr705 of another STAT3 molecule and vice versa.52 The STAT3 dimers then translocate to the nucleus to regulate the expression a number of genes. The activation of JAK/STAT3 signaling by IL-6 is tightly regulated by SOCS (suppressor of cytokine signaling) and PIAS (protein inhibitor of activated STAT).53 In addition to the activation of JAK/STAT3 pathway, IL-6 also mediates its signaling by activating PI3K/AKT, RAS/MAPK and Wnt signaling pathways.20, 54, 55
Fig. 1.
IL-6 signaling pathways. IL-6 can mediate its intracellular signal by using both membrane-bound IL-6 receptor (IL-6 classical signaling) or soluble IL-6 receptor (IL-6 trans-signaling). In the classical IL-6 signaling, IL-6 binds to membrane-bound IL-6R (non-signaling receptor) and gp130 (signaling arm of IL-6 receptor) to form a complex. A complete functional complex consists of dimers of IL-6, IL-6R and gp130. IL-6 trans-signaling occurs in cells that lacks IL-6R. In this pathway, IL-6 first binds to soluble IL-6R (sIL-6R) to form a complex and then this complex binds to gp130 at the cell surface to mediate the intracellular signaling. A soluble form of IL-6 is released from cell surfaces by proteolytic cleavage (ADAM 17) or splicing of IL-6R mRNA. In both types of signaling, JAKs binds to Box1 and 2 of gp130 which leads to activation of JAKs. STATs also binds to gp130 but are phosphorylated by JAKs. IL-6 also activate PI3K/Akt pathway via JAKs. Both SOCS and SHP2 bind to gp130 via tyrosine 759. SOCS negatively regulate IL-6/STAT3 signaling by inhibiting both JAKs and STATs. SHP2 plays an important role in relating IL-6-mediated MAPK signaling.
Although gp130 is universally expressed in human cells, IL-6R (gp126) expression is highly restricted to a few types of cells.56 Recent studies have shown that IL-6 is still able to mediate its signaling in the cells that lack IL-6R expression through an alternate signaling mechanism known as ‘trans-signaling’.57, 58 In the ‘classical signaling’ pathway, IL-6 binds to membrane-bound IL-6R, whereas in ‘trans-signaling’ circulating IL-6 binds to the soluble form of IL-6R (sIL-6R) and then this complex binds to membrane-bound gp130 and mediates the downstream signaling (Fig. 1). The significance of trans-signaling was highlighted by the presence of the biologically active form of sIL-6R in the blood samples from cancer patients.59, 60 The importance of the sIL-6R is highlighted by the finding that 70% of the sIL-6R forms complexes with IL-6 in the blood and then binds directly to the membrane-bound gp130.61 In addition, sIL-6R can function as a carrier protein for IL-6, thereby markedly prolonging the plasma half-life and signaling of IL-6.62
A number of studies have shown that IL-6 mediates persistent activation of the STAT3 pathway and up regulation of downstream target genes in head and neck cancers leading to increased tumor cell proliferation, migration, survival, invasion, epithelial to mesenchymal transition (EMT), cancer stem cell expansion, and chemoresistance.20, 37, 63 In addition to IL-6, STAT3 can also be activated by EGFR that is often overexpressed in head and neck cancer cells.64 However, recent studies have shown that the persistent activation of STAT3 in HNSCC is predominantly mediated by IL-6 and not by the EGFR pathway.65, 66 Sriuranpong et al65 examined the interplay between STAT3, IL-6 and EGFR pathways using a panel of HNSCC cell lines. They showed that STAT3 was active in most of the cell lines, although only 3 out 10 HNSCC cell lines were moderately to strongly positive for activated EGFR. Even in EGFR-positive cell lines, STAT3 activation was not dependent on EGFR activation, as activated STAT3 persisted after treatment with AG1478 (EGFR inhibitor). In contrast, immunodepletion of IL-6 or blocking of gp130 (the signaling unit of the IL-6R complex) abolished STAT3 phosphorylation, thereby confirming IL-6-dependent and EGFR-independent STAT3 activation. In a similar study, Squarize et al66 showed an increased activity of IL-6 promoter in HNSCC cells, which was dependent on the presence of an intact NFkappaB site. Higher IL-6 expression in turn promoted STAT3 activation in the EGFR-independent manner. The IL-6/STAT3 pathway has also been shown to promote cell survival in HNSCC cells by modulating the expression of serpin B3/B4, known as squamous cell carcinomas antigens 1 and 2.67 A study by Ahmed et al67 showed that targeting of IL-6/STAT3 signaling by a novel antibody against gp130 significantly inhibited STAT3 activation leading to prompt disappearance of cysteine proteases of serpin B3/B4 mRNAs and markedly enhancing tumor cell apoptosis. Recent studies have also shown that IL-6 modulates HNSCC tumorigenesis through epigenetic gene silencing via altering the CpG promoter methylation and repressing a number of tumor suppressor genes including CHFR, GATA5 and PAX6.68
Epithelial-mesenchymal transition (EMT) is a key process in tumor metastatic cascade that is characterized by the loss of cell–cell junctions and cell polarity, resulting in the acquisition of migratory and invasive properties.69, 70, 71 We have recently shown that IL-6 promotes EMT in head and neck cancer cells by repressing E-cadherin expression via the JAK/STAT3 signaling pathway.20 STAT3 knockdown in tumor cells or normal keratinocytes significantly decreased IL-6-mediated cell scattering, cell motility and reversal of EMT phenotype. Furthermore, tumor cells overexpressing IL-6 showed marked increase in lymph node and lung metastasis in a SCID mouse xenograft model. IL-6 has also been shown to modulate tumor cell motility by a number of downstream mediators. In our study, we show that IL-6/STAT3 signaling modulate cell motility by regulating Focal Adhesion Kinase (FAK) activation.20 Whereas Su et al63 have shown that IL-6 enhances head and neck tumor cell motility by stabilizing Twist via the activation of casein kinase 2. Similarly, IL-6 has been shown to enhance head and neck cancer cell invasiveness by enhancing the expressions of MMP-1 and MMP-9.72, 73, 74 In addition, IL-6 also promotes tumor growth, metastasis and chemoresistance by enhancing tumor stem cell phenotype.25, 28 We have recently shown that RhoC modulates cancer stem cell phenotype in head and neck cancer cells by regulating the expression of key stem cell transcription factors (nanog, oct3/4 and sox2) via the IL-6/STAT3 signaling pathway.25 IL-6/STAT3 signaling cascade was also shown to enhance cancer stem cell survival in HNSCC.28 Blocking of IL-6 signaling by tocilizumab (humanized anti-IL-6R antibody) significantly decreased cancer stem cell population and markedly enhanced the anti-tumor effects of conventional chemotherapy.75
IL-6/STAT3 signaling as a potential target to treat HNSCC patients
A number of clinical studies have shown that IL-6 levels are directly associated with poor overall survival, higher tumor stage, tumor recurrence and metastasis in a number of cancer types including HNSCC.6, 7, 8, 26, 36, 72 Therefore, targeting IL-6 signaling is a potential therapeutic strategy for the treatment of patients with HNSCC. Because IL-6 has been shown to use multiple downstream molecules to mediate its biological function, it comes as no surprise that molecules with diverse modes of action have been tried as therapeutics to block IL-6 signaling (Table 1). Johnson et al76 employed high-content imaging (HCS) assays to screen 1726 compounds from the Library of Pharmacologically Active Compounds and identified 51 inhibitors of IL-6-induced pSTAT3 activation. However, only three of these inhibitors selectively inhibited STAT3 as compared with STAT1. They subsequently confirmed Azelastine, an H1 receptor antagonist, as a selective inhibitor of IL-6-induced pSTAT3 activation that also reduced the growth of HNSCC cell lines. In the subsequent study, the same group screened a library of 94,491 compounds from the Molecular Library Screening Center Network (MLSCN) for the ability to inhibit IL-6-induced pSTAT3 activation.77 Two hundred and three concentration-dependent inhibitors of IL-6-induced pSTAT3 activation were identified from this library and finally four chemical series (Guanidinoquinazolines, Triazolothiadiazines, Amino alcohols, and Oxazole-piperazine singleton) progressed to lead optimization stage.
Table 1.
Drugs designed to target IL-6 signaling.
| Drug | Activity |
|---|---|
| Ruxolitinib | Inhibitor of JAK1 and JAK287 |
| AZD1480 | Inhibitor of JAK1 and JAK288 |
| BMS-911543 | Selective JAK2 inhibitor89 |
| STAT3 decoy | Highly specific inhibitor of STAT385, 86 |
| Tocilizumab | Humanized anti-human IL-6R antibody28 |
| Siltuximab | Anti-IL-6 antibody91, 92 |
| G-quartet Oligodeoxynucleotides | Disrupts STAT3 DNA binding96 |
| mAb 1339 | Anti-IL-6 monoclonal antibody97 |
| BE-8 | Anti-IL-6 monoclonal antibody90 |
| Eriocalyxin B (EB) | Inhibits constitutive and IL-6-mediated STAT3 activation98 |
| Curcumin (diferuloylmethane) Curcumin analog (H-4073) |
Inhibits STAT3 phosphorylation and nuclear translocation80 Inhibits STAT3 phosphorylation and chemoresistance81 |
| Azelastine | Inhibits IL-6-mediated STAT3 activation76 |
| Guanidinoquinazolines, Triazolothiadiazines, Amino alcohols, and Oxazole-piperazine singleton | Inhibits IL-6-mediated STAT3 activation77 |
Recent studies have demonstrated that curcumin, the constituent of the spice turmeric, in addition to its anti-inflammatory function also show potent anti-proliferative property in several tumor types. Curcumin has been shown to mediate its anti-tumor effects by inhibiting a number of signaling pathways including IL-6/STAT3 pathway.78, 79 However, use of curcumin has been limited due to its poor bio-absorption.80 In order to overcome the poor bio-absorption of curcumin, we have recently developed a novel class of curcumin analogs, based on diarylidenylpiperidones (DAP), by incorporating a piperidone link to the beta-diketone structure and fluoro substitutions on the phenyl groups.81 This compound (H-4073) showed >5 fold higher cellular uptake in head and neck cancer cell lines as compared to curcumin and inhibited cell proliferation in a dose dependent manner. H-4073 mediated its anti-tumor effects by inhibiting JAK/STAT3, FAK, Akt and VEGF signaling pathways that play important roles in cell proliferation, migration, survival and angiogenesis. In addition, pre-treatment of cisplatin-resistant HNSCC cell lines with H-4073 significantly reversed chemoresistance. In the SCID mouse xenograft model, H-4073 significantly enhanced the anti-tumor and anti-angiogenesis effects of cisplatin, with no added systemic toxicity. Another plant based natural compound (−)-epigallocatechingallate (EGCG) has been shown to inhibit IL-6-mediated STAT3 phosphorylation.82, 83, 84 Lin et al82 showed that EGCG induces Fas/CD95-mediated apoptosis in head and neck squamous cell carcinoma cells by inhibiting constitutive and IL-6-induced JAK/STAT3 signaling. EGCG was also able to block EGFR signaling and enhanced the anti-tumor effects of 5-flurouracil based chemotherapy.83 Recently, a chemically modified cyclic STAT3 decoy oligonucleotide with improved serum and thermal stability was designed and tested in a nude mouse model. STAT3 decoy demonstrated a significant decrease in tumor volume compared with the control groups (mutant cyclic STAT3 decoy or saline) in conjunction with down modulation of STAT3 target gene expression without any significant side effects.85 This STAT3 decoy oligonucleotide was further tested in phase 0 clinical trials.86 Intravenous injection of the cyclic STAT3 decoy inhibited tumor growth and downregulated STAT3 target genes in the tumors.
Although targeting of the IL-6/STAT3 pathway in the head and neck cancer seems very promising, it has been challenging so far to target STAT3 using small molecule inhibitors in humans. What appears more promising is the use of JAK receptor antagonists. IL-6 phosphorylates STAT3 through Janus kinases (JAK) 1 & 2. Ruxolitinib is the first FDA approved drug that targets JAK1 and JAK2 kinases for myelofibrosis.87 Other drugs including AZD1480, a preclinical JAK1/2 inhibitor, suppressed IL-6-induced STAT3 phosphorylation and showed subsequent human tumor growth suppression.88 Additionally, BMS-911543, a JAK2 selective inhibitor, showed similar suppression of tumor growth.89 These drugs have not been tested in HNSCC yet. However, their potential benefits by blocking IL-6/STAT3 signaling do warrant HNSCC studies.
Another strategy to target IL-6/STAT3 signaling is to block IL-6 binding to IL-6R through neutralizing antibodies. Antibodies targeting IL-6 prevent the association of the ligand with its receptor, leading to prevention of STAT3 activation. Most of the studies using anti-IL-6 antibodies so far have been carried out using murine or humanized monoclonal antibodies.90, 91, 92 BE-8, a murine anti-IL-6 antibody, was developed by Diaclone and showed limited anti-tumor response.90 It was shown that BE-8 could not block the daily IL-6 production >8 mg.90 In addition BE-8 had a short half-life (3–4 days) and there is a natural drawback of murine antibodies inducing host anti-mouse antibody response.93 In contrast Siltuximab is a chimeric humanized monoclonal antibody that was developed by Centocor and has a much longer half-life (2 weeks).94 Siltuximab is currently in a phase II clinical trials for treatment in prostate cancer and multiple myeloma.91, 95 Recently, tocilizumab, a humanized anti-IL-6R antibody (developed by Hoffmann-La Roche and Chugai), was tested in head and neck cancer model and was shown to disrupt primary human tumor initiation mediated by cancer stem cells.28
Concluding remarks
In this review, we examined the role of IL-6 as a prognostic marker for patients with HNSCC, the importance of IL-6 in HNSCC tumor proliferation and metastasis, and finally the therapeutic potential of targeting the IL-6/STAT3 pathway. There is extensive data available to associate IL-6 levels in patients with clinicopathological parameters and oncological outcomes in HNSCC. Although IL-6 receptor is not expressed universally in different cell types, IL-6 can still mediate its intracellular signaling in these cells through trans-signaling mechanisms. As a result, IL-6/STAT3 signaling pathway is examined as an important therapeutic target. There has been extensive progress made in designing and testing novel drugs that target IL-6 signaling (Table 1), but we still need continued efforts to develop novel drugs which could effectively block this signaling pathway without significant adverse effects.
Funding program
NIH/NCI-CA178649 (P. Kumar) and The Ohio State University Comprehensive Cancer Center.
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Leemans C.R., Braakhuis B.J., Brakenhoff R.H. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011;11:9–22. doi: 10.1038/nrc2982. [DOI] [PubMed] [Google Scholar]
- 2.Mehanna H., Paleri V., West C.M., Nutting C. Head and neck cancer – part 1: epidemiology, presentation, and prevention. BMJ. 2010;341:c4684. doi: 10.1136/bmj.c4684. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R., Naishadham D., Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 4.Kalavrezos N., Bhandari R. Current trends and future perspectives in the surgical management of oral cancer. Oral Oncol. 2010;46:429–432. doi: 10.1016/j.oraloncology.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Worden F.P., Kumar B., Lee J.S. Chemoselection as a strategy for organ preservation in advanced oropharynx cancer: response and survival positively associated with HPV16 copy number. J Clin Oncol. 2008;26:3138–3146. doi: 10.1200/JCO.2007.12.7597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duffy S.A., Taylor J.M., Terrell J.E. Interleukin-6 predicts recurrence and survival among head and neck cancer patients. Cancer. 2008;113:750–757. doi: 10.1002/cncr.23615. [DOI] [PubMed] [Google Scholar]
- 7.Chen Z., Malhotra P.S., Thomas G.R. Expression of proinflammatory and proangiogenic cytokines in patients with head and neck cancer. Clin Cancer Res. 1999;5:1369–1379. [PubMed] [Google Scholar]
- 8.Riedel F., Zaiss I., Herzog D., Götte K., Naim R., Hörmann K. Serum levels of interleukin-6 in patients with primary head and neck squamous cell carcinoma. Anticancer Res. 2005;25:2761–2765. [PubMed] [Google Scholar]
- 9.Kumar B., Brown N.V., Swanson B.J. High expression of myoferlin is associated with poor outcome in oropharyngeal squamous cell carcinoma patients and is inversely associated with HPV-status. Oncotarget. 2016;7:18665–18677. doi: 10.18632/oncotarget.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moskowitz H.S., Gooding W.E., Thomas S.M. Serum biomarker modulation following molecular targeting of epidermal growth factor and cyclooxygenase pathways: a pilot randomized trial in head and neck cancer. Oral Oncol. 2012;48:1136–1145. doi: 10.1016/j.oraloncology.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirano T., Yasukawa K., Harada H. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986;324:73–76. doi: 10.1038/324073a0. [DOI] [PubMed] [Google Scholar]
- 12.Yoshizaki K., Nakagawa T., Fukunaga K., Tseng L.T., Yamamura Y., Kishimoto T. Isolation and characterization of B cell differentiation factor (BCDF) secreted from a human B lymphoblastoid cell line. J Immunol. 1984;132:2948–2954. [PubMed] [Google Scholar]
- 13.Yasukawa K., Hirano T., Watanabe Y. Structure and expression of human B cell stimulatory factor-2 (BSF-2/IL-6) gene. EMBO J. 1987;6:2939–2945. doi: 10.1002/j.1460-2075.1987.tb02598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gauldie J., Richards C., Harnish D., Lansdorp P., Baumann H. Interferon beta 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc Natl Acad Sci USA. 1987;84:7251–7255. doi: 10.1073/pnas.84.20.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Snick J., Cayphas S., Szikora J.P. cDNA cloning of murine interleukin-HP1: homology with human interleukin 6. Eur J Immunol. 1988;18:193–197. doi: 10.1002/eji.1830180202. [DOI] [PubMed] [Google Scholar]
- 16.Woloski B.M., Fuller G.M. Identification and partial characterization of hepatocyte-stimulating factor from leukemia cell lines: comparison with interleukin 1. Proc Natl Acad Sci USA. 1985;82:1443–1447. doi: 10.1073/pnas.82.5.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bethin K.E., Vogt S.K., Muglia L.J. Interleukin-6 is an essential, corticotropin-releasing hormone-independent stimulator of the adrenal axis during immune system activation. Proc Natl Acad Sci USA. 2000;97:9317–9322. doi: 10.1073/pnas.97.16.9317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kopf M., Baumann H., Freer G. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature. 1994;368:339–342. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- 19.Stahl E.A., Raychaudhuri S., Remmers E.F. Genome-wide association study meta-analysis identifies seven new rheumatoid arthritis risk loci. Nat Genet. 2010;42:508–514. doi: 10.1038/ng.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yadav A., Kumar B., Datta J., Teknos T.N., Kumar P. IL-6 promotes head and neck tumor metastasis by inducing epithelial-mesenchymal transition via the JAK-STAT3-SNAIL signaling pathway. Mol Cancer Res. 2011;9:1658–1667. doi: 10.1158/1541-7786.MCR-11-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sironi M., Breviario F., Proserpio P. IL-1 stimulates IL-6 production in endothelial cells. J Immunol. 1989;142:549–553. [PubMed] [Google Scholar]
- 22.Watson J.M., Sensintaffar J.L., Berek J.S., Martínez-Maza O. Constitutive production of interleukin 6 by ovarian cancer cell lines and by primary ovarian tumor cultures. Cancer Res. 1990;50:6959–6965. [PubMed] [Google Scholar]
- 23.Andersson U., Sander B., Andersson J., Möller G. Concomitant production of different lymphokines in activated T cells. Eur J Immunol. 1988;18:2081–2084. doi: 10.1002/eji.1830181232. [DOI] [PubMed] [Google Scholar]
- 24.Lederle W., Depner S., Schnur S. IL-6 promotes malignant growth of skin SCCs by regulating a network of autocrine and paracrine cytokines. Int J Cancer. 2011;128:2803–2814. doi: 10.1002/ijc.25621. [DOI] [PubMed] [Google Scholar]
- 25.Islam M., Sharma S., Teknos T.N. RhoC regulates cancer stem cells in head and neck squamous cell carcinoma by overexpressing IL-6 and phosphorylation of STAT3. PLoS One. 2014;9:e88527. doi: 10.1371/journal.pone.0088527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jinno T., Kawano S., Maruse Y. Increased expression of interleukin-6 predicts poor response to chemoradiotherapy and unfavorable prognosis in oral squamous cell carcinoma. Oncol Rep. 2015;33:2161–2168. doi: 10.3892/or.2015.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu C.C., Lin J.H., Hsu T.W. IL-6 enriched lung cancer stem-like cell population by inhibition of cell cycle regulators via DNMT1 upregulation. Int J Cancer. 2015;136:547–559. doi: 10.1002/ijc.29033. [DOI] [PubMed] [Google Scholar]
- 28.Krishnamurthy S., Warner K.A., Dong Z. Endothelial interleukin-6 defines the tumorigenic potential of primary human cancer stem cells. Stem Cells. 2014;32:2845–2857. doi: 10.1002/stem.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mojtahedi Z., Khademi B., Hashemi S.B. Serum interleukine-6 concentration, but not interleukine-18, is associated with head and neck squamous cell carcinoma progression. Pathol Oncol Res. 2011;17:7–10. doi: 10.1007/s12253-010-9261-y. [DOI] [PubMed] [Google Scholar]
- 30.Lathers D.M., Achille N.J., Young M.R. Incomplete Th2 skewing of cytokines in plasma of patients with squamous cell carcinoma of the head and neck. Hum Immunol. 2003;64:1160–1166. doi: 10.1016/j.humimm.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 31.Sparano A., Lathers D.M., Achille N., Petruzzelli G.J., Young M.R. Modulation of Th1 and Th2 cytokine profiles and their association with advanced head and neck squamous cell carcinoma. Otolaryngol Head Neck Surg. 2004;131:573–576. doi: 10.1016/j.otohns.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 32.Tartour E., Deneux L., Mosseri V. Soluble interleukin-2 receptor serum level as a predictor of locoregional control and survival for patients with head and neck carcinoma: results of a multivariate prospective study. Cancer. 1997;79:1401–1408. [PubMed] [Google Scholar]
- 33.Wang Y.F., Chang S.Y., Tai S.K., Li W.Y., Wang L.S. Clinical significance of interleukin-6 and interleukin-6 receptor expressions in oral squamous cell carcinoma. Head Neck. 2002;24:850–858. doi: 10.1002/hed.10145. [DOI] [PubMed] [Google Scholar]
- 34.Allen C., Duffy S., Teknos T. Nuclear factor-kappaB-related serum factors as longitudinal biomarkers of response and survival in advanced oropharyngeal carcinoma. Clin Cancer Res. 2007;13:3182–3190. doi: 10.1158/1078-0432.CCR-06-3047. [DOI] [PubMed] [Google Scholar]
- 35.De Schutter H., Landuyt W., Verbeken E., Goethals L., Hermans R., Nuyts S. The prognostic value of the hypoxia markers CA IX and GLUT 1 and the cytokines VEGF and IL 6 in head and neck squamous cell carcinoma treated by radiotherapy +/− chemotherapy. BMC Cancer. 2005;5:42. doi: 10.1186/1471-2407-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Argiris A., Lee S.C., Feinstein T. Serum biomarkers as potential predictors of antitumor activity of cetuximab-containing therapy for locally advanced head and neck cancer. Oral Oncol. 2011;47:961–966. doi: 10.1016/j.oraloncology.2011.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stanam A., Love-Homan L., Joseph T.S., Espinosa-Cotton M., Simons A.L. Upregulated interleukin-6 expression contributes to erlotinib resistance in head and neck squamous cell carcinoma. Mol Oncol. 2015;9:1371–1383. doi: 10.1016/j.molonc.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duffy S.A., Teknos T., Taylor J.M. Health behaviors predict higher interleukin-6 levels among patients newly diagnosed with head and neck squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2013;22:374–381. doi: 10.1158/1055-9965.EPI-12-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang C.H., Hsiao C.F., Yeh Y.M. Circulating interleukin-6 level is a prognostic marker for survival in advanced nonsmall cell lung cancer patients treated with chemotherapy. Int J Cancer. 2013;132:1977–1985. doi: 10.1002/ijc.27892. [DOI] [PubMed] [Google Scholar]
- 40.Okada S., Okusaka T., Ishii H. Elevated serum interleukin-6 levels in patients with pancreatic cancer. Jpn J Clin Oncol. 1998;28:12–15. doi: 10.1093/jjco/28.1.12. [DOI] [PubMed] [Google Scholar]
- 41.Wong V.W., Yu J., Cheng A.S. High serum interleukin-6 level predicts future hepatocellular carcinoma development in patients with chronic hepatitis B. Int J Cancer. 2009;124:2766–2770. doi: 10.1002/ijc.24281. [DOI] [PubMed] [Google Scholar]
- 42.Nakashima J., Tachibana M., Horiguchi Y. Serum interleukin 6 as a prognostic factor in patients with prostate cancer. Clin Cancer Res. 2000;6:2702–2706. [PubMed] [Google Scholar]
- 43.Zhang G.J., Adachi I. Serum interleukin-6 levels correlate to tumor progression and prognosis in metastatic breast carcinoma. Anticancer Res. 1999;19:1427–1432. [PubMed] [Google Scholar]
- 44.Hibi M., Murakami M., Saito M., Hirano T., Taga T., Kishimoto T. Molecular cloning and expression of an IL-6 signal transducer, gp130. Cell. 1990;63:1149–1157. doi: 10.1016/0092-8674(90)90411-7. [DOI] [PubMed] [Google Scholar]
- 45.Taga T., Hibi M., Hirata Y. Interleukin-6 triggers the association of its receptor with a possible signal transducer, gp130. Cell. 1989;58:573–581. doi: 10.1016/0092-8674(89)90438-8. [DOI] [PubMed] [Google Scholar]
- 46.Heinrich P.C., Behrmann I., Haan S., Hermanns H.M., Müller-Newen G., Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heinrich P.C., Behrmann I., Müller-Newen G., Schaper F., Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J. 1998;334:297–314. doi: 10.1042/bj3340297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamasaki K., Taga T., Hirata Y. Cloning and expression of the human interleukin-6 (BSF-2/IFN beta 2) receptor. Science. 1988;241:825–828. doi: 10.1126/science.3136546. [DOI] [PubMed] [Google Scholar]
- 49.Skiniotis G., Boulanger M.J., Garcia K.C., Walz T. Signaling conformations of the tall cytokine receptor gp130 when in complex with IL-6 and IL-6 receptor. Nat Struct Mol Biol. 2005;12:545–551. doi: 10.1038/nsmb941. [DOI] [PubMed] [Google Scholar]
- 50.Hunter C.A., Jones S.A. IL-6 as a keystone cytokine in health and disease. Nat Immunol. 2015;16:448–457. doi: 10.1038/ni.3153. [DOI] [PubMed] [Google Scholar]
- 51.Yu H., Lee H., Herrmann A., Buettner R., Jove R. Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nat Rev Cancer. 2014;14:736–746. doi: 10.1038/nrc3818. [DOI] [PubMed] [Google Scholar]
- 52.Levy D.E., Lee C.K. What does Stat3 do? J Clin Invest. 2002;109:1143–1148. doi: 10.1172/JCI15650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kershaw N.J., Murphy J.M., Lucet I.S., Nicola N.A., Babon J.J. Regulation of Janus kinases by SOCS proteins. Biochem Soc Trans. 2013;41:1042–1047. doi: 10.1042/BST20130077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim M.S., Lee W.S., Jeong J., Kim S.J., Jin W. Induction of metastatic potential by TrkB via activation of IL6/JAK2/STAT3 and PI3K/AKT signaling in breast cancer. Oncotarget. 2015;6:40158–40171. doi: 10.18632/oncotarget.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Linnskog R., Jönsson G., Axelsson L., Prasad C.P., Andersson T. Interleukin-6 drives melanoma cell motility through p38α-MAPK-dependent up-regulation of WNT5A expression. Mol Oncol. 2014;8:1365–1378. doi: 10.1016/j.molonc.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wolf J., Rose-John S., Garbers C. Interleukin-6 and its receptors: a highly regulated and dynamic system. Cytokine. 2014;70:11–20. doi: 10.1016/j.cyto.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 57.Rose-John S. IL-6 trans-signaling via the soluble IL-6 receptor: importance for the pro-inflammatory activities of IL-6. Int J Biol Sci. 2012;8:1237–1247. doi: 10.7150/ijbs.4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rose-John S., Scheller J., Elson G., Jones S.A. Interleukin-6 biology is coordinated by membrane-bound and soluble receptors: role in inflammation and cancer. J Leukoc Biol. 2006;80:227–236. doi: 10.1189/jlb.1105674. [DOI] [PubMed] [Google Scholar]
- 59.Jurczyszyn A., Czepiel J., Biesiada G. HGF, sIL-6R and TGF-β1 play a significant role in the progression of multiple myeloma. J Cancer. 2014;5:518–524. doi: 10.7150/jca.9266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Won H.S., Kim Y.A., Lee J.S. Soluble interleukin-6 receptor is a prognostic marker for relapse-free survival in estrogen receptor-positive breast cancer. Cancer Invest. 2013;31:516–521. doi: 10.3109/07357907.2013.826239. [DOI] [PubMed] [Google Scholar]
- 61.Gaillard J.P., Mani J.C., Liautard J., Klein B., Brochier J. Interleukin-6 receptor signaling. I. gp80 and gp130 receptor interaction in the absence of interleukin-6. Eur Cytokine Netw. 1999;10:43–48. [PubMed] [Google Scholar]
- 62.Peters M., Jacobs S., Ehlers M. The function of the soluble interleukin 6 (IL-6) receptor in vivo: sensitization of human soluble IL-6 receptor transgenic mice towards IL-6 and prolongation of the plasma half-life of IL-6. J Exp Med. 1996;183:1399–1406. doi: 10.1084/jem.183.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Su Y.W., Xie T.X., Sano D., Myers J.N. IL-6 stabilizes Twist and enhances tumor cell motility in head and neck cancer cells through activation of casein kinase 2. PLoS One. 2011;6:e19412. doi: 10.1371/journal.pone.0019412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rubin Grandis J., Zeng Q., Drenning S.D. Epidermal growth factor receptor-mediated stat3 signaling blocks apoptosis in head and neck cancer. Laryngoscope. 2000;110:868–874. doi: 10.1097/00005537-200005000-00016. [DOI] [PubMed] [Google Scholar]
- 65.Sriuranpong V., Park J.I., Amornphimoltham P., Patel V., Nelkin B.D., Gutkind J.S. Epidermal growth factor receptor-independent constitutive activation of STAT3 in head and neck squamous cell carcinoma is mediated by the autocrine/paracrine stimulation of the interleukin 6/gp130 cytokine system. Cancer Res. 2003;63:2948–2956. [PubMed] [Google Scholar]
- 66.Squarize C.H., Castilho R.M., Sriuranpong V., Pinto D.S., Jr., Gutkind J.S. Molecular cross-talk between the NFkappaB and STAT3 signaling pathways in head and neck squamous cell carcinoma. Neoplasia. 2006;8:733–746. doi: 10.1593/neo.06274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ahmed S.T., Darnell J.E., Jr. Serpin B3/B4, activated by STAT3, promote survival of squamous carcinoma cells. Biochem Biophys Res Commun. 2009;378:821–825. doi: 10.1016/j.bbrc.2008.11.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gasche J.A., Hoffmann J., Boland C.R., Goel A. Interleukin-6 promotes tumorigenesis by altering DNA methylation in oral cancer cells. Int J Cancer. 2011;129:1053–1063. doi: 10.1002/ijc.25764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kalluri R. EMT: when epithelial cells decide to become mesenchymal-like cells. J Clin Invest. 2009;119:1417–1419. doi: 10.1172/JCI39675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee M.Y., Chou C.Y., Tang M.J., Shen M.R. Epithelial-mesenchymal transition in cervical cancer: correlation with tumor progression, epidermal growth factor receptor overexpression, and snail up-regulation. Clin Cancer Res. 2008;14:4743–4750. doi: 10.1158/1078-0432.CCR-08-0234. [DOI] [PubMed] [Google Scholar]
- 71.Mandal M., Myers J.N., Lippman S.M. Epithelial to mesenchymal transition in head and neck squamous carcinoma: association of Src activation with E-cadherin down-regulation, vimentin expression, and aggressive tumor features. Cancer. 2008;112:2088–2100. doi: 10.1002/cncr.23410. [DOI] [PubMed] [Google Scholar]
- 72.Kanazawa T., Nishino H., Hasegawa M. Interleukin-6 directly influences proliferation and invasion potential of head and neck cancer cells. Eur Arch Otorhinolaryngol. 2007;264:815–821. doi: 10.1007/s00405-007-0264-6. [DOI] [PubMed] [Google Scholar]
- 73.Nishino H., Miyata M., Kitamura K. The effect of interleukin-6 on enhancing the invasiveness of head and neck cancer cells in vitro. Eur Arch Otorhinolaryngol. 1998;255:468–472. doi: 10.1007/s004050050101. [DOI] [PubMed] [Google Scholar]
- 74.Sundelin K., Roberg K., Grénman R., Håkansson L. Effects of cytokines on matrix metalloproteinase expression in oral squamous cell carcinoma in vitro. Acta Otolaryngol. 2005;125:765–773. doi: 10.1080/00016480510027484. [DOI] [PubMed] [Google Scholar]
- 75.Mochizuki D., Adams A., Warner K.A. Anti-tumor effect of inhibition of IL-6 signaling in mucoepidermoid carcinoma. Oncotarget. 2015;6:22822–22835. doi: 10.18632/oncotarget.4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Johnston P.A., Sen M., Hua Y. High-content pSTAT3/1 imaging assays to screen for selective inhibitors of STAT3 pathway activation in head and neck cancer cell lines. Assay Drug Dev Technol. 2014;12:55–79. doi: 10.1089/adt.2013.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Johnston P.A., Sen M., Hua Y. HCS campaign to identify selective inhibitors of IL-6-induced STAT3 pathway activation in head and neck cancer cell lines. Assay Drug Dev Technol. 2015;13:356–376. doi: 10.1089/adt.2015.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Goel A., Kunnumakkara A.B., Aggarwal B.B. Curcumin as “Curecumin”: from kitchen to clinic. Biochem Pharmacol. 2008;75:787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 79.Adams B.K., Cai J., Armstrong J. EF24, a novel synthetic curcumin analog, induces apoptosis in cancer cells via a redox-dependent mechanism. Anticancer Drugs. 2005;16:263–275. doi: 10.1097/00001813-200503000-00005. [DOI] [PubMed] [Google Scholar]
- 80.Anand P., Kunnumakkara A.B., Newman R.A., Aggarwal B.B. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 81.Kumar B., Yadav A., Hideg K., Kuppusamy P., Teknos T.N., Kumar P. A novel curcumin analog (H-4073) enhances the therapeutic efficacy of cisplatin treatment in head and neck cancer. PLoS One. 2014;9:e93208. doi: 10.1371/journal.pone.0093208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lin H.Y., Hou S.C., Chen S.C. (−)-Epigallocatechin gallate induces Fas/CD95-mediated apoptosis through inhibiting constitutive and IL-6-induced JAK/STAT3 signaling in head and neck squamous cell carcinoma cells. J Agric Food Chem. 2012;60:2480–2489. doi: 10.1021/jf204362n. [DOI] [PubMed] [Google Scholar]
- 83.Masuda M., Suzui M., Weinstein I.B. Effects of epigallocatechin-3-gallate on growth, epidermal growth factor receptor signaling pathways, gene expression, and chemosensitivity in human head and neck squamous cell carcinoma cell lines. Clin Cancer Res. 2001;7:4220–4229. [PubMed] [Google Scholar]
- 84.Chang C.M., Chang P.Y., Tu M.G. Epigallocatechin gallate sensitizes CAL-27 human oral squamous cell carcinoma cells to the anti-metastatic effects of gefitinib (Iressa) via synergistic suppression of epidermal growth factor receptor and matrix metalloproteinase-2. Oncol Rep. 2012;28:1799–1807. doi: 10.3892/or.2012.1991. [DOI] [PubMed] [Google Scholar]
- 85.Sen M., Paul K., Freilino M.L. Systemic administration of a cyclic signal transducer and activator of transcription 3 (STAT3) decoy oligonucleotide inhibits tumor growth without inducing toxicological effects. Mol Med. 2014;20:46–56. doi: 10.2119/molmed.2013.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sen M., Thomas S.M., Kim S. First-in-human trial of a STAT3 decoy oligonucleotide in head and neck tumors: implications for cancer therapy. Cancer Discov. 2012;2:694–705. doi: 10.1158/2159-8290.CD-12-0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vaddi K., Sarlis N.J., Gupta V. Ruxolitinib, an oral JAK1 and JAK2 inhibitor, in myelofibrosis. Expert Opin Pharmacother. 2012;13:2397–2407. doi: 10.1517/14656566.2012.732998. [DOI] [PubMed] [Google Scholar]
- 88.Hedvat M., Huszar D., Herrmann A. The JAK2 inhibitor AZD1480 potently blocks Stat3 signaling and oncogenesis in solid tumors. Cancer Cell. 2009;16:487–497. doi: 10.1016/j.ccr.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Purandare A.V., McDevitt T.M., Wan H. Characterization of BMS-911543, a functionally selective small-molecule inhibitor of JAK2. Leukemia. 2012;26:280–288. doi: 10.1038/leu.2011.292. [DOI] [PubMed] [Google Scholar]
- 90.Trikha M., Corringham R., Klein B., Rossi J.F. Targeted anti-interleukin-6 monoclonal antibody therapy for cancer: a review of the rationale and clinical evidence. Clin Cancer Res. 2003;9:4653–4665. [PMC free article] [PubMed] [Google Scholar]
- 91.Fizazi K., De Bono J.S., Flechon A. Randomised phase II study of siltuximab (CNTO 328), an anti-IL-6 monoclonal antibody, in combination with mitoxantrone/prednisone versus mitoxantrone/prednisone alone in metastatic castration-resistant prostate cancer. Eur J Cancer. 2012;48:85–93. doi: 10.1016/j.ejca.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 92.Puchalski T., Prabhakar U., Jiao Q., Berns B., Davis H.M. Pharmacokinetic and pharmacodynamic modeling of an anti-interleukin-6 chimeric monoclonal antibody (siltuximab) in patients with metastatic renal cell carcinoma. Clin Cancer Res. 2010;16:1652–1661. doi: 10.1158/1078-0432.CCR-09-2581. [DOI] [PubMed] [Google Scholar]
- 93.Beck J.T., Hsu S.M., Wijdenes J. Brief report: alleviation of systemic manifestations of Castleman's disease by monoclonal anti-interleukin-6 antibody. N Engl J Med. 1994;330:602–605. doi: 10.1056/NEJM199403033300904. [DOI] [PubMed] [Google Scholar]
- 94.van Zaanen H.C., Lokhorst H.M., Aarden L.A. Chimaeric anti-interleukin 6 monoclonal antibodies in the treatment of advanced multiple myeloma: a phase I dose-escalating study. Br J Haematol. 1998;102:783–790. doi: 10.1046/j.1365-2141.1998.00835.x. [DOI] [PubMed] [Google Scholar]
- 95.Orlowski R.Z., Gercheva L., Williams C. A phase 2, randomized, double-blind, placebo-controlled study of siltuximab (anti-IL-6 mAb) and bortezomib versus bortezomib alone in patients with relapsed or refractory multiple myeloma. Am J Hematol. 2015;90:42–49. doi: 10.1002/ajh.23868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jing N., Zhu Q., Yuan P., Li Y., Mao L., Tweardy D.J. Targeting signal transducer and activator of transcription 3 with G-quartet oligonucleotides: a potential novel therapy for head and neck cancer. Mol Cancer Ther. 2006;5:279–286. doi: 10.1158/1535-7163.MCT-05-0302. [DOI] [PubMed] [Google Scholar]
- 97.Fulciniti M., Hideshima T., Vermot-Desroches C. A high-affinity fully human anti-IL-6 mAb, 1339, for the treatment of multiple myeloma. Clin Cancer Res. 2009;15:7144–7152. doi: 10.1158/1078-0432.CCR-09-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yu X., He L., Cao P., Yu Q. Eriocalyxin B inhibits STAT3 signaling by covalently targeting STAT3 and blocking phosphorylation and activation of STAT3. PLoS One. 2015;10:e0128406. doi: 10.1371/journal.pone.0128406. Edited by Xin Jin. [DOI] [PMC free article] [PubMed] [Google Scholar]