Abstract
Sporadic vestibular schwannoma (acoustic neuroma) is a benign tumor arising from cochleovestibular nerve. Nowadays, various specialties and medical centers are treating this disease, and the multidisciplinary collaboration is the trend. In an effort to promote a uniform standard for reporting clinical results, even for treatment indications, the mainly controversies were posed and discussed during the 7th International Conference on acoustic neuroma, and the agreement was summarized by the Committee of this conference. The main symptoms grading and tumor stage should note its name of classification for making them comparable. The goal of the modern managements for vestibular schwannoma is to improve the quality of life with lower mortality, lower morbidity and better neurological function preservation. The experience of surgical team and their preference might be a major factor for the outcome. Because of lacking of long-term follow-up large data after radiotherapy, and with the development of microsurgery, radiotherapy is now less recommended except for recurrent cases or elderly patients.
Keywords: Sporadic acoustic neuroma, Vestibular schwannoma, Management, Symptoms grading, Tumor stage, Microsurgery, Radiotherapy
Introduction
The 7th International Conference on acoustic neuroma was held on April 12–15, 2015 in Shanghai, China. This series conference, where gathers the outstanding experts worldwide, is the most remarkable meeting in the field of acoustic neuroma. The 7th conference was co-hosted by the Xinhua Hospital, People's Liberation Army General Hospital, Tiantan Hospital, and Huashan Hospital. There were more than 700 participants, including 345 foreign attendees from 41 countries, composed of neurosurgeons, neurotologists, radiotherapists, neuro-radiologists, audiologists, plastic surgeons, and basic researchers. After several multidisciplinary discussions, some ancient controversies reached an agreement, and this consensus summarized by the committee of this conference.
Nomenclature
Acoustic neuroma (AN) is also known as vestibular schwannoma, since this benign tumor almost originates from superior or inferior vestibular branch of the cochleovestibular nerve in the internal auditory canal (IAC).1 Moreover, the tumor is schwannoma in pathology rather than neuroma. The two nomenclatures are both accepted, however, vesctibular schwannoma (VS) is preferable.
Sporadic Vestibular Schwannoma is basically distinct from Neurofibromatosis type 2 (NF2). If it is not specifically noted, VS refers to the sporadic vestibular schwannoma in the context.
Cystic vestibular schwannnoma (CVS) should be distinguish from solid vestibular schwannoma (SVS) because of the their variant clinical, radiological, histopathological features and surgical outcomes.2, 3, 4, 5 CVS can be peripherally located thin-walled tumors, and centrally located thick-walled tumors based on CT or MRI images. CVS frequently presents rapid progression of symptoms with facial nerve involvement.
Standardization of main symptoms grading
The purpose of standardizing the grading of the main symptoms is to unify the description of patients' status, and then to make analyzing management strategy and outcome more precisely. Classically, the AAO-HNS Hearing Classification System,6 House-Brackmann Facial Nerve Grading System,7 Tinnitus Handicap Inventory8 and Dizziness Handicap Inventory9 are widely accepted and used for VS. But the two latters are in the form of questionnaire which is more complicated, this consensus attempts to classify them into four grades as alternatives (Table 1, Table 2).
Table 1.
Tinnitus grading system for acoustic neuromas.
| Grade | Descriptions |
|---|---|
| Ⅰ | No tinnitus |
| Ⅱ | Intermittent or mild tinnitus, can only be heard when the ambient noise is low |
| Ⅲ | Persistent or moderate tinnitus, can be heard every day |
| Ⅳ | Persistent and severe tinnitus, interfere with work and sleep |
Table 2.
Dizziness grading system for acoustic neuromas.
| Grade | Descriptions |
|---|---|
| Ⅰ | No dizziness or imbalance |
| Ⅱ | Occasional and mild dizziness or imbalance |
| Ⅲ | Persistent or moderate vertigo or imbalance |
| Ⅳ | Persistent and severe dizziness or imbalance, disturbing daily life |
Tumor size and stages
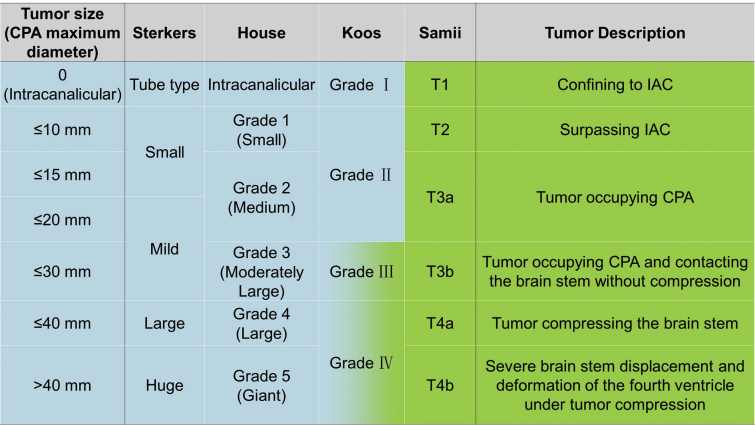
Several stage grading systems have been reported according to tumor size.10, 11, 12, 13 Generally, the tumor size should be measured on MRI images, and the maximum diameter (also called tumor diameter) means the one measured in cerebellopontine angle (CPA) along the long axis of tumor. The type of tumor within the IAC should be classified separately. Four commonly used tumor grading are Sterkers classification, House classification, Koos classification and Samii classification (Fig. 1).
Fig. 1.
Main grading systems for acoustic neuromas. The classifications on the left side (blue area) are mainly based on tumor size, while those on the right side (green area) are based on the anatomical relationship around the tumor. Koos classification combines the tumor size and anatomical relationship for larger tumors.
As an alternative, rather than using a particular staging systems mentioned above, indicative of intracanalicular type (with size in millimeters) and simple reference to tumor size in the CPA in 10 mm increments might simplify the VS grading and render more consistent tumor size reporting from all centers.
Management goal and strategy
The goal of the modern managements for VS is to improve the quality of life with lower mortality, lower morbidity and better neurological function preservation. VS management is no longer simply limited to surgical resection. The conception of “wait and scan” is accepted worldwide, especially for the small, primary and sporadic tumor.14, 15, 16, 17 Radiotherapy is accepted as mainstream method for the patients with surgical contra-indication.18, 19 The management strategy becomes individual, which mainly depends on the radiological features (cystic or not, tumor size and extension), the biologic feature (tumor growth rate), the severity of symptoms (ipsilateral and contralateral hearing, facial function, other complications), the patient's age, and the general situation and expectations.17, 20, 21, 22, 23, 24, 25, 26
Small tumors with useful hearing
Several long term follow-up studies have found that, during the follow-up of small tumor, the possibility of hearing loss after 5 years was about 70% whether a tumor was increased or not.27, 28, 29 For the small tumor, the hearing preservation probability after surgery is about 60% in experienced institutes if the fundus of IAC free of tumor involvement.15, 30, 31, 32, 33, 34, 35 Attending experts in the 7th conference agreed that for younger patients, with grade B or grade A hearing level and free IAC fundus, surgical intervention can be considered earlier. However, for small tumors involving the IAC fundus, the hearing preservation rate is lower than 50% even in experienced institute.36 Therefore, this consensus proposes that a planned follow-up should be the first choice under this circumstance in consideration of quality of life. However, surgical procedure is reasonable to perform in those patients who are well-informed and willing to take the risk of surgery regarding the situation and options for management.
Small tumors with refractory vertigo or imbalance
In such a situation, regular treatment and observation must go through for 6 months and whether the quality of life of such patient is affected by vertigo or imbalance is determined. If vertigo cannot be alleviated in the short term, surgical intervention should be taken into account.
Small tumors without useful hearing in young people
It has been reported that VS grows slowly. According to a large number of reports, facial paralysis rate after surgery for small tumors with no useful hearing in young people was 10%.37, 38, 39, 40, 41 Therefore, young patients who might be expected to live 25–30 years (or longer) with slow growing tumor and without hearing might be recommended to be followed for at least one year. However, surgical procedure is reasonable to perform in those patients who are well-informed and willing to take the risk of surgery regarding their situation and options for management.
CVS
VS with cystic degeneration or cystic degeneration appears during follow-up often means rapid tumor growth.15, 42, 43 Moreover, this type of VS is less sensitive to radiotherapy has been documented.44, 45 Therefore, the optimal choice of treatment is surgery for these patients and should be performed as soon as possible.
Difference among medical centers
More important, not all centers where manage VS patients achieve comparable results in terms of surgical treatment. The experience of surgical team and their preference might be a major factor. The ratio of post-operative hearing preservation for small tumors can be apparent various among centers, also for the relationship between hearing preservation and internal auditory canal (IAC) fundal involvement. Management strategy should be specific for a patient with VS in any center, in this way the patient could make an informed decision in their particular situation.
Surgical approaches
There are three main surgical approaches, including translabyrinthine approach, retrosigmoid approach, and middle fossa approach. The selection of approach should defer to surgeon's preference and experience. It is generally accepted that the retrosigmoid approach is recommended when hearing preservation is considered. Nowadays, in virtue of endoscopic technique and advanced surgical experience, the extent or/and IAC fundus involvement are no longer the opposition for this approach because some centers achieve excellent hearing outcomes in patients with small tumors with fundus involvement.46 The translabyrinthine, or enlarged translabyrinthine,47, 48 or modified translabyrinthine approach, as well as in combination with a retrosigmoid exposure,49 is appropriate to removal of VSs for any size. Because of the endoscope assisted technique in the retrosigmoid approach, the middle fossa approach becomes less selected for hearing preservation, however, this approach is still the main approach for moderate or small tumor in some centers with excellent outcome.32, 50
Evaluation of tumor resection
Tumor resection only includes total resection, near total resection, subtotal resection, and partial resection. Total resection means no tumor residue. Near-total resection (NTR) was assigned when a small piece of tumor remnant (size was no greater than 25 mm2 and 2 mm thick, and could not be detected by routine MRI) was intentionally left in situ in an effort to preserve neural integrity. Subtotal resection (STR) was used to describe any situation where less than NTR was performed.51 Partial resection (PR) was defined and used a percentage of the original tumor when Tumor residues greater than >5%.52
The size of the residual tumor is indicated by the vertical diameter of each other. Meanwhile, the location of the residual tumor should be documented, for example, residue in IAC, in CPA, on brainstem surface, or on cerebellar surface, etc.
Evaluation and follow-up after radiotherapy
Long-term follow-up is mandatory after tumor radiotherapy which just controls tumor growth. So far, there is a lack of long-term follow-up large data. It has been documented that in longer term follow up after fractionated stereotactic radiotherapy, 30% of tumors continued to grow (defined as at least 15% increase in tumor volume).53 Furthermore, either the tumor did or did not increase in size after the typical 18 month time frame after radiation in which tumor edema may have occurred.54, 55 Thus, for the young people with VS, radiotherapy is not recommended. Radiotherapy is applied to recurrent cases after surgery or elderly patients. The grade of radiotherapy outcome is shown in Table 3.
Table 3.
Radiotherapy outcome for acoustic neuromas.
| Grade | Description |
|---|---|
| 1 | Tumor control, tumor diameter is reduced by more than 2 mm, and the volume is reduced by more than 10% |
| 2 | Tumor stability, tumor diameter reduction is less than 2 mm, and the volume reduction is less than 10% |
| 3 | Tumor growth, the tumor does not shrink or tumor size re-increases after shrinking |
Footnotes
Peer review under responsibility of Chinese Medical Association.
Contributor Information
Hao Wu, Email: wuhao622@sina.cn.
Liwei Zhang, Email: zlwtt@aliyun.com.
Dongyi Han, Email: hdy301@263.net.
Ying Mao, Email: maoying@fudan.edu.cn.
References
- 1.Pineda A., Feder B.H. Acoustic neuroma: a misnomer. Am Surg. 1967;33:40–43. [PubMed] [Google Scholar]
- 2.Benech F., Perez R., Fontanella M.M., Morra B., Albera R., Ducati A. Cystic versus solid vestibular schwannomas: a series of 80 grade III-IV patients. Neurosurg Rev. 2005;28:209–213. doi: 10.1007/s10143-005-0380-y. [DOI] [PubMed] [Google Scholar]
- 3.Moon K.S., Jung S., Seo S.K. Cystic vestibular schwannomas: a possible role of matrix metalloproteinase-2 in cyst development and unfavorable surgical outcome. J Neurosurg. 2007;106:866–871. doi: 10.3171/jns.2007.106.5.866. [DOI] [PubMed] [Google Scholar]
- 4.Wandong S., Meng L., Xingang L. Cystic acoustic neuroma. J Clin Neurosci. 2005;12:253–255. doi: 10.1016/j.jocn.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 5.Tang I.P., Freeman S.R., Rutherford S.A., King A.T., Ramsden R.T., Lloyd S.K. Surgical outcomes in cystic vestibular schwannoma versus solid vestibular schwannoma. Otol Neurotol. 2014;35:1266–1270. doi: 10.1097/MAO.0000000000000435. [DOI] [PubMed] [Google Scholar]
- 6.Committee on hearing and equilibrium guidelines for the evaluation of hearing preservation in acoustic neuroma (vestibular schwannoma) Otolaryngol Head Neck Surg. 1995;113:179–180. doi: 10.1016/S0194-5998(95)70101-X. [DOI] [PubMed] [Google Scholar]
- 7.House J.W., Brackmann D.E., House J.W., Brackmann D.E. Facial nerve grading system. Otolaryngol Head Neck Surg. 1985;93:146–147. doi: 10.1177/019459988509300202. [DOI] [PubMed] [Google Scholar]
- 8.Newman C.W., Jacobson G.P., Spitzer J.B. Development of the tinnitus handicap index. Arch Otolaryngol Head Neck Surg. 1996;122:143–148. doi: 10.1001/archotol.1996.01890140029007. [DOI] [PubMed] [Google Scholar]
- 9.Jacobson G.P., Newman C.W. Development of the dizziness handicap index. Arch Otolaryngol Head Neck Surg. 1990;116:424–427. doi: 10.1001/archotol.1990.01870040046011. [DOI] [PubMed] [Google Scholar]
- 10.Sterkers J.M., Morrison G.A., Sterkers O., El-Dine M.M. Preservation of facial, cochlear, and other nerve functions in acoustic neuroma treatment. Otolaryngol Head Neck Surg. 1994;110:146–155. doi: 10.1177/019459989411000202. [DOI] [PubMed] [Google Scholar]
- 11.Hitselberger W.E., House W.F. Classification of acoustic neuromas. Arch Otolaryngol. 1966;84:245–246. [PubMed] [Google Scholar]
- 12.Koos W.T., Day J.D., Matula C., Levy D.I. Neurotopographic considerations in the microsurgical treatment of small acoustic neurinomas. J Neurosurg. 1998;88:506–512. doi: 10.3171/jns.1998.88.3.0506. [DOI] [PubMed] [Google Scholar]
- 13.Samii M., Matthies C. Management of 1000 vestibular schwannomas (acoustic neuromas): surgical management and results with an emphasis on complications and how to avoid them. Neurosurgery. 1997;40:11–21. doi: 10.1097/00006123-199701000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Silverstein H., McDaniel A., Norrell H., Wazen J. Conservative management of acoustic neuroma in the elderly patient. Laryngoscope. 1985;95:766–770. [PubMed] [Google Scholar]
- 15.Bozorg Grayeli A., Kalamarides M., Ferrary E. Conservative management versus surgery for small vestibular schwannomas. Acta Otolaryngol. 2005;125:1063–1068. doi: 10.1080/00016480510038013. [DOI] [PubMed] [Google Scholar]
- 16.Hoistad D.L., Melnik G., Mamikoglu B., Battista R., O'Connor C.A., Wiet R.J. Update on conservative management of acoustic neuroma. Otol Neurotol. 2001;22:682–685. doi: 10.1097/00129492-200109000-00021. [DOI] [PubMed] [Google Scholar]
- 17.Mackeith S.A., Kerr R.S., Milford C.A. Trends in acoustic neuroma management: a 20-year review of the oxford skull base clinic. J Neurol Surg B Skull Base. 2013;74:194–200. doi: 10.1055/s-0033-1342919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pollock B.E., Driscoll C.L., Foote R.L. Patient outcomes after vestibular schwannoma management: a prospective comparison of microsurgical resection and stereotactic radiosurgery. Neurosurgery. 2006;59:77–85. doi: 10.1227/01.NEU.0000219217.14930.14. [DOI] [PubMed] [Google Scholar]
- 19.Boari N., Bailo M., Gagliardi F. Gamma Knife radiosurgery for vestibular schwannoma: clinical results at long-term follow-up in a series of 379 patients. J Neurosurg. 2014;121:123–142. doi: 10.3171/2014.8.GKS141506. [DOI] [PubMed] [Google Scholar]
- 20.Lee C.C., Wu H.M., Chung W.Y., Chen C.J., Pan D.H., Hsu S.P. Microsurgery for vestibular schwannoma after Gamma Knife surgery: challenges and treatment strategies. J Neurosurg. 2014;121:150–159. doi: 10.3171/2014.8.GKS141312. [DOI] [PubMed] [Google Scholar]
- 21.Muzevic D., Legcevic J., Splavski B., Cayé-Thomasen P. Stereotactic radiotherapy for vestibular schwannoma. Cochrane Database Syst Rev. 2014;12 doi: 10.1002/14651858.CD009897.pub2. CD009897–CD009897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Régis J., Carron R., Park M.C. Wait-and-see strategy compared with proactive Gamma Knife surgery in patients with intracanalicular vestibular schwannomas: clinical article. J Neurosurg. 2013;119:105–111. doi: 10.3171/2010.8.GKS101058. [DOI] [PubMed] [Google Scholar]
- 23.Zygourakis C.C., Oh T., Sun M.Z., Barani I., Kahn J.G., Parsa A.T. Surgery is cost-effective treatment for young patients with vestibular schwannomas: decision tree modeling of surgery, radiation, and observation. Neurosurg Focus. 2014;37:E8. doi: 10.3171/2014.8.FOCUS14435. [DOI] [PubMed] [Google Scholar]
- 24.Bittencourt A.G., Alves R.D., Ikari L.S., Burke P.R., Gebrim E.M., Bento R.F. Intracochlear schwannoma: diagnosis and management. Int Arch Otorhinolaryngol. 2014;18:322–324. doi: 10.1055/s-0033-1364170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van de Langenberg R., Hanssens P.E., Van Overbeeke J.J. Management of large vestibular schwannoma. Part I. Planned subtotal resection followed by Gamma Knife surgery: radiological and clinical aspects. J Neurosurg. 2011;115:875–884. doi: 10.3171/2011.6.JNS101958. [DOI] [PubMed] [Google Scholar]
- 26.Van de Langenberg R., Hanssens P.E., Verheul J.B. Management of large vestibular schwannoma. Part II. Primary Gamma Knife surgery: radiological and clinical aspects. J Neurosurg. 2011;115:885–893. doi: 10.3171/2011.6.JNS101963. [DOI] [PubMed] [Google Scholar]
- 27.Breivik C.N., Nilsen R.M., Myrseth E. Conservative management or gamma knife radiosurgery for vestibular schwannoma: tumor growth, symptoms, and quality of life. Neurosurgery. 2013;73:48–56. doi: 10.1227/01.neu.0000429862.50018.b9. discussion 56–57. [DOI] [PubMed] [Google Scholar]
- 28.Fayad J.N., Semaan M.T., Lin J., Berliner K.I., Brackmann D.E. Conservative management of vestibular schwannoma: expectations based on the length of the observation period. Otol Neurotol. 2014;35:1258–1265. doi: 10.1097/MAO.0000000000000285. [DOI] [PubMed] [Google Scholar]
- 29.Combs S.E., Welzel T., Kessel K. Hearing preservation after radiotherapy for vestibular schwannomas is comparable to hearing deterioration in healthy adults and is accompanied by local tumor control and a highly preserved quality of life (QOL) as patients' self-reported outcome. Radiother Oncol. 2013;106:175–180. doi: 10.1016/j.radonc.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 30.Samii M., Gerganov V., Samii A. Hearing preservation after complete microsurgical removal in vestibular schwannomas. Prog Neurol Surg. 2008;21:136–141. doi: 10.1159/000156900. [DOI] [PubMed] [Google Scholar]
- 31.Khrais T., Sanna M. Hearing preservation surgery in vestibular schwannoma. J Laryngol Otol. 2006;120:366–370. doi: 10.1017/S002221510600332X. [DOI] [PubMed] [Google Scholar]
- 32.Arts H.A., Telian S.A., El-kashlan H., Thompson G.B. Hearing preservation and facial nerve outcomes in vestibular schwannoma surgery: results using the middle cranial fossa approach. Otol Neurotol. 2006;27:234–241. doi: 10.1097/01.mao.0000185153.54457.16. [DOI] [PubMed] [Google Scholar]
- 33.Woodson E.A., Dempewolf R.D., Gubbels S.P. Long-term hearing preservation after microsurgical excision of vestibular schwannoma. Otol Neurotol. 2010;31:1144–1152. doi: 10.1097/MAO.0b013e3181edb8b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamakami I., Yoshinoir H., Saeki N., Wada M., Oka N. Hearing preservation and intraoperative auditory brainstem response and cochlear nerve compound action potential monitoring in the removal of small acoustic neuroma via the retrosigmoid approach. J Neurol Neurosurg Psychiatry. 2009;80:218–227. doi: 10.1136/jnnp.2008.156919. [DOI] [PubMed] [Google Scholar]
- 35.Nguyen Q.T., Wu A.P., Mastrodimos B., Cueva R.A. Impact of fundal extension on hearing after surgery for vestibular schwannomas. Otol Neurotol. 2012;33:455–458. doi: 10.1097/MAO.0b013e318245cf01. [DOI] [PubMed] [Google Scholar]
- 36.Goddard J.C., Schwartz M.S., Friedman R.A. Fundal fluid as a predictor of hearing preservation in the middle cranial fossa approach for vestibular schwannoma. Otol Neurotol. 2010;31:1128–1134. doi: 10.1097/MAO.0b013e3181e8fc3f. [DOI] [PubMed] [Google Scholar]
- 37.Samii M., Gerganov V., Samii A. Improved preservation of hearing and facial nerve function in vestibular schwannoma surgery via the retrosigmoid approach in a series of 200 patients. J Neurosurg. 2006;105:527–535. doi: 10.3171/jns.2006.105.4.527. [DOI] [PubMed] [Google Scholar]
- 38.Brackmann D.E., Cullen R.D., Fisher L.M. Facial nerve function after translabyrinthine vestibular schwannoma surgery. Otolaryngol Head Neck Surg. 2007;136:773–777. doi: 10.1016/j.otohns.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 39.Anaizi A.N., Gantwerker E.A., Pensak M.L., Theodosopoulos P.V. Facial nerve preservation surgery for koos grade 3 and 4 vestibular schwannomas. Neurosurgery. 2014;75:671–675. doi: 10.1227/NEU.0000000000000547. discussion 676–677; quiz 677. [DOI] [PubMed] [Google Scholar]
- 40.Dunn I.F., Bi W.L., Erkmen K. Medial acoustic neuromas: clinical and surgical implications. J Neurosurg. 2014;120:1095–1104. doi: 10.3171/2014.1.JNS131701. [DOI] [PubMed] [Google Scholar]
- 41.Springborg J.B., Fugleholm K., Poulsgaard L., Cayé-Thomasen P., Thomsen J., Stangerup S.E. Outcome after translabyrinthine surgery for vestibular schwannomas: report on 1244 patients. J Neurol Surg B Skull Base. 2012;73:168–174. doi: 10.1055/s-0032-1301403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Charabi S., Mantoni M., Tos M., Thomsen J. Cystic vestibular schwannomas: neuroimaging and growth rate. J Laryngol Otol. 1994;108:375–379. doi: 10.1017/s0022215100126854. [DOI] [PubMed] [Google Scholar]
- 43.Nutik S.L., Babb M.J. Determinants of tumor size and growth in vestibular schwannomas. J Neurosurg. 2001;94:922–926. doi: 10.3171/jns.2001.94.6.0922. [DOI] [PubMed] [Google Scholar]
- 44.Delsanti C., Regis J. Cystic vestibular schwannomas. Neurochirurgie. 2004;50:401–406. [PubMed] [Google Scholar]
- 45.Link M.J., Driscoll C.L., Foote R.L., Pollock B.E. Radiation therapy and radiosurgery for vestibular schwannomas: indications, techniques, and results. Otolaryngol Clin North Am. 2012;45:353–366. doi: 10.1016/j.otc.2011.12.006. viii–ix. [DOI] [PubMed] [Google Scholar]
- 46.Kari E., Friedman R.A. Hearing preservation: microsurgery. Curr Opin Otolaryngol Head Neck Surg. 2012;20:358–366. doi: 10.1097/MOO.0b013e3283579673. [DOI] [PubMed] [Google Scholar]
- 47.Wu H., Sterkers J. Translabyrinthine removal of large acoustic neuromas in young adults. Auris Nasus Larynx. 2000;27:201–205. doi: 10.1016/s0385-8146(00)00057-2. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Z., Wang Z., Huang Q., Yang J., Wu H. Removal of large or giant sporadic vestibular schwannomas via translabyrinthine approach: a report of 115 cases. ORL J Otorhinolaryngol Relat Spec. 2012;74:271–277. doi: 10.1159/000343791. [DOI] [PubMed] [Google Scholar]
- 49.Zou P., Zhao L., Chen P. Functional outcome and postoperative complications after the microsurgical removal of large vestibular schwannomas via the retrosigmoid approach: a meta-analysis. Neurosurg Rev. 2014;37:15–21. doi: 10.1007/s10143-013-0485-7. [DOI] [PubMed] [Google Scholar]
- 50.Wang A.C., Chinn S.B., Than K.D. Durability of hearing preservation after microsurgical treatment of vestibular schwannoma using the middle cranial fossa approach. J Neurosurg. 2013;119:131–138. doi: 10.3171/2013.1.JNS1297. [DOI] [PubMed] [Google Scholar]
- 51.Carlson M.L., Van Abel K.M., Driscoll C.L. Magnetic resonance imaging surveillance following vestibular schwannoma resection. Laryngoscope. 2012;122:378–388. doi: 10.1002/lary.22411. [DOI] [PubMed] [Google Scholar]
- 52.Schwartz M.S., Kari E., Strickland B.M. Evaluation of the increased use of partial resection of large vestibular schwanommas: facial nerve outcomes and recurrence/regrowth rates. Otol Neurotol. 2013;34:1456–1464. doi: 10.1097/MAO.0b013e3182976552. [DOI] [PubMed] [Google Scholar]
- 53.Kapoor S., Batra S., Carson K. Long-term outcomes of vestibular schwannomas treated with fractionated stereotactic radiotherapy: an institutional experience. Int J Radiat Oncol Biolphys. 2011;81:647–653. doi: 10.1016/j.ijrobp.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hasegawa T., Kida Y., Kato T., Iizuka H., Kuramitsu S., Yamamoto T. Long-term safety and efficacy of stereotactic radiosurgery for vestibular schwannomas: evaluation of 440 patients more than 10 years after treatment with Gamma Knife surgery. J Neurosurg. 2013;118:557–565. doi: 10.3171/2012.10.JNS12523. [DOI] [PubMed] [Google Scholar]
- 55.Maniakas A., Saliba I. Microsurgery versus stereotactic radiation for small vestibular schwannomas: a meta-analysis of patients with more than 5 years' follow-up. Otol Neurotol. 2012;33:1611–1620. doi: 10.1097/MAO.0b013e31826dbd02. [DOI] [PubMed] [Google Scholar]