Abstract
Transoral robotic surgery is a exciting field that continues to develop and push the boundaries of current procedural ability and challenges historical treatment paradigms. With the first use of a surgical robot in 1985, to the first clinical use of the robot transorally in 2005, there was some lag in adoption of robotic techniques in the head and neck region. However, since 2005 transoral robotic surgery has rapidly gained momentum amongst head and neck surgeons. With FDA approval of the da Vinci robot in 2009, transoral robotic surgery is currently offered as a treatment modality for malignant and nonmalignant disease of the head and neck region. This new technology is being used to reconsider historical treatment paradigms for malignancies of the upper aerodigestive tract due to the fact that minimally invasive surgical access to the oropharynx and larynx has been improved. Along with this enhanced access have come innovative procedures and uses of the technology for multiple facets of head and neck disease. Technology continues to improve and innovation in surgical robotics is expected to continue as more companies attempt to capture this market. This article aims to provide a view at the landscape of transoral robotic surgery and explore the future frontiers.
Keyword: Robotic surgery
Introduction
The use of robotic surgical systems in the operating room first began in 1985 when a motorized arm, named Puma 560, was used to perform targeted neurosurgical procedures.1 Throughout the 1980's the interest in the use of robotic systems continued to grow and technology advanced to the point where several commercial systems were developed, namely the AESOP robotic camera positioning device for endoscopes,2 and the da Vinci surgical system. As time went on, the da Vinci system became the leader in the field and is the most prevalent robotic system in use today. The surgical robot has been widely applied in multiple specialties including urologic, gynecologic, orthopedic, thoracic, general surgery, cardiothoracic, and head and neck surgery.
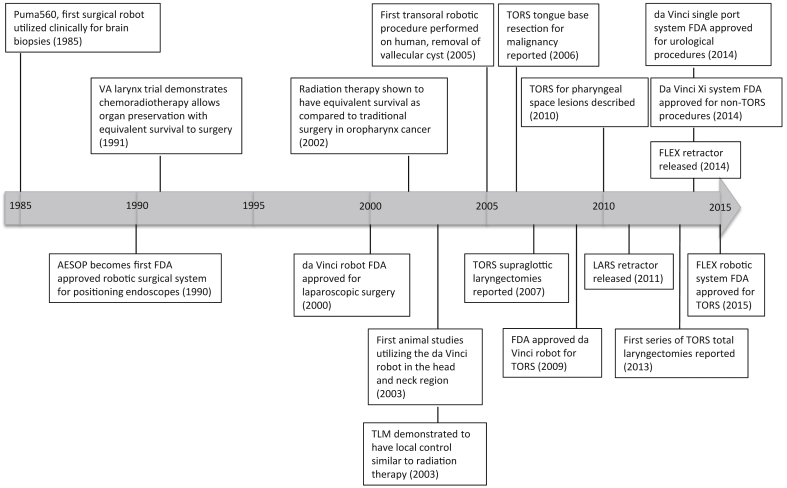
The adoption of robotic technology by the head and neck surgery community did not occur until 2003 (Fig. 1), when Haus et al3 studied the use of the robot for head and neck procedures (submandibular gland excision, parotidectomy, neck dissection, and thymectomy) in pigs. It was in 2005 that McLeod and Medler4 first used the robot in a human patient to remove a vallecular cyst. The University of Pennsylvania had also conducted preclinical studies using the surgical robot for pharyngeal and laryngeal surgery in cadavers and canines in the early 2000's,5, 6, 7 and in 2006 a case series of three patients who underwent TORS for tongue base squamous cell carcinoma was reported by O'Malley et al8 demonstrating safety and efficacy of TORS. Since then there have been numerous reports from multiple institutions demonstrating the safety and efficacy of TORS for radical tonsillectomy, tongue base resection, and supraglottic laryngectomy. These procedures are being offered as treatment options for resectable malignancies in these subsites. As technology extends the ability of the surgeon to safely and effectively operate in the oropharynx, larynx, and hypopharynx, the role of surgery in the management of lesions in these subsites is being reevaluated.
Fig. 1.
Timeline of transoral robotic surgery.
Discussion
Treatment frontiers
There have been several recent developments that have led to a renewed interest in surgically managing oropharyngeal malignancies. First, TORS allows for improved visualization and the ability to resect lesions en bloc in the oropharynx. Second, the biology of oropharynx cancer is better understood. As we continue to gain insights on the role of the human papilloma virus (HPV) in oropharyngeal cancer the role of surgical management of this disease remains to be fully understood.
In 1991 the VA larynx study which demonstrated the equal survival between patients receiving chemoradiation therapy vs. patients undergoing laryngectomy followed by radiation.9 This lead to increased interest in avoiding surgery in order to preserve function and these protocols were applied to other subsites in the head and neck. In 2002, Mendenhall et al conducted a large retrospective study of patients treated for oropharyngeal cancer at 51 institutions. This landmark study demonstrated equal effectiveness of radiation therapy to surgery in the treatment of oropharynx cancer and increased severe or fatal complications in patients that underwent surgery and radiation therapy.10 This solidified the place of radiation therapy as the treatment of choice for oropharyngeal cancer and somewhat marginalized the role of surgery.
However, in smaller studies, transoral laser microsurgery (TLM), a precursor to TORS, was shown by Steiner et al11 in 2003 to have local control rates around 85% at 5 years and spared patients radiation 52% of the time. Haughey et al12 published 5 year survival of 79% and local control rate of 97% in 2011 in a series of 204 patients. This suggests that this type surgical therapy might be as effective as radiation therapy and eliminate the associated toxicities. There have been no randomized controlled studies of surgery vs. radiation therapy in the treatment of oropharynx cancer. At this time, work began at the University of Pennsylvania on utilizing the da Vinci robot for transoral robotic surgery and several small series of patients with oropharynx and supraglottic cancers resected via a TORS approach were published. By 2009, the FDA granted approval for the use of the da Vinci robot for use in TORS. Since that time several case series from major institutions have been published. Overall results suggest adequate safety, equivalent oncologic outcomes, and improved functional outcomes as compared to open surgery and radiation therapy.13, 14, 15, 16, 17, 18
As the connection between HPV and oropharyngeal cancer emerged, there was increasing awareness that the population of patients affected by HVP positive oropharyngeal cancer was young and healthy and these patients have increased survival as compared to patients with HPV negative disease (82% vs. 57% respectively).19 These tumors respond well to radiation therapy, however there is increasing concern of the long-term effects of radiation therapy in this young and otherwise healthy cohort. Therefore interest in being able to treat these patients surgically and sparing them adjuvant radiation therapy has been renewed. Recently, Olsen et al reported a series of 18 patients with HPV positive, T1–T3, N0—N2a, oropharyngeal cancer treated with TORS and neck dissection alone. These patients had 3-year survival of 100% and recurrence free survival of 91%.20 Deintensification of therapy in HPV positive patients is currently being studied in the ECOG 3311 trial. The results of this important trial should help to direct therapy that is appropriate while limiting the functional deficits caused by treatment. The trial sets a framework for future studies investigating the issue of deintensification of therapy.
Perhaps the most compelling data evaluating TORS vs. radiation therapy is a 2014 systematic published by Genden et al.21 8 IMRT and 12 TORS case series were included in the analysis. They found equivalent 2-year survival ranging from 84% to 96% in the IMRT studies and 82%–94% in the TORS studies. Thus indicating TORS may be equivalent to IMRT. Further investigation with a prospective trial as well as longer follow up of these patients is needed to better understand the survival and functional outcomes of TORS.
As clinical experience with the robot has grown, surgeons have been working to better understand the indications for its use, and large centers have been operating on more advanced disease and performing more extensive dissection. Weinstein et al22 published a prospective cohort study of 47 patients from the University of Pennsylvania on TORS outcomes in patients with advanced (stage III and VI) oropharyngeal cancers. This study included 9 patients with T3 and 2 patients with T4 disease. Overall disease free survival was 90% at two years. 18 of 47 (38%) patients were spared chemotherapy, and 5 of 47 (11%) patients were spared radiation therapy as well. Only 1 patient remained PEG tube dependent at the end of 1 year.
Another area that TORS has been shown to be potentially useful is in carcinomas of unknown primary sites (CUP). Despite thorough workup of patients who present with metastatic carcinoma in cervical lymph nodes, about 2% of patients will not have a localizable primary site of disease.23, 24 Determining the origin of the carcinoma allows for narrowing of radiation fields and reducing doses, thus limiting toxicity of treatment. Some patients may also be candidates for surgical management alone if the primary is resected with negative margins and the neck disease can be managed surgically. Karni et al25 showed in a 2011 study that the use of TLM allowed identification of the unknown primary site in 95% of patients. Nagel et al26 published a slightly lower rate of primary site detection of 86%, but identification of the primary is far better with these surgical methods as compared to imaging and standard direct laryngoscopy with biopsy alone. With similar principles in mind, several studies have now been published using TORS as part of the workup and treatment algorithm for CUP. The robot allows improved 3D visualization of the collapsible tissues in the oropharynx and allows for enhanced visualization of subtle mucosal changes associated with sub-centimeter primary lesions. Mehta et al27 showed that in patients with CUP, when all other workup was negative (PET/CT, tonsillectomy, and thorough endoscopy with biopsies), robotic tongue base resection located 90% of the previously unknown primaries in the tongue base. Patel et al28 demonstrated a similar benefit of TORS for identifying elusive primary lesions. Utilizing a TORS approach, the authors were able to identify 72.2% of primary sites in patients with no radiographic or clinical evidence of disease. The Ohio State University group reported a series of 22, that 13/17 patients whose tumors were identified using a TORS approach were removed with negative margins. Prior studies of CUP, not utilizing TLM or TORS, have shown that use of panendoscopy, tonsillectomy, and directed biopsies have only led to the identification of a primary site in about 20% of patients with no clinical or radiological evidence of disease.29, 30 A cost effectiveness study from the University of Pittsburgh indicates that including a TORS tongue base resection may be a cost effective procedure during the initial exam under anesthesia.31 Again, larger series and prospective studies are needed to further define the role of TORS in CUP, however based on these initial findings, the technique appears to be a valuable tool for both working up and treating these patients.
Procedural frontiers
One of the advantages of TORS has been the ability to resect lesions of the upper aerodigestive tract without entering the neck, often eliminating the need for reconstructive procedures and mitigating the risk of pharyngocutaneous fistula. However, there may be a role for reconstruction in these patients to help the ultimately achieve better functional outcomes and to protect exposed structures, such as the carotid artery, from oral secretions. Traditionally, the tongue base, oropharynx, and supraglottic larynx have been difficult areas to reconstruct and open techniques for access often require mandibulotomy and lip splitting incisions. With the robot, surgeons have been able to visualize these areas and successfully inset free flaps in a minimally invasive manner. The indications and the benefits of transoral robotic reconstruction remain for further investigation.32, 33, 34, 35, 36, 37
While TORS tonsillectomy, tongue base resection, and supraglottic laryngectomy are the three most common robotic applications in the head and neck malignancy, case reports and small series describing resection in other anatomic subsites have been reported. In 2008, Ozer and Waltonen38 were the first to describe nasopharyngectomy in a cadaver, an approach which also allows access to the clivus and upper cervical vertebrae. O'Malley et al39 were the first to publish on a TORS approach to pharyngeal space lesions in 2010. Other authors have further shown that the placement of transcervical suprahyoid oropharyngeal ports can further expand the surgical access to include the skull base.40, 41, 42, 43
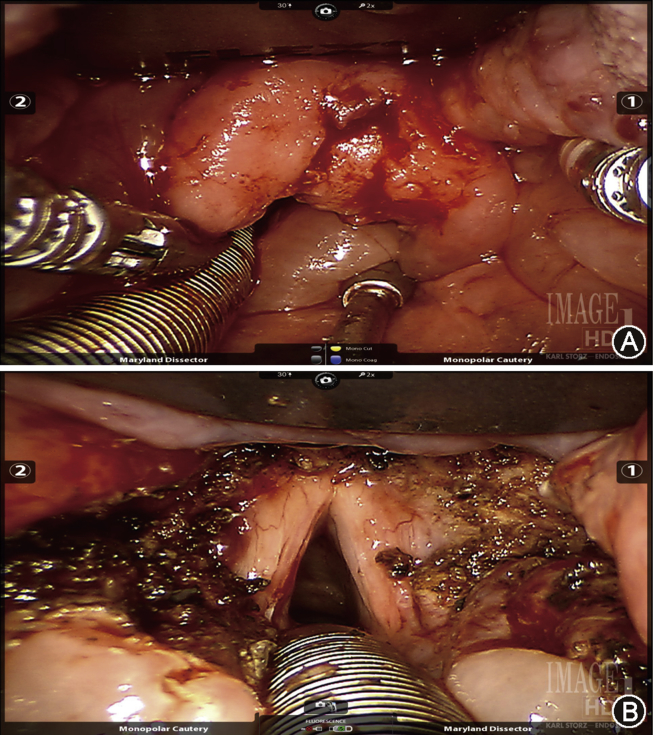
Robotic supraglottic laryngectomy (Fig. 2) has now become a relatively standard procedure amongst TORS surgeons, however compared to oropharyngeal TORS there is substantially less data. Ozer et al44 published a case series of 13 patients who underwent TORS supraglottic laryngectomy demonstrating safety and good functional outcomes. Survival data in this population is limited, with Mendelsohn et al45 reporting 2 year survival data in 18 patients (local regional control 83%, disease specific survival 100%, overall all survival 89%). Park et al46 showed a 2-year disease free survival rate of 91%. These patients were matched to a cohort of patients who underwent open supraglottic laryngectomy and the TORS group demonstrated earlier oral feeding, decreased time to decannulation, and decreased hospital stay.
Fig. 2.
A. Intraoperative view of supraglottic squamous cell carcinoma on the laryngeal surface of the epiglottis. This tumor extended from the tip of the epiglottis to just superior to the anterior commissure. B. Postoperative appearance after TORS supraglottic laryngectomy showing preserved true vocal cords anteriorly and arytenoids posteriorly. The epiglottis, aryepiglottic folds, false vocal cords, and ventricular mucosa has been resected.
Several reports of expanding the use of the robot in laryngeal cancer beyond the supraglottic laryngectomy have now been published. In 2013 Smith et al published a multi institutional case series of 7 patients who underwent attempted transoral robotic laryngectomy. Five of the procedures were completed successfully, while two required conversion to an open approach. The authors suggested that this procedure might be particularly valuable in surgical salvage patients and in patients with nonfunctional larynx after radiation therapy. The limited dissection is proposed to potentially lead to fewer wound healing complications.47, 48 Further study is required before this technique will become widely endorsed.
What is the limit to the frontier of transoral robotic surgery? Weinstein et al49 suggested limits in 2015 with a published set of contraindications for TORS. They suggest vascular, functional, oncological and non-oncological contraindications to TORS based on their experience with 1400 TORS cases. They assert that TORS is not a modality designed to push the surgical envelope; rather TORS should be used as a tool. TORS procedures being performed by head and neck surgeons should be reproducible, teachable, and have predictable outcomes.
Technological frontiers
There is continued interest in expanding the role of robotic surgery within head and neck surgery, and many of the limitations have been technical. Commonly used mouth gags for exposure are the Feyh-Kastenbauer-Weinstein-O'Malley retractor or the Crowe-Davis mouth gag. New retractors designed specifically for TORS are hitting the market. The LARS retractor by FENTEXmedicala and the MedRobotics Flex retractorb are now available, and other retractors are in development. Having several retractor options may improve the ability to expose the surgical site in a wider variety of patients.
Several new robotic platforms are emerging at this time. In the daVinci line of robots, the S and Si platforms have been widely utilized for TORS. In 2014, da Vinci released a new robotic system, the Xi, which has a different arm configuration that allows for more freedom in positioning the robotic arms.c This system has already been found useful in abdominal and thoracic procedures, however its use in the head and neck has been limited by lack of FDA approval for TORS. Several centers are investigating its use and are optimistic that it may have advantages as compared to the S and Si systems. Furthermore, this system has the ability to perform fluorescence imaging; a feature that with specially designed pharmaceuticals injected intravenously prior to surgery or applied topically may help with identification of tumors and improve the ability to visualize tumor margins. Several preclinical studies have shown feasibility of such an approach.50, 51, 52
With high volumes of abdominal and pelvic robotic surgery as compared to TORS, many of the technological developments have been aimed to improve these procedures. The desire to limit the number of access ports required for these procedures has led to the development of a single port system by da Vinci. This system consists of a single rigid port from which four flexible arms extend, three instruments arms and one flexible endoscope. The instruments are steered into position by the surgeon. The single port is much larger than any of the currently utilized da Vinci instruments, at 24 mm, however may be beneficial for transoral procedures.d The ability to successfully perform oropharyngeal surgery using this system has been demonstrated in cadaver studies. The authors also noted that the single port design increased the room at the head of the bed so the assistant can better access the surgical site.53 Currently, this device is only FDA approved for specific urological procedures, but the approval is expected to broaden to cover head and neck application.
Another surgical robot has also recently entered the market to compete with the Da Vinci robot. The Flex robotic system from MedRobotics was FDA approved for transoral use in July of 2015. This robotic system is unique as it is a flexible snake like device that can be steered to the operative site rather than having rigid arms like the da Vinci robot. Once in position, there are two dissection arms, on either side of a high definition endoscope, which can be used to manipulate tissue.e This robot has had limited use thus far, however there are increasing case reports and series being reported since its FDA approval.54, 55
Titan Medical, a Canadian based company, has also developed a single port robotic system, the SPORT Surgical System. They are expected to enter the commercial market in the US in 2017.f Google and Ethicon also announced a collaboration to develop a surgical robotics system with a start up company coined Verb.g A key feature of their proposed robotic system is haptic feedback, which is a feature the da Vinci does not provide.
There are several other useful technologies yet to be incorporated in commercially available surgical robotic systems. One such feature would be the use of image guidance and navigation features such as 3D overlays on the endoscopic images to identify vascular, neural, or other key structures. End effectors able to remove bone such as drills or ultrasonic devices would enhance the ability of the robot to be used transorally at the skull base and cervical spine, thus further expanding the usefulness of these systems.40
Conclusion
With further innovation, the ability of robotic surgical systems to assist surgeons will continue to expand. With the ECOG 3311 trial setting the groundwork for surgical trials investigating TORS and TLM, further clinical studies will be key in better defining the role of these techniques in treatment of patients. As surgical experience utilizing the robot and technological capabilities grow, new procedures and approaches will be developed that further the scope of transoral robotic surgery. Several competitors have now emerged in the field of surgical robotics. Companies will have to continue to innovate in order to compete and competition may help control cost and improve the quality of these products.
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Kwoh Y.S., Hou J., Jonckheere E.A., Hayati S. A robot with improved absolute positioning accuracy for CT guided stereotactic brain surgery. IEEE Trans Biomed Eng. 1988;35:153–160. doi: 10.1109/10.1354. [DOI] [PubMed] [Google Scholar]
- 2.Begin E., Gagner M., Hurteau R., de Santis S., Pomp A. A robotic camera for laparoscopic surgery: conception and experimental results. Surg Laparosc Endosc. 1995;5:6–11. [PubMed] [Google Scholar]
- 3.Haus B.M., Kambham N., Le D., Moll F.M., Gourin C., Terris D.J. Surgical robotic applications in otolaryngology. Laryngoscope. 2003;113:1139–1144. doi: 10.1097/00005537-200307000-00008. [DOI] [PubMed] [Google Scholar]
- 4.McLeod I.K., Melder P.C. Da Vinci robot-assisted excision of a vallecular cyst: a case report. Ear Nose Throat J. 2005;84:170–172. [PubMed] [Google Scholar]
- 5.O'Malley B.W., Jr., Weinstein G.S., Hockstein N.G. Transoral robotic surgery (TORS): glottic microsurgery in a canine model. J Voice. 2006;20:263–268. doi: 10.1016/j.jvoice.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Weinstein G.S., O'malley B.W., Jr., Hockstein N.G. Transoral robotic surgery: supraglottic laryngectomy in a canine model. Laryngoscope. 2005;115:1315–1319. doi: 10.1097/01.MLG.0000170848.76045.47. [DOI] [PubMed] [Google Scholar]
- 7.Hockstein N.G., O'Malley B.W., Jr., Weinstein G.S. Assessment of intraoperative safety in transoral robotic surgery. Laryngoscope. 2006;116:165–168. doi: 10.1097/01.mlg.0000199899.00479.75. [DOI] [PubMed] [Google Scholar]
- 8.O'Malley B.W., Jr., Weinstein G.S., Snyder W., Hockstein N.G. Transoral robotic surgery (TORS) for base of tongue neoplasms. Laryngoscope. 2006;116:1465–1472. doi: 10.1097/01.mlg.0000227184.90514.1a. [DOI] [PubMed] [Google Scholar]
- 9.The Department of Veterans Affairs Laryngeal Cancer Study Group Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. N Engl J Med. 1991;324:1685–1690. doi: 10.1056/NEJM199106133242402. [DOI] [PubMed] [Google Scholar]
- 10.Parsons J.T., Mendenhall W.M., Stringer S.P. Squamous cell carcinoma of the oropharynx: surgery, radiation therapy, or both. Cancer. 2002;94:2967–2980. doi: 10.1002/cncr.10567. [DOI] [PubMed] [Google Scholar]
- 11.Steiner W., Fierek O., Ambrosch P., Hommerich C.P., Kron M. Transoral laser microsurgery for squamous cell carcinoma of the base of the tongue. Arch Otolaryngol Head Neck Surg. 2003;129:36–43. doi: 10.1001/archotol.129.1.36. [DOI] [PubMed] [Google Scholar]
- 12.Haughey B.H., Hinni M.L., Salassa J.R. Transoral laser microsurgery as primary treatment for advanced-stage oropharyngeal cancer: a United States multicenter study. Head Neck. 2011;33:1683–1694. doi: 10.1002/hed.21669. [DOI] [PubMed] [Google Scholar]
- 13.Leonhardt F.D., Quon H., Abrahão M., O'Malley B.W., Jr., Weinstein G.S. Transoral robotic surgery for oropharyngeal carcinoma and its impact on patient-reported quality of life and function. Head Neck. 2012;34:146–154. doi: 10.1002/hed.21688. [DOI] [PubMed] [Google Scholar]
- 14.Iseli T.A., Kulbersh B.D., Iseli C.E., Carroll W.R., Rosenthal E.L., Magnuson J.S. Functional outcomes after transoral robotic surgery for head and neck cancer. Otolaryngol Head Neck Surg. 2009;141:166–171. doi: 10.1016/j.otohns.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 15.Hurtuk A.M., Marcinow A., Agrawal A., Old M., Teknos T.N., Ozer E. Quality-of-life outcomes in transoral robotic surgery. Otolaryngol Head Neck Surg. 2012;146:68–73. doi: 10.1177/0194599811421298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weinstein G.S., O'Malley B.W., Jr., Magnuson J.S. Transoral robotic surgery: a multicenter study to assess feasibility, safety, and surgical margins. Laryngoscope. 2012;122:1701–1707. doi: 10.1002/lary.23294. [DOI] [PubMed] [Google Scholar]
- 17.Dziegielewski P.T., Teknos T.N., Durmus K. Transoral robotic surgery for oropharyngeal cancer: long-term quality of life and functional outcomes. JAMA Otolaryngol Head Neck Surg. 2013;139:1099–1108. doi: 10.1001/jamaoto.2013.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore E.J., Olsen K.D., Kasperbauer J.L. Transoral robotic surgery for oropharyngeal squamous cell carcinoma: a prospective study of feasibility and functional outcomes. Laryngoscope. 2009;119:2156–2164. doi: 10.1002/lary.20647. [DOI] [PubMed] [Google Scholar]
- 19.Ang K.K., Harris J., Wheeler R. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olsen S.M., Moore E.J., Laborde R.R. Transoral surgery alone for human-papillomavirus-associated oropharyngeal squamous cell carcinoma. Ear Nose Throat J. 2013;92:76–83. [PubMed] [Google Scholar]
- 21.de Almeida J.R., Byrd J.K., Wu R. A systematic review of transoral robotic surgery and radiotherapy for early oropharynx cancer: a systematic review. Laryngoscope. 2014;124:2096–2102. doi: 10.1002/lary.24712. [DOI] [PubMed] [Google Scholar]
- 22.Weinstein G.S., O'Malley B.W., Jr., Cohen M.A., Quon H. Transoral robotic surgery for advanced oropharyngeal carcinoma. Arch Otolaryngol Head Neck Surg. 2010;136:1079–1085. doi: 10.1001/archoto.2010.191. [DOI] [PubMed] [Google Scholar]
- 23.Grau C., Johansen L.V., Jakobsen J., Geertsen P., Andersen E., Jensen B.B. Cervical lymph node metastases from unknown primary tumours. Results from a national survey by the Danish Society for Head and Neck Oncology. Radiother Oncol. 2000;55:121–129. doi: 10.1016/s0167-8140(00)00172-9. [DOI] [PubMed] [Google Scholar]
- 24.Waltonen J.D., Ozer E., Hall N.C., Schuller D.E., Agrawal A. Metastatic carcinoma of the neck of unknown primary origin: evolution and efficacy of the modern workup. Arch Otolaryngol Head Neck Surg. 2009;135:1024–1029. doi: 10.1001/archoto.2009.145. [DOI] [PubMed] [Google Scholar]
- 25.Karni R.J., Rich J.T., Sinha P., Haughey B.H. Transoral laser microsurgery: a new approach for unknown primaries of the head and neck. Laryngoscope. 2011;121:1194–1201. doi: 10.1002/lary.21743. [DOI] [PubMed] [Google Scholar]
- 26.Nagel T.H., Hinni M.L., Hayden R.E., Lott D.G. Transoral laser microsurgery for the unknown primary: role for lingual tonsillectomy. Head Neck. 2014;36:942–946. doi: 10.1002/hed.23372. [DOI] [PubMed] [Google Scholar]
- 27.Mehta V., Johnson P., Tassler A. A new paradigm for the diagnosis and management of unknown primary tumors of the head and neck: a role for transoral robotic surgery. Laryngoscope. 2013;123:146–151. doi: 10.1002/lary.23562. [DOI] [PubMed] [Google Scholar]
- 28.Patel S.A., Magnuson J.S., Holsinger F.C. Robotic surgery for primary head and neck squamous cell carcinoma of unknown site. JAMA Otolaryngol Head Neck Surg. 2013;139:1203–1211. doi: 10.1001/jamaoto.2013.5189. [DOI] [PubMed] [Google Scholar]
- 29.Durmus K., Rangarajan S.V., Old M.O., Agrawal A., Teknos T.N., Ozer E. Transoral robotic approach to carcinoma of unknown primary. Head Neck. 2014;36:848–852. doi: 10.1002/hed.23385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang S.Y., Dziegielewski P.T., Old M.O., Ozer E. Transoral robotic surgery for carcinoma of unknown primary in the head and neck. J Surg Oncol. 2015;112:697–701. doi: 10.1002/jso.24027. [DOI] [PubMed] [Google Scholar]
- 31.Byrd J.K., Smith K.J., de Almeida J.R. Transoral robotic surgery and the unknown primary: a cost-effectiveness analysis. Otolaryngol Head Neck Surg. 2014;150:976–982. doi: 10.1177/0194599814525746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Selber J.C., Robb G., Serletti J.M., Weinstein G., Weber R., Holsinger F.C. Transoral robotic free flap reconstruction of oropharyngeal defects: a preclinical investigation. Plast Reconstr Surg. 2010;125:896–900. doi: 10.1097/PRS.0b013e3181cb6568. [DOI] [PubMed] [Google Scholar]
- 33.Selber J.C. Transoral robotic reconstruction of oropharyngeal defects: a case series. Plast Reconstr Surg. 2010;126:1978–1987. doi: 10.1097/PRS.0b013e3181f448e3. [DOI] [PubMed] [Google Scholar]
- 34.de Almeida J.R., Park R.C., Genden E.M. Reconstruction of transoral robotic surgery defects: principles and techniques. J Reconstr Microsurg. 2012;28:465–472. doi: 10.1055/s-0032-1313762. [DOI] [PubMed] [Google Scholar]
- 35.Bonawitz S.C., Duvvuri U. Robot-assisted oropharyngeal reconstruction with free tissue transfer. J Reconstr Microsurg. 2012;28:485–490. doi: 10.1055/s-0032-1313758. [DOI] [PubMed] [Google Scholar]
- 36.Mukhija V.K., Sung C.K., Desai S.C., Wanna G., Genden E.M. Transoral robotic assisted free flap reconstruction. Otolaryngol Head Neck Surg. 2009;140:124–125. doi: 10.1016/j.otohns.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 37.Genden E.M., Kotz T., Tong C.C. Transoral robotic resection and reconstruction for head and neck cancer. Laryngoscope. 2011;121:1668–1674. doi: 10.1002/lary.21845. [DOI] [PubMed] [Google Scholar]
- 38.Ozer E., Waltonen J. Transoral robotic nasopharyngectomy: a novel approach for nasopharyngeal lesions. Laryngoscope. 2008;118:1613–1616. doi: 10.1097/MLG.0b013e3181792490. [DOI] [PubMed] [Google Scholar]
- 39.O'Malley B.W., Jr., Quon H., Leonhardt F.D., Chalian A.A., Weinstein G.S. Transoral robotic surgery for parapharyngeal space tumors. ORL J Otorhinolaryngol Relat Spec. 2010;72:332–336. doi: 10.1159/000320596. [DOI] [PubMed] [Google Scholar]
- 40.Ozer E., Durmus K., Carrau R.L. Applications of transoral, transcervical, transnasal, and transpalatal corridors for robotic surgery of the skull base. Laryngoscope. 2013;123:2176–2179. doi: 10.1002/lary.24034. [DOI] [PubMed] [Google Scholar]
- 41.McCool R.R., Warren F.M., Wiggins R.H., 3rd, Hunt J.P. Robotic surgery of the infratemporal fossa utilizing novel suprahyoid port. Laryngoscope. 2010;120:1738–1743. doi: 10.1002/lary.21020. [DOI] [PubMed] [Google Scholar]
- 42.Kim G.G., Zanation A.M. Transoral robotic surgery to resect skull base tumors via transpalatal and lateral pharyngeal approaches. Laryngoscope. 2012;122:1575–1578. doi: 10.1002/lary.23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Malley B.W., Jr., Weinstein G.S. Robotic anterior and midline skull base surgery: preclinical investigations. Int J Radiat Oncol Biol Phys. 2007;69:S125–S128. doi: 10.1016/j.ijrobp.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 44.Ozer E., Alvarez B., Kakarala K., Durmus K., Teknos T.N., Carrau R.L. Clinical outcomes of transoral robotic supraglottic laryngectomy. Head Neck. 2013;35:1158–1161. doi: 10.1002/hed.23101. [DOI] [PubMed] [Google Scholar]
- 45.Mendelsohn A.H., Remacle M., Van Der Vorst S., Bachy V., Lawson G. Outcomes following transoral robotic surgery: supraglottic laryngectomy. Laryngoscope. 2013;123:208–214. doi: 10.1002/lary.23621. [DOI] [PubMed] [Google Scholar]
- 46.Park Y.M., Kim W.S., Byeon H.K., Lee S.Y., Kim S.H. Surgical techniques and treatment outcomes of transoral robotic supraglottic partial laryngectomy. Laryngoscope. 2013;123:670–677. doi: 10.1002/lary.23767. [DOI] [PubMed] [Google Scholar]
- 47.Smith R.V., Schiff B.A., Sarta C., Hans S., Brasnu D. Transoral robotic total laryngectomy. Laryngoscope. 2013;123:678–682. doi: 10.1002/lary.23842. [DOI] [PubMed] [Google Scholar]
- 48.Dowthwaite S., Nichols A.C., Yoo J. Transoral robotic total laryngectomy: report of 3 cases. Head Neck. 2013;35:E338–E342. doi: 10.1002/hed.23226. [DOI] [PubMed] [Google Scholar]
- 49.Weinstein G.S., O'Malley B.W., Jr., Rinaldo A., Silver C.E., Werner J.A., Ferlito A. Understanding contraindications for transoral robotic surgery (TORS) for oropharyngeal cancer. Eur Arch Otorhinolaryngol. 2015;272:1551–1552. doi: 10.1007/s00405-014-3331-9. [DOI] [PubMed] [Google Scholar]
- 50.Rosenthal E.L., Kulbersh B.D., Duncan R.D. In vivo detection of head and neck cancer orthotopic xenografts by immunofluorescence. Laryngoscope. 2006;116:1636–1641. doi: 10.1097/01.mlg.0000232513.19873.da. [DOI] [PubMed] [Google Scholar]
- 51.Kulbersh B.D., Duncan R.D., Magnuson J.S., Skipper J.B., Zinn K., Rosenthal E.L. Sensitivity and specificity of fluorescent immunoguided neoplasm detection in head and neck cancer xenografts. Arch Otolaryngol Head Neck Surg. 2007;133:511–515. doi: 10.1001/archotol.133.5.511. [DOI] [PubMed] [Google Scholar]
- 52.Rosenthal E.L., Kulbersh B.D., King T., Chaudhuri T.R., Zinn K.R. Use of fluorescent labeled anti-epidermal growth factor receptor antibody to image head and neck squamous cell carcinoma xenografts. Mol Cancer Ther. 2007;6:1230–1238. doi: 10.1158/1535-7163.MCT-06-0741. [DOI] [PubMed] [Google Scholar]
- 53.Holsinger F.C. A flexible, single-arm robotic surgical system for transoral resection of the tonsil and lateral pharyngeal wall: next-generation robotic head and neck surgery. Laryngoscope. 2016;126:864–869. doi: 10.1002/lary.25724. [DOI] [PubMed] [Google Scholar]
- 54.Johnson P.J., Rivera Serrano C.M., Castro M. Demonstration of transoral surgery in cadaveric specimens with the medrobotics flex system. Laryngoscope. 2013;123:1168–1172. doi: 10.1002/lary.23512. [DOI] [PubMed] [Google Scholar]
- 55.Remacle M., M N Prasad V., Lawson G., Plisson L., Bachy V., Van der Vorst S. Transoral robotic surgery (TORS) with the Medrobotics Flex™ System: first surgical application on humans. Eur Arch Otorhinolaryngol. 2015;272:1451–1455. doi: 10.1007/s00405-015-3532-x. [DOI] [PubMed] [Google Scholar]
Web resources
- http://www.gracemedical.com/sites/488/uploaded/files/LARS_Brochure.pdf. Accessed 2.22.16.
- http://medrobotics.com/flex-retractor/. Accessed 2.22.16.
- http://www.intuitivesurgical.com/products/da-vinci-xi/. Accessed 2.22.16.
- http://investor.intuitivesurgical.com/phoenix.zhtml?c=122359&p=irol-newsArticle&ID=1920546. Accessed 2.22.16.
- http://medrobotics.com/gateway/flex-system-int/. Accessed 2.22.16.
- http://www.titanmedicalinc.com. Accessed 2.22.16.
- http://spectrum.ieee.org/automaton/robotics/medical-robots/google-verily-johnson-johnson-verb-surgical-medical-robots.“Google and Johnson & Johnson Conjugate to Create Verb Surgical, Promise Fancy Medical Robots”. Ackerman, Evan. IEEE SPECTRUM. 12/17/2015. Accessed 2.22.16.