Abstract
Inactivation of the tumor suppressor p53 is the predominant pathogenetic event in head and neck squamous cell carcinoma (HNSCC). The p53 pathway in HNSCC can be compromised through multiple mechanisms including gene mutations, hyperactivation of endogenous negative p53 regulators and by the human papillomavirus E6 protein. Inactivation of p53 is associated with poor clinical response and outcome; therefore, restoration of the p53 signaling cascade may be an effective approach to ablate HNSCC cells. Viral approaches to restore p53 activity in HNSCC have been well-studied and shown modest activity in clinical trials. Recent work has focused on high-throughput screens and rational designs to identify and develop small molecules to rescue p53 function. Several p53-targeting small molecules have demonstrated very promising activity in pre-clinical studies but have yet progressed to the clinical setting. Further development of p53 therapies, in particular chemical approaches, should be prioritized and evaluated in the HNSCC setting.
Keywords: Head and neck cancer, Tumor suppressor p53, Gene therapy, Human papillomavirus, p53 mutations
Introduction
Worldwide, head and neck squamous cell carcinoma (HNSCC) is the sixth most common cancer with an annual incidence of 600,000 and an overall mortality rate of 50%–60%. In the United States, 53,640 new cases were reported in 2013, accounting for 3% of all cancers.1 All head and neck malignancies appear to arise from the mucosal surfaces at various sites, including the oral cavity, pharynx and larynx. Despite their common histologic origin, HNSCC are heterogeneous in behavior and genetics. The main risk factors for the development of HNSCC include prolonged exposure to tobacco products and alcohol. More recently, infection with human papillomavirus (HPV) has emerged as a major etiologic factor for HNSCC.2 Survival in patients with HPV-associated HNSCC is significantly better than for patients with HPV-negative HNSCC.3, 4 Though there is relatively good prognosis for patients with HPV-associated HNSCC, clinical outcomes for HPV-negative HNSCC patients remain poor and has not changed in the past 3 decades despite advances in clinical management.5 A common genetic alterations that is indispensable in both HPV-negative and HPV-associated HNSCC is the inactivation of the tumor suppressor p53. In HPV-negative HSNCC, inactivation of p53, either through mutations or dysregulation of endogenous p53 regulators, is believed to be one of the earliest genetic alterations to predispose cells to initiate the tumorigenesis cascade.6, 7 In contrast, p53 is almost universally wildtype in HPV-associated HNSCC, however, p53 function is compromised by the HPV oncogene E6.2 Functional inactivation of the p53 pathway accounts for about 80% of HSNCC,6 therefore, treatments targeting the restoration of p53 function in HPV-negative and HPV-associated HNSCC as a therapeutic strategy has received intense focus. In this chapter, p53-based therapies for HNSCC will be reviewed. This will include gene therapy to delivery wildtype p53, the development of viruses designed to target carcinoma cells without functional p53, small molecules to restore p53 function in mutant p53 carcinoma cells and small molecules to disrupt endogenous or exogenous inactivation of wildtype p53 (Fig. 1, Fig. 2).
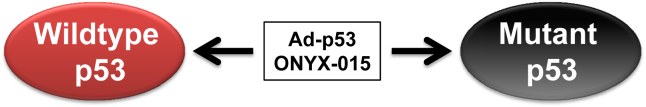
Fig. 1.
Virus-based therapeutics to modulate p53 in HNSCC. Adenoviral p53 (Ad-p53) and ONYX-015 therapies are not selective and have activity against wildtype and mutant p53 HNSCC cells.
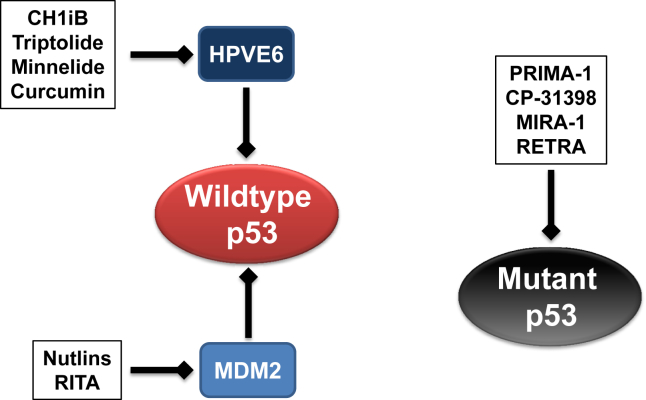
Fig. 2.
Small molecule-based therapeutics to modulate p53 in HNSCC. Wildtype p53 is inactivated through MDM2 dysregulation or exogenous HPVE6 oncogene in HNSCC. CH1iB and natural products, triptolide, Minnelide and curcumin, target the HPVE6 oncogene to reactivate p53 in HPV-associated carcinomas including HNSCC. Nutlins and RITA block the p53-MDM2 interaction to reactivate p53 in HPV-negative HNSCC. PRIMA-1, CP-31390, MIRA-1 and RETRA are chemical molecules that restore the transactivation activity of mutant p53.
Adenovirus p53 gene therapy
The tumor suppressor p53 and its target genes are essential regulators of cell cycle control and induction of apoptosis. The p53 signaling cascade modulates cell cycle and DNA repair to maintain the genetic integrity of cells. If irreparable DNA damages occur, p53 activates cellular apoptotic pathways to eliminate genetically damaged cells.8 p53 is one of the most common genetic alteration and mutations occurs in up to two-thirds of HNSCC. p53 mutations in HNSCC are also associated with resistance to cisplatin-based chemotherapy and radiation resulting in inferior prognosis compared to HNSCC patients with wildtype p53.9, 10 To overcome inactivating p53 mutations, an approach to reintroduce wildtype p53 has been developed in which an adenovirus is used as a vehicle to transport the wildtype p53 gene into carcinoma cells.11 This live virus could then selectively target and kill cancer cells. Adenoviral vector system was selected as an ideal approach for gene therapy based on several key reasons. Adenovirus is able to infect both dividing and quiescent cells and the adenovirus genome remains episomal and does not integrate in the host cells. In addition, high levels of the desired transgene product can be achieved in host cells and large scale manufacturing is possible with the adenoviral system.12 Liu et al,13 was one of the first to incorporate the wildtype p53 gene into a first generation adenoviral backbone (Ad-p53). In vitro studies with Ad-p53 virus demonstrated that p53 transduction could induce apoptosis and decrease cell proliferation in various carcinoma cell lines, including HNSCC.13 Another group showed that introduction of Ad-p53 into HNSCC cells containing mutated p53, sensitized the tumor cells to radiation therapy.14 Impressively, Ad-p53 virus was active in vivo and reduced tumor growth in xenograft models of HNSCC.15
Based on these successful pre-clinical studies, several clinical trials assessing the safety and efficacy of Ad-p53 were conducted in the HNSCC population. A Phase I clinical trial using p53 adenovirus gene therapy, INGN 201, was performed in 33 patients with recurrent HNSCC.16 INGN 201 was designed to be a replication-defective virion and contained p53 cDNA in the El region of the virus. All enrolled HNSCC patients had incurable disease with loco-regional recurrence. INGN 201 was dose-escalated in log increments from 106 to 109 plaque-forming units (pfu) and in half-log increments from 109 to 1011 pfu, delivered via intratumor injections. All HNSCC patients received at least one course of INGN 201 consisting of 6 total administrations given three times per week (every other day) for 2 weeks. Patients with resectable disease (n = 15) received one full course of injections pre-operatively followed by two additional; one during surgery after tumor resection in the site of microscopic residual disease and one 72 h after surgery via retrograde catheter instillation. In this resectable cohort, 27% of HNSCC patients remained disease-free with a median follow-up time of 18 months. Of the 17 patients with non-resectable disease, 2 patients responded with a greater than 50% reduction in tumor size by CT scan, 6 patients had stable disease and 9 patients had disease progression. However, the duration of stable disease in these six patients was very modest and only lasted 1–3.5 months. Multiple courses of direct intra-tumoral injections of INGN 201 were well tolerated with no dose-limiting toxicity or serious adverse events. Injection site pain was the most common reported adverse event and resolved within 24 h p53 expression was detected in post-treatment tumor biopsies providing proof of principle that adenoviral delivery of wildtype p53 to HNSCC tumors can be achieved with a safe toxicity profile.
Different schedules of viral gene therapy were evaluated in Phase II trials to determine the optimal intratumoral dose of p53 adenoviral therapy in HNSCC. In the T201 trial, doses ranged from 5 × 1010 viral particles (vp) to 2.5 × 1012 vp with a median dose of 1.2 × 1011 vp.17 HNSCC patients were stratified to two cohorts with different dosing schedules; one group received 3 doses every 28 days and another group received 6 doses in 28 days. Objective response was only reported in 6 out of 106 enrolled patients in both cohorts. In another Phase II trial, using a starting dose that was 50 times less than used in the T201 study, 20% of the high-dose cohort demonstrated a durable response of greater than 3 months compared to 14% of patients in the low-dose cohort.17 The median survival was 6 months versus 3.5 months and mortality was reported as 60% versus 40% at 150 days in the high-dose and low-dose cohorts, respectively. Both studies concluded that p53 adenoviral therapy was safe and well-tolerated.
In a Phase III randomized clinical trial, 116 patients with recurrent HNSCC were enrolled and treated with Ad-p53 gene therapy or methotrexate.18 Study endpoints included p53 biomarkers profiles to determine favorable versus unfavorable characteristics and which type of HNSCC patients would respond to therapy. Interestingly, this study found that most of the responders to Ad-p53 therapy had wildtype p53 in which p53 was inactivated by overexpression of the p53 inhibitors MDM2/MDM4 or had low expression of mutant p53. HNSCC patients with the favorable p53 profile had a significant increase in survival compared with patients with an unfavorable p53 profile (high expression of mutant p53). Survival for patients with a favorable p53 profile was 7.2 months compared to 2.7 months in the unfavorable p53 profile group (P < 0.0001). In contrast, most patients with response to methotrexate (87%) had high expression of mutated p53. The authors concluded that there was no significant difference in clinical benefit between patients treated with Ad-p53 and methotrexate; however, Ad-p53 therapy may be less toxic than methotrexate.
Virus that targets p53-deficient carcinoma cells
Another viral approach that has been evaluated is the delivery of a modified adenovirus that preferentially targets p53-deficient carcinoma cells. It is well documented that an active p53 signaling pathway negatively regulates the replication of adenoviruses. Adenoviruses evade this negative control mechanism by producing the E1B protein to bind and inactivate wildtype p53 function in infected host cells.19 ONYX-015 is a chimeric virus consisting of 2 species C adenoviruses genomes, serotypes 5 (Ad5) and serotype 2 (Ad2). This virus was designed to not express E1B. As a result of this deletion, ONYX-015 was proposed to efficiently replicate in and lyse p53-deficient cell, while not affect cells containing wildtype p53. Two studies confirmed that ONYX-015 induced lysis in p53 mutant carcinoma cells after exposure, in a replication-dependent manner, both in vitro and in vivo.20, 21 These studies demonstrated the selective destruction of cancer cells, however, it was later found that ONYX-015 had anti-tumor activity against tumor cells that expressed wildtype p53, via a unknown mechanism.22
Despite the contradictory pre-clinical data, the efficacy of ONYX-015 as monotherapy or in combination of standard chemotherapeutics was assessed in HNSCC patients. A Phase I trial was initiated to evaluate intratumoral ONYX-015 in recurrent HNSCC patients.23 p53 status was assessed in all the patients enrolled (n = 22); 16 patients had mutant p53, 5 patients had wildtype p53 and 1 patient could not be categorized. Overall, intratumoral injection of ONYX-015 was well-tolerated with no dose-limiting toxicity observed. Dose escalation was halted due to limitation of virus manufacturing capacity encountered during the active patient recruitment phase. In any event, viral doses were escalated to 1 × 1011 plaque-forming units (pfu) given daily once every 3 weeks or 1 × 1010 pfu for 5 consecutive days every 3 weeks. The most frequent symptoms were low grade fever and pain at the injection site. One patient experienced grade 2 symptoms of tracheal obstruction that may be attributable to ONYX-015. All patients in the study progressed; however, it should be noted that all tumors were not injected with ONYX-015 in each patient. To account for this study design limitation, data was evaluated only on tumors that received ONYX-015. A common finding was that the injected tumor became soft and fluctuant after treatment. One patient had a 50% decrease in the size of the tumor while another showed a 75% reduction in the size of the tumor by radiological imaging. These preliminary results show that intratumoral administration of ONYX-015 is feasible, well tolerated and associated with some biological activity. However, there was no association between tumor response and tumor p53 status in this Phase I study cohort.
A Phase II trial in recurrent or refractory HNSCC patients that received conventional therapy (n = 40) was performed to further evaluate the clinical activity of ONYX-015.24 For each patient, a single tumor was identified for intratumoral ONYX-015 treatment. If more than one possible injectable tumor was seen the most symptomatic and/or largest lesion was treated with ONYX-015. For the initial phase of the study, tumor injections were performed once daily for 5 consecutive days (standard schedule) then repeated every 3 weeks or until tumor progression. After documentation of safety with the standard treatment regimen with the first 30 patients, a more aggressive hyperfractionated schedule was performed on the next group of patients. For the second cohort of patients, injections were performed twice daily for 5 days during each of the first 2 weeks, the same regimen was repeated after a 1-week rest period and subsequently, maintenance treatment was given every 3 weeks. In the standard dosing group, 4 patients (14%) achieved a partial or complete regression at the injection site, 12 (41%) had stable disease, and 13 (45%) progressed. In the hyperfractionated patients, 1 patient had complete regression, 4 (58%) achieved stable disease, and 2 (29%) progressed. In contrast to the Phase I study, a correlation was demonstrated between tumor response and mutant p53 status.
In vitro and in vivo pre-clinical data provided compelling evidence that ONYX-015 potentiated the anti-tumor efficacy of standard chemotherapeutics.20 Based on these findings, two studies evaluated the combination of ONYX-015 with cisplatin/5-flurouracil (5-FU) in the recurrent HNSCC setting.25, 26 Khuri et al reported that the combination regimen was highly active and reduced the size of tumors in 25 out of the 30 cases. Objective response, with a decrease of 50% or more in tumor size, was reported in 63% of patients with injected tumors. The addition of ONYX-015 to cisplatin/5-FU was suggested to be highly beneficial since the overall injected-tumor response rate observed was superior to published data from multi-center, randomized trials evaluating cisplatin/5-FU in HNSCC. However, similar to the Phase I trial, no correlation between tumor response and tumor p53 status was reported in this Phase II combination regimen trial.
These reports show that ONYX-015 had limited effects when used alone, however, a select group of patients receiving ONYX-015 combined with chemotherapy did better than standard treatment alone. Identifying such patients was not completely clear. Analysis of the data showed that p53 selectivity did not always occur. Some patients with wild type p53 did better than patients with mutant p53 tumors. As a result of these findings in US based clinical trials and funding issues of the manufactures of ONYX-015, continued Phase III studies were stopped. The technology was licensed to a Chinese company that modified ONYX-015 to form another oncolytic adenovirus H101 (Oncorine).27 In Phase III clinical trials done in China, Oncorine plus chemotherapy was reported to have 79% response rate in nasopharyngeal carcinoma compared 40% with chemotherapy alone.28 In 2005, H101 received approval in China for treatment of nasopharyngeal cancer. Though this a milestone for the development of oncolytic virus, limitations still occur due to the fact that both ONYX-01529 and H101 have to be given intratumor and both viruses have not been able to be given systemically. Continued research into oncolytic therapy may be able to overcome these issues using other viruses instead of adenoviral vectors.29
Molecules that target mutant p53
Mutations in p53 are a frequent event and occur in about two-thirds of all HNSCC cases. p53 mutations are predominantly localized in the DNA binding domain which effectively block mutant p53 from binding to response elements of transactivate target genes. Considerable effort has been made to discover molecules that can alter and stabilize the 3-dimensional confirmation of mutant p53 to restore function. A screen using a library of >100,000 compounds identified CP-31398 as a small molecule which enhanced the conformational stability of the DNA binding domain in wildtype and mutant p53.30 CP-31398 was shown to enhance the transcriptional activity of mutant p53 and induce the p21, a p53-regulated gene, levels in Saos-2 osteocarcinoma cells expressing V173A or R249S mutant p53.30 Moreover, in vivo tumor growth of p53 mutant carcinoma cells, A375.S2 melanoma (249 mutation) and DLD-1 colon carcinoma (241 mutation), were retarded with systemic delivery of CP-31398.30 Another molecule, p53 reactivation and induction of massive apoptosis (PRIMA-1), was identified from a chemical library screen to selectively inhibit the proliferation of cells in a mutant p53-dependent manner.31 Impressively, PRIMA-1 restored the ability of a cadre of p53 mutants to bind to DNA confirming that PRIMA-1 can modify the 3-dimensional confirmation of mutant p53.31 PRIMA-1 was reported to be degraded to reactive products with thiol-modifying activity.32 These reactive products form adducts with thiols on one or several cysteine residues in the core domain of mutant p53 to restore p53 function.32
The anti-tumor activity of CP-31398 and PRIMA-1 has been widely studied in a wide variety of carcinomas over that past decade. However, to date, only one study has examined the response of HNSCC cells to these two mutant p53 modifying molecules. In a small panel of wildtype and mutant p53 HNSCC cell lines, CP-31398 and PRIMA-1 was shown to be more active in three mutant p53 HNSCC cell lines than in a wildtype p53 HNSCC cell line.33 The combination regimen of PRIMA-1 and cisplatin was more active than either single-agent treatment to induce the levels of p53-regulated pro-apoptotic genes, p21, Bax, PUMA and NOXA and suppress cell proliferation in p53 mutant (Y220C) UMSCC22A HNSCC cells.33 To improve on the activity of PRIMA-1, Bykov et al synthesized PRIMA-1 analogs and found that a methylated analog of PRIMA-1, PRIMA-1MET, was more active than PRIMA-1 to block the proliferation and induce active caspase-3 in mutant p53 cells.34 The anti-tumor efficacy of cisplatin was potentiated with PRIMA-1MET in mutant p53 (H175) H1299 lung carcinoma cells in vitro and in vivo.34 In addition, PRIMA-1MET cooperated with cisplatin to induce Bax and PUMA in a mutant p53-dependent context.34 The development of PRIMA-1MET has progressed rapidly and a Phase Ib/II clinical trial opened in 2014 (NCT02098343) to study PRIMA-1MET as single-agent or in combination with a platinum-based chemotherapeutic in recurrent ovarian carcinoma. Since PRIMA-1MET is already in clinical development, pre-clinical studies to assess the activity of PRIMA-1MET in HNSCC in vitro and in animal models should be prioritized in the near future.
Although PRIMA-1 and PRIMA-1MET were shown to restore mutant p53 function, there is accumulating experimental evidence that these molecules have p53-independent anti-tumor activity. PRIMA-1 induced autophagy in wildtype and mutant p53 carcinoma cells.35 Two different groups demonstrated that the anti-tumor effect of PRIMA-1MET in multiple myeloma was due primarily to induction of p73 or modulation of the glutathione/reactive oxygen species balance.36, 37 These findings indicate that PRIMA-1 and PRIMA-1MET have multiple mechanisms of action and suggest that these molecules should not be limited to the mutant p53 setting.
Other novel mutant p53 reactivation molecules, such as mutant p53 reactivation and induction of rapid apoptosis (MIRA-1) and reactivation of transcriptional reporter activity (RETRA), have been developed but have not been studied in HSNCC. MIRA-1 was identified using the same screening approach as PRIMA-1; selective cell proliferation inhibition in mutant p53 cells.38 MIRA-1 altered the 3-dimensional conformation of a small set of p53 mutants to restore DNA binding to the consensus p53 response element.38 It should be noted that MIRA-1 was not as robust as PRIMA-1 and only restored DNA binding to 3/13 p53 mutants whereas PRIMA-1 restored DNA binding to 10/13 mutants.31, 38 A high-throughput p53 reporter activity screen showed that RETRA selectively restore p53 activity in p53 mutant (H273) A431 lung carcinoma cells.39 RETRA enhanced p53 transcriptional activity in a panel of carcinoma cells lines with various p53 mutations including R273H, R248W, G266E and R280L.39 Interestingly, the primary mechanism of action for RETRA was not to restore mutant p53 function but rather to release p73 sequestered by mutant p53 to augment p73 activity.39 The finding suggests that activation of p53 family members, such as p73, may be an alternative therapeutic strategy to ablate mutant p53 carcinoma cells.
Molecules that target negative endogenous p53 regulators
p53 is dynamically and tightly controlled by endogenous p53 regulators to modulate p53 function in response to diverse cellular conditions. Mouse double minute 2 homolog (MDM2) is the prototypical negative p53 regulator and can suppress p53 through several distinct mechanisms. MDM2 binds to and masks the transactivation domain of p53 to repress p53 transcriptional activity. In addition, MDM2 functions as an E3 ubiquitin ligase to mark p53 for degradation through the ubiquitin-proteasome pathway.40 MDM2 levels are elevated in numerous solid malignancies, including HNSCC, and thus, rescue of p53 function by targeting the p53-MDM2 interaction may be an effective approach to eliminate wildtype p53 carcinoma cells.
A class of small molecules, nutlins, was identified from a diverse chemical library to displace the binding of wildtype p53 from MDM2 with high potency and selectivity.41 Nutlins bind to the p53-binding pocket of MDM2 to stabilize p53 resulting in p53 pathway activation in wildtype p53, but not in mutant p53, carcinoma cells.41 Another research group identified reactivation of p53 and induction of tumor cell apoptosis (RITA) from a cell-based screen to selectively inhibit the growth of wildtype p53 HCT116 carcinoma cells in comparison to the isogenic p53 null HCT 116 carcinoma cells.42 RITA directly binds to the N-terminal domain of wildtype p53 and is postulated to induce a conformational change in wildtype p53 to prevent association with MDM2.42 Wildtype p53 carcinoma cells treated with RITA had an increase in p53 accumulation and activity and moreover, high expression of p53 target genes, GADD45α and PUMA.42 In a panel of wildtype and mutant p53 HNSCC cell lines, nutlin-3 and RITA were found to be more active in wildtype p53 HNSCC cells than in mutant p53 HNSCC cells.33 Nutlin-3 and RITA increased p53 accumulation and apoptosis and potentiated the anti-tumor efficacy of cisplatin in wildtype p53 JHU-028 HNSCC cells.33, 43
Recent evidence indicates that p53-MDM2 protein–protein inhibitors (PPIs), such as nutlins and RITA, have anti-tumor activity in the setting of mutant or null p53. RITA was shown to induce senescence in HNSCC cells in a p53-independent mechanism.44 In mutant or null p53 HNSCC cells, RITA promoted senescence through inhibition of silent information regulator T1 (SIRT1), a histone deacetylase recognized as a negative regulator of cellular senescence.44, 45 Moreover, RITA augmented that activity of tenovin 6, a SIRT1 inhibitor, and radiation in mutant p53 HN31 HNSCC cells.44 Another study showed that nutlin-3 augments chemotherapy-induced apoptosis in mutant and null p53 carcinoma cells by releasing E2F1 from the E2F1-MDM2 complex.46 Also, nutlin-3 was reported to disrupt p73-MDM2 interaction to enhance p73 activity in mutant p53 SK-N-BE2 neuroblastoma and null p53 Saos-2 osteosarcoma cells.47 These results provide initial evidence that nutlin-3 and RITA may have clinical utility as part of a combination regimen in mutant p53 HNSCC cells.
Molecules that target negative exogeneous p53 regulators
The traditional risk factors for HNSCC are alcohol and tobacco abuse. However, there is ample evidence that the human papillomavirus (HPV) is an etiological factor for HNSCC, particularly oropharyngeal SCC. HPV16 is the most prevalent subtype and accounts for ∼90% of HPV-associated HNSCC.48, 49 Epidemiological data indicate that the prevalence of HPV-associated HNSCC has increased by ∼3-fold in the past three decades in the United States and Europe.50, 51, 52 Data obtained from the Swedish Cancer Registry showed a 2.8-fold increase in the incidence of oropharyngeal SCC in the Stockholm area between 1970 and 2002. Interestingly, over the same time period, the incidence of HPV-associated oropharyngeal SCC increased by ∼3-fold from 23% in the 1970s to 68% in the 2000s.53 Based on these alarming numbers, it has been suggested that an epidemic of HPV-associated HNSCC will emerge in the near future.52, 53 HPV-associated HNSCC patients are often managed with radiation or concurrent platinum-based chemoradiation. These strategies are effective against HPV-associated HNSCC but come at a cost of high patient morbidity. Molecularly-targeted therapies that ablate HPV-associated HNSCC with better toxicity profiles are critically needed to manage this growing patient population.
In HPV-associated HNSCC, p53 is almost universally wildtype but inactivated by the HPV oncogene E6. HPVE6 forms a trimeric protein complex with E6AP and p53 to facilitate E6AP-mediated ubiquitination and degradation of p53 through the proteasomal pathway.54, 55 In addition to promoting p53 degradation via the proteasome, a less recognized but perhaps more important mechanism is that HPVE6 can bind to the p300 transcriptional co-activator to sequester p300 function. Several lines of evidence indicate that HPVE6 associates with p300 to inhibit p300-mediated p53 acetylation.32, 56, 57 Acetylation controls p53 function at multiple levels including enhancing p53 stability, tetramerization, DNA binding and co-activator recruitment.58, 59, 60 Since inactivation of p53 by HPVE6 is indispensable for HPV-mediated tumorigenesis, targeting the HPVE6-p300 interaction may be a novel molecular approach to reactivate p53 and ablate HPV-associated carcinoma cells. Several lines of evidence show that HPV16E6 binds to the CH1 domain of p300.57, 61 Therefore, CH1-like ligands may serve as competitive inhibitors to mask the HPV16E6 binding site on p300 and disrupt HPV16E6-p300 interaction in HPV-associated HNSCC. A rationally designed small molecule CH1 ligand, CH1iB, blocked the HPV16E6-p300 interaction and reactivated the p53 pathway in HPV16-associated HNSCC cell lines, UMSCC47 and UPCI-SCC090.62 CH1iB induced the expression of p53-regulated genes, p21, miR-34a and miR-200c, promoted a pleotropic anti-tumor effect and potentiated the efficacy of cisplatin in HPV16-associated HNSCC cells.62 These results demonstrate that targeting the HPV16-p300 interaction is a fresh and tractable approach to reactivate p53 in HPV16-associated HNSCC.
Several anti-cancer natural products have been reported to degrade HPVE6 and/or reactivate p53 in HPV-associated carcinoma cells. Triptolide, a derivative from the Chinese herb Tripterygium wilfordii, reduced HPV16E6 expression and increased p53 levels and transcriptional activity in HPV16-associated UMSCC47 and 93-VU-147 HNSCC cells.63 In a HPV16-associated HNSCC xenograft animal model, a water-soluble analog of triptolide, Minnelide, enhanced p53 activation, inhibited tumor growth and increased apoptosis.63 The major yellow pigment from the rhizomes of turmeric, curcumin, reduced HPVE6 levels and induced p53 accumulation in HPV16- and HPV18-associated cervical carcinoma cells.64, 65, 66 Curcumin sensitized HPV-associated cervical carcinoma cells to radiation through an increase in reactive oxygen species production.67 Another laboratory showed that curcumin failed to sensitize HPV-associated HNSCC cells to radiation suggesting that the radiosensitizing actions of curcumin may be cell type-dependent.68 Interestingly, in silico docking analysis revealed that several natural products, including curcumin, have the potential to dock to the p53-binding site of E6, however, with varying binding affinities.69 The natural products, carrageenan (sulfated polysaccharide extracted from red algae) and withaferin A (active component of the medicinal plant Withania somnifera), have much higher binding affinities than curcumin and interacts with amino acids Ala53, Leu117 and Lys122 in the E6 p53-binding pocket.69 Carrageenan and withaferin A have not been evaluated in HNSCC and work should be performed to determine the activity and mechanism of action of these two natural products for HPV16-associated HNSCC.
Conclusion
The standard of care to manage HPV-negative and HPV-associated HNSCC patients has evolved over the years and currently consists of a multi-disciplinary treatment approach, including surgery, radiation and/or chemotherapy. Despite treatment advances, the clinical outcome of HPV-negative HNSCC remains dismal and has not changed in the past several decades. HPV-associated HNSCC patients respond well to the standard of care; however, this intense treatment regimen is difficult to tolerate and associated with high morbidity. Novel therapeutic strategies, with higher efficacy and lower toxicity, are needed to better manage the HPV-negative and HPV-associated HNSCC population.
Since loss of p53 function is the predominant pathogenetic event in HNSCC, restoration of p53 activity may be an optimal approach to eliminate HNSCC cells with high specificity. Adenoviral therapeutic strategies, such as Ad-p53 and ONYX-015, have progressed to clinical trials in the HNSCC setting but have shown modest activity. In addition to limited clinical activity, there are several issues that argue against additional research investment into these two adenoviral therapies. One major limitation of Ad-p53 and ONYX-015 is the requirement of repeated intratumoral injections into each tumor lesion and thus, the adoptability and availability of these two therapies may be limited to major academic centers. Another confounding issue with Ad-p53 and ONYX-015 is target cell specificity. Ad-p53 was designed to target mutant p53 carcinoma cells; however, data from a Phase III clinical trial indicate that Ad-p53 is more active in the wildtype but inactivated p53 setting. Similarly, ONYX-015 is non-selective and has activity in both wildtype and mutant p53 HNSCC. Recent work has focused on chemical approaches to restore p53 function in HPV-negative and HPV-associated HNSCC. Several of these molecules, such as PRIMA-1 for HPV-negative HNSCC and CH1iB for HPV-associated HNSCC, have shown very promising activity in the pre-clinical realm but has yet to be proven in the clinical setting. Additional resources to fast-track the development of chemical molecules that restore p53 function in HPV-negative and HPV-associated HNSCC is warranted and should be prioritized in the near future.
Grant support
This work was supported in part by the National Institutes of Health (R01CA193590; R01DE023555; R01GM117921); and Arthur G. James Cancer Hospital and Richard J. Solove Research Institute, The Ohio State University Comprehensive Cancer Center.
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Siegel R., Naishadham D., Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Rautava J., Syrjänen S. Biology of human papillomavirus infections in head and neck carcinogenesis. Head Neck Pathol. 2012;6:S3–S15. doi: 10.1007/s12105-012-0367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fakhry C., Westra W.H., Li S. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 4.Fakhry C., Zhang Q., Nguyen-Tan P.F. Human papillomavirus and overall survival after progression of oropharyngeal squamous cell carcinoma. J Clin Oncol. 2014;32:3365–3373. doi: 10.1200/JCO.2014.55.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaturvedi A.K., Engels E.A., Pfeiffer R.M. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leemans C.R., Braakhuis B.J., Brakenhoff R.H. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011;11:9–22. doi: 10.1038/nrc2982. [DOI] [PubMed] [Google Scholar]
- 7.Oliner J.D., Pietenpol J.A., Thiagalingam S., Gyuris J., Kinzler K.W., Vogelstein B. Oncoprotein MDM2 conceals the activation domain of tumour suppressor p53. Nature. 1993;362:857–860. doi: 10.1038/362857a0. [DOI] [PubMed] [Google Scholar]
- 8.Pflaum J., Schlosser S., Müller M. p53 family and cellular stress responses in cancer. Front Oncol. 2014;4:285. doi: 10.3389/fonc.2014.00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohnishi K., Ota I., Takahashi A., Yane K., Matsumoto H., Ohnishi T. Transfection of mutant p53 gene depresses X-ray- or CDDP-induced apoptosis in a human squamous cell carcinoma of the head and neck. Apoptosis. 2002;7:367–372. doi: 10.1023/a:1016131614856. [DOI] [PubMed] [Google Scholar]
- 10.Poeta M.L., Manola J., Goldwasser M.A. TP53 mutations and survival in squamous-cell carcinoma of the head and neck. N Engl J Med. 2007;357:2552–2561. doi: 10.1056/NEJMoa073770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Malley B.W., Jr., Chen S.H., Schwartz M.R., Woo S.L. Adenovirus-mediated gene therapy for human head and neck squamous cell cancer in a nude mouse model. Cancer Res. 1995;55:1080–1085. [PubMed] [Google Scholar]
- 12.Berkner K.L. Development of adenovirus vectors for the expression of heterologous genes. Biotechniques. 1988;6:616–629. [PubMed] [Google Scholar]
- 13.Liu T.J., el-Naggar A.K., McDonnell T.J. Apoptosis induction mediated by wild-type p53 adenoviral gene transfer in squamous cell carcinoma of the head and neck. Cancer Res. 1995;55:3117–3122. [PubMed] [Google Scholar]
- 14.Pirollo K.F., Hao Z., Rait A. p53 mediated sensitization of squamous cell carcinoma of the head and neck to radiotherapy. Oncogene. 1997;14:1735–1746. doi: 10.1038/sj.onc.1201116. [DOI] [PubMed] [Google Scholar]
- 15.Clayman G.L., El-Naggar A.K., Roth J.A. In vivo molecular therapy with p53 adenovirus for microscopic residual head and neck squamous carcinoma. Cancer Res. 1995;55:1–6. [PubMed] [Google Scholar]
- 16.Clayman G.L., El-Naggar A.K., Lippman S.M. Adenovirus-mediated p53 gene transfer in patients with advanced recurrent head and neck squamous cell carcinoma. J Clin Oncol. 1998;16:2221–2232. doi: 10.1200/JCO.1998.16.6.2221. [DOI] [PubMed] [Google Scholar]
- 17.Nemunaitis J., Nemunaitis J. Head and neck cancer: response to p53-based therapeutics. Head Neck. 2011;33:131–134. doi: 10.1002/hed.21364. [DOI] [PubMed] [Google Scholar]
- 18.Nemunaitis J., Clayman G., Agarwala S.S. Biomarkers predict p53 gene therapy efficacy in recurrent squamous cell carcinoma of the head and neck. Clin Cancer Res. 2009;15:7719–7725. doi: 10.1158/1078-0432.CCR-09-1044. [DOI] [PubMed] [Google Scholar]
- 19.Hall A.R., Dix B.R., O'Carroll S.J., Braithwaite A.W. p53-dependent cell death/apoptosis is required for a productive adenovirus infection. Nat Med. 1998;4:1068–1072. doi: 10.1038/2057. [DOI] [PubMed] [Google Scholar]
- 20.Bischoff J.R., Kirn D.H., Williams A. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373–376. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- 21.Heise C., Sampson-Johannes A., Williams A., McCormick F., Von Hoff D.D., Kirn D.H. ONYX-015, an E1B gene-attenuated adenovirus, causes tumor-specific cytolysis and antitumoral efficacy that can be augmented by standard chemotherapeutic agents. Nat Med. 1997;3:639–645. doi: 10.1038/nm0697-639. [DOI] [PubMed] [Google Scholar]
- 22.Goodrum F.D., Ornelles D.A. p53 status does not determine outcome of E1B 55-kilodalton mutant adenovirus lytic infection. J Virol. 1998;72:9479–9490. doi: 10.1128/jvi.72.12.9479-9490.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ganly I., Kirn D., Eckhardt G. A phase I study of Onyx-015, an E1B attenuated adenovirus, administered intratumorally to patients with recurrent head and neck cancer. Clin Cancer Res. 2000;6:798–806. [PubMed] [Google Scholar]
- 24.Nemunaitis J., Khuri F., Ganly I. Phase II trial of intratumoral administration of ONYX-015, a replication-selective adenovirus, in patients with refractory head and neck cancer. J Clin Oncol. 2001;19:289–298. doi: 10.1200/JCO.2001.19.2.289. [DOI] [PubMed] [Google Scholar]
- 25.Khuri F.R., Nemunaitis J., Ganly I. A controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat Med. 2000;6:879–885. doi: 10.1038/78638. [DOI] [PubMed] [Google Scholar]
- 26.Lamont J.P., Nemunaitis J., Kuhn J.A., Landers S.A., McCarty T.M. A prospective phase II trial of ONYX-015 adenovirus and chemotherapy in recurrent squamous cell carcinoma of the head and neck (the Baylor experience) Ann Surg Oncol. 2000;7:588–592. doi: 10.1007/BF02725338. [DOI] [PubMed] [Google Scholar]
- 27.Garber K. China approves world's first oncolytic virus therapy for cancer treatment. J Natl Cancer Inst. 2006;98:298–300. doi: 10.1093/jnci/djj111. [DOI] [PubMed] [Google Scholar]
- 28.Yu W., Fang H. Clinical trials with oncolytic adenovirus in China. Curr Cancer Drug Targets. 2007;7:141–148. doi: 10.2174/156800907780058817. [DOI] [PubMed] [Google Scholar]
- 29.Shilpa P., Kaul R., Bhat S., Sultana N., Pandeshwar P. Oncolytic viruses in head and neck cancer: a new ray of hope in the management protocol. Ann Med Health Sci Res. 2014;4:S178–S184. doi: 10.4103/2141-9248.141953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foster B.A., Coffey H.A., Morin M.J., Rastinejad F. Pharmacological rescue of mutant p53 conformation and function. Science. 1999;286:2507–2510. doi: 10.1126/science.286.5449.2507. [DOI] [PubMed] [Google Scholar]
- 31.Bykov V.J., Issaeva N., Shilov A. Restoration of the tumor suppressor function to mutant p53 by a low-molecular-weight compound. Nat Med. 2002;8:282–288. doi: 10.1038/nm0302-282. [DOI] [PubMed] [Google Scholar]
- 32.Lambert J.M., Gorzov P., Veprintsev D.B. PRIMA-1 reactivates mutant p53 by covalent binding to the core domain. Cancer Cell. 2009;15:376–388. doi: 10.1016/j.ccr.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 33.Roh J.L., Kang S.K., Minn I., Califano J.A., Sidransky D., Koch W.M. p53-Reactivating small molecules induce apoptosis and enhance chemotherapeutic cytotoxicity in head and neck squamous cell carcinoma. Oral Oncol. 2011;47:8–15. doi: 10.1016/j.oraloncology.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bykov V.J., Zache N., Stridh H. PRIMA-1(MET) synergizes with cisplatin to induce tumor cell apoptosis. Oncogene. 2005;24:3484–3491. doi: 10.1038/sj.onc.1208419. [DOI] [PubMed] [Google Scholar]
- 35.Russo D., Ottaggio L., Foggetti G. PRIMA-1 induces autophagy in cancer cells carrying mutant or wild type p53. Biochim Biophys Acta. 2013;1833:1904–1913. doi: 10.1016/j.bbamcr.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 36.Saha M.N., Jiang H., Yang Y., Reece D., Chang H. PRIMA-1Met/APR-246 displays high antitumor activity in multiple myeloma by induction of p73 and Noxa. Mol Cancer Ther. 2013;12:2331–2341. doi: 10.1158/1535-7163.MCT-12-1166. [DOI] [PubMed] [Google Scholar]
- 37.Tessoulin B., Descamps G., Moreau P. PRIMA-1Met induces myeloma cell death independent of p53 by impairing the GSH/ROS balance. Blood. 2014;124:1626–1636. doi: 10.1182/blood-2014-01-548800. [DOI] [PubMed] [Google Scholar]
- 38.Bykov V.J., Issaeva N., Zache N. Reactivation of mutant p53 and induction of apoptosis in human tumor cells by maleimide analogs. J Biol Chem. 2005;280:30384–30391. doi: 10.1074/jbc.M501664200. [DOI] [PubMed] [Google Scholar]
- 39.Kravchenko J.E., Ilyinskaya G.V., Komarov P.G. Small-molecule RETRA suppresses mutant p53-bearing cancer cells through a p73-dependent salvage pathway. Proc Natl Acad Sci U S A. 2008;105:6302–6307. doi: 10.1073/pnas.0802091105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Honda R., Tanaka H., Yasuda H. Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53. FEBS Lett. 1997;420:25–27. doi: 10.1016/s0014-5793(97)01480-4. [DOI] [PubMed] [Google Scholar]
- 41.Vassilev L.T., Vu B.T., Graves B. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303:844–848. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- 42.Issaeva N., Bozko P., Enge M. Small molecule RITA binds to p53, blocks p53-HDM-2 interaction and activates p53 function in tumors. Nat Med. 2004;10:1321–1328. doi: 10.1038/nm1146. [DOI] [PubMed] [Google Scholar]
- 43.Roh J.L., Ko J.H., Moon S.J., Ryu C.H., Choi J.Y., Koch W.M. The p53-reactivating small-molecule RITA enhances cisplatin-induced cytotoxicity and apoptosis in head and neck cancer. Cancer Lett. 2012;325:35–41. doi: 10.1016/j.canlet.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 44.Chuang H.C., Yang L.P., Fitzgerald A.L. The p53-reactivating small molecule RITA induces senescence in head and neck cancer cells. PLoS One. 2014;9:e104821. doi: 10.1371/journal.pone.0104821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu T., Liu P.Y., Marshall G.M. The critical role of the class III histone deacetylase SIRT1 in cancer. Cancer Res. 2009;69:1702–1705. doi: 10.1158/0008-5472.CAN-08-3365. [DOI] [PubMed] [Google Scholar]
- 46.Ambrosini G., Sambol E.B., Carvajal D., Vassilev L.T., Singer S., Schwartz G.K. Mouse double minute antagonist Nutlin-3a enhances chemotherapy-induced apoptosis in cancer cells with mutant p53 by activating E2F1. Oncogene. 2007;26:3473–3481. doi: 10.1038/sj.onc.1210136. [DOI] [PubMed] [Google Scholar]
- 47.Lau L.M., Nugent J.K., Zhao X., Irwin M.S. HDM2 antagonist Nutlin-3 disrupts p73-HDM2 binding and enhances p73 function. Oncogene. 2008;27:997–1003. doi: 10.1038/sj.onc.1210707. [DOI] [PubMed] [Google Scholar]
- 48.Gillison M.L., Koch W.M., Capone R.B. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000 May 3;92:709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 49.Klussmann J.P., Gültekin E., Weissenborn S.J. Expression of p16 protein identifies a distinct entity of tonsillar carcinomas associated with human papillomavirus. Am J Pathol. 2003;162:747–753. doi: 10.1016/S0002-9440(10)63871-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Licitra L., Rossini C., Bossi P., Locati L.D. Advances in the changing patterns of aetiology of head and neck cancers. Curr Opin Otolaryngol Head Neck Surg. 2006;14:95–99. doi: 10.1097/01.moo.0000193170.23956.5f. [DOI] [PubMed] [Google Scholar]
- 51.Shiboski C.H., Schmidt B.L., Jordan R.C. Tongue and tonsil carcinoma: increasing trends in the U.S. population ages 20–44 years. Cancer. 2005;103:1843–1849. doi: 10.1002/cncr.20998. [DOI] [PubMed] [Google Scholar]
- 52.Sturgis E.M., Cinciripini P.M. Trends in head and neck cancer incidence in relation to smoking prevalence: an emerging epidemic of human papillomavirus-associated cancers? Cancer. 2007;110:1429–1435. doi: 10.1002/cncr.22963. [DOI] [PubMed] [Google Scholar]
- 53.Hammarstedt L., Lindquist D., Dahlstrand H. Human papillomavirus as a risk factor for the increase in incidence of tonsillar cancer. Int J Cancer. 2006;119:2620–2623. doi: 10.1002/ijc.22177. [DOI] [PubMed] [Google Scholar]
- 54.Huibregtse J.M., Scheffner M., Howley P.M. Localization of the E6-AP regions that direct human papillomavirus E6 binding, association with p53, and ubiquitination of associated proteins. Mol Cell Biol. 1993;13:4918–4927. doi: 10.1128/mcb.13.8.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scheffner M., Huibregtse J.M., Vierstra R.D., Howley P.M. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell. 1993;75:495–505. doi: 10.1016/0092-8674(93)90384-3. [DOI] [PubMed] [Google Scholar]
- 56.Zimmermann H., Degenkolbe R., Bernard H.U., O'Connor M.J. The human papillomavirus type 16 E6 oncoprotein can down-regulate p53 activity by targeting the transcriptional coactivator CBP/p300. J Virol. 1999;73:6209–6219. doi: 10.1128/jvi.73.8.6209-6219.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patel D., Huang S.M., Baglia L.A., McCance D.J. The E6 protein of human papillomavirus type 16 binds to and inhibits co-activation by CBP and p300. EMBO J. 1999;18:5061–5072. doi: 10.1093/emboj/18.18.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li M., Luo J., Brooks C.L., Gu W. Acetylation of p53 inhibits its ubiquitination by Mdm2. J Biol Chem. 2002;277:50607–50611. doi: 10.1074/jbc.C200578200. [DOI] [PubMed] [Google Scholar]
- 59.Thomas M.C., Chiang C.M. E6 oncoprotein represses p53-dependent gene activation via inhibition of protein acetylation independently of inducing p53 degradation. Mol Cell. 2005;17:251–264. doi: 10.1016/j.molcel.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 60.Gu W., Roeder R.G. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell. 1997;90:595–606. doi: 10.1016/s0092-8674(00)80521-8. [DOI] [PubMed] [Google Scholar]
- 61.Bernat A., Avvakumov N., Mymryk J.S., Banks L. Interaction between the HPV E7 oncoprotein and the transcriptional coactivator p300. Oncogene. 2003;22:7871–7881. doi: 10.1038/sj.onc.1206896. [DOI] [PubMed] [Google Scholar]
- 62.Xie X., Piao L., Bullock B.N. Targeting HPV16 E6-p300 interaction reactivates p53 and inhibits the tumorigenicity of HPV-positive head and neck squamous cell carcinoma. Oncogene. 2014;33:1037–1046. doi: 10.1038/onc.2013.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Caicedo-Granados E., Lin R., Fujisawa C., Yueh B., Sangwan V., Saluja A. Wild-type p53 reactivation by small-molecule Minnelide™ in human papillomavirus (HPV)-positive head and neck squamous cell carcinoma. Oral Oncol. 2014;50:1149–1156. doi: 10.1016/j.oraloncology.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Divya C.S., Pillai M.R. Antitumor action of curcumin in human papillomavirus associated cells involves downregulation of viral oncogenes, prevention of NFkB and AP-1 translocation, and modulation of apoptosis. Mol Carcinog. 2006;45:320–332. doi: 10.1002/mc.20170. [DOI] [PubMed] [Google Scholar]
- 65.Maher D.M., Bell M.C., O'Donnell E.A., Gupta B.K., Jaggi M., Chauhan S.C. Curcumin suppresses human papillomavirus oncoproteins, restores p53, Rb, and PTPN13 proteins and inhibits benzo[a]pyrene-induced upregulation of HPV E7. Mol Carcinog. 2011;50:47–57. doi: 10.1002/mc.20695. [DOI] [PubMed] [Google Scholar]
- 66.Debata P.R., Castellanos M.R., Fata J.E. A novel curcumin-based vaginal cream Vacurin selectively eliminates apposed human cervical cancer cells. Gynecol Oncol. 2013;129:145–153. doi: 10.1016/j.ygyno.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 67.Javvadi P., Segan A.T., Tuttle S.W., Koumenis C. The chemopreventive agent curcumin is a potent radiosensitizer of human cervical tumor cells via increased reactive oxygen species production and overactivation of the mitogen-activated protein kinase pathway. Mol Pharmacol. 2008;73:1491–1501. doi: 10.1124/mol.107.043554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tuttle S., Hertan L., Daurio N. The chemopreventive and clinically used agent curcumin sensitizes HPV (−) but not HPV (+) HNSCC to ionizing radiation, in vitro and in a mouse orthotopic model. Cancer Biol Ther. 2012;13:575–584. doi: 10.4161/cbt.19772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kumar S., Jena L., Galande S., Daf S., Mohod K., Varma A.K. Elucidating molecular interactions of natural inhibitors with HPV-16 E6 oncoprotein through docking analysis. Genomics Inform. 2014;12:64–70. doi: 10.5808/GI.2014.12.2.64. [DOI] [PMC free article] [PubMed] [Google Scholar]