Abstract
Objective
To provide an overview of the healthcare and societal consequences and costs of untreated obstructive sleep apnea syndrome.
Data sources
PubMed database for English-language studies with no start date restrictions and with an end date of September 2014.
Methods
A comprehensive literature review was performed to identify all studies that discussed the physiologic, clinical and societal consequences of obstructive sleep apnea syndrome as well as the costs associated with these consequences. There were 106 studies that formed the basis of this analysis.
Conclusions
Undiagnosed and untreated obstructive sleep apnea syndrome can lead to abnormal physiology that can have serious implications including increased cardiovascular disease, stroke, metabolic disease, excessive daytime sleepiness, work-place errors, traffic accidents and death. These consequences result in significant economic burden. Both, the health and societal consequences and their costs can be decreased with identification and treatment of sleep apnea.
Implications for practice
Treatment of obstructive sleep apnea syndrome, despite its consequences, is limited by lack of diagnosis, poor patient acceptance, lack of access to effective therapies, and lack of a variety of effective therapies. Newer modes of therapy that are effective, cost efficient and more accepted by patients need to be developed.
Keywords: Obstructive sleep apnea syndrome, Cost, Continuous positive airway pressure, Mandibular advancement device
Introduction
Sleep disorders and specifically obstructive sleep apnea syndrome (OSAS) have been increasingly recognized as significant health problems in the last two decades.1 OSAS is a condition in which repetitive episodes of airway occlusion occur during sleep, resulting in apneas or hypopneas and related arousals. These events lead to intermittent hypoxemia, increased sympathetic tone, cytokine production, metabolic abnormalities, and abnormal sleep structure. This abnormal physiology results in increased health risks, most notably those related to the cardiovascular and cerebrovascular systems. It also leads to neurocognitive abnormalities, which have significant societal consequences.
The term obstructive sleep apnea (OSA) generally refers to objective laboratory findings. The term obstructive sleep apnea syndrome (OSAS) refers to the combination of the laboratory finding of OSA plus clinical consequences related to the events such as excessive daytime sleepiness. A diagnosis of OSAS is generally derived from one of three diagnostic modalities: full-night polysomnography (PSG), split-night PSG and unattended portable home monitoring. Unattended portable home sleep tests are being used with growing frequency, although full-night PSGs are still considered the gold standard for evaluation of OSAS. The number of apneas and hypopneas per hour of sleep is termed the apnea–hypopnea index (AHI). This paper is primarily focused on moderate OSAS, defined as AHI ≥15 and severe OSAS defined as AHI ≥30.2
Research has established links between OSAS and several important co-morbidities. Recent studies have shown a clear association of OSAS with the development of hypertension,3, 4, 5 type II diabetes,6, 7 stroke,8, 9, 10, 11 congestive heart failure,12 coronary artery disease,8, 13, 14, 15, 16 cardiac arrhythmias17 and even early mortality.18, 19, 20 OSAS remains significantly under recognized, and it is estimated that only 40% of those with OSAS have been diagnosed.2 In addition, the obesity epidemic in the United States potentiates explosive growth of this disease entity.
The consequences of undiagnosed and untreated OSAS are medically serious and economically costly.21 Continuous positive airway pressure (CPAP) is considered the gold standard of treatment for OSAS. When adherence is optimal, CPAP improves sleep quality, reduces the risk of OSAS-related co-morbidities and improves patient quality of life.14, 22, 23, 24, 25 However, in spite of many technological advances of the CPAP apparatus and the patient–device interface, adherence remains a significant problem.26, 27
Surgical treatment options for OSAS, other than tracheostomy, can be effective for some properly selected patients, but overall, results are equivocal. In addition to mixed results, OSAS surgeries often involve high morbidity, long recovery times, and low patient acceptance.28, 29 Recently, there has been examination of other treatments (e.g. mandibular advancement devices (MAD), and hypoglossal nerve stimulation in reducing risk of co-morbidities).30, 31, 32
In this review, we discuss the physiologic consequences and the resultant economic burden related to the health problems of OSAS.
Methods
A comprehensive search was performed in the PubMed database with no date restrictions. The search term used included “OSA,” “OSAS,” or “sleep apnea” to search for OSAS. To search for consequences and costs, the following search terms were used: “consequences,” “transportation,” “crashes,” “accidents,” “job,” “depression,” “heart failure,” “stroke,” “arrhythmia,” “diabetes,” “cost,” “heart attack,” and “hospitalization.” Articles were chosen for recency and relevance to the specific topics discussed below.
Results
With the above criteria, we found the following number of articles for each section that informed the basis of this review: Medical consequences, 4025 articles; Transportation consequences, 81 articles; Workplace consequences 66 articles; Economic consequences 466 articles and treatment options, 5758 articles. The authors chose data from 106 exemplary articles as the basis of this review.
Prevalence
The Wisconsin Sleep Cohort Study is a landmark study that evaluated the prevalence of sleep apnea in state employees. It demonstrated that 24% of men and 9% of women had mild to severe OSAS (AHI ≥ 5).2 This translates to about 29.5 million people over the age of 30 in the United States. It also showed that 4% of women and 9% of men have moderate to severe OSAS (AHI ≥ 15), which translates to about 13 million people over the age of 30 in the U.S.2 In addition, the prevalence is much higher in certain patient populations. For example, 55% of patients with documented coronary artery disease and 37% of patients with diabetes (type I and II) have moderate to severe OSAS.33, 34
Medical consequences of untreated OSAS
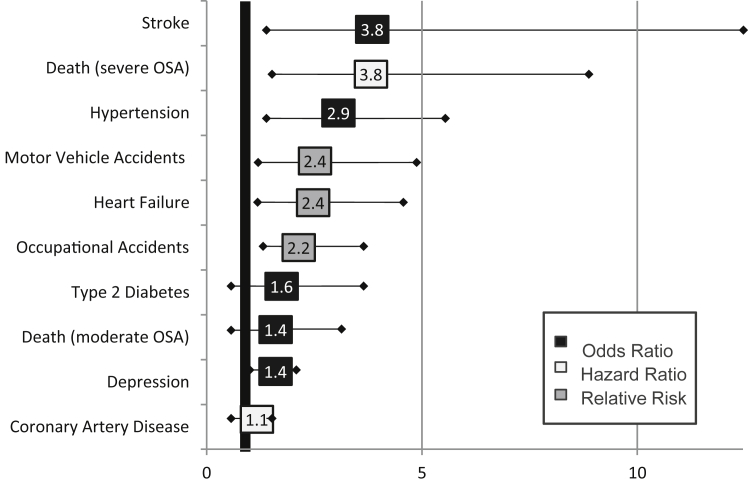
The consequences of undiagnosed and untreated OSAS are numerous and serious (see Fig. 1 and Table 135, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50). During sleep, OSAS patients experience recurrent airway obstruction (in the form of apneas and hypopneas). An increase in heart rate and a surge in blood pressure are seen during these events and, as a result, the events cause repeated strain on the heart and circulatory system throughout the night.51, 52 The arousals, which terminate the airway occlusion, are accompanied by a sympathetic nervous system response.53 Evidence suggests that this sympathetic activation from nocturnal events persists during the daytime as well.54, 55 Consequently, OSAS patients tend to have higher heart rates, less heart rate variability, higher blood pressure and increased arterial stiffness compared to healthy controls.51, 56 It is not surprising that multiple studies have found OSAS patients to be at increased risk for cardiovascular morbidities and hypertension.3, 4, 5, 8, 10, 11, 12, 14, 15 These patients are also at an increased risk for early all-cause mortality, and, as one might expect, cardiovascular mortality risk is high (adjusted hazard ratio = 5.2 (95% CI 1.4, 19.2)).18, 19, 20 OSAS has been associated with metabolic disorders,57, 58, 59, 60 and elevated risk for cancer and mortality among OSAS patients, particularly for those with severe OSAS (AHI ≥ 30).61, 62 Perioperative complications including need for prolonged intubation, need for re-intubation, pneumonias, aspiration, arrhythmias and cardiac arrest are increased in those with untreated sleep apnea.63, 64, 65 Recent evidence suggests that when sleep apnea is diagnosed prior to surgery, the risk of cardiac and neurovascular events is decreased by about 50%, and it is comparable to those individuals that do not have sleep apnea.50
Fig. 1.
Increased risk in obstructive sleep apnea syndrome.
Table 1.
Untreated OSAS puts patients at increased risk for the following health conditions.
| Condition | Quantified risk | Quantified costs | Reference |
|---|---|---|---|
| Hypertension | Sleep apnea is an independent risk factor for hypertension. 89% of patients with resistant hypertension have moderate sleep apnea. About 50% of essential hypertension patients have sleep apnea; about 50% of apnea patients have hypertension. | In 2010 high blood pressure cost the US $93.5 billion in health care services (Centers for Disease Control). In 2008 the cost of treating hypertension in the US was $50.6 billion (American Heart Association (AHA)). Patients with OSAS are 2–3 times more likely to be taking antihypertensive medications than controls (more prescriptions, more doses, greater cost). | Pepperd 20003 Nieto 20004 Otake 200244 Young 20092 Lurie 201135 Pedrosa 201136 Roger 201284 Phillips 20135 Lloberes 201498 Schein 201423 |
| Heart failure | Male patients with OSAS are 6 times or more likely to be treated for congestive heart failure compared to controls. OSAS patients have reduced left ventricular ejection fraction. | It has been estimated by the AHA that the cost of treating heart failure in the USA in 2015 will be $44.6 billion. | Shahar 200112 Smith 200271 Roger 201284 Sun 201337 |
| Arrhythmias | Atrial fibrillation is very common in sleep apnea. Untreated sleep apnea independently predicts atrial fibrillation recurrence in patients undergoing ablation therapy. Patients with severe OSAS are less likely to respond to antiarrhythmic drug therapy. OSAS patients who had a stroke had higher rates of atrial fibrillation. | The annual direct cost of treating a single patient with atrial fibrillation is $8705. The annual cost to the USA is $26 billion. | Gami 200838 Albuquerque 201239 Bitter 201240 Monahan 201242 Mansukhani 201241 Roger 201284 Goyal 201499 Vizzardi 201417 |
| Coronary artery disease | Men with sleep apnea are more likely to have coronary heart disease, and are more likely to have fatal and nonfatal cardiovascular events if untreated. Women with sleep apnea also have increased risk of coronary heart disease, and their risk is attenuated by use of CPAP. | The cost of coronary heart disease in the US is about $190 billion per year. The average cost for treating acute myocardial infarction in 2006 was about $14,000 per patient. | Selim 201015 Marin 201214 Roger 201284 Loo 201413 Campos-Rodriguez 20148 |
| Stroke | There is a strong association between sleep apnea and the subsequent development of ischemic stroke, which can be attenuated with CPAP use. | The cost of treating stroke in the US in 2008 was $34.3 billion (AHA). The lifetime cost of treating a single patient with stroke is likely between $100,000 and $300,000. | Taylor 199685 Yaggi 200511 Redline 201010 Roger 201284 Cho 201343 Campos-Rodriguez 20148 |
| Metabolic syndrome | Sleep apnea is associated with the metabolic syndrome in women and men. | The age-adjusted prevalence for metabolic syndrome is 35.1% for men and 32.6% for women. Each component of the syndrome is associated with increased costs for hypertension ($550), obesity ($366), low high-density lipoprotein ($363), and high triglycerides ($317) (P < 0.001 for all). | Nichols 201159 Hall 201258 Roger 201284 Schmitt 201360 Lin 201457 |
| Type II diabetes | In adults without known diabetes, increasing OSAS severity is independently associated with impaired glucose metabolism, higher HbA1c values, increasing them to higher risks of diabetes. Sleep apnea is associated with impairments in insulin sensitivity, glucose effectiveness, and pancreatic beta-cell function. | In 2007, the direct ($116 billion) and indirect ($58 billion) cost attributable to diabetes was $174 billion. | Reichmuth 20056 Punjabi 20097 CDC 201145 Priou 201247 Guest 2014100 |
| Depression | Depression is more than two times more common in females with sleep apnea than the general population. Depressive symptoms may improve with apnea treatment. | The cost of depression was $ 83.1 billion dollars in 2000: $ 26.1 billion (31%) were direct medical costs, $5.4 billion (7%) were suicide-related mortality costs, and $51.5 billion (62%) were workplace costs. | Smith 200271 Greenberg 200346 El-Sherbini 201170 Gagnadoux 2014101 |
| Adverse perioperative event | Untreated sleep apnea patients are much more likely to develop serious perioperative events including cardiac arrest and respiratory failure that require ICU stays. | Mean intensive care unit cost and length of stay were $31,574 and 14.4 days for patients requiring mechanical ventilation and $12,931 and 8.5 days for those not requiring mechanical ventilation. | Dasta 200549 Vasu 201248 Gaddam 2013102 Memtsoudis 201464 Stundner 201463 Mutter 201450 |
| Cancer | Cancer rate is increased particularly in patients with hypoxemia and with sleep apnea. Cancer-related mortality increases with hypoxemia and severity of sleep apnea in younger patients. | Unknown | Rich 201119 Nieto 201261 Martinez-Garcia 2014103 Chen 2014104 Marshall 2014105 |
| Death | Death rate is increased, particularly in patients less than 50 years of age. | Not applicable | Young 200818 Rich 201119 Marshall 2014105 |
OSAS patients experience daytime sleepiness, decreased cognitive function and are at an increased risk for co-morbidities and accidents. Not surprisingly, studies have consistently found OSAS patients report lower quality of life than non-OSAS patients (see Table 2).66, 67, 68 The bed partners of OSAS patients also assess the quality of life for their partner to be lower than individuals without OSAS.69 The prevalence of depression in this population has been sited to be as high as 50%.70, 71 When the patient's OSAS is treated, quality of life and symptoms of depression improve.22, 70 The challenge, as mentioned above, is long-term adherence with CPAP. If CPAP is not used as directed, lowered quality of life and possibly depression will persist.
Table 2.
Untreated OSAS significantly diminishes patient quality of life.
| Condition | Quantified risk | Quantified costs | Reference |
|---|---|---|---|
| Home | Patients with sleep apnea have an impaired quality of life that affects both the patient and family members. Quality of life improves with treatment. | Not applicable | Finn 199866 Baldwin 200167 Breugelmans 200469 Siccoli 200822 Warkentin 2014106 |
| Workplace | Patients with OSAS are about ten times more likely to have workplace disability. This has the potential of increasing costs to employers in the realms of reduced productivity, healthcare costs and liability in the event of accidents. | The cost of absenteeism is difficult to calculate but, Hoffman et al. found that treating OSAS patients with CPAP reduced the % of Commercial Drivers taking short-term disability by 50% | Omachi 200981 Vennelle 201068 Hoffman 201090 |
Transportation consequences of untreated OSAS
Sleep-related respiratory events interrupt physiological sleep structure and inhibit sleep-based functional recovery. This leads to excessive daytime sleepiness, impaired vigilance, decreased ability to adequately perform daily tasks and ultimately, accidents. The increased risk of motor vehicle crashes among patients suffering from OSAS has been well documented (see Table 3).72, 73, 74, 75, 76 Although specific risk factors for automobile crashes among those with OSAS have yet to be determined, improvement in vigilance and motor vehicle crash rates with effective CPAP therapy has been documented.73, 77, 78 The costs (in lives and dollars) specifically related to OSAS-related motor vehicle crashes are significant. In 2004, Sassani and colleagues estimated that 810,000 motor vehicle crashes a year are attributable to OSAS, resulting in 1400 fatalities and costing roughly $15.9 billion.79 Their research also concluded that treating the same OSAS sufferers with CPAP, assuming 70% adherence, would prevent roughly 500,000 collisions, save 1000 lives and reduce cost by $11.1 billion. The cost of CPAP was factored into the dollars saved.79
Table 3.
Untreated OSAS puts patients and the public at increased risk for accidents.
| Condition | Quantified risk | Quantified costs | Reference |
|---|---|---|---|
| Motor Vehicle Accidents | The rate of vehicle crashes (personal and commercial) is at least double in OSAS patients compared to controls. These crashes often result in personal injury. Many drivers in head-on crashes are found to have OSAS. Treatment reduces the accident rate. This improvement with treatment is most evident in the most severely affected patients. | In 2000, conservative calculations found that more than 800,000 drivers were involved in OSAS-related motor-vehicle collisions in the United States. These collisions cost $15.9 billion and 1400 lives. In the United States, treating all drivers suffering from OSAS with CPAP would cost 3.18 billion dollars, save 11.1 billion dollars in collision costs, and save 980 lives annually | Sassani 200479 Ellen 200675 Tregear 200976 Antonopoulos 201174 Karimi 201473 |
| Work Related Accidents | Workplace accidents are particularly more common in OSAS patients when they are in the transportation industry. | The costs of workplace accidents are difficult to quantitate precisely because of the direct and indirect costs. However, OSAS is now considered a public health hazard given its pervasive effects in the workplace. | Lindberg 200180 Vennelle 201068 Leger 201272 |
Workplace consequences of untreated OSAS
Consistent with the findings on motor vehicle crashes, OSAS sufferers are also at an increased risk for workplace accidents. Lindberg and colleagues found that survey respondents reporting both snoring and excessive daytime sleepiness over a ten-year period were at a greater risk for workplace accidents, odds ratio of 3.1 (1.5–6.4).80 A limitation of the Lindberg study is that they only categorized subjects based on snoring and daytime sleepiness: PSG was not performed to confirm a diagnosis of OSAS. In addition, their study was a retrospective study that relied on patient self-reports of errors made. However, it seems reasonable to assume that many of those reporting snoring and excessive daytime sleepiness were reporting symptoms that resulted from OSAS. The negative impact on workplace disability resulting from OSAS is also important to consider. Omachi and colleagues found that individuals suffering from OSAS and reporting excessive daytime sleepiness experience a significantly greater risk of workplace disability than those with no OSAS and no daytime sleepiness, odds ratio of 13.7 (95% confidence interval [CI], 3.9–48). These same individuals also experience an increased risk of long-term duty modification as a result of their OSAS.81 A more recent study by Guglielmi and group evaluated the relationship between severity of OSAS by polysomnographic measures and sleepiness and job satisfaction in individuals with and without OSAS.82 They found that subjective sleepiness in OSAS patient did influence job satisfaction and burnout regardless of OSAS severity by polysomnographic parameters. The same group reported in another paper that absenteeism and productivity were worse among OSAS patients.83
Economic consequences of untreated OSAS
The increased risk of health complications, work-place errors and traffic accidents in OSAS patients conveys significant costs in terms of healthcare dollars and the overall economy. Case in point is the cost of care for two of the most significant consequences of untreated OSAS: stroke and myocardial infarction. Broadly, the cost of coronary heart disease in the United States is estimated at roughly $190 billion per year and the average cost for treating acute myocardial infarction in 2006 was roughly $14,000 per patient.84 The cost of treating stroke in the United States in 2008 was $34.3 billion and the lifetime cost of treating a single patient with stroke is estimated between $100,000 and $300,000.84, 85
Case-controlled studies have specifically evaluated the cost impact of OSAS and have demonstrated that overall healthcare utilization costs are higher for undiagnosed OSAS patients. Three such studies include Kapur et al., 1999, Tarasiuk et al., 2005 and Albarrak et al., 2005.86, 87, 88 After normalizing the results to a common currency (US$) and adjusting for inflation, it is estimated that increased healthcare spending to treat undiagnosed OSAS patients is between $1950 and $3,899, per patient, per year. Taking an estimated prevalence of 29.5 million people extrapolated from the Wisconsin Sleep Cohort data, if 60% remain undiagnosed, an estimate of the added burden on the healthcare system is between $34 billion and $69 billion annually (Table 4).
Table 4.
Untreated OSAS has a significant economic burden on society and employers.
| Condition | Quantified risk | Quantified costs | Reference |
|---|---|---|---|
| Economic burden of OSAS | Patients with undiagnosed OSAS have higher medical costs than non-OSAS patients. | Undiagnosed OSAS patients cost $1950 to $3899 per year more than non-OSAS patients. | Kapur 199988 Albarrak 200586 Tarasiuk 200587 Berger 200689 Hoffman 201090 |
| Patients with diagnosed but, untreated OSAS, have higher medical costs compared to patients not receiving treatment for their OSAS. | OSAS patients that are treated with CPAP cost $2700-$5200 less per year, than OSAS patients not receiving treatment | Berger 200689 Hoffman 201090 |
Several additional studies were reviewed that specifically evaluated the effect of treating OSAS versus not treating. It should be noted here that commercial motor vehicle operators appear to be early adopters of a well-organized strategy to address OSAS in their workforce populations. Because of this, perhaps two of the most useful studies looking at the impact of appropriately treating OSAS originate within the commercial motor vehicle population.
The first study was a retrospective analysis conducted by Berger et al. in 2006.89 The authors analyzed healthcare costs for a population of 337 commercial vehicle drivers, before and after the initiation of CPAP treatment. They found an overall reduction in healthcare costs of 48%, from $906.28 per member, per month to $472.69 per member, per month. They also found a significant reduction in the accident rate from 93% pre-CPAP treatment to 25% post-CPAP treatment.89 Although the authors ended their analysis here, improvements in cost and worker productivity most certainly followed this reduction in workplace accidents.
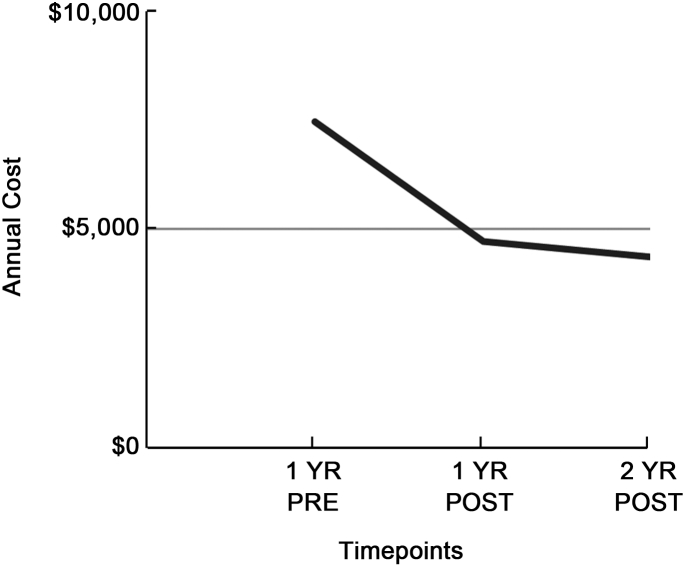
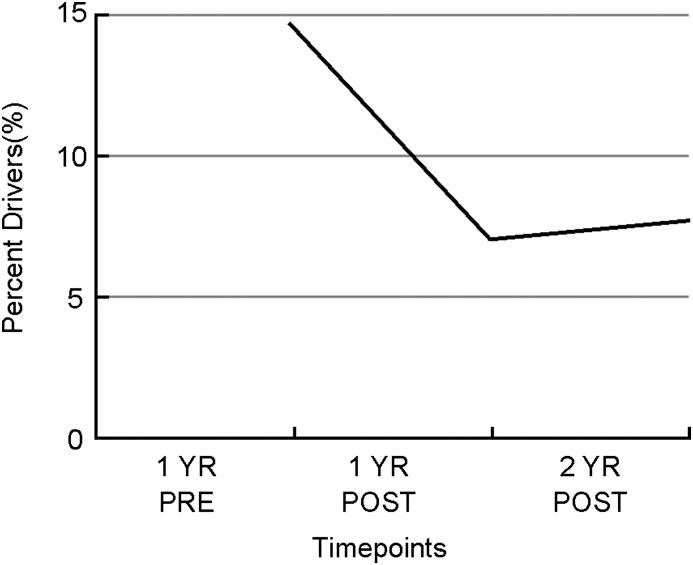
The second study, published by Hoffman et al. in 2010, was a retrospective analysis of commercial motor vehicle drivers that compared diagnosed OSAS patients receiving treatment via CPAP (n = 156) to a control group (n = 92) that was not receiving treatment. They found that annual healthcare costs decreased by 37% one year post treatment and noted further decrease to by 41% decrease in annual healthcare costs when comparing the second year with treatment to pre-treatment healthcare costs (see Fig. 2).90 They also found that the percentage of drivers taking short-term disability leave decreased by about 50% in the 2 years following treatment compared to a year prior to treatment (see Fig. 3).90
Fig. 2.
Health plan costs for treated drivers.
Fig. 3.
Percent of drivers taking short term disability leave.
OSAS treatment options
According to the American Academy of Sleep Medicine, OSAS is a chronic disease that requires long-term multidisciplinary management. Conservative therapeutic approaches are generally initial recommendations that include: CPAP or mandibular advancement devices, weight loss and measures to improve sleep hygiene. CPAP is offered for all patients with moderate to severe disease and to patients with mild disease and clinical evidence of daytime sleepiness. In recent work, Kim et al. defined CPAP treatment success as reducing AHI to <10 in patients (n = 151) with moderate and severe OSAS. By this definition, 92% of non-obese patients (BMI <25) and 79% of obese patients received successful treatment with CPAP. Furthermore, among non-obese patients, 82% of patients with moderate OSAS and 94% of patients with severe OSAS patients were adherent to CPAP for more than four hours per day for more than 70% of days. In obese patients, adherence by this definition was 94% in moderate OSAS and 76% in severe OSAS.91 In a contrasting study, Kuna et al. showed a lower adherence to CPAP patients with moderate to severe apnea. In their study, adherence was 52% in the 105 patients who underwent home sleep testing and 49% in the 96 who underwent in-laboratory polysomnogram.
In the event that CPAP is not effective or is not tolerated by the patient, there are alternate therapies, which include surgical procedures to implement varying levels of airway reconstruction. Uvulopalatopharyngoplasty (UPPP) involves excision of the tonsils, posterior soft palate and uvula, and closure of the tonsillar pillars. Success, defined as a reduction in AHI by 50% and less than 20, was noted to be much lower with UPPP than that of CPAP by Kim et al.; in 70 patients with AHI >15 who underwent UPPP and/or tongue base radiofrequency ablation, they found that 48% of obese and 43% of non-obese patients achieved treatment success at one year.91 The relative failure of UPPP was echoed in a recent meta-analysis.92 The incidence of serious nonfatal complications and 30-day mortality after UPPP are 1.5% and 0.2%, respectively, in a large cohort of UPPP patients at veteran hospitals.93 Maxillo-mandibular advancement (MMA) is a skeletal surgery designed to enlarge the velo-orohypopharyngeal airway by advancing the anterior pharyngeal tissues attached to the maxilla, mandible, and hyoid bone. In a meta-analysis of surgical OSAS treatments via MMA, 234 subjects with severe OSAS and a mean AHI of 54.4 per hour had an overall reduction in AHI of 87% (95% CI 80%–92%) with a mean postoperative AHI of 7.7.92 Despite its efficacy, acceptance of MMA has been variable due to patient's concerns over the invasiveness of the procedure, and poor insurance coverage for maxillofacial related procedures. Surgical placement of a tracheostomy was one of the earliest methods of treating obstructive sleep apnea. It has been shown to be effective in reducing the number of respiratory events, decreasing sleepiness and reducing mortality.94 However, its use is limited obvious cosmetic concerns and patient acceptance.
Mandibular advancement devices (MADs) reposition the lower jaw and/or the tongue to increase the dimensions of the retroglossal airway lumen. These devices are generally recommended for patients with less severe sleep apnea and are considered better tolerated than positive airway pressure therapy. However, these devices do not achieve the same reduction in AHI as other modalities.95 In a cross-over study of 126 patients with moderate to severe OSAS who were sequentially treated with CPAP followed by MAD (or the reverse), CPAP was more efficacious than MAD in reducing AHI (CPAP AHI, 4.5 ± 6.6/h; MAD AHI, 11.1 ± 12.1/h; P < 0.01) but reported adherence was higher on MAD (MAD, 6.50 ± 1.30 h per night vs. CPAP, 5.20 ± 2.00 h per night; P < 0.00001).95 Moreover, most insurance companies do not cover the cost of MAD, leaving the patients to bear the cost themselves. Because the cost of these devices can be high, especially with possibility of poor efficacy, it may deter patients from their use.
More recent studies have demonstrated the efficacy of implantable unilateral hypoglossal nerve stimulation as treatment for moderate and severe OSAS is a very select group of patients.32, 96, 97 This modality involves the implantation of a device with a stimulation electrode over the hypoglossal nerve and a sensing electrode near the intercostal muscles to sense respiratory effort. The objective is to stimulate the hypoglossal nerve to recruit tongue protrusion in order to maintain airway patency during sleep while ensuring synchrony with respiratory effort. Strollo et al. evaluated the utility of this device in patients with an AHI of 20–50/hour on baseline polysomnogram and a body-mass index less than 32 kg/m2 who could not tolerate CPAP. They showed that there was a reduction of AHI from a baseline value of 29.3 to 9.0 events per hour. In addition, they demonstrated an improvement in the oxygen desaturation index, functional outcomes defined by responses to a sleep questionnaire and the Epworth Sleepiness Scale. However, the anatomic features of the patients enrolled in this study were very specific, and therefore this treatment may not be generalizable. Further data is also needed on improvement in other health outcomes with the use of this approach to treatment of OSAS.
Limitations of this review
Despite the apparent impact of OSAS, there are very few studies that quantitatively evaluate the true cost of the disease. Perhaps this is due to the recency of the recognition of OSAS as a public health problem, or perhaps due to the large number of detrimental and sometimes interacting outcomes of untreated OSAS. As just one of many possible examples OSAS is associated with arterial hypertension, which in turn is associated with coronary artery disease. Many of the existing studies and a summary of their findings were discussed above. However, this review is limited by the scarcity of studies to evaluate this problem.
Implications for practice
The consequences of undiagnosed and untreated OSAS are serious, and the medical and societal costs are very high. Identification of those patients with yet undiagnosed sleep apnea and treating them is therefore vital. The current gold standard of treatment for OSAS is CPAP therapy, which has been shown to improve sleep quality, reduce the risk of co-morbidities and improve patient quality of life. However, adherence to CPAP remains poor in many patients, and this remains a significant problem. When a patient fails CPAP, alternate therapies including oral devices and surgical therapies should be considered. Surgical treatment options for OSAS can be effective for properly selected patients, but overall, accessibility, acceptability and clinical results are mixed. Oral devices are effective in only a selected and generally mildly to moderately affected patient populations. In order to address these unmet therapy needs, the medical community needs new treatment options to tackle OSAS, reduce the risk of dangerous co-morbidities and improve quality of life for people suffering from this debilitating condition.
Disclosure
This manuscript did not have financial support.
Off-label and investigational use of medications is not discussed within this manuscript.
Footnotes
Peer review under responsibility of Chinese Medical Association.
Contributor Information
Melissa Knauert, Email: melissa.knauert@yale.edu.
Sreelatha Naik, Email: sreelatha.naik@yale.edu.
M. Boyd Gillespie, Email: gillesmb@musc.edu.
Meir Kryger, Email: meir.kryger@yale.edu.
References
- 1.Young T., Palta M., Dempsey J. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Young T., Palta M., Dempsey J. Burden of sleep apnea: rationale, design, and major findings of the Wisconsin Sleep Cohort study. WMJ – Off Publ State Med Soc Wis. 2009;108:246–249. [PMC free article] [PubMed] [Google Scholar]
- 3.Peppard P.E., Young T., Palta M. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 4.Nieto F.J., Young T.B., Lind B.K. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA – J Am Med Assoc. 2000;283:1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 5.Phillips C.L., O'Driscoll D.M. Hypertension and obstructive sleep apnea. Nat Sci Sleep. 2013;5:43–52. doi: 10.2147/NSS.S34841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reichmuth K.J., Austin D., Skatrud J.B. Association of sleep apnea and type II diabetes: a population-based study. Am J Respir Crit Care Med. 2005;172:1590–1595. doi: 10.1164/rccm.200504-637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Punjabi N.M., Beamer B.A. Alterations in glucose disposal in sleep-disordered breathing. Am J Respir Crit Care Med. 2009;179:235–240. doi: 10.1164/rccm.200809-1392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campos-Rodriguez F., Martinez-Garcia M.A., Reyes-Nunez N. Role of sleep apnea and continuous positive airway pressure therapy in the incidence of stroke or coronary heart disease in women. Am J Respir Crit Care Med. 2014;189:1544–1550. doi: 10.1164/rccm.201311-2012OC. [DOI] [PubMed] [Google Scholar]
- 9.Arzt M., Young T., Finn L. Association of sleep-disordered breathing and the occurrence of stroke. Am J Respir Crit Care Med. 2005;172:1447–1451. doi: 10.1164/rccm.200505-702OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Redline S., Yenokyan G., Gottlieb D.J. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med. 2010;182:269–277. doi: 10.1164/rccm.200911-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yaggi H.K., Concato J., Kernan W.N. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 12.Shahar E., Whitney C.W., Redline S. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 13.Loo G., Tan A.Y., Koo C.Y. Prognostic implication of obstructive sleep apnea diagnosed by post-discharge sleep study in patients presenting with acute coronary syndrome. Sleep Med. 2014;15:631–636. doi: 10.1016/j.sleep.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 14.Marin J.M., Agusti A., Villar I. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA – J Am Med Assoc. 2012;307:2169–2176. doi: 10.1001/jama.2012.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Selim B., Won C., Yaggi H.K. Cardiovascular consequences of sleep apnea. Clin Chest Med. 2010;31:203–220. doi: 10.1016/j.ccm.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 16.Gottlieb D.J., Yenokyan G., Newman A.B. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;122:352–360. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vizzardi E., Sciatti E., Bonadei I. Obstructive sleep apnoea-hypopnoea and arrhythmias: new updates. J Cardiovasc Med. 2014 doi: 10.2459/JCM.0000000000000043. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Young T., Finn L., Peppard P.E. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31:1071–1078. [PMC free article] [PubMed] [Google Scholar]
- 19.Rich J., Raviv A., Raviv N. An epidemiologic study of snoring and all-cause mortality. Otolaryngol Head Neck Surg – Off J Am Acad Otolaryngol Head Neck Surg. 2011;145:341–346. doi: 10.1177/0194599811402475. [DOI] [PubMed] [Google Scholar]
- 20.Campos-Rodriguez F., Pena-Grinan N., Reyes-Nunez N. Mortality in obstructive sleep apnea-hypopnea patients treated with positive airway pressure. Chest. 2005;128:624–633. doi: 10.1378/chest.128.2.624. [DOI] [PubMed] [Google Scholar]
- 21.Banno K., Ramsey C., Walld R. Expenditure on health care in obese women with and without sleep apnea. Sleep. 2009;32:247–252. doi: 10.1093/sleep/32.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siccoli M.M., Pepperell J.C., Kohler M. Effects of continuous positive airway pressure on quality of life in patients with moderate to severe obstructive sleep apnea: data from a randomized controlled trial. Sleep. 2008;31:1551–1558. doi: 10.1093/sleep/31.11.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schein A.S., Kerkhoff A.C., Coronel C.C. Continuous positive airway pressure reduces blood pressure in patients with obstructive sleep apnea, a systematic review and meta-analysis with 1000 patients. J Hypertens. 2014;32:1762–1773. doi: 10.1097/HJH.0000000000000250. [DOI] [PubMed] [Google Scholar]
- 24.Sun X., Luo J., Xiao Y. Continuous positive airway pressure is associated with a decrease in pulmonary artery pressure in patients with obstructive sleep apnoea: a meta-analysis. Respirology. 2014;19:670–674. doi: 10.1111/resp.12314. [DOI] [PubMed] [Google Scholar]
- 25.Xu H., Yi H., Guan J. Effect of continuous positive airway pressure on lipid profile in patients with obstructive sleep apnea syndrome: a meta-analysis of randomized controlled trials. Atherosclerosis. 2014;234:446–453. doi: 10.1016/j.atherosclerosis.2014.03.034. [DOI] [PubMed] [Google Scholar]
- 26.Engleman H.M., Wild M.R. Improving CPAP use by patients with the sleep apnoea/hypopnoea syndrome (SAHS) Sleep Med Rev. 2003;7:81–99. doi: 10.1053/smrv.2001.0197. [DOI] [PubMed] [Google Scholar]
- 27.Weaver T.E., Sawyer A.M. Adherence to continuous positive airway pressure treatment for obstructive sleep apnoea: implications for future interventions. Indian J Med Res. 2010;131:245–258. [PMC free article] [PubMed] [Google Scholar]
- 28.Maurer J.T., Van de Heyning P., Lin H.S. Operative technique of upper airway stimulation: an implantable treatment of obstructive sleep apnea. Oper Tech Otolaryngol. 2012;23:227–233. [Google Scholar]
- 29.Won C.H., Li K.K., Guilleminault C. Surgical treatment of obstructive sleep apnea: upper airway and maxillomandibular surgery. Proc Am Thorac Soc. 2008;5:193–199. doi: 10.1513/pats.200708-121MG. [DOI] [PubMed] [Google Scholar]
- 30.Vanderveken O.M., Maurer J.T., Hohenhorst W. Evaluation of drug-induced sleep endoscopy as a patient selection tool for implanted upper airway stimulation for obstructive sleep apnea. J Clin Sleep Med – JCSM – Off Publ Am Acad Sleep Med. 2013;9:433–438. doi: 10.5664/jcsm.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dieltjens M., Vanderveken O.M., Hamans E. Treatment of obstructive sleep apnea using a custom-made titratable duobloc oral appliance: a prospective clinical study. Sleep Breath = Schlaf Atmung. 2013;17:565–572. doi: 10.1007/s11325-012-0721-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strollo P.J., Soose R.J., Maurer J.T. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370:139–149. doi: 10.1056/NEJMoa1308659. [DOI] [PubMed] [Google Scholar]
- 33.Hetzenecker A., Buchner S., Greimel T. Cardiac workload in patients with sleep-disordered breathing early after acute myocardial infarction. Chest. 2013;143:1294–1301. doi: 10.1378/chest.12-1930. [DOI] [PubMed] [Google Scholar]
- 34.Schober A.K., Neurath M.F., Harsch I.A. Prevalence of sleep apnoea in diabetic patients. Clin Respir J. 2011;5:165–172. doi: 10.1111/j.1752-699X.2010.00216.x. [DOI] [PubMed] [Google Scholar]
- 35.Lurie A. Cardiovascular disorders associated with obstructive sleep apnea. Adv Cardiol. 2011;46:197–266. doi: 10.1159/000325110. [DOI] [PubMed] [Google Scholar]
- 36.Pedrosa R.P., Drager L.F., Gonzaga C.C. Obstructive sleep apnea: the most common secondary cause of hypertension associated with resistant hypertension. Hypertension. 2011;58:811–817. doi: 10.1161/HYPERTENSIONAHA.111.179788. [DOI] [PubMed] [Google Scholar]
- 37.Sun H., Shi J., Li M. Impact of continuous positive airway pressure treatment on left ventricular ejection fraction in patients with obstructive sleep apnea: a meta-analysis of randomized controlled trials. PLoS One. 2013;8:e62298. doi: 10.1371/journal.pone.0062298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gami A.S., Somers V.K. Implications of obstructive sleep apnea for atrial fibrillation and sudden cardiac death. J Cardiovasc Electrophysiol. 2008;19:997–1003. doi: 10.1111/j.1540-8167.2008.01136.x. [DOI] [PubMed] [Google Scholar]
- 39.Albuquerque F.N., Calvin A.D., Sert Kuniyoshi F.H. Sleep-disordered breathing and excessive daytime sleepiness in patients with atrial fibrillation. Chest. 2012;141:967–973. doi: 10.1378/chest.11-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bitter T., Nolker G., Vogt J. Predictors of recurrence in patients undergoing cryoballoon ablation for treatment of atrial fibrillation: the independent role of sleep-disordered breathing. J Cardiovasc Electrophysiol. 2012;23:18–25. doi: 10.1111/j.1540-8167.2011.02148.x. [DOI] [PubMed] [Google Scholar]
- 41.Mansukhani M.P., Calvin A.D., Kolla B.P. The association between atrial fibrillation and stroke in patients with obstructive sleep apnea: a population-based case-control study. Sleep Med. 2013;14:243–246. doi: 10.1016/j.sleep.2012.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monahan K., Brewster J., Wang L. Relation of the severity of obstructive sleep apnea in response to anti-arrhythmic drugs in patients with atrial fibrillation or atrial flutter. Am J Cardiol. 2012;110:369–372. doi: 10.1016/j.amjcard.2012.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cho E.R., Kim H., Seo H.S. Obstructive sleep apnea as a risk factor for silent cerebral infarction. J Sleep Res. 2013;22:452–458. doi: 10.1111/jsr.12034. [DOI] [PubMed] [Google Scholar]
- 44.Otake K., Delaive K., Walld R. Cardiovascular medication use in patients with undiagnosed obstructive sleep apnoea. Thorax. 2002;57:417–422. doi: 10.1136/thorax.57.5.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.CDC. CDC.gov. Available at: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf Accessed 01.07.12.
- 46.Greenberg P.E., Kessler R.C., Birnbaum H.G. The economic burden of depression in the United States: how did it change between 1990 and 2000? J Clin Psychiatry. 2003;64:1465–1475. doi: 10.4088/jcp.v64n1211. [DOI] [PubMed] [Google Scholar]
- 47.Priou P., Le Vaillant M., Meslier N. Independent association between obstructive sleep apnea severity and glycated hemoglobin in adults without diabetes. Diabetes Care. 2012;35:1902–1906. doi: 10.2337/dc11-2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vasu T.S., Grewal R., Doghramji K. Obstructive sleep apnea syndrome and perioperative complications: a systematic review of the literature. J Clin Sleep Med – JCSM – Off Publ Am Acad Sleep Med. 2012;8:199–207. doi: 10.5664/jcsm.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dasta J.F., McLaughlin T.P., Mody S.H. Daily cost of an intensive care unit day: the contribution of mechanical ventilation. Crit Care Med. 2005;33:1266–1271. doi: 10.1097/01.ccm.0000164543.14619.00. [DOI] [PubMed] [Google Scholar]
- 50.Mutter T.C., Chateau D., Moffatt M. A matched cohort study of postoperative outcomes in obstructive sleep apnea. Anesthesiology. 2014;121:707–718. doi: 10.1097/ALN.0000000000000407. [DOI] [PubMed] [Google Scholar]
- 51.Narkiewicz K., Montano N., Cogliati C. Altered cardiovascular variability in obstructive sleep apnea. Circulation. 1998;98:1071–1077. doi: 10.1161/01.cir.98.11.1071. [DOI] [PubMed] [Google Scholar]
- 52.Luthje L., Andreas S. Obstructive sleep apnea and coronary artery disease. Sleep Med Rev. 2008;12:19–31. doi: 10.1016/j.smrv.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 53.Somers V.K., Dyken M.E., Clary M.P. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–1904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carlson J.T., Hedner J., Elam M. Augmented resting sympathetic activity in awake patients with obstructive sleep apnea. Chest. 1993;103:1763–1768. doi: 10.1378/chest.103.6.1763. [DOI] [PubMed] [Google Scholar]
- 55.Fletcher E.C. Sympathetic over activity in the etiology of hypertension of obstructive sleep apnea. Sleep. 2003;26:15–19. doi: 10.1093/sleep/26.1.15. [DOI] [PubMed] [Google Scholar]
- 56.Seetho I.W., Parker R.J., Craig S. Obstructive sleep apnea is associated with increased arterial stiffness in severe obesity. J Sleep Res. 2014 doi: 10.1111/jsr.12156. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 57.Lin Q.C., Chen L.D., Yu Y.H. Obstructive sleep apnea is associated with metabolic syndrome and inflammation. Eur Arch Otorhinolaryngol. 2014;271:825–831. doi: 10.1007/s00405-013-2669-8. [DOI] [PubMed] [Google Scholar]
- 58.Hall M.H., Okun M.L., Sowers M. Sleep is associated with the metabolic syndrome in a multi-ethnic cohort of midlife women: the SWAN Sleep Study. Sleep. 2012;35:783–790. doi: 10.5665/sleep.1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nichols G.A., Moler E.J. Metabolic syndrome components are associated with future medical costs independent of cardiovascular hospitalization and incident diabetes. Metab Syndr Relat Disord. 2011;9:127–133. doi: 10.1089/met.2010.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schmitt A.C., Cardoso M.R., Lopes H. Prevalence of metabolic syndrome and associated factors in women aged 35 to 65 years who were enrolled in a family health program in Brazil. Menopause. 2013;20:470–476. doi: 10.1097/gme.0b013e318272c938. [DOI] [PubMed] [Google Scholar]
- 61.Nieto F.J., Peppard P.E., Young T. Sleep-disordered breathing and cancer mortality: results from the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med. 2012;186:190–194. doi: 10.1164/rccm.201201-0130OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Campos-Rodriguez F., Martinez-Garcia M.A., Martinez M. Association between obstructive sleep apnea and cancer incidence in a large multicenter Spanish cohort. Am J Respir Crit Care Med. 2013;187:99–105. doi: 10.1164/rccm.201209-1671OC. [DOI] [PubMed] [Google Scholar]
- 63.Stundner O., Chiu Y.L., Ramachandran S.K. Sleep apnea adversely affects the outcome in patients who undergo posterior lumbar fusion: a population-based study. Bone Joint J. 2014;96-B:242–248. doi: 10.1302/0301-620X.96B2.31842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Memtsoudis S.G., Stundner O., Rasul R. The impact of sleep apnea on post-operative utilization of resources and adverse outcomes. Anesth Analg. 2014;118:407–418. doi: 10.1213/ANE.0000000000000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kaw R., Chung F., Pasupuleti V. Meta-analysis of association between obstructive sleep apnoea and postoperative outcome. Br J Anaesth. 2012;109:897–906. doi: 10.1093/bja/aes308. [DOI] [PubMed] [Google Scholar]
- 66.Finn L., Young T., Palta M. Sleep-disordered breathing and self-reported general health status in the Wisconsin Sleep Cohort Study. Sleep. 1998;21:701–706. [PubMed] [Google Scholar]
- 67.Baldwin C.M., Griffith K.A., Nieto F.J. The association of sleep-disordered breathing and sleep symptoms with quality of life in the Sleep Heart Health Study. Sleep. 2001;24:96–105. doi: 10.1093/sleep/24.1.96. [DOI] [PubMed] [Google Scholar]
- 68.Vennelle M., Engleman H.M., Douglas N.J. Sleepiness and sleep-related accidents in commercial bus drivers. Sleep Breath = Schlaf Atmung. 2010;14:39–42. doi: 10.1007/s11325-009-0277-z. [DOI] [PubMed] [Google Scholar]
- 69.Breugelmans J.G., Ford D.E., Smith P.L. Differences in patient and bed partner-assessed quality of life in sleep-disordered breathing. Am J Respir Crit Care Med. 2004;170:547–552. doi: 10.1164/rccm.200310-1421OC. [DOI] [PubMed] [Google Scholar]
- 70.El-Sherbini A.M., Bediwy A.S., El-Mitwalli A. Association between obstructive sleep apnea (OSA) and depression and the effect of continuous positive airway pressure (CPAP) treatment. Neuropsychiatric Dis Treat. 2011;7:715–721. doi: 10.2147/NDT.S26341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smith R., Ronald J., Delaive K. What are obstructive sleep apnea patients being treated for prior to this diagnosis? Chest. 2002;121:164–172. doi: 10.1378/chest.121.1.164. [DOI] [PubMed] [Google Scholar]
- 72.Leger D., Bayon V., Laaban J.P. Impact of sleep apnea on economics. Sleep Med Rev. 2012;16:455–462. doi: 10.1016/j.smrv.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 73.Karimi M., Eder D.N., Eskandari D. Impaired vigilance and increased accident rate in public transportation operators is associated with sleep disorders. Accid Anal Prev. 2013:208–214. doi: 10.1016/j.aap.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 74.Antonopoulos C.N., Sergentanis T.N., Daskalopoulou S.S. Nasal continuous positive airway pressure (nCPAP) treatment for obstructive sleep apnea, road traffic accidents and driving simulator performance: a meta-analysis. Sleep Med Rev. 2011;15:301–310. doi: 10.1016/j.smrv.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 75.Ellen R.L., Marshall S.C., Palayew M. Systematic review of motor vehicle crash risk in persons with sleep apnea. J Clin Sleep Med – JCSM – Off Publ Am Acad Sleep Med. 2006;2:193–200. [PubMed] [Google Scholar]
- 76.Tregear S., Reston J., Schoelles K. Obstructive sleep apnea and risk of motor vehicle crash: systematic review and meta-analysis. J Clin Sleep Med – JCSM – Off Publ Am Acad Sleep Med. 2009;5:573–581. [PMC free article] [PubMed] [Google Scholar]
- 77.George C.F.P. Reduction in motor vehicle collisions following treatment of sleep apnoea with nasal CPAP. Thorax. 2001;56:508–512. doi: 10.1136/thorax.56.7.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Karimi M., Hedner J., Lombardi C. Driving habits and risk factors for traffic accidents among sleep apnea patients – a European multi-centre cohort study. J Sleep Res. 2014 Dec;23:689–699. doi: 10.1111/jsr.12171. [DOI] [PubMed] [Google Scholar]
- 79.Sassani A., Findley L.J., Kryger M. Reducing motor-vehicle collisions, costs, and fatalities by treating obstructive sleep apnea syndrome. Sleep. 2004;27:453–458. doi: 10.1093/sleep/27.3.453. [DOI] [PubMed] [Google Scholar]
- 80.Lindberg E., Carter N., Gislason T. Role of snoring and daytime sleepiness in occupational accidents. Am J Respir Crit Care Med. 2001;164:2031–2035. doi: 10.1164/ajrccm.164.11.2102028. [DOI] [PubMed] [Google Scholar]
- 81.Omachi T.A., Claman D.M., Blanc P.D. Obstructive sleep apnea: a risk factor for work disability. Sleep. 2009;32:791–798. doi: 10.1093/sleep/32.6.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Guglielmi O., Jurado-Gamez B., Gude F. Job stress, burnout, and job satisfaction in sleep apnea patients. Sleep Med. 2014;15:1025–1030. doi: 10.1016/j.sleep.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 83.Jurado-Gamez B., Guglielmi O., Gude F. Workplace accidents, absenteeism and productivity in patients with sleep apnea. Arch Bronconeumol. 2015 May;51:213–218. doi: 10.1016/j.arbres.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 84.Roger V.L., Go A.S., Lloyd-Jones D.M. Heart disease and stroke statistics – 2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Taylor T.N., Davis P.H., Torner J.C. Lifetime cost of stroke in the United States. Stroke – J Cereb Circ. 1996;27:1459–1466. doi: 10.1161/01.str.27.9.1459. [DOI] [PubMed] [Google Scholar]
- 86.Albarrak M., Banno K., Sabbagh A.A. Utilization of healthcare resources in obstructive sleep apnea syndrome: a 5-year follow-up study in men using CPAP. Sleep. 2005;28:1306–1311. doi: 10.1093/sleep/28.10.1306. [DOI] [PubMed] [Google Scholar]
- 87.Tarasiuk A., Greenberg-Dotan S., Brin Y.S. Determinants affecting health-care utilization in obstructive sleep apnea syndrome patients. Chest. 2005;128:1310–1314. doi: 10.1378/chest.128.3.1310. [DOI] [PubMed] [Google Scholar]
- 88.Kapur V., Blough D.K., Sandblom R.E. The medical cost of undiagnosed sleep apnea. Sleep. 1999;22:749–755. doi: 10.1093/sleep/22.6.749. [DOI] [PubMed] [Google Scholar]
- 89.Berger M.B., Sullivan W., Owen R. Schneider National Inc., Precision Pulmonary Diagnostics Inc., and Definity Health Corp.; Houston, TX: 2006. A Corporate Driven Sleep Apnea Detection and Treatment Program: Results and Challenges. [Google Scholar]
- 90.Hoffman B., Wingenbach D.D., Kagey A.N. The long-term health plan and disability cost benefit of obstructive sleep apnea treatment in a commercial motor vehicle driver population. J Occup Environ Med/Am Coll Occup Environ Med. 2010;52:473–477. doi: 10.1097/JOM.0b013e3181dbc8ab. [DOI] [PubMed] [Google Scholar]
- 91.Kim H., Kim M.S., Lee J.E. Treatment outcomes and compliance according to obesity in patients with obstructive sleep apnea. Eur Arch Otorhinolaryngol. 2013 doi: 10.1007/s00405-013-2397-0. [DOI] [PubMed] [Google Scholar]
- 92.Caples S.M., Rowley J.A., Prinsell J.R. Surgical modifications of the upper airway for obstructive sleep apnea in adults: a systematic review and meta-analysis. Sleep. 2010;33:1396–1407. doi: 10.1093/sleep/33.10.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kezirian E.J., Weaver E.M., Yueh B. Incidence of serious complications after uvulopalatopharyngoplasty. Laryngoscope. 2004;114:450–453. doi: 10.1097/00005537-200403000-00012. [DOI] [PubMed] [Google Scholar]
- 94.Camacho M., Certal V., Brietzke S.E. Tracheostomy as a treatment for adult obstructive sleep apnea: a systematic review and meta-analysis. Laryngoscope. 2014 doi: 10.1002/lary.24433. [DOI] [PubMed] [Google Scholar]
- 95.Phillips C.L., Grunstein R.R., Darendeliler M.A. Health outcomes of continuous positive airway pressure versus oral appliance treatment for obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med. 2013;187:879–887. doi: 10.1164/rccm.201212-2223OC. [DOI] [PubMed] [Google Scholar]
- 96.Eastwood P.R., Barnes M., Walsh J.H. Treating obstructive sleep apnea with hypoglossal nerve stimulation. Sleep. 2011;34:1479–1486. doi: 10.5665/sleep.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kezirian E.J., Goding G.S., Malhotra A. Hypoglossal nerve stimulation improves obstructive sleep apnea: 12-month outcomes. J Sleep Res. 2014;23:77–83. doi: 10.1111/jsr.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lloberes P., Gabriel S., Espinel E. A randomized controlled study of CPAP effect on plasma aldosterone concentration in patients with resistant hypertension and obstructive sleep apnea. J Hypertens. 2014:32. doi: 10.1097/HJH.0000000000000238. [DOI] [PubMed] [Google Scholar]
- 99.Goyal S.K., Wang L., Upender R. Severity of obstructive sleep apnea influences the effect of genotype on response to anti-arrhythmic drug therapy for atrial fibrillation. J Clin Sleep Med – JCSM – Off Publ Am Acad Sleep Med. 2014;10:503–507. doi: 10.5664/jcsm.3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Guest J.F., Panca M., Sladkevicius E. Clinical outcomes and cost-effectiveness of continuous positive airway pressure to manage obstructive sleep apnea in patients with type 2 diabetes in the U.K. Diabetes Care. 2014;37:1263–1271. doi: 10.2337/dc13-2539. [DOI] [PubMed] [Google Scholar]
- 101.Gagnadoux F., Le Vaillant M., Goupil F. Depressive symptoms before and after long-term CPAP therapy in patients with sleep apnea. Chest. 2014;145:1025–1031. doi: 10.1378/chest.13-2373. [DOI] [PubMed] [Google Scholar]
- 102.Gaddam S., Gunukula S., Mador M. Post-operative outcomes in adult obstructive sleep apnea patients undergoing non-upper airway surgery: a systematic review and meta-analysis. Sleep Breath = Schlaf Atmung. 2013 doi: 10.1007/s11325-013-0925-1. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 103.Martinez-Garcia M.A., Campos-Rodriguez F., Duran-Cantolla J. Obstructive sleep apnea is associated with cancer mortality in younger patients. Sleep Med. 2014;15:742–748. doi: 10.1016/j.sleep.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 104.Chen J.C., Hwang J.H. Sleep apnea increased incidence of primary central nervous system cancers: a nationwide cohort study. Sleep Med. 2014;15:749–754. doi: 10.1016/j.sleep.2013.11.782. [DOI] [PubMed] [Google Scholar]
- 105.Marshall N.S., Wong K.K., Cullen S.R. Sleep apnea and 20-year follow-up of all-cause mortality, stroke, cancer incidence and mortality in Busselton Health Study cohort. J Clin Sleep Med – JCSM – Off Publ Am Acad Sleep Med. 2014;10:355–362. doi: 10.5664/jcsm.3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Warkentin L.M., Majumdar S.R., Johnson J.A. Predicters of health-related quality of life in 500 severely obese patients. Obesity (Silver Spring) 2014;22:1367–1372. doi: 10.1002/oby.20694. [DOI] [PubMed] [Google Scholar]