Abstract
Objective
To compare and contrast our experience with middle cranial fossa approach (MFR) and transmastoid approach with capping of the dehiscence (TMR) of superior semicircular canal dehiscence and to determine guidelines to help guide management of these patients.
Methods
All patients from 2005 to 2014 with symptomatic superior semicircular canal dehiscence syndrome with dehiscence demonstrated on CT scan of the temporal bone who underwent surgical repair and had a minimum 3 months of follow up. Surgical repair via the MFR or TMR, preoperative CT temporal bone, preoperative, and postoperative cervical vestibular evoked myogenic potential (cVEMP) testing and anterior canal video head thrust testing (vHIT). Success of repair was stratified as complete success, moderate success, mild success, or failure based on resolution of all symptoms, the chief complaint, some symptoms, or no improvement, respectively.
Results
A total of 29 ears in 27 patients underwent surgical repair of canal dehiscence. Complete or moderate success was seen in 71% of the MFR group compared to 80% of the TMR group. There were zero failures with the MFR group and no major intracranial complications. There were 2 failures out of 15 ears that underwent the TMR. Residual symptoms were most commonly vertigo or disequilibrium in the MFR and aural fullness or autophony in the TMR groups, respectively. MFR hospital stay was approximately 2 days longer. Average cVEMP threshold shifted 18 dB with surgical correction in the MFR group. A 29 dB average shift was seen in the TMR group. The MFR group had a significant reduction in their anterior canal gain compared to the TMR group.
Conclusions
TMR is a less invasive alternative to MFR. However, in our series, we have not seen any intracranial complications (aphasia, stroke, seizures, etc.) in our MFR patients. Interestingly, vestibular symptoms were better addressed than audiological symptoms by the TMR suggesting its usefulness as a less invasive option for patients with primarily vestibular complaints. Residual auditory symptoms in TMR patients may be due to the flow of acoustic energy from the superior canal to the mastoid cavity through an incompletely sealed third window.
Keywords: Superior canal dehiscence, Plugging, Middle fossa, Transmastoid, Cartilage graft
Introduction
Minor and colleagues formally characterized patients with superior semicircular canal dehiscence in 1998.1 These individuals present with vertigo and imbalance aggravated by intense sound, Valsalva maneuvers, and other maneuvers that alter intracranial pressure. They describe autophony and sensitivity to bone-conducted sounds such as jaw movement, eye movement, and even movement in the joints of the extremities.2
Patients with superior semicircular canal dehiscence may display nystagmus with tragal pressure or pneumotoscopy. Audiological testing reveals supra threshold conductive hearing loss with decreased bone conduction thresholds and intact stapedius reflexes. This has been characterized as via an inner ear conductive hearing loss with air-conducted acoustic energy shunted away from the organ of Corti and decreased phase cancellation of bone-conducted acoustic energy.3 The hearing loss is typically low frequency in nature with greatest severity below 2000 Hz.4 Occasionally patients have associated encephaloceles and even more rarely they are at risk for otitic meningitis and brain abscess through the presence of a dehiscent middle fossa floor and direct communication between the mastoid and middle fossa.5, 6
Minor treated these patients with a MFR with resurfacing and/or plugging of the affected canal resulting in restoration of the normal flow of acoustic energy to the cochlea and resolution of symptoms.1, 7
As understanding of this syndrome has evolved so has its management. Decreased cervical vestibular evoked myogenic potential (cVEMP) thresholds were shown to increase specificity of diagnosis and have become the key diagnostic test for physiologic dehiscence with high resolution CT scan used to demonstrate anatomic dehiscence. The latter may overestimate physiologic dehiscence by a factor of 5–10.8 New options for surgical management arose including the TMR with cartilage capping of the affected canal and round window plugging.9, 10
Typical surgical management is the MFR with plugging of the dehiscent canal although some reports also describe resurfacing via a middle fossa approach. This approach typically allows direct visual confirmation of the dehiscence and its repair. This approach does involve a craniotomy with temporal lobe retraction adding risk of morbidity.11, 12 Also, while ablation of the affected canal may improve symptoms of canal dehiscence, this carries its own set of risks including sensorineural hearing loss and vestibular deficits. For this reason, some advocate cartilage capping of the affected canal via a TMR as a more conservative treatment with shorter hospital stay and less disruption of the inner ear especially for patients with bilateral disease.13
Currently there is variability in management without a consensus or even guidelines on which approach should be used for given patients. The purpose of this series is to review a volume of SSCD repair cases performed at a single institution comparing the TMR and the MFR. Specifically, we were interested in postoperative complications, length of hospital course, and effectiveness of treatment.
Methods
Between 2005 and 2014, 27 patients underwent surgical repair of symptomatic superior semicircular canal dehiscence. Retrospective chart review was performed with special attention to clinical presentation, demographics, imaging modalities, cVEMP thresholds, canal gain determined by vHIT, pre- and postoperative hearing testing, dehiscence characteristics, surgical technique, and postoperative course. Retrospective date collection and analysis was approved by the institutional IRB.
All symptoms and signs were recorded at both pre- and postoperative appointments by eliciting thorough history and comprehensive neurotologic examination. Audiometric testing (air and bone thresholds 250–8000 Hz), cVEMP testing was carried out at the pre- and postoperative visits. Change in air bone gap was evaluated by analyzing the pre- and postoperative air bone gap at 500 Hz. This frequency was picked to maximize the number of patients in whom a change could be recorded. vHIT testing was performed retrospectively on 10/14 patients treated with the middle fossa approach and 11/15 patient treated with transmastoid resurfacing. Symptoms were stratified as either chief complaint or associated symptoms. They were further typed as either auditory, vestibular, or both. Patients underwent axial CT scans with 0.6 mm slices that were reconstructed in the sagittal plane oblique to the superior canal using Osirix software. The dehiscence size was measured to the nearest 0.5 mm by drawing a line from one end of the dehiscence to the other. Comparison of pre- and postoperative cVEMP was performed where available. At our institution cVEMP responses <70 dB are considered abnormal.
cVEMPs were obtained by the following protocol. The patient's skin was prepared for the electrodes. After exfoliation of the skin on the forehead and neck, two electrodes were placed on the forehead. These serve as the ground and reference electrodes. The active electrode was placed over the midportion of the sternocleidomastoid muscle(SCM). The patient was reclined at a 45° angle. Stimulus was then presented to the ipsilateral ear. Tone bursts were presented at 90 dB HL. They were decreased at 10 dB HL increments until the p13, n23 wave tracing on the ipsilateral SCM is lost. At this point bursts were increased 5 dB HL. The threshold is defined in dB HL as the least intense tone burst that resulted in the presence of a p13, n23 biphasic waveform in the ipsilateral SCM. Seventy dB HL was the lower limit of normal.
Video head impulse test (vHIT): vHIT testing allows analysis of individual semicircular canal function. Testing was carried out using a commercially available unit (ICS impulse, Otometrics Inc.). To assess each of the canal planes the subject's head was positioned in a way so that the delivery of the head impulse is coplanar to the paired canals in question. In the case of the left and right anterior/posterior planes, the head was tilted ∼45° to the right and left respectively for the delivery of head shakes in the pitch plane. VOR gains for the all canals on the operative side were recorded at a separate postoperative visit.
Success of repair was stratified into four categories: complete, moderate, mild, and failed repairs. Complete success was defined as complete resolution of all auditory and vestibular symptoms. Moderate success was achieved if patients had resolution of their preoperative chief complaint but some minor residual symptoms. Mild success was defined as improvement in symptoms such that patients feel subjectively better than before surgery but still are symptomatic, chief complaint not adequately addressed. Failure was defined as residual symptoms with subjective feeling of the same or worse as the preoperative state. Hospital stay, complications, and residual symptoms were also compared among the different repair types.
Repair
Patients underwent either transmastoid or middle fossa craniotomy based on surgeon preference. One author prefers exclusively middle fossa craniotomy while another has exclusively performed the transmastoid approach since adopting the technique in 2010 prior to which he performed middle fossa based repairs.
Transmastoid approach to the superior canal
Patients were given a dose of intravenous cefazolin for skin incision prophylaxis. One g/kg mannitol and 10 mg dexamethasone were given to decrease intracranial pressure. A post auricular incision was made followed by a musculoperiosteal incision. Mastoidectomy was then performed with skeletonization of the tegmen, sigmoid sinus, and horizontal semicircular canal. Drilling medial and superior to the lateral semicircular canal but below the tegmen allowed identification of the superior semicircular canal. The tegmen lateral to the superior semicircular canal was removed gently with a diamond drill. The dura over the temporal lobe was carefully elevated away from the tegmen over the superior semicircular canal through the mastoid. The dehiscence was then resurfaced (see below).
Middle fossa approach to the superior canal
After induction of general anesthesia, the patients were treated with intravenous cefazolin for skin incision prophylaxis. One g/kg mannitol and 10 mg dexamethasone were given to decrease intracranial pressure. After the temporalis muscle was identified and reflected antero-inferiorly and the root of the zygoma was identified. A craniotomy flap at least 3.5 cm × 3.5 cm was created at least 2/3 anterior to the level of the external auditory canal was developed and removed, exposing the temporal lobe dura. The temporal lobe dura is elevated off the floor of the middle cranial fossa. The position of the superior canal was identified in or near the area of the arcuate eminence. The dehiscence repaired (see below). The temporal lobe was then allowed to relax back into position, typically over a small piece of dural substitute. Craniotomy flap was replaced and secured using titanium miniplates.
Methods of dehiscence repair
After localization of the dehiscence, repair was carried out with one of the following. In all cases of middle fossa surgeries, the entire dehiscence was visualized and packed with bone wax. The middle fossa was then resurfaced with Stryker bone source™(hydroxyapatite), to cover the plugged canal and repair any air cells that were open into the middle ear/mastoid.
For transmastoid repairs a tragal cartilage graft was harvested. The cartilage was carved into a trapezoid with the shorter base of the trapezoid fashioned to fit over the SSC as described by Lundy.14 The wider portion of the graft was designed to be slightly wider than the exposed portion of dura to prevent intracranial displacement. The narrower (8 mm wide) portion of the graft was placed over the superior canal, and the temporal lobe was allowed to relax over the graft. The wider portion of the graft remained within the mastoid cavity. It was then secured in place with bone cement as part of the reconstruction of the tegmen.
Results
Preoperative analysis
There were a total of 29 ears included in the review. There were 18 female ears and 11 male ears. The median age was 48 years with a range of 37–61 years. Patients typically presented with multiple auditory and vestibular symptoms. The most common chief complaint was vertigo in 59% of patients. Vertigo was aggravated by Valsalva maneuver in 22% of patients. It was aggravated by sound, nose blowing, or external ear manipulation in another 22% of patients. Chronic disequilibrium was also a common chief complaint. The chief complaint was purely auditory in 15% and purely vestibular in 55% of patients. The remaining 30% had mixed hearing and balance chief complaints.
Most commonly patients carried a diagnosis of Meniere's disease or otosclerosis upon initial presentation. This was true in 11% and 7% of patients, respectively. Other prior diagnoses included vestibular migraines, mal de debarquement syndrome, head trauma, vascular loop syndrome, and familial cerebellar ataxia.
CT scanning was positive for at minimum unilateral dehiscence in all patients. An additional 55% of patients had bilateral dehiscence. The average length of dehiscence was 4.2 mm, ranging from 1.5 mm to 6.5 mm. The average dehiscence sizes for complete, moderate, and success were 4.6 ± 2.0, 3.1 ± 1.8, and 2.0 ± 1.5 mm, respectively. Failed repairs had an average dehiscence of 2.1 ± 1.0 mm.
Evaluation of pre- and postoperative hearing: 9/15 and 10/14 patients showed a preoperative low frequency air bone gap. The average change in the air bone gap at 500 Hz for the MFR group was 6.2 ± 2.0 dB and for the TMR group 3.4 ± 2.0 dB (P = 0.94, Mann–Whitney U test). There were no incidences of sensorineural hearing loss in either group.
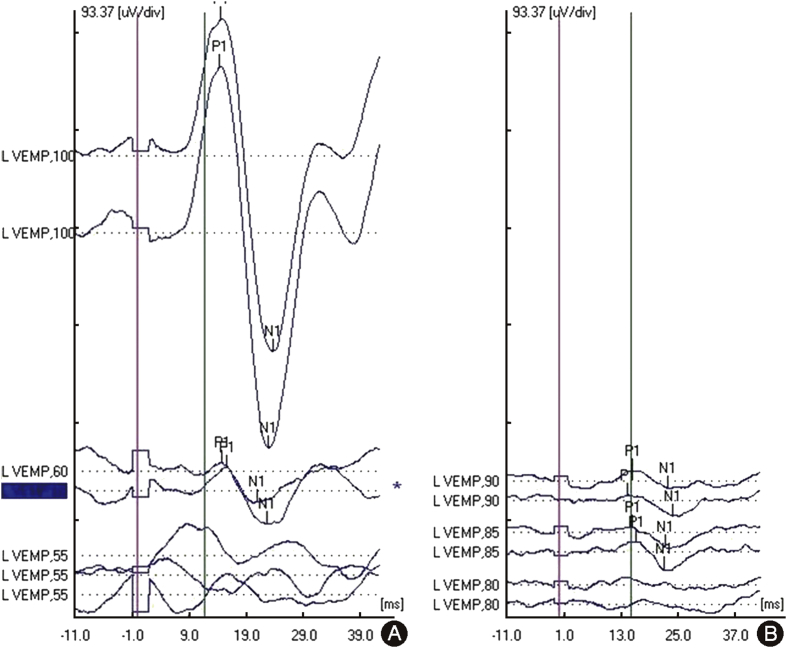
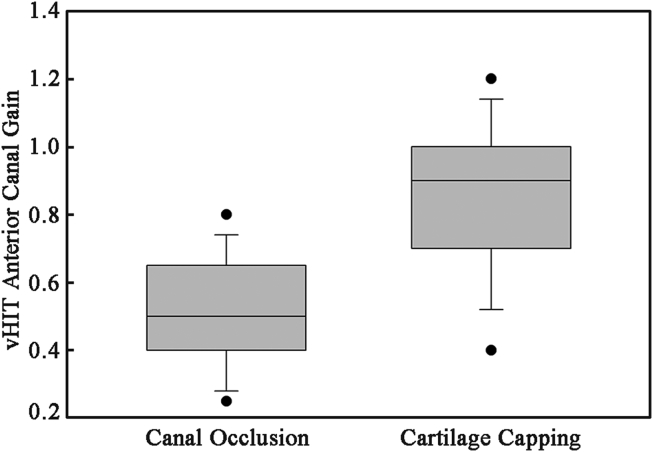
Vestibular test results: Average cVEMP threshold in this patient population was 70.4 dB ranging from 55 dB to 80 dB. Additionally, we had 1 patient with absent cVEMP. Average thresholds shifted 18 dB with surgical correction in the MFR group. A 29 dB average shift was seen in the TMR group. Postoperative cVEMPs averaged 87.3 ± 3.0 dB and 89.1 ± 2.0 dB (P = 0.17). Fig. 1 shows a patient's preoperative and postoperative cVEMP testing from the TMR group. The average gain of the superior canal for the canal occlusion group was 0.52 ± 0.12 and for the TMR group was 0.92 ± 0.23 (P < 0.01, Mann–Whitney U test) (Fig. 2). The average gains for the ipsilateral horizontal and posterior canals were within normal limits for both the MFR (0.88 ± 0.03, 0.86 ± 0.42) and the TMR (0.88 ± 0.16, 1.12 ± 0.71) groups.
Fig. 1.
Postoperative cVEMP threshold normalization. TMR technique results in similar postoperative cVEMP threshold normalization above 80 dB HL as MFR technique.
Fig. 2.
Box plot of postoperative superior canal gain as measured by vHIT. Patients treated with canal occlusion averaged lower VOR gain in the treated canal. This was not reflected in patient symptoms.
Postoperative analysis
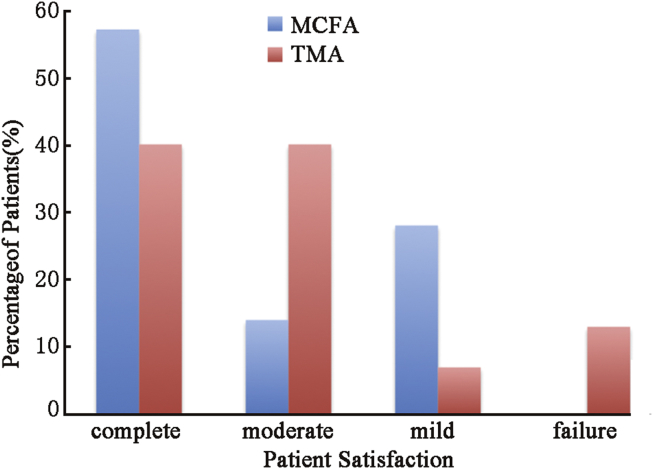
Success of repair was judged by degree of resolution of symptoms. TMR cartilage capping resulted in 80% of patients experiencing complete (40%) or moderate (40%) success. MFR ablation resulted in 71% of patients experiencing complete (57%) or moderate (14%) success. The rate at which patients experience satisfactory resolution (complete + moderate success rates) of their chief complaint in our series was 76% (22/29 ears) by all methods. Fig. 3 illustrates each success rate for different techniques.
Fig. 3.
Success rate for each technique. MFR group experienced no failures and more complete successes. Eighty percent of TMR patients experienced either complete or moderate success.
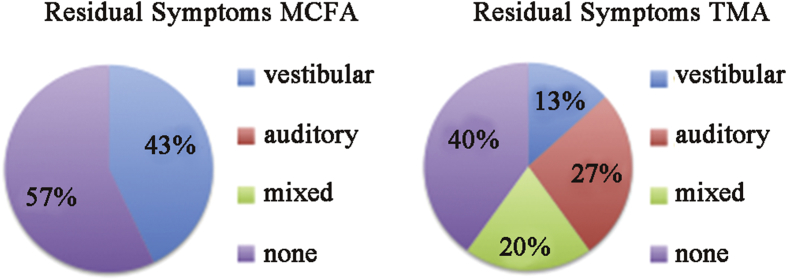
The most common residual symptoms after the TMR and the MFR were aural fullness/autophony and chronic disequilibrium, respectively. Moreover, 47% of patients in the TMR cartilage capping group had symptoms classified as auditory compared to 0% in the MFR ablation group. Fig. 4 shows the relative incidence residual symptoms categorized as auditory of vestibular.
Fig. 4.
Residual symptoms. MFR patients experienced only residual vestibular symptoms. Many had no residual symptoms. The TMR group commonly experienced residual auditory symptoms.
The most common postoperative complication was positional nystagmus, due to canalithiasis or cupulolithiasis in 14% and 7%, respectively. There were no cases of stroke, temporal lobe hemorrhage, CSF leak, meningitis, facial nerve injury, or sensorineural hearing loss in this series. One patient in the TMR group struggled with prolonged postoperative vertigo resulting in a 6-day postoperative course requiring intravenous fluids, scheduled benzodiazepines, and corticosteroids. Mobility was severely limited requiring extensive work with the physical and occupational therapy team. A second patient in the TMR group required a revision TMR plugging procedure after recurrence of symptoms associated with head trauma. Repeat CT scanning revealed displacement of the cartilage cap. The mean length of stay varied for the MFR ablation and TMR cartilage capping groups was 3.29 ± 0.76 and 1.87 ± 1.40 days (P < 0.05, Mann–Whitney U test).
Discussion
Understanding of superior semicircular canal dehiscence syndrome has continued to evolve since its original description. Innovative surgeons have introduced new techniques aimed at decreasing morbidity and increasing efficacy of treatment. One such technique is the TMR cartilage capping technique.14 As previously discussed this approach has the advantages of avoiding temporal lobe retraction and ablation of vestibular function. Disadvantages may include residual symptoms. The goal of reporting our cases is to address these advantages and disadvantages as well as gain further understanding of canal dehiscence syndrome.
In our experience, the most common preoperative symptom was vertigo. Other common symptoms included chronic disequilibrium, aural fullness, and Tullio phenomenon. The symptoms profile and their incidence are similar to previous studies.2 Other than Tullio phenomenon, these are all nonspecific findings that may lead physicians to assign another diagnosis. The most common prior diagnosis was Meniere's disease that was refractory to medications and surgical procedures such as endolymphatic sac decompressions. In these cases, the key distinguishing symptoms were conductive hearing loss, autophony, and hypersensitive bone conduction of joint/eye movements. These symptoms were also crucial in distinguishing canal dehiscence from vestibular migraines. Another common alternative diagnosis was otosclerosis that led to middle ear explorations without abnormality. Acoustic reflexes allowed for distinguishing the true diagnosis in these cases. As previously shown, SSCD is often demonstrated on CT scans of patients previously undergoing surgery for otosclerosis and is a contraindication to stapedectomy.15 In a series of patients undergoing exploratory tympanotomy for conductive hearing loss with normal ear exams, 12% had inner ear abnormalities and 4% had SSCD. None of these patients experienced improvement in hearing.16 Rarer alternative diagnoses were mal de debarquement (MDD) syndrome, vascular loop syndrome, and laxity of the oval and round window membranes. As one might expect, patients with incorrect diagnoses of otosclerosis, Meniere's disease, and vestibular migraine all experienced complete or moderate improvement after recognition and repair of SSCD syndrome. Comparatively, patients who had true diagnoses of MDD or familial cerebellar ataxia with concurrent canal dehiscence rarely experienced moderate improvement, and more often experienced mild improvement or failed procedures.
cVEMP threshold shift was examined as an objective measure of surgical correction. The average shift in threshold was larger in the TMR group. The physiologic significance of this finding is unclear. The TMR starting threshold for the TMR group was much lower on average. Both groups had postoperative averages above the normal value consistent with physiologic correction of the defect and were not statistically different. While canal plugging has previously been shown very effective in cVEMP threshold normalization,17 our study shows transmastoid cartilage capping also results in normalization of cVEMP threshold. There was no significant difference in the change in air bone gap at 500 Hz when comparing the two groups. Postoperative measures of the VOR gain of the repaired canal (Fig. 2) showed that canal occlusion reduced the average gain of the treated canal compared to the TMR procedure. Future studies will evaluate bilaterally treated patients over time to see if there is a difference in patient outcomes that would influence our surgical approach.
As previously outlined, success of surgery was determined in subjective manner based on patient satisfaction and residual symptoms at postoperative visits. For the TMR group satisfaction rates are similar to previous series on cartilage capping.14 For the MFR group, satisfaction rates are lower than rates reported by Minor.7 In our series, TMR patients have a significant rate of residual symptoms that have not been characterized by previous studies.
The residual symptoms experienced by TMR patients are most commonly auditory or mixed in nature while those in MFR patients are fewer and more commonly vestibular in nature. Moreover, the main residual vestibular symptom in the TMR group is the Tullio phenomenon. Residual symptoms in the TMR group tend to be due to vestibulofugal flow of acoustic energy and those in the MFR group tend to be from vestibulopetal flow of barometric energy. We suspect that after TMR cartilage capping acoustic energy no longer flows through the third window between the canal and the middle cranial fossa but still flows from the canal to the intracranial space resulting in increased bone conduction and Tullio phenomenon. The two-way flow of energy may be reduced to one-way flow in these patients with residual auditory symptoms. Residual symptoms in the MFR group can be explained by persistent flow of energy from middle fossa through the incompletely closed third window into the superior canal resulting in vertigo with Valsalva maneuvers and chronic disequilibrium. The most common symptoms after MFR were chronic disequilibrium. This symptom often occurred despite normalization of cVEMP threshold. There are reports of transformation of peripheral end-organ dizziness to psychogenic dizziness after surgical repair.18
While the MFR ablation technique has the advantage of fewer residual symptoms than the TMR cartilage capping, patients did experience a longer ICU stay and overall length of stay after undergoing a craniotomy. Although our series suggests this is negligible when compared to TMR cartilage capping length of stay (approximately 2 versus 3 days), a closer look at our results suggests that most TMR patients only stay overnight with 2 outlier patients, both requiring revision surgeries staying 3–6 nights over their 2 admissions. This was due to postoperative vertigo/disequilibrium necessitating vestibular suppression and physical therapy. When these patients taken into account the mean LOS for the TMR cartilage capping group is 1.2 days. TMR cartilage capping can even be done as an outpatient procedure as demonstrated by Deschenes.19
The size of dehiscence showed an inverse correlation to the rate of success. We suspect this finding is explained by two phenomena. First, patients with large third windows experience more severe preoperative symptoms. Closure of these defects result in relatively larger improvement in symptom detection by patients. Second, larger defects are more apparent at the time of surgery and therefore more likely to be comprehensively repaired.
The major limitation of this series is the subjective measure of success of therapy. Ideally, future studies seeking to compare management of SSCD patients should use outcome measures less prone to bias. Standardized measures of success could be obtained by use of dizziness handicap inventory before and after every intervention. This has been achieved for MFR with plugging but not for TMR cartilage capping.20 cVEMPs before and after each intervention would be useful for study purposes. Audiograms should be obtained after procedures as canal plugging may cause threshold shifts in hearing. Video head impulse testing may also prove to be a useful marker of objective success pre- and postoperatively as well.
Our series is also limited by small size, making subset analysis difficult. For example, when comparing patient satisfaction for different sizes of dehiscences, it would be interesting to look at these trends for each technique. Due to the small number of patients in this series, data from both surgical groups was pooled to exam the relationship between size of dehiscence and patient satisfaction.
In addition, the use of length of dehiscence as a surrogate measure for size of dehiscence limits the accuracy of dehiscence size.
Ideally, patients could be grouped according to specific dehiscence characteristics including total area, location, and shape. Efficacies of each technique for a given dehiscence characteristic could then be quantified. For example, the TMR is relatively blind especially when addressing medial defects of the superior canal. Perhaps grouping patients based on dehiscence characteristics would allow for preferred approach recommendations to be made for arcuate eminence dehiscences versus medial superior petrosal sinus dehiscences. It would also be interesting to stratify patients into groups based on preoperative symptoms to see if there were preferred methods of repair based on preoperative symptoms.
Finally, we are comparing MFR plugging to TMR cartilage capping. The ideal study would also include TMR plugging, as both the approach and the repair are ultimately factors in patient satisfaction. Perhaps TMR plugging is a more definitive approach than TMR capping. Because the capping technique is done with a tragal cartilage graft including perichondrium, there may be some degree of partial canal occlusion. It would be interesting to evaluate TMR plugging versus capping not only in efficacy, but also with regard to postoperative hearing threshold shifts and fluid signal on MRI.
Conclusion
Surgical management of SSCD is currently evolving. The MFR and TMR can be combined with capping, resurfacing, and plugging in a variety of ways resulting in individual plans for each patient. Resurfacing and capping of the dehiscences prevents ablation of vestibular function associated with plugging of the dehiscence. This can be effectively accomplished using tragal cartilage graft. Although a reasonably efficacious approach, some residual symptoms are to be expected. In our experience, these symptoms are likely related to residual third window phenomena between the semicircular canal and the intracranial cavity. Our series indicates that while MFR ablation is useful for patients with primarily vestibular chief complaints and TMR cartilage capping is useful for patients with primarily auditory chief complaints, both have disadvantages and neither is particularly suited to every patient. While an ideal approach, which is minimally invasive while comprehensively effective for canal dehiscence patients, does not exist, we believe our data useful in tailoring management based on patient symptoms. Additionally, our series highlights the need for further studies on SSCD repair.
Edited by Xin Jin
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Minor L.B., Solomon D., Zinreich J.S., Zee D.S. Sound- and/or pressure-induced vertigo due to bone dehiscence of the superior semicircular canal. Arch Otolaryngol Head Neck Surg. 1998;124:249–258. doi: 10.1001/archotol.124.3.249. [DOI] [PubMed] [Google Scholar]
- 2.Chi F.L., Ren D.D., Dai C.F. Variety of audiologic manifestations in patients with superior semicircular canal dehiscence. Otol Neurotol. 2010;31:2–10. doi: 10.1097/mao.0b013e3181bc35ce. [DOI] [PubMed] [Google Scholar]
- 3.Merchant S.N., Rosowski J.J. Conductive hearing loss caused by third-window lesions of the inner ear. Otol Neurotol. 2008;29:282–289. doi: 10.1097/mao.0b013e318161ab24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McEvoy T.P., Mikulec A.A., Armbrecht E.S., Lowe M.E. Quantification of hearing loss associated with superior semi-circular canal dehiscence. Am J Otolaryngol. 2013;34:345–349. doi: 10.1016/j.amjoto.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Manara R., Lionello M., de Filippis C., Citton V., Staffieri A., Marioni G. Superior semicircular canal dehiscence: a possible pathway for intracranial spread of infection. Am J Otolaryngol. 2012;33:263–265. doi: 10.1016/j.amjoto.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Lim Z.M., Friedland P.L., Boeddinghaus R., Thompson A., Rodrigues S.J., Atlas M. Otitic meningitis, superior semicircular canal dehiscence, and encephalocele: a case series. Otol Neurotol. 2012;33:610–612. doi: 10.1097/MAO.0b013e3182536de7. [DOI] [PubMed] [Google Scholar]
- 7.Minor L.B. Clinical manifestations of superior semicircular canal dehiscence. Laryngoscope. 2005;115:1717–1727. doi: 10.1097/01.mlg.0000178324.55729.b7. [DOI] [PubMed] [Google Scholar]
- 8.Masaki Y. The prevalence of superior canal dehiscence syndrome as assessed by temporal bone computed tomography imaging. Acta Otolaryngol. 2011;131:258–262. doi: 10.3109/00016489.2010.526145. [DOI] [PubMed] [Google Scholar]
- 9.Silverstein H., Van Ess M.J. Complete round window niche occlusion for superior semicircular canal dehiscence syndrome: a minimally invasive approach. Ear Nose Throat J. 2009;88:1042–1056. [PubMed] [Google Scholar]
- 10.Fiorino F., Barbieri F., Pizzini F.B., Beltramello A. A dehiscent superior semicircular canal may be plugged and resurfaced via the transmastoid route. Otol Neurotol. 2010;31:136–139. doi: 10.1097/MAO.0b013e3181b76b9e. [DOI] [PubMed] [Google Scholar]
- 11.Glasscock M.E., 3rd, Hughes G.B., Davis W.E., Jackson C.G. Labyrinthectomy versus middle fossa vestibular nerve section in Menière's disease. A critical evaluation of relief of vertigo. Ann Otol Rhinol Laryngol. 1980;89:318–324. doi: 10.1177/000348948008900405. [DOI] [PubMed] [Google Scholar]
- 12.McElveen J.T., Jr., House J.W., Hitselberger W.E., Brackmann D.E. Retrolabyrinthine vestibular nerve section: a viable alternative to the middle fossa approach. Otolaryngol Head Neck Surg. 1984;92:136–140. doi: 10.1177/019459988409200203. [DOI] [PubMed] [Google Scholar]
- 13.Amoodi H.A., Makki F.M., McNeil M., Bance M. Transmastoid resurfacing of superior semicircular canal dehiscence. Laryngoscope. 2011;121:1117–1123. [Google Scholar]
- 14.Lundy L., Zapala D., Moushey J. Cartilage cap occlusion technique for dehiscent superior semicircular canals. Otol Neurotol. 2011;32:1281–1284. doi: 10.1097/MAO.0b013e31822e5b27. [DOI] [PubMed] [Google Scholar]
- 15.Whetstone J., Nguyen A., Nguyen-Huynh A., Hamilton B.E. Surgical and clinical confirmation of temporal bone CT findings in patients with otosclerosis with failed stapes surgery. AJNR Am J Neuroradiol. 2014;35:1195–1201. doi: 10.3174/ajnr.A3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim S.C., Lee W.S., Kim M., Jeon J.H., Kim J., Choi J.Y. Third windows as a cause of failure in hearing gain after exploratory tympanotomy. Otolaryngol Head Neck Surg. 2011;145:303–308. doi: 10.1177/0194599811403076. [DOI] [PubMed] [Google Scholar]
- 17.Rinaldi V., Portmann D. Vestibular-evoked myogenic potentials after superior semicircular canal obliteration. Rev Laryngol Otol Rhinol (Bord) 2011;132:85–87. [PubMed] [Google Scholar]
- 18.McCaslin D.L., Jacobson G.P., Burrows H.L., Littlefield P., Haynes D.S. Transforming superior canal dehiscence to chronic subjective dizziness: from SCD to CSD. J Am Acad Audiol. 2010;21:293–300. doi: 10.3766/jaaa.21.5.2. [DOI] [PubMed] [Google Scholar]
- 19.Deschenes G.R., Hsu D.P., Megerian C.A. Outpatient repair of superior semicircular canal dehiscence via the transmastoid approach. Laryngoscope. 2009;119:1765–1769. doi: 10.1002/lary.20543. [DOI] [PubMed] [Google Scholar]
- 20.Crane B.T., Minor L.B., Carey J.P. Superior canal dehiscence plugging reduces dizziness handicap. Laryngoscope. 2008;118:1809–1813. doi: 10.1097/MLG.0b013e31817f18fa. [DOI] [PubMed] [Google Scholar]