Abstract
Objective
To assess the normal levels of nasal nitric oxide (NNO) and fractional exhaled nitric oxide (FENO) in healthy Chinese young people, and to determine whether the obtained values were associated with age, sex, height, weight, BMI (body mass index) or BSA (body surface area).
Methods
One hundred and twenty healthy people were selected from a total of 436 Chinese young people based on their answers to a questionnaire. An electrochemical analyzer (NIOX MINO system) was used to measure NNO and FENO. The relationship between NNO, FENO and age, sex, height, weight, BMI, BSA was analyzed using SPSS software.
Results
The values of NNO were normal distributed (mean 273.5 ppb; SD 112.3). The values of FENO were non-normally (Skewed) distributed (median: 14.00 ppb; interquartile range: 7.00 ppb). The obtained NNO values were independent of age, sex, height, weight, BMI and BSA, but were positively correlated to lnFENO (FENO log base e); lnFENO values were also independent of age, height, weight, BMI and BSA, but correlated with NNO and sex.
Conclusions
NNO values positively correlate with lnFENO in healthy people and the levels of each may be predicted by the other. The results of this study are expected to serve as a reference for future studies in China.
Keywords: Nitric oxide, Nasal cavity, Lower respiratory tract, Normal value, Healthy people
Introduction
Since the discovery of its function as an endothelium-derived relaxing factor in 1987, nitric oxide (NO) has been further documented to be present in the exhaled air of rabbits, guinea pigs, and humans.1 Nasal nitric oxide (NNO) refers to NO measured in air aspirated from the nasal cavity. The main source of this gaseous molecule is the paranasal sinus epithelium. The physiological role of NNO is to modulate ciliary motility, and to serve as an aerocrine mediator that helps to maintain adequate ventilation perfusion matching in the lung and nasal cavity. NO can kill bacteria, viruses, fungi, and tumor cells at high concentrations. In addition, inflammatory factors can activate nitric oxide synthetase (NOS), which can lead to a rapid increase in NNO concentration. NNO was recommended in Europe as the preferred first-line test for Primary Ciliary Dyskinesia (PCD) prior to performing confirmatory diagnostic tests in 2009.2 Patients with severe nasal polyps have low levels of NNO, which increase markedly following therapy.3 In patients with allergic rhinitis, NNO levels tend to be high and decrease following treatment.4 High levels of NNO almost certainly rule out the presence of cystic fibrosis.5 FENO refers to NO measured in air exhaled from the lower airway. FENO is produced by the human lung and is present in exhaled breath. The measurement of FENO has been standardized for clinical use and has been implicated in the pathophysiology of lung diseases, including asthma.6 NO is usually measured by chemiluminescence or electrochemical analyzers, which are secure, non-invasive, repeatable, and feasible methods. The development of sensitive techniques for the instantaneous measurement and monitoring of NO concentrations in human airways has spurred many clinical studies focused on monitoring diseases and the effects of treatments.
Although some progress has been made, the use of NNO as a marker of upper airway diseases within clinical practice still needs to be developed. In contrast to FENO, there is no current consensus on the normal values of NNO. In China, there have only been two reports about normal NNO levels; however, they reported different and inconsistent results.7, 8 Therefore, in this study, we aimed to assess the normal values of NNO and FENO in healthy young people in China, and to determine the factors that affect NNO and FENO levels.
Materials and methods
Subjects
In total, 436 Chinese young people (aged 9–22 years) recruited from one college and one middle school were invited to participate in this study. The inclusion criteria were: aged between 9 and 22 years and written consent from themselves or their parents. The subjects were first asked if they had experienced any nasal or lower respiratory symptoms, including itching, rhinorrhea, sneezing, obstruction, cough, wheezing, and asthma, in the last four weeks. Subjects experiencing any nasal or lower respiratory symptoms were excluded. The remaining subjects were then asked to fill out an extensive questionnaire, which included potential confounding factors (sex, age, height, weight, BMI, BSA) and the exclusion criteria: history of respiratory diseases including rhinitis, deviation of nasal septum, nasal polyps, sinusitis, asthma, cough, wheezing, chronic pharyngitis, systemic or nasal administration of medications within two weeks; consumption of caffeine-containing beverages such as coffee, tea or colas; consumption of foods rich in nitrogen such as sausage, various animal offal, lettuce, and spinach within 2 h; respiratory tract infection within two weeks; related history of nasal operation; physical exercise within two hours of NO measurement; active or passive smoking.
The Ethical Committee of the Chinese People's Liberation Army General Hospital (Beijing, China) approved the study protocol.
Methods
NNO and FENO measurements
NNO and FENO were measured online according to the NIOX MINO manual (Aerocrine AB, Solna, Sweden). NIOX MINO does require ambient NO measurement. The NO measurement unit was parts per billion (ppb). The NNO measurement range was 5–1700 ppb, and the FENO measurement range was 5–300 ppb. The machine automatically recorded and stored the FENO and NNO values.
The measurement of NNO: The subjects rested for at least 30 min before measurement. A NO-inert olive tip was blocked firmly against the right nostril and was connected to NIOX MINO through a sampling tube. The flow rate was 5 ml/s. Subjects were asked to breathe normally and then hold their breath for 45 s. The concentration of NNO was automatically calculated by the NIOX MINO system.
The measurement of FENO: The subjects rested for 15 min after NNO measurement. The subjects were then asked to stand and exhale to residual volume. A mouthpiece was then inserted, the patient inhaled to total lung capacity and then exhaled for 10 s at a constant flow rate of about 50 ml/s. The NIOX MINO automatically reported the detected FENO concentrations.
Statistical analysis
All data were entered into a computer twice by two individuals. A one-Sample Kolmogorov–Smirnov Test was performed to analyze the distribution of baseline variables, which were described as the mean ± standard deviation (SD). FENO values and age were non-normally distributed, and they were expressed as the median (interquartile range, IQR). Logarithmic transformations were performed for FENO values and expressed as lnFENO. Correlation was examined using the Pearson and Spearman correlation analysis, and linear regression analysis was used to derive the prediction rules for NNO and FENO values. A P < 0.05 was considered statistically significant. All statistical procedures were performed using SPSS software, version 13.0.
Results
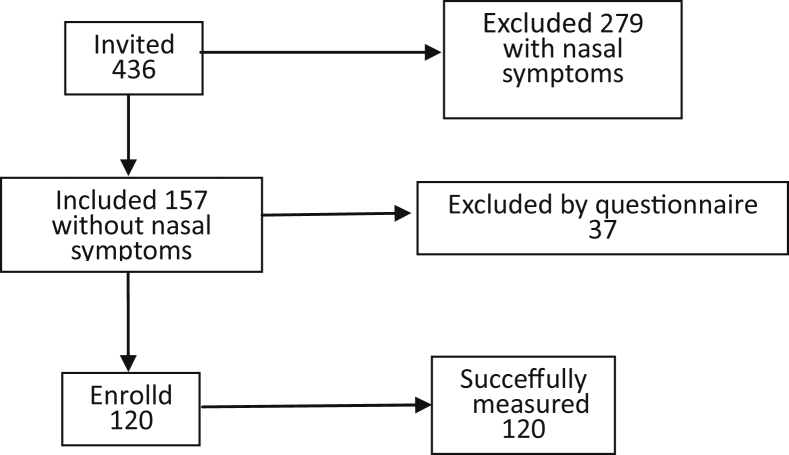
In total, 436 Chinese young people (aged 8–22 years) were willing to participate in this study, including 200 males (45.9%) and 236 females (54.1%). Of these volunteers, 279 were excluded due to nasal or lower respiratory symptoms. An additional 37 people were excluded after the second, more detailed, questionnaire. In total, 120 (27.5%) subjects (aged 9–22, average age 15.1 years) satisfied our requirements, and were selected to undergo NNO and the FENO measurement (Fig. 1). Of these subjects, 49 were male (40.8%) and 71 were female (59.2%). The general characteristics of the participants and their NNO and FENO results are displayed in Table 1.
Fig. 1.
Flow chart of the study.
Table 1.
General characteristics of subjects and NNO/FENO levels (n = 120).
| Minimum | Maximum | Mean ± SD or median (IQR) | Distribution | |
|---|---|---|---|---|
| NNO (ppb) | 16 | 582 | 273.50 ± 112.28 | Normal |
| FENO (ppb) | 5 | 73 | 14.0 (7.0) | Non-Normal |
| Age (year) | 9 | 22 | 15.0 (5.0) | Non-Normal |
| Height (cm) | 133.400 | 191.00 | 163.05 ± 10.76 | Normal |
| Weight (kg) | 30.00 | 82.00 | 51.88 ± 10.94 | Normal |
| BMI (kg/m2) | 12.527 | 27.042 | 19.37 ± 2.77 | Normal |
| BSA (m2) | 1.08 | 2.007 | 1.64 ± 0.19 | Normal |
IQR, interquartile range; SD, standard deviation; BMI, body mass index; BSA, body surface area.
NNO values
NNO values were normally distributed [(273.50 ± 112.28) ppb, 95% CI, 253.20–293.80 ppb] with a 95% reference value range (prediction interval) of 53.43–493.57 ppb, and an absolute range from 16 to 582 ppb. Correlation analysis revealed that the NNO values were independent of age, sex, height, weight, BMI and BSA, but correlated positively with lnFENO (r = 0.283, P = 0.002), Table 2.
Table 2.
Correlations among lnFENO, NNO, and other factors.
| NNO |
lnFENO |
|||
|---|---|---|---|---|
| r | P | R | P | |
| Sex | 0.094 | 0.307 | 0.248 | 0.006a |
| Age | 0.025 | 0.789 | −0.081 | 0.377 |
| Height | −0.051 | 0.577 | 0.144 | 0.117 |
| Weight | 0.045 | 0.622 | 0.123 | 0.181 |
| BMI | 0.091 | 0.323 | 0.042 | 0.646 |
| BSA | 0.020 | 0.828 | 0.147 | 0.110 |
| lnFENO | 0.283 | 0.002a | / | / |
| NNO | / | / | 0.283 | 0.002a |
lnFENO, FENO log base e; SD, standard deviation; BMI, body mass index; BSA, body surface area; r, correlation coefficient.
Correlation is significant at the 0.01 level (2-tailed).
FENO values
The FENO values were not normally distributed. The median FENO value was 14.00 ppb (IQR, 7.00 ppb). The maximum was 73 ppb and the minimum 5 ppb with a 95% reference value range of 5.93–35.87 ppb. After logarithmic base e transformations of the FENO values, the resulting lnFENO values were normally distributed. Correlation analyses revealed that lnFENO values were independent of age, height, weight, BMI, and BSA. However lnFENO values were correlated positively with NNO and sex (male = 2, female = 1, r = 0.248, P = 0.006). A prediction rule for lnFENO and FENO values in healthy patients can be derived from NNO measurements:
| lnFENO = 2.084 + 0.001063650672482 × NNO + 0.2150389683607 × sex. |
| FENO = e2.084 + 0.001063650672482 × NNO + 0.2150389683607 × sex. |
For example, if a girl's NNO is 300 ppb, then the predicted FENO concentration would be 13.71 ppb (=e2.084 + 0.001063650672482 × 300 + 0.2150389683607 × 1). NNO can also be predicted from FENO values as follows: NNO = −141.426 + 355.594 × lnFENO − 113.267 × lnFENO2 + 14.254 × lnFENO3. For example, if FENO = 20 ppb and lnFENO = 3, then the approximate NNO concentration would be 290.811 ppb (=−141.426 + 355.594 × 3–113.267 × 32 + 14.254 × 33).
Discussion
The official ATS (American Thoracic Society) clinical practice guidelines for the interpretation of FENO levels for clinical applications was issued in 2012.6 However, standardization of measurements, the establishment of reference values, and reference equations for NNO have not yet been developed. The measurement of NNO as a non-invasive method will be of great value in the field of rhinology. Since its first description in 1993, more studies have focused on FENO rather than NNO. The few studies reporting NNO values were variable and do not agree with one another (Table 3). In China specifically, only two reports have measured NNO levels, and their results were not in agreement.7, 8 NNO levels are more susceptible than FENO to factors such as season,9 time of day,10 and the analyzer used,1 and NNO has a high degree of inter-individual variability among healthy people. For these reasons, NNO seems to be used less frequently than FENO in clinical settings. However, most researchers agree that extremely low levels of NNO can predict PCD or cystic fibrosis.2, 5, 11, 12, 13 In addition, NNO varies between individuals over time, meaning that 20%–25% of the normal variation seen is not reflective of changes in disease status or response to medication. Therefore, in the same patient, NNO values can change more than 25% without the influence of medications or disease status.14
Table 3.
Reported NNO levels in healthy people and association with other factors.
| Year | First author | Healthy people | NNO levels in ppb | NNO associated |
|---|---|---|---|---|
| 2002 | Colantonio D19 | n = 20 | 740.9 ± 148.1 | |
| 2002 | Narang I20 | n = 53 | 716 | |
| 2003 | Wodehouse T12 | n = 16 | 759 ± 145.8 | |
| 2005 | Struben VM16 | n = 340 | 449 ± 115 | With age (<12 yrs), but not sex, passive smoking, or BMI |
| 2007 | Stark H9 |
n = 18 | Winter 133.5 ± 29.7 | |
| n = 17 | Summer 138.0 ± 47.6 | |||
| n = 21 | Autumn 121.0 ± 37.5 | |||
| 2008 | Dressel H10 |
n = 19 | 7 AM–9 AM: 1505 ± 113 | |
| 11 AM–1 PM: 1736 ± 109 | ||||
| 1 PM–3 PM: 1730 ± 105 | ||||
| 3 PM–5 PM: 1740 ± 95 | ||||
| 5 PM–7PM: 1670 ± 103 | ||||
| 11 AM–1 PM: 1736 ± 109 | ||||
| 2009 | Zhang L7 | n = 80 | 819 ± 211 | With age and gender, but not height or BMI |
| 2010 | Williamson PA15 | n = 41 | 878.1 (807.0–955.6) ppb | Without age, sex |
| 2010 | Kramer MF21 | n = 11 | 1380 (988–2097) | |
| 2010 | Piacentini GL17 | n = 43 (cooperative) | 396.9 ± 25.0 | With age and weight, but not gender, height, or BMI |
| 2011 | Zhou H22 | n = 35 | 303 ± 90 | |
| 2011 | Marthin JK13 | n = 52 | 534 ± 30 | |
| 2012 | Leng YG8 | n = 182 | 79 ± 35 | |
| 2012 | Lee KJ23 | n = 34 | 276 ± 88 | |
| 2012 | Irander K24 | n = 7 | 79 ± 33 | No relationship with age, gender, height, BMI, measure time, or right-left side |
| 2013 | Marthin JK11 | n = 21 | MINO5, TB (nVC): 340 ± 23 | |
| n = 21 | MINO2, TB (nVC): 752 ± 59 | |||
| n = 8 | NIOX MINO5, BH (VC): 603 ± 42 | |||
| n = 21 | NIOX FLEX, TB (nVC): 486 ± 34 | |||
| n = 21 | CLD 88 sp, TB (nVC): 499 ± 35 | |||
| n = 21 | NIOX FLEX, BH (VC): 890 ± 62 | |||
| n = 21 | CLD 88 sp1(VC): 799 ± 57 |
BMI, body mass index; BSA, body surface area; MINO5: sampling rate 5 ml/s; MINO2: sampling rate 2 ml/s; TB: tidal breathing; BH: breathing hold; nVC: on-velum closure; VC: velum closure; NIOX (Aerocrine AB, Solna, Sweden): NIOX (hand-hold), NIOX MINO Nasal(hand-hold), NIOX FLEX(stationary); CLD 88 sp and CLD 88 sp1 (ECO MEDICSH AG, Duernten, Switzerland).
The healthy subjects measured in our study were strictly selected based on ATS/ERS (European Respiratory Society) recommendations. A strict screening of all 436 subjects through questionnaire survey identified those with potentially confounding factors including food, drink, medication, smoking, sports, respiratory diseases, and surgery. In addition, subjects with nasal or respiratory symptoms including itching, rhinorrhea, sneezing, obstruction, cough, wheezing, and asthma were also excluded. Finally, 120 healthy young students that satisfied all requirements were enrolled and successfully completed NNO and FENO measurement. Standard operating procedures (SOP) for NIOX MINO were used to measure NNO and FENO levels in order to guarantee that our results were objective and reliable.
General subject characteristics including age, sex, height, weight, BMI, and BSA may affect NNO and FENO levels (Table 3, Table 4). We found that NNO values were independent of age, sex, height, weight, BMI, and BSA, but correlated positively with lnFENO. The correlation between NNO and lnFENO (FENO) means that one value may be calculated from the other, which has not been previously reported, and may be helpful in the diagnosis and treatment of respiratory system diseases. Williamson PA et al15 found that NNO and FENO were correlated in healthy volunteers (r = 0.44, P = 0.004), which is consistent with the results of our study. Multiple linear regressions of the whole cohort demonstrated that after diagnosis, age, sex, and inhaled corticosteroids were taken into account, NNO was significantly associated with FENO (P = 0.02). Zhang L et al7 found that NNO values were associated with age and gender, but not height or BMI. Leng G et al8 came to a similar conclusion as that of the present study, namely that NNO levels in healthy people were independent of age, sex, height, weight, and BMI. However, Struben VM et al16 found that NNO concentrations were not associated with sex, passive smoking, or BMI, but that in children aged <12 yrs, NNO correlated positively with age. Piacentini GL et al17 reported that age and NNO concentration were significantly related in pre-school children and that children older than 6 years had levels of NNO that were about 100 ppb higher than those of children aged 3 or under. This can be explained by the accelerated pneumatization of developing paranasal sinuses that occurs during childhood. Similarly to other studies,18 we also found significantly higher FENO levels in males compared to females. The relationship between FENO and gender may be due to geometrical factors such as the total airway mucosal surface area and the airway caliber, both of which can affect FENO output. Interestingly, in our study, FENO did not correlate with the age, which is not consistent with the majority of previous studies (Table 4). Age has been found to be a strong factor that effects FENO concentration in children younger than 12 yrs. These inconsistent results may be explained by the fact that the majority of subjects were aged >12 yrs in this study, and that only 9.16% of subjects were aged <12 yrs. Struben VM et al16 found that FENO correlated positively with age in children aged <12 yrs, but not in children aged >12 yrs, and that ambient NO was the only significant modifier in older children. The different outcomes of the various studies may be due to the following four possible explanations. First, individual differences depend on the sinus development, and sinus pneumatization is differs between all individuals. Second, some experts have found that the NNO levels of healthy subjects were lower in the morning than in the afternoon.9, 10 Third, different equipment and different sampling flow rates may yield different results. Marthin JK et al11 found that using different instruments lead to different results. Measurements acquired at a sampling rate of 5 ml/s were superior to those acquired at 2 ml/s using the same instrument. Last, ambient NO levels may influence NNO levels. Although our instrument is unaffected by ambient NO, the instruments used in other studies may have been affected by it. High concentrations of ambient NO may reduce the gradient for NO diffusion from the nasal epithelium to the nasal cavity. Struben VM et al16 found that the level of ambient NO (5–182 ppb) heavily influenced the measured levels of NNO in their study.
Table 4.
Reported FENO levels in healthy people and their association with other factors.
| Year | First author | Healthy people number and years | FENO levels in ppb mean ± SD or mean (range) | FENO associated |
|---|---|---|---|---|
| 2005 | Wong GK25 | n = 258(Chinese) (14.0 ± 1.6) yrs |
Male: 10.8 (7.8–17.6) Female: 9.1 (7.5–11.9) |
With gender, but not age, height, weight, BMI, or BSA |
| 2006 | Malmberg LP26 | n = 114 11.3 (7.2–15.7) yrs |
10.3 | With age, height, weight, and BSA, but not gender |
| 2008 | Kovesi T27 | n = 657 (10.8 ± 0.89) yrs |
14.0 ± 13.4 | Not with age, gender, or BMI |
| 2008 | Hervás D28 | n = 15 9.5 (6–16) yrs |
7.9 (5.9–10.6) | With gender and age |
| 2009 | Banovcin P29 | n = 27 (12 ± 4) yrs |
12.4 ± 1.2 | With gender and age. |
| 2010 | Matsunaga K30 | n = 240 (18–74) yrs |
16.9 (6.5–35.0) | Not with age, gender, or height |
| 2012 | Janahi L31 | n = 203 (12–18) yrs |
14.7 (5.0–90.5) | Negatively associated with age and gender BUT height, weight, BMI |
| 2013 | Zhang H32 | n = 300 (6–14) yrs |
Male:13, IQR 9–18 Female:10, IQR 8–14 |
With gender and age |
BMI, body mass index; BSA, body surface area; IQR, interquartile range.
In conclusion, NNO values were independent of age, sex, height, weight, BMI, and BSA, but correlated positively with lnFENO. FENO values were also independent of age, height, weight, BMI and BSA. However, lnFENO values were correlated positively with NNO and sex. Although the relationship between NNO and lnFENO should be studied further, we hope that the results of this study may be helpful in the future clinical application of NNO.
Acknowledgements
The authors would like to thank the college and the middle school, and all the people who participated in our study. This project was supported by Clinical research fund (No. 2012FC-TSYS-2010) of the Chinese People's Liberation Army General Hospital.
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Gustafsson L.E., Leone A.M., Persson M.G., Wiklund N.P., Moncada S. Endogenous nitric oxide is present in the exhaled air of rabbits, guinea pigs and humans. Biochem Biophys Res Commun. 1991;181:852–857. doi: 10.1016/0006-291x(91)91268-h. [DOI] [PubMed] [Google Scholar]
- 2.Barbato A., Frischer T., Kuehni C.E. Primary ciliary dyskinesia: a consensus statement on diagnostic and treatment approaches in children. Eur Respir J. 2009;34:1264–1276. doi: 10.1183/09031936.00176608. [DOI] [PubMed] [Google Scholar]
- 3.Carraro S., Cutrone C., Cardarelli C., Zanconato S., Baraldi E. Clinical application of nasal nitric oxide measurement. Int J Immunopathol Pharmacol. 2010;23:50–52. [PubMed] [Google Scholar]
- 4.Serrano C., Valero A., Picado C. Nasal nitric oxide. Arch Bronconeumol. 2004;40:222–230. doi: 10.1016/s1579-2129(06)70088-x. [DOI] [PubMed] [Google Scholar]
- 5.Walker W.T., Liew A., Harris A., Cole J., Lucas J.S. Upper and lower airway nitric oxide levels in primary ciliary dyskinesia, cystic fibrosis and asthma. Respir Med. 2013;107:380–386. doi: 10.1016/j.rmed.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 6.Dweik R.A., Boggs P.B., Erzurum S.C. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184:602–615. doi: 10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang L., Luo X.R., Liu C.Y., Zhao Y., Han D.M. Measurement of exhaled nitric oxide in healthy Chinese. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2009;44:302–306. [PubMed] [Google Scholar]
- 8.Leng G., Li Z., Wang Q. Detection of exhaled nitric oxide of healthy in Nanjing. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2012;26:769–771. [PubMed] [Google Scholar]
- 9.Stark H., Purokivi M., Kiviranta J., Randell J., Tukiainen H. Short-term and seasonal variations of exhaled and nasal NO in healthy subjects. Respir Med. 2007;101:265–271. doi: 10.1016/j.rmed.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Dressel H., Bihler A., Jund F. Diurnal variation of nasal nitric oxide levels in healthy subjects. J Investig Allergol Clin Immunol. 2008;18:316–317. [PubMed] [Google Scholar]
- 11.Marthin J.K., Nielsen K.G. Hand-held tidal breathing nasal nitric oxide measurement–a promising targeted case-finding tool for the diagnosis of primary ciliary dyskinesia. PLoS One. 2013;8:e57262. doi: 10.1371/journal.pone.0057262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wodehouse T., Kharitonov S.A., Mackay I.S., Barnes P.J., Wilson R., Cole P.J. Nasal nitric oxide measurements for the screening of primary ciliary dyskinesia. Eur Respir J. 2003;21:43–47. doi: 10.1183/09031936.03.00305503. [DOI] [PubMed] [Google Scholar]
- 13.Marthin J.K., Nielsen K.G. Choice of nasal nitric oxide technique as first-line test for primary ciliary dyskinesia. Eur Respir J. 2011;37:559–565. doi: 10.1183/09031936.00032610. [DOI] [PubMed] [Google Scholar]
- 14.Bartley J., Fergusson W., Moody A., Wells A.U., Kolbe J. Normal adult values, diurnal variation, and repeatability of nasal nitric oxide measurement. Am J Rhinol. 1999;13:401–405. doi: 10.2500/105065899781367528. [DOI] [PubMed] [Google Scholar]
- 15.Williamson P.A., Vaidyanathan S., Clearie K., Stewart M., Lipworth B.J. Relationship between fractional exhaled nitric oxide and nasal nitric oxide in airways disease. Ann Allergy Asthma Immunol. 2010;105:162–167. doi: 10.1016/j.anai.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 16.Struben V.M., Wieringa M.H., Mantingh C.J. Nasal NO: normal values in children age 6 through to 17 years. Eur Respir J. 2005;26:453–457. doi: 10.1183/09031936.05.00015205. [DOI] [PubMed] [Google Scholar]
- 17.Piacentini G.L., Bodini A., Peroni D.G. Nasal nitric oxide levels in healthy pre-school children. Pediatr Allergy Immunol. 2010;21:1139–1145. doi: 10.1111/j.1399-3038.2010.00989.x. [DOI] [PubMed] [Google Scholar]
- 18.Grasemann H., Storm van's Gravesande K., Buscher R., Drazen J.M., Ratjen F. Effects of sex and of gene variants in constitutive nitric oxide synthases on exhaled nitric oxide. Am J Respir Crit Care Med. 2003;167:1113–1116. doi: 10.1164/rccm.200211-1342OC. [DOI] [PubMed] [Google Scholar]
- 19.Colantonio D., Brouillette L., Parikh A., Scadding G.K. Paradoxical low nasal nitric oxide in nasal polyposis. Clin Exp Allergy. 2002;32:698–701. doi: 10.1046/j.1365-2222.2002.01379.x. [DOI] [PubMed] [Google Scholar]
- 20.Narang I., Ersu R., Wilson N.M., Bush A. Nitric oxide in chronic airway inflammation in children: diagnostic use and pathophysiological significance. Thorax. 2002 Jul;57:586–589. doi: 10.1136/thorax.57.7.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kramer M.F., Olzowy B., Bihler A. Exhaled and nasal nitric oxide in laryngectomized patients. BMC Pulm Med. 2010;10:4. doi: 10.1186/1471-2466-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou H., Zou B., Hazucha M., Carson J.L. Nasal nitric oxide and lifestyle exposure to tobacco smoke. Ann Otol Rhinol Laryngol. 2011;120:455–459. doi: 10.1177/000348941112000706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee K.J., Cho S.H., Lee S.H. Nasal and exhaled nitric oxide in allergic rhinitis. Clin Exp Otorhinolaryngol. 2012;5:228–233. doi: 10.3342/ceo.2012.5.4.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Irander K., Palm J.P., Borres M.P., Ghafouri B. Clara cell protein in nasal lavage fluid and nasal nitric oxide – biomarkers with anti-inflammatory properties in allergic rhinitis. Clin Mol Allergy. 2012;10:4. doi: 10.1186/1476-7961-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong G.W., Liu E.K., Leung T.F. High levels and gender difference of exhaled nitric oxide in Chinese schoolchildren. Clin Exp Allergy. 2005;35:889–893. doi: 10.1111/j.1365-2222.2005.02263.x. [DOI] [PubMed] [Google Scholar]
- 26.Malmberg L.P., Petäys T., Haahtela T. Exhaled nitric oxide in healthy nonatopic school-age children: determinants and height-adjusted reference values. Pediatr Pulmonol. 2006;41:635–642. doi: 10.1002/ppul.20417. [DOI] [PubMed] [Google Scholar]
- 27.Kovesi T., Dales R. Exhaled nitric oxide and respiratory symptoms in a community sample of school aged children. Pediatr Pulmonol. 2008;43:1198–1205. doi: 10.1002/ppul.20927. [DOI] [PubMed] [Google Scholar]
- 28.Hervás D., Milán J.M., Garde J. Differences in exhaled nitric oxide in atopic children. Allergol Immunopathol. 2008;36:331–335. doi: 10.1016/s0301-0546(08)75865-8. [DOI] [PubMed] [Google Scholar]
- 29.Banovcin P., Jesenak M., Michnova Z. Factors attributable to the level of exhaled nitric oxide in asthmatic children. Eur J Med Res. 2009;4:9–13. doi: 10.1186/2047-783X-14-S4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsunaga K., Hirano T., Kawayama T. Reference ranges for exhaled nitric oxide fraction in healthy Japanese adult population. Allergol Int. 2010;59:363–367. doi: 10.2332/allergolint.10-OA-0197. [DOI] [PubMed] [Google Scholar]
- 31.Janahi I., Saadoon A., Tuffaha A., Panneerselvam B. Effects of age, gender, and environmental exposures on exhaled nitric oxide level in healthy 12 to 18 years Qatari children. Ann Thorac Med. 2012;7:98–103. doi: 10.4103/1817-1737.94532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang H., Shu L., Cai X. Gender and age affect the levels of exhaled nitric oxide in healthy children. Exp Ther Med. 2013;5:1174–1178. doi: 10.3892/etm.2013.922. [DOI] [PMC free article] [PubMed] [Google Scholar]