Abstract
Worldwide, about 600,000 head and neck squamous cell carcinoma (HNSCC) are detected annually, many of which involve high risk human papilloma virus (HPV). Surgery is the primary and desired first treatment option. Following surgery, the existence of cancer cells at the surgical margin is strongly associated with eventual recurrence of cancer and a poor outcome. Despite improved surgical methods (robotics, microsurgery, endoscopic/laparoscopic, and external imaging), surgeons rely only on their vision and touch to locate tumors during surgery. Diagnostic imaging systems like computed tomography (CT), magnetic resonance imaging (MRI), single-photon emission computed tomography (SPECT) and positron-emission tomography (PET) are too large, slow and costly to use efficiently during most surgeries and, ultrasound imaging, while fast and portable, is not cancer specific. This purpose of this article is to review the fundamental technologies that will radically advance Precision Otolaryngology practices to the benefit of patients with HNSCC. In particular, this article will address the potential for tumor-targeting peptides to enable more precise diagnostic imaging while simultaneously advancing new therapeutic paradigms for next generation image-guided surgery, tumor-specific chemotherapeutic delivery and tumor-selective targeted radiotherapy (i.e., theranostic).
Keywords: Squamous cell carcinoma, Peptide, Optical surgical navigation, Diagnostic imaging, Theranostic
Abbreviations
- 18F-FDG
Fluorine-18-fluorodeoxyglucose
- 64Cu
Copper-64
- 68Ga
Gallium-68
- 89Zr
Zirconium-89
- 90Y
Yttrium-90
- 111In
Indium-111
- 123I
Iodine-123
- 124I
Iodine-124
- 131I
Iodine-131
- 177Lu
Lutetium-177
- CLI
Cerenkov luminescence imaging
- CPP
Cell-penetrating peptide
- CR
Cerenkov radiation
- CT
Computed tomography
- Cy5
Cyanine 5 dye
- DT
Diphtheria toxin
- EGF
Epidermal growth factor
- FITC
Fluorescein isothiocyanate
- GC
Gamma camera
- HN-J
HN-Jumbled
- HNSCC
Head and neck squamous cell carcinoma
- HPV
Human papilloma virus
- ICG
Indocyanine green
- MRI
Magnetic resonance imaging
- NIR
Near-infrared
- OSN
Optical surgical navigation
- PET
Positron-emission tomography
- PKCε
Protein kinase Cε
- SPECT
Single-photon emission computed tomography
- siRNA
Small interfering RNA
Squamous cell carcinoma and the roles of conventional imaging and advanced molecular imaging
There are approximately 600,000 new cases of HNSCC worldwide each year consisting of both high risk HPV+ and HPV− malignancies.1, 2 Routine surgical resection is used for definitive treatment of HNSCC tumors and is currently guided only by the surgeon's vision and palpation. The current treatment paradigm begins with pre-operative surgical planning using conventional diagnostic imaging (CT, MRI and/or PET) imaging followed by targeted visual resection of the lesion(s) as well as concerning lymph nodes. Due to the associated complex anatomy, complete resection of HNSCC is often arduous with little or no real-time verification of complete surgical resection. The standard histopathologic assessment of resected surgical specimens and their margins can take several days to complete. Not uncommonly, surgeons are notified days later that positive surgical margins exist in the resected specimens. Following definitive unguided surgical resection, recurrent malignancy occurs in 10%–26% of HNSCC patients within 5 years, which suggests that some degree of residual malignancy remained after surgery.3 The presence of residual malignancy within the surgical bed indicates that local tumor recurrence is likely and is a poor prognostic factor.4 In addition, the total number of histologically malignant lymph nodes removed during the concurrent neck dissections is a risk factor for local recurrent malignancy as well as distant metastases.3, 5 The overall survival rate for HNSCC patients has not significantly improved during the last 20 years with locoregional tumor recurrence/relapse and distant metastases representing the primary causes of morbidity and mortality.6
The ability to detect and completely resect the primary malignancy as well as adequately evaluate for associated metastatic lymph nodes are critical factors in determining patient prognosis and subsequent adjuvant therapies. With respect to conventional imaging assessment for patients with HNSCC, contrast-enhanced CT or MRI of the site of primary malignancy in the head and neck is performed, as well as chest imaging, as part of the initial clinical evaluation. Advanced molecular imaging using Fluorine-18-fluorodeoxyglucose (18F-FDG) PET/CT or PET/MRI may also be performed to evaluate the primary tumor and assess for locoregional and distant metastatic disease. 18F-FDG PET is used for the diagnosis, staging, and restaging of HNSCC patients. Unfortunately 18F-FDG PET has a false positive rate of 23%–30% in HNSCC patients,7, 8, 9, 10 which may necessitate further diagnostic evaluation and tissue sampling as well as potentially delaying therapy. These current limitations and the critical need for improved diagnostic, quantitative and prognostic stratification of HNSCC patients before, during and after definitive therapy are recognized by the interdisciplinary treatment team.
Peptides and antibodies as potential HNSCC imaging agents
A promising approach to improve both diagnostic sensitivity/specificity and definitive therapy is the evolving field of tumor-targeting peptides and antibodies. The angiogenic cyclic RGD peptide cilengitide was one of the first promising peptides which specifically targeted the αvβ3 integrin receptor associated with highly vascular tumors like HNSCC.11, 12 Recent work in BcaCD885 tumor-bearing mice continues to advance on this track with RGD peptides conjugated with 800 nm emission quantum dots. These results demonstrate tumor-to-background ratios of 4.5 compared to 1 with untargeted quantum dots.13
Another promising approach is antibody-mediated targeting of Epidermal Growth Factor (EGF).14 Two antibodies, cetuximab and panitumumab, have been studied as conjugates with the near-infrared (NIR) dye, IRDye800, which is an 800 nm emitting dye in use in a number of optical surgical navigation (OSN) preclinical agents.15, 16 Antibodies are strong target binders, generally, and these two antibodies are typical with <1 nM kd values. Peak signal intensity in tumors with adequate tumor to background ratios require longer injection-to-imaging times with the large antibodies (e.g., 48–72 h post administration) whereas small peptides like RGD demonstrate peak signal intensity in less than 24 h. Antibodies also tend to be excreted through the hepatobiliary system which can limit their diagnostic utility when used to evaluate for liver and gastrointestinal lesions but not for the head and neck. Using orthotopic HNSCC tongue tumor mice, tumor and metastatic lymph node visualization was judged to be equivalent with IRdye800-labeled cetuximab and panitumumab.16 Response to cetuximab therapy could also be predicted in several HNSCC xenograft tumor mice when using cyanine 5 dye (Cy5)-labeled cetuximab.17
Once identified, tumor-targeting peptides and antibodies can be adapted and used for both therapeutic and diagnostic purposes (i.e., theranostic). The term ‘theranostic’ describes any agent which possesses therapeutic and diagnostic attributes and is derived from ‘therapeutic’ and ‘diagnostic’. Theranostic agents are an important part of the evolving field of precision medicine as it enables personalized patient management for the treatment of various diseases like HNSCC.18, 19
HN-1: a prototype tumor-targeting peptide for HNSCC
The 12 amino acid peptide, HN-1, has been described as a targeted peptide agent which binds to and is internalized into human HNSCC cells. It was discovered through the screening of phage-based peptide libraries using human HNSCC tumor cells. Since the goal was to identify cancer-specific peptides for tumor cell internalization and potentially drug delivery, screening was performed at 37 °C to facilitate the underlying tumor-associated endocytotic pathways. Furthermore, the screening was performed using serum-containing medium to identify those peptides which were stable in serum and would not subsequently degrade when developed and administered as theranostic agents. These efforts lead to the isolation and description of a novel peptide sequence (TSPLNIHNGQKL) which was designated HN-1.20
As initial proof of concept that HN-1 can potentially be used for tumor-targeted drug delivery, HN-1 has been conjugated with fluorescein isothiocyanate (FITC) with photon excitation and emission wavelengths of ∼495 nm and ∼519 nm, respectively, and Cy5 with photon excitation and emission wavelengths of ∼650 nm and ∼670 nm, respectively. Neither FITC nor Cy5 is imageable by commercial fluorescent imaging system designed for NIR imaging at the 800 nm emission. Both FITC-labeled and Cy5-labeled HN-1 agents maintain their stability and highly specific binding to HNSCC tumor cells,6, 20 as demonstrated qualitatively using fluorescence optical imaging. It should be noted that in these studies, FITC and Cy5 were reported to be attached to the N-terminal end of the HN-1 peptide,6, 20 but there were two distinct labeling sites for the dyes on HN-1 and the original synthetic chemistry described for fluorescent labeling was not site-selective. In terms of cell targeting studies, HN-1 agents have been used in a variety of malignant/metastatic HNSCC, non-HNSCC, and non-malignant cells lines (Table 1). FITC-labeled HN-1 when incubated in μM concentration with a variety of human HNSCC tumor cell lines demonstrated internal fluorescence within viable cells and this was both dose-dependent and time-dependent. Fluorescence uptake was noted by 24–48 h following administration.6, 20
Table 1.
Cell lines used for HN-1 experiments.
| Cell lines | Fluorescent uptake | Reference |
|---|---|---|
| HNSCC Cells | ||
| MDA Tu167a | Visualized | 20 |
| MDA Tu177 | Visualized | 20 |
| MDA Tu138 | Visualized | 20 |
| MDA Tu159 | Visualized | 20 |
| MDA Tu686 | Visualized | 20 |
| MDA Tu1986 | Visualized | 20 |
| UMSCC1 | Visualized | 6 |
| UMSCC36 | Visualized | 6 |
| UMB-SCC-745 | Little or no uptake | 43 |
| UT-SCC-36 | Little or no uptake | 43 |
| UT-SCC-38 | Little or no uptake | 43 |
| SCC-25 | Visualized | 22 |
| Detroit 562 | Visualized | 22 |
| Non-HNSCC Cells | ||
| DU145 | Little or no uptake | 20 |
| SW480 | Little or no uptake | 20 |
| U373 MG | Little or no uptake | 20 |
| MDA-MB231 | Visualized | 6 |
| SKBR3 | Visualized | 6 |
| A549 lung carcinoma | Little or no uptake | 43 |
| MCF-7 | Visualized | 21 |
| MDA-MB-468 | Visualized | 21 |
| ZR-75-1 | Visualized | 21 |
| Control Cells | ||
| HPV-Immortalized human oral keratinocytes (HOK16B) | Little or no uptake | 20 |
| Immortalized normal oral epithelial cells (E6/E7-NOE) | Little or no uptake | 6 |
| MCF10A non-tumorigenic mammary epithelial cells | Little or no uptake | 6 |
| NHDF – Normal Human Dermal Fibroblasts | Little or no uptake | 21 |
Apparently the MDA Tu167 cell line is genetically identical to the UMSCC-1 cell line and MDA Tu167 likely represents a UMSCC-1 contamination.62
Further characterization of FITC-labeled HN-1 indicates that its intracellular fluorescence can be blocked when incubated with greater than 200 molar excess (>500 μM) of unlabeled HN-1 peptide whereas incubation with an irrelevant peptide had no effect on intracellular fluorescence. Likewise, incubating human HNSCC cells with both fluorescein dye and unlabeled HN-1 yielded little detected fluorescent labeling suggests that intracellular fluorescence is not mediated by free dye. Additional characterization of internalized FITC-labeled HN-1 and Texas red-labeled HN-1 within cells suggested that these HN-1 agents may be compartmentalized within the cytoplasm.20
In terms of the amino acid sequence for HN-1-mediated cell targeting (Table 2), it has been shown that the addition of amino acids to both the N-terminal and C-terminal ends of HN-1 (i.e., HN-2) or simply the N-terminus of HN-1 (i.e., HN-3 and HN-1TYR) did not prevent internalization of FITC-labeled HN-2, HN-3 or HN-1TYR agents.20, 21 On the other hand, rearranging HN-1's amino acids into clustered groups of polar amino acids at the N-terminus and apolar amino acids at the C-terminus (i.e., HN-J or HN-Jumbled) prevented detection of internalized fluorescence.20 Although these findings suggested that HN-1-mediated cell targeting was sequence-dependent, it was shown that alternatively scrambling HN-1's sequence while maintaining its overall polar-apolar-polar distribution pattern (i.e., HN-1-scr) yields intracellular fluorescence comparable to HN-1.22 These results suggest that the specific amino acid sequence of HN-1 is not essential for tumor-specific binding and internalization despite the isolation of this specific peptide sequence during human HNSCC tumor cell screening. Therefore, it may be that a sequence-specific peptide receptor is not the tumor-specific target but rather some other tumor-mediated internalization process. Further evaluation of the secondary structure of the HN-1 and HN-1-scr peptides compared to HN-J did not elucidate any new insights into the potential tumor target since the structural motifs were distinctly different.22
Table 2.
Amino acid sequences of various peptides used in HN-1 related studies.
| Peptide name | Sequence | Reference |
|---|---|---|
| HN-1 based peptides | ||
| HN-1 | TSPLNIHNGQKL | 20 |
| HN-2 | GGG-TSPLNIHNGQKL-GGGS | 20 |
| HN-3 | GSRRASV-TSPLNIHNGQKL | 20 |
| HN-1TYR | YY-TSPLNIHNGQKL | 21 |
| Control peptides | ||
| HN-J | NQHSKNTLLIGP | 20 |
| HN-1-scr | LNKQTHGLIPNS | 22 |
| Irrelevant peptide | GIGKFLHSAKKFGKAFVGEIMNS | 20 |
| Irrelevant peptide | GGGRHAYHMHPHHG | 22 |
The polar amino acids are labeled in bold.
Despite the lack of an identified HNSCC tumor target for HN-1, preclinical studies using xenograft tumor mice have demonstrated that both FITC-labeled HN-1 (administered via tail vein injection and imaged at 48 h) and Cy5-labeled HN-1 agents (administered intraperitoneally and imaged at 24 h) are specifically internalized within human HNSCC tumors in vivo (Table 3).6, 20 In FITC-labeled HN-1 fluorescing tumors, there was little labeling of the tumor nuclei which again supports its internalization into the cytoplasmic compartment. Further histopathologic evaluation of the FITC-labeled HN-1 tumors demonstrated fluorescently labeled cells throughout the tumor and not just at the periphery. Control studies using (1) fluorescein dye only, (2) fluorescein dye with unlabeled HN-1 peptide, and (3) FITC-labeled HN-J control peptide demonstrated little fluorescent labeling of tumors. In addition, xenograft mice bearing only prostate cancer (i.e., non-HNSCC) tumors did not show fluorescent labeling of the tumors following administration with FITC-labeled HN-1. In terms of the biodistribution for FITC-labeled HN-1, there was little fluorescence labeling noted in the brain, heart, lungs, kidneys and liver for both HNSCC-bearing and non-tumor-bearing mice at 48 h.20 Subsequent xenograft studies using Cy5-labeled HN-1 agents also demonstrated selective labeling of HNSCC tumors while very little uptake was noted in non-tumor tissues.6
Table 3.
Xenograft tumor models used influorescent HN-1 imaging studies to date.
| Cell lines | Fluor | HN-1 uptake | Reference |
|---|---|---|---|
| MDA Tu177 | FITC | Visualized | 20 |
| MDA Tu167a | FITC | Visualized | 20 |
| UMSCC-1 | Cy5 | Visualized | 6 |
| DU145 | FITC | Not visualized | 20 |
Apparently the MDA Tu167 cell line is genetically identical to the UMSCC-1 cell line and MDA Tu167 likely represents a UMSCC-1 contamination.62
Potential for advanced molecular imaging and theranostics with tumor-targeting peptides
Cerenkov imaging – potential for novel optical imaging with PET radioisotopes
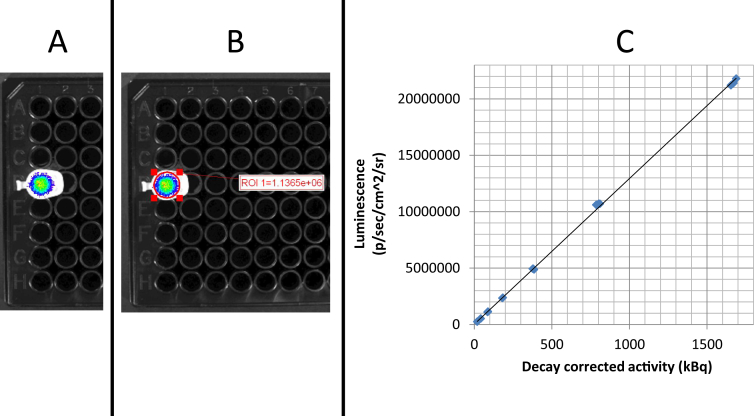
Arecent and innovative approach for real-time imaging of lesions containing positron-emitting radioisotopes is the optical detection of Cerenkov radiation (CR). CR is the visible and ultraviolet light produced during the process of either positron- or high-energy therapeutic betaparticleemission.23, 24, 25 Quite simply, CR is produced as positrons or electrons travel faster than the speed of light through the aqueous medium of cells, tissues and organs. As these particles travel through the aqueous medium, the local electromagnetic field of the water is disrupted and electrons in the atoms of the water molecules become displaced. These atoms are polarized by the passing electromagnetic field of the positron or electron. As the displaced electrons in the water molecules restore themselves to equilibrium, ultraviolet and visible light photons are emitted and these photons can be detected using bioluminescence imaging technology. The optical detection of CR has been recently designated as Cerenkov luminescence imaging (CLI).26 Detectable CLI signals have been described in vitro for a number of medical positron-emitting radioisotopes (e.g., 18F) and the therapeutic betaparticle emitting radioisotope (e.g., Iodine-131 (131I)).27, 28, 29 Fig. 1 demonstrates the feasibility of CLI of the positron-emitting radioisotope Gallium-68 in vitro.
Fig. 1.
Cerenkov luminescence imaging of the PET radioisotope 68Ga in vitro. The positron-emitter 68Ga was eluted from a generator (Eckert & Ziegler, Berlin, Germany) and its activity was measured using a calibrated dose calibrator (Capintec CRC 212). The manufacturer specified setting and calibration factor for 68Ga was used for all measurements. Clear Eppendorf tubes containing various activity concentrations of 68Ga were placed within the imaging chamber of a commercial bioluminescence imaging system (IVIS 100; Perkin Elmer, Waltham, MA). The IVIS system consists of a cryogenically-cooled charged couple device (CCD) camera, operating at −90 °C, and a temperature controlled, light-tight imaging chamber. Two sets of images were collected; a white-light image and a luminescence image that captured photon output due to Cerenkov luminescence. Photon flux was quantified by means of proprietary software (Living Image version 2.5) using custom regions of interest based on the outlines of the Eppendorf tube caps visible on the white-light image. A. CLI image of 89 kBq of 68Ga. B. ROI analysis of photon flux demonstrated 1136,500 p/sec/cm2/sr. C. There is a positive linear relationship between the 68Ga activity and measured luminescence (R2 = 0.9996). The 68Ga activities ranged from 19 to 1689 kBq and were imaged in triplicate using the IVIS 100 high resolution (4) setting, FOV15, f1, open filter for 30 s (Courtesy of CLW, MVK and MFT).
The CLI approach represents new clinical and surgical opportunities for the real-time detection PET radioactivity in vitro and in vivo. In particular, images with high spatial resolution can be obtained within a matter of seconds using CLI as opposed to several minutes forconventional or intraoperative nuclear medicine imaging approaches. Current CLI systems are also smaller and less costly than conventional nuclear medicine imaging systems. Clinical applications for human CLI have recently been described using 131I and 18F-FDG and represent a new and evolving industry for surgical/interventional imaging systems.30, 31 Furthermore, CLI technology will help to facilitate the pre-clinical development of PET-labeled theranostic agents like tumor-targeting peptides (e.g., HN-1 and related peptides).
Oncologic SPECT and PET imaging approaches using radiolabeled tumor-targeted imaging peptides
Although 18F-FDG PET/CT is routinely used to detect and stage patients with HNSCC, FDG is not cancer specific.32 At present, no studies have been published on the radiolabeling of the HN-1 peptide for diagnostic nuclear medicine imaging using conventional gamma-emitting (planar scintigraphy, SPECT, SPECT/CT) or positron-emitting (PET/CT, PET/MRI) medical radioisotopes. On the other hand, there are several radiolabeled peptide imaging agents specific for somatostatin receptor expressing tumors (e.g., neuroendocrine tumors, carcinoid tumors, and paragangliomas of the head and neck) which have been developed and are in clinical use. The most commonly used tumor-targeting peptide imaging agent is Indium-111 (111In)-labeled pentetreotide which binds to these somatostatin-receptor expressing tumors. 111In-labeled pentetreotide can be imaged intraoperatively using portable gamma camera imaging,33 or it can be imaged conventionally with planar scintigraphy, SPECT and SPECT/CT imaging.34 This approach requires injection-to-scan times ranging from 4 to 48 h.
More recently, several 68Ga-labeled DOTA-peptides (e.g., DOTATOC, DOTATATE, DOTANOC) have been developed as PET imaging analogs for various somatostatin receptor expressing tumors.35, 36, 37, 38, 39, 40 The 68Ga-DOTA-peptide agents enable much faster imaging with injection-to-scan times ranging from 45 to 90 min as well as markedly improved image resolution for better lesion detectability. Another PET-labeled agent, Copper-64 (64Cu)-labeled DOTATATE, has been recently developed and used clinically for the assessment of neuroendocrine tumors.41, 42 As expected, both 68Ga- and 64Cu-labeled PET-labeled DOTA-peptides facilitate faster imaging with better lesion detectability and lesion characterization (especially for small lesions) when compared with standard 111In-labeled pentetreotide approaches. Moreover, both 68Ga- and 64Cu-labeled DOTA-peptide agents can be alternatively radiolabeled with therapeutic radioisotopes like Lutetium-177 (177Lu) or Yttrium-90 (90Y) and administered as Peptide Receptor Radionuclide Therapy (PRRT) for somatostatin-receptor-avid lesions.
It remains to be seen if HNSCC-targeting peptides can be conjugated with DOTA moieties for subsequent radiolabeling with 68Ga or 64Cu and still maintain ideal tumor-specific binding and internalization. Given the relatively short half-lives for 68Ga and 64Cu (1.1 h and 12.7 h, respectively) when compared with the published uptake times for FITC-labeled and Cy5-labeled HN-1 (24–48 h), this type of approach might not be feasible with HN-1. Alternatively, longer lived PET radioisotopes like Zirconium-89(89Zr, half-life of 3.3 d) and Iodine-124 (124I, half-life of 4.2 d) may be more appropriate strategies for developing diagnostic PET-labeled HN-1 agents.
Theranostics and precision otolaryngology
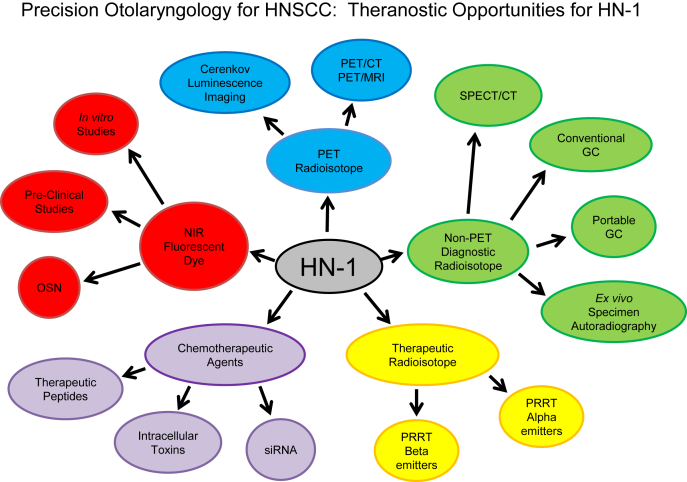
The current clinical management of HNSCC patients involves a multimodality treatment approach consisting of surgery, systemic chemotherapy, and radiation therapy. The relative lack of tumor-targeted approaches for each of these modalities can contribute to disease recurrence and/or treatment-related toxicities in HNSCC patients.43 The paradigm of Precision Otolaryngology incorporates new tumor-specific targeting strategies to improve the diagnostic imaging and quantification of malignant/metastatic disease burden while enhancing the multimodality therapeutic options for individual patients, reducing disease recurrence/relapse rates and minimizing treatment-related toxicities. We have already discussed that HNSCC tumor-targeting peptides like RGDs and HN-1 and various antibodies have the capability to selectively target tumor cells when conjugated dye molecules like FITC, Cy5, IRDye800 and Texas red. This conjugation of optical or fluorescent dyes to the targeting moiety enables new approaches beyond the optical detection of tumor (e.g., new fluorescent image-guided resection of tumor lesions), such as photodynamic therapies. Therefore, the targeted entities have the potential to become a new class of theranostic agents for HNSCC. Although new image-guided surgical applications can easily be envisioned, the range of possible therapeutic approaches is not limited to surgery. If tumor-targeting peptides can also be conjugated to small therapeutic molecules or therapeutic radioisotopes, it may enable more precise delivery of chemotherapeutics and/or therapeutic radioactivity. Fig. 2 illustrates the theranostic opportunities for HN-1 and other tumor-targeting peptides to enable Precision Otolaryngology for HNSCC.
Fig. 2.
Precision Otolaryngology for HNSCC. The HN-1 peptide as a the ranostic agent (i.e., demonstrates both therapeutic and diagnostic attributes). HN-1 (center) can potentially be conjugated with NIR fluorescence dyes, PET radioisotopes and non-PET diagnostic radioisotopes for tumor-specific diagnostic imaging applications. In terms of therapeutics, HN-1 can potentially be conjugated with various types of chemotherapeutic agents like peptides, toxins and siRNA or therapeutic radioisotopes for PRRT mediated via alpha or beta particle emissions. In fact, HN-1 has already demonstrated its potential for labeling with multiple diagnostic and therapeutic moieties. Dual-labeling of HN-1 with fluorescent dyes and chemotherapeutic agents has already been described in the literature and evaluated in vitro and, to some extent, in vivo. There is potential for dual-labeling HN-1 with both fluorescent dyes and diagnostic radioisotopes to enable more precise image-guided surgery or fluorescence-augmented radioguided surgery. There is also potential for dual-labeling HN-1 with diagnostic radioisotopes and chemotherapeutic agents to precisely localize and quantify the delivery of the theranostic agent to the target lesions. Dual-labeled HN-1 with therapeutic radioisotopes and chemotherapeutic radiosensitizers is another approach to precisely deliver a high-yield chemoradiotherapy payload to target tumor lesions.
New surgical approaches enabled with optical surgical navigation
Although useful for in vitro and pre-clinical fluorescence imaging, FITC and Cy5 are suboptimal for clinical fluorescence imaging applications but a NIR fluorescent emission dye like IR800 dye (photon excitation and emission wavelengths of ∼780 nm and ∼800 nm, respectively) is ideal due to low tissue auto-fluorescence, better tissue penetration, and minimal in vivo toxicity.15, 44 In addition, there are approximately 7000 optical imaging systems approved for imaging indocyanine green (ICG), a dicyanine fluorescent blood pool marking agent that emits in the NIR just above 800 nm.45 The application of intraoperative fluorescent optical imaging (i.e., OSN) with agents targeted to cancer cells (e.g., small molecules, peptides or antibodies) will enhance the surgeon's detection and resection of primary tumors and metastatic lesions in real-time, improve the complete tumor resection rates, and thereby improve progression-free survival.14, 46, 47 The accelerating development of new OSN technology using cancer specific NIR imaging agents is being driven by the unmet clinical need to more precisely identify, in real-time, tumors and lesions than is possible with unguided surgical approaches.
The recent development of new OSN systems has triggered a technical evolution away from conventional unguided surgical resection to new image-guided surgical approaches.48, 49 Consequently, there is a current need for the development of fluorescent tumor-targeting agents to facilitate clinical translation and take advantage of this emerging technology. This technology enables real-time detection of fluorescent lesions within the surgical field of view, facilitates image-guided resection and improved evaluation of the surgical bed for any residual fluorescence activity (indicating residual tumor). Given the demonstrated ability to visualize cancer specific NIR imaging agents, both in real-time and within the surgical field of view, it is unlikely that OSN will be replaced by other non-optical surgical approaches. Existing OSN imaging cameras have the capability to image fluorescent tumors during resection, but adoption of this technology is constrained by the lack of targeted fluorescent imaging agents which demonstrate accurate imaging concordance with current diagnostic imaging agents like 18F-FDG. Successful clinical translation of fluorescent-only imaging agents is therefore fundamentally limited by differences in mechanisms of tumor uptake, limits of detection at depths exceeding a few mm (dues to attenuation and scattering of light), administered doses in the μmol/kg range (compared to >100 fold lower for radionuclides) and injection-to-imaging times when compared with standard diagnostic imaging agents like 18F-FDG.
New therapeutic approaches using small peptides, molecules and cytotoxic agents
HN-1-PKCε
It has been noted that protein kinase Cε (PKCε) contributes to aggressive HNSCC tumor phenotypes and therefore inhibiting PKCε impairs the invasiveness and motility of tumor cells.6 As a potential theranostic agent, FITC-labeled HN-1 (diagnostic) was conjugated to an inhibitory PKCε peptide (therapeutic) and was found to inhibit proliferation, motility and invasion in HNSCC cell lines but had minimal effect on the control immortalized normal oral epithelial cells. In principle, these results demonstrate that HN-1 represents a promising theranostic entity that can maintain its tumor selectivity despite conjugation of multiple diagnostic/therapeutic moieties as well as facilitate the internalization or intracellular delivery of potential therapeutics.
Cy5-labeled HN-1-PKCε was subsequently used for preclinical studies in HNSCC-bearing xenograft mice. Minimal fluorescence was noted in HNSCC tumors or normal tissues when treated with control Cy5-labeled-PKCε (i.e., no HN-1). Likewise, there was minimal autofluorescence in HNSCC tumors or normal tissues in untreated mice. On the other hand, HNSCC tumor fluorescence was greater than 8-fold higher than normal tissues in mice treated with Cy5-labeled HN-1-PKCε. Cy5-labeled HN-1-PKCε also reduced overall HNSCC tumor growth when compared with Cy5-labeled-PKCε treated or untreated tumor-bearing mice. Again, these results demonstrate that the HN-1 peptide can serve as a theranostic agents for HNSCC.6
Although FITC-labeled HN-1 did not demonstrate significant fluorescent internalization with DU145 (prostate cancer), SW480 (colon cancer) and U373 MG (astrocytoma) cells,20 FITC-labeledHN-1-PKCε demonstrated significant fluorescent internalization when applied to two breast cancer cell lines. Much lower fluorescent internalization was noted when FITC-labeledHN-1-PKCε was applied to non-tumorigenic mammary epithelial cells.6 This was the first observation that HN-1 may not be tumor-specific or organ-specific but rather cancer-specific. As a cancer-selective targeting peptide as opposed to a HNSCC-selective targeting peptide, the potential theranostic applications for HN-1 increase.
Diptheria toxin/HN-1
In another study, HN-1 was conjugated to diphtheria toxin (DT) and was found to have cytotoxic effects on the HNSCC cell lines used whereas the non-HNSCC cells were resistant to the cytotoxic effects of DT/HN-1. The DT/HN-1 agent demonstrated a 4-fold increase in tumor cell apoptosis when compared with tumor cells treated with DT alone. DT/HN-1 also contributed to dose-dependent inhibition of HNSCC tumor cell proliferation. This suggests that HN-1 conjugated with DT can selectively deliver DT to HNSCC cells and its subsequent internalization facilitates its therapeutic effect. Interestingly, if the translocation domain of DT is deleted but conjugated to HN-1 (DTΔT/HN-1), the resultant cytotoxicity on HNSCC cells was reduced which suggests that although HN-1 facilitates tumor cell internalization (possibly via endosomes) it cannot further translocate DT into the cytoplasm.43 To date, no preclinical studies have been reported that further evaluate the impact of DT/HN-1 on tumors in xenograft or orthotopic models.
HN-1TYR-anti-hRRM2 siRNAR
Another study evaluated the feasibility of using modified HN-1 constructs to both target cancer cells and deliver small interfering RNA (siRNA) molecules for anticancer therapy. In this study, HN-1 was first specifically modified with the addition of 2 tyrosine residues at the N-terminus (i.e., HN-1TYR). FITC-labeled HN-1TYR demonstrated fluorescent internalization when incubated with presumed head and neck cancer KB cells (ATCC CCL-17) but not with NHDF (normal human dermal fibroblasts) cells.21 Of note, KB cells were initially believed to be derived from oral epidermal carcinoma but were subsequently found to represent HeLa cell contamination (i.e., epithelial cervical adenocarcinoma) according to the ATCC website.50 Although no other HNSCC cell lines were tested with the FITC-labeled HN-1TYR agent in this study,21 this correction implies that the HN-1 peptide may also represent a potential tumor-targeting peptide agent for cervical adenocarcinoma but further studies are needed to validate this.
Given that HN-1 internalization had been previously noted in human breast cancer cells,6 the potential for FITC-labeled HN-1TYR to serve as a tumor-specific delivery mechanism for discrete siRNA molecules was evaluated in human breast cancer cells.21 The intracellular target was ribonucleotide reductase and therefore anti-hRRM2 siRNA was conjugated to FITC-labeled HN-1TYR (i.e., HN-1TYR-anti-hRRM2 siRNAR). The in vitro application of HN-1TYR-anti-hRRM2 siRNAR partially suppressed the normal expression levels of hRRM2 in the treated breast cancer cells which suggests that HN-1 may help to facilitate the selective internalization of potential anticancer siRNA molecules into tumor cells. To date, no preclinical studies have been reported that have advanced this promising possibility of FITC-labeled HN-1TYR or HN-1TYR-anti-hRRM2 siRNAR localization to breast tumor-bearing xenograft/orthotopic models.
Current challenges and future opportunities for advancing theranostics and precision otolaryngology
At present, no ‘HN-1 receptor’ has been identified and since a scrambled HN-1 peptide (i.e., HN-1-scr) demonstrated comparable intracellular fluorescence to the native HN-1,22 it is possible that strict sequence-dependence is not essential for HN-1-mediated cellular internalization. In addition, HN-1 has been shown to target/internalize into other non-HNSCC tumor types. Collectively these findings suggest that HN-1 may not represent a true tumor-specific receptor ligand but rather a tumor cell-penetrating peptide (CPP). If HN-1 is a CPP, some potential tumor entry mechanisms for HN-1 may include membrane glycosaminoglycans, clathrin-dependent endocytosis, caveolin-associated lipid rafts, and actin/microtubule-mediated transport processes. Needless to say, additional studies will be needed to specifically address and elucidate the membrane target(s) or cellular mechanism(s) that HN-1 peptides exploit for tumor cell entry.51
Despite the fact that HPV has been strongly associated with the prevalence of HNSCC worldwide, no studies have yet demonstrated the feasibility of HN-1-mediated uptake and internalization into HPV+ HNSCC cells or tumors. All HN-1 studies to date have exclusively used HPV− negative HNSCC cell lines and tumors. At present, there is no rationale to expect HN-1 mediated uptake and internalization would be significantly different for HPV+ and HPV− HNSCC tumors but it will be important for future HN-1 studies to address this for future clinical translation. The observation that FITC-labeled HN-1TYR internalized into HeLa cells, a HPV+ cervical adenocarcinoma cell line, provides initial evidence that the utility of HN-1 will extend to HPV+ carcinomas.
In the future, the clinical promise and translational significance of a dual NIR fluorescent and radioactively labeled theranostic HN-1 agent targeted to HNSCC is that it will enable:
-
(1)
both pre- and post-surgical advanced molecular imaging using gamma/PET imaging systems to specifically detect and precisely localize HN-1-avid malignant and metastatic lesions. It is conceivable that such diagnostic HN-1 imaging agents may improve the staging of HNSCC and more accurately triage patients to or away from surgery.
-
(2)
Real-time intraoperative NIR fluorescent detection of HN-1-avid lesions during image-guided resection using one of the more than 7000 FDA-approved optical surgical imaging systems. A caveat is that these optical imaging systems are not all of equivalent sensitivity and, at the moment, FDA approval for these systems are specific to ICG. This will necessitate Phase III trials with these imaging systems and interest groups are actively investigating these and other logistic and regulatory issues.52
-
(3)
Alternatively, if the HN-1 agent is labeled with a diagnostic non-PET radioisotope (e.g., 111In or Iodine-123 (123I)) then intraoperative optical techniques using NIR dye could initially guide a surgeon's resection in real-time with subsequent intraoperative gamma imaging and/or hand-held radioactivity probing of the surgical field to verify complete resection or redirect the surgeon to areas of persistent radioactivity (i.e., residual tumor or tumor which is too deep for adequate optical detection). Radioguided surgical approaches have been demonstrated and are clinically effective.33, 53, 54, 55
-
(4)
Ex vivo gamma/PET and fluorescent imaging of resected specimens for more rapid and precise radiologic–pathologic correlation and assessment.
-
(5)
Following surgical resection, any residual microscopic malignancy or metastatic lesions could be further treated using a therapeutic radioisotope (e.g., 90Y, 131I, 177Lu) conjugated to the HN-1 peptide agent. Since external radiation therapy is often used in the treatment of HNSCC, this targeted approach for subsequent targeted PRRT for residual microscopic disease maybe a useful adjunct to existing therapy and improve patient progression-free survival.
Accurate clinical correlation of the gamma/PET radioactivity and NIR fluorescent signals could be achieved most reliably when the same targeted HN-1 molecule be dual-labeled and used even though the limits of detection and useful imaging concentrations for PET and fluorescent imaging are orders of magnitude different. Targeted dual-labeled HN-1 agents are transformative technology that will enable new diagnostic and therapeutic paradigms for HNSCC and potentially other cancer patients. While this concept of dual-modality cancer agents has been previously reviewed,56, 57 true dual-function molecular cancer agents are rare.58, 59, 60, 61
Conclusions
The treatment of HNSCC is complex and necessitates close collaboration among various medical specialties including nuclear medicine, interventional and diagnostic radiology, otolaryngology, pathology, surgical/medical oncology and radiation oncology. There is an unmet clinical need to both improve non-invasive diagnostic imaging and image-guided surgical methodologies as well as targeted chemotherapeutic and radiotherapeutic approaches for HNSCC patients (i.e., Precision Otolaryngology). Theranostic tumor targeting peptides represent transformative technology and will enable consistent, quantifiable in vivo imaging that can be clinically translated and easily implemented for diagnostic/therapeutic assessment by the treatment team.
Conflict of interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Acknowledgments
CLW is supported by (1) Grant no. IRG-67-003-50 from the American Cancer Society, (2) Award no. UL1TR000090 from the National Center For Advancing Translational Sciences (The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Advancing Translational Sciences or the National Institutes of Health), (3) Richard P. & Marie R. Bremer Medical Research Fund and William H. Davis Endowment for Basic Medical Research from The Ohio State University Medical Center (The remarks and opinions are the sole responsibility of the authors and do not necessarily reflect the views of the Davis/Bremer Research Fund or The Ohio State University Medical Center), and (4) the National Institutes of Health (NIH)/National Cancer Institute (NCI), Clinical Loan Repayment Program.
CLW, MVK and MFT are also supported by the Wright Center of Innovation in Biomedical Imaging and Ohio TECH 10-012.
Footnotes
Peer review under responsibility of Chinese Medical Association.
Contributor Information
Chadwick L. Wright, Email: Chadwick.Wright@osumc.edu.
Quintin Pan, Email: Quintin.Pan@osumc.edu.
Michael V. Knopp, Email: Michael.Knopp@osumc.edu.
Michael F. Tweedle, Email: Michael.Tweedle@osumc.edu.
References
- 1.Kamangar F., Dores G.M., Anderson W.F. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 2.Ramqvist T., Dalianis T. An epidemic of oropharyngeal squamous cell carcinoma (OSCC) due to human papillomavirus (HPV) infection and aspects of treatment and prevention. Anticancer Res. 2011;31:1515–1519. [PubMed] [Google Scholar]
- 3.Leemans C.R., Tiwari R., Nauta J.J., van der Waal I., Snow G.B. Recurrence at the primary site in head and neck cancer and the significance of neck lymph node metastases as a prognostic factor. Cancer. 1994;73:187–190. doi: 10.1002/1097-0142(19940101)73:1<187::aid-cncr2820730132>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 4.Liao C.T., Chang J.T., Wang H.M. Analysis of risk factors of predictive local tumor control in oral cavity cancer. Ann Surg Oncol. 2008;15:915–922. doi: 10.1245/s10434-007-9761-5. [DOI] [PubMed] [Google Scholar]
- 5.Leemans C.R., Tiwari R., Nauta J.J., van der Waal I., Snow G.B. Regional lymph node involvement and its significance in the development of distant metastases in head and neck carcinoma. Cancer. 1993;71:452–456. doi: 10.1002/1097-0142(19930115)71:2<452::aid-cncr2820710228>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 6.Bao L., Gorin M.A., Zhang M. Preclinical development of a bifunctional cancer cell homing, PKCepsilon inhibitory peptide for the treatment of head and neck cancer. Cancer Res. 2009;69:5829–5834. doi: 10.1158/0008-5472.CAN-08-3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nayak J.V., Walvekar R.R., Andrade R.S. Deferring planned neck dissection following chemoradiation for stage IV head and neck cancer: the utility of PET-CT. Laryngoscope. 2007;117:2129–2134. doi: 10.1097/MLG.0b013e318149e6bc. [DOI] [PubMed] [Google Scholar]
- 8.Keski-Säntti H., Mustonen T., Schildt J., Saarilahti K., Mäkitie A.A. FDG-PET/CT in the assessment of treatment response after oncologic treatment of head and neck squamous cell carcinoma. Clin Med Insights Ear Nose Throat. 2014;7:25–29. doi: 10.4137/CMENT.S16399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schöder H., Fury M., Lee N., Kraus D. PET monitoring of therapy response in head and neck squamous cell carcinoma. J Nucl Med. 2009;50:74S–88S. doi: 10.2967/jnumed.108.057208. [DOI] [PubMed] [Google Scholar]
- 10.Abgral R., Querellou S., Potard G. Does 18F-FDG PET/CT improve the detection of posttreatment recurrence of head and neck squamous cell carcinoma in patients negative for disease on clinical follow-up? J Nucl Med. 2009;50:24–29. doi: 10.2967/jnumed.108.055806. [DOI] [PubMed] [Google Scholar]
- 11.Nothelfer E.M., Zitzmann-Kolbe S., Garcia-Boy R. Identification and characterization of a peptide with affinity to head and neck cancer. J Nucl Med. 2009;50:426–434. doi: 10.2967/jnumed.108.058123. [DOI] [PubMed] [Google Scholar]
- 12.Burke P.A., DeNardo S.J., Miers L.A., Lamborn K.R., Matzku S., DeNardo G.L. Cilengitide targeting of alpha(v)beta(3) integrin receptor synergizes with radioimmunotherapy to increase efficacy and apoptosis in breast cancer xenografts. Cancer Res. 2002;62:4263–4272. [PubMed] [Google Scholar]
- 13.Huang H., Bai Y.L., Yang K., Tang H., Wang Y.W. Optical imaging of head and neck squamous cell carcinoma in vivo using arginine-glycine-aspartic acid peptide conjugated near-infrared quantum dots. Onco Targets Ther. 2013;6:1779–1787. doi: 10.2147/OTT.S53901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heath C.H., Deep N.L., Sweeny L., Zinn K.R., Rosenthal E.L. Use of panitumumab-IRDye800 to image microscopic head and neck cancer in an orthotopic surgical model. Ann Surg Oncol. 2012;19:3879–3887. doi: 10.1245/s10434-012-2435-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marshall M.V., Draney D., Sevick-Muraca E.M., Olive D.M. Single-dose intravenous toxicity study of IRDye 800CW in Sprague-Dawley rats. Mol Imaging Biol. 2010;12:583–594. doi: 10.1007/s11307-010-0317-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Day K.E., Sweeny L., Kulbersh B., Zinn K.R., Rosenthal E.L. Preclinical comparison of near-infrared-labeled cetuximab and panitumumab for optical imaging of head and neck squamous cell carcinoma. Mol Imaging Biol. 2013;15:722–729. doi: 10.1007/s11307-013-0652-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Helman E.E., Newman J.R., Dean N.R., Zhang W., Zinn K.R., Rosenthal E.L. Optical imaging predicts tumor response to anti-EGFR therapy. Cancer Biol Ther. 2010;10:166–171. doi: 10.4161/cbt.10.2.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee D.Y., Li K.C. Molecular theranostics: a primer for the imaging professional. AJR Am J Roentgenol. 2011;197:318–324. doi: 10.2214/AJR.11.6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie J., Lee S., Chen X. Nanoparticle-based theranostic agents. Adv Drug Deliv Rev. 2010;62:1064–1079. doi: 10.1016/j.addr.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hong F.D., Clayman G.L. Isolation of a peptide for targeted drug delivery into human head and neck solid tumors. Cancer Res. 2000;60:6551–6556. [PubMed] [Google Scholar]
- 21.Un F., Zhou B., Yen Y. The utility of tumor-specifically internalizing peptides for targeted siRNA delivery into human solid tumors. Anticancer Res. 2012;32:4685–4690. [PubMed] [Google Scholar]
- 22.Dudas J., Idler C., Sprinzl G., Bernkop-Schnuerch A., Riechelmann H. Identification of HN-1-peptide target in head and neck squamous cell carcinoma cells. ISRN Oncol. 2011;2011:140316. doi: 10.5402/2011/140316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holland J.P., Normand G., Ruggiero A., Lewis J.S., Grimm J. Intraoperative imaging of positron emission tomographic radiotracers using Cerenkov luminescence emissions. Mol Imaging. 2011;10:177–186. 1–3. [PMC free article] [PubMed] [Google Scholar]
- 24.Robertson R., Germanos M.S., Li C., Mitchell G.S., Cherry S.R., Silva M.D. Optical imaging of Cerenkov light generation from positron-emitting radiotracers. Phys Med Biol. 2009;54:N355–N365. doi: 10.1088/0031-9155/54/16/N01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spinelli A.E., D'Ambrosio D., Calderan L., Marengo M., Sbarbati A., Boschi F. Cerenkov radiation allows in vivo optical imaging of positron emitting radiotracers. Phys Med Biol. 2010;55:483–495. doi: 10.1088/0031-9155/55/2/010. [DOI] [PubMed] [Google Scholar]
- 26.DLj Thorek, Robertson R., Bacchus W.A. Cerenkov imaging – a new modality for molecular imaging. Am J Nucl Med Mol Imaging. 2012;2:163–173. [PMC free article] [PubMed] [Google Scholar]
- 27.Liu H., Ren G., Miao Z. Molecular optical imaging with radioactive probes. PLoS One. 2010;5:e9470. doi: 10.1371/journal.pone.0009470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruggiero A., Holland J.P., Lewis J.S., Grimm J. Cerenkov luminescence imaging of medical isotopes. J Nucl Med. 2010;51:1123–1130. doi: 10.2967/jnumed.110.076521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu Y., Liu H., Cheng Z. Harnessing the power of radionuclides for optical imaging: Cerenkov luminescence imaging. J Nucl Med. 2011;52:2009–2018. doi: 10.2967/jnumed.111.092965. [DOI] [PubMed] [Google Scholar]
- 30.Spinelli A.E., Ferdeghini M., Cavedon C. First human Cerenkography. J Biomed Opt. 2013;18:20502. doi: 10.1117/1.JBO.18.2.020502. [DOI] [PubMed] [Google Scholar]
- 31.Thorek D.L., Riedl C.C., Grimm J. Clinical Cerenkov luminescence imaging of (18)F-FDG. J Nucl Med. 2014;55:95–98. doi: 10.2967/jnumed.113.127266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong R.J. Current status of FDG-PET for head and neck cancer. J Surg Oncol. 2008;97:649–652. doi: 10.1002/jso.21018. [DOI] [PubMed] [Google Scholar]
- 33.Hall N.C., Nichols S.D., Povoski S.P. Intraoperative use of a portable large field of view gamma camera and handheld gamma detection probe for radioguided localization and prediction of complete surgical resection of gastrinoma: proof of concept. J Am Coll Surg. 2015;221:300–308. doi: 10.1016/j.jamcollsurg.2015.03.047. [DOI] [PubMed] [Google Scholar]
- 34.Whiteman M.L., Serafini A.N., Telischi F.F., Civantos F.J., Falcone S. 111In octreotide scintigraphy in the evaluation of head and neck lesions. AJNR Am J Neuroradiol. 1997;18:1073–1080. [PMC free article] [PubMed] [Google Scholar]
- 35.Henze M., Schuhmacher J., Hipp P. PET imaging of somatostatin receptors using [68GA]DOTA-D-Phe1-Tyr3-octreotide: first results in patients with meningiomas. J Nucl Med. 2001;42:1053–1056. [PubMed] [Google Scholar]
- 36.Koukouraki S., Strauss L.G., Georgoulias V. Evaluation of the pharmacokinetics of 68Ga-DOTATOC in patients with metastatic neuroendocrine tumours scheduled for 90Y-DOTATOC therapy. Eur J Nucl Med Mol Imaging. 2006;33:460–466. doi: 10.1007/s00259-005-0006-1. [DOI] [PubMed] [Google Scholar]
- 37.Janssen I., Chen C.C., Taieb D. 68Ga-DOTATATE PET/CT in the localization of head and neck paragangliomas compared with other functional imaging modalities and CT/MRI. J Nucl Med. 2016;57:186–191. doi: 10.2967/jnumed.115.161018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deppen S.A., Liu E., Blume J.D. Safety and efficacy of 68Ga-DOTATATE PET/CT for diagnosis, staging, and treatment management of neuroendocrine tumors. J Nucl Med. 2016;57:708–714. doi: 10.2967/jnumed.115.163865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Binnebeek S., Vanbilloen B., Baete K. Comparison of diagnostic accuracy of (111)In-pentetreotide SPECT and (68)Ga-DOTATOC PET/CT: a lesion-by-lesion analysis in patients with metastatic neuroendocrine tumours. Eur Radiol. 2016;26:900–909. doi: 10.1007/s00330-015-3882-1. [DOI] [PubMed] [Google Scholar]
- 40.Hope T.A., Pampaloni M.H., Nakakura E. Simultaneous (68)Ga-DOTA-TOC PET/MRI with gadoxetate disodium in patients with neuroendocrine tumor. Abdom Imaging. 2015;40:1432–1440. doi: 10.1007/s00261-015-0409-9. [DOI] [PubMed] [Google Scholar]
- 41.Pfeifer A., Knigge U., Binderup T. 64Cu-DOTATATE PET for neuroendocrine tumors: a prospective head-to-head comparison with 111In-DTPA-octreotide in 112 patients. J Nucl Med. 2015;56:847–854. doi: 10.2967/jnumed.115.156539. [DOI] [PubMed] [Google Scholar]
- 42.Pfeifer A., Knigge U., Mortensen J. Clinical PET of neuroendocrine tumors using 64Cu-DOTATATE: first-in-humans study. J Nucl Med. 2012;53:1207–1215. doi: 10.2967/jnumed.111.101469. [DOI] [PubMed] [Google Scholar]
- 43.Potala S., Verma R.S. Targeting head and neck squamous cell carcinoma using a novel fusion toxin-diphtheria toxin/HN-1. Mol Biol Rep. 2011;38:1389–1397. doi: 10.1007/s11033-010-0242-8. [DOI] [PubMed] [Google Scholar]
- 44.Hilderbrand S.A., Weissleder R. Near-infrared fluorescence: application to in vivo molecular imaging. Curr Opin Chem Biol. 2010;14:71–79. doi: 10.1016/j.cbpa.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 45.Olive M. Olive Consulting; Lincoln, NE: 2014. Image-guided Surgery. Dye Chemistries, Targeting Agents, Camera Systems, Preclinical Preparations, and Market Overview. [Google Scholar]
- 46.Loja M.N., Luo Z., Greg Farwell D. Optical molecular imaging detects changes in extracellular pH with the development of head and neck cancer. Int J Cancer. 2013;132:1613–1623. doi: 10.1002/ijc.27837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Dam G.M., Themelis G., Crane L.M. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: first in-human results. Nat Med. 2011;17:1315–1319. doi: 10.1038/nm.2472. [DOI] [PubMed] [Google Scholar]
- 48.van den Berg N.S., Brouwer O.R., Klop W.M. Concomitant radio- and fluorescence-guided sentinel lymph node biopsy in squamous cell carcinoma of the oral cavity using ICG-(99m)Tc-nanocolloid. Eur J Nucl Med Mol Imaging. 2012;39:1128–1136. doi: 10.1007/s00259-012-2129-5. [DOI] [PubMed] [Google Scholar]
- 49.van der Vorst J.R., Schaafsma B.E., Verbeek F.P. Near-infrared fluorescence sentinel lymph node mapping of the oral cavity in head and neck cancer patients. Oral Oncol. 2013;49:15–19. doi: 10.1016/j.oraloncology.2012.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.http://www.atcc.org/products/all/CCL-17.aspx#characteristics
- 51.Heitz F., Morris M.C., Divita G. Twenty years of cell-penetrating peptides: from molecular mechanisms to therapeutics. Br J Pharmacol. 2009;157:195–206. doi: 10.1111/j.1476-5381.2009.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosenthal E.L., Warram J.M., de Boer E. Successful translation of fluorescence navigation during oncologic surgery: a consensus report. J Nucl Med. 2016;57:144–150. doi: 10.2967/jnumed.115.158915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hall N.C., Plews R.L., Agrawal A. Intraoperative scintigraphy using a large field-of-view portable gamma camera for primary hyperparathyroidism: initial experience. Biomed Res Int. 2015;2015:930575. doi: 10.1155/2015/930575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Povoski S.P., Neff R.L., Mojzisik C.M. A comprehensive overview of radioguided surgery using gamma detection probe technology. World J Surg Oncol. 2009;7:11. doi: 10.1186/1477-7819-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sickle-Santanello B.J., O'Dwyer P.J., Mojzisik C. Radioimmunoguided surgery using the monoclonal antibody B72.3 in colorectal tumors. Dis Colon Rectum. 1987;30:761–764. doi: 10.1007/BF02554623. [DOI] [PubMed] [Google Scholar]
- 56.Azhdarinia A., Ghosh P., Ghosh S. Dual-labeling strategies for nuclear and fluorescence molecular imaging: a review and analysis. Mol Imaging Biol. 2012;14:261–276. doi: 10.1007/s11307-011-0528-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lütje S., Rijpkema M., Helfrich W., Oyen W.J., Boerman O.C. Targeted radionuclide and fluorescence dual-modality imaging of cancer: preclinical advances and clinical translation. Mol Imaging Biol. 2014;16:747–755. doi: 10.1007/s11307-014-0747-y. [DOI] [PubMed] [Google Scholar]
- 58.Azhdarinia A., Wilganowski N., Robinson H. Characterization of chemical, radiochemical and optical properties of a dual-labeled MMP-9 targeting peptide. Bioorg Med Chem. 2011;19:3769–3776. doi: 10.1016/j.bmc.2011.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wong C.C., Tse A.P., Huang Y.P. Lysyl oxidase-like 2 is critical to tumor microenvironment and metastatic niche formation in hepatocellular carcinoma. Hepatology. 2014;60:1645–1658. doi: 10.1002/hep.27320. [DOI] [PubMed] [Google Scholar]
- 60.Li C., Wang W., Wu Q. Dual optical and nuclear imaging in human melanoma xenografts using a single targeted imaging probe. Nucl Med Biol. 2006;33:349–358. doi: 10.1016/j.nucmedbio.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 61.Banerjee S.R., Pullambhatla M., Byun Y. Sequential SPECT and optical imaging of experimental models of prostate cancer with a dual modality inhibitor of the prostate-specific membrane antigen. Angew Chem Int Ed Engl. 2011;50:9167–9170. doi: 10.1002/anie.201102872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao M., Sano D., Pickering C.R. Assembly and initial characterization of a panel of 85 genomically validated cell lines from diverse head and neck tumor sites. Clin Cancer Res. 2011;17:7248–7264. doi: 10.1158/1078-0432.CCR-11-0690. [DOI] [PMC free article] [PubMed] [Google Scholar]