Abstract
Objective
To develop a three-dimensional study tool of the membranous labyrinth in order to study the pathophysiology, diagnostic workup and treatment of benign paroxysmal positional vertigo (BPPV). BPPV is the most common cause of peripheral vertigo. Its diagnosis and treatment depend on an understanding of the anatomy of the vestibular labyrinth and its position relative to the head. To date, many illustrations have been made to explain principals of diagnosis and treatment of BPPV, but few have been based on anatomical studies of the membranous labyrinth.
Methods
A cadaveric human membranous labyrinth was axially sectioned at 20 μm resolution, stained and segmented to create a high-resolution digital model. The model was cloned to create an enantiomeric pair of labyrinths. These were associated a 3D model of a human skull, segmented from MRI data, and were oriented according to established anatomic norms. Canal markers representing otoliths were created to mark canalith position during movement of the model within the 3D environment.
Results
The model allows visualization of true membranous labyrinth anatomy in both ears simultaneously. The dependent portion of each semicircular duct and of the utricle can easily be visualized in any head position. Moveable markers can mark the expected progress of otolith debris with changes in head position and images can be captured to document simulations. The model can be used to simulate pathology as well as diagnostic maneuvers and treatment procedures used for BPPV. The model has great potential as a teaching tool.
Conclusion
A simple model based on human anatomy has been created to allow careful study of BPPV pathophysiology and treatment. Going forward, this tool could offer insights that may lead to more accurate diagnosis and treatment of BPPV.
Keywords: Benign paroxysmal positional vertigo, Modeling, Anatomy, Histology, Model, 3D
Introduction
Benign paroxysmal positional vertigo (BPPV) is the most common cause of vestibular symptoms in man. Initially thought to be caused by vertebro-basilar insufficiency. Dix and Hallpike proved that symptoms could be produced without head on body rotation.1 Attention was then diverted to histologic evidence of cupulolithiasis2 and finally, after 20 years, canalithiasis was established as the cause of most symptoms of BPPV.3
In contrast to classical cases, clinical BPPV can be confusing. Any canal can be involved with cupulo-or canalithiasis. Multiple canals may be involved in the same patient causing eye movements with different intensities, directions and time courses to be superimposed on one another. Treatment maneuvers for repositioning of misplaced otoliths have proliferated and have generated considerable confusion among practitioners. Illustrations offered by the authors of new maneuvers often bear no relation to true anatomy of the membranous labyrinth. Also, illustrations often use the anatomy of the bony labyrinth to illustrate problems in otolith disease when there are distinct morphological differences between semicircular canal and semicircular duct anatomy that have implications for the diseases being described.4 In short, clarity in scientific communication has been compromised because of the absence of a reference model for labyrinthine anatomy.
While the anatomy of the bony labyrinth has been studied for more than a century using a variety of techniques,5, 6 it is the morphology of the membranous labyrinth that is most important to the study of BPPV. Studies of the morphology of the human membranous labyrinth have been more recent and limited. As the labyrinth cannot be practically delineated with current MRI or clinical CT, the most detailed of these studies have employed histologic techniques. These recent investigations have focused on canal plane analysis, estimates of endolymph volume, measures of canal cross-sections and sensory epithelia, biomechanical modeling of semicircular canal function and an anatomical model of the temporal bone for teaching.7, 8, 9, 10, 11, 12
An anatomic model of BPPV based on human membranous labyrinthine anatomy correctly associated with the human head can clarify communications related to BPPV and result in better teaching, innovation and refinements of treatment. We have created a 3D model of a human vestibular labyrinth for the study of BPPV. The method of its creation and details of its functionality are described herein.
Subjects and methods
The materials and methods for preparation of the surface model of the membranous labyrinth and vestibular organs followed the same technique as reported by Wang, Northrop et al for the creation of the Downloadable Virtual Model of the Temporal Bone12 and are summarized here:
Histologic sections and digitization
This model was created from histologic sections from a one-day, forty-weeks gestation female. The temporal bone was harvested two hours postmortem. Following fixation in formalin, the specimen was decalcified using 5% trichloroacetic acid, embedded in celloidin, and serially sectioned in the axial plane at a thickness of 20 μm. Each of 780 sections was stained with hematoxylin and eosin and mounted on glass slides. Each stained section was digitized using a Nikon Super Cool Scan 4000 ED at 5590 × 3473 pixel resolution. As the size of each color image was large (approximately 60 MB) the color images were converted into gray scale, and the resolution of each image was reduced to 1397 × 868 pixels, which diminishes data size sufficiently to allow us to fit the complete image stack into our workstation memory and into Amira 5.2.2.
Alignment
The digitized two-dimensional (2D) sections were then registered pair by pair using Amira's interactive alignment tool. Every two consecutive sections were displayed simultaneously with half-opacity. Amira's “quality function optimization method” was used to auto-align the pair, which was a “least square best fit” algorithm computed from minimizing the squared gray-scale value differences of the pixels in the two consecutive slices.
Segmentation
After alignment, Amira's semi-automated segmentation tools were used to extract the membranous vestibular labyrinth cristae and otolith organs. These tools included “thresholding,” “lasso,” “magic wand”, “contour fitting”, and others. These steps simplified and accelerated the process of segmentation. A tedious manual technique of segmentation was required for the cristae and otolith organs in which the indistinct segmentation boundary on the digital image was guided by direct observation of the corresponding histologic section on a microscope mounted next to the workstation.
Surface reconstruction, simplification, and smoothing
Amira's “simplification” algorithm was applied to the surface models of the labyrinth, otolith organs and semicircular canal cristae that removed redundant vertices and smoothed the models by reducing the number of triangles in each one. Two to five iterations of the smoothing algorithm were applied to each surface.
Surface refinement: interpolation
Mechanical distortions introduced in mounting individual temporal bone sections on glass slides, such as stretching, curved mounting or folding of a section, and can result in artefactual distortions of the reconstructed surface model of an anatomic structure. The technique of “interpolation” was used to overcome this problem. If section n was distorted, it was not segmented; rather, it was replaced by an interpolation between data from section n+1 and n−1. The amount of interpolation needed varied among different structures.
Coloring and further smoothing
In order to enhance the visual effect of our model and clearly separate the individual semicircular canals from each other, we completed the smoothing of the model and added different colors to each of the canals using 3DSMax Software (Autodesk Inc. San Rafael, CA) (Fig. 1).
Fig. 1.

Final smoothing and editing of the model and addition of colors to each of the canals was done using 3DSMax Software (Autodesk Inc. San Rafael, CA).
Cloning of the labyrinth
Once the final design of the 3D model was obtained, it was cloned to create an enantiomeric (mirror image) left labyrinth using Amira. The method of this transformation was the change of all x-axis point definitions in the labyrinth from negative to positive.
Fusion with magneticresonance imaging study
An MRI was obtained using a standard internal auditory canal protocol from a male cadaveric specimen. The skull and soft tissue surfaces were extracted on the MRI workstation and the DICOM images uploaded into Amira. In the Amira 3D environment, the membranous labyrinths were aligned with the MRI images of the skull using anatomic norms averaged from 21 subjects13 (Fig. 2).
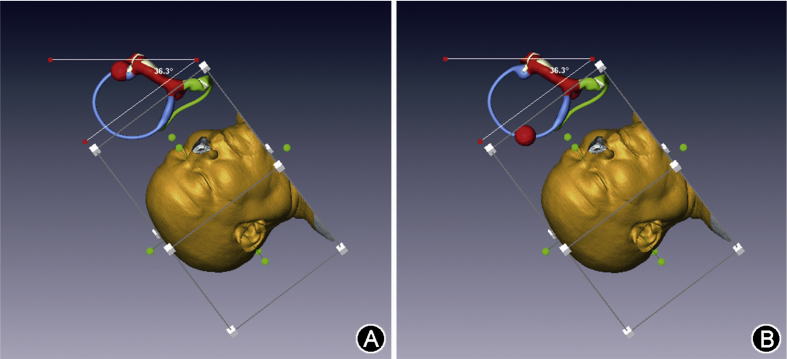
Fig. 2.
(A) Cloned labyrinths associated with skull model extracted from MRI data. (B) Addition of skin surface to the model and demonstration of anterior to anterior canal angle of 104°. (C) Demonstration of posterior canal to posterior canal angle of 83°. (D) Demonstration of horizontal canal to horizontal canal angle of 155°.
Creation of canalith markers
The Amira function for “creation of parametric surfaces” was used to create marker spheres that can be moved within the 3D environment. Spheres were positioned around the circumference of all 6 semicircular canals at the macula of the utricle but can be moved to any position within the model. These spheres can be manually turned on and off as needed to mark the expected most-dependent position of the otolith mass during movement of the model. This method is sufficient for recording images that are easily and instantly understandable to a viewer (Fig. 3).
Fig. 3.
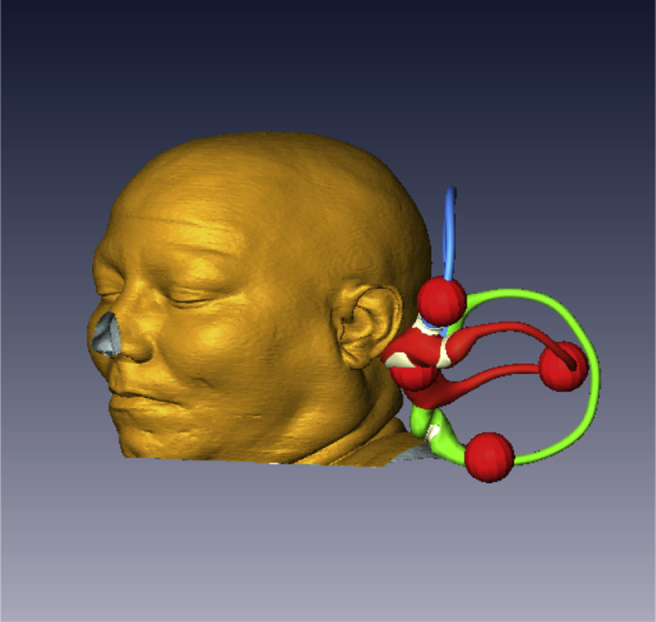
Canal markers indicating the expected position of an otolith mass in any position can be moved to any position on the labyrinth.
Results
The resulting model functions within the 3D Amira environment on a PC laptop workstation (Fig. 4). The head and its 2 membranous labyrinths can be rotated in any plane as a unit as in nature. Amira's measuring tools allow measurement of the angles of rotation in any position within the working environment. The canal markers can be moved to any position within the environment to mark and to illustrate the dependent portions of the labyrinths in any position, and the position of free otolith debris. Sequential screenshots allow a record of problem analysis (Fig. 5).
Fig. 4.
Desktop view of the Amira viewer with the model in the working environment.
Fig. 5.
(A) Right superior canalithiasis after left Dix-Hallpike demonstrating the angle below horizontal needed to provoke otolith movement. (B) The final expected position of the otolith mass.
Because transparency can be added to the labyrinth the otolith marker can be reduced in size to fit and be visualized within the membranous labyrinth if analysis requires it (Fig. 6).
Fig. 6.
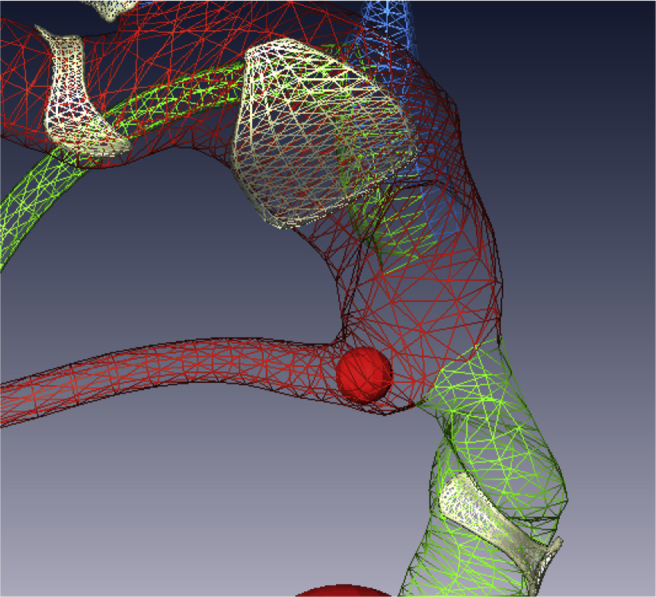
The otolith marker is shown within the membranous labyrinth at the nonampullated end of the right horizontal semicircular canal.
Discussion
The evolution of understanding of BPPV by clinicians over the last 50 has been hampered by the lack of an accurate reference that all clinicians can refer to for conceptualization and communication. The 3D BPPV model is both accurate and detailed. Based on human membranous labyrinthine anatomy obtained from histologic sections, the model can be used for analysis in 2D mode. The creation of the 3D BPPV model in a 3D environment ensures the configuration accuracy of the model.
This model will allow a clear analysis of many different problems encountered by clinicians interested in BPPV. It can be used for consideration of simultaneous phenomenon in multiple canals and on both sides in addition to unilateral phenomenon alone. Using this model, newer more efficient repositioning strategies may be devised that will improve treatment for patients. Optimization of existing maneuvers can be proposed.
A clear model allows finer distinctions to be made between predicted phenomena and observed clinical phenomena. There is no better example than the history of the understanding of BPPV itself to illustrate this point. An initial conceptual model of BPPV as cupulolithiasis existed for 30 years despite dramatic discrepancies between results predicted by the cupulolithiasis theory and observed clinical behavior. During this period, confusing and contradictory clinical observations that were essential to progress were discarded, and delayed the conceptualization of canalithiasis as the predominant form of otolith disease. While based on a human membranous labyrinth the model is based on only a single labyrinth. It resides within the bony labyrinth which has small but significant variations of position within the human skull. As such the model may not be said to be a final predictor of all possible otolith movement phenomenon related to BPPV.
The assumption the model makes that all otoliths assume the most dependent position in any head position or in response to movement may be wrong. Sophisticated mathematical modeling of BPPV, or clinical experience may contradict model predictions. In these cases, there will be an opportunity for greater insight.
The 3D BPPV Model is not yet widely available. It is, in many ways a draft of a more functional model that can be created for all investigators to use. Future development will allow a downloadable version to be available to any interested clinician. A future programming challenge will be the creation of gravity dependent movement of otoliths loaded at any point within the labyrinth. The model may be improved to allow better visualization of simulated disease. The current model does not allow removal of individual canals if they interfere with critical observation from a specific angle of view and requires that the entire labyrinth be made transparent, or the model to be rotated to make a specific observation. The model could also serve as accurate repository of techniques of BPPV diagnosis and treatment if it can be animated and programmed to store sequences of head movements and associated otolith movements. These improvements are relatively simple and require programming effort and expertise. A more complicated version of the model could include biomechanical modeling of otolith and cupula movement based on known physical parameters such as otolith mass, duct diameter and endolymph viscosity.
Conclusion
A simple model based on human anatomy has been created to allow careful study of BPPV pathophysiology and treatment. Going forward, this tool could offer insights that may lead to more accurate diagnosis and treatment of BPPV. Future development of this model into a version available to all investigators will enhance communication and support innovation in BPPV diagnosis and treatment.
Acknowledgments
The 3D model that we used was created under the direction of Clarinda Northrop and the assistance of Haobing Wang and Stephen Levine. We would like to acknowledge their hard work in creating this model, as well as their help with the modified description of its creation for our BPPV model.
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Dix M.R., Hallpike C.S. The pathology symptomatology and diagnosis of certain common disorders of the vestibular system. Proc R Soc Med. 1952;45:341–354. doi: 10.1177/003591575204500604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schuknecht H.F. Cupulolithiasis Arch Otolaryngol. 1969;90:765–778. doi: 10.1001/archotol.1969.00770030767020. [DOI] [PubMed] [Google Scholar]
- 3.Epley J.M. The canalith repositioning procedure: for treatment of benign paroxysmal positional vertigo. Otolaryngol Head Neck Surg. 1992;107:399–404. doi: 10.1177/019459989210700310. [DOI] [PubMed] [Google Scholar]
- 4.Hashimoto S., Naganuma H., Tokumasu K., Itoh A., Okamoto M. Three-dimensional reconstruction of the human semicircular canals and measurement of each membranous canal plane defined by Reid's stereotactic coordinates. Ann Otol Rhinol Laryngol. 2005;114:934–938. doi: 10.1177/000348940511401207. [DOI] [PubMed] [Google Scholar]
- 5.Siebenmann F. Bergmann; Wiesbaden: 1890. Die Korrosions-Anatomie des knˆchernen Labyrinthes des menschlichen ohres. [Google Scholar]
- 6.Teixido M.T., Kirkilas G., Seymour P. Normative inner ear volumetric measurements. J Craniofac Surg. 2015;26:251–254. doi: 10.1097/SCS.0000000000001204. [DOI] [PubMed] [Google Scholar]
- 7.Curthoys I.S., Markham C.H., Curthoys E.J. Semicircular duct and ampulla dimensions in cat, guinea pig and man. J Morphol. 1977;151:17–34. doi: 10.1002/jmor.1051510103. [DOI] [PubMed] [Google Scholar]
- 8.Curthoys I.S., Oman C.M. Dimensions of the horizontal semicircular duct, ampulla and utricle in the human. Acta Otolaryngol. 1987;103:254–261. [PubMed] [Google Scholar]
- 9.Igarashi M. Dimensional study of the vestibular apparatus. Laryngoscope. 1967;77:1806–1817. doi: 10.1288/00005537-196710000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Igarashi M., Ohashi K., Ishii M. Morphometric comparison of endolymphatic and perilymphatic spaces in human temporal bones. Acta Otolaryngol. 1986;101:161–164. doi: 10.3109/00016488609132823. [DOI] [PubMed] [Google Scholar]
- 11.Ifediba M.A., Rajguru S.M., Hullar T.E., Rabbitt R.D. The role of 3-canal biomechanics in angular motion transduction by the human vestibular labyrinth. Ann Biomed Eng. 2007;35:1247–1263. doi: 10.1007/s10439-007-9277-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang H., Northrop C., Burgess B., Liberman M.C., Merchant S.N. Three-dimensional virtual model of the human temporal bone: a stand-alone, downloadable teaching tool. Otol Neurotol. 2006;27:452–457. doi: 10.1097/01.mao.0000188353.97795.c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Della Santina C.C., Potyagaylo V., Migliaccio A.A., Minor L.B., Carey J.P. Orientation of human semicircular canals measured by three-dimensional multiplanar CT reconstruction. J Assoc Res Otolaryngol. 2005;6:191–206. doi: 10.1007/s10162-005-0003-x. [DOI] [PMC free article] [PubMed] [Google Scholar]