Abstract
The majority of patients with head and neck cutaneous squamous cell carcinoma (cSCC) are successfully treated with surgical treatment of the primary site. While only a minority of patients is at risk for regional metastasis, these patients have significantly worse outcomes. Tumor and patient factors that place patients at high risk for development of regional metastasis have been identified. Advancing treatment of cSCC requires identifying and escalating treatment in this high risk patient population, while avoiding overtreatment of the majority of cSCC patients that do not develop regional metastasis. Sentinel lymph node biopsy has emerged as a promising technique in cSCC to detect micrometastasis and allow early surgical treatment of regional disease. Future directions involve genomic characterization of metastatic and nonmetastatic cSCC to identify genomic alterations causing metastasis that may be used to predict disease behavior.
Keywords: Squamous cell carcinoma, Cutaneous, Head and neck cancer
Introduction
Cutaneous squamous cell carcinoma (cSCC) is the second most common cancer diagnosed in the USA annually with approximately 700,000 cases of cSCC each year. Exposure to ultraviolet radiation is the most important risk factor for development of cSCC.1 Most patients with cSCC elicit a history of prolonged lifetime sunlight exposure, and history of severe sunburns are a critical risk factor.2 Ultraviolet radiation typically produces the formation of thymidine dimers in the p53 tumor-suppressor gene and failure to recognize and repair these mutations results in development of cSCC.1
Only a minority of patients with cSCC will develop regional metastasis and subsequently require treatment to the regional lymph node basin. The classic number that is commonly cited regarding the percentage of patients that develop regional metastasis is 5%.1, 3, 4 As such, the majority of patients with cSCC can be successfully treated with surgical excision and Mohs' micrographic surgery. However, the rate of regional metastasis may be as high as 21% in tertiary care centers.5 In these patients, development of regional metastasis results in 3-year disease free survival rate of 56% and 5-year overall survival of 25%–35%4, 6, 7; 10-year overall survival is less than 20%.1
Identifying patients with cSCC at risk for regional metastasis and delivering early, therapeutic treatment to the regional lymph node basin in this patient population is critical. Importantly, the majority of patients with cSCC do not develop regional metastasis, and it is paramount to avoid overtreating this patient population. This article reviews critical data and articles that have advanced the management of head and neck cSCC and in particular, the high risk patient with cSCC.
Defining the “high risk” population
Brantsch et al.8 prospectively analyzed 615 patients with cSCC, analyzing factors predicting regional lymph node metastasis. In this study, all patients were treated with surgical excision and confirmed negative margins. They were subsequently followed every 6 months for 2 years, followed by surveillance every 24 months. Mean and median follow up was 43 months, and 26 patients (4%) developed regional metastasis. Brantsch et al.8 identified 4 key prognostic factors on multivariate analysis predictive of regional metastasis:
-
1.
Increased tumor thickness (HR 4.79)
-
2.
Localization at the ear (HR 3.61)
-
3.
Increased tumor diameter (HR 2.22)
-
4.
Presence of immunosuppression (HR 4.32)
Specifically, increased tumor thickness showed a hazard ratio of 4.79. Importantly, patients in this series with 2.0 mm thickness or less never developed regional metastasis. In contrast, 16% of patients with greater than 6.0 mm thickness developed regional metastasis and 3.8% of patients with intermediate tumor thickness of 2.1–6.0 mm developed regional metastasis. Localization at the ear resulted in a hazard ratio of 3.61 as 10% of patients with primary ear cSCC developed regional metastasis. Similar to tumor thickness, increasing tumor diameter resulted in greater metastatic potential; patients with tumors 2 cm or less in diameter metastasized less than 2% of the time. In contrast, tumor diameter greater than 5.0 cm metastasized 20% of the time, and those with intermediate tumor diameter 2.1–5.0 cm metastasized 8% of the time. Finally, presence of immunosuppression resulted in a hazard ratio of 4.32, with 16% of immunosuppressed patients developing regional metastasis. Findings from the Bransch study suggest that tumor size (thickness and diameter) are important features in metastasis prediction.
Veness9 published a review article highlighting several other high risk features: incomplete excision/recurrence, poor differentiation, location on lip and/or drainage to the parotid basin, perineural and lymphovascular invasion.3, 10
TNM staging
American Joint Committee on Cancer (AJCC) TNM staging classification11 for cSCC was updated in 2010 and is shown in Table 1. The latest update added several high risk features to the T classification staging system and sub-classified nodal staging into 5 categories (N1, N2a, N2b, N2c, and N3). Previously, nodal staging was either positive (N1) or negative (N0).
Table 1.
AJCC staging system for cutaneous squamous cell carcinoma.
| AJCC tumor staging system for cSCC | |
|---|---|
| T classification | |
| Tx | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ |
| T1 | Tumor ≤2 cm in greatest dimension with <2 high-risk features |
| T2 | Tumor >2 cm in greatest dimension or any size with ≥2 high-risk features |
| T3 | Tumor with invasion of maxilla, mandible, orbit, or temporal bone |
| T4 | Tumor with invasion of skeleton (axial or appendicular) or perineural invasion of skull base |
| N classification | |
| Nx | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis in a single ipsilateral lymph node, ≤3 cm |
| N2 | Metastasis in a single ipsilateral lymph node, >3 cm but ≤6 cm in greatest dimension; or in bilateral or contralateral lymph nodes, none greater than 6 cm |
| N2a | Metastasis in a single ipsilateral lymph node >3 cm but ≤6 cm |
| N2b | Metastasis in multiple ipsilateral lymph nodes, none >6 cm |
| N2c | Metastasis in bilateral or contralateral lymph nodes, none >6 cm |
| N3 | Metastasis in a lymph node >6 cm in greatest dimension |
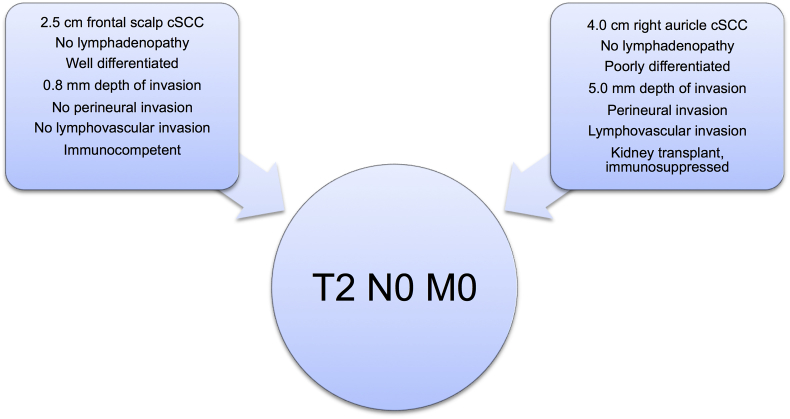
Despite these changes, the TNM classification system continues to have significant weaknesses.12 For example, consider Patient #1, who has a 2.5 cm well differentiated cSCC on the frontal scalp, 1 mm depth of invasion, no perineural or lymphovascular invasion, no palpable lymphadenopathy, and no history of immunosuppression. On the other hand, consider Patient #2, who has a 4.0 cm poorly differentiated cSCC on the right ear, 5 mm depth of invasion, with perineural and lymphovascular invasion, with a history of renal transplantation and immunosuppression, but no palpable lymphadenopathy. Based on the current staging system, both of these patients would be staged as T2 N0 M0 (Fig. 1) despite distinct risk profiles for recurrence and regional adenopathy.12 Furthermore, Brunner et al.13 showed no difference in survival between the newly designated N1 and N2 subgroups, while patients with N3 disease had significantly worse overall survival.
Fig. 1.
In the current staging system, two patients with distinct risk profiles for recurrence and regional metastasis are placed into the same TNM stage classification.
Treatment
The primary treatment options for cSCC of the head and neck are surgical excision and Mohs micrographic surgery. The optimal treatment modality depends on the tumor location, stage, comorbidities, and patient choice. Mohs micrographic surgery provides a 97.4% local control rate14 while preserving as much normal tissue as possible. The downside of Mohs micrographic surgery is the additional time required as well as a significant increase in cost.15 Furthermore, in high risk cases, sentinel lymph node biopsy cannot be performed in lesions undergoing Mohs micrographic surgery. It is important to note that while both methods are considered standard of care in the management of cSCC, there is no data to support superiority of Mohs micrographic surgery or standard surgical excision.
Surgical margins are dependent on the relative risk of local recurrence. There is a lack of recent data regarding surgical margins in cSCC. One frequently cited study stated that obtaining a 4 mm margin for lesions with a diameter of 2 cm or less would result in a 95% negative margin rate, and a 6 mm margin for lesions with a diameter of greater than 2 cm.16 While 1 cm margins are recommended for lesions >2 cm,17 this can be difficult to achieve in cosmetically sensitive subsites. These authors recommend 4–6 mm margins for low risk cSCC, and 1 cm margins for tumors exhibiting high risk features.17 In the setting of positive margins, re-resection is recommended.17 Radiation treatment is typically reserved for adjuvant treatment but can be used in cases where surgery is contraindicated or margin clearance is not possible.17
Sentinel node biopsy in the N0 neck
Gore et al.18 published the largest series of sentinel lymph node biopsy (SLNB) in head and neck cSCC. This study included 57 patients, all of whom had at least one of the following high risk features: tumor size >2 cm, invasion into subcutaneous fat or tumor thickness >5 mm, poor differentiation, perineural invasion/lymphovascular invasion, local recurrence in setting of adequate initial resection, ear or lip location, immunosuppressed, and carcinoma arising in a preexisting scar. In this population, 12.3% of patients had a positive sentinel lymph node biopsy, leading to immediate therapeutic neck dissection. In the patients undergoing neck dissection, 20% had additional nodal disease identified by neck dissection. There were two regional recurrences, one patient who had a positive sentinel node, immediate therapeutic neck dissection, and adjuvant radiation treatment, and one patient who could not undergo SLNB because the sentinel node could not be identified by lymphoscintigraphy or by neck exploration. Thus, the false negative rate SLNB in this series was 0%. Perineural invasion, lymphovascular invasion, and number of high risk factors were predictors of regional metastasis. As expected, patients with positive SLNB had significant worse disease specific survival as compared to those with negative SLNB.
Gore et al.18 pooled the SLNB in head and neck for cSCC in the existing literature, citing 82 cases19, 20, 21, 22 with a 10% SLNB positivity rate and 98% negative predictive value. This, along with their series, suggests that SLNB for head and neck cSCC is a feasible, accurate staging procedure that carries significant prognostic information. However, more data are needed to answer the critical question of whether early intervention in patients with micrometastatic disease and positive SLNB in the form of immediate therapeutic neck dissection provides survival benefit over observation and delayed therapeutic neck dissection.
Management of the N+ neck
Patients that present with palpable cervical lymphadenopathy or parotid disease should undergo upfront, therapeutic selective neck dissection and/or parotidectomy.23 The authors recommend dissection of the clinically involved nodal basin and those nodal basins at risk given the location of the cSCC. For anterior lesions of the face, the superficial parotid and levels I–IV are at risk for nodal involvement.23 For posterior lesions of the scalp, the postauricular, suboccipital, and levels II–V at risk.23 The authors, as a general guideline, use the coronal plane of the external ear canal to divide the at risk nodal basin to an anterior field including the superficial parotid and levels I–IV, and a posterior field involving postauricular and suboccipital lymph nodes as well as levels II–V. It remains critical to always resect the external jugular lymph node chain, which is at high risk for nodal spread.24 Patients presenting with a parotid metastasis have a 30% chance of harboring cervical neck metastases,24 so the cervical lymph node basin should always be treated either with selective neck dissection or adjuvant radiation treatment when parotid lymph nodes are involved.
Adjuvant treatment
While there are no universally adopted guidelines for adjuvant treatment in cSCC,17 commonly accepted indications for adjuvant radiation treatment include two or more positive lymph nodes, extracapsular spread, lymph nodes greater than 3 cm in size, perineural invasion, dermal or in transit metastases, bone invasion, and close or positive margins.17, 23
Future directions: MicroRNA, epigenetic alterations, and next-generation sequencing
Basic science research in the field of tumor metastasis has illuminated the molecular basis of metastasis. The metastatic cascade by which tumor cells detach from the primary tumor and enter the lymphatic or circulatory system, survive during transport, and proliferate at regional or distant sites, requires an exquisite coordination of temporal gene expression.25 Noncoding regulatory RNA genes, known as microRNA (miRNA), have been identified as key regulators of tumor metastasis, having pro-metastatic and anti-metastatic effects.25 Ma et al. reported the first miRNA related to metastasis promotion in breast cancer, identifying miR-10b as a metastasis promoter in breast cancer.9 Identification of miRNAs in cSCC that promote tumor metastasis has tremendous potential for clinical translation, as these markers would be highly useful for prognosis, risk stratification of metastatic disease, and potential targeted therapies.26
Gillespie et al.27 investigated microRNA (miRNA) expression in metastatic cSCC relative to non-metastatic primary cSCC to identify candidates for targeted therapy and/or biomarkers that predict metastasis. miRNA expression analysis was performed in non-metastatic primary cSCC and in metastatic cSCC at the primary site and the regional lymph node. Expression of multiple miRNAs showed significant differences at the regional lymph node metastasis versus the primary cSCC, with up-regulation of miR-4286, miR-200a-3p and miR-148-3p and down-regulation of miR-1915-3p, miR-205-5p, miR-4516 and miR-150-5p, suggesting that these may be useful predictors of regional metastasis or represent therapeutic targets.
Epigenetic modifications are also known to play a role in tumor metastasis.28 Darr et al.28 investigated epigenetic profiles of metastatic and non-metastatic cSCC to identify unique patterns that may predict the development of metastatic cSCC. While widespread differences in methylation patterns were not seen, distinct epigenetic profiles were identified in the promoter region of FRZB in metastatic cSCC compared to their non-metastatic counterparts and this site represented the most differentially methylated site. FRZB, a known regulator of bone development, was found to be extensively hypermethylated in metastatic (median methylation: 46.7%) versus non-metastatic cSCC (median methylation 4.7%). Methylation of FRZB has also been associated with worse outcomes in bladder cancer,29 and these results suggest that in cSCC, FRZB may represent a biomarker of the metastatic phenotype.
Li et al.30 performed next generation sequencing on cSCC lymph node metastases to identify recurrent patterns of genomic alterations that may lead to new clinical trials and therapeutic agents. In this study of 29 patients that developed metastatic cSCC, 11 patients exhibited recurrence within an average of 24 months. However the time to recurrence ranged from 1 to 78 months, while other patients remained recurrence free at 130 months, emphasizing the heterogeneous nature of this cohort of patients with metastatic cSCC and the need for advancing treatment options in this patient population. Next generation sequencing identified a heterogenous degree of genomic alterations and mutations were recurrently identified across the entire cohort in four major categories: RAS/RTK/PI3K, squamous differentiation, cell cycle, and chromatin remodeling pathway gene pathways. The top three altered genes were TP53, CDKN2A, and NOTCH1/2/4. No single gene significantly predicted survival which is unsurprising as these genes are frequently mutated in all SCCs. However, oncogenic alterations activating the RAS/RTK/PI3K pathway, which were present in 45% of samples, were significantly correlated with worse progression free survival. Numerous tumors exhibited activating mutations of receptor tyrosine kinases, downstream kinases, and genes in the PI3K/AKT pathway, suggesting that agents currently under investigation in other cancer sites (MEK, mTOR, FGFR, BRAF, PI3K inhibitors) should be considered in this population of patients with metastatic cSCC.30
Conclusions
Surgical excision remains the mainstay of treatment of cutaneous squamous cell carcinoma, whether by Mohs micrographic surgery or standard surgical resection. While the majority of patients are cured with local surgical excision, a subset of patients is at high risk for developing recurrence and regional lymph node metastasis. Clinical features placing patients at high risk for regional metastasis such as size and perineural invasion have been established. These patients may be ideal candidates for sentinel lymph node biopsy, although the survival impact of SLNB and early therapeutic neck dissection versus observation of the neck and delayed neck dissection remains to be seen. Future directions involve the use of next-generation sequencing and microRNA analysis to identify biomarkers that predict development of regional metastasis that may guide upfront treatment and provide therapeutic targets for adjuvant treatment.
Acknowledgments
The authors thank Diane Dziewatkowski for her assistance in preparation of the manuscript.
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Alam M., Ratner D. Cutaneous squamous-cell carcinoma. N Engl J Med. 2001;344:975–983. doi: 10.1056/NEJM200103293441306. [DOI] [PubMed] [Google Scholar]
- 2.Suchniak J.M., Baer S., Goldberg L.H. High rate of malignant transformation in hyperkeratotic actinic keratoses. J Am Acad Dermatol. 1997;37:392–394. doi: 10.1016/s0190-9622(97)70137-3. [DOI] [PubMed] [Google Scholar]
- 3.Karia P.S., Han J., Schmults C.D. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J Am Acad Dermatol. 2013;68:957–966. doi: 10.1016/j.jaad.2012.11.037. [DOI] [PubMed] [Google Scholar]
- 4.Johnson T.M., Rowe D.E., Nelson B.R., Swanson N.A. Squamous cell carcinoma of the skin (excluding lip and oral mucosa) J Am Acad Dermatol. 1992;26:467–484. doi: 10.1016/0190-9622(92)70074-p. [DOI] [PubMed] [Google Scholar]
- 5.Moore B.A., Weber R.S., Prieto V. Lymph node metastases from cutaneous squamous cell carcinoma of the head and neck. Laryngoscope. 2005;115:1561–1567. doi: 10.1097/01.mlg.0000173202.56739.9f. [DOI] [PubMed] [Google Scholar]
- 6.Kraus D.H., Carew J.F., Harrison L.B. Regional lymph node metastasis from cutaneous squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 1998;124:582–587. doi: 10.1001/archotol.124.5.582. [DOI] [PubMed] [Google Scholar]
- 7.Kwa R.E., Campana K., Moy R.L. Biology of cutaneous squamous cell carcinoma. J Am Acad Dermatol. 1992;26:1–26. doi: 10.1016/0190-9622(92)70001-v. [DOI] [PubMed] [Google Scholar]
- 8.Brantsch K.D., Meisner C., Schönfisch B. Analysis of risk factors determining prognosis of cutaneous squamous-cell carcinoma: a prospective study. Lancet Oncol. 2008;9:713–720. doi: 10.1016/S1470-2045(08)70178-5. [DOI] [PubMed] [Google Scholar]
- 9.Ma L., Teruya-Feldstein J., Weinberg R.A. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007;449:682–688. doi: 10.1038/nature06174. [DOI] [PubMed] [Google Scholar]
- 10.Schmults C.D., Karia P.S., Carter J.B., Han J., Qureshi A.A. Factors predictive of recurrence and death from cutaneous squamous cell carcinoma: a 10-year, single-institution cohort study. JAMA Dermatol. 2013;149:541–547. doi: 10.1001/jamadermatol.2013.2139. [DOI] [PubMed] [Google Scholar]
- 11.Edge S.B., Compton C.C. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 12.Veness M.J. Time to rethink TNM staging in cutaneous SCC. Lancet Oncol. 2008;9:702–703. doi: 10.1016/S1470-2045(08)70185-2. [DOI] [PubMed] [Google Scholar]
- 13.Brunner M., Ng B.C., Veness M.J., Clark J.R. Assessment of the new nodal classification for cutaneous squamous cell carcinoma and its effect on patient stratification. Head Neck. 2015;37:336–339. doi: 10.1002/hed.23602. [DOI] [PubMed] [Google Scholar]
- 14.Leibovitch I., Huilgol S.C., Selva D., Hill D., Richards S., Paver R. Cutaneous squamous cell carcinoma treated with Mohs micrographic surgery in Australia I. Experience over 10 years. J Am Acad Dermatol. 2005;53:253–260. doi: 10.1016/j.jaad.2005.02.059. [DOI] [PubMed] [Google Scholar]
- 15.Smeets N.W., Krekels G.A., Ostertag J.U. Surgical excision vs Mohs' micrographic surgery for basal-cell carcinoma of the face: randomised controlled trial. Lancet. 2004;364:1766–1772. doi: 10.1016/S0140-6736(04)17399-6. [DOI] [PubMed] [Google Scholar]
- 16.Brodland D.G., Zitelli J.A. Surgical margins for excision of primary cutaneous squamous cell carcinoma. J Am Acad Dermatol. 1992;27:241–248. doi: 10.1016/0190-9622(92)70178-i. [DOI] [PubMed] [Google Scholar]
- 17.Porceddu S.V., Veness M.J., Guminski A. Nonmelanoma cutaneous head and neck cancer and Merkel cell carcinoma: current concepts, advances, and controversies. J Clin Oncol. 2015;33:3338–3345. doi: 10.1200/JCO.2014.60.7333. [DOI] [PubMed] [Google Scholar]
- 18.Gore S.M., Shaw D., Martin R.C. Prospective study of sentinel node biopsy for high-risk cutaneous squamous cell carcinoma of the head and neck. Head Neck. 2016;38:E884–E889. doi: 10.1002/hed.24120. [DOI] [PubMed] [Google Scholar]
- 19.Civantos F.J., Moffat F.L., Goodwin W.J. Lymphatic mapping and sentinel lymphadenectomy for 106 head and neck lesions: contrasts between oral cavity and cutaneous malignancy. Laryngoscope. 2006;112:1–15. doi: 10.1097/01.mlg.0000200750.74249.79. [DOI] [PubMed] [Google Scholar]
- 20.Nouri K., Rivas M.P., Pedroso F., Bhatia R., Civantos F. Sentinel lymph node biopsy for high-risk cutaneous squamous cell carcinoma of the head and neck. Arch Dermatol. 2004;140:1284. doi: 10.1001/archderm.140.10.1284-a. [DOI] [PubMed] [Google Scholar]
- 21.Wagner J.D., Evdokimow D.Z., Weisberger E. Sentinel node biopsy for high-risk nonmelanoma cutaneous malignancy. Arch Dermatol. 2004;140:75–79. doi: 10.1001/archderm.140.1.75. [DOI] [PubMed] [Google Scholar]
- 22.Altinyollar H., Berberoğlu U., Celen O. Lymphatic mapping and sentinel lymph node biopsy in squamous cell carcinoma of the lower lip. Eur J Surg Oncol. 2002;28:72–74. doi: 10.1053/ejso.2001.1206. [DOI] [PubMed] [Google Scholar]
- 23.Martin RCaC, Jonathan . Hodder and Stoughton Ltd; 2012. Non-melanoma and Melanoma Skin Cancer. [Google Scholar]
- 24.O'Brien C.J., McNeil E.B., McMahon J.D., Pathak I., Lauer C.S. Incidence of cervical node involvement in metastatic cutaneous malignancy involving the parotid gland. Head Neck. 2001;23:744–748. doi: 10.1002/hed.1106. [DOI] [PubMed] [Google Scholar]
- 25.Hurst D.R., Edmonds M.D., Welch D.R. Metastamir: the field of metastasis-regulatory microRNA is spreading. Cancer Res. 2009;69:7495–7498. doi: 10.1158/0008-5472.CAN-09-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma L., Weinberg R.A. MicroRNAs in malignant progression. Cell Cycle. 2008;7:570–572. doi: 10.4161/cc.7.5.5547. [DOI] [PubMed] [Google Scholar]
- 27.Gillespie J., Skeeles L.E., Allain D.C. MicroRNA expression profiling in metastatic cutaneous squamous cell carcinoma. J Eur Acad Dermatol Venereol. 2016;30:1043–1045. doi: 10.1111/jdv.13098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Darr O.A., Colacino J.A., Tang A.L. Epigenetic alterations in metastatic cutaneous carcinoma. Head Neck. 2015;37:994–1001. doi: 10.1002/hed.23701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marsit C.J., Houseman E.A., Christensen B.C. Identification of methylated genes associated with aggressive bladder cancer. PLoS One. 2010;5:e12334. doi: 10.1371/journal.pone.0012334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y.Y., Hanna G.J., Laga A.C., Haddad R.I., Lorch J.H., Hammerman P.S. Genomic analysis of metastatic cutaneous squamous cell carcinoma. Clin Cancer Res. 2015;21:1447–1456. doi: 10.1158/1078-0432.CCR-14-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]